Abstract
OBJECTIVES:
Existing algorithms predicting the risk of colorectal cancer (CRC) assign a fixed score for family history of CRC. Whether the increased CRC risk attributed to family history of CRC was higher in younger patients remains inconclusive. We examined the risk of CRC associated with family history of CRC in first‐degree relative (FDR) according to the age of index subjects (<40 vs. ≥40; <50 vs. ≥50; and <60 vs. ≥60 years).
METHODS:
Ovid Medline, EMBASE, and gray literature from the reference lists of all identified studies were searched from their inception to March 2017. We included case‐control/cohort studies that investigated the relationship between family history of CRC in FDR and prevalence of CRC. Two reviewers independently selected articles according to the PRISMA guideline. A random effects meta‐analysis pooled relative risks (RR).
RESULTS:
We analyzed 9.28 million subjects from 63 studies. A family history of CRC in FDR confers a higher risk of CRC (RR = 1.76, 95% CI = 1.57‐1.97, p < 0.001). This increased risk was higher in younger individuals (RR = 3.29, 95% CI = 1.67‐6.49 for <40 years versus RR = 1.42, 95% CI = 1.24‐1.62 for ≥40 years, p = 0.017; RR = 2.81, 95% CI = 1.94‐4.07 for <50 years versus RR = 1.47, 95% CI = 1.28‐1.69 for ≥50 years, p = 0.001). No publication bias was identified, and the findings are robust in subgroup analyses.
CONCLUSIONS:
The increase in relative risk of CRC attributed to family history was found to be higher in younger individuals. Family history of CRC could be assigned a higher score for younger subjects in CRC risk prediction algorithms. Future studies should examine if such approach may improve their predictive capability.
INTRODUCTION
Globally, colorectal cancer (CRC) is the third commonest diagnosed cancer, and the second leading cause of cancer‐related mortality [1]. It accounted for 10% of all malignancies and 8% of all cancer deaths. Its incidence and mortality are rising rapidly in Asia Pacific countries, and poses substantial public health challenges in terms of its healthcare costs incurred [2]. Most CRC are sporadic with one‐fifth associated with familial clustering and 6% attributed to familial adenomatous polyposis syndrome and hereditary nonpolyposis colorectal cancer [3].
In the past decade, the concept of personalized medicine and targeted screening has become increasingly popular [4]. Risk scores devised and validated to predict the risk of CRC could improve detection yield and optimize screening efficiency. Clinically, it allows individuals to be more aware of their own risk, make informed decisions on choices of screening tests, and tailor the intensity of screening or prevention approaches to the predicted level of risk. From a public health perspective, the implementation of risk stratification strategies may better justify allocation and utilization of colonoscopic resources, facilitate resource planning in the formulation of population‐based screening programs, and reduce healthcare costs. There are several at‐risk groups who should receive earlier screening, and colonoscopy is more preferred among the increased risk individuals.
A recent systematic review provided a comprehensive analysis of risk prediction tools for risk of CRC in asymptomatic individuals within the general population [5]. A total of 52 risk models have been identified, and among them 33 included family history of CRC as a major predictor. Among these predictors, the risk increase is strongest for subjects with first‐degree relatives (FDRs) with CRC, where their risk of CRC could be increased by two‐ to three‐fold [6, 7]. However, current risk scoring systems assigned a fixed score for a positive family history of CRC, irrespective of the age of the screening participant. There have been three metaanalyses that assessed the association between familial CRC risks in FDR of CRC according to the nature of family history [8,9,10]. A 2001 meta‐analysis was limited by the modest number of studies, making comparison of CRC risk according to age of screening subjects who reported family history of CRC underpowered [8]. Two subsequent meta‐analyses showed the relative risk (RR) of CRC among FDRs decreased with age of CRC diagnosis in the proband [9, 10]. Since then, many large‐scale studies have been published, including a multinational, multicenter study from our expert panels in the Asia Pacific Working Group for CRC [11]. It is still uncertain if the increased risk of CRC conferred by a family history of CRC in FDR is significantly higher in younger individuals than older subjects. An updated meta‐analysis is needed to address this important knowledge gap.
The objective of this systematic review and meta‐analysis is to quantify the risk of CRC in asymptomatic subjects with a FDR with CRC compared with those with no such family history. We tested the a priori hypothesis that the increased CRC risk associated with a family history of CRC in FDR was not higher in younger subjects compared to older subjects. If this null hypothesis is rejected, the finding would serve as an evidence to recommend that a higher score should be assigned to family history of CRC in younger individuals.
METHODS
Search strategy and selection criteria
This systematic review and meta‐analysis was performed with adherence to the PRISMA (Preferred Reporting Items for Systematic reviews and meta‐Analyses) statement [12], performed according to a pre‐determined protocol. Ovid Medline and EMBASE were searched from their inception to March 2017, as were gray literature from the reference lists of all identified studies. Three main categories of the search terms were used: “colorectal cancer”, “family”, and “study design”. All searches were limited to English language. The following search terms were used: (1) AND (2) AND (3) AND (4):
(1) “colon”, “colonic”, “rectal”, “rectum”, “colorectal”, “colorectum”, “bowel”
(2) “neoplas”, “cancer”, “carcinoma”, “adenoma”, “adenocarcinoma”, “polyp”
(3) “family”, “familial”, “parent”, “father”, “mother”, “paternal”, “maternal”, “offspring”, “child”, “children”, “son”, “daughter”, “sibling”, “brother”, “sister”
(4) “case control”, “cohort”, “cross‐sectional”, “observational”, “epidemiologic”, “prospective”, and “retrospective”. Supplementary Table 1 shows detailed search strategy.
All articles extracted from the databases were filtered. Reference lists of eligible studies and related meta‐analyses were hand searched to identify further relevant studies. Two reviewers (CHC and JL) independently screened all abstracts identified in the initial search and excluded studies not fulfilling the eligible criteria. The search was restricted to case‐control and cohort studies that investigated the relationship between family history of CRC in FDR and incidence/prevalence of CRC. The following types of studies were excluded:
1. Studies that recruited symptomatic patients;
2. Studies with duplicated data;
3. Studies not specifying the type of family history;
4. Studies that did not define subjects in their “non‐exposure group” as those without family history of CRC in FDRs.
A third reviewer (JLWH) reviewed all selected studies to ensure they met the inclusion criteria.
Data extraction
Data from summary estimates of all eligible studies were obtained. CHC and JL extracted data from all selected full‐text articles reviewed in duplicate, and in the case of disagreement, consensus was reached via referral to a third reviewer (MCSW). The information on study population and study design was extracted into a database. All estimations of the RR and their precision were collected. If they were not provided, the estimations of RR were calculated as frequencies of the exposed and non‐exposed among cases and controls. The odds ratios (ORs) were extracted directly with an assumption that ORs are good estimates of RR [13], since CRC is relatively rare among asymptomatic subjects (prevalence <1%) [14]. Adjusted risk estimates were used if they were adjusted for relevant effect moderators; otherwise unadjusted estimates or raw data were collected to calculate the relative risks. When data were shown separately in strata, Mantel‐Haenzel method was used to pool raw data [15]. The inverse variance method was used for data presented as relative risks and confidence intervals (CIs). Stratified data were also recorded and categorized according to potential effect modifiers for subgroup analysis.
Quality assessment
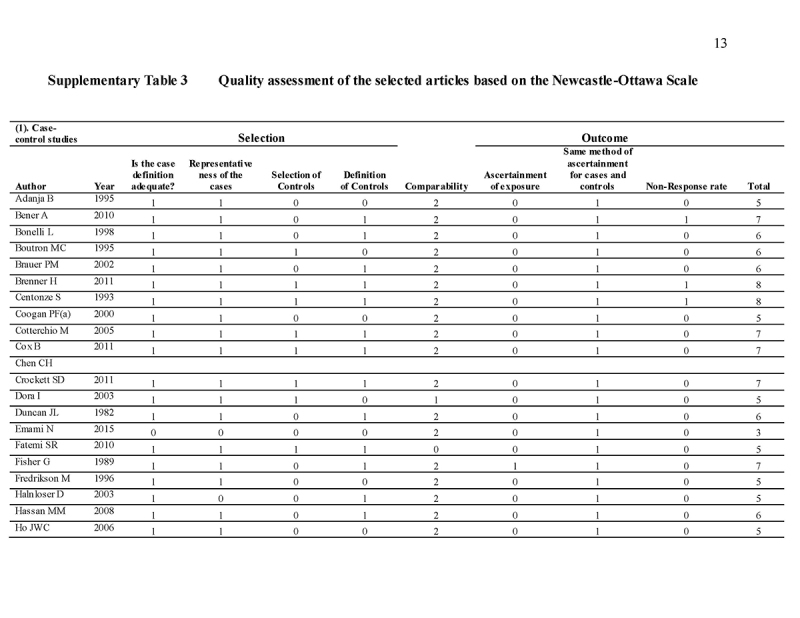
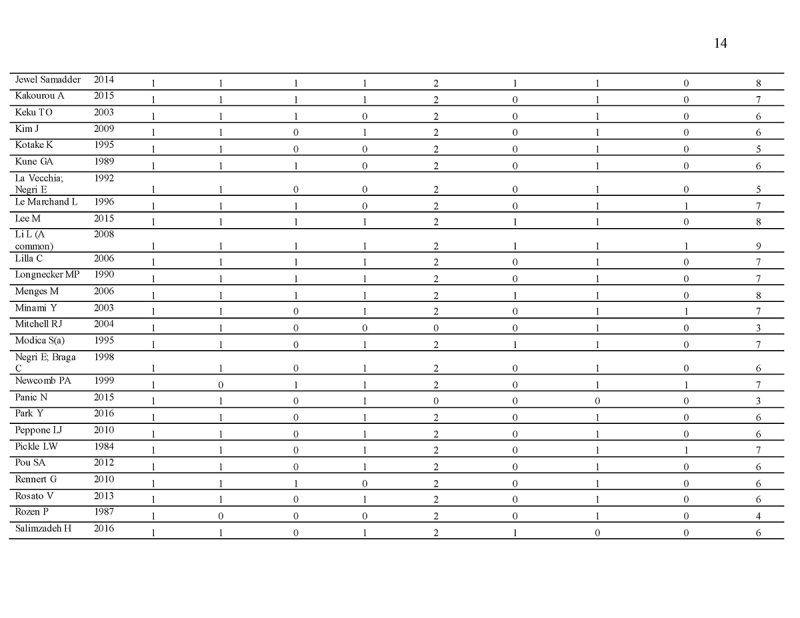
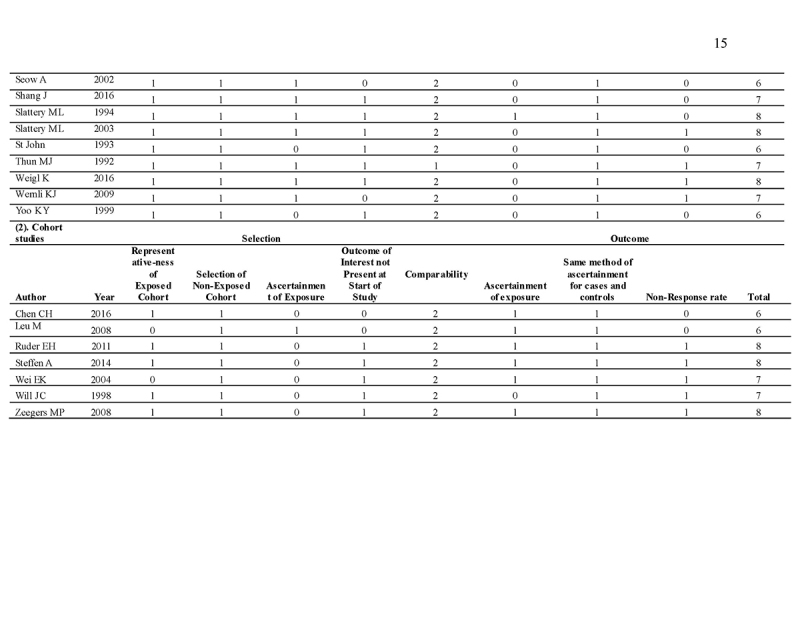
The Newcastle‐Ottawa Scale (NOS) is used as a quality assessment tool for observational studies [16, 17]. It is a 9‐point scale with eight items on the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively. The quality of studies was scored based on these eight items. The main items for study quality scoring were as follows: (1) representativeness of the exposed cohort: one point was assigned if the subjects represent the general population; no points were assigned if samples are special population groups (e.g., veteran) or not mentioned; (2) selection of the non‐exposed cohort: one point was assigned if the non‐exposed cohort (i.e., subjects without family history) was drawn from the same community as the exposed cohort (i.e., subject with family history); (3) ascertainment of exposure: one point was assigned if family history was ascertained by healthcare professionals; (4) demonstration that outcome of interest was not present at study commencement: one point was assigned for stating exclusion of subjects with CRA (colorectal adenoma)/advanced CRA/CRC or stating subjects have no history of CRA/advanced CRA/CRC; (5) comparability of cohorts on the basis of the design or analysis: two points were assigned for studies that adjusted for covariates in the statistical analysis, and one point was given if there was no adjustment; (6) assessment of outcome by colonoscopy and histological examination: one point was assigned if it was based on medical records; (7) follow‐up duration: one point was assigned for all eligible studies if the follow‐up period is long enough to detect the outcome of interest; (8) adequacy of follow‐up among cohorts: one point was assigned for completion of at least 90% follow‐up. Scores ranged from 0 (lowest) to 9 (highest). Similar to a previous meta‐analysis [18], studies with scores ≥7 and <7 were classified as “high” and “low” quality, respectively.
Statistical analysis
The relative risk was used as the effect measure of outcomes. Random effects meta‐analysis was performed using the inverse variance method. Between‐study variance in the random effects model was estimated by a restricted maximum‐likelihood estimator [19]. Heterogeneity between the included studies was assessed using the I2 statistic from the standard chi‐square test [20]. This describes the percentage of the variability in the effect estimates resulting from heterogeneity. I2 > 50% was considered to reflect significant statistical heterogeneity and in such cases the random effects model was used, otherwise the fixed effects model was used.
Owing to heterogeneity and possible between‐factor interactions, subgroup analyses were conducted by random effects models with separate estimates for between‐study variance across different subgroups [21]. The subgroup differences were tested by the Q‐tests. Meta‐regression was used to investigate the effect of any potential confounders. Univariate meta‐regression analysis was used and followed by multivariate modeling for covariates with p < 0.2. We compared the risk of CRC in subjects at different age with affected FDRs, namely, ≥40 years versus <40 years; ≥50 years versus <50 years; and ≥60 years versus <60 years. To explore possible publication bias, a funnel plot was used as a graphical presentation of the data. Begg's and Egger's regression tests were performed to examine funnel plot asymmetry by Comprehensive Meta Analysis (version 2.2, Biostat, Inc., 2011) [22]. The funnel plot is a scatter plot of the effect estimate from every study enrolled in the meta‐analysis against the measure of its precision (1/standard error). If either the Begg's or Egger's test indicated publication bias, the Trim and Fill method would be used to estimate the number of missing studies and the treatment effect after adjustment for small‐study effect [23]. All of these analyses were conducted by using R version 3.3.2 with package meta ver 4.6‐0 and metafor ver 1.9‐9.
Subgroup analysis
Since the data were expected to be heterogeneous, we performed 10 additional subgroup analyses on the risk of CRC according to the following characteristics: (1) number of FDR affected: one versus ≥1 versus ≥2 FDRs; (2) age of affected relatives; (3) type of family history exposure: parents versus siblings; (4) site of CRC in the proband: colon versus rectum; (5) gender of the proband; (6) gender of exposed relatives; (7) study design: cohort studies versus case‐control studies versus nested case‐control studies at different settings; (8) outcomes: incidence versus mortality; (9) geographic region of study: Western Pacific versus America vs. Eastern Mediterranean versus Europe; and (10) method of assessment of family history: medical records versus self‐reported history. These subgroup analyses are important as we perceived them as potential effect modifiers of the present meta‐analysis. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
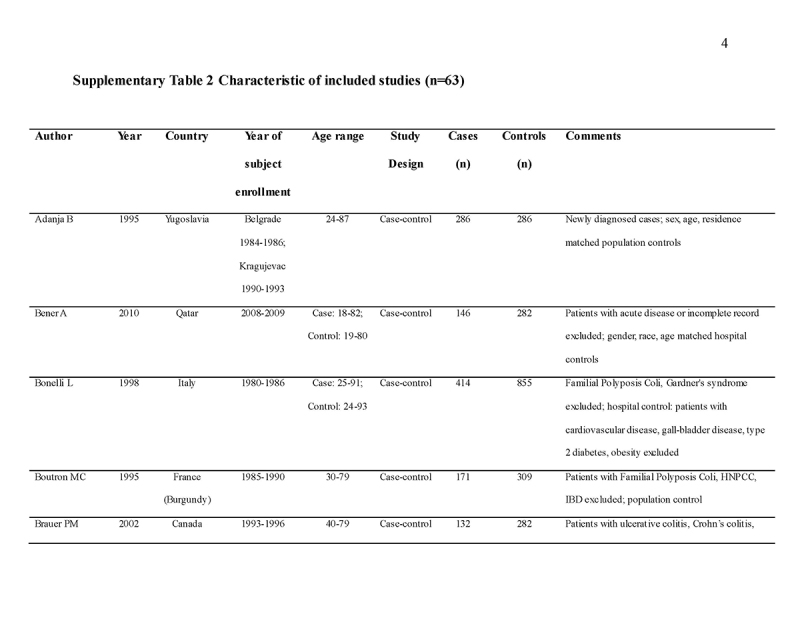
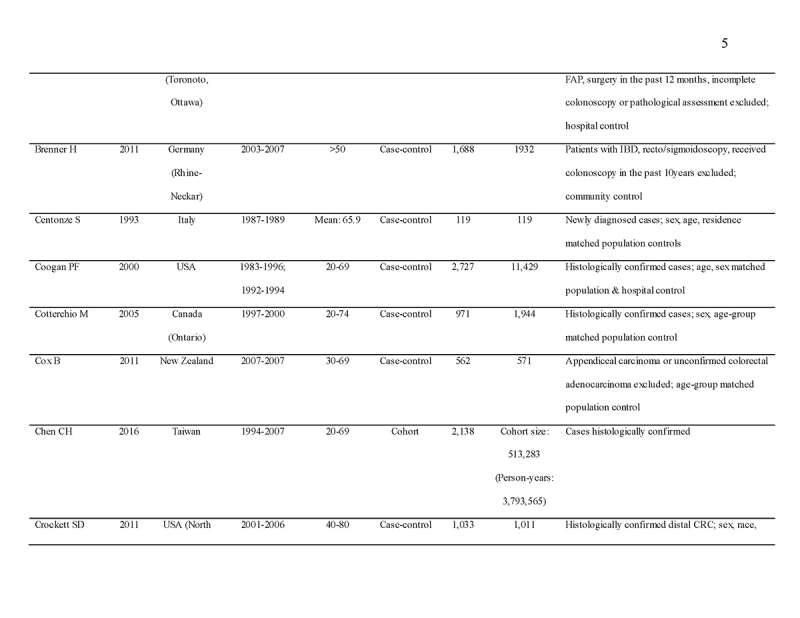
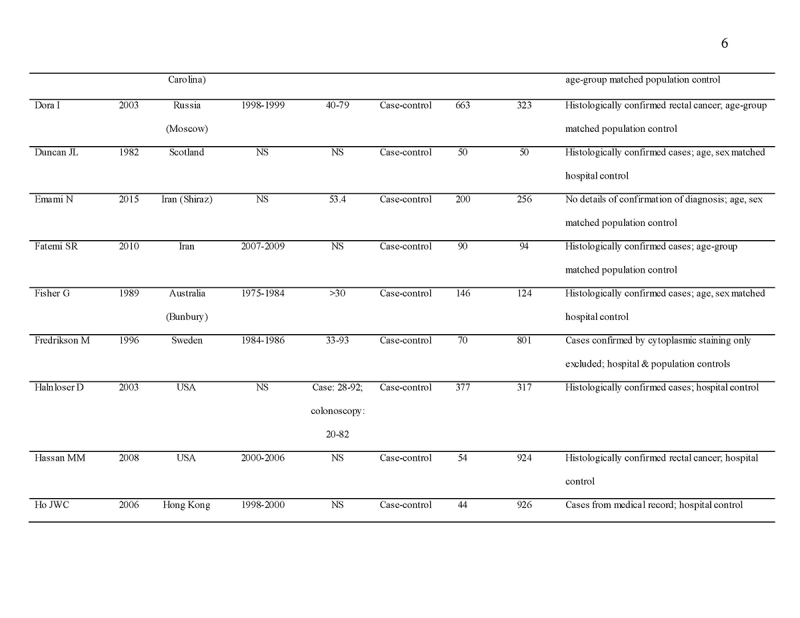
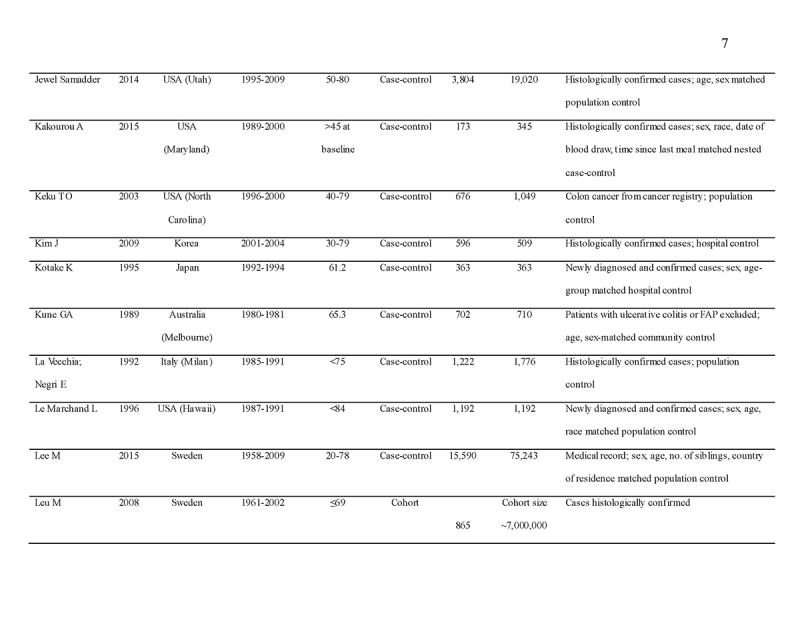
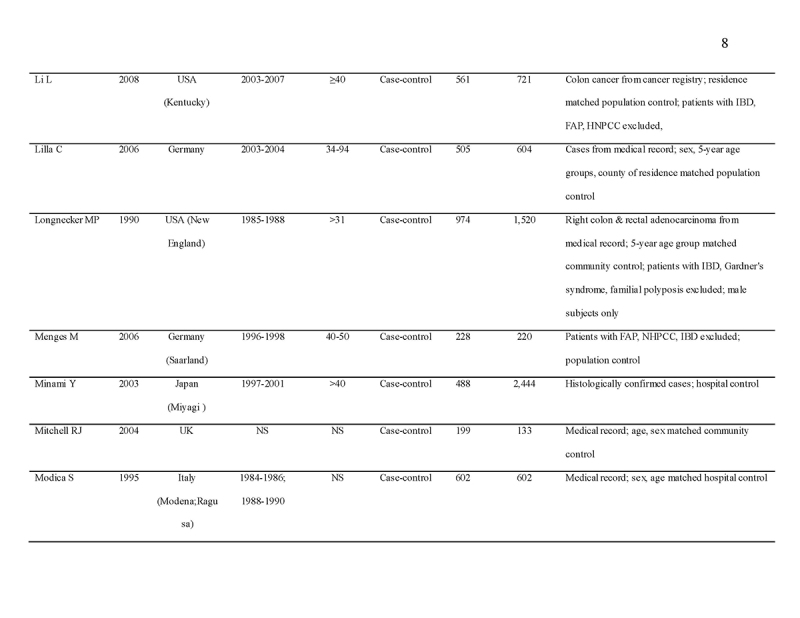
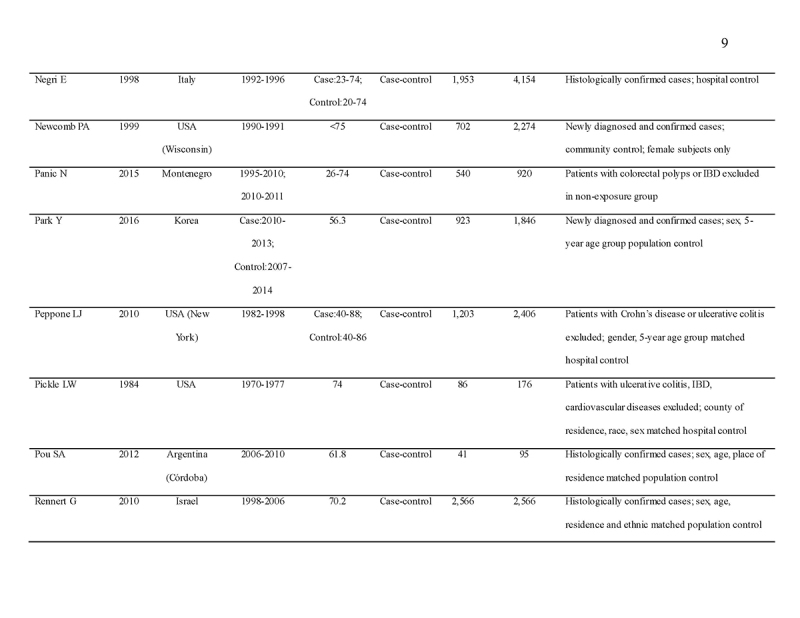
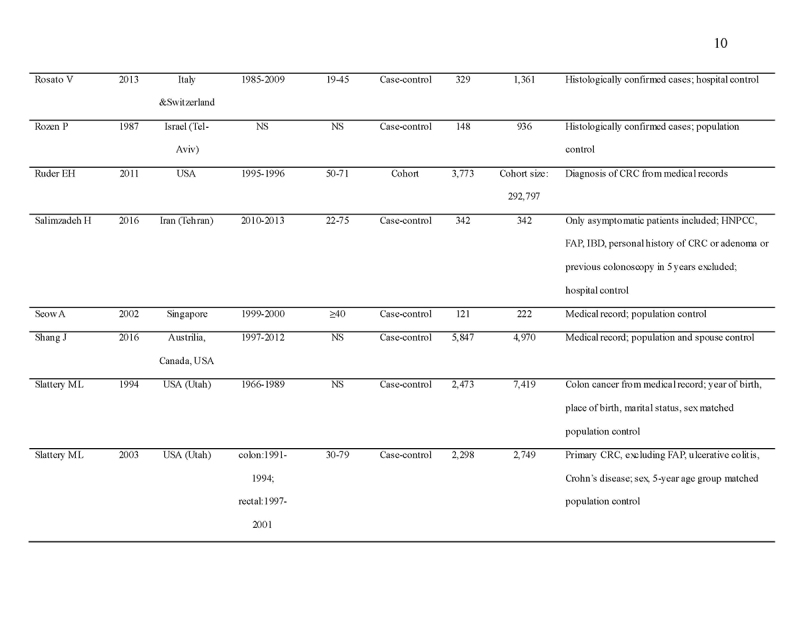
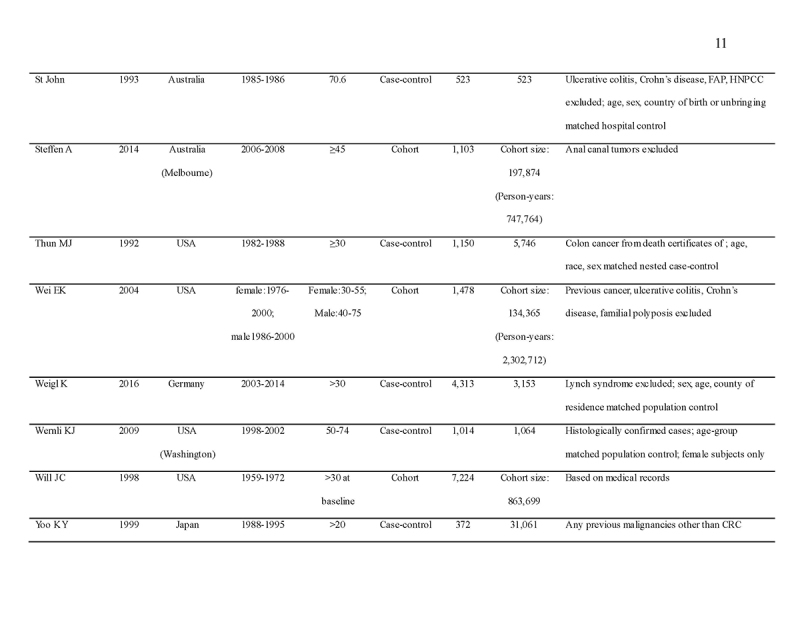
We identified 4119 articles from our search strategy (Fig. 1). The following articles were excluded: duplicates (n = 863) or irrelevance to the present research question (n = 2444). The full texts of the remaining 812 articles were reviewed, with 45 articles fulfilling our eligibility criteria being selected, and 18 additional studies selected from reference lists of eligible articles. Therefore, a total of 63 articles were included in this meta‐analysis, including 9,284,074 patients (Supplementary Table 2) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. The earliest calendar year of subject enrollment was 1982. Among these, 43, 13, and 7 studies were conducted in western countries, Western Pacific nations, and eastern Mediterranean, respectively. Most of the selected studies were case‐control studies (n = 56), examined risk of CRC in at least one FDR affected by CRC (n = 63), assessed family history of CRC based on self‐reports or surveys (n = 54), and provided incidence estimates of CRC (n = 62). Based on NOS score of ≥7, a total of 30 studies were classified as high quality (Supplementary Table 3).
Fig. 1.
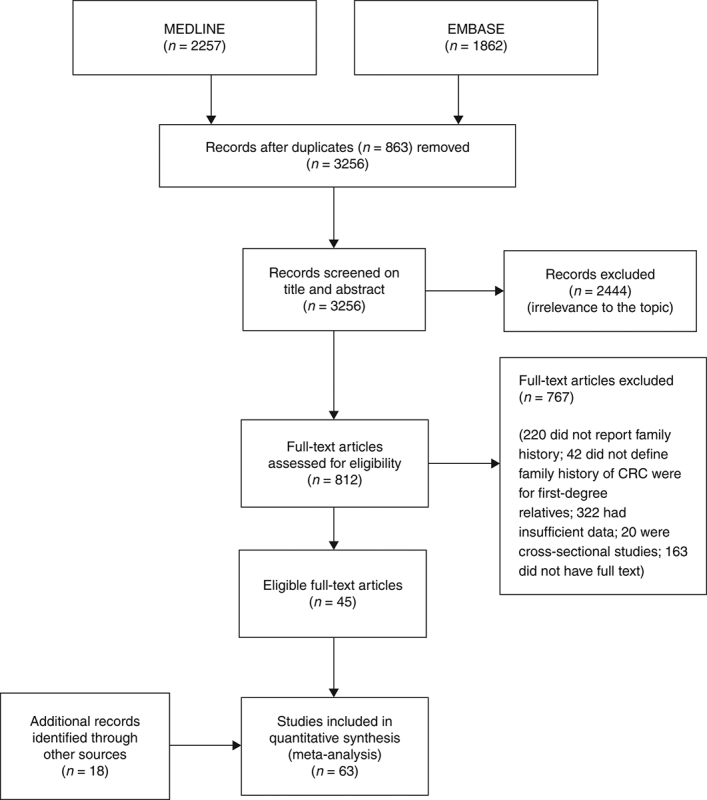
Flow diagram of study selection
From available data among the selected studies, subjects with a FDR with CRC were significantly more likely to have CRC (RR = 1.76, 95% CI = 1.57‐1.97, p < 0.001) compared to subjects with no family history (Fig. 2). A total of 50 studies showed significantly positive association. The heterogeneity was high (I2 = 95.7%; Q (df = 62) = 756.9, p < 0.001). No publication bias was observed (Begg's regression test p = 0.133; Egger's regression test p = 0.078; see funnel plot in Fig. 3).
Fig. 2.
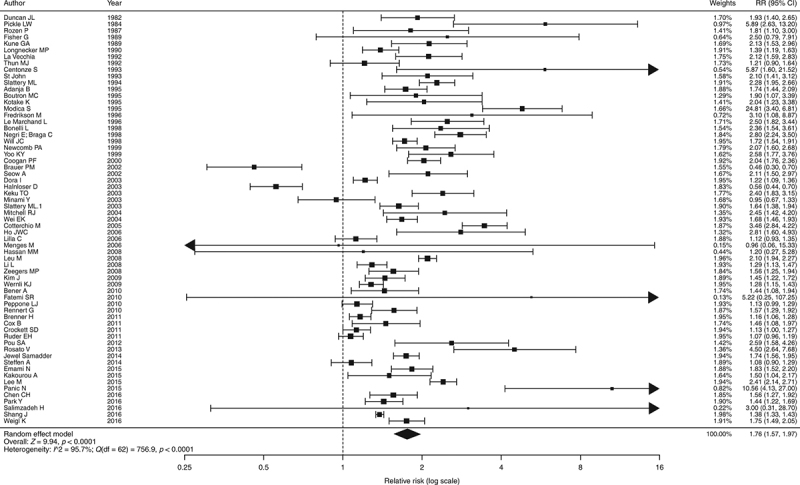
Pooled odds ratios of colorectal cancer in individuals with family history of colorectal cancer compared with control
Fig. 3.
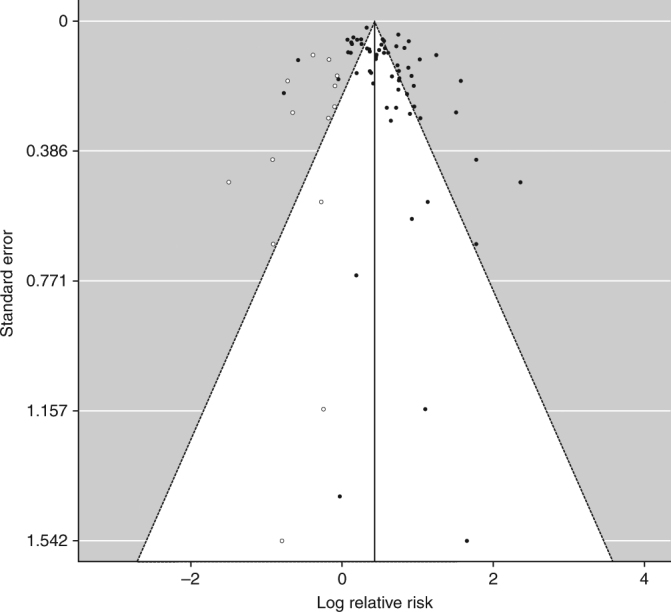
Funnel plot for publication bias
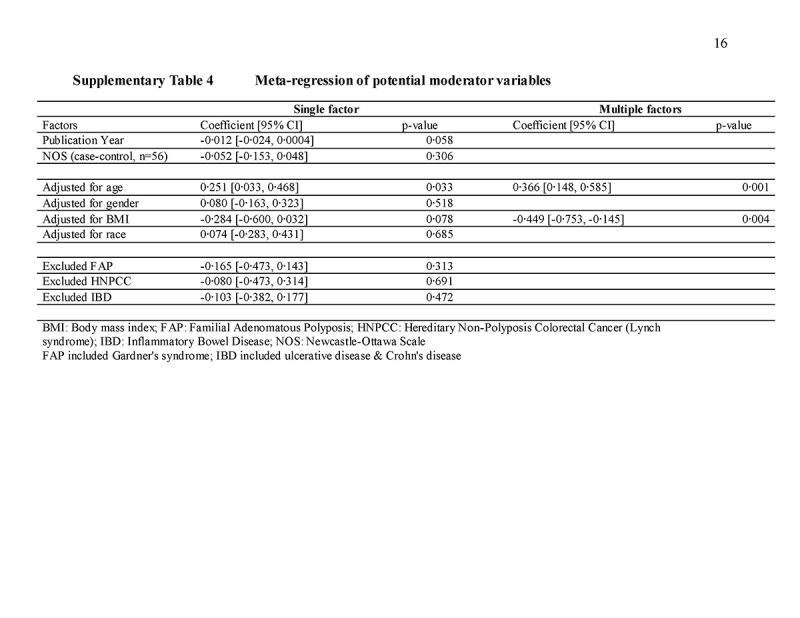
The relative risk of CRC among those with a FDR with CRC was further analyzed according to the age of the index subject, age of affected relatives, number of FDRs affected, type of family history exposure (parents versus siblings), site of CRC (colon versus rectum) in the affected FDR, gender of the index subject and affected FDR, assessment method of family history, study design, clinical outcome of CRC (incidence versus mortality), geographic area of study, and study quality (Fig. 4). The impact of family history on risk of CRC was significantly stronger when the index subject was aged <40 years (RR = 3.29, 95% CI = 1.67‐6.49, I2 = 78.4% versus RR = 1.42, 95% CI = 1.24‐1.62, I2 = 95%, p = 0.017 for ≥40 years) and <50 years (RR = 2.81, 95% CI = 1.94‐6.07, I2 = 81.0% versus RR = 1.47, 95% CI = 1.28‐1.69, I2 = 91.8%, p < 0.001 for ≥50 years). Individuals with ≥2 FDRs affected had higher risk of CRC than those with one FDR affected (RR = 2.68, 95% CI = 1.92‐3.74, I2 = 75.0% versus RR = 1.82, 95% CI = 1.51‐2.18, I2 = 76.3%), but the difference did not reach statistical significance as demonstrated by an overlap of their 95% CIs. Other subgroup analysis did not identify inter‐group differences. No significant difference was observed between studies that reported relative risks and odds ratios (p = 0.12); between crude and adjusted effect size (p = 0.12); and studies that were classified as high (NOS score ≥7) and low quality (NOS score <7). From meta‐regression analysis, it was found that age (coefficient = 0.366, 95% CI = 0.148, 0.585, p = 0.001) and body mass index (coefficient = ‐0.449, 95% CI = ‐0.753, ‐0.145, p = 0.004) accounted for the heterogeneity detected in this study (Supplementary Table 4).
Fig. 4.
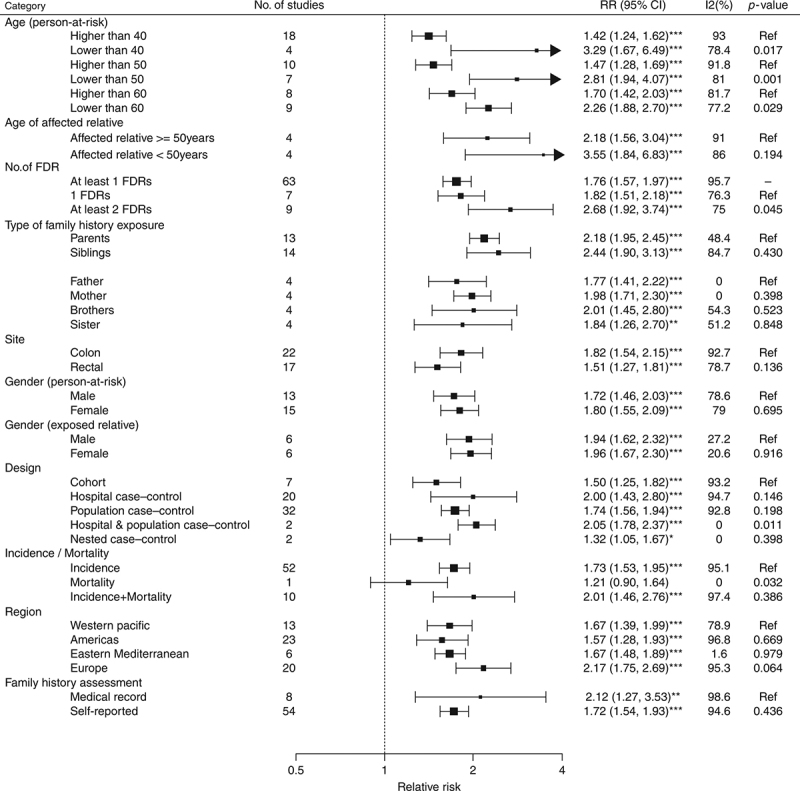
Subgroup analysis—association between family history of colorectal cancer in a first‐degree relative and incidence/prevalence of colorectal cancer. The “*” indicate different significant levels of z tests in meta‐analysis. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
DISCUSSION
The main findings of this systematic review of 63 studies involving more than 9 million patients show that: (1) a FDR with a history of CRC conferred a 1.76‐fold increased risk of CRC compared to those without such a family history; (2) the increased risk of CRC associated with a family history of CRC in FDR was significantly higher when the index subject is younger than 50 years old than in subjects older than 50 years; and (3) the higher risk of CRC for index subjects with two or more affected FDRs compared to those with only one affected FDR was marginal.
Thus far, there were three published meta‐analyses that investigated the association between family history and risk of CRC [8,9,10]. The pooled risk estimate of developing CRC given at least one affected FDR ranged between 2.26 and 2.28, which was higher than the relative risk (1.76) reported in this study. This may be due to the smaller number of studies (n = 27 and 20, respectively) included in the two previous meta‐analyses [8, 10] compared with this study, difference in calendar years where the included studies were published (up to late 1990 and early 2000 in previous meta‐analyses), and the inclusion of cross‐sectional studies in one meta‐analysis [9]. It should also be noted that some studies presented type I relative risk, which refers to risk of CRC in an index person given a specific family history of CRC, whereas some reported type II relative risk, which signifies risk of CRC in a relative given a specific index person is affected [10].
In our meta‐analysis, 13 out of 63 studies showed no significantly increased risk of CRC among individuals with family history of CRC [28, 39, 40, 42, 43, 56, 58, 59, 66, 72, 73, 79, 80], compared with other 50 studies that reported increased relative risk. Some possible explanations for this difference include the accuracy of self‐reported family history, varying quality of colonoscopy and experience of endoscopists across studies, the number of risk factors of CRC among subjects included in these studies, and differences in their region of residence. Most of the studies included in the present meta‐analysis did not match between those having family history and those without, recruited consecutive patients who had CRC symptoms as controls, and included individuals' FDRs who were not examined by colonoscopies for ascertainment of the presence/absence of CRC. In addition, differences in the methodological design of these studies could lead to differences in conclusions drawn. For instance, relative risk estimates from prospective cohort studies are in general lower than that from retrospective studies due to the presence of recall bias in the latter [9].
The reasons why the additional risk of CRC conferred by family history was higher in younger subjects remain speculative. Early age at diagnosis has been thought to be indicative of inherited genetic mutations. One might hypothesize that low‐penetrant genes interacting with diet and lifestyle factors contribute differentially to individuals of different ages when the family history of CRC is reported.
Study limitations
Our meta‐analysis has a number of strengths. Firstly, it included asymptomatic, average‐risk subjects pooled from all selected studies conducted in both western and Asian countries. In the literature, the retrospective observational studies of the effectiveness of screening on CRC incidence reduction are all struggling with the contamination of screening and diagnostic examinations. Hence, the application of its findings based on asymptomatic participants is more generalizable to screening practices when compared with previous colonoscopy studies. Secondly, this meta‐analysis includes a large number of screening participants recruited from all eligible studies. Thirdly, various effect modifiers were addressed in subgroup analyses. Also, quality assessment was based on an internationally recognized NOS scale for all the selected articles in a systematic manner.
Several limitations should be noted. Firstly, informal reports might have been omitted by our search strategy. Secondly, a high level of heterogeneity was observed, but this could be partially explained by adjusting for age and body mass index. Thirdly, not all studies have been adjusted for potential confounders such as dietary habits and physical inactivity. In addition, prevalence and distribution of colorectal neoplasia varied in people of different ethnicities yet the present studies recruited subjects from certain continents only. The heterogeneity of the study was reduced by subgroup analysis based on ethnicity. Ascertainment of both family history and the outcomes might have improved over time. Finally, the data on the age for the diagnosis of the FDR were limited in the current analysis. One of the most important questions in clinical practice is when to start CRC screening for individuals who have family history of CRC in a FDR. The current consideration that may trigger a colonoscopy in an individual aged <40 years with a family history of CRC is that the proband should receive colonoscopy 10 years younger than the FDR with CRC. The relationship between the age of diagnosis of the FDR and CRC risk is also important, and few studies have examined this issue. For instance, Lee et al. [53] reported that CRC risks were highest in siblings who were diagnosed at a younger age, with incident rate ratios of 9.1, 6.0, 2.6, 2.2, and 1.9 for the 30‐40, 50‐60, 60‐70, and 70‐75 age groups, respectively. Also, Slattery et al. [77] also found greater associations when the FDR was diagnosed with CRC at the age of less than 50 years or younger. The odds ratios were 2.1, 1.6, and 1.0, respectively, for age of diagnosis at <50 years, 51‐64 years, and ≥65 years, respectively. These findings imply that the younger the FDR with CRC, the earlier the proband should be investigated with colonoscopy. Nevertheless, there have been few studies that have directly addressed whether probands of age 40‐50 years who had CRC were related in age to the FDR who were in their fifth decade of life. The correlation between the ages of FDRs and the ages of the probands should be studied in future evaluations.
Our meta‐analysis shows that among subjects with family history of CRC in FDR, the increased risk of CRC associated with the family history was higher in young individuals (<50 years) than in older individuals (>50 years). This finding implies that family history of CRC in FDR could be assigned a higher score for younger subjects in CRC risk prediction algorithms. Future studies should examine if such approach may improve their predictive capability.
ACKNOWLEDGEMENTS
We wish to express our gratitude to the statistical advice from the Division of Biostatistics of the School of Public Health and Primary Care, Chinese University of Hong Kong.
CONFLICT OF INTEREST
Guarantor of the article: CH Chan and Jiayan Lin.
Specific author contributions: Martin CS Wong had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Martin CS Wong, John CT Wong, Justin CY Wu, Francis KL Chan; acquisition, analysis, or interpretation of data: Martin CS Wong, CH Chan, Jiayan Lin, Jason LW Huang, Junjie Huang; drafting of the manuscript: Martin CS Wong, Justin CY Wu, Francis KL Chan; critical revision of the manuscript for important intellectual content: John CT Wong, Gary Tse, Justin CY Wu, Francis KL Chan; statistical analysis: CH Chan, Jiayan Lin, Jason LW Huang, Junjie Huang, Yuan Fang, Wilson WL Cheung; administrative, technical, or material support: Martin CS Wong, CH Chan, Jiayan Lin, Jason LW Huang, Junjie Huang; study supervision: Justin CY Wu, Francis KL Chan.
Financial support: None.
Potential competing interests: None.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
Current risk scoring systems assigned a fixed score for a positive family history of colorectal cancer (CRC), irrespective of the age of the screening participant.
However, whether the increased CRC risk associated with family history was higher in younger patients remains inconclusive.
Previous meta‐analyses are limited by the modest number of studies, making comparison of CRC risk according to age of screening participants who reported family history of CRC underpowered.
We examined the risk of CRC associated with family history of CRC in first‐degree relative (FDR) according to the age of index subjects.
WHAT IS NEW HERE
In this meta‐analysis including 63 studies (9.28 million asymptomatic screening participants), the increased CRC risk associated with family history was higher in younger individuals.
No publication bias was observed and the findings are robust in subgroup analysis.
Family history of CRC could be assigned a higher score for younger subjects in CRC risk prediction algorithms.
Future studies should examine if such approach may improve their predictive capability.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://doi.org/10.1038/s41395‐018‐0075‐y
Correspondence: M.C.S.W. (email: wong_martin@cuhk.edu.hk) or F.K.L.C. (email: fklchan@cuhk.edu.hk)
published online 5 June 2018
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Sung JJY, Lau JYW, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6: 871–6. [DOI] [PubMed] [Google Scholar]
- 3.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851–60. [DOI] [PubMed] [Google Scholar]
- 4.Ma GK, Ladabaum U. Personalizing colorectal cancer screening: a systematic review of models to predict risk of colorectal neoplasia. Clin Gastroenterol Hepatol. 2014;12:1624–34. [DOI] [PubMed] [Google Scholar]
- 5.Usher-Smith JA, Walter FM, Emery JD. Risk prediction models for colorectal cancer: a systematic review. Cancer Prev Res. 2016;9:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kune GA, Kune S, Read A, et al. Colorectal polyps, diet, alcohol, and family history of colorectal cancer: a case-control study. Nutr Cancer. 1991;16:25–30. [DOI] [PubMed] [Google Scholar]
- 7.Tung SY, Wu CS. Risk factors for colorectal adenomas among immediate family members of patients with colorectal cancer in Taiwan: a case-control study. Am J Gastroenterol. 2000;95:3624–8. [DOI] [PubMed] [Google Scholar]
- 8.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–27. [DOI] [PubMed] [Google Scholar]
- 10.Baglietto L, Jenkins MA, Severi G, et al. Measures of familial aggregation depend on definition of family history: a meta-analysis for colorectal cancer. J Clin Epidemiol. 2006;59:114–24. [DOI] [PubMed] [Google Scholar]
- 11.Wong MC, Ching JY, Chiu HM, et al. Risk of colorectal neoplasia in individuals with self-reported family history: a prospective colonoscopy study from Asia-Pacific regions. Am J Gastroenterol. 2016;111:1621–9. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 13.Bonita R, Beaglehole R, Kjellström T. Basic epidemiology. World Health Organization, Geneva, Switzerland; 2006. [Google Scholar]
- 14.Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol. 1982;116:547–53. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014). Accessed 14 July 2017.
- 18.Okabayashi K, Ashrafian H, Hasegawa H, et al. Body mass index category as a risk factor for colorectal adenomas: a systematic review and metaanalysis. Am J Gastroenterol. 2012;107:1175–85. [DOI] [PubMed] [Google Scholar]
- 19.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30:261–93. [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JPT. Introduction to meta- analysis. Oxford: Wiley-Blackwell (an imprint of John Wiley & Sons Ltd); 2009. [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta- analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 24.Adanja B, Vlajinac H, Jarebinski M, Pantovic V. Malignant tumors in family members of colorectal cancer patients. Neoplasm. 1995;42:155–7. [PubMed] [Google Scholar]
- 25.Bener A, Moore MA, Ali R, et al. Impacts of family history and lifestyle habits on colorectal cancer risk: a case-control study in Qatar. Asian Pac J Cancer Prev. 2010;11:963–8. [PubMed] [Google Scholar]
- 26.Bonelli L, Martines H, Conio M, et al. Family history of colorectal cancer as a risk factor for benign and malignant tumours of the large bowel. A case-control study. Int J Cancer. 1988;41:513–7. [DOI] [PubMed] [Google Scholar]
- 27.Boutron MC, Faivre J, Quipourt V, et al. Family history of colorectal tumours and implications for the adenoma-carcinoma sequence: a case control study. Gut. 1995;37:830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brauer PM, McKeown-Eyssen GE, Jazmaji V, et al. Familial aggregation of diabetes and hypertension in a case-control study of colorectal neoplasia. Am J Epidemiol. 2002;156:702–13. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 30.Centonze S, Boeing H, Leoci C, et al. Familial risk of colo-rectal cancer in a low incidence area in southern Italy. Eur J Epidemiol. 1993;9:26–32. [DOI] [PubMed] [Google Scholar]
- 31.Chen CH, Wen CP, Tsai MK. Fecal immunochemical test for colorectal cancer from a prospective cohort with 513,283 individuals: providing detailed number needed to scope (NNS) before colonoscopy. Medicine (Baltimore). 2016;95:e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coogan PF, Rosenberg L, Louik C, et al. NSAIDs and risk of colorectal cancer according to presence or absence of family history of the disease. Cancer Causes Control. 2000;11:249–55. [DOI] [PubMed] [Google Scholar]
- 33.Cotterchio M, Manno M, Klar N, et al. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control. 2005;16:865–75. [DOI] [PubMed] [Google Scholar]
- 34.Cox B, Sneyd MJ. School milk and risk of colorectal cancer: a national case-control study. Am J Epidemiol. 2011;173:394–403. [DOI] [PubMed] [Google Scholar]
- 35.Crockett SD, Long MD, Dellon ES, et al. Inverse relationship between moderate alcohol intake and rectal cancer: analysis of the North Carolina colon cancer study. Dis Colon Rectum. 2011;54:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dora I, Arab L, Martinchik A, et al. Black tea consumption and risk of rectal cancer in Moscow population. Ann Epidemiol. 2003;13:405–11. [DOI] [PubMed] [Google Scholar]
- 37.Duncan JL, Kyle J. Family incidence of carcinoma of the colon and rectum in north-east Scotland. Gut. 1982;23:169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emami N, Saadat I, Omidvari S. Susceptibility to colorectal cancer and two genetic polymorphisms of XRCC4. Pathol Oncol Res. 2015;21:881–5. [DOI] [PubMed] [Google Scholar]
- 39.Fatemi SR, Shivarani S, Malek FN, et al. Colonoscopy screening results in at risk Iranian population. Asian Pac J Cancer Prev. 2010;11:1801–4. [PubMed] [Google Scholar]
- 40.Fisher G, Armstrong B. Familial colorectal cancer and the screening of family members. Med J Aust. 1989;150:22–25. [DOI] [PubMed] [Google Scholar]
- 41.Fredrikson M, Axelson O, Sun XF, et al. A pilot study on risk factors and p53 gene expression in colorectal cancer. Br J Cancer. 1996;73:1428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahnloser D, Petersen GM, Rabe K, et al. The APC E1317Q variant in adenomatous polyps and colorectal cancers. Cancer Epidemiol Biomark Prev. 2003;12:1023–8. [PubMed] [Google Scholar]
- 43.Hassan MM, Phan A, Li D, et al. Family history of cancer and associated risk of developing neuroendocrine tumors: a case-control study. Cancer Epidemiol Biomark Prev. 2008;17:959–65. [DOI] [PubMed] [Google Scholar]
- 44.Ho JWC, Yuen ST, Lam TH. A case-control study on environmental and familial risk factors for colorectal cancer in Hong Kong: chronic illnesses, medication and family history. Hong Kong Med J. 2006;12:S14–6. [Google Scholar]
- 45.Samadder J, Curtin NK, Tuohy TMF, et al. Increased risk of colorectal neoplasia among family members of patients with colorectal cancer: a population-based study in Utah. Gastroenterology. 2014;147:814–21.e815. [DOI] [PubMed] [Google Scholar]
- 46.Kakourou A, Koutsioumpa C, Lopez DS, et al. Interleukin-6 and risk of colorectal cancer: results from the CLUE II cohort and a meta-analysis of prospective studies. Cancer Causes Control. 2015;26:1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keku TO, Millikan RC, Martin C, et al. Family history of colon cancer: what does it mean and how is it useful? Am J Prev Med. 2003;24:170–6. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Kim DH, Lee BH, et al. Folate intake and the risk of colorectal cancer in a Korean population. Eur J Clin Nutr. 2009;63:1057–64. [DOI] [PubMed] [Google Scholar]
- 49.Kotake K, Koyama Y, Nasu J, et al. Relation of family history of cancer and environmental factors to the risk of colorectal cancer: a case-control study. Jpn J Clin Oncol. 1995;25:195–202. [PubMed] [Google Scholar]
- 50.Kune GA, Kune S, Watson LF. The role of heredity in the etiology of large bowel cancer: data from the Melbourne Colorectal Cancer Study. World J Surg. 1989;13:124–9. [DOI] [PubMed] [Google Scholar]
- 51.La Vecchia C, Negri E, Franceschi S, et al. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70:50–55. [DOI] [PubMed] [Google Scholar]
- 52.Le Marchand L, Zhao LP, Quiaoit F, et al. Family history and risk of colorectal cancer in the multiethnic population of Hawaii. Am J Epidemiol. 1996;144:1122–8. [DOI] [PubMed] [Google Scholar]
- 53.Lee M, Czene K, Rebora P, et al. Patterns of changing cancer risks with time since diagnosis of a sibling. Int J Cancer. 2015;136:1948–56. [DOI] [PubMed] [Google Scholar]
- 54.Leu M, Reilly M, Czene K. Evaluation of bias in familial risk estimates: a study of common cancers using Swedish population-based registers. J Natl Cancer Inst. 2008;100:1318–25. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Plummer SJ, Thompson CL, et al. A common 8q24 variant and the risk of colon cancer: a population-based case-control study. Cancer Epidemiol Biomark Prev. 2008;17:339–42. [DOI] [PubMed] [Google Scholar]
- 56.Lilla C, Verla-Tebit E, Risch A, et al. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomark Prev. 2006;15:99–107. [DOI] [PubMed] [Google Scholar]
- 57.Longnecker MP. A case-control study of alcoholic beverage consumption in relation to risk of cancer of the right colon and rectum in men. Cancer Causes Control. 1990;1:5–14. [DOI] [PubMed] [Google Scholar]
- 58.Menges M, Fischinger J, Gartner B, et al. Screening colonoscopy in 40- to 50-year-old first-degree relatives of patients with colorectal cancer is efficient: a controlled multicentre study. Int J Colorectal Dis. 2006;21: 301–7. [DOI] [PubMed] [Google Scholar]
- 59.Minami Y, Tateno H. Associations between cigarette smoking and the risk of four leading cancers in Miyagi Prefecture, Japan: a multi-site case-control study. Cancer Sci. 2003;94:540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell R, Brewster D, Campbell H, et al. Accuracy of reporting of family history of colorectal cancer. Gut. 2004;53:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Modica S, Roncucci L, Benatti P, et al. Familial aggregation of tumors and detection of hereditary non-polyposis colorectal cancer in 3-year experience of 2 population-based colorectal-cancer registries. Int J Cancer. 1995;62:685–90. [DOI] [PubMed] [Google Scholar]
- 62.Negri E, Braga C, La Vecchia C. Family history of cancer and risk of colorectal cancer in Italy. Br J Cancer. 1998;77:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newcomb PA, Taylor JO, Trentham-Dietz A. Interactions of familial and hormonal risk factors for large bowel cancer in women. Int J Epidemiol. 1999;28:603–8. [DOI] [PubMed] [Google Scholar]
- 64.Panic N, Rosch T, Smolovic B, et al. Colorectal cancer screening in a low-incidence area: general invitation versus family risk targeting: a comparative study from Montenegro. Eur J Gastroenterol Hepatol. 2015;27: 1222–5. [DOI] [PubMed] [Google Scholar]
- 65.Park Y, Lee J, Oh JH, et al. Dietary patterns and colorectal cancer risk in a Korean population. Medicine (Baltimore). 2016;95:e3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peppone LJ, Reid ME, Moysich KB, et al. The effect of secondhand smoke exposure on the association between active cigarette smoking and colorectal cancer. Cancer Causes Control. 2010;21:1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pickle LW, Greene MH, Ziegler RG, et al. Colorectal cancer in rural Nebraska. Cancer Res. 1984;44:363–9. [PubMed] [Google Scholar]
- 68.Pou SA, Diaz Mdel P, Osella AR. Applying multilevel model to the relationship of dietary patterns and colorectal cancer: an ongoing case-control study in Cordoba, Argentina. Eur J Nutr. 2012;51:755–64. [DOI] [PubMed] [Google Scholar]
- 69.Rennert G, Rennert HS, Pinchev M, et al. A case-control study of levothyroxine and the risk of colorectal cancer. J Natl Cancer Inst. 2010;102:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24:335–41. [DOI] [PubMed] [Google Scholar]
- 71.Rozen P, Lynch HT, Figer A, et al. Familial colon cancer in the Tel-Aviv area and the influence of ethnic origin. Cancer. 1987;60:2355–9. [DOI] [PubMed] [Google Scholar]
- 72.Ruder EH, Thiébaut ACM, Thompson FE, et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2011;94:1607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salimzadeh H, Bishehsari F, Amani MR, et al. Advanced colonic neoplasia in the first degree relatives of colon cancer patients: a colonoscopy-based study. Int J Cancer. 2016;139:2243–51. [DOI] [PubMed] [Google Scholar]
- 74.Seow A, Quah SR, Nyam D, et al. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95:2390–6. [DOI] [PubMed] [Google Scholar]
- 75.Shang J, Reece JC, Buchanan DD, et al. Cholecystectomy and the risk of colorectal cancer by tumor mismatch repair deficiency status. Int J Colorectal Dis. 2016;31:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst. 1994;86:1618–26; erratum 86, 1802 (1994). [DOI] [PubMed] [Google Scholar]
- 77.Slattery ML, Levin TR, Ma K, et al. Family history and colorectal cancer: predictors of risk. Cancer Causes Control. 2003;14:879–87. [DOI] [PubMed] [Google Scholar]
- 78.St John DJ, McDermott FT, Hopper JL, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med. 1993;118:785–90. [DOI] [PubMed] [Google Scholar]
- 79.Steffen A, MacInnis RJ, Joshy G, et al. Development and validation of a risk score predicting risk of colorectal cancer. Cancer Epidemiol Biomark Prev. 2014;23:2543–52. [DOI] [PubMed] [Google Scholar]
- 80.Thun MJ, Calle EE, Namboodiri MM, et al. Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst. 1992;84:1491–500. [DOI] [PubMed] [Google Scholar]
- 81.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weigl K, Jansen L, Chang-Claude J, et al. Family history and the risk of colorectal cancer: the importance of patients' history of colonoscopy. Int J Cancer. 2016;139:2213–20. [DOI] [PubMed] [Google Scholar]
- 83.Wernli KJ, Wang Y, Zheng Y, et al. The relationship between gravidity and parity and colorectal cancer risk. J Women's Health. 2009;18: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Will JC, Galuska DA, Vinicor F, et al. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol. 1998;147:816–25. [DOI] [PubMed] [Google Scholar]
- 85.Yoo KY, Tajima K, Inoue M, et al. Reproductive factors related to the risk of colorectal cancer by subsite: a case-control analysis. Br J Cancer. 1999;79:1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeegers MP, Schouten LJ, Goldbohm RA, et al. A compendium of familial relative risks of cancer among first degree relatives: a population-based study. Int J Cancer. 2008;123:1664–73. [DOI] [PubMed] [Google Scholar]