Abstract
OBJECTIVES:
Most cost‐effectiveness analyses of colorectal cancer (CRC) screening assume Medicare payment rates and a lifetime horizon. Our aims were to examine the implications of differential payment levels and time horizons for commercial insurers vs. Medicare on the cost‐effectiveness of CRC screening.
METHODS:
We used our validated Markov cohort simulation of CRC screening in the average risk US population to examine CRC screening at ages 50‐64 under commercial insurance, and at ages 65‐80 under Medicare, using a health‐care sector perspective. Model outcomes included discounted quality‐adjusted life‐years (QALYs) and costs per person, and incremental cost/QALY gained.
RESULTS:
Lifetime costs/person were 20‐44% higher when assuming commercial payment rates rather than Medicare rates for people under 65. Most of the substantial clinical benefit of screening at ages 50‐64 was realized at ages ≥65. For commercial payers with a time horizon of ages 50‐64, fecal occult blood testing (FOBT) and fecal immunochemical testing (FIT) were cost‐effective (<$61,000/QALY gained), but colonoscopy was costly (>$185,000/QALY gained). Medicare experienced substantial clinical benefits and cost‐savings from screening done at ages <65, even if screening was not continued. Among those previously screened, continuing FOBT and FIT under Medicare was cost‐saving and continuing colonoscopy was highly cost‐effective (<$30,000/QALY gained), and initiating any screening in those previously unscreened was highly effective and cost‐saving.
CONCLUSIONS:
Modeling suggests that CRC screening is highly cost‐effective over a lifetime even when considering higher payment rates by commercial payers vs. Medicare. Screening may appear relatively costly for commercial payers if only a time horizon of ages 50‐64 is considered, but it is predicted to yield substantial clinical and economic benefits that accrue primarily at ages ≥65 under Medicare.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer‐related death in the United States [1]. Screening with guaiac‐based fecal occult blood testing (gFOBT) [2] or sigmoidoscopy [3] decreases CRC incidence and mortality, and greater benefits are expected from fecal immunochemical testing (FIT) [4,5,6,7] and colonoscopy [8,9,10,11].
Multiple studies have explored the cost‐effectiveness of CRC screening, usually concluding that screening is cost‐effective, and potentially cost‐saving [12]. Most US studies have been conducted using a single payer or societal perspective [13], and have measured costs based on Medicare payments [12], which are believed to reflect true costs more closely than other measures. In the United States, however, people usually change insurers over their lifetime and most adults have private insurance or Medicaid while they are younger than 65 and Medicare when they are 65 and over.
In this analysis, we explored two aspects of taking an insurer or payer perspective when evaluating the cost‐effectiveness of CRC screening under a health‐care sector perspective [14]. First, we examined the implications of differences in prices paid by different types of insurers. Payments by commercial insurers, who cover the vast majority of people under age 65, often substantially exceed Medicare payment rates [15,16,17].
Second, commercial insurers and Medicare have different time horizons due to the transition in coverage at age 65. Because most CRCs arise from adenomas or serrated lesions over many years [18, 19], the removal of CRC precursors between ages 50 and 64 may translate into prevented CRCs once people become Medicare beneficiaries at ages 65 and older. Thus, commercial insurers may bear the screening costs that yield benefits under Medicare in the form of increased quality‐adjusted life‐years (QALYs) as well as reductions in spending for CRC care.
Our aims were to assess the implications of differential payment levels between commercial insurers and Medicare when assessing the cost‐effectiveness of CRC screening from the insurer, third‐party payer perspective, considering insurer‐specific time horizons, and to evaluate the distribution of costs and benefits of CRC screening between commercial insurers and Medicare in analyses with lifetime perspectives. Given our aims, we focused primarily on the incremental costs/QALY gained by screening vs. no screening under various scenarios, but we also report the incremental costs/QALY gained between competing screening strategies.
METHODS
General study design
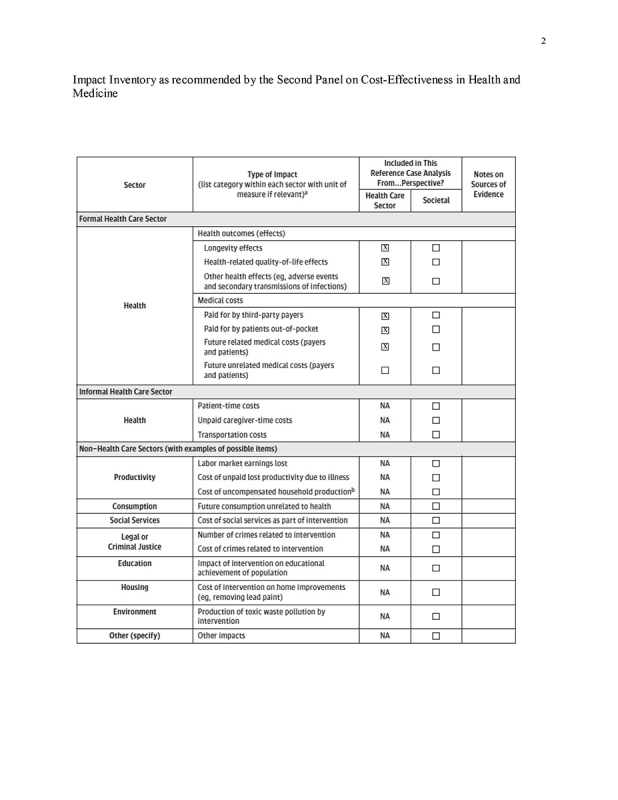
We conducted a cost‐effectiveness analysis under a health‐care sector perspective as recommended by the Second Panel on Cost‐Effectiveness in Health and Medicine, but focusing on CRC‐related screening, complication, and treatment costs (Impact Inventory, Supplement) [14]. Given our aims, a comprehensive societal perspective analysis [14] was not applicable in this study.
We adapted our validated decision analytic Markov cohort model of CRC screening in the general US population [20, 21] to explore screening under differential payment rates for persons under age 65 (reflecting commercial insurance payments) vs. age ≥65 (reflecting Medicare payments). We modeled several currently established and accepted screening strategies [22, 23], including annual FOBT or FIT, sigmoidoscopy every 5 years (FS), colonoscopy every 10 years (COLO), FS/FOBT, and FS/FIT, with screening eligibility from age 50 until age 80 (the mid‐point of the age range 76‐85 for which the U.S. Preventive Services Task Force recommends individualizing screening decisions) [22]. Based on the results of randomized controlled trials, we assumed that the mortality benefit of flexible sigmoidoscopy was restricted to the distal colon and rectum [24,25,26].
We did not consider computed tomography (CT) colonography or a multi‐target stool DNA assay [22], or methylated Septin 9 DNA because we did not have detailed primary data on commercial payments for these tests. However, we have previously considered the potential cost‐effectiveness of these modalities [20,27,28,29,30].
We explored six screening scenarios representing different combinations of time horizons, compliance with recommended screening, and payment rates by age (Table 1):
Table 1.
Perspectives, time horizons, use of screening, and payment rates considered in cost‐effectiveness analyses

1“Lifetime Horizon ‐ Insurer‐Specific Rates”: Age 50 until death, with screening for ages 50‐80, with commercial payments under age 65 and Medicare payments at ages ≥65.
2“Lifetime Horizon ‐ Medicare Rates”: Age 50 until death, with screening for ages 50‐80, with Medicare payments at all ages. Our purpose was to compare our results to those of previous studies, which often use only Medicare payments.
3“Commercial Perspective”: Age 50‐64, with screening at ages 50‐64, with commercial payments. This scenario takes the perspective of commercial insurers by restricting the time horizon and focusing on commercial rates.
4“Medicare Perspective ‐ Screened Ages 50‐64”: Age 65 until death in persons previously screened at ages 50‐64, with screening for ages 65‐80, with Medicare payments. This scenario reflects Medicare's perspective on continuing screening in previously screened persons.
5“Medicare Perspective ‐ Unscreened Ages 50‐64”: Age 65 until death in persons not previously screened, with screening for ages 65‐80, with Medicare payments. This scenario reflects Medicare's perspective on initiating screening in previously unscreened persons.
6“Medicare Perspective ‐ Screened Ages 50‐64 and Not 65‐80”: Age 65 until death in persons previously screened at ages 50‐64, with no further screening for ages 65‐80, with Medicare payments. This scenario highlights the benefits realized by Medicare that are attributable to screening under commercial insurance before age 65. In this case, Medicare's payments were restricted to diagnostic testing and CRC care.
Decision analytic model and time horizons of simulations
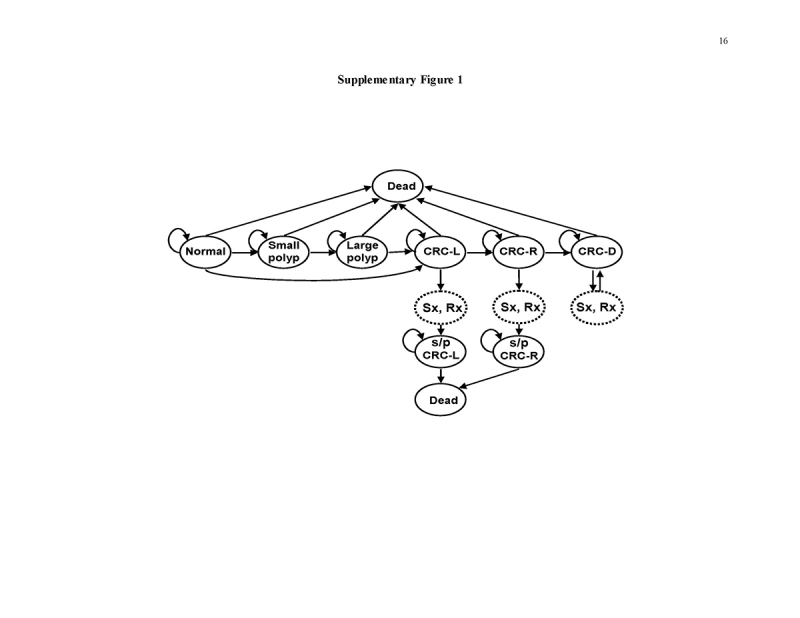
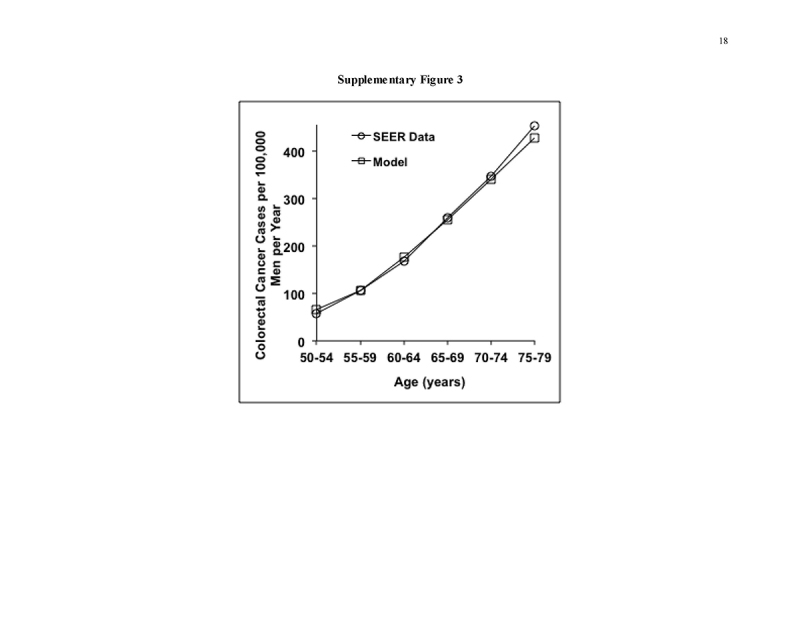
Our original model, data sources, and validations against the Minnesota Colon Cancer Control Study [31, 32], United Kingdom Flexible Sigmoidoscopy Trial [24], SCORE Trial [25], and PLCO Cancer Screening Trial [26] have been detailed previously [20, 21, 29] (Supplementary Figure 1 and Table 2). The validations are, by necessity, limited to the time horizons of available clinical studies, but the model projects the benefits of all strategies over a lifetime. Model calibrations for analyses stratified by gender are described in the Supplement. The model reproduces the natural history of CRC precursors and CRC in the general US population without screening. Persons transition between health states of normal, small polyp, large polyp, localized, regional, disseminated CRC, and dead, in 1‐year cycles. Screening is superimposed on the natural history module, resulting in CRC prevention or early detection as determined by screening test performance characteristics, and patient participation [20, 21, 29]. The model inputs are derived from autopsy data on polyp prevalence; Surveillance, Epidemiology, and End Results (SEER) data on CRC incidence and stage distribution from dates preceding widespread CRC screening; clinical studies on test performance characteristics and complication rates, outcomes after CRC treatment, and CRC‐related quality of life; U.S. Life Tables data; and commercial and Medicare payments rates (Table 2 and Supplement).
Table 2.
Model inputs

Table 2.
Continued.

In our first set of analyses, persons entered the model at age 50 and screening and surveillance were offered from age 50 to 80, with persons followed until death (age 100 maximum) (analyses with “Lifetime Horizon,” Table 1). In our analyses reflecting the “Commercial Perspective” only (Table 1), persons entered the model at age 50 and screening and surveillance could be offered from age 50 to 64, with persons followed until age 64 or death. In our analyses reflecting the “Medicare Perspective” only (Table 1), persons entered the model at age 65, either previously screened or unscreened, and were followed until age 100 or death, either with or without screening under Medicare at ages 65‐80 (Table 1). The primary analyses included both genders. We performed analyses stratified by gender to explore scenarios in which cost‐effectiveness differed by gender.
Cost inputs
Payment rates were derived from national average Medicare reimbursement rates [33,34,35,36,37] and CRC care costs [38] for persons age ≥65, and from commercial payment rates for colorectal testing in persons aged 50‐64 [15], accounting for colonoscopy site of service [15, 38]. The rates include the insurer and patient liabilities associated with the service. In the base case, we measured payment rates in 2009 dollars, reflecting the year from which commercial payment data were available [15]. In sensitivity analyses, we updated cost inputs to 2015 dollars; we used the year‐specific new Medicare rates, and updated all other costs by the medical component of the consumer price index (Table 2 and Supplement). Based on our observed mean ratio of commercial to Medicare payment rates for colorectal tests of 1.35 [15], we assumed base case CRC care and complication costs for persons aged 50‐64 that were 1.35‐fold those of persons age ≥65, and we varied this in sensitivity analyses.
Clinical and economic outcomes
The principal model outputs were quality‐adjusted life‐years (QALYs) and costs per person [13, 39]. Future QALYs and costs were discounted by 3% annually [40]. Health state utilities for CRC by stage (Table 2) were used to calculate QALYs by applying these for 5 years after CRC diagnosis. We estimated CRC cases by stage and CRC deaths, and lifetime number of tests/person in cohorts of 100,000 persons.
Cost‐effectiveness analyses
Analyses from the perspective of the third‐party payers were performed in TreeAge Pro (TreeAge Software, Inc., Williamstown, MA) and Excel 2010 (Microsoft Corporation, Redmond, WA), reflecting a hybrid‐payer longitudinal perspective as well as time horizon‐specific perspectives for commercial insurers and Medicare as detailed above. Incremental cost‐effectiveness ratios (ICERs) were calculated [13, 39]. In discussing whether strategies were “cost‐effective,” we adopted the commonly used willingness‐to‐pay threshold of $100,000/QALY gained, but because there is no universal consensus on a threshold, we emphasize costs/QALY gained.
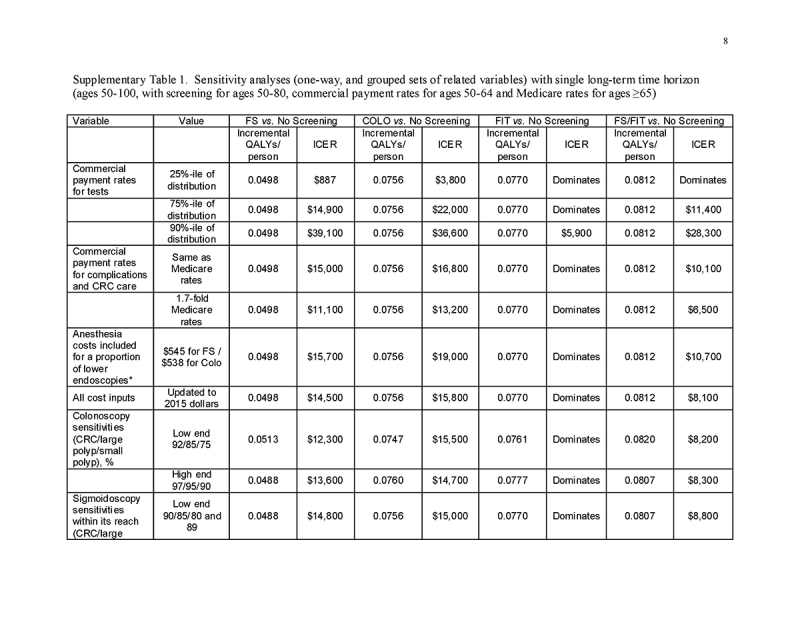
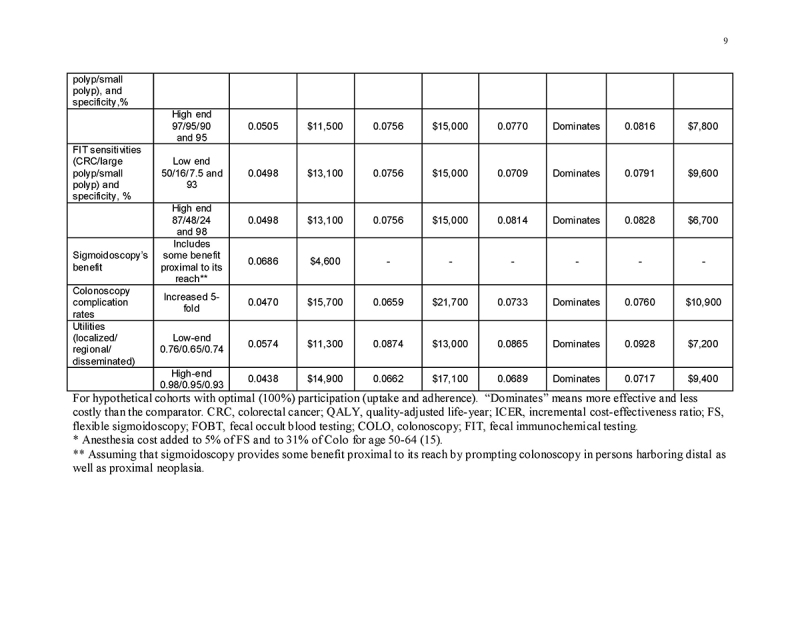
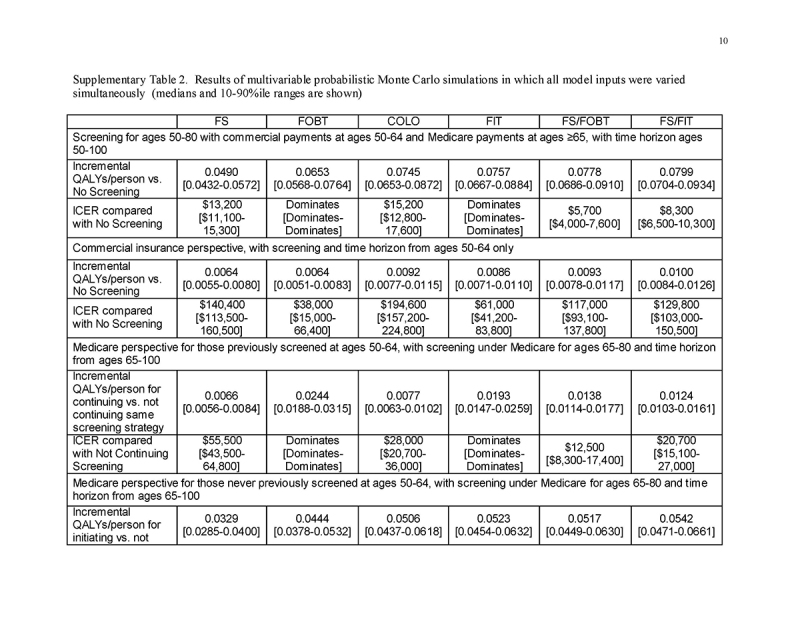
Sensitivity analyses (one‐way and on grouped sets of related variables) were used to examine all model inputs. We considered the potentially higher costs of lower endoscopy when anesthesia‐related costs are incurred, as observed in our recent study [15]. In threshold analyses, we examined test cost requirement to achieve costs of $100,000/QALY gained. We performed probabilistic Monte Carlo simulations with 10,000 trials. In the first Monte Carlo simulation, we varied only commercial payment rates within the distributions observed in our recent study [15]. In a second Monte Carlo simulation, we varied all model inputs simultaneously, with commercial payments rates as in the first simulation, and using beta distributions for probabilities derived from means, standard deviations and ranges in the literature [41]. For commercial payment inputs, we used gamma distributions, with means and standard deviations [15] used to approximate alpha and lambda. Costs of screening under Medicare were varied together within a range of ±20% of the base case value, and costs of care were varied together but independently from costs of screening within a range of ±20%.
RESULTS
Lifetime horizon ‐ insurer‐specific rates
Under a single long‐term time horizon spanning ages 50 until death, with screening for ages 50‐80 with commercial payment rates for ages 50‐64 and Medicare rates for ages ≥65 (“Lifetime Horizon ‐ Insurer‐Specific Rates”), FOBT and FIT were cost‐saving, COLO and FS cost ≤$15,000/QALY gained, and FS/FOBT and FS/FIT cost ≤$8,300/QALY gained when compared with no screening (Fig. 1a and Table 3).
Fig. 1.
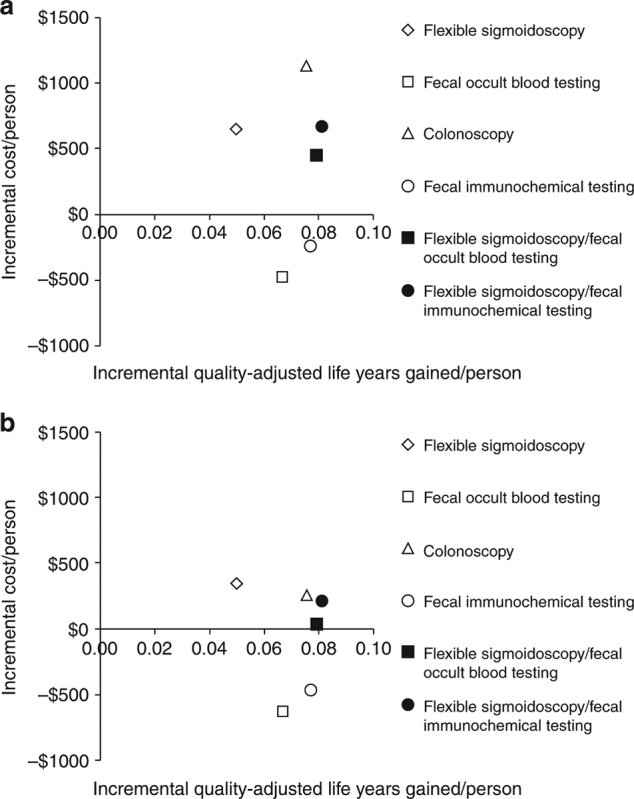
Incremental discounted quality‐adjusted life‐years gained and discounted costs per person with screening for ages 50‐80, and time horizon until death, assuming commercial payment rates for ages 50‐64 and Medicare payment rates starting at age 65 (a), or Medicare payment rates at all ages (b)
Table 3.
Results for 100,000‐person cohort with single long‐term time horizon (ages 50‐100, with screening for ages 50‐80)

In incremental comparisons between strategies, FOBT and FIT were both more effective and less costly than FS, and FIT was more effective and less costly than COLO; FIT cost $22,400/QALY gained vs. FOBT; and COLO cost $179,000/QALY gained vs. FOBT (Fig. 1a and Table 3).
Lifetime horizon ‐ Medicare rates
The lifetime costs/person with screening were 20‐44% higher when applying commercial payments rates for people aged 50‐64 (“Lifetime Horizon ‐ Insurer‐Specific Rates,” above) than when applying Medicare rates for all ages (“Lifetime Perspective ‐ Medicare Rates”) (Table 3). With Medicare rates for all ages, FOBT and FIT were cost‐saving relative to no screening, and other strategies cost ≤$7,000/QALY gained (Table 3). FIT continued to be more effective and less costly than FS and COLO (Fig. 1b vs. Fig. 1a and Table 3).
Commercial insurer perspective
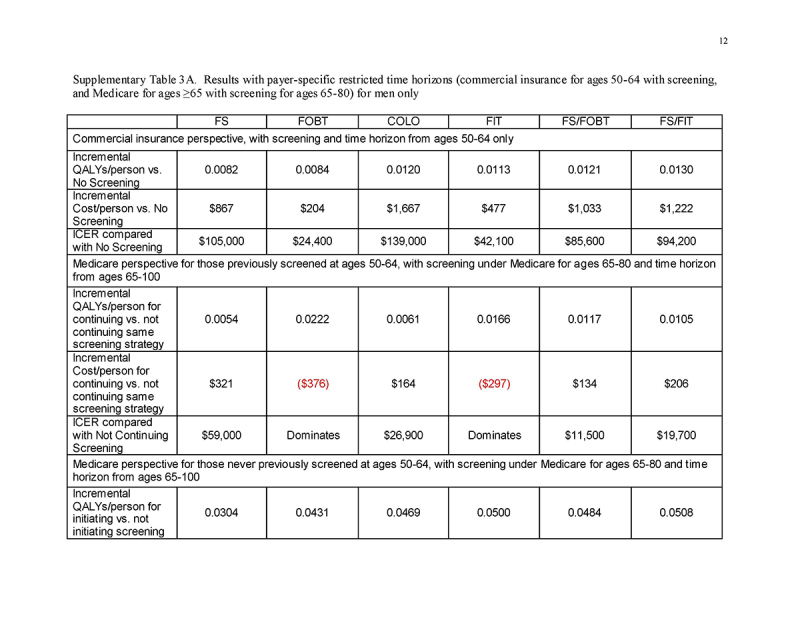
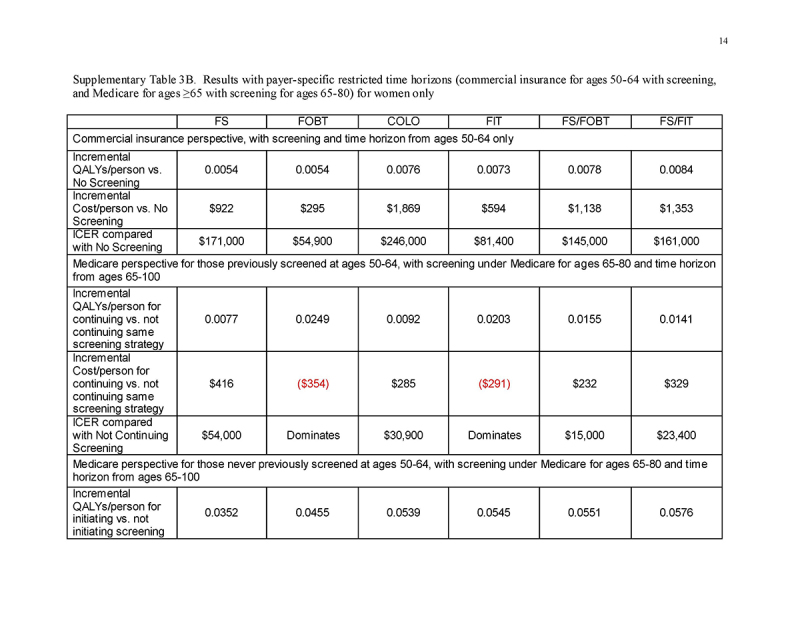
Under a restricted time horizon spanning ages 50‐64 and commercial payment rates (“Commercial Perspective”), no strategy was cost‐saving and each strategy was less cost‐effective than under either lifetime horizon. FOBT and FIT cost <$61,000/QALY gained; FS, FS/FOBT, and FS/FIT cost <$140,000/QALY gained; and COLO cost $189,000/QALY gained (Table 4 and Fig. 2a). The costs/QALY gained were more favorable for men (Supplementary Table 3A) than for women (Supplementary Table 3B), but COLO remained relatively costly at $139,000/QALY gained.
Table 4.
Results with payer‐specific restricted time horizons (commercial insurance for ages 50‐64 with screening, and Medicare for ages ≥65 with screening for ages 65‐80)

Fig. 2.
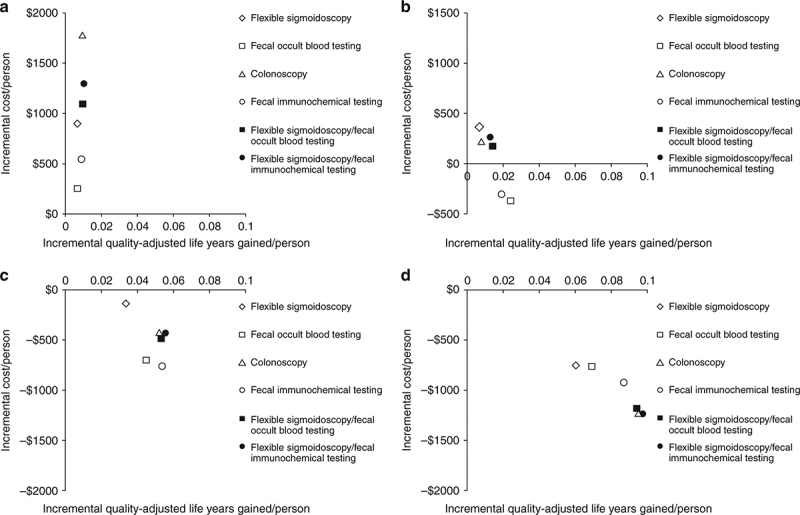
Incremental discounted quality‐adjusted life‐years gained and discounted costs per person from the perspective and time horizon of commercial payers compared with Medicare. a Commercial payer perspective, screening for ages 50‐64. b Medicare perspective, continuing screening for ages 65‐80 in those previously screened at ages 50‐64 under commercial insurance, compared with stopping screening. c Medicare perspective, initiating screening for ages 65‐80 in those previously unscreened at ages 50‐64 under commercial insurance, compared with not initiating screening. d Medicare perspective, no further screening for ages 65‐80 in those previously screened at ages 50‐64 under commercial insurance, compared with no screening for ages 65‐80 in those previously not screened at ages 50‐64
Medicare perspective: continuing screening in those previously screened
Under a restricted time horizon spanning ages ≥65, and assuming that persons entering Medicare had been screened previously under commercial insurance (“Medicare Perspective ‐ Screened Ages 50‐64”), continuing FOBT and FIT were more effective and less costly than stopping screening; continuing COLO, FS/FOBT, and FS/FIT cost <$30,000/QALY gained compared with stopping screening; and continuing FS cost $53,600/QALY gained compared with stopping screening (Table 4 and Fig. 2b). The results did not differ substantively by gender (Supplementary Tables 3A and 3B).
Medicare perspective: initiating screening in those previously unscreened
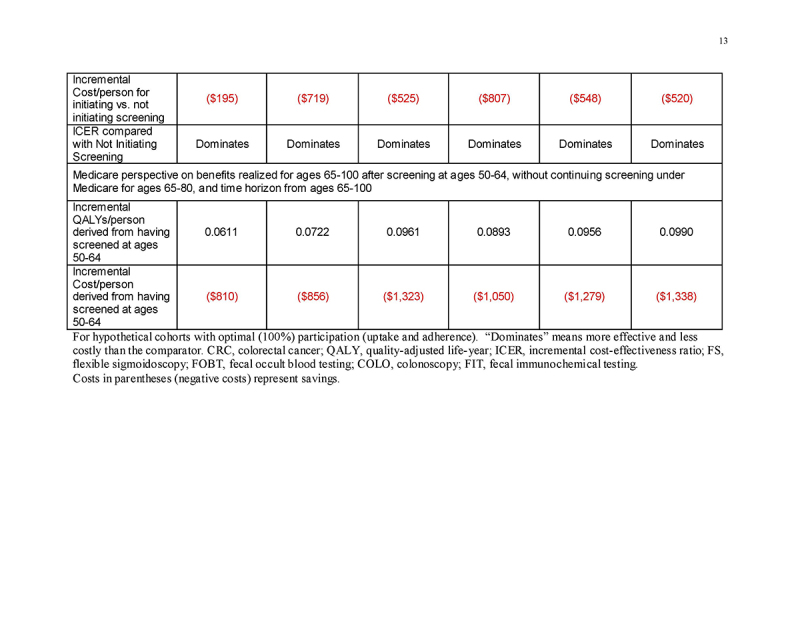
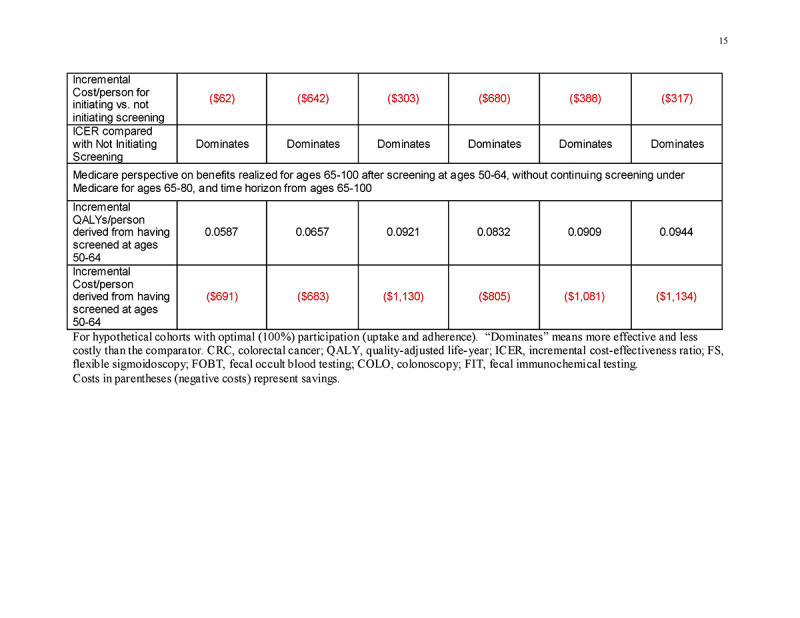
Under a restricted time horizon spanning ages ≥65, and assuming that persons entering Medicare never had been screened previously under commercial insurance (“Medicare Perspective ‐ Unscreened Ages 50‐64”), initiating any screening strategy was more effective (mean 0.0337‐0.0557 QALYs gained/person, depending on the screening strategy) and was cost‐saving (mean $137‐$755 saved/person, depending on the screening strategy) compared with not initiating screening (Table 4 and Fig. 2c). This pattern applied to all strategies for both genders (Supplementary Tables 3A and 3B).
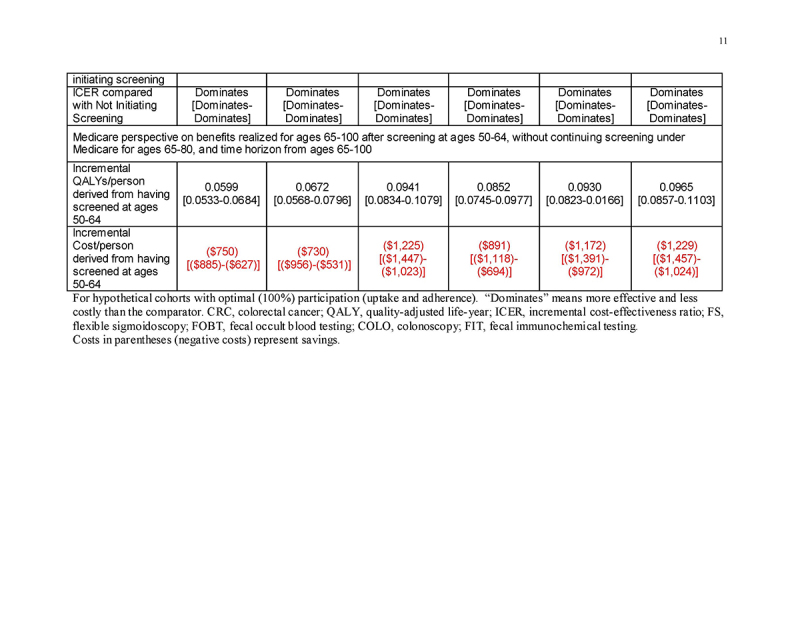
Medicare perspective: benefits to Medicare of screening under commercial insurance
In persons entering Medicare having been screened previously under commercial insurance at ages 50‐64, but then not continuing screening under Medicare (“Medicare Perspective ‐ Screened Ages 50‐64 and Not 65‐80”), the previous screening under commercial insurance yielded substantial gains in QALYs/person (mean 0.0605‐0.0976 QALYs gained/person, depending on the screening strategy) as well as cost‐savings (mean $750‐$1234 saved/person, depending on the screening strategy) for Medicare at ages ≥65 (Table 4 and Fig. 2d). This pattern applied to all strategies for both genders (Supplementary Tables 3A and 3B).
Sensitivity analyses
The most influential variables on the cost/QALY gained by screening were the commercial payment rates (Supplementary Table 1). Varying test performance characteristics, complication rates, utilities, and the cost of complications and CRC care, adding anesthesia costs to lower endoscopy, and updating costs to 2015 dollars had minimal impact on the results (Supplementary Table 1).
Focusing on the commercial insurer perspective with a restricted time horizon spanning ages 50‐64 and commercial payment rates, we calculated test costs at which COLO would be considered cost‐effective relative to no screening at a threshold of <$100,000/QALY gained. The resulting private insurer costs for colonoscopy and colonoscopy with lesion removal were $759 and $1,015, respectively. The respective values in analyses stratified by gender were $614 and $821 for women, and $956 and $1,279 for men.
In a Monte Carlo simulation of the “Lifetime Horizon ‐ Insurer‐Specific Rates” scenario in which only commercial payments were varied within the distributions observed in our recent study [15], the upper 90% confidence intervals for the costs/QALY gained by screening vs. no screening were <$35,000/QALY gained for all strategies: FS $8,500 [dominates‐$34,800], FOBT dominates [dominates‐dominates], COLO $13,000 [$685‐$31,900], FIT dominates [dominates‐$2,700], FS/FOBT $3,600 [dominates‐$ 18,500] and FS/FIT $6,500 [dominates‐$20,900].
The results of Monte Carlo simulations in which all model inputs were varied simultaneously, considering payer‐specific perspectives and time horizons, are shown in Supplementary Table 2. The 10‐90th percentile ranges around the median costs/QALY gained were narrow and the conclusions were not affected. From the perspective of commercial insurers with a restricted time horizon of ages 50‐64, screening gained fewer QALYs at higher cost/QALY gained for all modalities except colonoscopy than did continuing screening from the perspective of Medicare in those ages 65‐80. There remained substantial gains in QALYs and substantial cost‐savings by Medicare at ages 65‐100 that were attributable to screening under commercial insurance at ages 50‐64. Instituting screening at age 65 under Medicare in those previously unscreened under commercial insurance remained highly effective and cost‐saving for all strategies.
DISCUSSION
This study of colorectal neoplasia screening in the United States focuses on the effects of differences between commercial insurer and Medicare payment rates and time horizons on the cost‐effectiveness of screening using a health‐care sector perspective. Many cost‐effectiveness analyses have used Medicare rates for cost inputs over a lifetime, but this does not account for the higher reimbursement rates often paid by commercial insurers, or the different time horizons for commercial insurers and Medicare.
We found that for a time horizon of age 50 until death, the estimated cost per person of screening was 20‐44% higher, depending on the screening modality, when conducting the analysis using commercial rather than Medicare payment rates for health care used by people under the age 65. While accounting for commercial payments at ages 50‐64 increased the costs of all strategies, FOBT and FIT remained cost‐saving, and colonoscopy remained highly cost‐effective compared with no screening ($15,000/QALY gained when commercial costs were considered, compared with $3,400/QALY gained when Medicare rates were used for all ages).
Our analyses of restricted time horizons reflecting the differing perspectives of commercial insurers and Medicare demonstrate how the costs and benefits of lifetime screening are distributed across different types of insurance. Restricting the time horizon to ages 50‐64, we found that screening under commercial insurance generated fewer additional QALYs/person during ages 50‐64 with all modalities, except colonoscopy, than continuing screening for ages 65‐80 under Medicare. From the perspective of commercial insurers, the cost/QALY gained of endoscopic screening was high if the impact on CRC outcomes was only counted for ages 50‐64, at a willingness‐to‐pay threshold of $100,000/QALY gained. However, prior screening at ages 50‐64 yielded gains in QALYs at ages 65‐100 that were several‐fold higher than those realized before age 65, and Medicare‐derived substantial savings for CRCs prevented by screening and polypectomies at ages 50‐64 under commercial insurance. In those previously unscreened, initiating screening under Medicare for ages 65‐80 was highly effective, yielding gains in QALYs/person that were several‐fold higher than those resulting from continuing screening under Medicare in previously screened persons. Furthermore, initiating screening at age 65 under Medicare was cost‐saving for all modalities.
Our analysis points to the importance of considering a lifetime perspective when using cost‐effectiveness analysis to inform coverage decisions. Only FOBT and FIT were cost‐effective relative to no screening using conventional thresholds (cost/QALY gained ≤$100,000) when evaluated from the commercial insurer perspective with a time horizon of ages 50‐64. This analysis, however, by definition does not consider the longer‐term benefits of screening. Making decisions based on this truncated time horizon could result in the inappropriate conclusion that services that are cost‐effective but characterized by short‐term costs and long‐term benefits should be excluded from coverage. Similar issues arise in other settings. For example, should state Medicaid programs assess the effectiveness and cost‐effectiveness of vaccinations among children over a time horizon of only a few years for those who subsequently transition to private insurance?
In this study, we did not explicitly examine the overall effect of switching between different screening modalities over a lifetime. These types of analyses have been conducted in other studies. We have previously found that FIT screening starting at age 50 and converting to colonoscopy at age 55 is likely to be highly cost‐effective in Germany [42]. A previous US‐based simulation estimated that FIT beginning at age 50 and then a single colonoscopy at age 66 delivers clinical and economic outcomes similar to those of single‐modality strategies, with a favorable impact on resources demand [43]. While it may be tempting to conclude, based on our results for payer‐specific time horizons, that stool‐based screening of younger adults followed by colonoscopy at older ages may be an optimal strategy, the strategies that are most cost‐effective for particular payers based on a truncated time horizon do not necessarily represent the most cost‐effective mixed modality strategy when analyzed over a lifetime perspective. Modeling such hybrid strategies was beyond the scope of the current study.
Our analyses extend the observations from previous studies. In a previous modeling study we found that, compared with national screening uptake of 40% for ages 50‐80, screening 75% of the US population for ages 50‐80 increased annual costs related to CRC care and testing from $3.6 billion to $5.0 billion for 50‐64‐year olds, but decreased annual costs from $5.9 billion to $5.6 billion for those aged 65 years and older [44]. However, in that study, the costs for commercial payers were likely underestimated, as we did not account for differential payment rates by commercial insurers.
More recent analyses similarly concluded that increased screening participation in the pre‐Medicare population could reduce CRC incidence and mortality, with the additional screening costs largely offset by long‐term Medicare treatment savings [45, 46]. In the first study, the unit costs for screening procedures and CRC treatments in the pre‐Medicare privately‐insured population were assumed to be 40% higher than in the Medicare population, with 74% of the pre‐Medicare population insured by private insurance, and the rest by Medicaid or Medicare, or uninsured [45]. The second study focused on colonoscopy, and adopted a cost perspective of a private insurer and a value perspective that included survival past age 65 years [46]. Our current analysis focused specifically on commercial payers before age 65, informed by actual payment rates by commercial insurers, and considered different time horizons as well as the commercial vs. Medicare perspectives [15].
Our study has limitations. First, we did not examine differential participation rates between strategies because that was not the focus of these analyses. We refer interested readers to our previous detailed explorations of this topic [29, 30]. Second, we did not consider complex scenarios in which persons could be screened with one modality under commercial insurance, and then with a different modality under Medicare at ages 65‐80. Third, while we used primary data for commercial payments for screening tests, we did not have such data available for CRC care or complications, and instead we made assumptions about the ratio of commercial to Medicare payments for those categories of costs. However, varying those assumptions within reasonable ranges did not affect our conclusions. Fourth, we did not capture more recent reductions in Medicare payments for endoscopic services occurring in 2016 and 2017, but this would make screening even more attractive from the perspective of Medicare. Fifth, a comprehensive analysis including future costs unrelated to CRC, or accounting for time, transportation and non‐health‐care costs [14] was beyond the scope of this study. Sixth, we did not consider CT colonography or the multi‐target stool DNA assay in this analysis.
In summary, accounting for the higher payment rates by commercial payers compared with Medicare leads to higher but still highly acceptable estimated costs/QALY gained by colorectal neoplasia screening for ages 50‐80. Commercial insurers paying for screening at ages 50‐64 provide substantial clinical benefits over the lifetime of a population, but most of this benefit is realized at ages 65 and older. While Medicare derives substantial clinical and economic benefits from screening performed under commercial insurance at ages 50‐64 with any of the established CRC screening methods, continuing screening is likely to yield substantial additional clinical benefit at acceptable costs, and initiating any form of screening in those previously unscreened is predicted to be highly effective as well as cost‐saving. As US efforts intensify to reach the goal of screening at least 80% of eligible adults, we emphasize that multiple cost‐effective screening methods are available, and that accommodating patient preferences can improve overall adherence with screening.
What are the implications of our findings for policy? Many interventions are likely to show the same delayed benefit as seen with CRC screening, including, for instance, control of hypertension, diabetes, and hypercholesterolemia. We believe that insurers should use a lifetime perspective when assessing the cost‐effectiveness of covered services. Using a payer‐specific perspective focused on short‐term cost‐effectiveness would lead to the underuse of services whose benefit is derived primarily in the distant future. For older adults under Medicare, CRC screening should remain a preventive health priority, particularly in those who have not undergone screening previously.
CONFLICT OF INTEREST
Guarantor of the article: Uri Ladabaum, MD, MS.
Specific author contributions: Study concept and design: UL; acquisition of data: UL, MKB, AM, and ZL; analysis and interpretation of data: UL, AM, and MKB; drafting of the manuscript: UL and MKB; critical revision of the manuscript for important intellectual content and final approval of this version: UL, AM, JVB, ZL, and MKB; statistical analysis: UL and AM; obtained funding: UL; technical or material support: AM; study supervision: UL and MKB.
Financial support: None.
Potential competing interests: UL: consultant, Medtronic/Given; past grant support, Exact Sciences; Advisory Board, Univeral Dx. JVB: consultant, Academy of Managed Care Pharmacy, American Cancer Society, Aries Pharma, Astellas Pharma US, Avella Specialty Pharmacy, Baronova, Braeburn Pharmaceuticals, Bristol Myers Squibb, Cardinal Health, Clear Health Quality Institute, Diopsys, Echosens, Emergency Response, Endogastric Solutions, EO2 Concepts, FAIR Health, GeneNews, GI Therapies, Gilead Sciences, Halt Medical, IMPAQ International, ImpediMed, Indivior Pharmaceuticals, InsightTec, Lumendi, Mallinckrodt Pharmaceuticals, Medtronic, Monteris Medical, Phosphorus, Proteus Digital Health, Rebiotix, Seno Medical, Senseonics, Sunovion, UCB Pharma; stock options/warrants in GeneNews, SonarMD.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
✓ Colorectal cancer (CRC) screening decreases CRC incidence and mortality and is estimated to be highly cost‐effective.
✓ Most CRC screening cost‐effectiveness analyses have used Medicare payment rates, which are substantially lower than commercial insurance rates.
✓ Many Americans have private insurance while they are younger, and Medicare coverage when they are 65 and older.
WHAT IS NEW HERE
✓ Although lifetime costs/person for CRC screening are up to 44% higher when assuming commercial payment rates for people under 65, CRC screening remains highly cost‐effective over a lifetime.
✓ While CRC screening may be relatively costly for commercial payers if only a time horizon of ages 50‐64 is considered, it yields substantial clinical and economic benefits that accrue primarily at ages ≥65 when patients are eligible for Medicare.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://doi.org/10.1038/s41395‐018‐0106‐8
Correspondence: U.L. (email: uri.ladabaum@stanford.edu)
Published online 15 June 2018
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Elmunzer BJ, Hayward RA, Schoenfeld PS, et al. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabeneck L, Rumble RB, Thompson F, et al. Fecal immunochemical tests compared with guaiac fecal occult blood tests for population-based colorectal cancer screening. Can J Gastroenterol. 2012;26:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi PG, Vicentini M, Sacchettini C, et al. Impact of screening program on incidence of colorectal cancer: a cohort study in Italy. Am J Gastroenterol. 2015;110:1359–66. [DOI] [PubMed] [Google Scholar]
- 7.Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter NN, Warren JL, Barrett MJ, et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 10.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SS, Kilgore ML. Cost effectiveness of colorectal cancer screening strategies. Cancer Control. 2015;22:248–58. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 14.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 15.Ladabaum U, Levin Z, Mannalithara A, et al. Colorectal testing utilization and payments in a large cohort of commercially insured US adults. Am J Gastroenterol. 2014;109:1513–25. [DOI] [PubMed] [Google Scholar]
- 16.Congressional Budget Office. An analysis of private-sector prices for physician services. https://www.cbo.gov/publication/52818. Accessed 28 August 2017.
- 17.Congressional Budget Office. An analysis of hospital prices for commercial and medicare advantage plans. https://www.cbo.gov/publication/52819. Accessed 28 August 2017.
- 18.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. [DOI] [PubMed] [Google Scholar]
- 19.Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. [DOI] [PubMed] [Google Scholar]
- 20.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151–62. [DOI] [PubMed] [Google Scholar]
- 21.Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol. 2013;108:120–32. [DOI] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2017;153:307–23. [DOI] [PubMed] [Google Scholar]
- 24.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. [DOI] [PubMed] [Google Scholar]
- 25.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial-SCORE. J Natl Cancer Inst. 2011;103:1310–22. [DOI] [PubMed] [Google Scholar]
- 26.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Clin Gastroenterol Hepatol. 2004;2:554–63. [DOI] [PubMed] [Google Scholar]
- 28.Song K, Fendrick AM, Ladabaum U. Fecal DNA testing compared to conventional colorectal cancer ccreening methods: a decision analysis. Gastroenterology. 2004;126:1270–9. [DOI] [PubMed] [Google Scholar]
- 29.Ladabaum U, Allen J, Wandell M, et al. Colorectal cancer screening with blood-based biomarkers: cost-effectiveness of methylated septin 9 DNA vs. current strategies. Cancer Epidemiol Biomark Prev. 2013;22:1567–76. [DOI] [PubMed] [Google Scholar]
- 30.Ladabaum U, Mannalithara A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology. 2016;151:427–439.e6. [DOI] [PubMed] [Google Scholar]
- 31.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. [DOI] [PubMed] [Google Scholar]
- 32.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule 2009. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/. Accessed 19 February 2017.
- 34.Centers for Medicare & Medicaid Services. Physician fee schedule 2009. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/. Accessed 19 February 2017.
- 35.Centers for Medicare & Medicaid Services. Inpatient prospective payment system 2009. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/. Accessed 19 February 2017.
- 36.Centers for Medicare & Medicaid Services. Hospital outpatient prospective payment system 2009. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. Accessed 19 February 2017.
- 37.Centers for Medicare & Medicaid Services. Ambulatory Surgical Center (ASC) payment 2009. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/index.html. Accessed 19 February 2017.
- 38.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, et al. Technology assessment: cost-effectiveness of DNA stool testing to screen for colorectal cancer. Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and Agency for Healthcare Research and Quality (US), Rockville, MD and SimCRC Models: 2007. [PubMed] [Google Scholar]
- 39.Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–41. [DOI] [PubMed] [Google Scholar]
- 40.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, et al. editors. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. p. 214–35. [Google Scholar]
- 41.Briggs A, Claxton K, Sculpher M. Making decision models probabilistic. Decision modelling for health economic evaluation. Cambridge, MA: Oxford University Press; 2006. p. 77–120. [Google Scholar]
- 42.Ladabaum U, Alvarez-Osorio L, Rosch T, et al. Cost-effectiveness of colorectal cancer screening in Germany: current endoscopic and fecal testing strategies versus plasma methylated Septin 9 DNA. Endosc Int Open. 2014;2:E96–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinh T, Ladabaum U, Alperin P, et al. Health Benefits and Cost-effectiveness of a Hybrid Screening Strategy for Colorectal Cancer. Clin Gastroenterol Hepatol. 2013;11:1158–66. [DOI] [PubMed] [Google Scholar]
- 44.Ladabaum U, Phillips KA. Colorectal cancer screening: Differential costs for younger versus older Americans. Am J Prev Med. 2006;30:378–84. [DOI] [PubMed] [Google Scholar]
- 45.Goede SL, Kuntz KM, van Ballegooijen M, et al. Cost-Savings to Medicare From Pre-Medicare Colorectal Cancer Screening. Med Care. 2015;53:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitch K, Pyenson B, Blumen H, et al. The value of colonoscopic colorectal cancer screening of adults aged 50 to 64. Am J Manag Care. 2015;21:e430–8. [PubMed] [Google Scholar]
- 47.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819–25. [DOI] [PubMed] [Google Scholar]
- 48.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–86. [DOI] [PubMed] [Google Scholar]
- 50.Arminski TC, McLean MD. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1,000 autopsy examinations. Dis Colon Rectum. 1964;7:249–61. [DOI] [PubMed] [Google Scholar]
- 51.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–57. [DOI] [PubMed] [Google Scholar]
- 52.Wagner JL, Tunis S, Brown M, et al. Cost-effectiveness of colorectal cancer screening in average risk adults. In: Young G, Rozen P, Levin B, editors. Prevention and early detection of colorectal cancer. Philadelphia: WB Saunders; 1996. p. 321–56. [Google Scholar]
- 53.Ries LAG, Kosary CL, Hankey BF, et al. , editors. SEER Cancer Statistics Review, 1973-94. NIH Pub. No. 97-2789. Bethesda, MD: National Cancer Institute; 1997. [Google Scholar]
- 54.Bernold DM, Sinicrope FA. Advances in chemotherapy for colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:808–21. [DOI] [PubMed] [Google Scholar]
- 55.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. [DOI] [PubMed] [Google Scholar]
- 56.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. [DOI] [PubMed] [Google Scholar]
- 57.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 58.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. [DOI] [PubMed] [Google Scholar]
- 59.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. [DOI] [PubMed] [Google Scholar]
- 60.Saltz LB, Meropol NJ, Loehrer PJ Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. [DOI] [PubMed] [Google Scholar]
- 61.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112: 594–642. [DOI] [PubMed] [Google Scholar]
- 62.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. [DOI] [PubMed] [Google Scholar]
- 63.Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162–9. [DOI] [PubMed] [Google Scholar]
- 64.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101: 343–50. [DOI] [PubMed] [Google Scholar]
- 65.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. [DOI] [PubMed] [Google Scholar]
- 66.Pignone M, Rich M, Teutsch SM, et al. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–41. [DOI] [PubMed] [Google Scholar]
- 67.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levin TR, Conell C, Shapiro JA, et al. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002;123:1786–92. [DOI] [PubMed] [Google Scholar]
- 69.Atkin WS, Cook CF, Cuzick J, et al. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291–300. [DOI] [PubMed] [Google Scholar]
- 70.Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. [DOI] [PubMed] [Google Scholar]
- 71.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95: 3418–22. [DOI] [PubMed] [Google Scholar]
- 72.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–906. [DOI] [PubMed] [Google Scholar]
- 73.Arora G, Mannalithara A, Singh G, et al. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–64. [DOI] [PubMed] [Google Scholar]
- 74.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–303. [PubMed] [Google Scholar]
- 75.Centers for Medicare & Medicaid Services. Physician fee schedule 2015. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/. Accessed 19 February 2016.
- 76.Centers for Medicare & Medicaid Services. Inpatient prospective payment system 2015. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/. Accessed 19 February 2016.