Abstract
PURPOSE
Pembrolizumab monotherapy has demonstrated durable antitumor activity in advanced programmed death ligand 1 (PD-L1)–expressing non‒small-cell lung cancer (NSCLC). We report 5-year outcomes from the phase Ib KEYNOTE-001 study. These data provide the longest efficacy and safety follow-up for patients with NSCLC treated with pembrolizumab monotherapy.
PATIENTS AND METHODS
Eligible patients had confirmed locally advanced/metastatic NSCLC and provided a contemporaneous tumor sample for PD-L1 evaluation by immunohistochemistry using the 22C3 antibody. Patients received intravenous pembrolizumab 2 mg/kg every 3 weeks or 10 mg/kg every 2 or 3 weeks. Investigators assessed response per immune-related response criteria. The primary efficacy end point was objective response rate. Overall survival (OS) and duration of response were secondary end points.
RESULTS
We enrolled 101 treatment-naive and 449 previously treated patients. Median follow-up was 60.6 months (range, 51.8 to 77.9 months). At data cutoff—November 5, 2018—450 patients (82%) had died. Median OS was 22.3 months (95% CI, 17.1 to 32.3 months) in treatment-naive patients and 10.5 months (95% CI, 8.6 to 13.2 months) in previously treated patients. Estimated 5-year OS was 23.2% for treatment-naive patients and 15.5% for previously treated patients. In patients with a PD-L1 tumor proportion score of 50% or greater, 5-year OS was 29.6% and 25.0% in treatment-naive and previously treated patients, respectively. Compared with analysis at 3 years, only three new-onset treatment-related grade 3 adverse events occurred (hypertension, glucose intolerance, and hypersensitivity reaction, all resolved). No late-onset grade 4 or 5 treatment-related adverse events occurred.
CONCLUSION
Pembrolizumab monotherapy provided durable antitumor activity and high 5-year OS rates in patients with treatment-naive or previously treated advanced NSCLC. Of note, the 5-year OS rate exceeded 25% among patients with a PD-L1 tumor proportion score of 50% or greater. Pembrolizumab had a tolerable long-term safety profile with little evidence of late-onset or new toxicity.
INTRODUCTION
For patients with non‒small-cell lung cancer (NSCLC), median overall survival (OS) and 5-year survival rates have historically been poor. In the United States, 5-year survival between 2008 and 2014 was 24% for all patients with NSCLC and 5.5% for those with distant metastases.1 The introduction of novel agents and use of predictive biomarkers have resulted in improved outcomes for patients with advanced/metastatic NSCLC2,3; however, the extent to which these new approaches have altered long-term survival outcomes has been uncertain.
The introduction of agents that promote tumor recognition by the immune system by inhibiting signaling between the programmed death-1 receptor and programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) has been an important recent advance in the treatment of NSCLC.4,5 Pembrolizumab is a monoclonal antibody that binds the programmed death-1 receptor and blocks its interaction with PD-L1 and PD-L2.5,6 Antitumor activity and acceptable toxicity of pembrolizumab monotherapy in patients with advanced NSCLC—treatment naive and previously treated—were first demonstrated in the phase Ib KEYNOTE-001 study.7 Of note, patients with a PD-L1 tumor proportion score (TPS) of 50% or greater achieved a higher objective response rate (ORR) and median OS compared with patients with lower/absent tumor PD-L1 expression. Results from the KEYNOTE-001 study led to accelerated approval of pembrolizumab in patients with TPS 50% or greater, approval of the assay to assess PD-L1, and the incorporation of pembrolizumab into NSCLC treatment guidelines.2,3
These results were validated in the KEYNOTE-010 study, which demonstrated improved OS with pembrolizumab versus docetaxel among patients with previously treated advanced NSCLC with PD-L1 TPS of 1% or greater.8 In the first-line setting, pembrolizumab improved OS versus platinum-based chemotherapy in patients with advanced NSCLC without EGFR/ALK alterations and PD-L1 TPS of 50% or greater (KEYNOTE-024)9 and PD-L1 TPS of 1% or greater (KEYNOTE-042).10
Pembrolizumab in combination with platinum doublet chemotherapy is now considered a standard-of-care first-line therapy based on improved OS with the combination versus platinum-based chemotherapy plus placebo in the KEYNOTE-189 (nonsquamous)11 and KEYNOTE-407 (squamous)12 studies. However, for patients with PD-L1 TPS of 50% or greater, pembrolizumab monotherapy is often used rather than pembrolizumab plus chemotherapy based on similar outcomes in cross-study comparisons.13 Because the KEYNOTE-001 study was the first to evaluate pembrolizumab in patients with advanced NSCLC, it provides the longest follow-up to date for pembrolizumab monotherapy in patients with advanced NSCLC. Herein we report 5-year efficacy and safety outcomes.
PATIENTS AND METHODS
Patients
Full details of the study design (with amendments) for the NSCLC cohorts of KEYNOTE-001 have been reported previously.7,14 Eligible patients for NSCLC cohorts (other cohorts enrolled patients with locally advanced/metastatic cancer or melanoma) were age 18 years or older with locally advanced/metastatic NSCLC, measurable disease per immune-related response criteria (irRC),15 Eastern Cooperative Oncology Group performance status of 1 or less, and a contemporaneous biopsy sample. Patients who were eligible for the treatment-naive cohort had no previous systemic treatment for advanced disease or adjuvant treatment within the previous year, no EGFR mutation and/or ALK translocation (with the exception of 11 patients who were enrolled before a protocol amendment on March 28, 2013), and a PD-L1 TPS of 1% or greater. Patients who were eligible for the previously treated cohorts had experienced treatment failure while receiving one or more or two or more systemic therapies for advanced disease, depending on the cohort. Patients were excluded if they had active CNS disease—unless clinically stable 4 or more weeks after treatment of brain metastases—or autoimmune disease that required systemic corticosteroids and/or immunosuppressive agents.
The protocol was approved by the institutional review board/ethics committee at each study site. All patients provided written informed consent.
Study Design and Treatment
The study design for the KEYNOTE-001 NSCLC cohorts has been reported previously.7,14 Patients received pembrolizumab intravenously at a dose of 2 mg/kg every 3 weeks or 10 mg/kg every 2 weeks or 3 weeks. Treatment continued until disease progression, intolerable toxicity, investigator decision, or patient withdrawal. After a protocol amendment in April 2016, all patients were switched from their assigned regimen to pembrolizumab 200 mg every 3 weeks, and patients who achieved a partial response or stable disease who had received 24 months of treatment could discontinue, with treatment resuming if they experienced disease progression/recurrence.
End Points and Assessments
The primary efficacy end point was ORR. Imaging was performed every 9 weeks in the first 2 years, every 16 weeks in year 3, and every 6 months thereafter. Response was assessed by independent central review per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,16 (until April 2016 when independent central assessment ceased in order to ease the burden on study sites) and by investigators per irRC.15 In this analysis, we report response per investigator-assessed irRC.
OS and duration of response (DOR; time from the first confirmed response to time of the first documentation of disease progression) were secondary efficacy end points. Survival was assessed every 2 months after the discontinuation of pembrolizumab. Adverse events (AEs) were recorded and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
PD-L1 expression on tumor biopsy samples was assessed using immunohistochemistry using antibody clone 22C3 (Merck & Co., Inc, Kenilworth, NJ).17 For study enrollment, positivity was quantified with a prototype assay, which defined positivity as membranous staining on 1% or more of tumor cells or a distinctive staining pattern caused by mononuclear inflammatory cell infiltrates.7,17 To determine correlations between PD-L1 expression and efficacy outcomes, PD-L1 was assessed by a clinical assay that defined positivity on the basis of tumor cell staining only.17 Although both assays used the same antibody, differences in evaluation occurred, including around the 1% cut point.7
Statistical Analysis
Efficacy and safety analyses included patients who received one or more pembrolizumab dose. Because efficacy and toxicity were similar regardless of dose or schedule,18 pembrolizumab dose groups were pooled for treatment-naive and previously treated patient cohorts. Among previously treated patients, there were multiple cohorts on the basis of PD-L1 expression and the number of prior therapies; these cohorts were also pooled. We estimated ORR and its 95% CI using the binomial exact method. We estimated OS and DOR using the Kaplan-Meier method. Median follow-up was calculated as the time from random assignment/first treatment to data cutoff for all patients.
RESULTS
Patients and Treatment
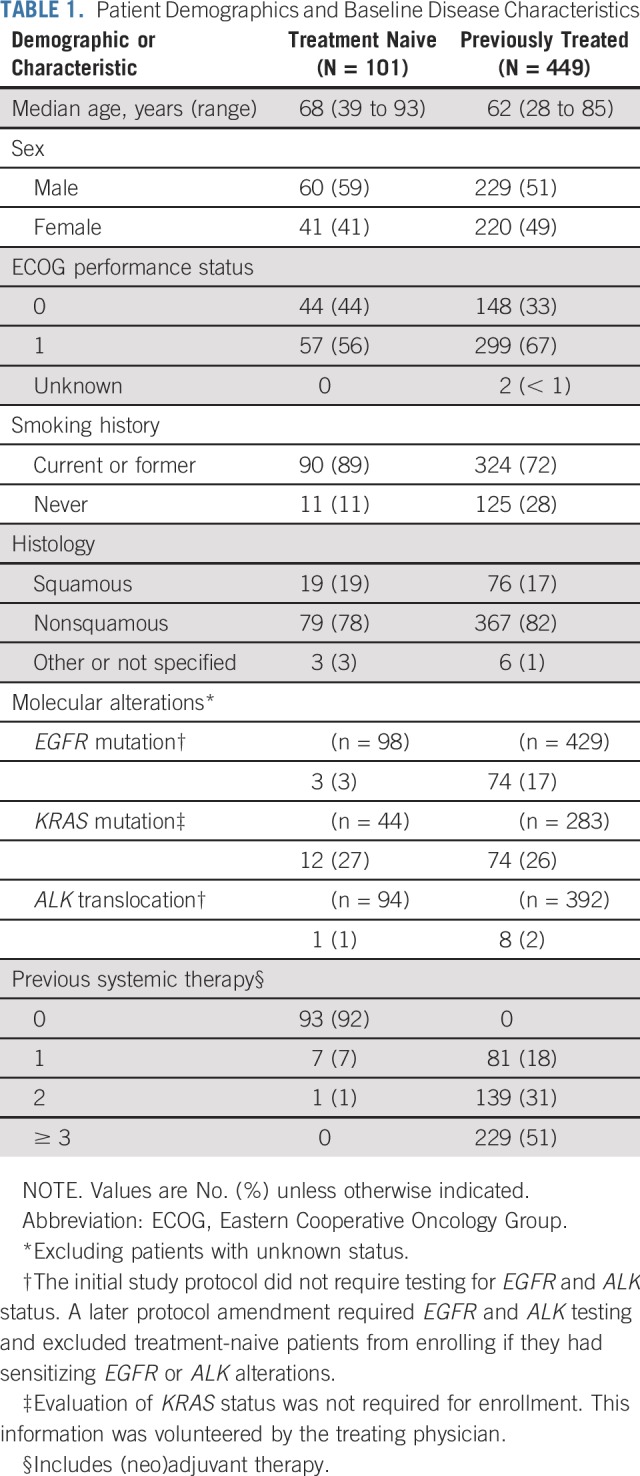
A total of 550 patients (101 treatment-naive and 449 previously treated) were enrolled between May 9, 2012, and July 13, 2014 (Data Supplement). Baseline demographic and clinical characteristics are listed in Table 1. As of the data cutoff—November 5, 2018—median follow-up was 60.6 months (range, 51.8 to 77.9 months). Median treatment duration was 3.3 months (range, 1 day to 75.9 months). One hundred patients were still alive at the time of analysis. Sixty patients—14 (14%) of 101 patients in the treatment-naive group and 46 (10%) of 449 patients in the previously treated group—received 2 or more years of treatment with pembrolizumab. Disposition of patients is described in the Data Supplement. One patient in the previously treated group ceased treatment after 44.4 months and upon experiencing disease progression at 47.2 months, began a second course of pembrolizumab, as permitted by the study protocol. The patient experienced a partial response during the second course of treatment, which continued until progressive disease occurred at 53.7 months.
TABLE 1.
Patient Demographics and Baseline Disease Characteristics
Efficacy
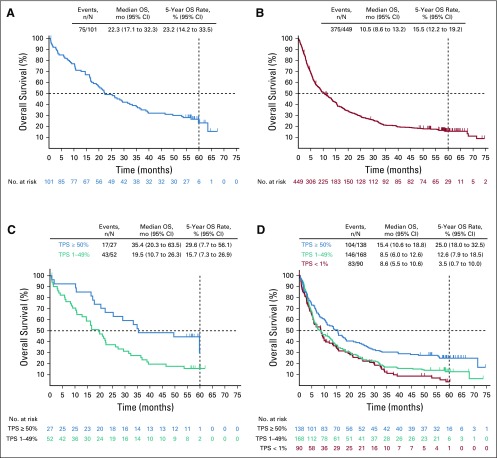
Seventy-five treatment-naive patients (74.3%) and 375 previously treated patients (83.5%) had died. Median OS was 22.3 months (95% CI, 17.1 to 32.3 months) for treatment-naive patients and 10.5 months (95% CI, 8.6 to 13.2 months) for previously treated patients (Figs 1A and 1B). The Kaplan-Meier estimate of OS at 5 years was 23.2% among treatment-naive patients and 15.5% among previously treated patients. Among treatment-naive patients, OS rates at 2 years, 3 years, and 4 years were 49.0%, 37.0%, and 31.0%, respectively. Among previously treated patients, OS rates at 2 years, 3 years, and 4 years were 30.1%, 20.9%, and 18.2%, respectively.
FIG 1.
Kaplan-Meier estimates of 5-year overall survival. (A) Treatment-naive patients. (B) Previously treated patients. (C) Treatment-naive patients by programmed death ligand 1 (PD-L1) tumor proportion score (TPS) status. (D) Previously treated patients by PD-L1 TPS status. NOTE. There were too few patients (n = 12) in the treatment-naive PD-L1 TPS < 1% group to evaluate overall survival (OS).
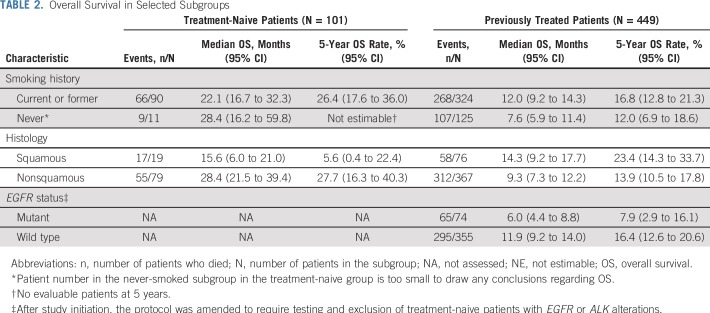
OS among select subgroups is summarized in Table 2. Outcomes by PD-L1 expression in EGFR-mutant patients are described in the Data Supplement.
TABLE 2.
Overall Survival in Selected Subgroups
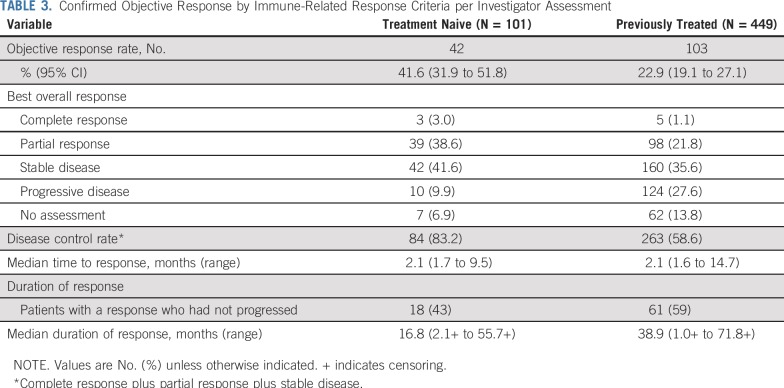
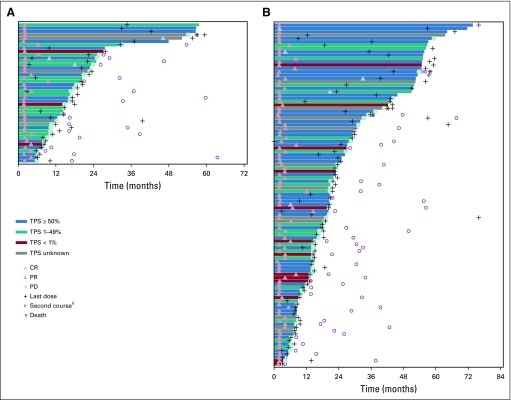
Forty-two patients (41.6% [95% CI, 31.9% to 51.8%]) in the treatment-naive group and 103 patients (22.9% [95% CI, 19.1% to 27.1%]) in the previously treated group had a confirmed objective response per irRC by investigator assessment (Table 3). Of note, ORR by investigator-assessed irRC was higher than that per central review by RECIST version 1.1 (as previously reported7), particularly among treatment-naive patients. Three patients achieved complete response in the treatment-naive group, and five achieved complete response in the previously treated group. Most responses occurred shortly after the initiation of therapy (Fig 2). Among 145 patients who experienced a response, 105 (72%) and 133 (92%) achieved response within 3 months and 6 months, respectively, of initiating treatment. Median time to response was 2.1 months (range, 1.7 to 9.5 months) and 2.1 months (range, 1.6 to 14.7 months) for treatment-naive and previously treated patients, respectively. Eighteen treatment-naive patients (43%) and 61 previously treated patients (59%) had an ongoing response. Among patients with an objective response, median DOR was 16.8 months (range, 2.1+ to 55.7+ months) in treatment-naive patients and 38.9 months (range, 1.0+ to 71.8+ months) in previously treated patients (Table 3; plus signs in ranges indicate non–disease progression at the last assessment [censored] for the patient with the minimum/maximum response duration).
TABLE 3.
Confirmed Objective Response by Immune-Related Response Criteria per Investigator Assessment
FIG 2.
Time to response and time to progression by immune-related response criteria (irRC) per investigator assessment. Bars indicate time to last follow-up or disease progression, whichever occurred earlier. Response per irRC per investigator assessment, rather than per RECIST version 1.1, is reported for this analysis because central assessment of response ceased in April 2016. If the investigator considered a patient to be experiencing clinical benefit with continued treatment after disease progression and the patient was clinically stable and tolerating pembrolizumab, he or she was permitted to continue on pembrolizumab with the approval of the study sponsor. (A) Treatment-naive patients. (B) Previously treated patients. (*) Four patients in the previously treated group and one patient in the treatment-naive group received 3 or more years of pembrolizumab therapy and experienced disease progression. Baseline characteristics for these patients are summarized in the Data Supplement. (§) One patient in the previously treated group (marked with a purple triangle) received a second course of pembrolizumab therapy. The patient experienced a partial response (PR) during the first course and continued therapy for 44.4 months. After progressive disease (PD) at 47.2 months, the patient initiated a second course of pembrolizumab at 48.0 months and achieved a PR at 50.9 months. The patient received his last dose of pembrolizumab at 53.0 months and subsequently experienced PD at 53.7 months and died at 55.8 months. CR, complete response; PD-L1, programmed death ligand 1; TPS, tumor proportion score.
PD-L1 TPS of 50% or greater was associated with longer OS.7 In the treatment-naive group, median OS was 35.4 months (95% CI, 20.3 to 63.5 months), and the 5-year OS rate was 29.6% among patients with PD-L1 TPS of 50% or greater. There were too few patients (n = 12) in the group of treatment-naive PD-L1 TPS less than 1% to evaluate OS; however, median OS was 19.5 months (95% CI, 10.7 to 26.3 months) with 5-year OS of 15.7% among patients with PD-L1 TPS of 1% to 49% (Fig 1C). Among previously treated patients with PD-L1 TPS of 50% or greater, median OS was 15.4 months (95% CI, 10.6 to 18.8 months) and the 5-year OS rate was 25.0% compared with 8.5 months (95% CI, 6.0 to 12.6 months) and 12.6% among patients with PD-L1 TPS of 1% to 49% and 8.6 months (95% CI, 5.5 to 10.6 months) and 3.5% among patients with PD-L1 TPS of 1% or less (Fig 1D). When OS and ORR were evaluated in quartiles defined by baseline PD-L1 expression—among all treated patients—higher PD-L1 expression was associated with longer median OS, higher 5-year OS rate, and higher ORR (Data Supplement). Clinicopathologic characteristics among patients with or without a response are summarized in the Data Supplement.
Among 100 patients who were alive at the time of data cutoff, 78 (78%) had experienced an objective response (20 [77%] of 26 patients in the treatment-naive group and 58 [78%] of 74 patients in the previously treated group). Among patients still alive, one in the treatment-naive group and five in the previously treated group achieved complete responses.
Of the 60 patients who received 2 or more years of treatment with pembrolizumab, 46 were alive at data cutoff, with an estimated 5-year OS rate of 78.6% in the treatment-naive group (n = 14) and 75.8% in the previously treated group (n = 46). Among patients who completed 2 or more years of pembrolizumab therapy, 12 (86%) of 14 patients in the treatment-naive group and 42 (91%) of 46 patients in the previously treated group experienced an objective response. Median DOR was 52.0 months (range, 10.2 to 55.7+ months) and not reached (range, 12.5 to 71.8+ months), respectively. Seven treatment-naïve patients (58%) and 30 previously treated patients (71%) had an ongoing response at data cutoff. In addition to the 54 patients with a response who completed 2 or more years of treatment (of 145 patients with a response), six patients with stable disease completed 2 or more years of treatment (202 patients with stable disease), two of whom had died.
Safety
Treatment-related AEs occurred in 388 (71%) of 550 patients. Grade 3 to 5 treatment-related AEs occurred in 69 patients (13%). Only three additional treatment-related grade 3 to 5 AEs occurred during follow-up after analysis at 3 years14: hypertension, glucose intolerance, and hypersensitivity reaction—all grade 3 and all resolved. Serious AEs occurred in 228 patients (42%), including 50 (9%) who had treatment-related serious AEs. Treatment-related AEs led to treatment discontinuation in 31 patients (6%), of whom nine are still alive and seven have an ongoing response. As previously reported,7,14 two patients died as a result of a treatment-related AE—interstitial lung disease (day 12) and cardiopulmonary arrest (day 32).
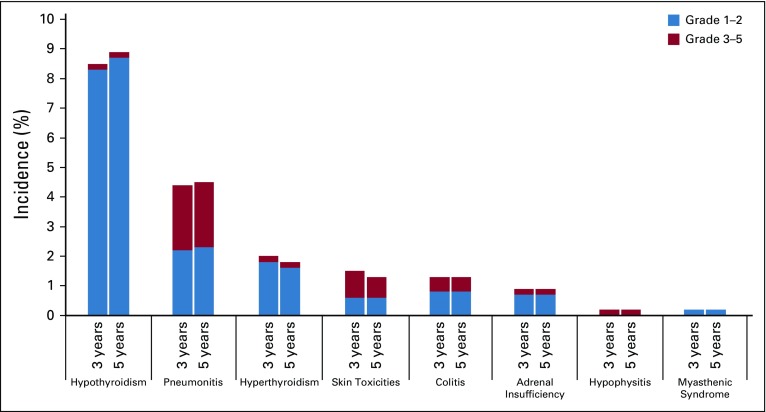
Overall, 92 patients (17%) experienced an immune-mediated AE, regardless of attribution to therapy by investigators, and 32 were still alive at data cutoff. Incidence of immune-mediated AEs at 3 and 5 years of follow-up is shown in Fig 3. The most common immune-mediated AEs (all grades) were hypothyroidism (9%), pneumonitis (5%), and hyperthyroidism (2%). Twenty-one patients (4%) had grade 3 to 5 immune-mediated AEs. The only grade 3 to 5 immune-mediated AEs to occur in more than one patient were pneumonitis (n = 12; 2%), severe skin reaction (n = 4; < 1%), and colitis (n = 3; < 1%).
FIG 3.
Immune-mediated adverse events at 3 years (September 6, 2016) and at 5 years (November 5, 2018) of follow-up. Immune-mediated AEs were classified based on a list of preferred terms identified by the sponsor as having an immune etiology. Because there were changes in events included in this list between the 3- and 5-year analyses, certain events classified as immune-mediated at 3 years may not have been so-classified at 5 years.
DISCUSSION
Five-year outcomes from the KEYNOTE-001 study represent the longest follow-up of patients with advanced/metastatic NSCLC who received pembrolizumab. Overall, it is clear that these outcomes represent a clinically meaningful improvement over the 5-year OS rate of 5.5% that was achieved with standard-of-care cytotoxic chemotherapies in the period immediately before the introduction of immunotherapies for the treatment of advanced/metastatic NSCLC.1 Given the single-arm nature of the KEYNOTE-001 study and the consequent absence of a comparator arm, the magnitude of any clinical benefit attributable to pembrolizumab cannot be quantified, and the predictive value of tumor PD-L1 expression cannot be fully evaluated in this exploratory analysis. However, efficacy outcomes in this study and the observed association between tumor PD-L1 expression and OS are consistent with data from randomized controlled trials with more limited follow-up, including KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042.8,19,20 Taken together, these data provide support for an association between PD-L1 TPS assessed using the PD-L1 immunohistochemistry 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA)21 and long-term outcomes among patients who received pembrolizumab monotherapy for advanced NSCLC. The results highlight the potential for long-term benefit that may be realized through individualized treatment selection.
The durability of responses achieved during pembrolizumab treatment appeared to be an important contributor to the OS outcomes. A subgroup of patients were not only alive at 5 years but also achieved durable responses, with some patients in both the treatment-naive and previously treated groups having response durations of 4 or more years. Among the 60 patients who received 2 or more years of pembrolizumab treatment, more than 85% experienced an objective response, and the 5-year OS rate exceeded 75%. Of note, among patients in this group, median DOR was 52.0 months for treatment-naive patients and had not been reached for previously treated patients. These results are consistent with findings from an analysis of the KEYNOTE-010 study in which the ORR (95%) and 3-year OS rate (98.7%) were high among patients who completed 2 years of treatment.20 The importance of achieving an objective response is further emphasized by the finding that the majority of patients who were still alive had achieved an objective response: among 100 patients who were alive at the time of analysis—of whom 46 had received 2 or more years of pembrolizumab therapy—78% had an objective response. No clinicopathologic characteristic appeared to differentiate between patients with a response who were alive at the data cutoff versus those who were not.
Long-term analyses from randomized controlled studies, albeit with shorter follow-up than in our analysis of KEYNOTE-001, have consistently demonstrated improved OS rates with pembrolizumab versus cytotoxic chemotherapy. In an updated analysis of the KEYNOTE-010 study in patients with previously treated NSCLC, among those with PD-L1 TPS of 50% or greater, the 3-year OS rate was 35% for patients who received pembrolizumab compared with 13% for patients who received docetaxel (median OS: 16.9 months v 8.2 months), whereas among those with PD-L1 TPS of 1% or greater, 3-year OS rates were 23% and 11%, respectively (median OS: 11.8 months v 8.4 months).20 In the first-line setting in patients with PD-L1 TPS of 50% or greater, an updated analysis of the KEYNOTE-024 study found a 2-year OS rate of 51.5% for pembrolizumab versus 34.5% for platinum-based chemotherapy (median OS: 30.0 months v 14.2 months).22 Data from randomized trials have thus shown data that are similar to that of the single-arm KEYNOTE-001 study at analogous time points and suggest that patients with PD-L1 TPS of 50% or greater may derive greater absolute benefit from pembrolizumab as a first-line therapy than as a second-line therapy.
Five-year OS rates were high and median OS was long across all subgroups evaluated, although assessment of these outcomes should be interpreted with caution given the limited numbers of patients in certain subgroups. Consistent with the KEYNOTE-001 results reported at 1 year and 3 years of follow-up,7,14 nonsquamous histology was associated with higher 5-year OS among treatment-naive patients, whereas the opposite was found for previously treated patients. Although a similar difference in outcome was observed for patients who used tobacco, with current or former smokers versus those associated with higher 5-year OS among previously treated patients, it should be noted that the number of never-smokers in the treatment-naive group was small (n = 11). The association between EGFR mutation and shorter median OS and lower 5-year OS rate among previously treated patients is consistent with the findings from a meta-analysis of three studies in previously treated patients (nivolumab v docetaxel [CheckMate-057]; pembrolizumab v docetaxel [KEYNOTE-010]; and atezolizumab v docetaxel [POPLAR]).23 Although it is anticipated that agents that target specific genomic abnormalities may increase the 5-year survival rate for appropriate populations, PD-L1 TPS of 50% or greater identifies a population of patients with NSCLC who may derive long-term survival benefit from pembrolizumab monotherapy. At present, it is unknown whether exploratory biomarkers, such as tumor mutational burden or the presence and/or characteristics of tumor-infiltrating lymphocytes, could aid in selecting long-term survivors. Research evaluating novel potential biomarkers is ongoing.
Safety data from this study after approximately 1 year and 3 years of follow-up have been reported previously.7,14 Updated safety data from the current analysis are consistent with the known safety profile of pembrolizumab and support the long-term tolerability of treatment of patients with advanced NSCLC with pembrolizumab. Overall incidence of treatment-related AEs and the nature and severity of immune-mediated AEs in this analysis were similar to those reported in prior analyses.7,14 The incidence of treatment-related and immune-mediated AEs was higher among patients who experienced a response versus those who had not (Data Supplement); however, the likelihood of significant confounding relationships between treatment exposure, objective response, and the incidence of AEs makes this finding difficult to interpret.
Five-year OS rates with pembrolizumab (treatment-naive, 23.2%; previously treated, 15.5%) are consistent with those reported for other anti‒PD-(L)1 agents. In a phase I study of nivolumab in patients with previously treated NSCLC (CA209-003), OS was 18% at 3 years and 16% at 5 years of follow-up.24,25 In long-term follow-up analyses of phase III studies that enrolled previously treated patients, 3-year OS for nivolumab has been reported as 17% versus 8% for doctaxel,26 whereas 3-year OS for atezolizumab and docetaxel was 19% versus 10% for docetaxel.27
Five-year outcomes from the KEYNOTE-001 study demonstrate that pembrolizumab provides long-term OS benefit and durable responses with tolerable safety for treatment-naive and previously treated patients with advanced PD-L1‒expressing NSCLC. In the context of low historical 5-year survival in advanced NSCLC, these data demonstrate the potential of pembrolizumab treatment to improve long-term outcomes for patients with advanced NSCLC.
ACKNOWLEDGMENT
The authors thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. The authors also thank Bilal Piperdi, MD, of Merck & Co., Inc., Kenilworth, NJ, for thoughtful review of the manuscript. Medical writing assistance was provided by Ali Hassan, PhD, and Mariana Ovnic, PhD, of C4 MedSolutions (Yardley, PA), a CHC Group company, which was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Footnotes
Scheduled to be presented at the 2019 Annual Meeting of the American Society for Clinical Oncology, Chicago, IL, May 31-June 4, 2019.
Supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., (Kenilworth, NJ).
Clinical trial information: NCT01295827.
See accompanying Editorial on page 2511
AUTHOR CONTRIBUTIONS
Conception and design: Edward B. Garon, Matthew D. Hellmann, Charu Aggarwal, Suresh S. Ramalingam, Debra A. Kush
Financial support: Debra A. Kush
Provision of study materials or patients: Enric Carcereny, Natasha B. Leighl, Myung-Ju Ahn, Joseph Paul Eder, Charu Aggarwal, Leora Horn, Amita Patnaik, Matthew Gubens, Suresh S. Ramalingam, Enriqueta Felip, Jonathan W. Goldman, Rina Hui
Collection and assembly of data: Edward B. Garon, Matthew D. Hellmann, Naiyer A. Rizvi, Enric Carcereny, Natasha B. Leighl, Joseph Paul Eder, Ani S. Balmanoukian, Leora Horn, Amita Patnaik, Matthew Gubens, Suresh S. Ramalingam, Enriqueta Felip, Jonathan W. Goldman, Cathie Scalzo, Debra A. Kush, Rina Hui
Data analysis and interpretation: Edward B. Garon, Matthew D. Hellmann, Natasha B. Leighl, Myung-Ju Ahn, Joseph Paul Eder, Leora Horn, Amita Patnaik, Suresh S. Ramalingam, Enriqueta Felip, Jonathan W. Goldman, Erin Jensen, Debra A. Kush, Rina Hui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Five-Year Overall Survival for Patients With Advanced Non–Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase 1 KEYNOTE-001 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Edward B. Garon
Consulting or Advisory Role: Dracen, EMD Serono
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Eli Lilly (Inst), Bristol-Myers Squibb (Inst), Mirati Therapeutics (Inst), Dynavax (Inst), Iovance Biotherapeutics (Inst), Neon Therapeutics (Inst)
Matthew D. Hellmann
Stock and Other Ownership Interests: Shattuck Labs
Honoraria: AstraZeneca, Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, AstraZeneca, MedImmune, Novartis, Janssen Pharmaceuticals, Nektar, Syndax, Mirati Therapeutics, Shattuck Labs
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Patent filed by Memorial Sloan Kettering (PCT/US2015/062208) for the use of tumor mutation burden for prediction of immunotherapy efficacy, licensed to Personal Genome Diagnostics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb
Naiyer A. Rizvi
Leadership: ARMO BioSciences
Stock and Other Ownership Interests: Gritstone Oncology, ARMO BioSciences, Bellicum Pharmaceuticals, Brooklyn ImmunoTherapeutics
Consulting or Advisory Role: AstraZeneca, MedImmune, Genentech, Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, Eli Lilly, AbbVie, Regeneron, Janssen Pharmaceuticals, EMD Serono, GlaxoSmithKline, NeoGenomics Laboratories
Research Funding: Bristol-Myers Squibb, Merck Sharp & Dohme
Patents, Royalties, Other Intellectual Property: Royalties related to patent filed by Memorial Sloan Kettering Cancer Center, determinants of cancer response to immunotherapy (PCT/US2015/062208), licensed to Personal Genome Diagnostics
Enric Carcereny
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Takeda, Pfizer
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche
Natasha B. Leighl
Research Funding: Guardant (Inst), Array (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, Nektar, Genentech, GlaxoSmithKline, Roche, AstraZeneca
Myung-Ju Ahn
Honoraria: AstraZeneca, Eli Lilly, MSD, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Takeda, Alpha Pharmaceutical
Joseph Paul Eder
Consulting or Advisory Role: Genentech, Celgene
Ani S. Balmanoukian
Speakers' Bureau: Merck, Genentech, AstraZeneca, Bristol-Myers Squibb
Charu Aggarwal
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Eli Lilly, Celgene, MedImmune
Research Funding: Genentech (Inst), Incyte (Inst), Macrogenics (Inst), Merck Sharp & Dohme (Inst), AstraZeneca (Inst), MedImmune (Inst)
Leora Horn
Consulting or Advisory Role: Merck, Xcovery, Genentech, Eli Lilly, AbbVie, AstraZeneca, Bristol-Myers Squibb, Incyte, EMD Serono, Tesaro
Research Funding: Boehringer Ingelheim (Inst), Xcovery (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Boehringer Ingelheim
Amita Patnaik
Consulting or Advisory Role: Bayer, Novartis, Genentech (I), Merck, Seattle Genetics, Merck (I), Bristol-Myers Squibb (I)
Research Funding: Merck (Inst), Pfizer (Inst), Eli Lilly (Inst), Plexxikon (Inst), Corvus Pharmaceuticals (Inst), Tesaro (Inst), AbbVie (Inst), Forty-Seven (Inst), Five Prime Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Proximagen (Inst), Pieris Pharmaceuticals (Inst), Surface Oncology (Inst), Livzon (Inst), Vigeo Therapeutics (Inst), Astellas Pharma (Inst), Klus Pharma (Inst), Symphogen (Inst), Syndax (Inst)
Matthew Gubens
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, AstraZeneca, Mersana, Heron, Boehringer Ingelheim, Takeda
Research Funding: Celgene (Inst), Merck (Inst), Novartis (Inst), Genentech (Inst), OncoMed (Inst)
Suresh S. Ramalingam
Consulting or Advisory Role: Amgen, Boehringer Ingelheim, Celgene, Genentech, Eli Lilly, ImClone, Bristol-Myers Squibb, AstraZeneca, AbbVie, Merck, Takeda, Tesaro, Nektar, Loxo
Research Funding: AbbVie (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca (Inst), MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Enriqueta Felip
Consulting or Advisory Role: Pfizer, Roche, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Celgene, Guardant Health, Novartis, Takeda, AbbVie, Blueprint Medicines, Eli Lilly, Merck, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol-Myers Squibb, Novartis, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Pfizer, AbbVie, Eli Lilly, Merck, Takeda
Research Funding: Fundación Merck Salud (Inst), EMD Serono (Inst)
Jonathan W. Goldman
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Genentech, Eli Lilly, Trovagene, Vortex Biosciences, Amgen, Celgene
Speakers' Bureau: Merck
Research Funding: Eli Lilly, Genentech, Astex Pharmaceuticals, Clovis Oncology, Bristol-Myers Squibb, AstraZeneca, MedImmune, Threshold Pharmaceuticals, Array BioPharma, Celgene, AbbVie, Astellas Pharma, Corvus Pharmaceuticals, Spectrum Pharmaceuticals, Merck
Cathie Scalzo
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Erin Jensen
Employment: Merck
Stock and Other Ownership Interests: Merck
Debra A. Kush
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Rina Hui
Honoraria: Merck Sharp & Dohme, Novartis, Roche, AstraZeneca, Bristol-Myers Squibb
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol-Myers Squibb, Novartis
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer (version 5.2018) https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 3.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 4.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. 2018;10:93–105. doi: 10.2217/imt-2017-0121. [DOI] [PubMed] [Google Scholar]
- 6.Merck . Keytruda (pembrolizumab): Full prescribing information. Whitehouse Station, NJ: Merck; 2019. [Google Scholar]
- 7.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 10.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1–expressing, locally advanced or metastatic non–small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 13.Lisberg A, Garon EB. Does platinum-based chemotherapy still have a role in first-line treatment of advanced non-small-cell lung cancer? J Clin Oncol. 2019;37:529–536. doi: 10.1200/JCO.18.01534. [DOI] [PubMed] [Google Scholar]
- 14.Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med. 2019;7:347–357. doi: 10.1016/S2213-2600(18)30500-9. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Dolled-Filhart M, Roach C, Toland G, et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med. 2016;140:1243–1249. doi: 10.5858/arpa.2015-0542-OA. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1291–1298. doi: 10.1093/annonc/mdw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl; abstr LBA4) [Google Scholar]
- 20.Herbst RS, Garon EB, Kim DW, et al. Long-term survival in patients (pts) with advanced NSCLC in the KEYNOTE-010 study overall and in pts who completed two years of pembrolizumab (pembro); European Society for Medical Oncology; Munich, Germany. October 19-23, 2018. [Google Scholar]
- 21.Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. doi: 10.1097/PAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 23.Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer: A meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J Clin Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 25.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 27.Mazières J, Park K, Lewanski C, et al. 3-year survival and duration of response in randomized phase II study of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC (POPLAR) J Thorac Oncol. 2018;13(abstr S79) [Google Scholar]