Abstract
INTRODUCTION:
The timing of prophylactic colorectal surgery in patients with familial adenomatous polyposis (FAP) is based on the immediacy of the colorectal cancer risk. The ability to predict the need for surgery may help patients and their families plan in the context of life events and CRC risk. We created a model to predict the likelihood of surgery within 2 and 5 years of first colonoscopy at our institution.
METHODS:
A single institution hereditary colorectal syndrome (CologeneTM) database was interrogated for all patients with FAP having a deleterious APC mutation. Patients with first colonoscopy after age 30 and before year 2000 were excluded. Cox regression analysis was done to assess multiple factors associated with surgery, followed by stepwise Cox regression analysis to select an optimal model. Receiver operator curve (ROC) analysis was performed to assess the model.
RESULTS:
A total of 211 (53% female) patients were included. Forty‐five percent underwent surgery after an average of 3.8 years of surveillance. The final model was created based on initial clinical characteristics (age, gender, BMI, family history of desmoids, genotype‐phenotype correlation), initial colonoscopic characteristics (number of polyps, polyp size, presence of high‐grade dysplasia); and on clinical events (chemoprevention and polypectomy). AUC was 0.87 and 0.84 to predict surgery within 2 and 5 years, respectively. The final model can be accessed at this website: http://app.calculoid.com/#/calculator/29638.
CONCLUSION:
This web‐based tool allows clinicians to stratify patients' likelihood of colorectal surgery within 2 and 5 years of their initial examination, based on clinical and endoscopic features, and using the philosophy of care guiding practice at this institution.
INTRODUCTION
Familial adenomatous polyposis (FAP) is a rare condition with a prevalence rate of ˜5 per 100,000 [1]. It is caused by a germ line mutation in the tumor suppressor gene APC situated on chromosome 5q21, and characterized by the early emergence of numerous colorectal adenomas [2]. Colorectal cancer (CRC) is almost inevitable in the untreated patient, and 60% of patients presenting with symptoms present with a cancer [1]. The average age of diagnosis of CRC is 39 years, and according to Jarvinen et al. [3], 93% of patients with classical FAP were diagnosed with CRC by age 50. Average life expectancy in patients with CRC in Jarvinen's study was 2.6 years, with an average age at death of 42 years. However, in patients who undergo colonoscopic surveillance and subsequent prophylactic surgery, cancer rates are very low and survival is equal to that of the general population [4].
Management of asymptomatic patients who present with FAP due to genetic diagnosis and screening colonoscopy is aimed primarily at preventing the development of cancer. A second priority is maintaining quality of life. Most of these patients are young, as colonoscopic surveillance begins at puberty, and major abdominal surgery would be a significant event in their lives and the lives of family members. Decisions relating to the timing and type of surgery must be made carefully and with due consideration of the implications of the decisions.
In order to help physicians with the timing of surgery over the course of surveillance, the American College of Gastroenterology (ACG) has endorsed guidelines stating that surgery is absolutely indicated for FAP patients with CRC or colorectal symptoms. Relative indications for prophylactic surgery include: multiple adenomas >6 mm, an adenoma with high‐grade dysplasia (HGD), inability to adequately survey the colon, and a significant increase in the number of adenomas [5]. A scoring system has been developed based on polyp number, degree of dysplasia, and size of polyps that can be used to suggest an intervention [6]. While these tools can help with the decision to offer surgery, there is no predictive tool that allows an estimation of the likely time that surgery will need to be done. Such a tool could help with life and financial decisions within the affected family and may even help compliance with surveillance. In this study, we develop and present a web‐based scoring system that can help predict the likelihood of surgery within 2 and 5 years in patients with newly diagnosed FAP.
METHODS
The Sanford R. Weiss, MD, Center for Hereditary Colorectal Neoplasia at the Cleveland Clinic maintains an Institutional Review Board‐approved database (CologeneTM). This database, which exists within the David G. Jagelman Inherited Colorectal Cancer Registry, was queried to identify FAP patients <30 years of age at the time of first colonoscopy. All patients enrolled in the registry sign an informed consent, which allows their anonymized data to be used for research. The registry is approved by the Institutional Review Board of the Cleveland Clinic yearly.
Because the goal of the analysis was to study contemporary surgical practices, patients who had their first colonoscopy before the year 2000 were excluded. Patients who were part of randomized clinical trials examining efficacy of chemo‐preventive agents were also excluded. Patients who did not have a personal or family APC mutation were excluded from the analysis. Data obtained from the database were verified by two authors (S.S. and M.M.) through review of individual patients' electronic medical record (EpicTM).
Baseline Patient Characteristics
Baseline demographic characteristics were summarized. Information about family history of cancers, desmoids, and FAP were obtained from the database and independently verified in the patient electronic medical record. Patients using sulindac, celecoxib, or other non‐steroidal anti‐inflammatory drugs (NSAIDs) for >3 months were considered as being exposed to chemo‐preventive medications. Genetic mutation information was entered into National Center for Biotechnology Information's Clinvar tool [7] or the International Society of Gastrointestinal Hereditary Tumors' (InSiGHT's) Leiden Open Variation Database (LOVD) [8] to obtain information about mutated codon location in the APC gene and type of mutation in those with point mutations (frameshift, missense, nonsense, splice site mutation). Information about de novo inheritance was obtained. A de novo mutation was based on either negative APC testing or lack of FAP phenotype (>10 adenomas, CRC, or need for colectomy by the age of 40) in parents.
Genotype‐phenotype correlations (GPC‐Loc) were made based on the location of the mutated APC codon as previously described by Nieuwenhuis et al. [9]. Codons 1‐157, alternatively spliced 9th exon, and 1596 to end were associated with attenuated phenotype; codons 158‐1249 and 1465‐1595 excluding alternatively spliced 9th exon were associated with intermediate (classical) phenotype; and finally, codons 1250‐1464 were associated with severe (profuse) phenotype. Large deletions spanning multiple exons or whole gene were coded separately.
The patients' first colonoscopy report was reviewed to identify the number of polyps present (grouped into 0‐20, 21‐99, or 100+ polyps), the site of the colon where polyps were most prominent, the number of rectal polyps, the presence of polyps >9 mm, and the percent of polyps resected during the colonoscopy. Polyp prominence was defined according to which segment of the colon had a majority of the polyps: right colon predominant, left colon predominant, or no predominance (equal left and right). Path ology reports were used to identify presence of HGD.
Surgical Indications
Information about date of first surgery, type of first surgery—colectomy with ileorectal anastomosis (IRA), total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA), and TPC with end ileostomy (TPC‐EI)—and indication for surgery were obtained from operative notes and progress notes. Colonoscopic data were used to corroborate the indication listed in the database. Frequency of each surgical indication was noted and divided by total number of patients who underwent surgery to obtain percentage of patients who underwent surgery for that indication. Frequencies of patients who underwent colectomy with IRA and TPC with IPAA were compared using χ2‐analysis in an omnibus and pair‐wise manner.
Time to Surgery and Modeling
In making these calculations, time to surgery was defined as the time from the first colonoscopy to the time of the first prophylactic colorectal surgery. In patients who did not undergo surgery, the censor point was chosen as the time of last colonoscopy. Univariate Cox regression analysis was done to assess the factors associated with surgery. Kaplan‐Meier (KM) curve was constructed to demonstrate time to surgery for all included patients along with life‐tables showing cumulative incidence with SE. KM curves were also constructed to demonstrate differences in time to surgery based on factors found to be significant (p < 0.05) on univariate analysis and by gender. All initial/baseline variables were considered for inclusion in the model and an automated forward stepwise variable selection method performed to choose the final model. To the best of our knowledge, we did not have any missing variables in our data. Multivariate Cox regression analysis was performed for variables chosen for the final model. For factors found to be significant at p < 0.05 in this final model, regression coefficients were used to construct a likelihood score for all individual patients. The regression equations were used to build a web‐based model: https://app.calculoid.com/#/calculator/29638.
Receiver operator curve (ROC) analysis was performed to assess the predictive ability of likelihood score from the final model to predict surgery within 2 and 5 years from the time of first colonoscopy. Internal validation of the final multivariate model was achieved using bootstrap resampling approach with 1000 replications. After likelihood scores of patients were ranked in an ascending order, patients were divided into four quartiles (>0‐25th percentile, 26th‐50th percentile, 51st‐75th percentile, and 76th‐>99th percentile). KM curve was created showing time to surgery during a 5‐year span differentiated by likelihood group assignment. In addition, the cumulative incidence of surgery over the 5‐year interval was calculated for each of the quartile‐based groups along with positive likelihood ratio of surgery within 2 and 5 years, respectively. All statistical analyses were conducted using Stata SE, version 15.0 (StataCorp, College Station, Texas, USA).
RESULTS
Baseline Characteristics of Included Patients
Two hundred and eleven patients representing a total of 611.3 patient‐years of surveillance were included for analysis. The mean age at first colonoscopy was 16.2 years, and 52.6% of patients were female. Forty‐five percent of the patients underwent surgery (22.3% IRA; 19.4% IPAA; and 3.3% TPC‐EI) at a mean age of 20.1 years. Thirty‐five (16.6%) patients were exposed to chemopreventive medications for >3 months: sulindac was prescribed in 20 (9.4%) patients, celecoxib in 14 (6.6%) patients, and other NSAIDs in 3 (1.4%) patients (these are not mutually exclusive categories). Colorectal cancer was diagnosed in 8 patients (3.8%), and 7 of these were diagnosed during baseline colonoscopy. One 29‐year‐old patient was a diagnosed with a stage IIIA rectal cancer. This was identified on a subsequent (second) colonoscopy performed almost 1 year after the initial colonoscopy. Other clinical and endoscopic characteristics of these patients are noted in Table 1. The Kaplan‐Meier curve demonstrating time to surgery, along with corresponding life‐table is shown in Fig. 1. The majority of the surgeries occurred within the first 5 years of initial colonoscopy.
Table 1.
Basic characteristics of all included patients
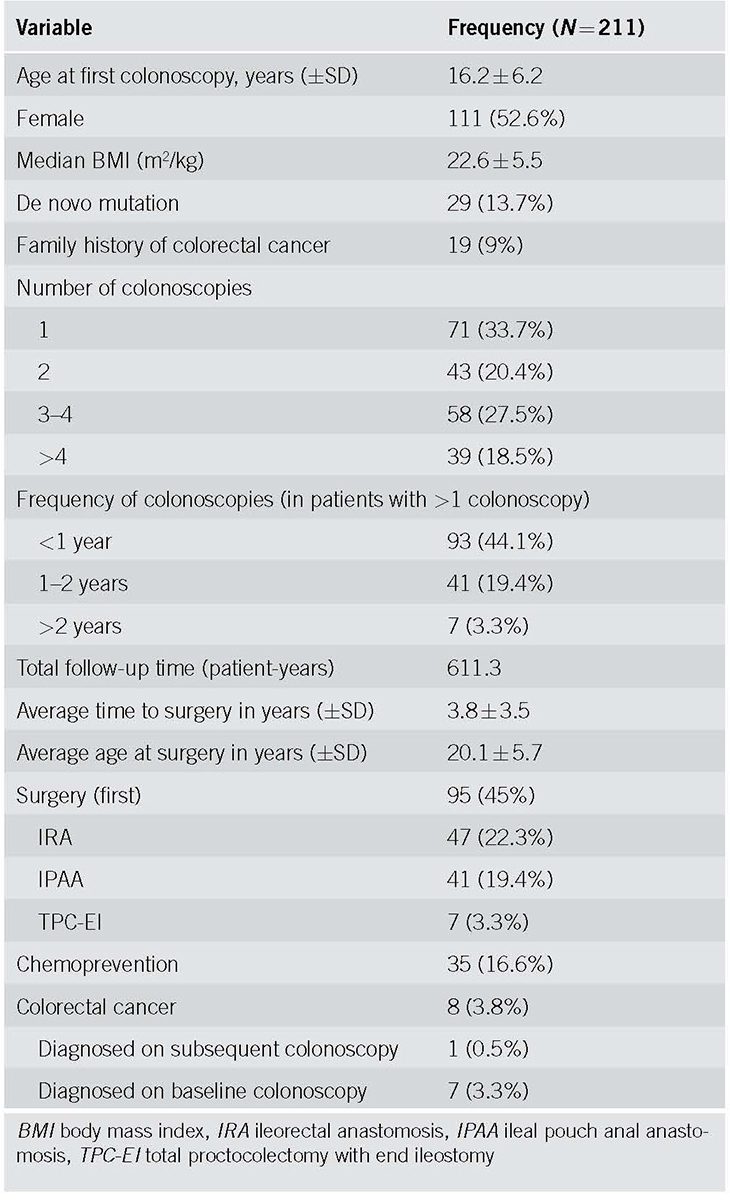
Fig. 1.
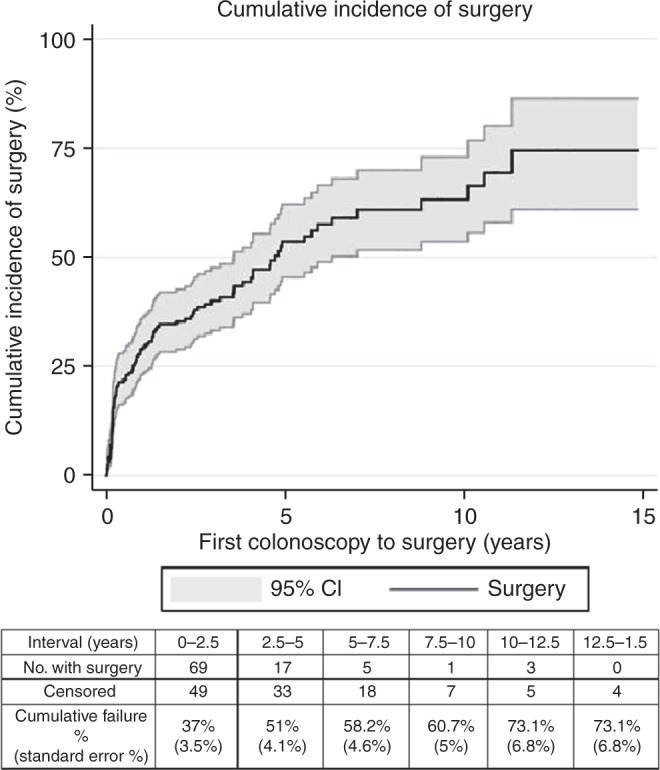
Time to surgery for all included patients along with 95% CI. CI confidence interval
Univariate Analysis of Factors Associated with Surgery
Unadjusted Cox regression analysis demonstrated multiple factors associated with surgery as summarized in Table 2. For every 1‐year increase in the age since the first colonoscopy, the likelihood of surgery increased by 9% (95% confidence interval (CI), 6‐10%; p = <0.001). For every 1 kg/m2 increase in body mass index at the time of first colonoscopy, the likelihood of surgery increased by 10% (95% CI, 7‐15%; p = <0.001). Compared to individuals with an attenuated FAP genotype (per GPC‐Loc), those with an intermediate genotype (hazard ratio (HR) = 2.3 (1.1, 4.7); p = 0.022), severe genotype (HR = 2.7 (1.3, 5.8); p = 0.009), and with large APC deletions (HR = 3.2 (1.3, 7.4); p = 0.008) had a shorter time to surgery. While patients without a family history of FAP had an increased likelihood of surgery (HR = 2.0 (1.3, 3.1); p = 0.001), those who were on chemoprevention medications had a significantly decreased likelihood of surgery (HR = 0.11 (0.04, 0.27); p = <0.001).
Table 2.
Analysis of factors associated with surgery
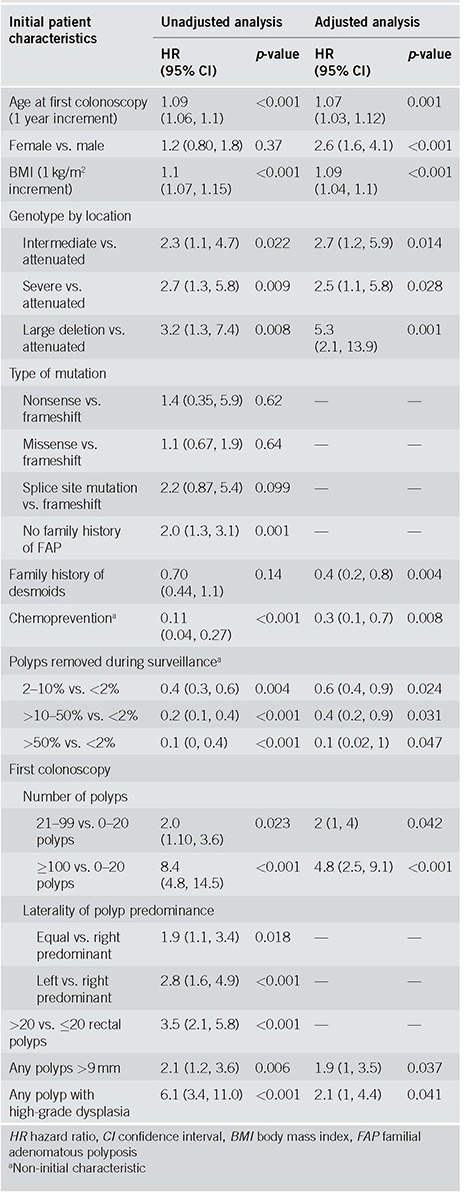
With regard to findings on initial colonoscopy, compared to patients with 0‐20 polyps, patients with 21‐99 polyps (HR = 2.0 (1.10, 3.6); p = 0.023) and ≥100 polyps (HR = 8.4 (4.8, 14.5); p = <0.001) had a higher likelihood of surgery. The segment of the colon with the predominant polyp burden also had an impact on surgical timing: patients with predominant burden on the left side (HR = 2.8 (1.6, 4.9); p = <0.001) or equal polyp burden on both sides of the colon (HR = 1.9 (1.1, 3.4); p = 0.018) were associated with a shorter time to surgery compared to those with predominantly right‐sided polyp burden. Patients with >20 rectal polyps (HR = 3.5 (2.1, 5.8); p = <0.001), any polyps >9 mm (HR = 2.1 (1.2, 3.6); p = 0.006), and polyps with HGD (HR = 6.1 (3.4, 11.0); p = <0.001) had an increased likelihood of surgery. Patients with removal of an aggregate of 2‐10% of polyps (HR = 0.4 (0.3, 0.6); p = <0.001), >10‐50% of polyps (HR = 0.2 (0.1, 0.4); p = <0.001), and >50% of polyps (HR = 0.1 (0, 0.4); p = 0.004) had a lower likelihood of surgery compared to patients who had <2% of polyps resected. Time to surgery based on selected characteristics is depicted in Fig. 2.
Fig. 2.
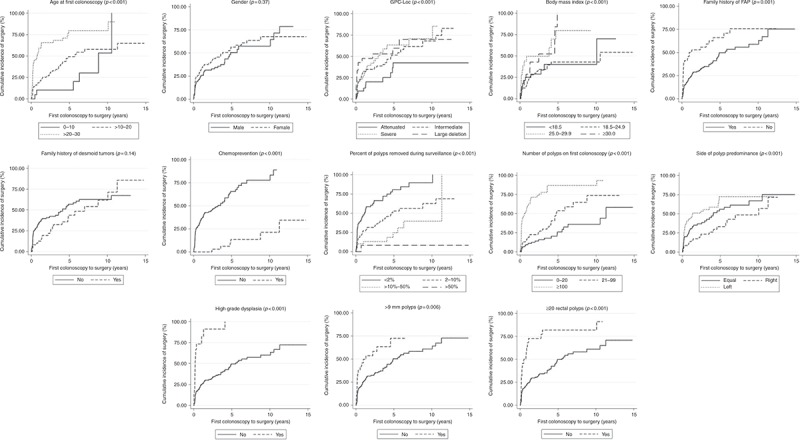
Kaplan‐Meier curves demonstrating time to surgery based on various factors found to be significant on univariate or multivariate analysis on surgical timing. GPC genotype‐phenotype correlation, FAP familial adenomatous polyposis
Multivariate Analysis of Factors Associated with Surgery
Results of stepwise multivariate Cox regression are displayed in Table 2. Higher age and body mass index (BMI) were associated with earlier surgery. Compared to males, females had higher likelihood of surgery. Compared to individuals with an attenuated genotype (per GPC‐Loc), those with intermediate genotype, severe genotype, and large APC deletions had a shorter time to surgery. Patients with a family history of desmoids or with exposure to chemo‐preventive medications had a lower likelihood of surgery. Removal of an aggregate of 2‐10% of polyps, >10‐50% of polyps, and >50% of polyps had lower likelihood of surgery compared to patients who had <2% of polyps treated.
With regard to findings on initial colonoscopy, compared to patients with 0‐20 polyps, patients with 21‐99 polyps and ≥100 polyps had higher likelihood of surgery. Having any polyps >9 mm, and HGD increased likelihood of surgery.
Web‐Based Model for Predicting Time to Surgery within 5 years of First Colonoscopy in Patients Diagnosed with FAP
Based on the regression coefficients obtained from the stepwise multivariate Cox regression, the following equation was derived to determine the likelihood of surgery:
Score = (0.06766 × age in years) + (0.95204, if female) + (0.0815 8 × BMI) + (0.98208, if intermediate genotype) + (0.92505, if severe genotype) + (1.67335, if large deletion genotype) + (‐0.82782, if family history of desmoids) + (‐1.30933, if chemoprevention) + (‐0.55339, if 2‐10% of polyps removed) + (‐0.84863, if >10‐50% of polyps removed) + (‐2.06357, if >50% polyps removed) + (0.70409, if 21‐99 polyps on initial scope) + (1.55857, if 100 or more polyps on initial scope) + (0.6471, if >9 mm polyps on initial scope) + (0.75142, if HGD on initial scope).
ROC analysis for the model revealed area under the ROC curve (AUC) of 0.8741 (0.8234‐0.9246), and 0.8402 (95% CI 0.7869‐0.8935) for predicting surgery within 2 and 5 years, respectively (Fig. 3a, b). The model was validated using 1000 resampling simulations with resultant AUC: 0.8741 (95% CI 0.8237‐0.9245), and 0.8402 (95% CI 0.7882‐0.8922) for 2‐ and 5‐year likelihood of surgery, respectively. This suggests excellent internal validity.
Fig. 3.
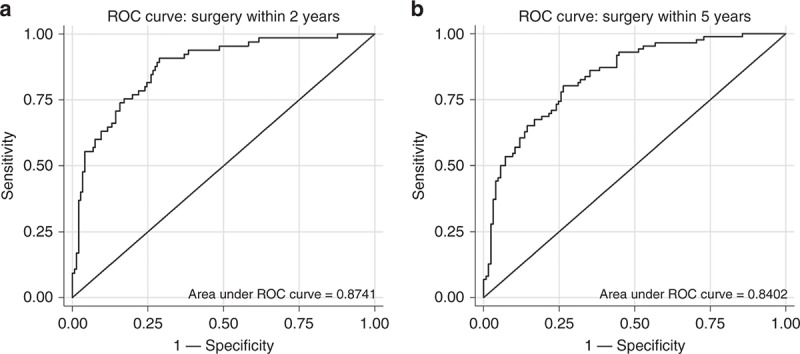
a ROC curve to assess predictive ability of final model to predict surgery in 2 years. AUC = 0.8741 (0.8234‐0.9246). Bootstrap resampling with 1000 replications for internal validity yielded AUC = 0.8741 (0.8237‐0.9245). b ROC curve to assess predictive ability of final model to predict surgery in 5 years. AUC = 0.8402 (0.7869‐0.8935). Bootstrap resampling of 1000 replications for internal validity yielded AUC = 0.8402 (0.7882‐0.8922). ROC receiver operator curve, AUC area under the ROC curve
Likelihood score of <3.18 was used to define the >0‐25th percentile group. The cumulative incidence of surgery for this group within two years was 2 ± 2% and within 5 years was 10.9 ± 6.7%. Positive likelihood ratio (+LR) for both 2 and 5 years for this group was 1.
Likelihood score between ≥3.18 and <4.434 was used to define the 26th to 50th percentile group. Cumulative incidence of surgery for this group within 2 years was 11 ± 4.7% and within 5 years was 53.8 ± 11.5%. For this group, +LR for surgery within 2 years was 1.51 and +LR for surgery within 5 years was 1.59.
Likelihood score between ≥4.434 and <5.852 was used to define the 51‐75th percentile group. Cumulative incidence of surgery for this group within 2 years was 45.1 ± 7.7% and within 5 years was 71.8 ± 10.6%. For this group, +LR for surgery within 2 years was 2.72 and +LR for surgery within 5 years was 2.82.
Likelihood score of ≥5.852 was used to define the 76‐>99th percentile group. Cumulative incidence of surgery for this group within 2 years was 82 ± 6% and within 5 years was 92.8 ± 4.6%. For this group, +LR for surgery within 2 years was 6.26 and +LR for surgery within 5 years was 7.11. Time to surgery for each of the four groups is depicted in Fig. 4.
Fig. 4.
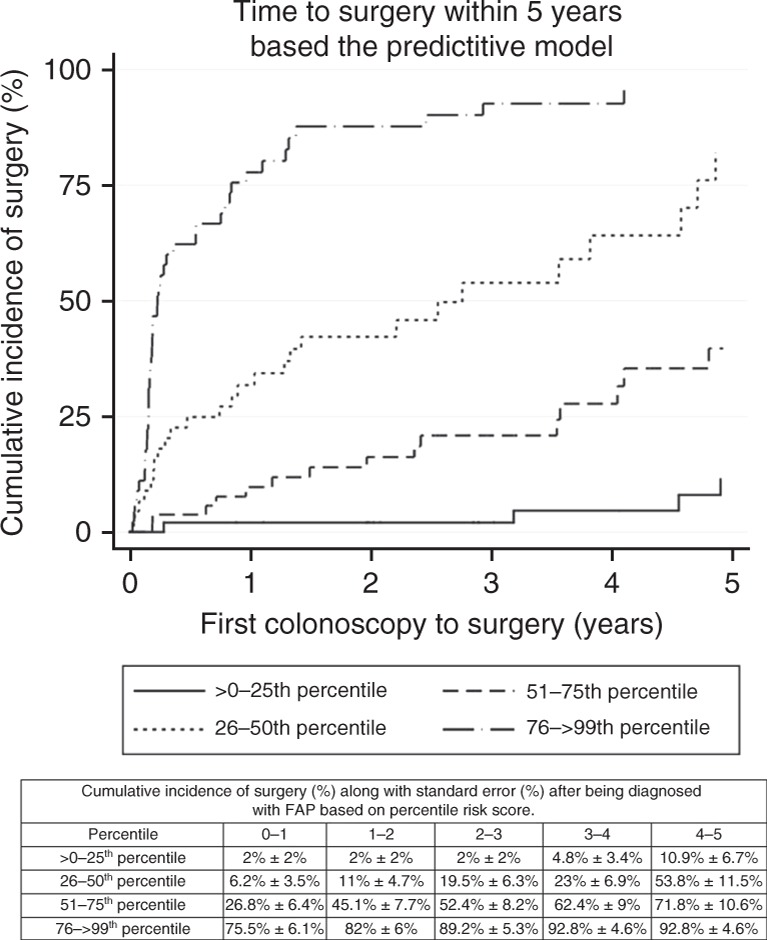
Kaplan‐Meier curve demonstrating time to surgery within 5 years based on percentiles corresponding to predictive score. FAP familial adenomatous polyposis
The above equation was used to create a web‐based scoring system to characterize likelihood of surgery in 5 years after first colonoscopy in patients newly diagnosed with FAP: http://app.calculoid.com/#/calculator/29638. Screenshot of this web‐based model, along with a fictional simulation is included (Fig. 5).
Fig. 5.

Screenshot of the web page with the model describing 5‐year risk of surgery in patients newly diagnosed with FAP (http://app.calculoid.com/#/calculator/29638). Included is a fictional simulation of this web‐based model. FAP familial adenomatous polyposis, BMI body mass index, LR likelihood ratio
SUPPLEMENTAL RESULTS
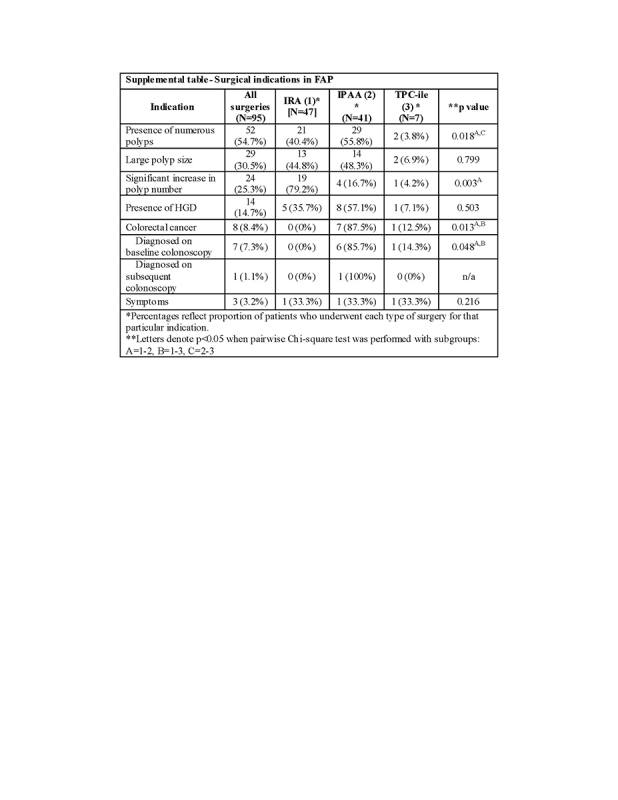
Supplemental table shows indications for surgery by surgery type. Most patients (54.7%) underwent surgery for numerous polyps. This was followed by large polyps (30.5%), significant increase in numbers of polyps between surveillance colonoscopies (25.3%), and presence of HGD (14.7%). Eight patients underwent surgery for the presence of colon cancer (8.4%); all except one of these patients had CRC diagnosed during the baseline colonoscopy. Fewer patients underwent surgery due to presence of colorectal symptoms (3.2%). It must be noted that these are not mutually exclusive indications. A greater proportion of patients underwent IPAA (55.8%) than IRA (40.4%) or TPC‐EI (3.8%) for the indication of a large number of polyps at initial exam (p = 0.018). However, a greater number of patients underwent IRA (79.2%) than IPAA (16.7%) for the indication of an increase in number of polyps during the course of surveillance (p = 0.003). Finally, a greater proportion of patients underwent IPAA (87.5%) or TPC‐EI (12.5%) for the diagnosis of CRC than IRA (0%; p = 0.013).
DISCUSSION
A new diagnosis of FAP creates anxiety and uncertainty for patients and family members, particularly regarding the need for surgery. Although colorectal surgery is almost certain, the type and timing of surgery vary according to multiple factors. This study identifies measurable factors and creates a model that could be used with patients and families to help predict the likelihood of surgery within 2 and 5 years from the time of first colonoscopy at our institution. This information could assist patients in planning various life events and allow them to prepare mentally and physically for the surveillance ahead of them.
This study reflects the philosophy of care of patients with FAP that is well established at the Cleveland Clinic. The basic premise of the philosophy is to balance CRC prevention with quality of life, for every patient, no matter the age or the background. Surgeons in different centers may approach the timing of surgery and type of operation in FAP based on provider preference and not on absolute and relative indications for surgery as recommended by the ACG guidelines. As we demonstrated in our paper, the majority of surgical decisions were based on the indications as set forth by the ACG guidelines on absolute and relative indications for surgery [5]. These include numerous polyps; large polyps; a significant increase in number of polyps between exams, and the presence of HGD. For that reason, we believe our data is valuable and the model generalizable both as a tool for gastroenterologists and an evidence‐based approach for surgeons. Gastroenterologists are often the first provider diagnosing FAP and it will help them generate a discussion with patients and families about the medical factors associated with timing of surgery. Other FAP centers of excellence may choose to develop similar internally validated predictive tools based on factors that reflect their own philosophies to assist their patients in planning timing of surgery.
Gender, GPC‐Loc, and BMI were identified as independent risk factors of surgical timing, probably because of their relationship with FAP phenotype. A large Swedish study has noted that female probands with FAP were diagnosed with symptoms and CRC significantly earlier than male probands [10]. Previous studies have shown that patients with APC mutations that correspond to severe or intermediate GPC‐Loc were noted to have profuse phenotype and an earlier time to second surgery than those with attenuated GPC‐Loc [9, 11]. Although patients with large deletions in the APC gene previously corresponded to the “classic” phenotype [12], our study demonstrates that these patients have greater likelihood of surgery than those with severe or intermediate GPC‐Loc. BMI was noted to have an inverse relationship with time to surgery. Although no research identifies BMI as a risk factor for severe phenotype in FAP, obesity and metabolic syndrome have been associated with adenoma development leading to higher rates of carcinoma [13, 14]. This effect is often balanced by a tendency to defer surgery in morbidly obese patients to allow a period of attempted weight reduction.
The effect of age at FAP diagnosis and family history of desmoid tumors on surgical timing likely reflects evolution of surgical practices based on accumulating evidence. Given that CRC is rare before age 20 [15], surgeons may favor surveillance over surgery in younger patients [16]. Several studies have demonstrated that family history of desmoid disease is a risk factor for desmoid tumor development in patients with FAP [17]. Given that desmoid disease is the primary cause of death in FAP patients post colorectal surgery [18] and that abdominal surgery is another independent risk factor for desmoid development [17], it is understandable that surgeons choose to delay surgery in FAP patients with family history of desmoids.
The initial colonoscopic characteristics that were associated with surgery roughly parallel some of the relative surgical indications outlined by ACG, including having large polyps, having numerous polyps, and presence of HGD [5]. Even the scoring system that was previously designed to help guide need for surgery after surveillance outlines the same factors as being important for surgical decision making [6]. Therefore, it is not surprising that these factors present on initial colonoscopy predict surgery. However, these are indeed relative indications—and as illustrated in our likelihood score—presence of only one of these factors is not sufficient to assign a patient with high likelihood for surgery.
As recognized at the InSiGHT (International Society for Gastrointestinal Hereditary Tumors) 2017 meeting [19], and elsewhere [20], there is a significant interest in examining the role of chemoprevention in delaying surgery in FAP. Previous studies have established the role of chemo‐preventive agents in reducing polyp burden [21,22,23,24], however, to date, no studies have examined time to surgery as an end point.
In our previous study, we found that use of chemoprevention was associated with reduced polyposis rate [25]. In this study, we demonstrate that the use of chemoprevention, an independent factor associated with a decreased likelihood of colorectal surgery at 2 and 5 years in patients with FAP, which has not been shown previously. In addition, the web‐based scoring system may allow for consideration of the use of chemoprevention as an adjunct to endoscopy to delay surgery based on the patients' complex risk profile if validated in other populations. While it may be considered there is bias regarding patients on chemoprevention self‐selecting delay in surgery, only 16% of patients were exposed to chemoprevention and surgeons were not typically involved in the prescribing of non‐steroidal anti‐inflammatories except in the case of patients with desmoids. Celecoxib use was primarily for the management of stage III duodenal polyposis.
Similar to the impact of chemoprevention, polypectomy has been associated with reduced polyp numbers. In a previous study, polypectomy of at least 30 adenomas was attempted to delay surgery in 21 patients with FAP. Most patients experienced significant decrease in polyp burden, with only two patients undergoing surgery during an average follow‐up of 11 months [26]. Our study, using a significantly larger sample size highlights the role of aggressive polypectomy as an independent factor in delaying surgery. Similar to above, the discussion regarding chemoprevention, our web‐based tool allows for confirmation of the utility of polypectomy (at various levels) in patients with varied risk profiles. However, the risk of deferring surgery by polypectomy is that advanced lesions may be missed, patient compliance may decrease, or polypectomy may be inefficient. Any of these events may lead to an interval cancer, and cancer prevention is the top priority of care. Polypectomy done to defer surgery must therefore be used with caution.
We recognize limitations of this study. As with any retrospective analysis, this study is prone to data omissions and misrepresentations. However, verifying data recorded in our polyposis registry with data in the electronic medical record by two independent investigators allowed a more accurate representation of patient data. Additionally, although all surgeries had a clear medical indication, patients' preference could have skewed the results of our study. Furthermore, the vast majority of the colonoscopies (CAB, JMC) and surgeries (JMC, MFK) were performed by three highly experienced physicians, which allowed for consistency in management and high quality, and detailed procedural reports.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
✓ Surgery is the mainstay of treatment for polyposis and prevention of colorectal cancer (CRC) in familial adenomatous polyposis.
✓ Colorectal surgery is often performed in teens and young adults during the formative years.
✓ Predicting the timing of surgery based on clinically accepted medical indications is not known.
WHAT IS NEW HERE
✓ This study identifies factors associated with the timing of surgery at a center of excellence.
✓ A model with excellent internal validity to predict the likelihood of surgery at 2 and 5 years was created.
HOW MIGHT IT IMPACT CLINICAL PRACTICE IN THE FUTURE
✓ Improving the understanding of factors associated with colorectal surgical timing in FAP.
✓ Assist patients and providers in planning life events around predicted surgical timing.
✓ Provide other institutions a model to consider within their practice consisting of the usual clinical factors known to impact surgical timing.
CONCLUSIONS
In patients with FAP, surgery continues to be the predominant choice of therapy to prevent or treat CRC. Using 16 years of FAP patient data at our institution, we developed an internally validated, accurate web‐based clinical tool to help clinicians to determine the likelihood of surgery within 2 and 5 years since first colonoscopy in patients with FAP based on readily available clinical and endoscopic factors. With this tool as an example of an accurate model in our institution, clinicians can help guide discussions regarding surgical timing in context of life events, and potentially the role of modifiable factors, such as chemoprevention and polypectomy in potentially delaying surgical intervention. External validation of our model in centers that use the medical indications for surgical timing, rather than patient or surgeon preference of timing is possible. But, ultimately, development of separate, internally validated models that reflects surgical practices at specific institutions is likely to be a more optimal solution.
CONFLICT OF INTEREST
Guarantor of the article: James M. Church, MD.
Specific author contributions: S.S.—study concept, study design, collecting data, data analysis, writing the manuscript, and approved final draft; C.A.B.—study concept, study design, critical edits, and approved final draft; R.L.—data analysis and approved final draft; M.M.—collecting data, critical edits, and approved final draft; B.H.L.—study design, critical edits, and approved final draft; L.L.—collecting data and approved final draft; M.O.—collecting data and approved final draft; M.F.K.—study design, critical edits, and approved final draft; J.M.C.—study concept, study design, critical edits, and approved final draft.
Financial support: C.A.B. has received research support from Cancer prevention pharmaceuticals, research support from Ferring pharmaceuticals, and consulting fees from Salix and Aries. None of these companies were involved in the study design, collection, analysis, and interpretation of the data and in the writing of the report.
Potential competing interests: C.A.B. received research support from Cancer Prevention Pharmaceuticals. The remaining authors declare that they have no conflict of interest.
Footnotes
Supplementary material accompanies this paper at https://doi.org/10.1038/s41395‐018‐0278‐2
Correspondence: J.M.C. (email: +216‐444‐9052churchj@ccf.org)
Published online 17 October 2018
REFERENCES
- 1.Bulow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn. 2013;15:31–43. [DOI] [PubMed] [Google Scholar]
- 3.Jarvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut. 1992;33:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiskanen I, Luostarinen T, Jarvinen HJ. Impact of screening examinations on survival in familial adenomatous polyposis. Scand J Gastroenterol. 2000;35:1284–7. [DOI] [PubMed] [Google Scholar]
- 5.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch PM, Morris JS, Wen S, et al. A proposed staging system and stage-specific interventions for familial adenomatous polyposis. Gastrointest Endosc. 2016;84:115–125.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W, Karapetyan K, Katz K, Liu C, Maddipatla Z, Malheiro A, McDaniel K, Ovetsky M, Riley G, Zhou G, Holmes JB, Kattman BL, Maglott DR. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;4. PubMed PMID: 29165669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aretz SWK, Spier I. Colon cancer gene variant database (adenomatous polyposis coli). Leiden Open Variation Database (LOVD). 2017. http://chromium.lovd.nl/LOVD2/colon_cancer/variants.php?action=search_unique&select_db=APC. Access date December 21, 2017.
- 9.Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153–61. [DOI] [PubMed] [Google Scholar]
- 10.Bjork J, Akerbrant H, Iselius L, Alm T, Hultcrantz R. Epidemiology of familial adenomatous polyposis in Sweden: changes over time and differences in phenotype between males and females. Scand J Gastroenterol. 1999;34:1230–5. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwenhuis MH, Mathus-Vliegen LM, Slors FJ, et al. Genotype-phenotype correlations as a guide in the management of familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2007;5:374–8. [DOI] [PubMed] [Google Scholar]
- 12.Michils G, Tejpar S, Thoelen R, et al. Large deletions of the APC gene in 15% of mutation-negative patients with classical polyposis (FAP): a Belgian study. Hum Mutat. 2005;25:125–34. [DOI] [PubMed] [Google Scholar]
- 13.Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenomacarcinoma sequence. Nutr Cancer. 2001;39:50–7. [DOI] [PubMed] [Google Scholar]
- 14.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a metaanalysis of cohort studies. World J Gastroenterol. 2007;13:4199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church JM, McGannon E, Burke C, Clark B. Teenagers with familial adenomatous polyposis: what is their risk for colorectal cancer? Dis Colon Rectum. 2002;45:887–9. [DOI] [PubMed] [Google Scholar]
- 16.Warrier SK, Kalady MF. Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin Colon Rectal Surg. 2012;25:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieuwenhuis MH, Lefevre JH, Bulow S, et al. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: an international cohort study. Dis Colon Rectum. 2011;54:1229–34. [DOI] [PubMed] [Google Scholar]
- 18.Belchetz LA, Berk T, Bapat BV, Cohen Z, Gallinger S. Changing causes of mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:384–7. [DOI] [PubMed] [Google Scholar]
- 19.Lynch PM. Lecture: Chemoprevention in hereditary GI cancer. Paper presented at: International Society for Gastrointestinal Hereditary Tumours (InSiGHT) July 2017; Florence, Italy. [Google Scholar]
- 20.Lynch PM. Chemoprevention of familial adenomatous polyposis. Fam Cancer. 2016;15:467–75. [DOI] [PubMed] [Google Scholar]
- 21.Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–9. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton SR. The adenoma-adenocarcinoma sequence in the large bowel: variations on a theme. J Cell Biochem Suppl. 1992;16G:41–6. [DOI] [PubMed] [Google Scholar]
- 23.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. [DOI] [PubMed] [Google Scholar]
- 24.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarvepalli S, Burke CA, Monachese M, et al. Natural history of colonic polyposis in young patients with familial adenomatous polyposis. Gastrointest Endosc. 2018;2 pii: S0016-5107(18)32753-6 10.1016/j.gie.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Patel NJ, Ponugoti PL, Rex DK. Cold snare polypectomy effectively reduces polyp burden in familial adenomatous polyposis. Endosc Int Open. 2016;4:E472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]