Abstract
OBJECTIVES:
Individuals with advanced adenomas or three or more adenomas have a higher risk of metachronous advanced neoplasia (AN) and are recommended to undergo surveillance colonoscopy at shorter intervals. However, it is questionable whether patients with multiple (three or more) non‐advanced diminutive adenomas should be considered as high‐risk.
METHODS:
We analyzed 5482 patients diagnosed with one or more adenomas during their first colonoscopy screening and who underwent a follow‐up colonoscopy. Patients were categorized into four groups based on adenoma characteristics at baseline: Group 1, 1‐2 non‐advanced adenomas; Group 2, ≥3 non‐advanced, diminutive (1 to 5 mm) adenomas; Group 3, ≥3 non‐advanced, small (6‐9 mm) adenomas; and Group 4, advanced adenomas.
RESULTS:
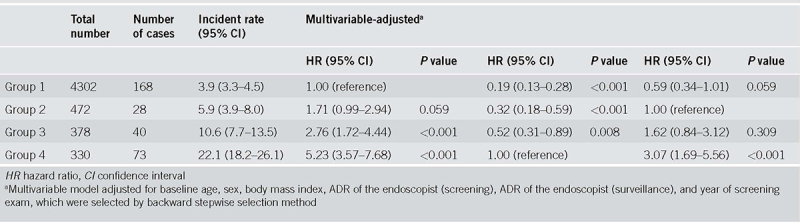
During a median follow‐up of 38 months, the incidence of metachronous AN at surveillance colonoscopy was 5.6%. The incidence of AN was 3.9% in group 1, 5.9% in group 2, 10.6% in group 3, and 22.1% in group 4. The adjusted hazard ratios (HRs) [95% confidence intervals (CIs)] for metachronous AN between group 2, group 3, and group 4, and low risk group 1 were 1.71 (0.99‐2.94), 2.76 (1.72‐4.44), and 5.23 (3.57‐7.68), respectively. Compared with group 4, the adjusted HRs (95% CIs) for group 1, group 2, and group 3 were 0.19 (0.13‐0.28), 0.32 (0.18‐0.59), and 0.52 (0.31‐0.89), respectively.
CONCLUSIONS:
We found that patients with three or more non‐advanced diminutive adenomas had a borderline increased risk of metachronous AN compared with patients with low risk adenomas.
INTRODUCTION
Colorectal cancer (CRC) is worldwide the fourth most frequently diagnosed cancer worldwide and the second leading cause of overall cancer‐related death [1]. The importance of early detection and removal of pre‐cancerous lesions has been emphasized because the majority of CRC occur from pre‐existing adenomatous polyps, after going through an adenoma‐carcinoma sequence [2, 3]. The performance of polypectomy during colonoscopy can prevent CRC and reduce mortality. Early detection as well as advanced neoplasia (AN) surveillance after polypectomy are important to reduce CRC mortality, because patients with previous adenomas are at a higher risk of metachronous lesions [2,3,4,5]. Therefore, post‐polypectomy surveillance has become a major indication for colonoscopy.
Guidelines recommend different follow‐up surveillance colonoscopy intervals based on the number and characteristics of the detected adenomas during colonoscopy screening [6, 7]. Several high‐risk factors are known to contribute to the development of AN, including the number of adenomas. As the number of adenomas increases, the risk of metachronous AN also increases. This trend is especially prevalent when there are three or more adenomas at index colonoscopy [8, 9]. In addition, any adenoma exhibiting high‐risk traits is referred to as an advanced adenoma. High‐risk traits include a size of 10 mm or more in diameter, and advanced histology of high‐grade dysplasia or villous component. In patients with advanced adenomas, current guidelines recommend a short‐term follow‐up surveillance colonoscopy in three years, compared with 5‐10 years for patients with 1 or 2 non‐advanced adenomas [6, 9].
However, it is questionable whether patients with three or more adenomas without any other high‐risk factors, especially when diminutive in size (≤5 mm), should also be considered at a high risk of metachronous AN. In this study, we subclassified patients at high risk based on adenoma size (diminutive vs small) and the presence of advanced adenoma. We then assessed the risk of metachronous AN among different groups.
METHODS
Study setting and participants
We conducted a retrospective cohort study of healthy individuals aged 50 years and older who underwent first‐time screening colonoscopy and subsequent surveillance colonoscopy at least 1 year apart between January 2006 and December 2017 at the Samsung Medical Center, South Korea (n = 18,091). We excluded 12,609 participants who met any of the following exclusion criteria: poor bowel preparation, incomplete colonoscopy, history of malignancy, history of colorectal surgery, inflammatory bowel disease, family history of colorectal CRC, no adenoma at screening, or sessile serrated adenoma/polyp or traditional serrated adenoma at screening. Finally, 5482 participants diagnosed with one or more colorectal adenomas at baseline and who underwent surveillance colonoscopy were included in this study (Fig. 1). The Institutional Review Board of the Samsung Medical Center approved this study and waived the requirement for informed consent because our data only consisted of de‐identified data that had been collected with clinical purpose as part of the health screening check‐up.
Fig. 1.
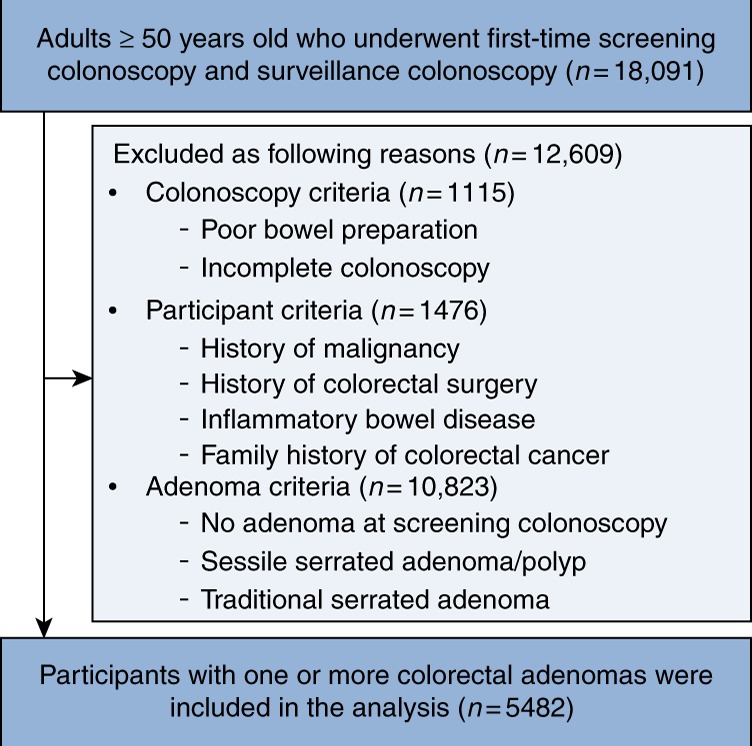
Flow chart of study participants
Measurement of variables
The comprehensive health‐screening program included demographic characteristics, anthropometric measurements, endoscopy, and a self‐administered health questionnaire on smoking status, alcohol intake, regular exercise, regular aspirin use, and family history of colon cancer. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants dressed in light clothing and bare feet. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Smoking status was categorized into three groups including never, former, and current smoker. Alcohol intake status was divided into mild (≤10 g/day) and modest (>10 g/day). Regular exercise was defined as exercising three or more times per week with moderate intensity.
Colonoscopies
Experienced board‐certified gastroenterologists performed the colonoscopies after bowel preparation with polyethylene glycol solutions. A complete examination was defined as one that reached the cecum. Endoscopists assessed the location and size of the detected colorectal polyp. Location between cecum to splenic flexure was defined as proximal, and descending colon to rectum as distal. The size of each polyp was routinely estimated using open biopsy forceps. Endoscopists routinely resected the detected polyps during colonoscopy and the histopathology of biopsy samples were assessed by qualified pathologists. The following information was recorded in the electronic medical record after the colonoscopy: number of polyps, locations and sizes of polyps, the time and result of the last colonoscopy, family history of CRC, bowel preparation (Aronchick scale), and colonoscopy completeness.
Variables and definitions
The primary outcome of interest was the development of metachronous colorectal AN at follow‐up colonoscopy. Advanced adenoma was defined as any adenoma 1‐cm or more in size, or containing ≥25% of villous component or high‐grade dysplasia. A diminutive adenoma was defined as an adenoma 1‐5 mm in size, and a small adenoma was defined as an adenoma 6‐9 mm in size. AN included advanced adenoma and invasive CRC. Baseline characteristics of participants including age, sex, BMI, smoking status, alcohol intake, regular exercise, regular aspirin use, and the adenoma detection rate (ADR) of the endoscopist were collected. ADR was used as a quality indicator of the colonoscopy. The ADR, determined by each endoscopist, was calculated from data of individuals ≥50‐years‐old who underwent a first‐time screening colonoscopy, except cases with poor bowel preparation.
We categorized patients into four risk groups based on the number and characteristics of the colorectal neoplasms detected during colonoscopy screening: Group 1, low risk, one or two non‐advanced adenomas; Group 2, three or more diminutive (1‐5 mm) non‐advanced adenomas; Group 3, three or more small (6‐9 mm) non‐advanced adenomas; and Group 4, high risk, advanced adenomas. The size was determined based on the largest adenoma.
Statistical analysis
Data were presented as mean ± standard deviation for continuous variables and percentages for categorical variables. The differences in baseline characteristics among the risk groups were compared using a one‐way ANOVA test or a t‐test for continuous variables and the χ2 test for categorical variables. We performed univariable Cox regression analysis to identify whether an association existed between the baseline factors and the development of metachronous AN. The Kaplan‐Meier method was used to describe the cumulative hazard of AN according to the risk groups. Further, we used multivariable Cox regression models to estimate adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for metachronous AN according to the risk groups. The multivariable model was selected by backward stepwise selection method based on the Akaike information criterion (AIC). The candidate factors included baseline age, sex, BMI, smoking status, alcohol consumption, regular exercise, regular aspirin use, bowel preparation (excellent/good/fair), ADR of the endoscopist (screening), ADR of the endoscopist (surveillance), and year of screening examination. In the multivariable Cox regression analysis, we performed pairwise comparison of the risk for metachronous AN by considering risk groups 1, 2, and 4 as references. For these multiple comparisons, Bonferroni correction was used to adjust both P‐value and the CIs of HR. A P‐value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics (n = 5482) are presented in Table 1. The mean age (standard deviation) of participants was 55.5 (5.7) years; 68.5% were men. When stratified alongside the pre‐defined risk groups, participants were categorized as follows: Group 1 (n = 4302), Group 2 (n = 472), Group 3 (n = 378), and Group 4 (n = 330). The low‐risk group (Group 1) had a significantly higher ratio of young, women, less obese individuals, and non‐smokers, as well as a longer surveillance interval, compared with the other groups. The number of baseline adenomas in groups with multiple adenomas of three or more, groups 2 and 3, showed a mean value of 3.7 and 4.0. In group 2, the proportion of the number of diminutive adenomas was 3 (61%), 4 (20.5%), 5 (11.9%), and 6‐10 (6.6%), respectively. The majority of patients in groups 2 and 3 had 3‐5 adenomas.
Table 1.
Baseline characteristics of risk groups based on adenoma characteristics at index colonoscopy
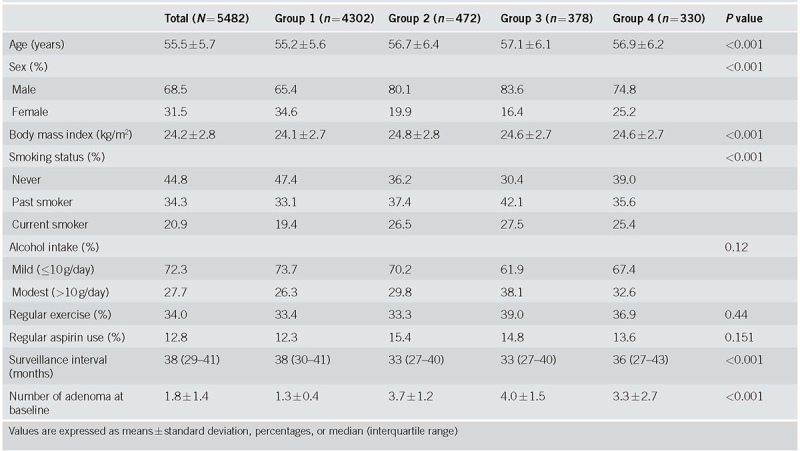
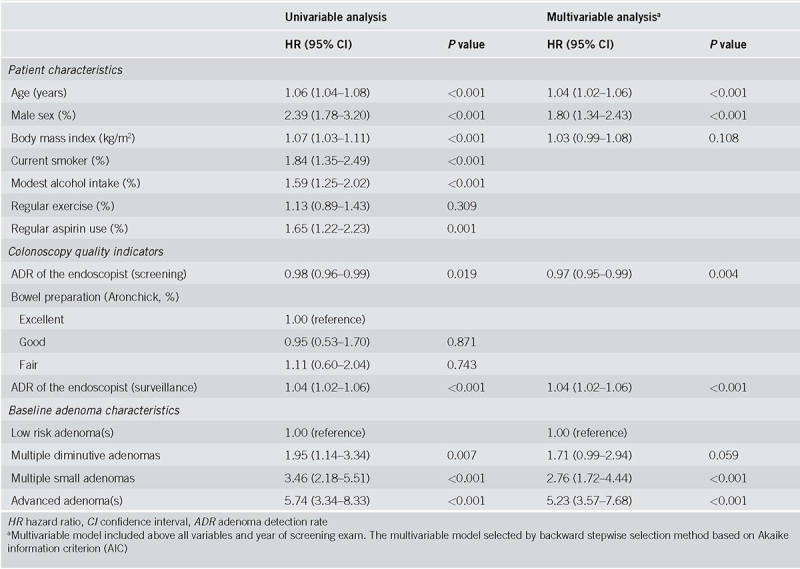
Table 2 presents the univariable and multivariable Cox regression analyses about baseline clinical factors associated with the development of metachronous AN. Among the patient factors, older age (adjusted HR, 1.04; 95% CI, 1.02‐1.06) and male sex (adjusted HR, 1.80; 95% CI, 1.34‐2.43) were significantly associated with the risk of metachronous AN. BMI was also a risk factor (unadjusted HR, 1.07; 95% CI 1.03‐1.11), albeit being insignificant (adjusted HR, 1.03; 95% CI 0.99‐1.08) in the multivariable analysis. ADR of the endoscopist was an independent predictor of metachronous AN. The risk of metachronous AN was decreased when screening was performed by an endoscopist with higher ADR (adjusted HR 0.97; 95% CI, 0.95‐0.99). The ADR of the endoscopist who performed surveillance colonoscopy was also retained as an independent predictor of metachronous AN (adjusted HR, 1.04; 95% CI, 1.02‐1.06).
Table 2.
Risk of metachronous advanced neoplasia in univariable and multivariable analyses
During a median follow‐up of 38 months, 309 AN developed in 5482 participants, with an incidence of 5.6%. The incidence of AN was 3.9% (168/4,302) in group 1, 5.9% (28/472) in group 2, 10.6% (40/378) in group 3, and 22.1% (73/330) in group 4 (Fig. 2). During the follow‐up period, five interval CRCs were developed. In group 1, two (0.05%) interval ascending colon cancers were developed. In group 2, one (0.21%) interval rectal cancer was developed. In group 3, one (0.26%) interval sigmoid colon cancer was developed. In group 4, one (0.3%) interval ascending colon cancer was developed. Figure 3 shows the cumulative hazard for metachronous AN according to the risk groups. Group 4, including patients with advanced adenoma, had the highest hazard for AN development among the risk groups. In contrast, group 2, including patients with multiple diminutive adenomas, had a slightly higher hazard for AN than did the group 1, which had a low‐risk. Table 3 presents the number of cases, incidence rate (95% CI), and the multivariable adjusted HR between the four prior categorized groups. Compared with group 1 (reference), adjusted HRs (95% CIs) were increased across the risk groups, demonstrating 1.71 (0.99‐2.94), 2.76 (1.72‐4.44), 5.23 (3.57‐7.68) for group 2, group 3, and group 4, respectively. This relationship was statistically significant for groups 3 and 4 (P < 0.001); by contrast, it was insignificant for group 2 (P = 0.059). Compared with group 4 (reference), adjusted HRs (95% CIs) for group 1, group 2, and group 3 were 0.19 (0.13‐0.28), 0.32 (0.18‐0.59), 0.52 (0.31‐0.89), respectively. In addition, compared with group 2 (reference), adjusted HRs (95% CIs) for group 1, group 3, and group 4 were 0.59 (0.34‐1.01), 1.62 (0.84‐3.12), and 3.02 (1.65‐5.51), respectively.
Fig. 2.
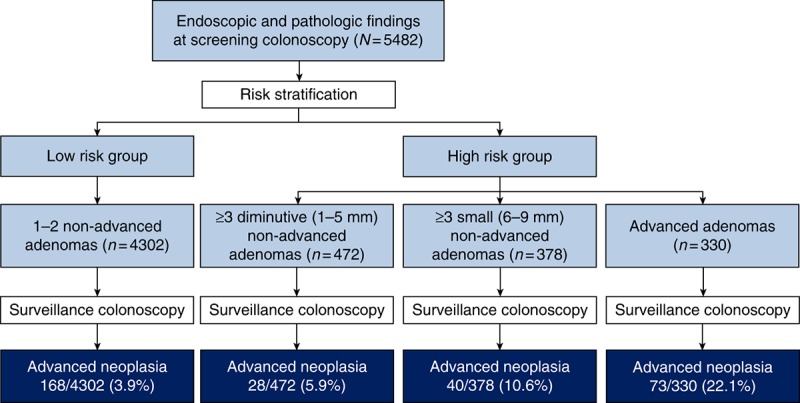
Incidence of advanced neoplasia according to endoscopic and pathologic findings at screening colonoscopy
Fig. 3.
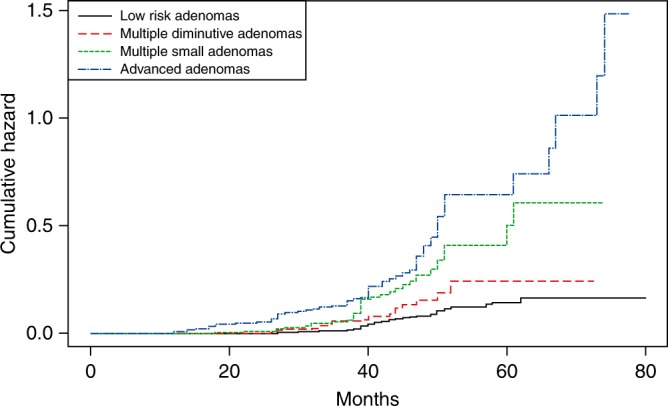
Cumulative hazard of advanced neoplasia according to baseline adenoma characteristics
Table 3.
Risk for metachronous advanced neoplasia based on baseline adenoma characteristics (different reference groups)
DISCUSSION
Current guidelines for surveillance colonoscopy in patients with high‐risk factors recommend a short‐term follow‐up surveillance colonoscopy of 3 years after screening, compared with 5‐10 years for patients with 1 or 2 non‐advanced adenomas [10, 11]. Size (≥ 10 mm), multiplicity (≥ 3), and presence of villous component or high‐grade dysplasia are independent risk factors for metachronous AN [12, 13]. The risk of metachronous AN is higher in high‐risk groups with any one of the factors mentioned above, but the contribution of each risk factor is less consistent [14, 15]. Indeed, we found that the risks of AN among individuals with high‐risk features vary according to baseline adenoma characteristics. Among the high‐risk group, patients with ≥3 diminutive (1‐5 mm in size) adenomas, ≥3 small (6‐9 mm in size) adenomas, and advanced adenomas developed AN at 5.9%, 10.6%, and 22.1%, respectively. In the multivariable analysis, patients with multiple diminutive adenomas had the lowest risk of AN among the subgroups with high‐risk.
There remains some doubt that patients with ≥3 diminutive non‐advanced adenomas really have an increased risk of AN at surveillance colonoscopy. The improvement of endoscopy image‐enhancing technology has increased the detection rate of diminutive polyps. Indeed, ADRs of 40% or more have been reported in more recent studies [16]. Most previous studies have not subclassified sub‐centimeter (<1 cm) adenomas into diminutive (1‐5 mm) and small (6‐9 mm) adenomas on screening colonoscopy [8, 15, 17]. In a recent study, researchers compared the risk of metachronous AN in patients whose largest adenoma was diminutive (1‐5 mm) versus small (6‐9 mm) [18]. The study revealed that patients with diminutive adenomas had a low risk of metachronous AN regardless of the number of adenomas. Our study also demonstrated that patients with multiple diminutive adenomas had a low incidence (5.9%) of metachronous AN and had a borderline increased risk compared to patients with low risk adenomas.
In the diminutive group, the proportion of the number of diminutive adenomas was 3 (61%), 4 (20.5%), 5 (11.9%), and 6‐10 (6.6%), respectively. The mean (standard deviation) number of adenomas was 3.7 ± 1.2 and most of them were distributed between 3 and 5. Because the sample size of 6‐10 diminutive adenomas was so small, it is difficult to extend our results to 6‐10 diminutive adenomas. Therefore, further studies assessing the risk of 6‐10 diminutive adenomas are needed using adequate sample size.
Among adenoma characteristics, the two most known significant factors contributing to the higher risk of AN are the adenoma's size and multiplicity. Large adenomas (10 mm or more) contributed to more than a two‐fold increase in the risk of AN, compared with sub‐centimeter adenomas (<10 mm) [5, 9]. Size is certainly an important factor although this study did not show significant results in the subgroup analysis comparing diminutive and small adenomas. However, patients with ≥3 diminutive adenomas, with the largest sized 1‐5 mm, had a significantly lower risk of metachronous AN than patients who had advanced adenomas at baseline.
In clinical practice, a majority of polyps detected and removed during screening colonoscopy are diminutive in size. More than 99% of diminutive adenomas are non‐advanced in terms of histology, and the rate of invasive cancer is scarce [19,20,21]. Post‐polypectomy surveillance guidelines should focus on patients with non‐diminutive adenomas with high‐risk factors (containing ≥25% of villous component or high‐grade dysplasia or ≥10 mm in size, or ≥3 adenomas) and recommend short‐term surveillance, because of the minimal risk associated with diminutive adenomas. Recent studies evaluating the time interval between screening and surveillance colonoscopy, have reported that the interval for low‐risk patients has been lengthened [22,23,24,25]. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend repeating colonoscopy after 10 years from the index colonoscopy in low‐risk patients with 1‐2 small adenomas and low grade dysplasia. The surveillance interval for patients with multiple diminutive adenomas may be lengthened. Our findings suggest precise surveillance interval for patients with multiple diminutive non‐advanced adenomas classified as intermediate risk from 3 to 5 years.
This study also provided important findings on colonoscopy quality. In the multivariable analysis, we considered the ADR of the endoscopists, which has been shown to predict the risk for interval caner after colonoscopy. The multivariable analysis indicated that the risk of metachronous AN was decreased when screening was performed by an endoscopist with a higher ADR. Our results underlined the importance of ADR as a prognostic factor for metachronous AN, to the same extent as patient‐ and adenoma‐related factors at baseline. These data suggest that ADR, possibly in combination with patient‐related factors other than just colonoscopy findings, could be used to design cost‐effective colonoscopic surveillance intervals [26].
Several limitations should be considered when interpreting our findings. First, misclassification may have occurred when the size of the polyp was roughly measured with an open biopsy as a reference. However, because endoscopists and pathologists were unaware of the study objectives, these measurement errors would be non‐differential and could possibly result in an underestimation of the association between adenoma characteristics and metachronous colorectal AN. Second, our study was a single‐center, retrospective study and included asymptomatic Korean men and women; therefore, it may be difficult to generalize our findings to other populations. Third, in this study, the median interval of surveillance was shorter than the intervals recommended in the current guidelines, especially in low‐risk patients. Surveillance colonoscopy was recommended at 5‐10 years in US at 5 years in Korean guidelines [6, 7] among low‐risk individuals. Early examination could have reduced the incidence of AN; thus, the incidence rate of AN may have been underestimated in these patients. Therefore, it is unclear whether there is a substantial difference in the risk of metachronous AN related to differences in surveillance intervals.
This study also has considerable strengths, including its cohort design, relatively large sample size, the inclusion of first‐time screening colonoscopies, and consideration of colonoscopy‐quality indicators, such as ADR of the endoscopists, bowel preparation and incompleteness of colonoscopy.
In conclusion, substantial variation in the risk of metachronous AN exists among patients with high‐risk factors based on adenoma characteristics such as size and advanced features. Among the high‐risk group, patients with advanced adenomas had the highest risk of metachronous AN, followed by those with ≥3 small adenomas, and those with ≥3 diminutive adenomas. Because patients with multiple diminutive adenomas have the lowest risk of metachronous AN among the high‐risk groups and they had a borderline increased risk compared to patients with low‐risk adenomas, the optimal surveillance interval for these patients may be lengthened to reflect differences in risk. Further studies are needed to verify our findings, because optimizing surveillance interval reduce underuse and overuse of surveillance colonoscopy, thus having substantial economic implications.
CONFLICT OF INTEREST
Guarantor of the article: Young‐Ho Kim, MD, PhD.
Specific author contributions: Study concept and design: Tae Jun Kim and Young‐Ho Kim. Acquisition, analysis, or interpretation of data: Jung Yoon Kim and Tae Jun Kim. Writing and drafting of the manuscript: Jung Yoon Kim and Tae Jun Kim. Critical revision of the manuscript for important intellectual content: Tae Jun Kim, Eun Ran Kim, Sung Noh Hong, Dong Kyung Chang, and Young‐Ho Kim. Statistical analysis: Sun‐Young Baek and Soo Hyun Ahn. Study supervision: Young‐Ho Kim. All authors approved the final submission.
Financial support: None.
Potential competing interests: None.
Study Highlights
WHAT IS CURRENT KNOWLEDGE
✓ Patients with advanced adenomas or ≥3 adenomas have a higher risk of advanced neoplasia (AN) or colorectal cancer and are recommended to undergo surveillance colonoscopy in 3 years after polypectomy.
✓ However, it is questionable whether patients with only multiple (three or more) non‐advanced diminutive adenomas should be considered as high‐risk.
WHAT IS NEW HERE
✓ Risks for AN among individuals with high‐risk features vary based on baseline adenoma characteristics.
✓ We found that patients with three or more non‐advanced diminutive adenomas had a borderline increased risk of metachronous AN compared with patients with low risk adenomas.
✓ The surveillance interval for patients with multiple diminutive adenomas may be lengthened from the recommended 3‐year interval that is used for high‐risk patients.
Footnotes
Correspondence: T.J.K. (email: taejunk91@gmail.com) or Y.‐H.K. (email: bowelkim@gmail.com)
Published online 3 August 2018
REFERENCES
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeve F, van Ballegooijen M, Snel P, et al. Colorectal cancer risk after colonoscopic polypectomy: a population-based study and literature search. Eur J Cancer. 2005;41:416–22. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–62. [DOI] [PubMed] [Google Scholar]
- 5.Bertario L, Russo A, Sala P, et al. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82–7. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–85. [DOI] [PubMed] [Google Scholar]
- 7.Hong SN, Yang D-H, Kim Y-H, et al. Korean guidelines for post-polypectomy colonoscopic surveillance. Korean J Gastroenterol. 2012;59:99–117. [DOI] [PubMed] [Google Scholar]
- 8.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537–43. [DOI] [PubMed] [Google Scholar]
- 10.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–51. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 12.Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61:1180–6. [DOI] [PubMed] [Google Scholar]
- 13.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–26. [DOI] [PubMed] [Google Scholar]
- 14.de Jonge V, Sint Nicolaas J, van Leerdam ME, et al. Systematic literature review and pooled analyses of risk factors for finding adenomas at surveillance colonoscopy. Endoscopy. 2011;43:560–72. [DOI] [PubMed] [Google Scholar]
- 15.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64:614–26. [DOI] [PubMed] [Google Scholar]
- 16.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–6. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. [DOI] [PubMed] [Google Scholar]
- 18.Sneh Arbib O, Zemser V, Leibovici Weissman Y, et al. Risk of advanced lesions at the first follow-up colonoscopy after polypectomy of diminutive versus small adenomatous polyps of low-grade dysplasia. Gastrointest Endosc. 2017;86:713–21 e2. [DOI] [PubMed] [Google Scholar]
- 19.Jeong YH, Kim KO, Park CS, et al. Risk factors of advanced adenoma in small and diminutive colorectal polyp. J Korean Med Sci. 2016;31:1426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Bansal A, Rao D, et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022–30. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Overhiser AJ, Chen SC, et al. Estimation of impact of American College of Radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol. 2009;104:149–53. [DOI] [PubMed] [Google Scholar]
- 22.Brenner H, Chang-Claude J, Seiler CM, et al. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol. 2011;29:3761–7. [DOI] [PubMed] [Google Scholar]
- 23.Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–24. [DOI] [PubMed] [Google Scholar]
- 24.Noshirwani KC, van Stolk RU, Rybicki LA, et al. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc. 2000;51:433–7. [DOI] [PubMed] [Google Scholar]
- 25.Ponugoti PL, Rex DK. Yield of a second screening colonoscopy 10 years after an initial negative examination in average-risk individuals. Gastrointest Endosc. 2017;85:221–4. [DOI] [PubMed] [Google Scholar]
- 26.Kim TJ, Kim ER, Hong SN, et al. Adenoma detection rate influences the risk of metachronous advanced colorectal neoplasia in low-risk patients. Gastrointest Endosc. 2018;87:809–17 e1. [DOI] [PubMed] [Google Scholar]


