Abstract
The rectum has distinctive anatomic and physiologic features, which increase the risk of local spread and recurrence among rectal cancers as compared to colon cancers. Essential to the management of rectal cancers is accurate endoscopic localization as well as preoperative imaging assessment of local and distant disease. Successful oncologic care is multidisciplinary including input from Gastroenterologists, Surgeons, Medical and Radiation Oncologists, Radiologists, and Pathologists. Extensive planning of curative intent is mandatory as failures of upfront treatment present great long‐term difficulty for patients and caregivers. Local recurrences are frequently associated with major morbidity including bowel and urinary obstruction, severe pain, and significantly diminished quality of life. Distant recurrence is associated with lower survival. Over the last two decades, there have been many advances in diagnostic imaging techniques as well as surgical techniques including transanal endoscopic microsurgery for very early stage cancers. Progress in curative management paradigms includes shorter courses of preoperative radiotherapy and chemotherapy doublet paradigms for perioperative treatment. This review describes the diagnosis, workup, and multimodality curative intent treatment of rectal cancers. It is emphasized that success begins in the hands and eyes of the gastroenterologist.
INTRODUCTION
Epidemiology and prognosis
In the US alone, there were an estimated 39,910 new diagnoses of rectal cancer in 2017 [1]. In the European Union, incidence of rectal cancer is 15‐25/100,000 population each year with approximately one third dying from the disease each year [2]. The 5 year overall survival (OS) for stages 2‐3 rectal cancers has remained relatively steady at approximately 65% [3] over the last 20 years. The incidence of rectal cancer has been decreasing as the widespread use of screening allows for the identification and treatment of premalignant lesions [4]; however, several recent studies have shown an increase in incidence of rectal cancers among young people. Data from the Surveillance, Epidemiology, and End Results (SEER) database show a significant increase in colorectal cancer diagnoses in patients <50 years old from 1974 to 2010 and predict that the incidence of rectal cancer in particular will increase by 124.2% for patients aged 20‐34 years by 2030 [5]. Currently the causes for this phenomenon are the topic of much interest but have yet to be identified.
Risk factors and screening
Age is the greatest risk factor for developing colorectal cancer, with 90% of cases occurring in those ≥50 years of age. This is why current guidelines recommend routine screening for those at average risk starting at age 50. Screening can be performed via colonoscopy, stool studies such as guaiac‐based, immunochemical‐based, or DNA‐based testing, a combination of flexible sigmoidoscopy with or without stool studies or a computedtomography colonography. The recommended interval of repeat testing depends on the modality chosen and the results of the initial screening study [6, 7].
Modifiable lifestyle factors can also contribute to an increased risk of colorectal cancer including a low activity level, a low fiber, high‐fat diet, a body mass index in the overweight or obese range, as well as alcohol and tobacco consumption [8]. A personal or family history of colorectal cancer increases one's risk as do known genetic syndromes such as familial adenomatous polyposis and Lynch Syndrome [9]. Coexisting medical conditions such as ulcerative colitis also increase the risk of colorectal cancer [10].
Clinical presentation
Although routine screening for colorectal cancer has increased the percentage of rectal cancers diagnosed incidentally, >70% of patients present to medical attention with symptoms from the local tumor [11]. Seventy‐five percent of patients have been reported to present with a change in bowel habits, 51% with bright red blood per rectum, 25% with a rectal mass, 10% with iron deficiency anemia, and 4% with abdominal pain [12]. Rectal cancers located low in the rectum can cause a sensation of incomplete bowel evacuation, rectal pain and tenesmus.
The role of the rectal examination
Rectal cancers can be missed by both rectal examination and endoscopy, if not properly performed. Hence a thorough physical examination, including a digital rectal examination (DRE), should be performed for all cases. The examiner should first inspect for any visible external lesions on the perianal skin including external hemorrhoids. Baseline sphincter function should be assessed and documented. The examiner should note the superior‐inferior extent, circumferential involvement, and distance from the anal verge of any palpable masses. The examiner should also note whether the mass appears fixed to the sphincter muscles, pelvic sidewall, or adjacent pelvic organs. A vaginal exam may be necessary to assess anterior invasion in women. Mid‐ to high‐rectal tumors may not be palpable on DRE depending on the dimensions of the examiner's finger, but when possible, careful documentation of the tumor on DRE at baseline is important for initial treatment planning as well as subsequently evaluating the response to neoadjuvant therapy, when administered.
Endoscopic diagnosis
Rectal cancers are defined endoscopically by occurrence distal to the most proximal of the three rectal folds (Fig. 1). Careful endoscopic evaluation is paramount for both establishing the diagnosis as well as planning eventual treatment. First and foremost, gastroenterologists have a key role in diagnosing rectal cancers and avoiding missing lesions. Adequate bowel preparation and careful mucosal visualization are essential to avoid missing flat lesions. It is important to carefully inspect both sides of folds. Retroflexion should be performed to avoid missing low‐lying rectal cancers occurring just above the dentate line.
Fig. 1.
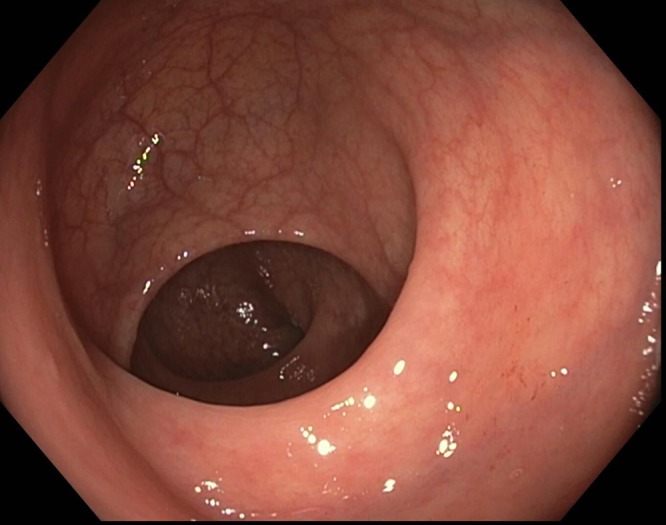
Endoscopic view of the three rectal folds
Because the decision to pursue a neoadjuvant treatment strategy partially hinges on the endoscopically determined location of the tumor, accurate reporting is necessary at the time of initial diagnostic endoscopy. These include above all, measured distance from the dentate line or anal verge, and in some cases with circumferential lesions, qualitative suspicion of risk for imminent obstruction. Patients with obstructing or near‐obstructing tumors should be considered for surgical diversion prior to neoadjuvant therapy for symptom management while not compromising ultimate oncologic outcomes [13].
Rigid proctosigmoidoscopy is recommended for accurate measurement of the tumor with respect to the anal verge [14]. One study showed colonoscopic reported localization of rectal tumors to be inaccurate in approximately 25% of patients, [15] which can impact clinical decision making and potential treatment options [16]. Most commonly, measurements of the tumor's location with respect to the anal verge are overestimated with colonoscopy when dealing with mid‐ to high‐rectal tumors. This can lead to erroneous surgical planning as well as neoadjuvant treatment recommendations.
To facilitate surgical planning, tattooing the lesion (usually the distal extent) may be helpful particularly as rates of complete response after preoperative treatment improve [17].
Obtaining tissue diagnosis
When obtaining a biopsy of a rectal mass, it is important to obtain sufficient tissue not only to establish the diagnosis, but also potentially to perform mutational analyses that can help guide future targeted therapy. While different Next Generation Sequencing assays require variable amounts of viable malignant cells, to enhance reliability and precision, enough of the tumor needs to be sampled to represent its entirety [18]. As such, at least five to six biopsies should be taken from the center and the margin of a lesion even with a classically appearing carcinoma on endoscopy. If biopsies are only taken from the outer periphery, it can miss the invasive component and only show the noninvasive adenomatous characteristics. If biopsies are only taken from the center, it may only show necrotic tissue without any definitive neoplastic cells [19]. Limited specimens also do not provide adequate tissue for specialized testing such as for Lynch Syndrome screening and for assessment of other molecular markers.
Biopsy interpretation
Careful analysis of the biopsy specimen is essential to establishing the diagnosis. The interpreting pathologist must confirm malignancy while excluding other entities from the differential diagnosis such as premalignant lesions, scarring diverticulitis, and solitary rectal ulcers with hyperplasia occurring due to prolapse. Squamous cell carcinoma of the anal canal can also invade proximally into the distal rectum, and metastatic disease from other types of adenocarcinomas (such as gastric or breast) should be considered. A malignant rectal lesion, by definition, invades through the muscularis mucosae. Typical invasive adenocarcinoma is the most common type of rectal cancer and can be identified by epithelial columnar cells arranged into glandular patterns. The prognostic markers, include grade of tumor, which is reported as well differentiated (>95% glandular structures), moderately differentiated (50‐95% glandular structures), poorly differentiated (5‐50% glandular structures), and undifferentiated (<5% glandular structures). The presence of mucin or signet ring cells is noted as is any observed tumor invasion into blood or lymphatic vessels [18].
Further workup and staging
Workup by the gastroenterologist. After the primary tumor is identified and thoroughly documented by endoscopy and even while the diagnosis of rectal cancer is being confirmed by biopsy, the staging workup should be initiated. The European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines both recommend a complete blood count, renal and liver function tests, carcinoembryonic antigen (CEA), and contrast‐enhanced CT scan of the chest and abdomen for complete staging. It is important to note that even in the setting of metastatic disease to the lungs and/or liver, the initial diagnostician should still refer on for complete staging and multidisciplinary review, as the sequencing of treatment and possibility of curative intent interventions requires careful team based consensus and is possible with limited metastatic disease (Fig. 2 PATHWAY DIAGRAM).
Fig. 2.
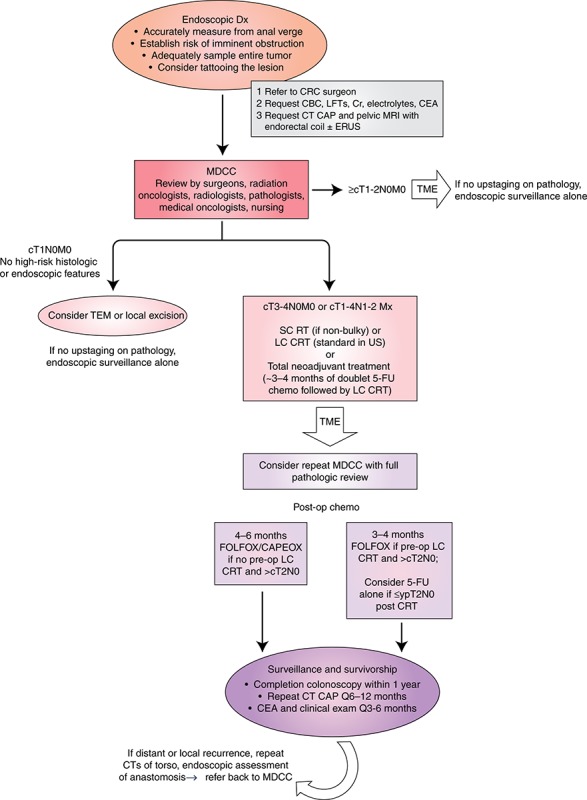
Pathway for diagnosis, staging, and treatment of rectal cancers. FOLFOX/CAPEOX fluorouracil, leucovorin, and oxaliplatin/capecitabine and oxaliplatin; LC long‐course; MDCC, multidisciplinary case conference; SC short‐course; TEM transanal endoscopic microsurgery; TME total mesorectal excision
Referrals: to whom and when? After the diagnosis has been established and the initial staging workup has been performed, the next referral is to a colorectal surgeon for further workup and management. In the setting of metastatic disease outside the pelvis, the first referral can instead be to a medical oncologist if the patient does not have any symptoms concerning for current or impending obstruction. For disease that appears localized on initial workup, the treating surgeon will order subsequent studies for further staging and treatment planning.
Preoperative clinical staging aims to describe the primary tumor (T‐stage), nodal status (N‐stage) and systemic status (M‐stage). Per the 8th edition of the American Joint Commission on Cancer (AJCC), T‐stage depends on the extent of tumor invasion through the rectal wall: T1 invades submucosa, T2 invades muscularis propria, T3 invades perirectal tissues, T4 invades through the peritoneum or directly invades or adheres to adjacent organs. N‐stage depends on the number of involved nodes: N1 involves 1‐3 nodes and N2 involves more than 3 nodes. M‐stage depends on the presence or absence of distant metastases: M1 with confirmed distant disease and M0 without [20].
Although pelvic magnetic resonance imaging (MRI) and endorectal ultrasound (ERUS) can both be used to determine the T‐stage of a rectal cancer by examining the extent of invasion through the rectal wall, as well as potentially involved lymph nodes, MRI has become the preferred strategy per the NCCN guidelines [21]. In some centers MRI and ERUS are used as complementary modalities in staging. As the use of endorectal coils and enhanced contrast agents becomes more widespread, MRI's advantages emerge. Although ERUS has been reported in some studies to have equal accuracy as MRI in evaluating perirectal lymph nodes and superior accuracy in evaluating local tumor penetration, it is highly operator‐dependent [22, 23]. Importantly, MRI provides valuable information to predict risk of positive circumferential resection margins [24] at the time of surgery, which has important prognostic value. [25] However, in the setting of a higher likelihood of positive nodal disease, MRI may overstage as it relies on size and signal intensity to describe nodal disease whereas ERUS can determine echogenicity and allow for diagnostic sampling if necessary (Fig. 3).
Fig. 3.
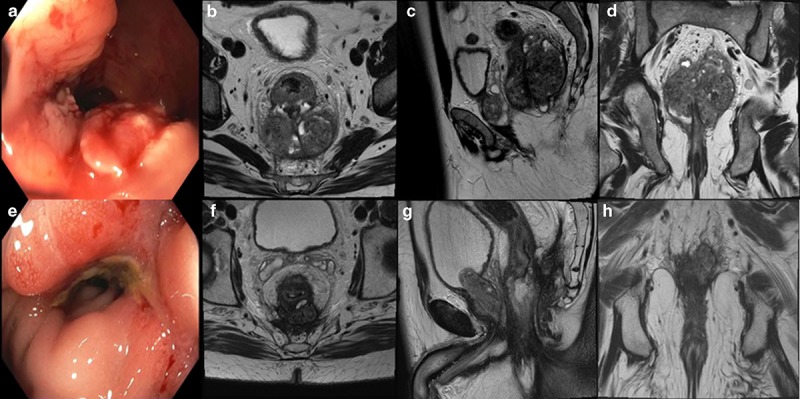
Staging workup information for a patient who was found to have a fungating, non‐obstructing rectal mass on a routine screening colonoscopy. A representative image from the in‐office sigmoidoscopy (a) shows the non‐obstructing mass situated on the posterior wall approximately 3 cm from the anal verge and extending 10 cm proximally. Representative axial (b), sagittal (c) and coronal (d) images from his baseline magnetic resonance imaging (MRI) show aT4aN2 tumor measuring 10 cm × 6 cm with the inferior edge extending through the levator into the ischiorectal fossa on the left, and the invading edge of the tumor involving the mesorectal fascia posteriorly with concern for extension beyond the mesorectal fascia superiorly and posteriorly. There were several suspicious mesorectal and external iliac lymph nodes present as well as extramural venous invasion. Due to the bulky nature of his primary tumor, he was dispositioned to neoadjuvant chemotherapy with four cycles of FOLFOX chemotherapy. Restaging MRI showed a nice response to chemotherapy, and he was then dispositioned to neoadjuvant chemoradiation. He received oral capecitabine twice daily on days he received radiation. Post‐treatment sigmoidoscopy (e) showed that the inferior tumor edge was now 4 cm proximal to the anal verge, and post‐treatment MRI (f, g, h) showed the tumor now measuring 5.5 × 3.9 cm with the degree of mesorectal fascial abutment significantly decreased. The pelvic adenopathy also decreased in size
Treatment of rectal cancer
Differences between Rectal and Colon Cancers. Similar to colon cancers, the vast majority of rectal cancers are adenocarcinomas and develop from a normal mucosa to adenoma to carcinoma in a sequence previously described by Vogelstein and others [26, 27]. Despite a similar sequence to invasion, rectal cancers differ from colon cancers in their risk for local recurrence, survival and management due to differing embryologic etiology, lymphovascular drainage basin, and molecular mutational burden even between sigmoid colon and rectum [28, 29].
Though the primary modality for curative intent treatment of all CRCs is a high‐quality oncologic surgical resection, recommendations for adjunct therapies are dictated by patterns of failure after definitive surgical management. The patterns of failure are highly dependent on the location of the tumor with respect to the peritoneal reflection in the pelvis. Colon cancers, defined by their location above the peritoneal reflection, proximal to the third rectal fold intraluminally and/or approximately 12‐15 cm above the anal verge, have a high incidence of distant failure within the abdominal cavity due to the proximity of the colonic wall to the peritoneal surface [30, 31].
In contrast, for rectal cancers—which are located below the peritoneal reflection—approximately 50‐60% of recurrences occur locally in the pelvis [32,33,34]. As local recurrences are difficult to salvage, [35, 36] escalating upfront (or neoadjuvant, i.e., prior to surgery) local therapy is the standard of care in the form of neoadjuvant chemoradiation (CRT) for patients with T3, T4 or node‐positive rectal cancer21. For colon cancers, adjuvant chemotherapy after curative intent surgery, is recommended for any node‐positive, T4N0 or high‐risk T3N0 disease, while radiation therapy (RT) is only considered for T4 disease that penetrates a fixed structure [37].
Surgical principles. Since the 1990s, it has been universally accepted that in conjunction with radiation, curative intent rectal surgery with a total mesorectal excision (TME) is standard of care reducing postoperative morbidity and local recurrence and enhancing long‐term survival [38, 39]. A completely intact mesorectal fascial envelope macroscopically (Fig. 4) and pathologically, portends a lower risk of local recurrence than an incomplete or non‐intact excision [40]. Negative margins are associated with lower risk of recurrence. There is also a decrease in recurrence risk when surgery is performed in high‐volume centers, and by high volume surgical teams demonstrating adherence to comprehensive multidisciplinary imaging, surgical and pathologic indicators [41].
Fig. 4.
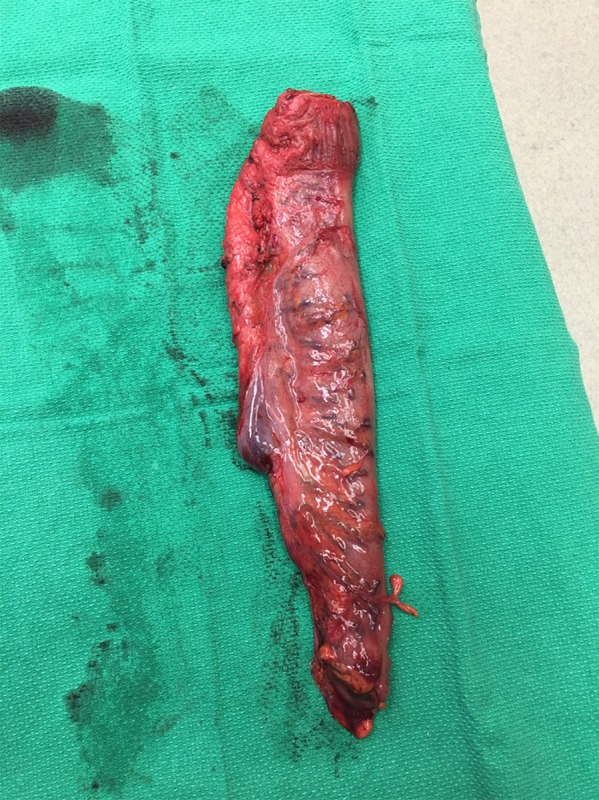
Intact Mesorectal envelope from successful TME for Stage 3 Rectal Cancer post‐neoadjuvant treatment
Depending on the tumor's location, this may include a sphincter preserving operation such as a low anterior resection with either colo‐anal or colo‐rectal anastomosis (restorative procedure) or with permanent end colostomy (Hartmann procedure), or an abdominoperineal resection (APR). These operations are done with an open procedure or with advanced laparoscopic techniques. With advances in transanal endoscopic microsurgery (TEM), select patients may be considered for this as an option.
When evaluating surgical approaches, issues regarding future long‐term ostomy care, quality of life, length of residual bowel, genitourinary, and sexual function are key elements of a patient centered surgical decision making. Various rectal cancer decision aids can be used [42]. Primary factors driving choice between an APR versus a restorative procedure are discussed in conjunction with patients while recognizing the location of the lesion. Avoiding a permanent colostomy depends on distance of tumor from the anal verge which influences the ability to attain a negative margin and discussion of the added risks of restorative procedures such as anastomotic leaks and the potential need for a temporary ileostomy. All of this has to be factored in with recognizing realistic functional outcomes with regards to the potential for long term altered bowel functioning (potential for fecal incontinence and fecal urgency) with a restorative procedure.
In addition to traditional open or laparoscopic TME, various high volume centers with access to advanced technology are now using robotic assisted and transanal approaches, shown to be potentially advantageous in technically difficult low‐lying lesions in certain situations, such as obese and male patients [43].
Surgery alone
For well‐selected, highly motivated individuals with very low‐risk tumors (T1N0) without high‐risk features, certain centers may offer local resection, commonly in the form of TEM. In expert hands with a full thickness excision and negative margins, this may be advantageous in terms of avoiding the significant morbidity of a pelvic dissection. However, local resection of rectal cancer is limited in its ability to assess loco‐regional lymph nodes. Furthermore, there is a higher local recurrence rate but this is in the face of lower perioperative mortality, lower major postoperative complications, and the lower need for a permanent stoma [44]. When comparing techniques for local resection of rectal cancer, TEM is favored over traditional trans‐anal excision as there are lower rates of specimen fragmentation and lower rates of recurrence with TEM. Furthermore, there are higher rates of negative margins [45]. TEM is also preferred over attempts at definitive resection via piecemeal polypectomy with colonoscopy. For those that remain pathologic T1N0, after TEM, with no high‐risk histologic features, discussions at Multidisciplinary Tumor Boards should take place to discuss the role of adjuvant treatment, which in most cases is not recommended.
Neoadjuvant chemoradiation
Rectal cancers have a high rate of local recurrence because of the absence of a serosa, close proximity of the rectal wall to other pelvic organs and the resultant difficulty obtaining wide surgical margins. To reduce the risk of local recurrence and the morbidity associated with salvage therapy after local recurrence, combined‐modality therapy is recommended by the NCCN for patients with stage II (T3‐4, node‐negative) and stage III (node‐positive) rectal cancer consisting of neoadjuvant CRT followed by surgical resection and then adjuvant chemotherapy with the total duration of perioperative therapy not to exceed 6 months21. Although radiation can be delivered with increased precision and accuracy using modern techniques, the side effects of treatment are not negligible. Short‐ and long‐term toxicity from CRT includes but is not limited to diarrhea, radiation enteritis, genitourinary dysfunction, and a small risk of secondary pelvic malignancies. Therefore, there is much interest in omitting radiation for a lower‐risk subset of patients who many have sufficiently low local recurrence rates with surgery alone and for whom CRT may represent overtreatment. Patients with T3N0 disease who have preoperative circumferential resection margins >1 mm have been shown in recent series to have local control rates between 2.5‐3.4% [46]. With careful evaluation of high‐resolution MRI studies at diagnosis, patients can be better stratified and the decision as to whether or not to offer neoadjuvant CRT for patients with low‐risk criteria by imaging can be better individualized42.
On the other end of the treatment spectrum, there is a group of higher‐risk patients for whom neoadjuvant treatment intensification is desired. The concept of “Total Neoadjuvant Treatment” [47] entails 5‐FU based doublet chemotherapy (mFOLFOX‐6) being administered either before or after CRT with all treatment delivered prior to curative surgery (Fig. 5). A potential benefit of this approach is treatment of micrometastatic disease in high‐risk patients, potential improved pathologic complete response rates, as well as better tolerance and completion rates of chemotherapy [48,49,50].
Fig. 5.
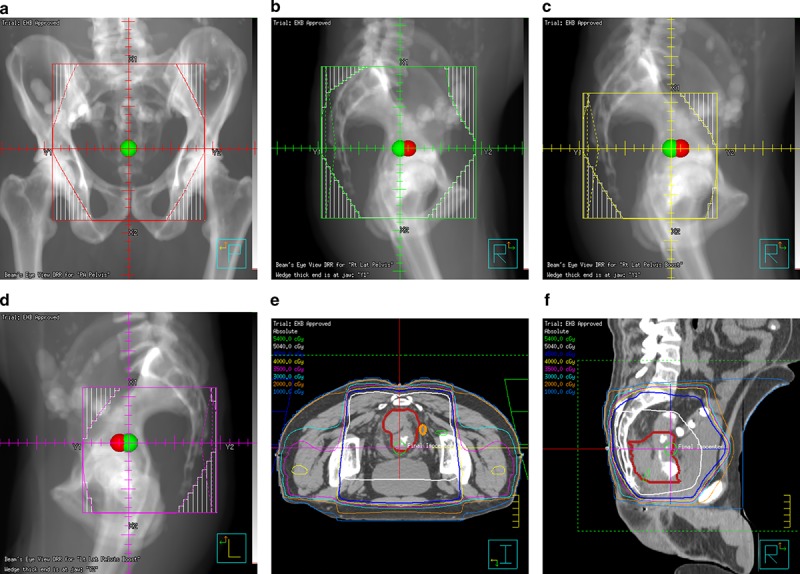
Representative images of a radiation treatment plan for a patient diagnosed on screening colonoscopy with a T4aN2 adenocarcinoma of the proximal rectum. Due to the bulky nature of his primary tumor, he was dispositioned to neoadjuvant chemotherapy with four cycles of FOLFOX chemotherapy. Restaging MRI showed a nice response to chemotherapy, and he was then dispositioned to neoadjuvant chemoradiation. He received oral capecitabine twice daily on days he received radiation. He was treated to 45 Gray in 25 fractions using a three‐field plan: a posterior‐anterior field (a) a left lateral field (b) and a right lateral field (not shown). A sequential boost of an additional 5.4 Gy in three fractions was delivered to the tumor plus margin using right (c) and left (d) lateral fields. Representative axial (e) and sagittal (f) images of the isodose distributions are displayed. He tolerated treatment well and underwent a complete combined abdominoperineal proctectomy with colostomy 6 weeks after completion of chemoradation. His pathologic stage was ypT3N0. All margins were negative and only 30% of the tumor specimen contained viable cells. He then went on to receive a final four cycles of adjuvant 5‐fluorouracil to complete his oncologic treatment
Although historic studies utilized postoperative CRT, [51, 52] preoperative therapy has been shown to be superior in terms of local control and less toxicity [53,54,55]. Adding concurrent chemotherapy to radiation further improves local control [56, 57], as such the current standard of care is to administer either oral capecitabine or infusional 5‐fluorouracil on days radiation is administered during standard long‐course RT (45‐50.4 Gray (Gy) in 25‐28 daily fractions). (see Fig. 5) Of note, the chemotherapy is provided with the primary aim as a radiosensitizer—making the tumor cells more vulnerable to damage by radiotherapy. To limit severe GI toxicity but maintain efficacy, dosing of this 5‐FU based IV or PO chemotherapy is typically 50‐60% of the standard dose of adjuvant 5‐FU monotherapy.
Although long‐course RT (45‐50.4 Gy in 25‐28 daily fractions) is the most commonly used regimen in the United States, short course (sc) RT (25 Gy in 5 daily fractions) is another evidenced‐based approach for neoadjuvant treatment [58]. Several European trials showed the local control (LC) benefit of radiation and surgery compared with surgery alone [59,60,61,62]. Preoperative sc‐RT also showed a LC benefit when compared with selective postoperative long‐course CRT [63]. More recently, the TROG 01.04 randomized trial compared preoperative long‐course CRT to preoperative sc‐RT followed by postoperative chemotherapy and found that, for patients with T3N0‐2M0 rectal adenocarcinoma, there was no difference in 3‐year LC, OS, late toxicity rates or health‐related quality of life [64, 65].
Chemotherapy alone as neoadjuvant therapy
PROSPECT [66], BACCHUS, [67], and FORWARC [68]are three ongoing international prospective clinical trials, amongst others, that are investigating the use of chemotherapy alone in the neoadjuvant setting for rectal cancer. These studies are allowing for the selective avoidance of short and long‐term morbidity from pelvic radiation with aims to determine a lack of detriment to long‐term recurrence and survival.
Postoperative chemotherapy
Finally, despite the lack of definitive evidence [69], the commonest practice worldwide is to offer at least 4 months of postoperative FOLFOX or other 5‐FU based treatment following conventional long course chemoradiotherapy (CRT) and curative TME. Much of the survival data and treatment regimens are extrapolated from the colon cancer literature. Most guidelines recommend postoperative chemotherapy with the caveat from ESMO that if there is any benefit it likely is in terms of Disease Free Survival as opposed to OS.
CRT alone
Illustrative of the global uncertainty regarding the disparate curative treatment options is the ongoing work of Drs. Habr‐Gama and Perez in Brazil who have reported long‐term data supporting organ preservation and the avoidance of definitive TME in highly selected patients after CRT alone. [70] This is currently in practice in an un‐controlled fashion in select centers in North America and Europe but decision making and outcomes are largely driven by patient specific factors. It is most successful in a setting where very short interval regular endoscopic and clinical surveillance can be performed. This is an experimental approach and not ready for routine practice; no guideline bodies currently endorse this paradigm despite its increasing use.
The role of the gastroenterologist in follow‐up and surveillance
Endoscopic long‐term surveillance and management of recurrence. After completion of all oncologic therapy, recommended surveillance includes follow‐up clinic visits every 3‐6 months for the first 2 years then every 6 months until 5 years post‐treatment. History and physical examination with CEA levels are to be performed at every visit; CT of the chest, abdomen and pelvis every 6‐12 months. Colonoscopy is recommended at 1 year and then after 3 years (i.e., 4 years after surgery or the first perioperative colonoscopy), and then after another 5 years (i.e., 9 years after surgery or perioperative colonoscopy) 37, [71]. Subsequent colonoscopies should occur at 5‐year intervals. If neoplastic polyps are detected, the intervals between colonoscopies should be in accordance with the published guidelines for polyp surveillance intervals. For the selected patients with rectal cancer who do not undergo TME (those with localized cancers who received TEMS, or those who complete CRT but who decline definitive surgery) local surveillance with flexible sigmoidoscopy or ERUS every 3‐6 months for the first 2‐3 years is recommended.
The future cumulative incidence of metachronous (subsequent new) colorectal cancers is estimated to be between 0.3 and 0.35% per year. [72] If local recurrence is suspected at the anastomosis or in the pelvis, full restaging information should be obtained with physical and endoscopic examination with biopsy, CEA level and CT of the chest, abdomen, and pelvis to rule out concomitant distant disease. If local‐only recurrence is confirmed, multidisciplinary evaluation and management should be pursued, as discussed above.
Management of recurrent and/or metastatic disease. Resectable local recurrence is typically treated with standard preoperative CRT if the patient is radiation‐naive. For patients who have been previously irradiated, hyperfractionated schedules are considered [73]. Surgical resection then occurs, with or without the use of intraoperative RT if there is concern for close or positive margins at the time of the operation. [74] Although historically, long‐term survival rates for recurrent rectal cancer were <10% [75], more recent series suggest that the 5‐year survival rate approaches 50%, with long‐term success rates associated with margin‐negative reresections [76]. Local recurrence frequently is associated with major morbidity including distal bowel and urinary obstruction, severe pain and significantly diminished quality of life.
Likewise, the modern hepatic and thoracic surgical literature, ESMO, and NCCN all support the use of multidisciplinary evaluation and the use of multimodality therapy in the curative intent treatment of oligometastatic or recurrent distant disease in the lungs and/or liver.
CONCLUSIONS
Rectal cancer is a relatively common and considerably morbid and lethal solid tumor malignancy. Epidemiologic data predict an increase in the number of cases over the coming decades. Though death rates and local recurrence rates have been declining since the widespread adoption of the TME approach in the 1990s, careful diagnosis and appropriate staging remain the lynchpin in successful treatment planning and care for people with rectal cancers. It is incumbent on the referring endoscopist to understand the correct pathways in the workup and subsequent management of such patients. A complete and careful endoscopy and workup by the gastroenterologist is necessary to drive effective multidisciplinary management of curable rectal cancers.
CONFLICT OF INTEREST
Guarantor of the article: Drs. Goldenberg and Singh are the guarantors of this article.
Specific author contributions: Drs. Goldenberg and Holliday wrote the first draft. All authors were involved in the interpretation of data and critical revision of the manuscript for important intellectual content.
Financial support: There was no external funding.
Potential competing interests: Dr. Singh has been on advisory board of Ferring and Merck Canada and has received research funding from Merck Canada.
Footnotes
Correspondence: H.S. (email: Harminder.Singh@umanitoba.ca)
published online 16 July 2018
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. (Supplement 4) [DOI] [PubMed] [Google Scholar]
- 3.Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of european randomized clinical trials. J Clin Oncol. 2011;29:3163–72. [DOI] [PubMed] [Google Scholar]
- 4.Henley SJ, Singh SD, King J, et al. Invasive cancer incidence and survival—United States, 2011. MMWR Morb Mortal Wkly Rep. 2015;64:237–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2020. JAMA Surg 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2017;153:307–23. [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 8.Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2016;2:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemminki K, Chen B. Familial risk for colorectal cancers are mainly due to heritable causes. Cancer Epidemiol Biomark Prev. 2004;13:1253–6. [PubMed] [Google Scholar]
- 10.Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroentereology. 2013;145:166–75. [DOI] [PubMed] [Google Scholar]
- 11.Moreno CC, Mittal PK, Sullivan PS, et al. Colorectal cancer initial diagnosis: screening colonoscopy, diagnostic colonoscopy, or emergent surgery, and tumor stage and size at initial presentation. Clin Colorectal Cancer. 2016;15:67–73. [DOI] [PubMed] [Google Scholar]
- 12.Thompson MR, O'Leary DP, Flashman K, et al. Clinical assessment to determine the risk of bowel cancer using symptoms, age, mass and iron deficiency anemia (SAMI). Br J Surg. 2017;104:1393. [DOI] [PubMed] [Google Scholar]
- 13.Koea JB, Guillem JG, Conlon KC, et al. Role of laparoscopy in the initial multimodality management of patients with near-obstructing rectal cancer. J Gastrointest Surg. 2000;4:105–8. [DOI] [PubMed] [Google Scholar]
- 14.Helewa RM, Park J. Surgery for locally advanced T4 rectal cancer: strategies and techniques. Clin Colon Rectal Surg. 2016;29:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piscatelli N, Hyman N, Osler T, Localiz colorectal cancer colon. Arch Surg Chic Ill 1960. 2005;140:932–5. [DOI] [PubMed] [Google Scholar]
- 16.Schoellhammer HF, Gregorian AC, Sarkisyan GG, et al. How important is rigid proctosigmoidoscopy in localizing rectal cancer? Am J Surg. 2008;196:904–8. [DOI] [PubMed] [Google Scholar]
- 17.Acuna SA, Elmi M, Shah PS, et al. Preoperative localization of colorectal cancer: a systematic review and meta-analysis. Surg Endosc. 2017;31:2366–79. [DOI] [PubMed] [Google Scholar]
- 18.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the american society for clinical pathology, college of american pathologists. Association for Molecular Pathology, and the American Society of Clinical Onclolgy. J Clin Oncol. 2017;35:1453–86. [DOI] [PubMed] [Google Scholar]
- 19.Wiech T, Werner M. Pathology of rectal cancer. MRI of rectal cancer. MRI of Rectal Cancer. Berlin, Heidelberg: Springer; 2009. [Google Scholar]
- 20.Amin MB, Edge S, Green F, et al. AJCC cancer staging manual. 8th ed Springer International Publishing; New York: 2017 [Google Scholar]
- 21.NCCN. National Comprehensive Cancer Network Guidelines Version 3.2017 Rectal Cancer. National Comprehensive Cancer Network. Accessed 08 Aug 2017. [Google Scholar]
- 22.Bipat S, Glas AS, Slors FJM, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232:773–83. [DOI] [PubMed] [Google Scholar]
- 23.Klessen C, Rogalla P, Taupitz M. Local staging of rectal cancer: the current role of MRI. Eur Radiol. 2007;17:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahaye MJ, Engelens SM, Nelemans PJ, et al. Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Seminars in ultrasound, CT and MRI, Vol. 26, issue 4; 2005. p. 259–68. [DOI] [PubMed] [Google Scholar]
- 25.Taylor FGM, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. [DOI] [PubMed] [Google Scholar]
- 26.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759. [DOI] [PubMed] [Google Scholar]
- 27.Bettington M, Walker N, Cluston AD, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–86. [DOI] [PubMed] [Google Scholar]
- 28.Tamas K, Wallenkamp AM, deVries EG, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41:671–9. [DOI] [PubMed] [Google Scholar]
- 29.Salerno G, Sinnatamby C, et al. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006;8:5–9. [DOI] [PubMed] [Google Scholar]
- 30.Willett CG, Tepper JE, Cohen AM, et al. Failure patterns following curative resection of colonic carcinoma. Ann Surg. 1984;200:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunderson LL, Sosin H, Levitt S. Extrapelvic colon—areas of failure in a reoperation series: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731–41. [DOI] [PubMed] [Google Scholar]
- 32.Malcolm AW, Perencevich NP, Olson RM, et al. Analysis of recurrence patterns following curative resection for carcinoma of the colon and rectum. Surg Gynecol Obstet. 1981;152:131–6. [PubMed] [Google Scholar]
- 33.Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–29. [DOI] [PubMed] [Google Scholar]
- 34.Pilipshen SJ, Heilweil M, Quan SH, et al. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984;53:1354–62. [DOI] [PubMed] [Google Scholar]
- 35.Wiig JN, Larsen SG, Dueland S, et al. Preoperative irradiation and surgery for local recurrence of rectal and rectosigmoid cancer. Prognostic factors with regard to survival and further local recurrence. Colorectal Dis. 2008;10:48–57. [DOI] [PubMed] [Google Scholar]
- 36.Bozzetti F, Bertario L, Rossetti C, et al. Surgical treatment of locally recurrent rectal carcinoma. Dis Colon Rectum. 1997;40:1421–4. [DOI] [PubMed] [Google Scholar]
- 37.NCCN. National Comprehensive Cancer Network Guidelines Version 2.2017 Colon Cancer. National Comprehensive Cancer Network. Accessed 08 August 2017. [Google Scholar]
- 38.Enker WE. Total mesorectal excision—the new golden standard of surgery for rectal cancer. Ann Med. 1997;29:127–33. [DOI] [PubMed] [Google Scholar]
- 39.Maurer CA, Renzulli P, Kull C, et al. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: long-term results. Ann Surg Oncol. 2011;18:1899. [DOI] [PubMed] [Google Scholar]
- 40.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wexner SD, Berho ME. The rationale for and reality of the New National Accreditation Program for Rectal Cancer. Dis Colon Rectum. 2017;60:595–602. [DOI] [PubMed] [Google Scholar]
- 42.Wu RC, Boushey RP, Scheer AS, et al. Evaluation of the rectal cancer patient decision aid: a before and after study. Dis Colon Rectum. 2016;59:165–72. (2016) [DOI] [PubMed] [Google Scholar]
- 43.Kuo LJ, Ngu JCY, Chen CC. Transanal total mesorectal excision: is it necessary in the era of robots? Int J Colorectal Dis. 2018;33:341. [DOI] [PubMed] [Google Scholar]
- 44.Kidane B, Chadi SA, Kanters S, et al. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:122–40. [DOI] [PubMed] [Google Scholar]
- 45.Clancy C, Burke JP, Albert MR, et al. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:254–61. [DOI] [PubMed] [Google Scholar]
- 46.Wang QX, Li SH, Zhang X, et al. Identification of locally advanced rectal cancer with low risk of local recurrence. PLoS One. 2015;28:10:e0117141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ludmir EB, Palta M, Willett CG, et al. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer. 2017;123:1497–506. [DOI] [PubMed] [Google Scholar]
- 48.Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2017;40:283–7. [DOI] [PubMed] [Google Scholar]
- 49.Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–8. [DOI] [PubMed] [Google Scholar]
- 51.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–304. [DOI] [PubMed] [Google Scholar]
- 52.Anonymous. Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1610–4. [PubMed] [Google Scholar]
- 53.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. [DOI] [PubMed] [Google Scholar]
- 54.Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst. Rev. 2009;21:CD006041. [DOI] [PubMed] [Google Scholar]
- 55.Sauer R, Becker H, Hohenberger W, et al. German Rectal Cancer Study Group Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:CD008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst. Rev. 2013;28:CD006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi81–88. Suppl 6 [DOI] [PubMed] [Google Scholar]
- 59.KCMJ Peeters, CAM Marijnen, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. [DOI] [PubMed] [Google Scholar]
- 60.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–50. [DOI] [PubMed] [Google Scholar]
- 61.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. [DOI] [PubMed] [Google Scholar]
- 62.Cedermark B, Johansson H, Rutqvist LE, et al. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal. Cancer Study Group Cancer. 1995;75:2269–75. [DOI] [PubMed] [Google Scholar]
- 63.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:3827–33. [DOI] [PubMed] [Google Scholar]
- 65.McLachlan S-A, Fisher RJ, Zalcberg J, et al. The impact on health-related quality of life in the first 12 months: A randomised comparison of preoperative short-course radiation versus long-course chemoradiation for T3 rectal cancer (Trans-Tasman Radiation Oncology Group Trial 01.04). Eur J Cancer. 2016;55:15–26. [DOI] [PubMed] [Google Scholar]
- 66.Alliance for Clinical Trials in Oncology. PROSPECT: Chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery ( NCT01515787). https://clinicaltrials.gov/ct2/show/
- 67.Glynne-Jones R, Hava N, Goh V, et al. Bevacizumab and Combination Chemotherapy in Rectal Cancer Until Surgery (BACCHUS): a phase II, multicentre, open-label, randomised study of neoadjuvant chemotherapy alone in patients with high-risk cancer of the rectum. BMC Cancer. 2015;15:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized 3 arm phase III trial. J Clin Oncol. 2016;34:3300–7. [DOI] [PubMed] [Google Scholar]
- 69.Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017;18:e354–e363. [DOI] [PubMed] [Google Scholar]
- 70.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the us multi-society task force on colorectal cancer. Am J Gastroenterol. 2016;111:337–46. [DOI] [PubMed] [Google Scholar]
- 72.Mulder SA, Kranse R, Damhuis RA, et al. The incidence and risk factors of metachronous colorectal cancer: an indication for follow-up. Dis Colon Rectum. 2012;55:522–31. [DOI] [PubMed] [Google Scholar]
- 73.Tao R, Tsai CJ, Jensen G, et al. Hyperfractionated accelerated reirradiation for rectal cancer: An analysis of outcomes and toxicity. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2017;122:146–51. [DOI] [PubMed] [Google Scholar]
- 74.Hyngstrom JR, Tzeng C-WD, Beddar S, et al. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: ten-year institutional experience. J Surg Oncol. 2014;109:652–8. [DOI] [PubMed] [Google Scholar]
- 75.Bozzetti F, Bertario L, Rossetti C, et al. Surgical treatment of locally recurrent rectal carcinoma. Dis Colon Rectum. 1997;40:1421–4. [DOI] [PubMed] [Google Scholar]
- 76.You YN, Skibber JM, Hu CY, et al. Impact of multimodal therapy in locally recurrent rectal cancer. Br J Surg. 2016;103:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]


