Goals:
The main aim of this study was to investigate the significance of a pale area via flexible spectral imaging color enhancement (FICE) in the diagnosis of esophageal dysplasia and cancer.
Background:
The early diagnosis of esophageal squamous cancer is challenging, and the indication of Lugol’s chromoendoscopy has not yet been well established.
Study:
The esophageal mucosa of patients at our endoscopic center were sequentially evaluated with white-light endoscopy and FICE during insertion of the endoscope, followed by staining with Lugol’s solution during withdrawal. Patients were divided into 2 groups depending on whether esophageal leukoplakia was detected by white-light endoscopy and 2 groups depending on whether a pale area was detected by FICE. We compared cases of patients with abnormal iodine staining, and cases of dysplasia or cancer in esophageal leukoplakia—or pale area—positive and negative groups.
Results:
Cases of abnormal staining in the esophageal leukoplakia—or pale area—positive group were far more numerous than cases without esophageal leukoplakia or pale area, respectively (P=0.000). Cases of esophageal dysplasia and cancer in the esophageal leukoplakia—or pale area—positive group were far more numerous than cases without esophageal leukoplakia or pale area, respectively (P=0.000).
Conclusions:
Iodine staining should be performed in patients with esophageal leukoplakia or pale areas. Esophageal dysplasia and early-stage cancer were more easily detected in those with esophageal leukoplakia or pale areas.
Key Words: esophageal squamous cell carcinoma, Fuji intelligent chromoendoscope, esophageal leukoplakia, Lugol’s solution
Esophageal cancer is the eighth most common cancer worldwide and the sixth leading cause of death from cancer. The 2 main histologic types are squamous cell carcinoma and adenocarcinoma. Approximately 90% of cases are esophageal squamous cell carcinoma (ESCC). The survival of patients with ESCC is very poor, largely due to the late development of symptoms and consequent late diagnosis.1,2
Endoscopy is a useful technique for the diagnosis of precancerous esophageal lesions and early cancer. Such lesions may be obscure during screening with white-light endoscopy (WLI), whereas chromoendoscopy techniques have been proven to improve performance of the test.3,4 Computed virtual chromoendoscopy from Fujifilm, called “Flexible spectral imaging color enhancement (FICE) or Fuji intelligent chromoendoscopy,” reconstructs the image and displays what the mucosa would look like if it were illuminated using a particular wavelength.5 Moreover, the simplest and most effective method for detecting precancerous lesions and early esophageal cancer is Lugol’s chromoendoscopy (LCE).6 Although LCE increases the sensitivity of WLI to >95%,7 it is uncomfortable for most patients and can cause inflammation of the esophageal mucosa. Above all, the indication of LCE has not yet been well established. It is therefore, important to make the indications for LCE clear and to apply them to high-risk groups.
Oral leukoplakia is an established precursor lesion associated with a high risk of oral squamous cell carcinoma,8 and oral leukoplakia is associated with an increased risk of ESCC, particularly in younger people.9,10 Esophageal leukoplakia presents as a “white patch” in the esophageal mucosa that cannot be washed out with water, and its significance has been underestimated. Esophageal leukoplakia is not an unusual phenomenon in Chinese elders during endoscopy screening. Routine LCE has not been performed in most endoscopic centers for each patient with esophageal leukoplakia. FICE has some advantages in determining the shape of a lesion and delineating the lesion’s margins. Pale areas via FICE nearly corresponded to esophageal leukoplakia via WLI in our earlier observations, and pale areas were more frequently detected than esophageal leukoplakia because the definition of FICE was higher than that of WLI. We tried to elucidate the necessity of LCE in patients with esophageal leukoplakia or pale areas.
MATERIALS AND METHODS
From May to August 2017, all general patients at our endoscopic center who fulfilled our inclusion criteria were enrolled for screening. All gave their informed consent. Endoscopic screening was performed with a single channel gastroscope (EG450; Fujifilm Co, Tokyo, Japan). The esophageal mucosa of each patient was initially studied with WLI and FICE sequentially during endoscopic insertion. The wavelengths of FICE were set as follows: Red, 550 nm (2); Green, 500 nm (2); Blue, 470 nm (3). During withdrawal, 20 to 40 mL of 1.5% iodine solution was sprayed onto the esophageal mucosa until it was evenly stained. The esophagus was divided into 3 segments—upper, middle, and lower—according to 2 landmarks 24 and 32 cm from the incisors. Endoscopic images of each lesion were captured 2 to 3 minutes after iodine staining. The number and location of esophageal leukoplakia and pale areas, abnormal staining regions in each segment, were immediately recorded by an assistant. Biopsies with clear margins were obtained from unstained regions. All biopsies were sent for pathologic diagnosis. The pathologist was blinded to the endoscopic results.
Inclusion Criteria
Age range from 40 to 80 years.
No melena, hematemesis, or shock.
No cardiac, pulmonary, hepatic, or renal dysfunction.
No coagulopathy.
No female patients who were pregnant or preparing for pregnancy.
No allergy to iodine.
We compared the following items in the esophageal leukoplakia-positive and leukoplakia-negative groups and in the pale area–positive and pale area–negative groups:
Common conditions.
Cases with abnormal staining regions.
Total number of abnormal staining regions in each patient.
Total biopsies in each patient.
Cases of dysplasia or early-stage cancer.
Statistical Analyses
The statistical analyses were performed using the SPSS 22.0 software package (SPSS, Chicago, IL). Comparisons between groups were performed using Student’s t test or the χ2 test. Total areas of abnormal staining and total biopsies in each patient were analyzed using the Mann-Whitney test. The sensitivity, specificity, and area under the receiver operating characteristic curve were used to assess the accuracy of classification.
RESULTS
A total of 201 patients were enrolled in the study.
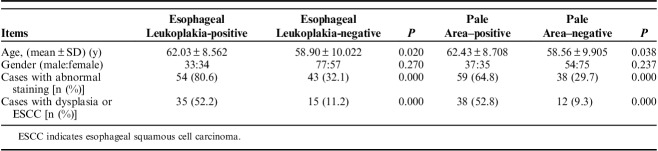
Table 1 showed demographic and histology data of the 201 patients and their WLI and FICE results.
TABLE 1.
Demographic and Histology Data of the 201 Patients and their WLI and FICE Results
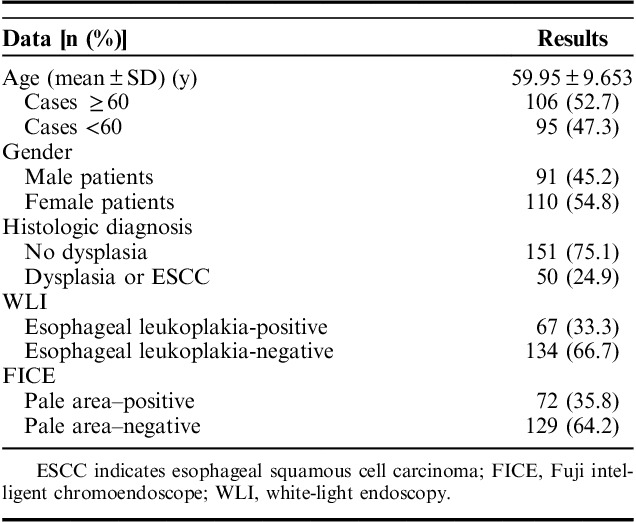
Table 2 showed distribution of esophageal leukoplakia, pale areas, dysplasia, and ESCC in the esophagus.
TABLE 2.
Distribution of Esophageal Leukoplakia, Pale Areas, Dysplasia, and ESCC in the Esophagus
Both esophageal leukoplakia and pale areas were more frequently detected in middle segment of esophagus. Esophageal leukoplakia in 68 patients and pale areas in 73 patients were distributed in ≥2 segments. Dysplasia of different grades and early-stage ESCC were most frequently found in the middle segment of the esophagus. The average age of patients with dysplasia or ESCC was greater than that of patients without dysplasia or ESCC (61.06±7.779 y vs. 59.58±10.194 y, P=0.004). There were no gender difference between patients with or without dysplasia or ESCC (37:35 vs. 54:75, P=0.237).
Figures 1–3 showed esophageal leukoplakia via WLI in the lower segment of the esophagus, pale area via FICE, and dark brown area via LCE in the same position, respectively.
FIGURE 1.
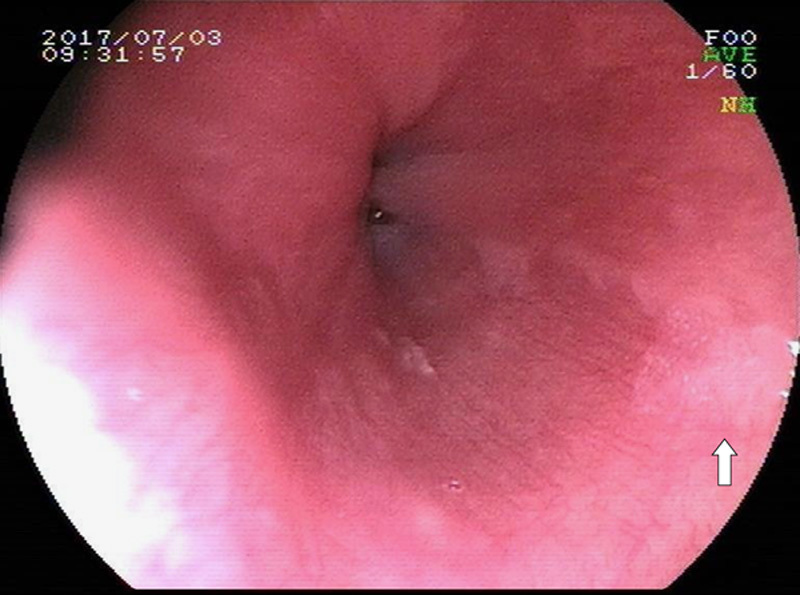
Esophageal leukoplakia via white-light endoscopy in the lower segment of the esophagus (see white solid arrow).
FIGURE 3.
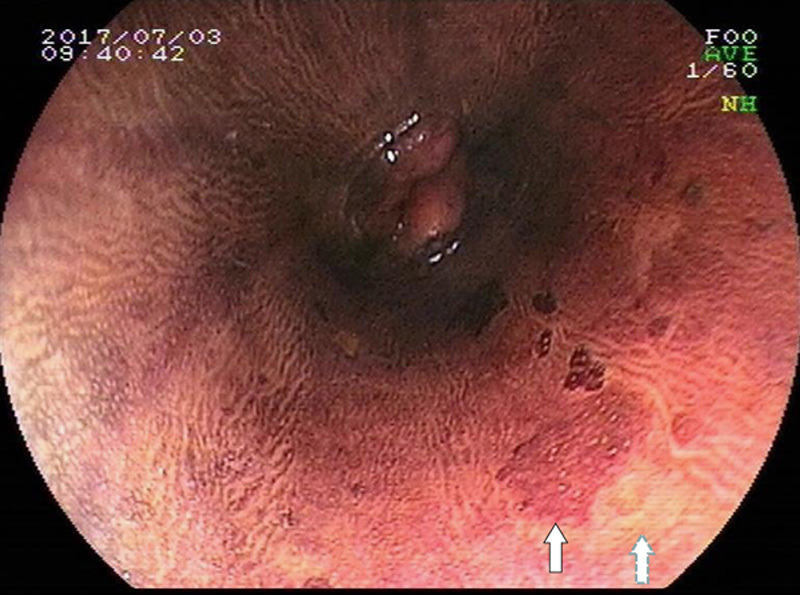
Dark brown area via Lugol’s chromoendoscopy in part of the same position (see white solid arrow); 2 understained regions and 1 unstained region can be detected close to the dark brown area. Pathologic diagnosis of biopsy from the unstained region (see white dotted arrow) showed moderate dysplasia.
FIGURE 2.
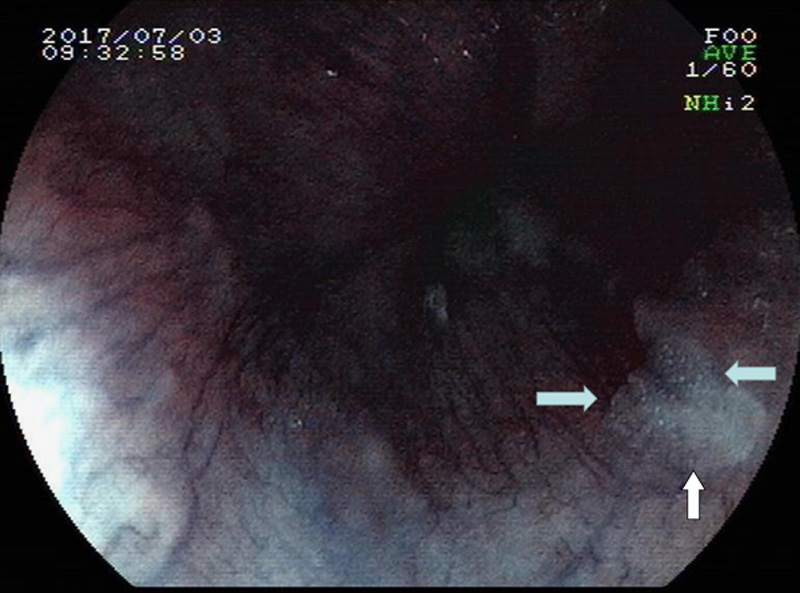
Pale area via FICE in the same position (see white solid arrows).
Table 3 showed the differences in groups with or without esophageal leukoplakia, or in groups with or without pale areas.
TABLE 3.
Differences in Groups With or Without Esophageal Leukoplakia, or in Groups With or Without Pale Areas
Areas of abnormal staining in the esophageal leukoplakia-positive group were significantly more numerous than those in the esophageal leukoplakia-negative group (Z=7.289, P=0.000); biopsied areas in the esophageal leukoplakia-positive group were significantly more numerous than those in the esophageal leukoplakia-negative group (Z=7.463, P=0.000). Areas of abnormal staining in the pale area–positive group were significantly more numerous than those in the pale area–negative group (Z=12.127, P=0.000); biopsied areas in the pale area–positive group were significantly more numerous than those in the pale area–negative group (Z=9.558, P=0.000).
Figure 4 showed receiver operating characteristic curves of esophageal leukoplakia and pale area for the detection of dysplasia and early-stage ESCC.
FIGURE 4.
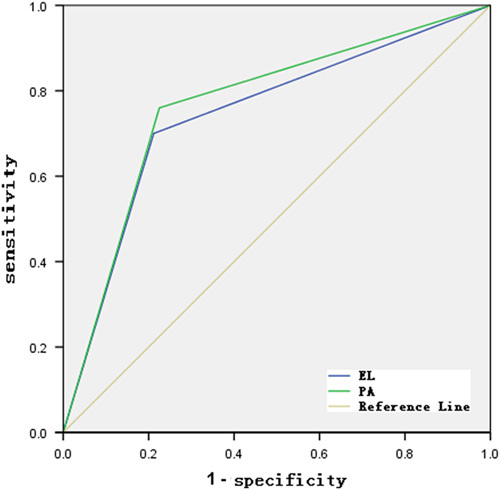
Receiver operating characteristic (ROC) curves of esophageal leukoplakia and pale area for the detection of dysplasia and early-stage esophageal squamous cell carcinoma. EL indicates esophageal leukoplakia; PA, pale area.
The sensitivity of esophageal leukoplakia for the detection of dysplasia and early-stage ESCC was 70.0% (35 and 50) with a specificity of 78.8%; the sensitivity of pale area for the detection of dysplasia and early-stage ESCC was 76.0% (38 and 50) with a specificity of 75.5%.
DISCUSSION
Esophageal cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of death from cancer in China.11,12 Endoscopy is the gold standard for the diagnosis of precancerous squamous lesions. Early detection of ESCC and precancerous lesions is directly related to an improved prognosis. Therefore, a major target of endoscopic screening is to identify these lesions, resect them endoscopically, and/or review them regularly. Unfortunately the early lesions are asymptomatic, and changes in the mucosa are subtle, so that these conditions can easily go unnoticed during a standard endoscopic examination. As a result, it has been suggested that chromoendoscopy techniques could improve diagnostic outcomes. The simplest and most effective way of detecting squamous dysplasia is via LCE.13–15 It can delineate the margin and significantly improve the endoscopic detection of precancerous or cancerous squamous lesions. The sensitivity and specificity of WLI for the detection of high-grade dysplasia and cancer are 62% and 79%, respectively, compared with the use of LCE, which has a much higher sensitivity of 96% at the expense of a slight loss of specificity of 63%, when using LCE.7 Sometimes endoscopists are reluctant to perform LCE because of patient discomfort. A low concentration of iodine can reduce the severity of esophagitis and increase patient compliance. Application of sodium thiosulfate solution can also reduce the adverse effects of LCE and accelerate color fading by reduction of the iodine.
Immediately after spraying of the iodine solution, normal mucosa turned brown, whereas the abnormal mucosa suggestive of dysplasia or cancer turned yellow. Within 2 to 3 minutes, some of the yellow areas changed to pink, which was defined as the “pink-color sign,” or positive.16 “Pink-color sign” is an important indication of high-grade dysplasia or cancer, however, this change in color may not appear with mild or moderate dysplasia. One study showed that esophageal squamous dysplasia seems to progress over months to many years depending on the grade. Among those in a high-risk Chinese population, untreated patients with biopsy-proven mild, moderate, and severe squamous dysplasia who were followed endoscopically, 5%, 27%, and 65%, respectively, developed clinically diagnosed ESCC after 3.5 years, and 24%, 50%, and 74% did so after 13.5 years, respectively. Another reported follow-up of patients with moderate or severe dysplasia showed that 22% with moderate dysplasia and 60% with severe dysplasia showed endoscopic (size) or histologic progression during the follow-up period.17–19 Therefore, based on the high cumulative incidence, it is probable that severe dysplasia requires prompt treatment, moderate dysplasia requires treatment or periodic endoscopic follow-up, and mild dysplasia can be followed over longer intervals.
It is known that a significant proportion (up to 62%) of oral squamous cell carcinomas arise from precursor oral potential malignant lesions, such as leukoplakia.8,20 Esophageal leukoplakia are also associated with esophageal cancer. In our experience, esophageal leukoplakia can be classified histologically as nondysplastic or dysplastic, including squamous hyperplasia, chronic inflammation, papillary epithelioma, eosinophilic esophagitis, and squamous dysplasia and early cancer squamous. Squamous hyperplasia is one of the most common benign types and present as “dark brown area” during LCE. The same as ESCC, esophageal leukoplakia, or pale areas, are more often detected in the middle segment of the esophagus. Frequently, esophageal cancer or precancerous lesions occurred close to or in the “dark brown area” in our endoscopic examination. Esophageal leukoplakia and pale areas can serve as markers for a field defect where unstained lesions are more common.
FICE digitally limits the wavelength range of the light and offers images with bright color and high light quality. Meanwhile, FICE can be activated during endoscopic screening without the need to interrupt the process.21 Pale areas, which have clear margins and bright colors, can be easily detected in the spot of esophageal leukoplakia with adequate wavelengths of FICE. In our research, we found pale areas are more sensitive than esophageal leukoplakia in predicting precancerous squamous lesions. Thus, FICE may have some advantages in screening ESCC.
Our study has some limitations. First, it is a research from a single endoscopic center in China. Second, it is not based on a crossover design. Nevertheless we can draw 2 definite conclusions: esophageal leukoplakia or pale areas are associated with precancerous squamous lesions and ESCC. At least in a high-risk population, LCE should be performed when pale areas are detected via FICE, or esophageal leukoplakia are detected via WLI.
ACKNOWLEDGMENTS
The authors would like to thank LetPub (http://www.letpub.com) for providing linguistic assistance during the preparation of this manuscript.
Footnotes
Y.L., C.Y., and Y.S. contributed equally.
The research were supported by Key clinical project of Peking University Third Hospital, No Y73480-03, and special fund project for the development of human resources in Tibet Autonomous region, No 20. Key Laboratory for Helicobacter pylori Infection and Upper Gastrointestinal Diseases in Beijing, No. BZ0371.
The study follows the Helsinki Declaration.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- 3.Tomie A, Dohi O, Yagi N, et al. Blue laser imaging-bright improves endoscopic recognition of superficial esophageal squamous cell carcinoma. Gastroenterol Res Pract. 2016;2016:6140854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uedo N, Fujishiro M, Goda K, et al. Role of narrow band imaging for diagnosis of early-stage esophagogastric cancer: currentconsensus of experienced endoscopists in Asia-Pacific region. Dig Endosc. 2011;23(suppl 1):58–71. [DOI] [PubMed] [Google Scholar]
- 5.Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014;26(suppl 1):105–115. [DOI] [PubMed] [Google Scholar]
- 6.Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lao-Sirieix P, Fitzgerald RC. Screening for oesophageal cancer. Nat Rev Clin Oncol. 2012;9:278–287. [DOI] [PubMed] [Google Scholar]
- 8.Schepman K, der Meij E, Smeele L, et al. Concomitant leukoplakia in patients with oral squamous cell carcinoma. Oral Dis. 1999;5:206–209. [DOI] [PubMed] [Google Scholar]
- 9.Ritter SB, Petersen G. Esophageal cancer, hyperkeratosis, and oral leukoplakia: follow-up family study. JAMA. 1976;236:1844–1845. [PubMed] [Google Scholar]
- 10.Liang H, Yang Z, Wang JB, et al. Association between oral leukoplakia and risk of upper gastrointestinal cancer death: a follow-up study of the Linxian General Population Trial. Thorac Cancer. 2017;8:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama A, Ohmori T, Makuuchi H, et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928–934. [DOI] [PubMed] [Google Scholar]
- 14.Shiozaki H, Tahara H, Kobayashi K, et al. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068–2071. [DOI] [PubMed] [Google Scholar]
- 15.Freitag CP, Barros SG, Kruel CD, et al. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191–195. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara R, Kanzaki H, Iishi H, et al. Pink-color sign in esophageal squamous neoplasia, and speculation regarding the underlying mechanism. World J Gastroenterol. 2013;19:4300–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian. China Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 19.Taylor PR, Abnet CC, Dawsey SM, et al. Squamous dysplasia—the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–1710. [PubMed] [Google Scholar]
- 21.Negreanu L, Preda CM, Ionescu D, et al. Progress in digestive endoscopy: Flexible Spectral Imaging Colour Enhancement (FICE)-technical review. J Med Life. 2015;8:416–422. [PMC free article] [PubMed] [Google Scholar]