Abstract
OBJECTIVES:
Colorectal cancer (CRC) screening using fecal immunochemical tests (FIT) may reduce CRC‐related mortality but its effectiveness is influenced by the limited accuracy of FIT. Identifying individuals at increased risk of a false FIT result could improve screening, but the available evidence is conflicting. We performed a systematic review and meta‐analysis on risk factors for false‐positive and false‐negative FIT results in CRC screening.
METHODS:
A systematic search in MEDLINE, EMBASE, and Cochrane Library identified publications (before 29 January 2017) on risk factors (known at time of FIT invitation) associated with false FIT results (presence/absence of advanced neoplasia) in a CRC screening setting. Risk of bias was assessed using QUIPS. In meta‐analysis, summary relative risk ratios and corresponding 95% confidence intervals were calculated for each risk factor.
RESULTS:
Of 518 records identified, 14 studies with 54,499 participants in total were included for analysis. In meta‐analysis, male sex was associated with a significantly lower risk of false‐positivity (RR 0.84, CI 0.74‐0.94), whereas participants using non‐steroidal anti‐inflammatory drugs (NSAIDs) had a higher risk (RR 1.16, CI 1.06‐1.27). The use of anticoagulants was most frequently studied, without a significant effect on FIT positivity. Males (RR 1.83, CI 1.53‐2.19), participants with a family history for CRC (RR 1.61, CI 1.19‐2.15), hyperglycemia (RR 1.29, CI 1.02‐1.65), hypertension (RR 1.50, CI 1.14‐1.98), obesity (RR 1.38, CI 1.11‐1.71), and (former) smokers (RR 1.93, CI 1.52‐2.45) were all at significantly higher risk for false‐negative results. Age was not found to have a systematic effect on either FIT false‐positivity or false‐negativity in meta‐analysis.
CONCLUSIONS:
Multiple risk factors, known at time of FIT invitation, are associated with false FIT results in CRC screening. This information can be used to identify populations risking false reassurance after a negative result or unnecessary colonoscopy after a positive result, and to further optimize CRC screening effectiveness.
INTRODUCTION
Population‐based colorectal cancer (CRC) screening using fecal occult blood tests has shown to be effective in reducing the high burden of worldwide CRC‐related mortality [1]. Fecal immunochemical tests (FIT) are replacing the former guaiac fecal occult blood (gFOBT) test as primary screening method because of their higher sensitivity, quantitative nature, and higher participation rates [2, 3]. Compared to gFOBT, FIT is easier to use as it is a one‐sample test and does not require dietary restrictions [3]. Yet, FIT is not a highly accurate test. Depending on the positivity cutoff used, no advanced neoplasia (AN) is detected at colonoscopy in 45‐70% of FIT‐positive participants [4,5,6]. Furthermore, up to 10% of participants with a negative FIT appear to have CRC or one of its precursor lesions [4, 7].
False‐negative FIT results can lead to false reassurance, which might delay CRC diagnosis if patients would develop symptoms. Negative FIT results could additionally decrease participation in successive rounds and may encourage participants to maintain an otherwise unhealthy lifestyle, due to the so‐called ‘certificate of health effect’ [8, 9]. False‐positive results can also be harmful because unnecessary colonoscopies are performed with a concomitant risk of complications, undeniable discomfort of the procedure, and healthcare costs. False‐positive results further may cause psychological distress in participants, in between receiving the FIT result and the colonoscopy findings [10, 11].
Evidence‐based knowledge on risk factors influencing FIT accuracy to detect AN may offer opportunities to increase the effectiveness of a CRC screening program. Multiple studies have shown that some participant‐related factors, such as sex, age, smoking habits and the presence of hemorrhoids could influence FIT‐positivity rates, lead to more false test results [12, 13]. The use of anticoagulants has been appointed as a potential source of false‐positive results [14].
The available evidence is however conflicting and a review is missing, hampering strong conclusions and the development of recommendations to improve FIT accuracy. We performed a systematic review and meta‐analysis of studies evaluating personal risk factors for either false‐positive or false‐negative FIT results, known at time of FIT invitation.
METHODS
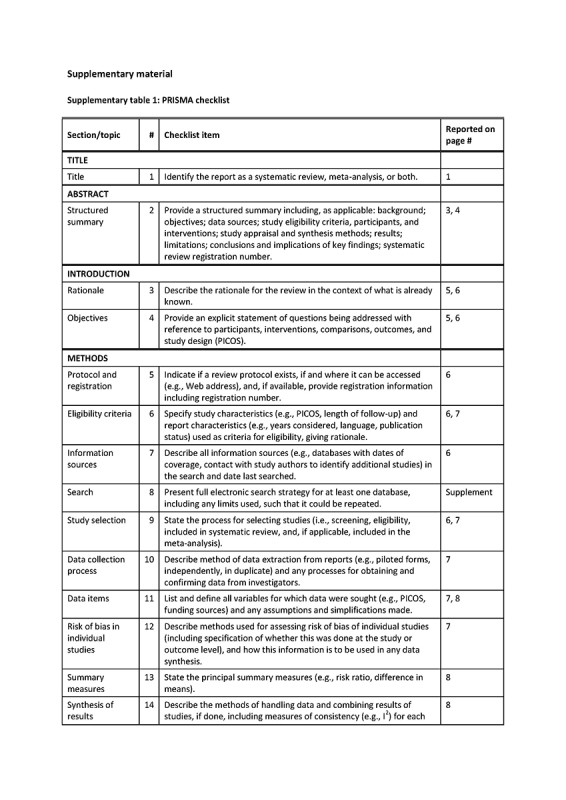
The review is described in accordance with the PRISMA guidelines (Supplementary table 1) [15]. The protocol of this study was not included in an accessible registry. This review addressed the question: do participants in population‐screening with a specific participant‐related factor more often have a false‐positive/false‐negative FIT result compared to participants without that risk factor. We planned to select the risk factors as reported in the individual studies.
Search strategy and eligibility criteria
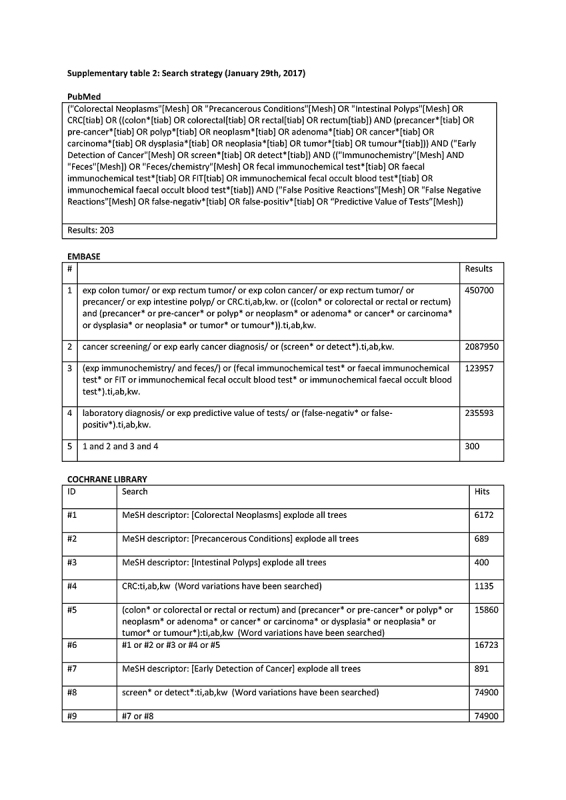
Databases Pubmed, Embase, and Cochrane were searched (29 January 2017) with the assistance of a specialized librarian, using MeSH terms and text words for colorectal cancer, screening, fecal immunochemical test, false‐positivity, false‐negativity, sensitivity, and specificity (Supplementary table 2).
Two reviewers (LV and CK) independently selected potentially eligible full‐text articles from the search results by scanning title and abstract of all identified records. Reference lists of selected articles and reviews were scanned for other relevant publications. Any disagreement was solved through discussion. Potentially eligible articles were read in full‐text to make final decisions about inclusion.
Eligible studies had assessed risk factors for false‐positive/false‐negative FIT results, defined as the presence or absence of AN (advanced adenoma and/or CRC) at colonoscopy; in a population‐based screening program, a pilot program, or in a similar research setting. These inclusion criteria were chosen to minimize the chance of missing articles that reported false results as secondary outcome. Excluded were studies only described in conference abstracts, letters to the editor, or in articles without quantitative results on the association of risk factors with false FIT results, which would preclude the calculation of relative risks. Also excluded were studies not available in full‐text report after contacting the corresponding author.
Since we aimed at personal factors known at time of screening, we also excluded studies assessing the effect of changing positivity cutoffs only, the effect of number of FITs performed in one screening round, the effect of sample return period or season, and studies assessing risk factors found at colonoscopy.
Data extraction
Two authors (LV and CK) independently reviewed included articles and extracted relevant data using a standardized extraction form. Data were collected on publication year, study setting, data collection period, number of included patients, type of FIT used, FIT cutoff value for positivity, definition of a false FIT result (detection/no detection of AN), evaluated risk factors and total numbers of positive FIT results, total number of participants with and without risk factors and total of participants with and without AN at colonoscopy. Positivity cutoff values were reported in μg hemoglobin (Hb)/g feces and, where needed, converted from ng Hb/ml buffer [16].
Risk of bias assessment
The risk of bias of included studies was assessed using the QUIPS tool for prognostic reviews [17]. QUIPS includes the domains ‘prognostic factor measurement’ and ‘outcome measurement’, which were considered more appropriate to assess risk of bias of studies on risk factors than the comparable domains in QUADAS ‘index test’ and ‘reference test’ [18]. The other evaluated QUIPS domains were participation, study attrition, study confounding and statistical analysis and reporting. Risk of bias on each domain was graded as low, intermediate or high, following the recommendations of the Cochrane Prognosis Methods Group.
Data analysis and synthesis
To allow for meta‐analysis, relative risks for reported risk factors were recalculated with the data in the study report. From 2 × 2 contingency tables, risk ratios for false‐positive FIT results were calculated by dividing the proportion of false‐positive results, relative to all FIT‐positives, in the group with the risk factor by the proportion of false‐positive results, relative to all FIT‐positives, in the group without that risk factor. Similarly, false‐negative relative risks were calculated using the false‐negative proportions in each group.
For each risk factor, we calculated a summary estimate of the relative risk in meta‐analysis, whenever this factor had been included in at least two study reports. We used Mantel‐Haenszel's random‐effects method, and calculated 95% confidence intervals to assess statistically significance, relying on a predefined significance level of p < 0.05 [19]. Forest plots were composed for each meta‐analysis. Heterogeneity was assessed with I2 statistics [20]. An I2 of <25% was considered low, 25‐50% moderate, and >50% high heterogeneity. Funnel plots were screened to assess publication bias. We used Review Manager version 5.3.5 (The Nordic Cochrane Centre, Copenhagen) for statistical (meta‐)analyses. For reporting, risk factors are grouped into the broader categories demographic factors, medication use, medical history/comorbidities. A sensitivity analysis was performed with studies using the same positivity cutoff level of 20 μg Hb/g feces.
RESULTS
Study selection
The search strategy yielded 518 records (Fig. 1). We removed 109 duplicates, 354 records based on title and abstract, 13 conference abstracts, 1 letter, 3 abstracts without full text, 7 articles without relevant results [21,22,23,24,25,26,27], 10 articles only evaluating risk factors found at colonoscopy [4,28,29,30,31,32,33] or organizational aspects [34,35,36], and 7 articles that reported insufficient data to calculate risk ratios [12,13,37,38,39,40,41]. This resulted in 14 articles included for review (Table 1) [14,42,43,44,45,46,47,48,49,50,51,52,53,54].
Fig. 1.

Study selection
Table 1.
Study characteristics

Study characteristics
Included studies and their characteristics are summarized in Table 1. All data had been collected within an existing or pilot screening program with a cohort design, except for one study [14] that was part of a randomized controlled trial comparing FIT with colonoscopy screening. OC‐Sensor was the most frequently used FIT brand. Positivity cutoffs varied between studies and between FIT brand (2 μg Hb/g feces to 50 μg Hb/g feces). The most frequently used cutoff, in six studies, was 20 μg Hb/g feces. Diagnostic accuracy of FIT was calculated based on the detection of AN in all studies except two that additionally included intermediate‐risk adenomas [43, 44].
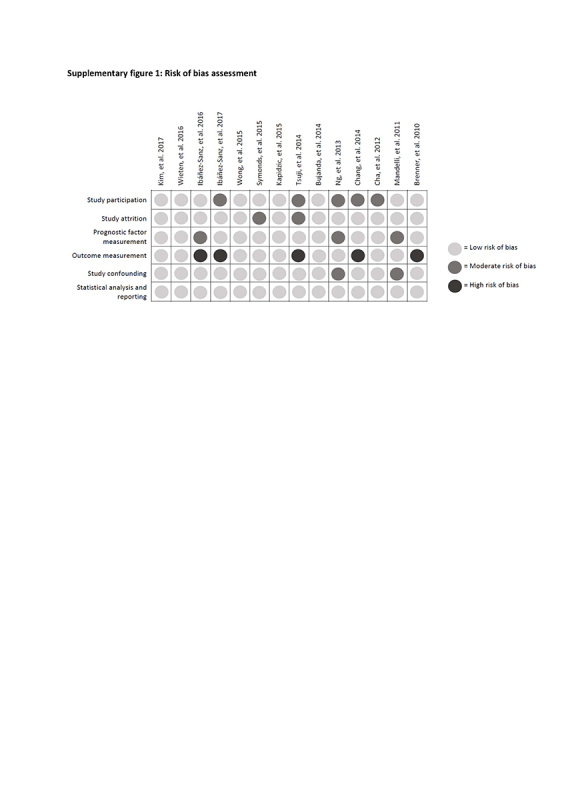
Risk of bias across studies
Risk of bias evaluations for each study are shown in Supplementary figure 1. A high risk of bias for outcome measurement was assigned to five studies because of an undefined FIT brand or cutoff level for positivity, but overall risk of bias was considered limited. The risk of bias assessment did not lead to any exclusion of or correction in studies.
Risk factors for false‐positive results
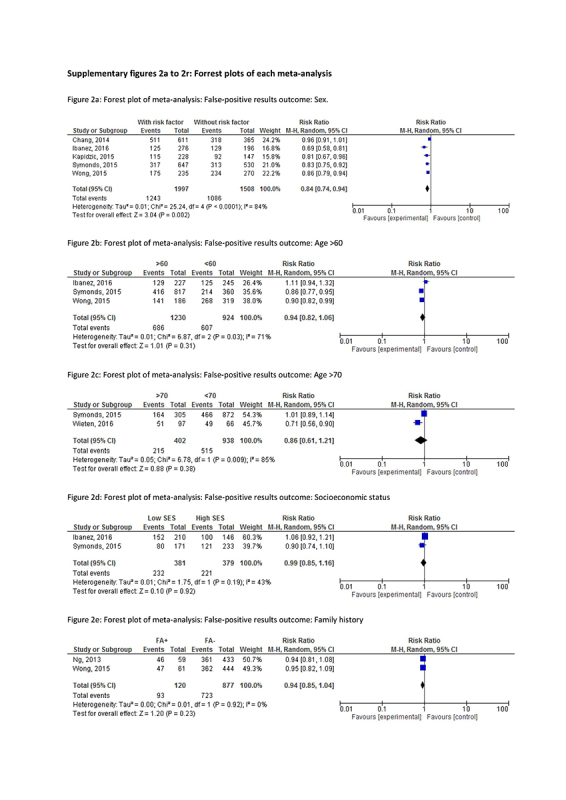
Risk factors and their association with false‐positive FIT results in each study are described in Table 2a and summary results are presented in Table 3 (forest plots in Supplementary figures 2a‐2l). The most frequently studied risk factors for false‐positive results are the use of anticoagulants and antithrombotic drugs (10 results: [14, 44, 47, 51, 54]), sex (5 results: [44, 46, 49, 50, 53]), and age (5 results: [44,45,46,53]).
Table 2a.
Individual risk ratios of risk factors for false‐positive FIT results
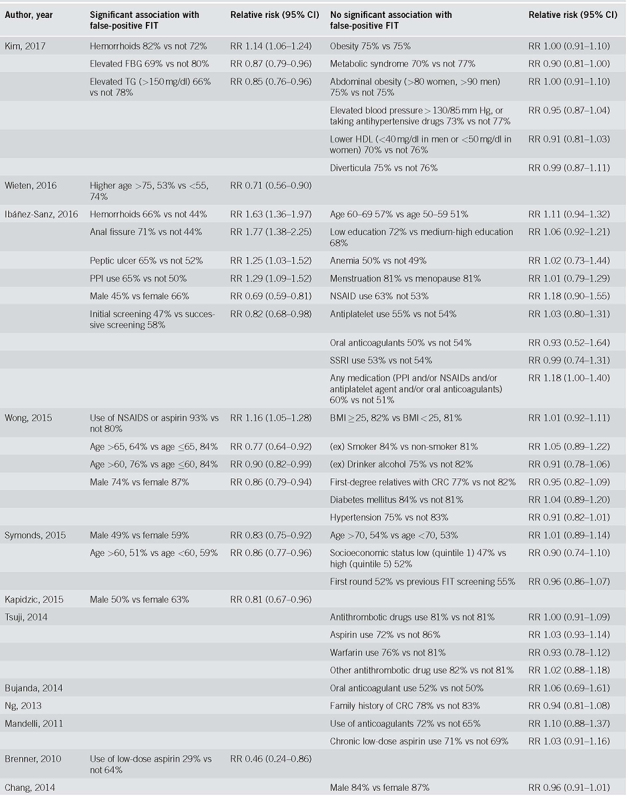
Table 3.
Meta‐analysis of risk ratios for false‐positive and false‐negative FIT results
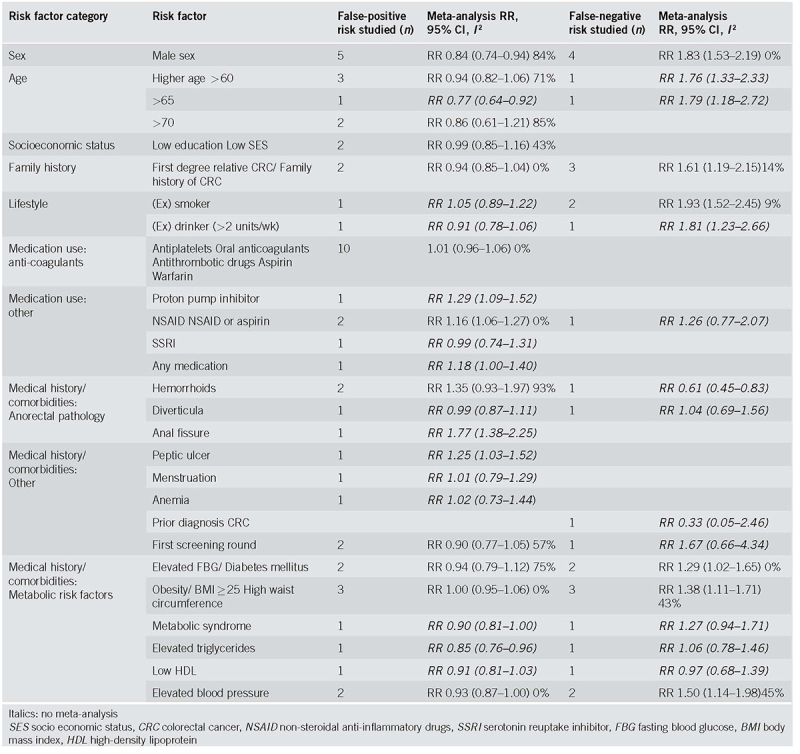
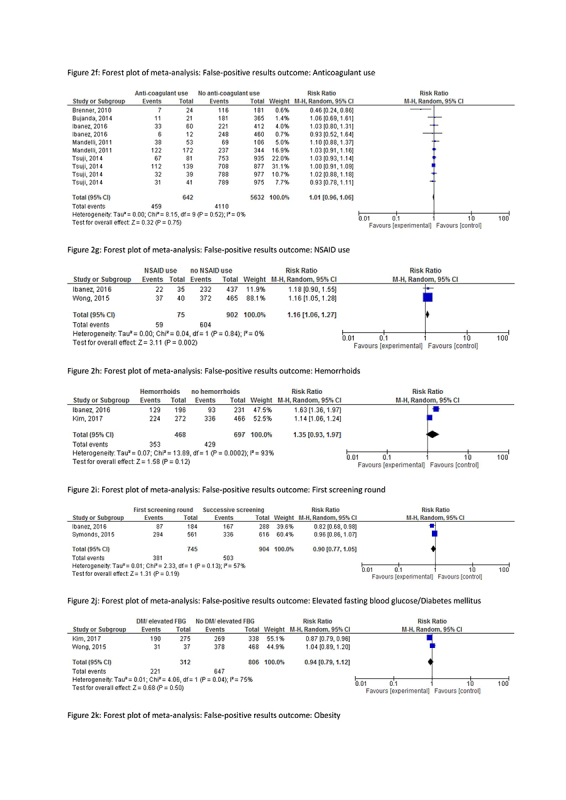
The risk factor that was associated with a lower risk of false‐positive results in meta‐analysis was male sex (RR 0.84, CI 0.74‐0.94, I2 84%), and, in one study, elevated triglycerides (RR 0.85, CI 0.76‐0.96) and age higher than 65 (RR 0.77, CI 0.65‐0.92). In contrast, a higher risk of false‐positive results was seen in participants using non‐steroidal anti‐inflammatory drugs (NSAIDs) (RR 1.16, CI 1.06‐1.27, I2 0%) and, in individual studies, in participants with an anal fissure (RR 1.77, CI 1.38‐2.25), a peptic ulcer (RR 1.25, CI 1.03‐1.52) and those using proton pump inhibitors (RR 1.29, CI 1.09‐1.52).
No significant associations were found for having hemorrhoids (RR 0.99, CI 0.93‐1.97, I2 93%, or the use of anticoagulants or antithrombotic drugs (RR 1.01, CI 0.96‐1.06, I2 0%). Only one study [47] reported a significantly lower risk of false‐positivity when using low dose of aspirin (RR 0.46, CI 0.24‐0.86), while in another study [46] the use of aspirin (or aspirin in combination with NSAIDs) was associated with a significantly higher risk of a false‐positive result (RR 1.16, CI 1.05‐1.28). Many other factors appeared to have no systematic effect on the proportion of false‐positive results: family history of CRC, socioeconomic status, obesity, diverticula, high blood pressure, hyperglycemia, low high‐density lipoprotein (HDL), metabolic syndrome, menstruation or menopause, anemia, use of a selective serotonin reuptake inhibitor (SSRI) or use of (combined) medication, (a history of) smoking or drinking alcohol and participating in prior screening rounds.
Risk Factors for False‐Negative Results
Risk factors for false‐negative FIT results as evaluated in each study are summarized in Table 2b and summary results are presented in Table 3 (forest plots in Supplementary figures 2m‐2r). Most frequently studied risk factors for false‐negative FIT results were: sex (4 results: [33, 42, 43, 48]), family history of CRC (3 results: [46, 48, 52]) and obesity (3 results: [42, 46]).
Table 2b.
Individual risk ratios of risk factors for false‐negative FIT‐results
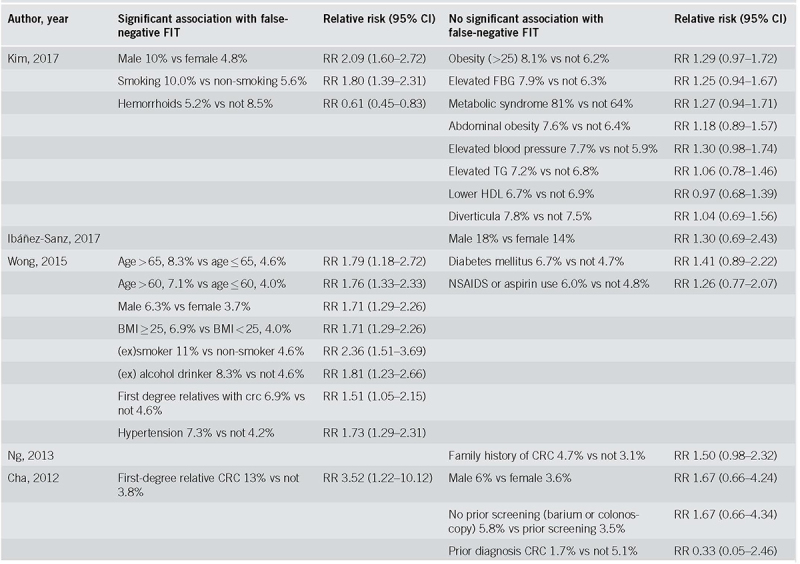
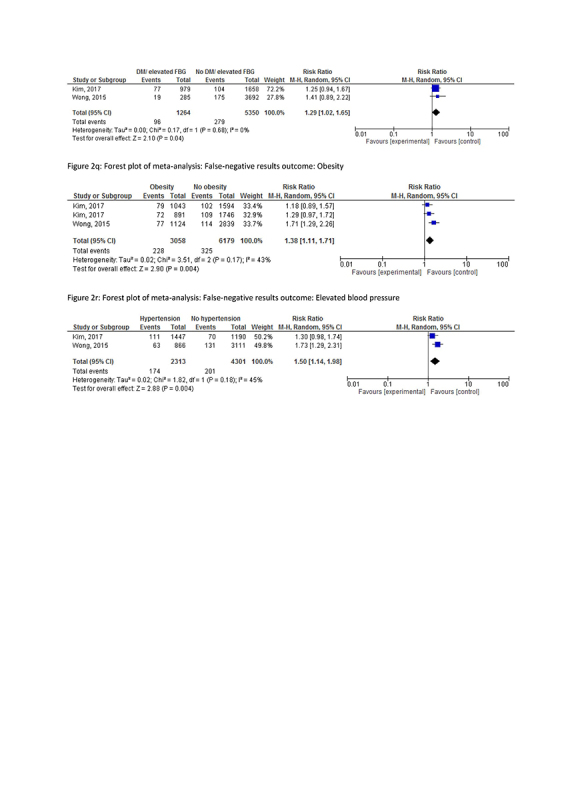
One study reported a significantly lower risk of false‐negative results for participants with hemorrhoids (RR 0.61, CI 0.45‐0.83). In meta‐analysis, a higher risk of false‐negative results was observed in males (RR 1.83, CI 1.53‐2.19, I2 0%), participants with a family history of CRC (RR 1.61, CI 1.19‐2.15, I2 14%), (former) smokers (RR 1.93, CI 1.52‐2.45, I2 9%), elevated fasting blood glucose (RR 1.29, CI 1.02‐1.65, I2 0%), obesity (RR 1.38, CI 1.11‐1.71, I2 43%), and hypertension (RR 1.50, CI 1.14‐1.98, I2 45%). Single studies have reported an effect of age > 60 (RR 1.76, CI 1.33‐2.33) and >65 (RR 1.79, CI 1.18‐2.72) and (a history of) drinking alcohol (RR 1.81, CI 1.23‐2.66).
No association with false‐negative results was found for (known) diverticulosis, other elements of metabolic syndrome, NSAID or aspirin use, prior screening, or a previous CRC.
Sensitivity Analysis of Studies Using an Equal Positivity Cutoff
A sensitivity analysis of the six studies that used a positivity cutoff of 20 μg Hb/gr feces [42,43,44,48,51,53], did not change the main conclusions. However, the analysis of NSAID use as risk factor was limited to one study, not showing a higher risk of a false‐positive result (RR 1.18, CI 0.90‐1.55). Similarly, hyperglycemia (RR 1.25, CI 0.94‐1.67) and hypertension (RR 1.30, CI 0.98‐1.74) did not lead to a higher risk of a false‐negative result. Some associations could not be evaluated because no studies with a 20 μg Hb/gr feces positivity cutoff included them: higher age and (a history of) drinking alcohol.
DISCUSSION
This systematic review shows that male sex is associated with a significantly lower risk of false‐positive FIT results, while using NSAIDs is associated with a higher risk of a false‐positive result. The use of anticoagulants has been studied frequently, but no systematic effect on FIT false‐positivity was observed. Males, participants with a family history of CRC, with elements of the metabolic syndrome (obesity, hyperglycemia, hypertension), and (former) smokers were found to be at increased risk of false‐negative results.
When interpreting this review several limitations should be acknowledged. First, data on FIT accuracy were compiled from different (pilot) screening programs, using different FIT brands and unequal cutoffs for positivity. Applying a higher cutoff level will lower sensitivity while increasing specificity, thus influencing the proportion of false‐negative and false‐positive screenees. However, since we compared the risks of any false result per study (proportion of participants with the risk factor vs. the proportion without that risk factor), differences in cutoff levels between studies should have little impact on the conclusions, as was shown in a sensitivity analysis of six studies using the same cutoff.
Seven studies that reported insufficient data (absolute numbers) to allow for relative risk calculation were excluded, limiting the precision of summary estimates. To allow for meta‐analysis, individual risk factors were clustered in groups, leading to possible loss of detail in characterizing risk factors. For example, all types of anticoagulant and antithrombotic drugs had to be clustered. Lastly, reporting bias is possible with regard to the prevalence of hemorrhoids, since these lesions are frequently not included in systematic colonoscopy reporting systems.
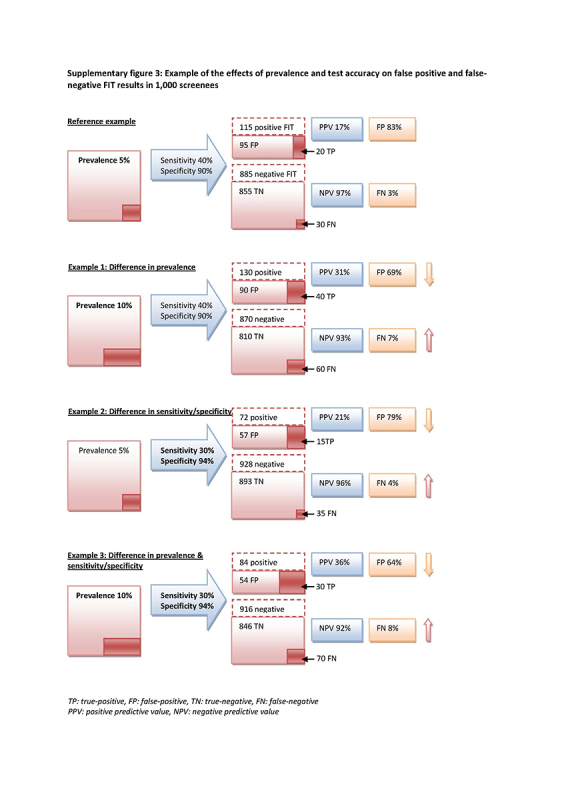
Differences between subgroups in the risk of a false‐negative FIT result and in the risk of a false‐positive FIT result could be due to differences in prevalence, differences in accuracy, or a combination. A higher CRC prevalence in one subgroup will result in a higher risk of a false‐negative result and a lower risk of a false‐positive result, even in the absence of a difference in accuracy. A lower FIT sensitivity in a subgroup will generate more false‐negatives, and a lower specificity will generate more false‐positives (see supplementary material for a worked‐out example). Due to the nature of the studies included in this review, we cannot distinguish between these causes as explanations for differences in the risk of false‐negatives and false‐positives.
Men are at lower risk of false‐positive results and at higher risk of false‐negative results. In single studies, similar conclusions have been drawn for participants at a higher age. This is likely related to the higher prevalence of CRC and AN in men and older individuals [55, 56], and raises the question whether the screening strategy should be optimized per subgroup. For men and older participants, the cutoff could be lowered to decrease the number of missed AN. Furthermore, the cutoff could be increased for women and younger participants to decrease the number of unnecessary colonoscopies in these populations. The most important question is the consequence of these modifications for the specificity of FIT in men and the sensitivity of FIT in women. Grobbee et al. assessed this balance and concluded that, for both sexes, a decrease in cutoff resulted in a relatively more rapid increase in sensitivity than the decline in specificity [57]. Alvarez et al. found similar results for men over 60 years old. In women younger than 60 years, a higher cutoff led to a significantly lower number of colonoscopies without modifying the detection rate of CRC [23]. Other than adjusting the cutoff for FIT‐positivity, effectiveness by gender and age may be improved by adjusting the starting age of screening. Brenner et al. found that women reached equivalent levels of CRC risk as men when they are 4‐8 years older, which might justify a postponed screening age for women [58]. Overall, this may support initiatives exploring the specific effect in specific screening populations of a lower cutoff for (older) men, a higher cutoff for (younger) women, or a postponed start of screening in women.
Currently, several research groups are investigating individual risk stratification for colonoscopy screening or colonoscopy referral after a positive FIT result. Among those are the Asia‐Pacific Colorectal Screening (APCS) score and the Dutch risk stratification model of Stegeman et al. [59, 60]. Both models include age, family history for CRC and smoking for risk assessment, and APCS additionally includes sex. In our analysis, the same personal factors appeared to be prone for false‐negative FIT results, which further supports the use of these factors in risk‐based strategies. Possibly, hyperglycemia, obesity and hypertension could additionally be explored for inclusion in colonoscopy referral stratification models in FIT‐based screening, since these factors are found to be related to more false‐negative results, and are so far only partially explained by a concomitant higher incidence of AN [61, 62]. The influence of combinations of risk factors could be of interest when these are likely to interact, such as aspects of metabolic syndrome, lifestyle and the use of medication. Because of the way they were reported in the study reports, the effect of combined risk factors could not be assessed in this review, but future original studies provide this opportunity.
Although meta‐analysis showed no significant increased risk of a false‐positive FIT result for invitees with hemorrhoids, this risk is commonly believed by patients, and was demonstrated in individual studies [42, 44]. An assumed higher chance of false‐positive results could restrain this group from participation in follow‐up colonoscopy when receiving a positive FIT result. GPs could play a role in informing this specific group. Especially because, in contrast with risk factors such as male sex and higher age, the incidence of AN in this subgroup is similar to those without hemorrhoids.
In line with the European Screening recommendation that anticoagulant drug restrictions in FIT screening are not indicated [3], we showed that the use of aspirin or anticoagulant drugs does not increase FIT false‐positivity in nine out of ten included studies. This may seem counter intuitive. One explanation could be that the absence of an effect is a consequence of both an increased and decreased risk of false‐positive results. Anticoagulants could increase positivity and decrease false‐positivity if AN is present but bleeds very little by itself. In contrast, anticoagulants could also increase false‐positivity if other non‐AN lesions are present and bleeding is provoked. Alternative explanations could point to molecular characteristics of AN and the exact mechanism of the anticoagulant used. For current practice, however, recent guidelines are adequate.
This study focused on personal risk factors and its influence on false FIT results. Other factors affecting FIT accuracy should also be acknowledged. Organizational aspects influencing FIT accuracy may for example include sample return times [34,35,36], season of return [34] or number of samples [33, 63]. Further, the colonic lesion itself may relate to the accuracy of FIT. For example, right‐sided AN is associated with more false‐negative FIT results than proximal lesions [33, 42, 64]. This is thought to be due to the degradation of hemoglobin during colon transit, the blood distribution through the stool [64], and a harder consistency of passing stool in the distal colon [65]. Similarly, the morphology of the lesion may relate to the (location specific) accuracy of FIT. FIT is less sensitive for the detection of sessile adenomas than for pedunculated adenomas [29, 32], and proximal adenomas are proportionately more often sessile than distal adenoma [66]. The relevance of studying other than personal factors should however be kept in mind, risks for a false FIT result evaluated during colonoscopy have limited value if risk‐based stratification for colonoscopy is intended.
In theory, if advanced adenomas are missed with FIT in a first round but detected in a following round before their progression to CRC, the effectiveness of the screening program is not affected, in contrast to interval cancer. The limited evidence on personal risk factors for FIT interval is conflicting. Higher interval cancer rates were found for women in a gFOBT screening program [67,68,69], but no significant differences for sex or age were found in other studies [70,71,72].
Multiple personal risk factors, known at time of FIT invitation, could influence the proportion of false FIT results in CRC screening. Personalized screening strategies might increase the sensitivity of FIT and reduce the number of unnecessary colonoscopies.
ACKNOWLEDGEMENTS
We thank Faridi van Etten‐Jamaludin for her support and expertise in searching the literature.
CONFLICTS OF INTEREST
Guarantor of the article: Prof. Evelien Dekker.
Specific author contributions: Conception and design: all authors. Literature search and data extraction: CK, LV. Data analysis and interpretation: CK, LV, PB. Drafting the manuscript: CK. Critical revision of the manuscript: all authors. Supervision: ED, PB.
Financial support: None.
Potential competing interests: None.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
✓ Colorectal cancer screening effectiveness is influenced by the suboptimal accuracy of FIT.
✓ Personal factors, such as sex, age, smoking habits, the presence of hemorrhoids, and the use of anti‐coagulants have been associated with an increased risk of a false‐positive or false‐negative FIT result.
WHAT IS NEW HERE
✓ This is the first systematic review and meta‐analysis summarizing the effect of personal factors on FIT accuracy.
✓ Male sex is associated with a lower risk of false‐positive FIT results and using NSAIDs with a higher risk.
✓ No systematic effect of the use of anticoagulants on FIT false‐positivity was found.
✓ Male sex, a family history of CRC, metabolic syndrome, and smoking are each associated with a higher risk of false‐negative results.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://doi.org/10.1038/s41395‐018‐0212‐7
Correspondence: E.D. (email: e.dekker@amc.uva.nl)
Published online 29 August 2018
REFERENCES
- 1.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. [DOI] [PubMed] [Google Scholar]
- 2.Rabeneck L, Rumble RB, Thompson F, Mills M, Oleschuk C, Whibley A, et al. Fecal immunochemical tests compared with guaiac fecal occult blood tests for population-based colorectal cancer screening. Can J Gastroenterol. 2012;26:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran SP, Launoy G, Zappa M. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition-Faecal occult blood testing. Endoscopy. 2012;44(Suppl 3):SE65–87. [DOI] [PubMed] [Google Scholar]
- 4.de Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570–8. [DOI] [PubMed] [Google Scholar]
- 5.Grobbee EJ, van der Vlugt M, van Vuuren AJ, Stroobants AK, Mundt MW, Spijker WJ, et al. A randomised comparison of two faecal immunochemical tests in population-based colorectal cancer screening. Gut. 2016;66:1975–82. [DOI] [PubMed] [Google Scholar]
- 6.Toes-Zoutendijk E, van Leerdam ME, Dekker E, van Hees F, Penning C, Nagtegaal I, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2016;152:767–75. [DOI] [PubMed] [Google Scholar]
- 7.Aniwan S, Ratanachu Ek T, Pongprasobchai S, Limsrivilai J, Praisontarangkul OA, Pisespongsa P, et al. The optimal cut-off level of the fecal immunochemical test for colorectal cancer screening in a country with limited colonoscopy resources: a multi-center study from Thailand. Asian Pac J Cancer Prev. 2017;18:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoff G, Thiis-Evensen E, Grotmol T, Sauar J, Vatn MH, Moen IE. Do undesirable effects of screening affect all-cause mortality in flexible sigmoidoscopy programmes? Experience from the Telemark Polyp Study 1983-1996. Eur J Cancer Prev. 2001;10:131–7. [DOI] [PubMed] [Google Scholar]
- 9.Cooper GC, Harvie MN, French DP. Do negative screening test results cause false reassurance? A systematic review. Br J Health Psychol. 2017;22:958–77. [DOI] [PubMed] [Google Scholar]
- 10.van der Velde JL, Blanker MH, Stegmann ME, de Bock GH, Berger MY, Berendsen AJ. A systematic review of the psychological impact of false-positive colorectal cancer screening: what is the role of the general practitioner? Eur J Cancer Care. 2017;26:1–10. [DOI] [PubMed] [Google Scholar]
- 11.Denters MJ, Deutekom M, Essink-Bot ML, Bossuyt PM, Fockens P, Dekker E. FIT false-positives in colorectal cancer screening experience psychological distress up to 6 weeks after colonoscopy. Support Care Cancer. 2013;21:2809–15. [DOI] [PubMed] [Google Scholar]
- 12.Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam M, van Ballegooijen M, Kraaijenhagen RA, et al. Risk factors for false-positive and for false-negative test results in screening with fecal occult blood testing. Int J Cancer. 2013;133:2408–14. [DOI] [PubMed] [Google Scholar]
- 13.Kim NH, Park JH, Park DI, Sohn CI, Choi K, Jung YS. Are hemorrhoids associated with false-positive fecal immunochemical test results? Yonsei Med J. 2017;58:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bujanda L, Sarasqueta C, Lanas A, Quintero E, Cubiella J, Hernandez V, et al. Effect of oral anticoagulants on the outcome of faecal immunochemical test. Br J Cancer. 2014;110:1334–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser CG, Allison JE, Halloran SP, Young GP. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst. 2012;104:810–4. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 19.Altman D. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakama H, Fukazawa K. Colorectal cancer risk in first-degree relatives of patients with colorectal adenomatous polyp. Hepatogastroenterology. 2002;49:157–9. [PubMed] [Google Scholar]
- 22.Nakama H, Zhang B, Fattah AS, Zhang X. Colorectal cancer in iron deficiency anemia with a positive result on immunochemical fecal occult blood. Int J Colorectal Dis. 2000;15:271–4. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Urturi C, Andreu M, Hernandez C, Perez-Riquelme F, Carballo F, Ono A, et al. Impact of age- and gender-specific cut-off values for the fecal immunochemical test for hemoglobin in colorectal cancer screening. Dig Liver Dis. 2016;48:542–51. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim NH, Yang HJ, Park SK, Park JH, Park DI, Sohn CI, et al. Does low threshold value use improve proximal neoplasia detection by fecal immunochemical test? Dig Dis Sci. 2016;61:2685–93. [DOI] [PubMed] [Google Scholar]
- 26.Auge JM, Pellise M, Escudero JM, Hernandez C, Andreu M, Grau J, et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology. 2014;147:628–36.e1. [DOI] [PubMed] [Google Scholar]
- 27.Kallenberg FG, Vleugels JL, de Wijkerslooth TR, Stegeman I, Stoop EM, van Leerdam ME, et al. Adding family history to faecal immunochemical testing increases the detection of advanced neoplasia in a colorectal cancer screening programme. Aliment Pharmacol Ther. 2016;44:88–96. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Sakaguchi K, et al. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am J Gastroenterol. 2007;102:2259–64. [DOI] [PubMed] [Google Scholar]
- 29.Abdul Fattah AS, Nakama H, Kamijo N. Clinico-pathological features of colorectal adenomatous polyps with negative results on immunochemical fecal occult blood test. Eur J Med Res. 1997;2:361–4. [PubMed] [Google Scholar]
- 30.Nakama H, Abdul Fattah AS, Zhang B, Kamijo N, Fujimori K, Miyata K. Detection rate of immunochemical fecal occult blood test for colorectal adenomatous polyps with severe dysplasia. J Gastroenterol. 1997;32:492–5. [DOI] [PubMed] [Google Scholar]
- 31.Nakama H, Kamijo N, Fujimori K, Horiuchi A, Fattah AS, Zhang B. Characteristics of colorectal cancer with false-negative result on immunochemical faecal occult blood test. J Med Screen. 1996;3:115–8. [DOI] [PubMed] [Google Scholar]
- 32.Chiu HM, Lee YC, Tu CH, Chen CC, Tseng PH, Liang JT, et al. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol. 2013;11:832–8.e1-2. [DOI] [PubMed] [Google Scholar]
- 33.Wong MC, Ching JY, Chan VC, Lam TY, Shum JP, Luk AK, et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but not number of specimens. Clin Gastroenterol Hepatol. 2015;13:1472–9. [DOI] [PubMed] [Google Scholar]
- 34.Dancourt V, Hamza S, Manfredi S, Drouillard A, Bidan JM, Faivre J, et al. Influence of sample return time and ambient temperature on the performance of an immunochemical faecal occult blood test with a new buffer for colorectal cancer screening. Eur J Cancer Prev. 2016;25:109–14. [DOI] [PubMed] [Google Scholar]
- 35.van Roon AH, Hol L, van Vuuren AJ, Francke J, Ouwendijk M, Heijens A, et al. Are fecal immunochemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am J Gastroenterol. 2012;107:99–107. [DOI] [PubMed] [Google Scholar]
- 36.van Rossum LG, van Rijn AF, van Oijen MG, Fockens P, Laheij RJ, Verbeek AL, et al. False-negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125:746–50. [DOI] [PubMed] [Google Scholar]
- 37.Chausserie S, Levillain R, Puvinel J, Ferrand O, Ruiz A, Raginel T, et al. Seasonal variations do not affect the superiority of fecal immunochemical tests over guaiac tests for colorectal cancer screening. Int J Cancer. 2015;136:1827–34. [DOI] [PubMed] [Google Scholar]
- 38.van Turenhout ST, Oort FA, Terhaar sive Droste JS, Coupe VM, van der Hulst RW, Loffeld RJ, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76:136–43. [DOI] [PubMed] [Google Scholar]
- 39.Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183:1474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. Am J Gastroenterol. 2010;105:2457–64. [DOI] [PubMed] [Google Scholar]
- 41.Chiang TH, Chuang SL, Chen SL, Chiu HM, Yen AM, Chiu SY, et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology. 2014;147:1317–26. [DOI] [PubMed] [Google Scholar]
- 42.Kim NH, Park JH, Park DI, Sohn CI, Choi K, Jung YS. Risk factors for false fecal immunochemical test results in colorectal cancer screening. J Clin Gastroenterol. 2017;51:151–9. [DOI] [PubMed] [Google Scholar]
- 43.Ibanez-Sanz G, Garcia M, Mila N, Rodriguez-Moranta F, Binefa G, Gomez-Matas J, et al. False-negative rate cannot be reduced by lowering the haemoglobin concentration cut-off in colorectal cancer screening using faecal immunochemical test. Eur J Cancer Prev. 2017;26:365–7. [DOI] [PubMed] [Google Scholar]
- 44.Ibanez-Sanz G, Garcia M, Rodriguez-Moranta F, Binefa G, Gomez-Matas J, Domenech X, et al. Prescription drugs associated with false-positive results when using faecal immunochemical tests for colorectal cancer screening. Dig Liver Dis. 2016;48:1249–54. [DOI] [PubMed] [Google Scholar]
- 45.Wieten E, Schreuders EH, Nieuwenburg SA, Hansen BE, Lansdorp-Vogelaar I, Kuipers EJ, et al. Effects of increasing screening age and fecal hemoglobin cutoff concentrations in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2016;14:1771–7. [DOI] [PubMed] [Google Scholar]
- 46.Wong MC, Ching JY, Chan VC, Lam TY, Luk AK, Ng SS, et al. Factors associated with false-positive and false-negative fecal immunochemical test results for colorectal cancer screening. Gastrointest Endosc. 2015;81:596–607. [DOI] [PubMed] [Google Scholar]
- 47.Brenner H, Tao S, Haug U. Low-dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA. 2010;304:2513–20. [DOI] [PubMed] [Google Scholar]
- 48.Cha JM, Lee JI, Joo KR, Shin HP, Park JJ, Jeun JW, et al. First-degree relatives of colorectal cancer patients are likely to show advanced colorectal neoplasia despite a negative fecal immunochemical test. Digestion. 2012;86:283–7. [DOI] [PubMed] [Google Scholar]
- 49.Chang LC, Wu MS, Tu CH, Lee YC, Shun CT, Chiu HM. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc. 2014;79:961–9. [DOI] [PubMed] [Google Scholar]
- 50.Kapidzic A, van der Meulen MP, Hol L, van Roon AH, Looman CW, Lansdorp-Vogelaar I, et al. Gender differences in fecal immunochemical test performance for early detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2015;13:1464–71.e4. [DOI] [PubMed] [Google Scholar]
- 51.Mandelli G, Radaelli F, Paggi S, Terreni N, Gola G, Gramegna M, et al. Anticoagulant or aspirin treatment does not affect the positive predictive value of an immunological fecal occult blood test in patients undergoing colorectal cancer screening: results from a nested in a cohort case-control study. Eur J Gastroenterol Hepatol. 2011;23:323–6. [DOI] [PubMed] [Google Scholar]
- 52.Ng SC, Ching JY, Chan V, Wong MC, Suen BY, Hirai HW, et al. Diagnostic accuracy of faecal immunochemical test for screening individuals with a family history of colorectal cancer. Aliment Pharmacol Ther. 2013;38:835–41. [DOI] [PubMed] [Google Scholar]
- 53.Symonds EL, Osborne JM, Cole SR, Bampton PA, Fraser RJ, Young GP. Factors affecting faecal immunochemical test positive rates: demographic, pathological, behavioural and environmental variables. J Med Screen. 2015;22:187–93. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji Y, Gunji T, Sato H, Ono A, Ito T, Ohata K, et al. Antithrombotic drug does not affect the positive predictive value of an immunochemical fecal occult blood test. Dig Endosc. 2014;26:424–9. [DOI] [PubMed] [Google Scholar]
- 55.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferlitsch M, Heinze G, Salzl P, Britto-Arias M, Waldmann E, Reinhart K, et al. Sex is a stronger predictor of colorectal adenoma and advanced adenoma than fecal occult blood test. Med Oncol. 2014;31:151. [DOI] [PubMed] [Google Scholar]
- 57.Grobbee EJ, Wieten E, Hansen BE, Stoop EM, de Wijkerslooth TR, Lansdorp-Vogelaar I, et al. Fecal immunochemical test-based colorectal cancer screening: The gender dilemma. United European Gastroenterol J. 2017;5:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY, Wu DC, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011;60:1236–41. [DOI] [PubMed] [Google Scholar]
- 60.Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam ME, Dekker E, van Ballegooijen M, et al. Combining risk factors with faecal immunochemical test outcome for selecting CRC screenees for colonoscopy. Gut. 2014;63:466–71. [DOI] [PubMed] [Google Scholar]
- 61.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. [DOI] [PubMed] [Google Scholar]
- 62.Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181:832–45. [DOI] [PubMed] [Google Scholar]
- 63.Guittet L, Bouvier V, Mariotte N, Vallee JP, Levillain R, Tichet J, et al. Performance of immunochemical faecal occult blood test in colorectal cancer screening in average-risk population according to positivity threshold and number of samples. Int J Cancer. 2009;125:1127–33. [DOI] [PubMed] [Google Scholar]
- 64.Haug U, Kuntz KM, Knudsen AB, Hundt S, Brenner H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer. 2011;104:1779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer. 2017;140:2015–22. [DOI] [PubMed] [Google Scholar]
- 66.Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–90. [DOI] [PubMed] [Google Scholar]
- 67.Zorzi M, Fedato C, Grazzini G, Stocco FC, Banovich F, Bortoli A, et al. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut. 2011;60:944–9. [DOI] [PubMed] [Google Scholar]
- 68.Gill MD, Bramble MG, Rees CJ, Lee TJ, Bradburn DM, Mills SJ. Comparison of screen-detected and interval colorectal cancers in the Bowel Cancer Screening Programme. Br J Cancer. 2012;107:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steele RJ, McClements P, Watling C, Libby G, Weller D, Brewster DH, et al. Interval cancers in a FOBTbased colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut. 2012;61:576–81. [DOI] [PubMed] [Google Scholar]
- 70.van der Vlugt M, Grobbee EJ, Bossuyt PMM, Bos A, Bongers E, Spijker W, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017;153:439–47.e2. [DOI] [PubMed] [Google Scholar]
- 71.Garcia M, Domenech X, Vidal C, Torne E, Mila N, Binefa G, et al. Interval cancers in a population-based screening program for colorectal cancer in catalonia, Spain. Gastroenterol Res Pract. 2015;2015:672410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Digby J, Fraser CG, Carey FA, Lang J, Stanners G, Steele RJ. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen. 2016;23:130–4. [DOI] [PubMed] [Google Scholar]