Background:
Routine cytology of biliary stricture brushings obtained during endoscopic retrograde cholangiopancreatography (ERCP) has suboptimal sensitivity for malignancy. We compared the individual and combined ability of cytology, fluorescence in situ hybridization (FISH) analysis and PCR-based mutation profiling (MP) to detect malignancy in standard biliary brushings.
Methods:
We performed a prospective study of patients undergoing ERCP using histology or 1 year follow-up to determine patient outcomes. MP was performed on free-DNA from biliary brushing specimens using normally discarded supernatant fluid. MP examined KRAS point mutations and tumor suppressor gene associated loss of heterozygosity mutations at 10 genomic loci. FISH examined chromosome specific gains or losses.
Results:
A total of 101 patients were included in final analysis and 69% had malignancy. Cytology had 26% sensitivity and 100% specificity for malignancy. Using either FISH or MP in combination with cytology increased sensitivity to 44% and 56%, respectively. The combination of all 3 tests (cytology, FISH, and MP) had the highest sensitivity for malignancy (66%). There was no difference in the specificity of cytology, FISH or MP testing when examined alone or in combination. MP improved diagnostic yield of each procedure from 22% to 100%; FISH improved yield to 90%. MP detected 21 malignancies beyond that identified by cytology; FISH detected an additional 13. The combination of FISH and MP testing detected an additional 28 malignancies.
Conclusions:
Both MP and FISH are complimentary molecular tests that can significantly increase detection of biliary malignancies when used in combination with routine cytology of standard biliary brush specimens.
Key Words: biliary stricture, mutation profiling, florescence in situ hybridization, endoscopic retrograde cholangiography
Despite our increasing understanding of the molecular and genetic landscape of cancers arising from the biliary tree and gallbladder and our reliance on this data for promising targeted therapies, our clinical ability to diagnose and evaluate biliary strictures remains challenging.1,2 endoscopic retrograde cholangiopancreatography (ERCP) continues to be the cornerstone of tissue acquisition for the diagnosis of indeterminate biliary strictures, and while several techniques including brush cytology, fine-needle aspiration, and forceps biopsy have been utilized, all fall short when it comes to sensitivity for detecting malignancy.3–7 Indeed, the oldest and most widely used technique of brush cytology has a high specificity of 100%, but its sensitivity ranges from only 20% to 45%.4,8,9
In an effort to improve upon conventional cytology, fluorescence in situ hybridization (FISH) has been utilized to analyze brush cytology specimens for chromosomal abnormalities in malignant cells by means of fluorescent labeled complementary DNA probes. FISH detects chromosomal changes that have been described in 80% of malignant biliary neoplasm, but prospective studies have shown that the combination of cytology and FISH testing only has 50% to 60% sensitivity for malignancy in biliary strictures.1,3,10,11 Regardless, routine testing for aneuploidy of chromosomes 3, 7, and 17 and loss or gain of chromosomal regions 1q21 (MCL1), 7p12 (EGFR), 8q24 (MYC), and 9p21 (CDKN2A) have been found useful in aiding in diagnosis.12–15 Furthermore, such molecular testing has been recommended by guideline associations for the diagnosis of malignancy in pancreaticobiliary lesions.16
Given the less than ideal characteristics of the currently available diagnostic methods, novel molecular approaches have been investigated to assist in this significant, unmet clinical need. Specifically, mutation profiling (MP) of DNA obtained from the biliary tract has recently been shown to have value in the diagnosis of pancreatic and biliary malignancy.17–20 This approach utilizes free DNA retrieved from the biliary tract during cytology brushing. Whereas cytology and FISH require cellular material adherent to a cytology brush, MP can be performed on the free DNA found in the preservative fluid after routine cytocentrifugation of cells from a brush specimen. Specifically, evaluation for KRAS oncogene mutations and tumor-suppressor gene associated loss of heterozygosity (LOH) mutations may allow for the detection of malignant strictures that would be otherwise undetectable by cytology performed in parallel. Tumor suppressor gene (TSG) associated alterations have been observed over 10 genomic loci in malignant strictures: 1p (CMM1, Lmyc), 3p (VHL, OGG1), 5q (MCC, APC), 9p (CDKN2A, CDKN2B), 10q (PTEN, MXI1), 17p (TP53), 17q (NME1, RNF34), 18q (SMAD4, DCC), 21q (TFF1, PSEN2), and 22q (NF2). Several studies have demonstrated that the addition of this free-DNA MP approach to cytology and FISH testing increases the sensitivity for biliary malignancy to ∼70%.17–20
The purpose of this prospective study was to compare the diagnostic utility of standard FISH testing of cells and/or MP of free-DNA when used in combination with routine cytology to detect malignancy. Histologic diagnosis (histology from biopsy or cytology for fine-needle aspiration) in conjunction with clinical and/or imaging follow-up was used as the gold standard for diagnosis of malignancy.
METHODS
Patients
This study was approved by the Institutional Review Board at Washington University of St. Louis (Approval # 201211068). For this prospective study, adult patients with biliary strictures who underwent ERCP at Barnes Jewish Hospital between November 2013 and February 2016 were invited to participate. Patients with the following criteria were excluded for study enrollment: (1) severe coagulopathy (INR>1.8) or thrombocytopenia (platelet count <50,000), (2) inability to cannulate the common bile duct, (3) presence of altered anatomy (Billroth II or Roux-en-Y reconstruction), (4) intrahepatic biliary strictures or strictures within 2 cm of the ampulla, (5) absence of stricture.
Specimen Collection
ERCP was performed using standard techniques with side-viewing endoscopes (TJF-160; Olympus Optical, Tokyo, Japan) and under sedation with intravenous midazolam and intravenous meperidine/fentanyl or intravenous propofol. Standard cannulation techniques were used to gain access to the common bile duct. An initial cholangiogram was obtained and a guide wire was passed into the common bile duct. The location and the length of the stricture were defined based on the cholangiogram. The diameter of the duodenoscope was used to determine the length of the biliary stricture. Two standard cytology brushings were performed on each biliary stricture. One brush was used for cytology and the other for FISH. Allocation of each brush to cytology or FISH was randomized using Microsoft excel.
Cytology
The biliary brushing was placed into 20 mL of CytoLyt (Hologic Inc., Malborough, MA). Cytology was evaluated by a cytopathologist per standard clinical procedures for evaluation of biliary strictures at Barnes Jewish Hospital/Washington University of St. Louis. Cytology results were analyzed over 2 scenarios. The first scenario considered cytology results as positive for malignancy when cytology reports indicated the presence of “malignant” cells. In this scenario all other cytology results (ie, “suspicious,” “accellular/indeterminate,” or “negative” cells) were considered negative for malignancy and deemed “nonmalignant” cytology results. In the second scenario, cytology results were considered positive for malignancy when cytology reports indicated the presence of either “suspicious” or “malignant” cells. In this scenario all other cytology results (ie, “accellular/indeterminate” or “negative” cells) were considered negative for malignancy and deemed “nonmalignant” cytology results.
FISH
The biliary brushing that was separate from that used for cytology was placed into 20 mL of PreservCyt (Hologic Inc.) and sent for FISH testing (Mayo Clinic, Rochester, MN). FISH testing was performed blinded to patient outcomes. FISH examined aneuploidy using CEP3, CEP7, CEP 17 centromere probes and loss or gain of genes using loci specific probes for 1q21 (MCL1), 7p12 (EGFR), 8q24 (MYC), and 9p21 (CDKN2A). “Suspicious” and “malignant” FISH reports were considered a positive FISH result, while “indeterminate” and “negative” reports were considered a negative FISH result.
DNA MP
The biliary brushing used for cytology or FISH testing was allowed to settle for 20 minutes, then vortexed at the speed of 8 twice for 30 seconds. A volume of 5 mL of each resulting suspension was aliquoted from each brush specimen and sent for DNA MP. MP testing was performed blinded to patient outcomes. MP (commercially known as PancraGEN, Interpace Diagnostics) was performed using standard operating procedures to test mutations in free-DNA from each biliary brush specimen using normally discarded supernatant preservative fluid that remained after cytocentrifugation of cells from each suspension.20 MP examined KRAS oncogene point mutations and TSG LOH mutations at 10 genomic loci via capillary gel electrophoresis: 1p (CMM1, Lmyc), 3p (VHL, OGG1), 5q (MCC, APC), 9p (CDKN2A, CDKN2B), 10q (PTEN, MXI1), 17p (TP53), 17q (NME1, RNF34), 18q (SMAD4, DCC), 21q (TFF1, PSEN2), and 22q (NF2). Any positive mutation detected among the oncogene and TSG panel was considered a positive MP result, while the absence of all detectable mutations was considered a negative MP result.
Patient Outcomes
Patient outcomes were determined through follow-up operative reports, histopathology, cytology results from a follow-up ERCP or endoscopic ultrasound (EUS) procedure, or clinical follow-up. Malignant outcomes were defined as malignancy or high grade dysplasia described on histology from surgery or biopsy, the presence of frank cytologic malignancy on follow up sampling or clinical evidence of malignant progression based on imaging/ tumor markers within 1 year after the initial procedure. Nonmalignant outcomes were defined as instances in which evidence for malignant outcome were not reported in surgical or biopsy pathology reports or in clinical follow-up records that occurred 1 year after the initial ERCP procedure.
Test and Combination Test Performance
Test performance (accuracy, sensitivity, and specificity) was calculated using 2×2 contigency tables. Cytology performance was examined alone and in combination with molecular tests for 2 scenarios: (i) when cytology results reporting “malignant” cells were considered a positive cytology result and (ii) when cytology results reporting “suspicious” or “malignant” cells were considered a positive cytology result. For analysis of individual tests, cytology was dichotomized as positive or nonmalignant, MP was dichotomized as positive or negative, and FISH was dichotomized as positive or negative. For analysis of cytology and MP combination testing, the presence of positive cytology and/or positive MP results (ie, at least 1 positive among the 2 tests) was considered positive for malignancy and the absence of both was considered negative. For analysis of cytology and FISH combination testing, the presence of positive cytology and/or positive FISH results (ie, at least 1 positive among the 2 tests) was considered positive for malignancy and the absence of both was considered negative. For analysis of cytology, MP, and FISH combination testing, the presence of positive cytology, positive MP results, and/or positive FISH results (ie, at least 1 positive among the 3 tests) were considered positive for malignancy, and the absence of all 3 was considered negative. Test sensitivity and specificity were compared using McNemar Test. A 2-sided P-value of <0.05 was considered significant. Statistical analysis was performed using R software (r-project.org).
RESULTS
A total of 122 patients, 39 (32%) female, with a mean age of 63±12 years were enrolled in the study (Fig. 1). However, 16 were excluded because initial specimen processing rendered the sample ineligible for MP testing. One patient with an indeterminate follow-up outcome and 4 patients with insufficient follow-up time (ie, <1 y) were also excluded from the study. Final analysis included 101 patients (29% female, mean age 63 y), of which 69% had a malignant stricture. Malignancy was confirmed with histology in 35 cases, fine-needle aspiration in 30, and clinical evidence of malignant progression in 4 cases. Cholangiocarcinoma was the etiology of 33/70 malignant strictures, followed by pancreatic cancer in 26/70 and metastatic malignancy with prior diagnosis of pancreatic or bile duct cancer in 11/70. Demographics are shown in Table 1.
FIGURE 1.
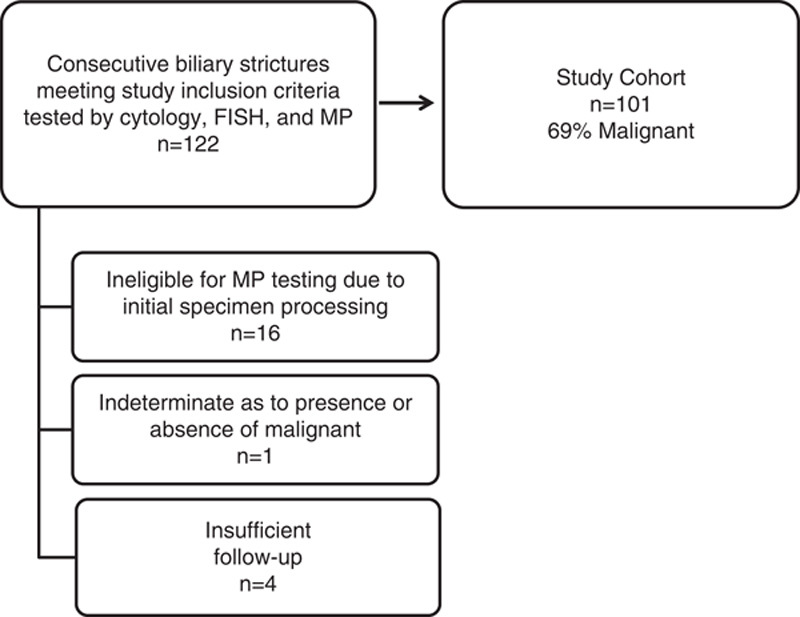
Study population. FISH indicates fluorescence in situ hybridization; MP, mutation profiling.
TABLE 1.
Patient Demographics
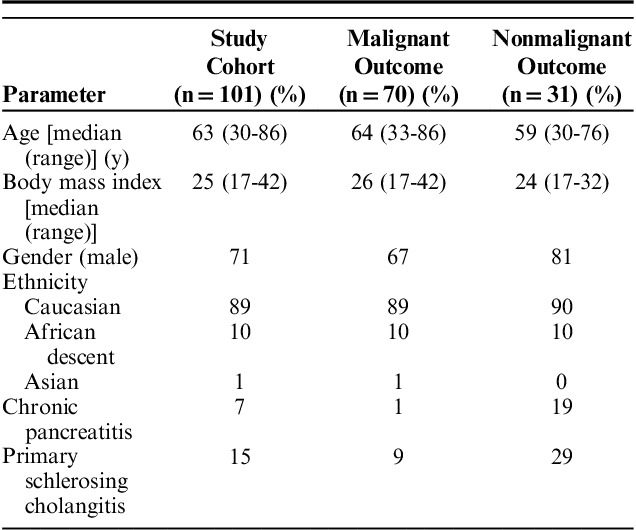
We examined the performance of cytology, FISH, and MP in the 101 patients included in the study cohort. The performance of cytology was determined for 2 scenarios: (i) when cytology results reporting “malignant” cells were considered a positive cytology result and (ii) when cytology results reporting “suspicious” or “malignant” cells were considered a positive cytology result. Of the 101 cases, 18 had “malignant” and 11 had “suspicious” cytology results. Cytology identified all cases of metastatic malignancy. “Malignant” cytology results identified malignant outcomes with 26% sensitivity and 100% specificity resulting in only 49% overall accuracy (Table 2). Sensitivity improved to 41% when “suspicious” or “malignant” cytology was considered positive for malignancy. Positive MP results had significantly higher sensitivity than “malignant” cytology (P<0.001), while positive FISH results did not (P=0.05) (Tables 2, 3). Use of the first brush or second brush specimen for cytology or FISH did not significantly impact the accuracy of either test (both P≥0.30). Neither FISH nor MP sensitivity was significantly different from that of using “suspicious” or “malignant” cytology results (both P>0.3) (Table 3). There was no statistical difference in the sensitivity of positive MP and positive FISH results (P=0.15) (Table 3). Both FISH and MP confirmed malignancy in all cases of metastatic disease, and all metatstatic disease was pancreatic or biliary in origin.
TABLE 2.
Diagnostic Performance of Cytology, FISH, and MP for Detecting Malignancy When Examined Independently and in Combination With One Another
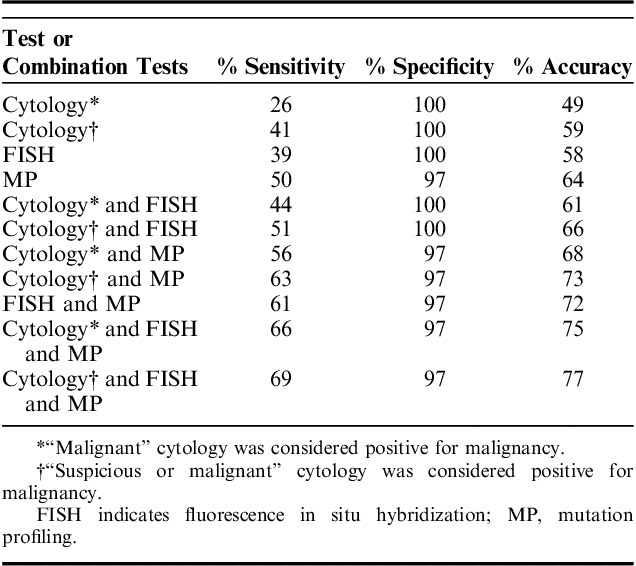
TABLE 3.
Comparisons of Sensitivity for Malignancy Between Tests and Test Combinations When “Malignant” Cytology Results Were Considered Positive for Malignancy and When “Suspicious” or “Malignant” Cytology Results Were Considered Positive for Malignancy
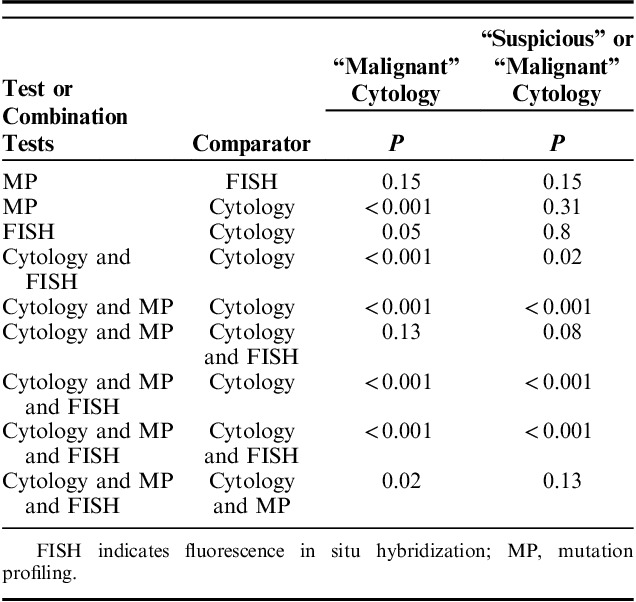
The combination of cytology and FISH or the combination of cytology and MP testing improved detection of malignancy compared with cytology alone, regardless of if “suspicious” and “malignant” cytology or only “malignant” cytology results were considered positive for malignancy (all P≤0.02) (Table 3). When either a positive FISH or a positive MP result was used to indicate malignancy when cytology was otherwise nonmalignant, sensitivity was increased to 44% and 56%, respectively, when “malignant” cells were considered a positive cytology result. FISH and MP further improved detection of malignancy to 51% and 63%, respectively, when “suspicious” or “malignant” cells were considered positive cytology (Table 2). There was no statistical difference in sensitivity achieved with a positive MP result compared with a positive FISH result used in combination with cytology (both P≥0.08) (Table 3). Using a combination of all 3 tests (cytology, FISH, and MP), with at least 1 positive among the 3, improved sensitivity (66%) and overall accuracy (75%) for malignancy compared with use of “malignant” cytology alone (P<0.001) and compared with use of a combination of “malignant” cytology with either MP (P<0.001) or FISH (P=0.02) (Tables 2, 3). However, use of only MP in combination with cytology achieved statistically similar sensitivity to that of all 3 tests when “suspicious” or “malignant” cytology results were considered positive (P=0.13) (Table 3). There was no significant difference in specificity for malignancy between cytology, FISH or MP when examined alone or in combination with one another (all P=1.0). Although not significantly different, one MP result was false positive for malignancy. This false positive was due to a reproducible KRAS mutation in a patient with chronic pancreatitis.
Cytology was considered nonmalignant due to lack of “malignant” cells in 83 patients, including those with “suspicious” (n=11), “accellular/indeterminate” (n=10), or “negative” (n=62) cytology. Among these cases, the diagnostic yield of FISH was 88%, while the diagnostic yield of MP was 100% (Table 4). Of these cases, 63% had malignant outcome due to primary cancer; there was no metastatic disease present in these cases. Positive MP results identified 21 additional cases of primary malignancy (Table 4). Positive FISH identified only 13 additional malignancies. In total, the presence of at least 1 positive test result when both MP and FISH were performed detected 28 additional malignancies, with MP and FISH separately identifying 7 different cases of malignancy. When cytology was considered nonmalignant due to lack of “suspicious” or “malignant” cells in patients with “accellular/indeterminate”, or “negative” cytology, the presence of at least one positive test result when both MP and FISH were performed detected 19 additional malignancies, with MP detecting 15 and FISH detecting 7 malignancies (data not shown).
TABLE 4.
Molecular Test Performance in the Subset of Patients Lacking Definitively “Malignant” Cytology Results
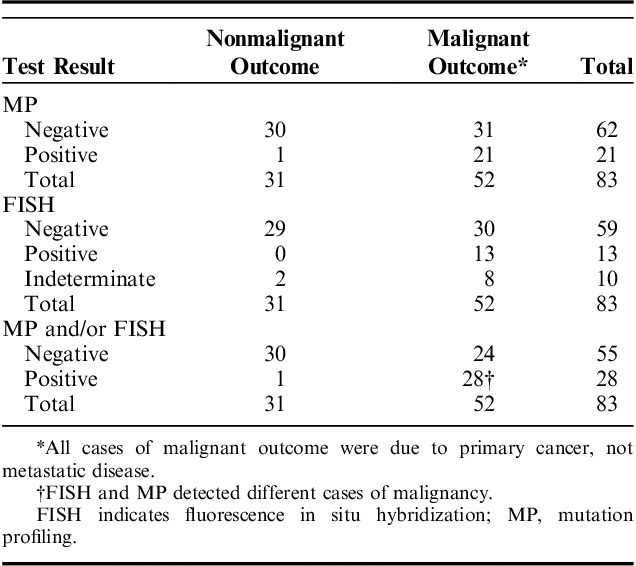
Of all patients in the study, 27 were positive by FISH testing and 35 were positive by MP testing. Eighteen of the positive FISH results had gains of 2 or more chromosomes. Two cases had loss of 9p21 (CDKN2A), and 7 cases indicated abnormal FISH results that were consistent with malignancy or high-grade dysplasia without specification of marker abnormalities. Among the 35 MP positive cases, half were positive for KRAS mutation and the other half were positive for at least one mutation among TSG associated LOH panel of 10 genomic loci. The most frequent TSG LOH mutation was at 9p (CDKN2A, CDKN2B), which occurred in 19% of MP positive specimens. The most infrequent LOH mutation was at 22q (NF2), which occurred in only 5% of MP positive specimens. All other TSG LOH mutations among the panel each occurred in 8% to 15% of MP positive specimens, including LOH at 1p (CMM1, Lmyc), 3p (VHL, OGG1), 5q (MCC, APC), 10q (PTEN, MXI1), 17p (TP53), 17q (NME1, RNF34), 18q (SMAD4, DCC), and 21q (TFF1, PSEN2).
DISCUSSION
Accurate clinical evaluation of biliary strictures remains highly challenging despite our advances in biliary imaging, sampling, and understanding of the molecular basis for biliary tract cancers. Submucosal patterns of tumor growth, low tumor cellularity and an anatomically challenging tumor site for clinical sampling are among the challenges in adequately sampling these lesions.5 Routine FISH testing for specific losses or gains in chromosomes has been shown to improve sensitivity of standard brush cytology.7,20,21 However, FISH relies on the presence of intact neoplastic cells in order to detect malignancy, and as such remains limited by the technical challenges of low tumor cellularity and clinically limited samples that are a hallmark of this disease process.
In this prospective study of patients undergoing ERCP for evaluation of biliary strictures, we found that the addition of PCR-based MP of free-DNA in fluid gathered during routine brushings had a significantly higher sensitivity (50%) for malignancy than standard “malignant” cytology results (26%, P<0.001) and comparable sensitivity to FISH testing (39%, P=0.15). All tests examined had similar specificity for malignancy. MP testing improved the overall diagnostic yield of each endoscopic procedure from 22% to 100%, while FISH improved the yield to 90%. Unlike cytology or FISH testing, MP does not require the presence of neoplastic cells in the specimen, but rather relies on identifying neoplastic free-DNA that is present in the normally discarded supernatant fluid of brushings that remains after cells are removed by centrifugation. This is particularly attractive given the hypocellular nature of specimens obtained by biliary brushings and the fact that additional procedure time or expertise is not required.17,20,21 In this scenario, MP provides superior diagnostic yield, which is an advantage over FISH testing and a cost-effective approach to obtaining diagnostic information given that repeat endoscopic procedures are not required to obtain additional cells for cytology or FISH analysis.
Importantly, we report that use of either MP or FISH testing significantly increased detection of pancreaticobiliary malignancy when cytology was otherwise nonmalignant. The combination of MP and cytology had similar sensitivity to that of the combination of FISH and cytology. Moreover, our results demonstrate that the highest sensitivity for malignancy was achieved when the combination of all 3 tests, cytology, MP, and FISH, were used (sensitivity 66% to 69%, accuracy 75% to 77%). Our results echo those of a recent study by Gonda et al.20 However, in our study, we also observed that use of only MP in combination with “suspicious” or “malignant” cytology results achieved statistically similar sensitivity (sensitivity 63%) to that of the combination of all three tests (P=0.13). While our patient population was different—with a higher proportion of malignant cases and particularly more patients with underlying cholangiocarcinoma—the overall sensitivity of MP for malignancy when used alone or in combination with cytology was similar between studies, emphasizing the utility of MP in cases of suspected cholangiocarcinoma.
Our results are also consistent with others describing increased sensitivity for pancreaticobiliary malignancy achieved when a combination of KRAS mutation and FISH testing is performed on cells from standard biliary brushings.22 However, in our study of free-DNA, KRAS mutation only accounted for half of all cases in which MP was positive. The other half of MP positive cases were due to LOH mutations among the TSG panel examined. Importantly, TSG-associated LOH mutations were detected among all 10 genomic loci in the panel over the various detected cases of malignancy, emphasizing the utility of these additional TSG LOH markers: 1p (CMM1, Lmyc), 3p (VHL, OGG1), 5q (MCC, APC), 9p (CDKN2A, CDKN2B), 10q (PTEN, MXI1), 17p (TP53), 17q (NME1, RNF34), 18q (SMAD4, DCC), 21q (TFF1, PSEN2), and 22q (NF2).
Interestingly, one malignant case in the study was found in an initially KRAS positive, primary sclerosing cholangitis (PSC) associated dominant stricture from a patient who 18 months later developed cholangiocarcinoma. As KRAS mutation is an early step in biliary carcinogenesis, we hypothesize that this mutation began in an otherwise occult precursor lesion, such as biliary intraepithelial neoplasia.23,24 This observation highlights the advantage of MP over other ancillary diagnostic tests, as it evaluates early neoplastic changes in free-DNA that are representative of the entirety of the biliary tree, rather than the focal area where cells are obtained.
In recent years, adjunctive methods for tissue acquisition of biliary structures have been shown to improve the diagnostic yield for malignancy. EUS-guided FNA (EUS-FNA) has a 75% to 90% sensitivity for malignancy when a visible mass is present on imaging or ultrasound; however, this sensitivity drops significantly when a discrete mass cannot be visualized.25–27 Moreover, EUS-FNA is contraindicated in patients with suspected hilar cholagniocarcinoma who are being considered for liver transplantation due to concern for tumor seeding in the needle tract.28 Video cholangioscopy with direct biopsy of the stricture is also a very helpful procedure for sampling biliary structures, and biopsies obtained in this manner have a 60% to 80% sensitivity for malignancy.29,30 However, video cholangioscopy is technically challenging and adds significant procedural costs making it a second line test at most centers. Our results demonstrate that ancillary molecular testing provides a similar sensitivity to that of EUS-FNA or cholanigoscopy-guided biopsies. Furthermore, MP testing has the advantage of requiring no additional facilities, staff training or equipment during ERCP.
There are several limitations to our study. First, this was a mixed cohort of patients with proximal and distal biliary structures resulting from multiple etiologies. Moreover, the overall number of patients with biliary strictures in the setting of inflammatory conditions (PSC and chronic pancreatitis) was relatively low. Interestingly, while KRAS is an early and critical mutation in the development of pancreatic cancer, oncogenic KRAS mutations can be found in up to 27% of patients with chronic pancreatitis.31 As such, the presence of only one false positive MP test attributable to KRAS is reassuring. Similarly, FISH aneuploidy is frequently seen in PSC patients likely due to the inflammatory landscape underlying this transformation.1 As a result, meta-analyses of sensitivity and specificity for FISH in cholangiocarcinoma in PSC patients is estimated at 68% and 70%, respectively.1 Because of potential inflammatory related false positive results, molecular information should be interpreted in the context of clinical and cytologic characteristics of each biliary stricture. Although whole exome sequencing on large cohorts of biliary tract cancer patients have recently been reported, PSC patients are under-represented in these cohorts and thus the mutational landscape in this subpopulation is less well characterized.1
In conclusion, in this prospective study we found that PCR-based MP of free-DNA obtained during routine brushings of biliary strictures improves detection of pancreaticobiliary malignancy when used as an ancillary molecular test to cytology. Use of either MP or FISH significantly increased detection of malignancy with similar sensitivity when cytology results were nonmalignant, with MP providing a superior diagnostic yield for each endoscopic procedure. The highest sensitivity and accuracy for detecting malignancy was achieved when both MP and FISH were used in combination with routine cytology. On the basis of this, we believe that when initially evaluating biliary structures, standard cytology, MP and FISH should be utilized in order to maximize diagnostic yields for malignancy and minimize the need for additional costly endoscopic procedures.
Footnotes
V.M.K., S.A.E., and S.D.F.: study design. V.M.K., S.A.E., S.D.F., N.A.T., and S.A.J.: manuscript preparation.
Supported by Interpace Diagnostic Corporation for the study at Washington University.
S.A.J., N.A.T., and S.D.F.: Employees of Interpace Diagnostic Corporation. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.Navaneethan U, Njei B, Venkatesh PG, et al. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:943–950. e3. [DOI] [PubMed] [Google Scholar]
- 2.Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Beers BE. Diagnosis of cholangiocarcinoma. HPB (Oxford). 2008;10:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowlus CL, Olson KA, Gershwin ME. Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol. 2016;13:28–37. [DOI] [PubMed] [Google Scholar]
- 6.Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaiteerakij R, Barr Fritcher EG, Angsuwatcharakon P, et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest Endosc. 2016;83:1228–1235. [DOI] [PubMed] [Google Scholar]
- 8.Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383–390. [DOI] [PubMed] [Google Scholar]
- 9.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783–789. [DOI] [PubMed] [Google Scholar]
- 10.Hartman DJ, Slivka A, Giusto DA, et al. Tissue yield and diagnostic efficacy of fluoroscopic and cholangioscopic techniques to assess indeterminate biliary strictures. Clin Gastroenterol Hepatol. 2012;10:1042–1046. [DOI] [PubMed] [Google Scholar]
- 11.Gonda TA, Glick MP, Sethi A, et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest Endosc. 2012;75:74–79. [DOI] [PubMed] [Google Scholar]
- 12.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritcher EG, Kipp BR, Halling KC, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136:2180–2186. [DOI] [PubMed] [Google Scholar]
- 14.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–1681. [DOI] [PubMed] [Google Scholar]
- 15.Barr Fritcher EG, Voss JS, Brankley SM, et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology. 2015;149:1813–1824. e1. [DOI] [PubMed] [Google Scholar]
- 16.Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: a review. Cancer Cytopathol. 2014;122:399–411. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein SD, Bibbo M, Loren DE, et al. Molecular analysis of centrifugation supernatant fluid from pancreaticobiliary duct samples can improve cancer detection. Acta Cytol. 2012;56:439–447. [DOI] [PubMed] [Google Scholar]
- 18.Deftereos G, Finkelstein SD, Jackson SA, et al. The value of mutational profiling of the cytocentrifugation supernatant fluid from fine-needle aspiration of pancreatic solid mass lesions. Mod Pathol. 2014;27:594–601. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra N, Jackson SA, Freed LL, et al. The added value of using mutational profiling in addition to cytology in diagnosing aggressive pancreaticobiliary disease: review of clinical cases at a single center. BMC Gastroenterol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonda TA, Viterbo D, Gausman V, et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin Gastroenterol Hepatol. 2017;15:913–919. [DOI] [PubMed] [Google Scholar]
- 21.Dudley JC, Zheng Z, McDonald T, et al. Next-generation sequencing and fluorescence in situ hybridization have comparable performance characteristics in the analysis of pancreaticobiliary brushings for malignancy. J Mol Diagn. 2016;18:124–130. [DOI] [PubMed] [Google Scholar]
- 22.Kipp BR, Fritcher EG, Clayton AC, et al. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. J Mol Diagn. 2010;12:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid MD, Lewis MM, Willingham FF, et al. The evolving role of pathology in new developments, classification, terminology, and diagnosis of pancreatobiliary neoplasms. Arch Pathol Lab Med. 2017;141:366–380. [DOI] [PubMed] [Google Scholar]
- 24.Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21:754–760. [DOI] [PubMed] [Google Scholar]
- 25.Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290–298. e1. [DOI] [PubMed] [Google Scholar]
- 26.De Moura DT, Moura EG, Bernardo WM, et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound. 2018;7:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104. [DOI] [PubMed] [Google Scholar]
- 28.Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608–614. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc. 2016;84:649–655. [DOI] [PubMed] [Google Scholar]
- 31.Luttges J, Diederichs A, Menke MA, et al. Ductal lesions in patients with chronic pancreatitis show K-ras mutations in a frequency similar to that in the normal pancreas and lack nuclear immunoreactivity for p53. Cancer. 2000;88:2495–2504. [DOI] [PubMed] [Google Scholar]


