Abstract
OBJECTIVES:
Follow‐up colonoscopy rates among persons with positive fecal occult blood test results (FOBT + ) remain suboptimal in many jurisdictions. In Ontario, Canada, primary care providers (PCPs) are responsible for arranging follow‐up colonoscopies. The objectives were to understand the reasons for a lack of follow‐up colonoscopy and any action plans to address follow‐up.
METHODS:
Semi‐structured interviews were conducted with 30 FOBT+ persons and 30 PCPs in Ontario. Eligible FOBT+ persons were identified through administrative databases and included those aged 50‐74, with a 6‐12 month old FOBT+, no follow‐up colonoscopy, and no prior colorectal cancer diagnosis or colectomy. Eligible PCPs had ≥1 rostered FOBT+ person without follow‐up colonoscopy. Transcripts were analyzed inductively using Nvivo 11 (QSR International Pty Ltd., 2015).
RESULTS:
Reasons for lack of follow‐up colonoscopy were: person and/or provider believed the FOBT + was a false positive; person was afraid of colonoscopy; person had other health issues; and breakdown in communication of FOBT+ results or colonoscopy appointments. PCPs who initially recommended follow‐up colonoscopy did not change the minds of the persons who dismissed the FOBT+ as a false positive and/or who were afraid of the procedure. These FOBT+ persons negotiated an alternative follow‐up action plan including repeating the FOBT or not following‐up.
CONCLUSIONS:
PCPs may not adequately counsel FOBT+ persons who believe the FOBT+ is a false positive and/or fear colonoscopy. PCPs may lack fail‐safe systems to communicate FOBT+ results and colonoscopy appointments. Using navigators may help address these barriers and increase follow‐up rates.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer‐related death worldwide [1]. CRC is amenable to early detection with earlier diagnosis improving prognosis [2,3,4]. Like many regions around the world, Canadian provincial screening programs use fecal occult blood tests (FOBTs) ‐guaiac or immunochemical, depending on the province‐ as the initial CRC screening test [5]. When FOBTs are positive (FOBT+), colonoscopy is required for a definitive CRC diagnosis [6]. Delays in obtaining follow‐up colonoscopy increase the risk of CRC, including advanced‐stage disease [7, 8], while non‐adherence considerably increases the risk of CRC death [9]. Timely receipt of follow‐up colonoscopy is therefore critical to reducing the burden of CRC at the population level.
Colon Cancer Check (CCC) is Ontario's organized CRC screening program and Canada's largest CRC screening program, serving just over 4 million eligible individuals [6]. CCC recommends biennial guaiac FOBT (Hema‐Screen, Immunostics Inc., NJ, USA) for persons ages 50‐74 at average CRC risk [10]. Primary care providers (PCPs) facilitate screening by dispensing FOBT kits, receiving test results and arranging follow‐up colonoscopy for persons with FOBT + results. While a follow‐up colonoscopy rate of 85‐90% is recommended [5, 11], Ontario's follow‐up rate remains suboptimal ‐78% at the time of the study, which means nearly 4,450 FOBT + Ontarians did not have follow‐up colonoscopy in 2015 [12]. Similar concerns exist for regions in Europe, the United States and elsewhere in Canada, where rates vary widely and can be as low as 50 [13], 58 [14], and 63% [5] respectively.
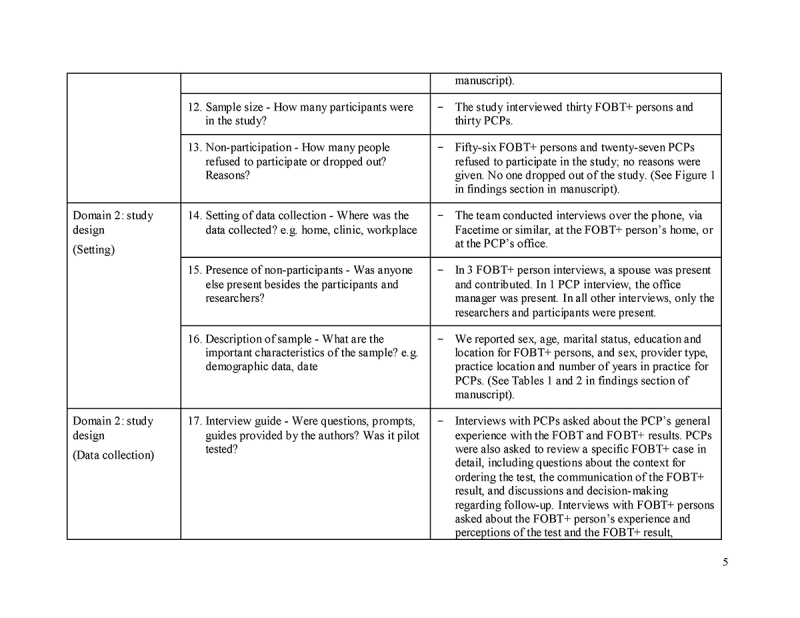
Understanding the reasons for a lack of follow‐up colonoscopy among FOBT+ persons is key to developing effective interventions to improve follow‐up. The extant literature provides some useful information for program planners[15,16,17,18,19,20,21]; however, prior studies have not collected first‐hand accounts from FOBT+ persons about the reasons for a lack of follow‐up colonoscopy, relying instead on medical records and administrative data [15,16,17,18,19,20,21].
In this study, we conducted interviews with Ontario FOBT+ persons who did not have follow‐up colonoscopy and with PCPs with at least one FOBT+ person in this situation to understand (1) the reasons for a lack of follow‐up colonoscopy; and (2) the action plans that were made to address follow‐up.
METHODS
Sunnybrook Research Institute's Research Ethics Board approved this qualitative study on 14 August 2014 (Project Identification Number 230‐2014).
Sampling and recruitment
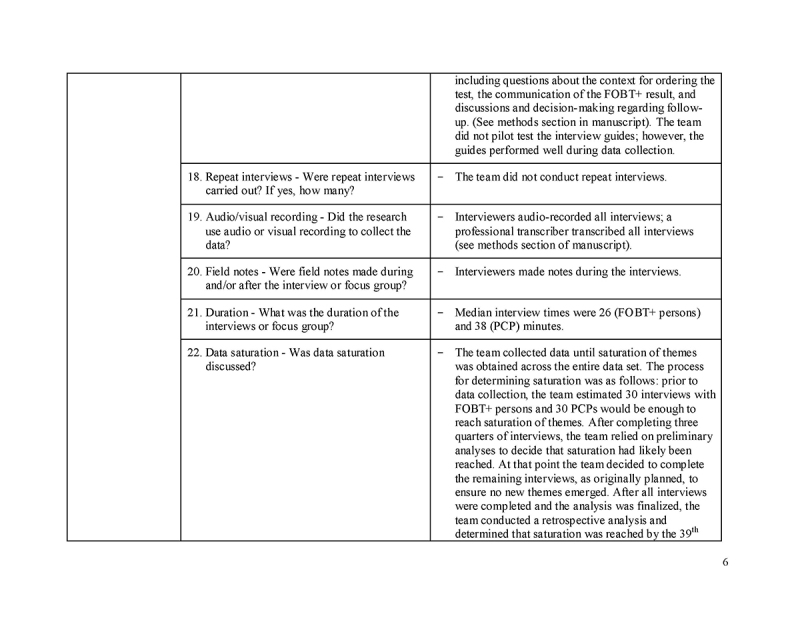
We used Cancer Care Ontario's (CCO) databases to identify all potentially eligible FOBT+ Ontarians (i.e. persons ages 50‐74 with a 6‐12 month old FOBT+ and no follow‐up colonoscopy within 6 months; excluding those with previous CRC diagnoses or colectomies, as per the Ontario Cancer Registry and Ontario Health Insurance Plan billing records, respectively). The six‐month follow‐up cut‐off was chosen because (1) in Ontario most follow‐up colonoscopies take place within this timeframe;[20] (2) CCO uses this timeframe for reporting purposes;[12] and (3) previous research suggests CRC risk increases after six months of the FOBT+ [7]. We also identified the PCPs who ordered these FOBTs. CCO staff then contacted a random subset of potentially eligible FOBT+ persons via mail to obtain consent to be contacted for the study. Study staff subsequently contacted interested individuals by telephone for further eligibility assessment and enrollment; FOBT+ persons were eligible if they correctly recalled having the test and that it was positive; FOBT+ persons were excluded if they had a follow‐up colonoscopy scheduled but not yet completed, and if they were unable to communicate in English. FOBT+ persons who were interviewed were asked for consent to invite their ordering PCP to participate in the study; we aimed to recruit as many matched PCP‐FOBT+ person pairs as possible. We also recruited PCPs not matched to a FOBT+ person in the study to increase the number of participating PCPs. All study respondents provided informed consent and received an honorarium for participating.
Data collection
We conducted semi‐structured interviews (telephone, Facetime or similar, or in‐person) from August‐December 2015. We relied on published research on barriers to screening to inform the interview guide. Interviews were audio‐recorded and transcribed. Matched PCPs were asked to describe the case of the FOBT+ person in the study. Non‐matched PCPs were asked to describe a FOBT+ person in their practice who had not had follow‐up colonoscopy. Following accepted standards of rigor in qualitative research, we collected data until saturation [22] was achieved across the entire data set.
Data analysis
We used a conventional content analysis approach [23]. We inductively analyzed transcripts to identify (1) reasons for a lack of follow‐up colonoscopy, and (2) action plans made to follow‐up on the FOBT+. We developed a codebook, which we refined iteratively. Coders analyzed transcripts independently and resolved discrepancies through discussion. Once all interviews were coded, we compared the reasons for a lack of colonoscopy reported by PCPs against those reported by FOBT+ persons to determine if any differences existed between them. Findings were discussed and consensus was achieved with all authors. We used NVivo 11 [24] for data management.
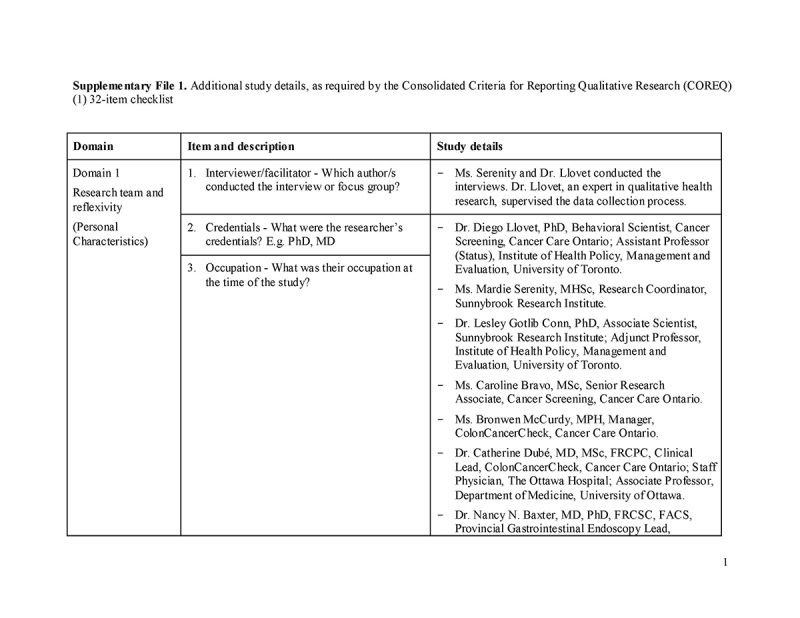
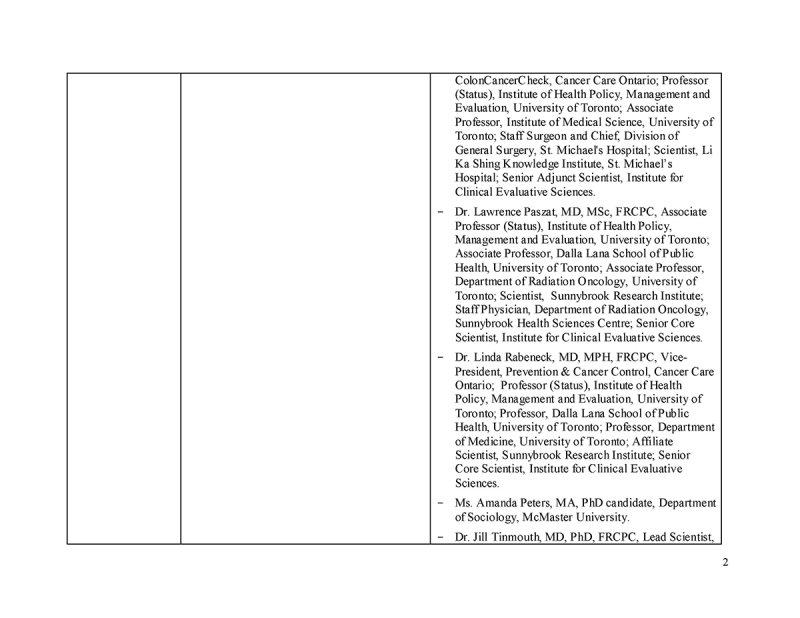
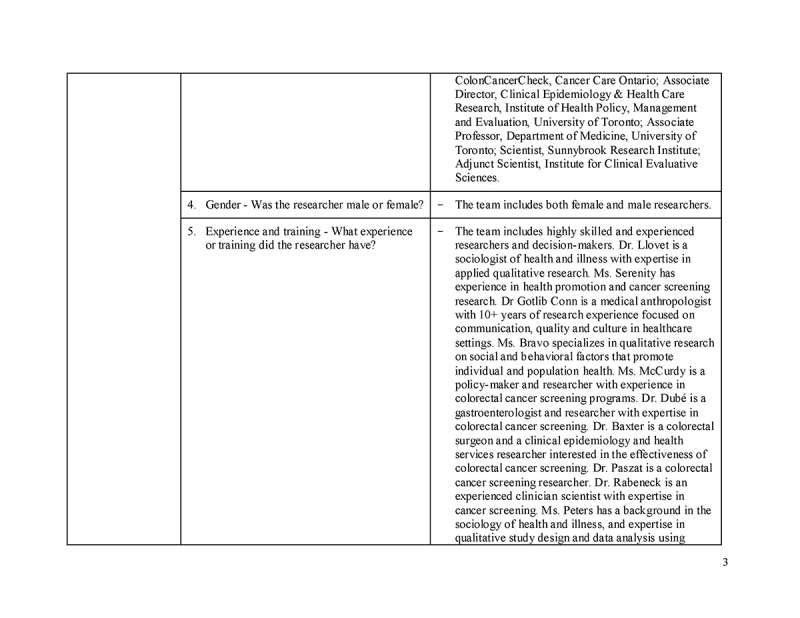
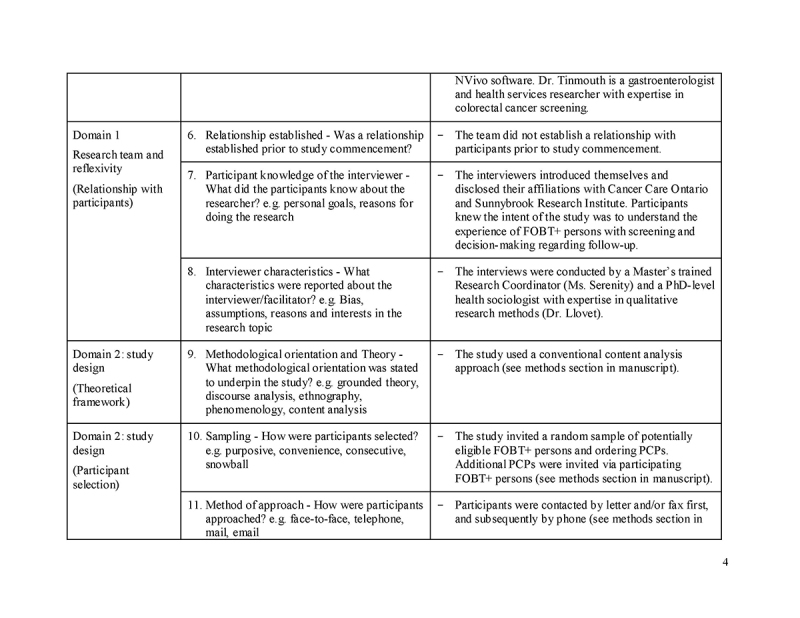
Please see Supplementary File 1 for additional details, as per the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist.
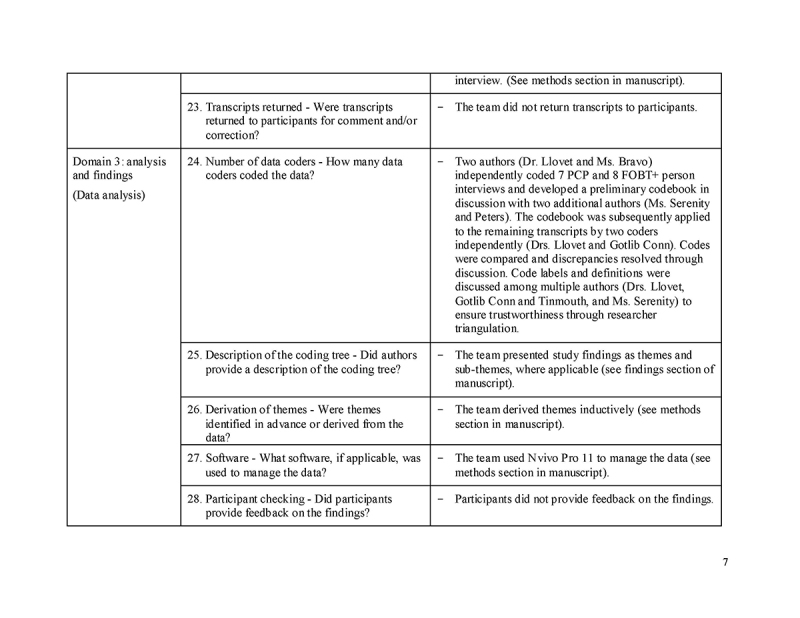
RESULTS
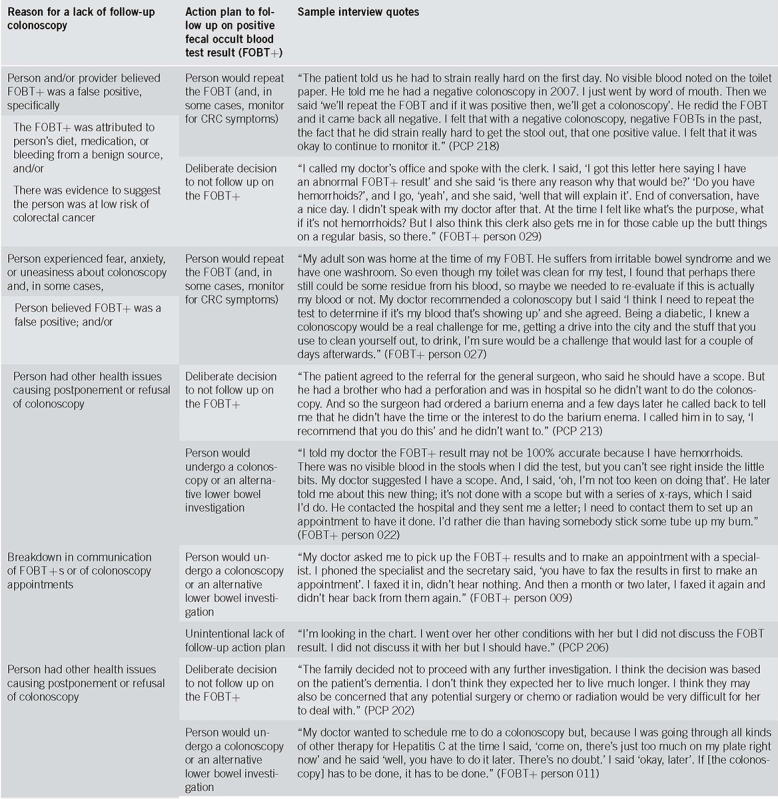
Thirty FOBT+ persons and 30 PCPs were interviewed, including 10 matched PCP‐FOBT+ person pairs. Figure 1 describes participant recruitment. Tables 1 and 2 present participant characteristics. We found four reasons for a lack of follow‐up colonoscopy and three action plans made to address the FOBT+; findings are presented narratively below and summarized in Table 3, with supporting quotes.
Fig. 1.
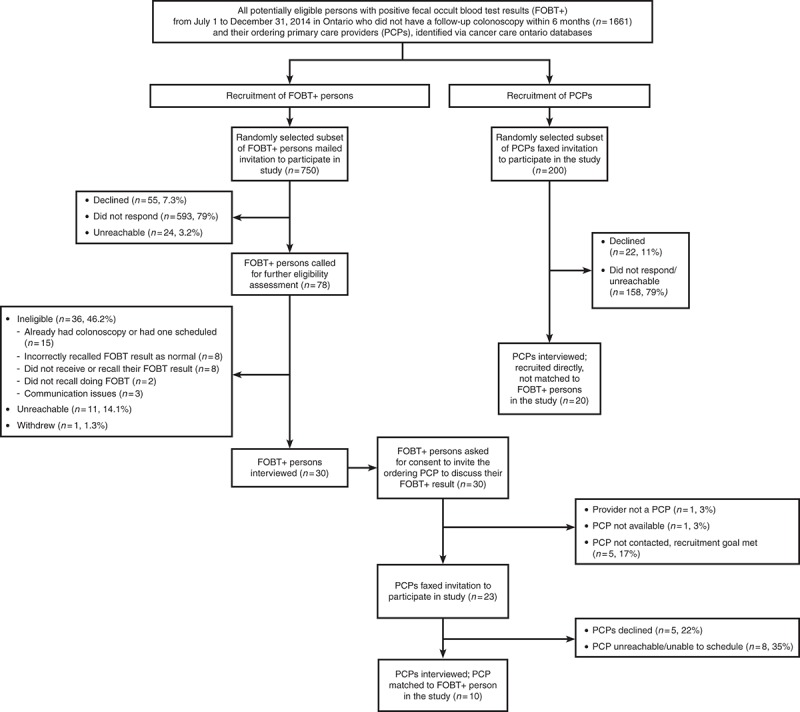
Recruitment of study participants
Table 1.
Participant characteristics: persons with positive fecal occult blood test results (n = 30), No
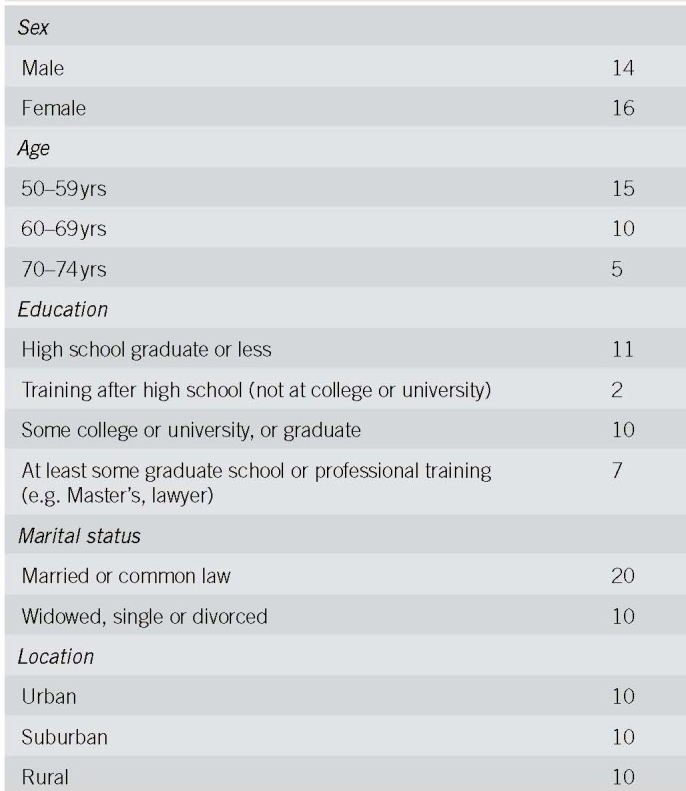
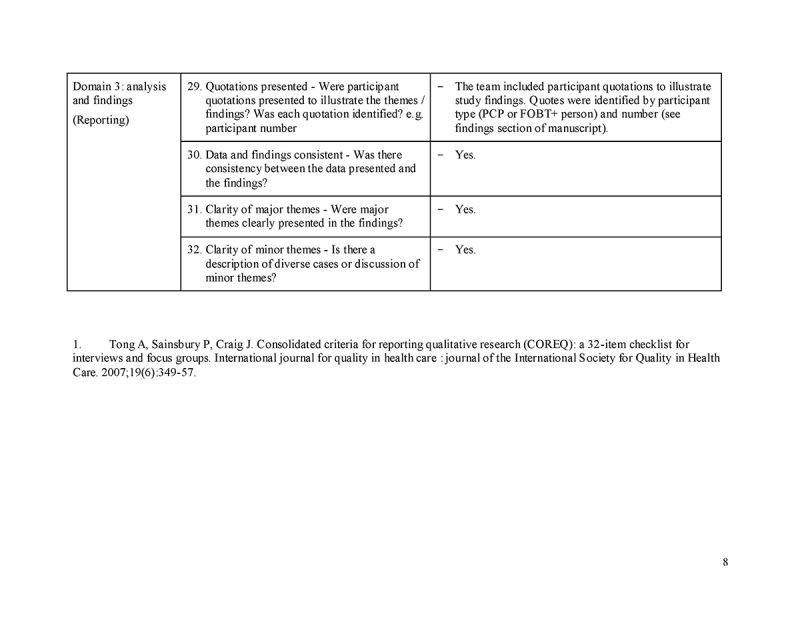
Table 2.
Participant characteristics: primary care providers (n = 30), No.
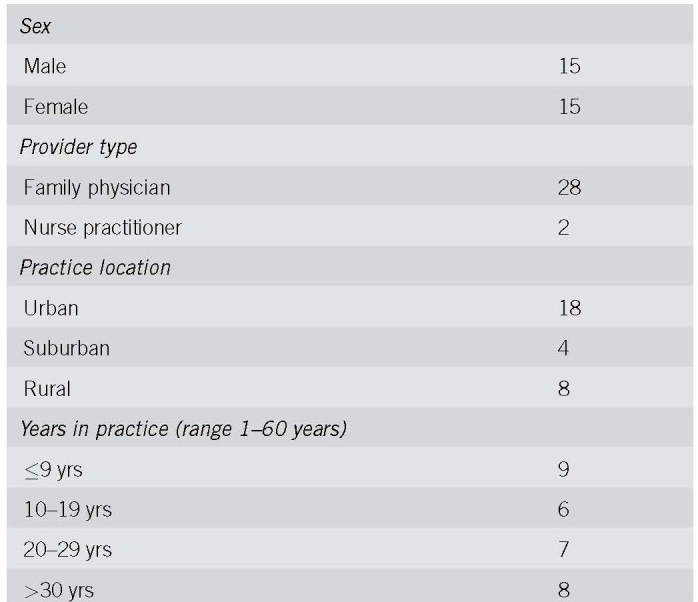
Table 3.
Thematic findings and sample interview quotes
No differences were found when comparing the reasons for a lack of follow‐up colonoscopy reported by PCP interviewees against those reported by FOBT+ participants. However, PCP participants (n = 10) reported unclear or unknown reasons more frequently than FOBT+ participants (n = 1). We also attempted to compare the reasons given by participants in the specific subset of 10 matched PCP‐FOBT+ person pairs but were unable to do so reliably because several of the PCP participants in this subset reported unclear or unknown reasons.
Reasons for a lack of follow‐up colonoscopy
Person and/or provider believed FOBT + was a false positive (n = 28/60; 11 PCP and 17 FOBT+ participants). This was the most reported reason for a lack of follow‐up colonoscopy. These participants attributed the FOBT+ to a benign source of bleeding (e.g. hemorrhoids, menstruation, and straining due to constipation), to diet (e.g. consuming beets, orange juice), or medication (e.g. blood thinner). While some FOBT+ persons knew at the time of sample collection that they were bleeding, in other cases FOBT+ persons and/or PCPs attributed the FOBT+ to benign bleeding after receiving the test result. Some PCPs and FOBT+ persons reported that having only one of three positive FOBT windows, along with the perceived existence of intermittent benign bleeding, helped them interpret the FOBT+ as a false positive.
Some PCPs and FOBT+ persons felt the risk of CRC was low because the person had had a recent normal colonoscopy prior to the FOBT+, or no other symptoms. Others questioned the FOBT+ by combining low CRC risk arguments with the attribution of the result to a benign source of bleeding, and/or the person's diet or medications.
In some cases, the idea that the FOBT+ was a false positive was suggested by providers; in others, it was suggested by FOBT+ persons.
Person experienced fear, anxiety, or uneasiness about colonoscopy (sometimes in combination with other reasons) (n = 12/60; 5 PCP and 7 FOBT+ participants). These FOBT+ persons were averse to the colonoscopy preparation, the procedure's invasiveness, or the possibility of experiencing harm and/or pain. Some had heard from loved ones who had bad colonoscopy experiences. Some participants reported fear of colonoscopy in combination with other reasons, such as a belief that the FOBT+ was a false positive and/or the need to prioritize other health concerns.
Breakdown in communication of FOBT + s or of colonoscopy appointments (n = 5/60; 2 PCP and 3 FOBT+ participants). Three types of communication breakdown prevented follow‐up colonoscopies: (1) the PCP was not aware of the FOBT+; (2) the PCP forgot to discuss the result with the FOBT+ person; and (3) the FOBT+ person was expecting but did not receive a colonoscopy appointment from the specialist office.
Person had other health issues causing postponement or refusal of colonoscopy (n = 4/60; 2 PCP and 2 FOBT+ participants). Competing health issues sometimes delayed plans for follow‐up colonoscopy (e.g., ongoing treatment for hepatitis C) or ruled it out altogether (e.g., terminal illness). The decision to postpone or not have a colonoscopy was sometimes made by the FOBT+ person or substitute decision maker, and in other cases, by the physician.
In 11 of 60 interviews, respondents reported unknown or unclear reasons for a lack of follow‐up colonoscopy.
Action plans to follow‐up on the FOBT+
Person would repeat the FOBT (and, in some cases, monitor for CRC symptoms) (n = 31/60; 13 PCP and 18 FOBT+ participants). Repeating the FOBT was the most commonly described action plan. In some cases, the PCP made the decision to repeat the FOBT without input from the FOBT+ person; in others, the decision resulted from a discussion between the two. In the latter case, FOBT+ persons shaped decision‐making by suggesting the FOBT+ may be a false positive and/or by stating their fear of colonoscopy. PCPs who had initially recommended a follow‐up colonoscopy did not pursue it based on the FOBT+ persons' perspective and/or concerns. While some of these PCPs initially attempted to counsel the FOBT+ person about the importance of having the recommended colonoscopy, others did not. In the latter case, PCPs readily accepted the FOBT+ persons' belief that the FOBT+ was a false positive, even when this belief appeared to be the result of speculation (e.g. FOBT+ person explained that his toilet could have been contaminated with someone else's blood, even though the toilet had been cleaned).
PCPs sometimes planned steps in addition to repeating the FOBT designed to detect possible CRC, including monitoring for new symptoms and resuming FOBT screening earlier (in 1 year instead of two).
Deliberate decision to not follow up on the FOBT + (n = 13/60; 8 PCP and 5 FOBT+ participants). Sometimes a deliberate decision was made to not follow up on the FOBT+ result. In some cases, the decision was made by the FOBT+ person (e.g. FOBT+ person refused the follow‐up colonoscopy recommended by the specialist, even after the PCP tried to persuade him to have it), or by the PCP or their designate (e.g. the clinic secretary asked the FOBT+ person to disregard the FOBT+ result as a false positive likely triggered by hemorrhoids). In others, the decision was the result of a negotiation between the FOBT+ person and their PCP (e.g. the PCP agreed with the FOBT+ person's plan to do nothing after checking that a prior colonoscopy had not shown polyps).
Person would undergo a colonoscopy or an alternative lower bowel investigation (n = 10/60; 4 PCP and 6 FOBT+participants). Participants sometimes reported plans for follow‐up colonoscopies that had not yet materialized. Reasons for this were: (1) PCP agreed to delay the follow‐up colonoscopy until the FOBT+ person completed another medical treatment; (2) FOBT+ person did not receive the colonoscopy appointment from the specialist office; and (3) FOBT+ person initiated the process to obtain colonoscopy (e.g. specialist visit) but was not sure she would complete it due to fear of the procedure. In one case, the FOBT+ person, fearful of colonoscopy, planned a CT colonography instead, a compromise suggested by his PCP.
Unintentional lack of follow‐up action plan (n = 2/60; 2 PCP participants). In some cases, FOBT+s remained unaddressed, likely unintentionally. This happened because there was no discussion of the FOBT+ (the PCP was not aware of it or forgot to discuss it with the FOBT+ person).
Three PCPs and one FOBT+ person reported not knowing, or being unclear about, the follow‐up action plan.
DISCUSSION
Through interviews with PCPs and FOBT+ persons, we identified four reasons for a lack of follow‐up colonoscopy, and three action plans that were made to address the FOBT+s. In a couple of cases, we found that there was no action plan. The belief that the FOBT+ was a false positive, and repeating the FOBT were the most reported reason and action plan, respectively.
In our study, FOBT+ persons sometimes believed their FOBT+ was a false positive. Psychological theory suggests this belief may represent defensive denial, a coping mechanism whereby individuals who become anxious after receiving threatening information (i.e. an FOBT+ may signal a health problem) can temporarily reduce their anxiety by denying that the threat exists [25, 26]. Prior studies have shown that recipients of positive FOBT results are more likely than recipients of negative results to experience higher levels of CRC‐related distress [27, 28]. While some FOBT+ persons had good reason to believe the FOBT+ was a false positive (e.g. the FOBT+ person irritated a hemorrhoid with the collection stick and transferred blood to the FOBT card), it is possible that other FOBT+ persons who questioned the validity of the FOBT+ on more speculative grounds (e.g. FOBT+ person believed that toilet was contaminated with someone else's blood even though the toilet had been cleaned) did so as a way to cope with their fear of CRC, even if they did not explicitly acknowledge this. Like Plumb et al. [19], we also found that fear of colonoscopy kept some FOBT+ persons from having the procedure. Unlike most previous research [15,16,17,18,20,21], our study emphasizes the importance of emotional distress (due to fear of colonoscopy, and possibly, of CRC) as a barrier to appropriate follow‐up.
Further, we found that decisions regarding follow‐up were sometimes the result of a negotiation between PCPs and FOBT+ persons, during which PCPs who had initially recommended a follow‐up colonoscopy later agreed to a repeat FOBT, an alternative lower bowel investigation, or no follow‐up. These negotiations were driven by the FOBT+ persons' belief that the FOBT+ was a false positive and/or stated fear of colonoscopy. In some cases, this negotiated approach was likely a reasonable way to address the concerns of FOBT+ persons (e.g., the FOBT+ person who was averse to colonoscopy and agreed instead to a CT colonography). In others, it appears that PCPs could have done more to counsel the FOBT+ person. This is especially true of cases where PCPs readily accepted the FOBT+ person's belief that the FOBT+ was a false positive (even when this belief appeared to be the result of speculation), rather than as a sign of possible emotional distress warranting further exploration.
Prior research has suggested that decisions to not pursue a follow‐up colonoscopy are the result of PCPs' lack of knowledge of guidelines [18, 20]. While this may sometimes be true, our study shows that in some cases these decisions may have less to do with the PCPs' lack of guideline knowledge (which they often have), and more to do with the challenges that PCPs face during sensitive conversations with FOBT+ persons who may be experiencing emotional turmoil. While provider knowledge of guidelines is critical to ensure adequate rates of follow‐up colonoscopy, ensuring providers employ effective counseling techniques [29,30,31,32] may be equally important.
Compared to FOBT‐ persons, FOBT+ persons are at increased risk of CRC; this risk may even extend to those who had a recent normal colonoscopy [33]. Failure to follow‐up after an abnormal FOBT is associated with a higher risk of CRC‐related death [9]. Therefore, it is imperative that providers discourage repeat FOBTs and avoid, where medically appropriate, supporting decisions to not follow up, as these actions may delay receipt of CRC treatment for FOBT+ persons with true positive findings, perhaps leading to diagnosis of later stage CRC [7]. Screening programs could support appropriate follow‐up by restricting the use of repeat FOBTs. Where guaiac FOBTs are used, refraining from providing information to PCPs and FOBT+ persons about the number of FOBT+ windows, which we and others have found to prompt questions about the validity of the test result [18, 20], may also help. By analogy, it may also be advisable for programs using quantitative fecal immunochemical tests (FIT) to communicate test results as either positive or negative and to avoid providing information about the hemoglobin concentration for positive tests (FIT+). Prior research shows that interventions that aim to educate PCPs on the definition of an FOBT+ (one having at least one positive window, in the case of the guaiac FOBT) and remind them of the need to pursue a follow‐up colonoscopy may not help increase follow‐up adherence [34].
Further, programs must develop better guidance to help PCPs select individuals for FOBT screening in the first place. Consistent with prior research [21], we found that some participants did not have a follow‐up colonoscopy on account of serious pre‐existing co‐morbidities (e.g. terminal illness); such individuals should not undergo screening. Improved program guidance for PCPs and participants should also extend to the use of FOBT in the presence of overt rectal bleeding, which we saw in our study. Better targeting of FOBT use at screening initiation may help reduce rates of non‐adherence among FOBT+ persons.
As in many jurisdictions, ColonCancerCheck currently relies on PCPs to arrange follow‐up for FOBT+ persons. While Ontario PCPs have been instrumental in helping the program achieve a relatively high follow‐up rate (compared to some other jurisdictions), it is possible that this same reliance on PCPs explains in part the program's suboptimal follow‐up rate. Our findings suggest some PCPs may not adequately counsel refractory FOBT+ persons. While this may be due to a PCP lack of patient‐centered communication skills [35,36,37,38,39,40], we also recognize that this may be due to a lack of time, given PCPs' heavy workload [41, 42]. We also showed some providers do not have fail‐safe information systems in place to communicate FOBT+s and colonoscopy appointments to FOBT+ persons. This is consistent with previous findings showing that primary care practices often lack office systems to support CRC screening [43].
Screening programs may want to consider a more centralized approach whereby a small group of specially trained navigators, and not PCPs, arrange follow‐up. Navigation is a promising strategy that has been shown to increase rates and timeliness of follow‐up colonoscopy [44, 45], and which has been successfully implemented in some Canadian and international jurisdictions. Trained navigators may be ideally suited to address the barriers we identified in our study, namely fear of colonoscopy, belief that the FOBT+ is a false positive, and breakdown in communication of FOBT+s and of colonoscopy appointments [44, 46]. Using centralized navigation to support, instead of replace, PCPs in arranging follow‐up (for example, in cases where the FOBT+ person has not had a follow‐up colonoscopy within a pre‐defined number of weeks of the FOBT+) may also be considered. PCP endorsement would, however, be essential to the success of any navigation intervention.
To our knowledge, our study was the first to directly interview FOBT+ persons who have not had follow‐up colonoscopy to understand the reasons for this lack of follow‐up. Previous studies have relied on chart reviews, which often report high rates of unknown or unclear reasons for a lack of colonoscopy [15,16,17,21], and administrative data, which identify correlates of a lack of follow‐up but shed no light on the motivations, circumstances and processes behind decision‐making [20]. Interviews with FOBT+ persons can supply some of this important information.
Our study has several limitations. First, non‐English speaking FOBT+ persons were excluded. Prior research has shown that language can be a barrier to participating in CRC screening [47,48,49]. This is a relevant consideration in the Ontario context, where 2.25% of the population is not able to converse in English, and where areas exist where PCPs are not proficient in the mother tongue of Non‐English speaking patients [50]. Second, we focused on the guaiac FOBT, as this is the test currently used in Ontario, while screening programs outside Ontario have begun to adopt FIT. However, our findings likely apply to FIT‐based programs. Because of the increased sensitivity of the test [51], FIT+ results may cause more anxiety than guaiac FOBT+ results, leading to a higher incidence of defensive denial preventing follow‐up. Our findings also likely apply to health care systems which, like most in the United States [52], take a more opportunistic approach to CRC screening and thus rely more heavily on PCPs to facilitate follow‐up. Third, we did not systematically collect information about the length of the relationship between FOBT+ persons and their PCPs, which may affect adherence to provider recommendations [53]. Fourth, we do not know how individuals who agreed to participate in the study were different from those who did not participate, so our findings may not be generalizable to the entire population of non‐adherent FOBT+ persons. We believe this limitation is offset by the strength of our qualitative design, which permitted a detailed exploration of important contextual factors affecting FOBT+ follow‐up that would not have been possible using other methods [54]. Fifth, our study relied on retrospective reports by participants, which may be subject to bias [55]. While direct observation of decision‐making regarding follow‐up may provide more accurate data, conducting prospective studies of this population is not feasible. Carefully collected retrospective self‐reports may therefore be the best option to qualitatively study the processes that result in non‐adherence to follow‐up colonoscopy. Further, we believe the risk of bias in our study is low because the themes from interviews with FOBT+ persons were consistent with those from PCP interviews.
In sum, our study identified four barriers to follow‐up colonoscopy: a belief that the FOBT+ was a false positive, fear of colonoscopy, other health issues, and a breakdown in the communication of FOBT+ results and of colonoscopy appointments. Study findings should also be transferable to screening programs using FIT instead of the guaiac FOBT, and to those relying on PCPs to arrange follow‐up. Using a combination of education and policy interventions along with trained program navigators may help overcome the barriers identified in this study.
ACKNOWLEDGEMENTS
We would like to acknowledge the contributions of the following individuals: Ms. Jessie Cunningham, Cancer Care Ontario's Medical Librarian, for knowledge synthesis and literature support services; Ms. Krystal Hartman, Senior Research Associate, Cancer Care Ontario, for formatting support; and Ms. Reena Manohar, Graphic Designer with the Digital & Visual Communications Department, Sunnybrook Health Sciences Centre, for design services.
CONFLICT OF INTEREST
Guarantor of the article: Diego Llovet, PhD.
Specific author contributions: Drs. Tinmouth, Llovet, Dubé, Baxter, Paszat, and Rabeneck, and Ms. Serenity and McCurdy contributed to the study concept and design. Dr. Llovet and Ms. Serenity collected the data. Drs. Llovet, Gotlib Conn and Tinmouth, and Ms. Serenity, Bravo, and Peters analyzed and interpreted the data. Drs. Llovet, Gotlib Conn, and Tinmouth drafted the manuscript, and all authors critically revised the manuscript for important intellectual content. Dr. Tinmouth obtained the funding to conduct the study. All authors approved the draft submitted.
Financial support: This study was funded by the Ontario Institute for Cancer Research (OICR), Health Services Research Program (PI: Dr. Tinmouth). OICR did not have a role in the design of this study, the collection, analysis, or interpretation of data, or the writing of this manuscript.
Potential competing interests: Dr. Llovet is a Behavioral Scientist in the Cancer Screening Unit, Cancer Care Ontario. Ms. Bravo is a Senior Research Associate in the Cancer Screening Unit, Cancer Care Ontario. Ms. McCurdy is the Manager for the ColonCancer‐Check program at Cancer Care Ontario. Dr. Dubé is the Clinical Lead for the ColonCancerCheck program. Dr. Baxter is Cancer Care Ontario's Provincial Gastrointestinal Endoscopy Lead. Dr. Rabeneck oversees the ColonCancerCheck program as Vice‐President, Cancer Prevention and Control. Dr. Tinmouth is the Lead Scientist for ColonCancerCheck. The remaining authors declare that they have no conflict of interest.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
✓ Screening with fecal occult blood tests (FOBT) can reduce colorectal cancer mortality only if persons with positive results (FOBT+) follow‐up with colonoscopy.
✓ Follow‐up colonoscopy rates remain suboptimal in many jurisdictions.
✓ In some jurisdictions, primary care providers (PCPs) are responsible for arranging follow‐up for FOBT+ persons.
✓ Previous research on the reasons for a lack of follow‐up colonoscopy has relied exclusively on chart reviews and administrative data.
WHAT IS NEW HERE
✓ Ours is the first study to collect first‐hand accounts from FOBT+ persons regarding the lack of follow‐up colonoscopy.
✓ Some PCPs did not address barriers to colonoscopy reported by FOBT+ persons, who negotiated inappropriate alternative follow‐up plans.
✓ This suggests PCPs' approach to counseling may be an important factor in the lack of follow‐up colonoscopy among FOBT+ persons.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://doi.org/10.1038/s41395‐018‐0381‐4
Correspondence: D.L. (email: diego.llovet@cancercare.on.ca)
Published online 25 October 2018
REFERENCES
- 1.World Health Organization. Cancer Fact Sheet. World Health Organization, 2018. [Google Scholar]
- 2.Mandel J, Bond J, Church T, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71. [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Partnership Against Cancer. Colorectal Cancer Screening in Canada: Monitoring and Evaluation of Quality Indicators - Results Report, January 2013 - December 2014. Canadian Partnership Against Cancer; Toronto; 2017. [Google Scholar]
- 6.Cancer Care Ontario. Ontario Cancer Screening Performance Report 2016. Toronto: Cancer Care Ontario; 2016. [Google Scholar]
- 7.Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5): djw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinmouth J, Vella E, Baxter N, et al. Colorectal cancer screening in average risk populations: evidence summary. Program in evidence-based care evidence summary No.: 15-14. Toronto: Cancer Care Ontario; 2015. [Google Scholar]
- 11.International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st ed. Luxembourg: Publications Office of the European Union; 2010. p. 454. [Google Scholar]
- 12.Cancer Quality Council of Ontario. Cancer system quality index: colorectal cancer screening follow-up. Toronto: Cancer Quality Council of Ontario; 2017. [Google Scholar]
- 13.Ponti A, Anttila A, Ronco G, et al. Cancer Screening in the European Union Report on the Implementation of the Council Recommendation on Cancer Screening. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 14.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to colonoscopy after positive fecal blood test in four U.S. health care systems. Cancer Epidemiol Biomark Prev. 2016;25(2):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oluloro A, Petrik AF, Turner A, et al. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Community Health. 2016;41(4):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimbo M, Myers RE, Meyer B, et al. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009;7(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130(1):93. e1-e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baig N, Myers RE, Turner BJ, et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol. 2003;98(9):2078–81. [DOI] [PubMed] [Google Scholar]
- 19.Plumb A, Ghanouni A, Rainbow S, et al. Patient factors associated with non-attendance at colonoscopy after a positive screening faecal occult blood test. J Med Screen. 2017;24(1):12–9. [DOI] [PubMed] [Google Scholar]
- 20.Correia A, Rabeneck L, Baxter NN, et al. Lack of follow-up colonoscopy after positive FOBT in an organized colorectal cancer screening program is associated with modifiable health care practices. Prev Med. 2015;76:115–22. [DOI] [PubMed] [Google Scholar]
- 21.Carlson CM, Kirby KA, Casadei MA, et al. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med. 2011;171(3):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morse J. The significance of saturation. Qual Health Res. 1995;5(2):147–9. [Google Scholar]
- 23.Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. [DOI] [PubMed] [Google Scholar]
- 24.NVivo. Version 11. 4.1.1064. QSR International Pty Ltd. Burlington, MA, 2015. [Google Scholar]
- 25.Ditto P, Lopez D. Motivated skepticism: use of differential decision criteria for preferred and nonpreferred conclusions. J Personal Soc Psychol. 1992;63(4):568–84. [Google Scholar]
- 26.Wiebe D, Korbel C. Defensive denial, affect, and the self-regulation of health threats. In: Cameron L, Leventhal H, editors. The Self-Regulation of Health and Illness Behaviour. New York: Routledge; 2003. p. 184–203. [Google Scholar]
- 27.Laing S, Bogart A, Chubak J, et al. Psychological distress after a positive fecal occult blood test result among members of an integrated healthcare delivery system. Cancer Epidemiol Biomark Prev. 2014;23(1):154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denters MJ, Deutekom M, Essink-Bot ML, et al. FIT false-positives in colorectal cancer screening experience psychological distress up to 6 weeks after colonoscopy. Support Care Cancer. 2013;21(10): 2809–15. [DOI] [PubMed] [Google Scholar]
- 29.Coulehan JL, Block MR. The medical interview: mastering skills for clinical practice. Philadelphia: F.A. Davis Company; 2006. [Google Scholar]
- 30.Ness DE, Ende J. Denial in the medical interview. Recognition and management. JAMA. 1994;272(22):1777–81. [PubMed] [Google Scholar]
- 31.Cole S, Bird J. The medical interview: the three function approach. Philadelphia: Elsevier, Saunders; 2014. [Google Scholar]
- 32.Balint M. The doctor's therapeutic function. Lancet. 1965;1(7397): 1177–80. [DOI] [PubMed] [Google Scholar]
- 33.Tinmouth J, Lim T, Kone A, et al. 968 Risk of colorectal cancer among those who are gFOBt positive but have had a recent prior colonoscopy: Experience from an organized screening program. Gastroenterology. 2015;148(4 Suppl):S-189-S-90. [Google Scholar]
- 34.Stock D, Rabeneck L, Baxter NN, et al. Mailed participant reminders are associated with improved colonoscopy uptake after a positive FOBT result in Ontario's ColonCancerCheck program. Implement Sci. 2015;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021–7. [DOI] [PubMed] [Google Scholar]
- 36.Ashbury F, Iverson D, Kralj B. Physician communication skills: results of a survey of general/family practitioners in Newfoundland. Med Educ Online. 2001;6(1):4521. [DOI] [PubMed] [Google Scholar]
- 37.Marvel M, Epstein RM, Flowers K, et al. Soliciting the patient's agenda: have we improved? JAMA. 1999;281(3):283–7. [DOI] [PubMed] [Google Scholar]
- 38.Levinson W, Lesser C, Epstein R. Developing physician communication skills for patient-centered care. Health Aff (Millwood). 2010;29(7): 1310–8. [DOI] [PubMed] [Google Scholar]
- 39.Tai-Seale M, McGuire T, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42(5):1871–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarnall K, Pollak K, Østbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manca DP, Varnhagen S, Brett-MacLean P, et al. Rewards and challenges of family practice: web-based survey using the Delphi method. Can Fam Physician. 2007;53(2):277–86. [PMC free article] [PubMed] [Google Scholar]
- 42.Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172(18):1377–85. [DOI] [PubMed] [Google Scholar]
- 43.Klabunde CN, Lanier D, Nadel MR, et al. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med. 2009;37(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raich PC, Whitley EM, Thorland W, et al. Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomark Prev. 2012;21(10):1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selby K, Baumgartner C, Levin T, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med. 2017;167(8):565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escoffery C, Fernandez ME, Vernon SW, et al. Patient navigation in a colorectal cancer screening program. J Public Health Manag Pract. 2015;21(5):433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson-Kozlow M, Roussos S, Rovniak L, et al. Colorectal cancer test use among Californians of Mexican origin: influence of language barriers. Ethn Dis. 2009;19(3):315–22. [PMC free article] [PubMed] [Google Scholar]
- 48.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168(12): 1317–24. [DOI] [PubMed] [Google Scholar]
- 49.Diaz JA, Roberts MB, Goldman RE, et al. Effect of language on colorectal cancer screening among Latinos and non-Latinos. Cancer Epidemiol Biomark Prev. 2008;17(8):2169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sears J, Khan K, Ardern CI, et al. Potential for patient-physician language discordance in Ontario. BMC Health Serv Res. 2013;13:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tinmouth J, Lansdorp-Vogelaar I, Allison J. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64(8):1327–37. [DOI] [PubMed] [Google Scholar]
- 52.Levin TR, Jamieson L, Burley DA, et al. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–10. [DOI] [PubMed] [Google Scholar]
- 53.Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–42. [DOI] [PubMed] [Google Scholar]
- 54.Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ. 1995;311(6996):42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz N. Retrospective and concurrent self-reports: the rationale for real-time data capture. In: Stone A, Shiffman SS, Atienza A & Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. New York: Oxford University Press. 2007;11:26. [Google Scholar]