Abstract
Docosahexaenoic acid (DHA) is an ω-3 dietary-derived polyunsaturated fatty acid of marine origin enriched in testes and necessary for normal fertility, yet the mechanisms regulating the enrichment of DHA in the testes remain unclear. Long-chain ACSL6 (acyl-CoA synthetase isoform 6) activates fatty acids for cellular anabolic and catabolic metabolism by ligating a CoA to a fatty acid, is highly expressed in testes, and has high preference for DHA. Here, we investigated the role of ACSL6 for DHA enrichment in the testes and its requirement for male fertility. Acsl6−/− males were severely subfertile with smaller testes, reduced cauda epididymal sperm counts, germ cell loss, and disorganization of the seminiferous epithelium. Total fatty acid profiling of Acsl6−/− testes revealed reduced DHA and increased ω-6 arachidonic acid, a fatty acid profile also reflected in phospholipid composition. Strikingly, lipid imaging demonstrated spatial redistribution of phospholipids in Acsl6−/− testes. Arachidonic acid–containing phospholipids were predominantly interstitial in control testes but diffusely localized across Acsl6−/− testes. In control testes, DHA-containing phospholipids were predominantly within seminiferous tubules, which contain Sertoli cells and spermatogenic cells but relocalized to the interstitium in Acsl6−/− testes. Taken together, these data demonstrate that ACSL6 is an initial driving force for germ cell DHA enrichment and is required for normal spermatogenesis and male fertility.
Keywords: fatty acid metabolism, polyunsaturated fatty acid (PUFA), spermatogenesis, phospholipid metabolism, testis, acyl-CoA synthetase, docosahexaenoic acid, male infertility
Introduction
Cellular membranes of the mammalian brain and testis are the most highly enriched (>3–4× over other tissues) with polyunsaturated fatty acids (1, 2). The three most abundant polyunsaturated fatty acids in the testes are ω-6 arachidonic acid (AA, 20:4n6),3 ω-6 docosapentaenoic acid (DPA, 22:5n6), and ω-3 docosahexaenoic acid (DHA, 22:6n3) (3). Because mammals cannot efficiently generate fatty acids with double bonds at the ω-3 or -6 position, these essential fatty acids must therefore be obtained from dietary sources. In Western diets, ω-6s are much more readily available than ω-3s (4). Although the precursor for DHA, α-linolenic acid (18:3n3), is consumed in small portions in certain land-based food sources, the rates of α-linolenic acid to DHA conversion are extremely low (5). Therefore, bodily DHA enrichment is best achieved by direct consumption of dietary DHA via marine-based foods. However, a majority of the world's population does not consume the recommended levels of DHA (6), and combined with its low rates of synthesis, a large proportion of the population is at risk for DHA deficiency. Multiple studies using mouse models and analyzing human populations have shown that DHA deficiency negatively impacts spermatogenesis and male fertility and that supplementation with DHA improves semen quality and fertility (7–11). However, little is known about the underlying mechanisms by which DHA is enriched in the mammalian testis.
The requisite first step in cellular fatty acid metabolism is the enzymatic activation of fatty acids by acyl-CoA synthetases (ACSs). The ACS reaction ligates a CoA to a fatty acid in an ATP-dependent manner, which both traps the fatty acid within the cell and activates it for downstream catabolic or anabolic metabolism. The ACS family consists of 26 isozymes in humans and 25 in rodents, each encoded by distinct genes exhibiting unique enzyme kinetics, expression patterns, and substrate preferences (12–15). Based on these specialized characteristics, each ACS enzyme is predicted to serve a discrete and nonredundant function in lipid biology and thus cannot functionally compensate for another.
We recently reported that one of the ACS enzymes, ACSL6 (long-chain acyl-CoA synthetase 6), is highly expressed in the central nervous system, where it facilitates the enrichment of DHA into membrane lipids (16, 17). Because ACSL6 is also abundant in testes, we examined its role in testis lipid biology, germ cell development, and male fertility. Here, we demonstrate that ACSL6 is predominantly expressed in germ cells, specifically in meiotic spermatocytes and postmeiotic spermatids. This suggests a role for DHA activation in the specialized processes of meiosis and spermatid morphogenesis (spermiogenesis). Acsl6 knockout (Acsl6−/−) male mice had reduced testis DHA content and were severely subfertile. Lipid imaging demonstrated that although Acsl6−/− mice exhibited DHA-containing phospholipid patterns in the testicular interstitium, DHA was excluded from germ cell membranes within the seminiferous tubules. This work implicates ACSL6 as a requisite for male fertility and the initial driving force that enriches germ cells with DHA.
Results
ACSL6 is expressed by meiotic spermatocytes and postmeiotic spermatids
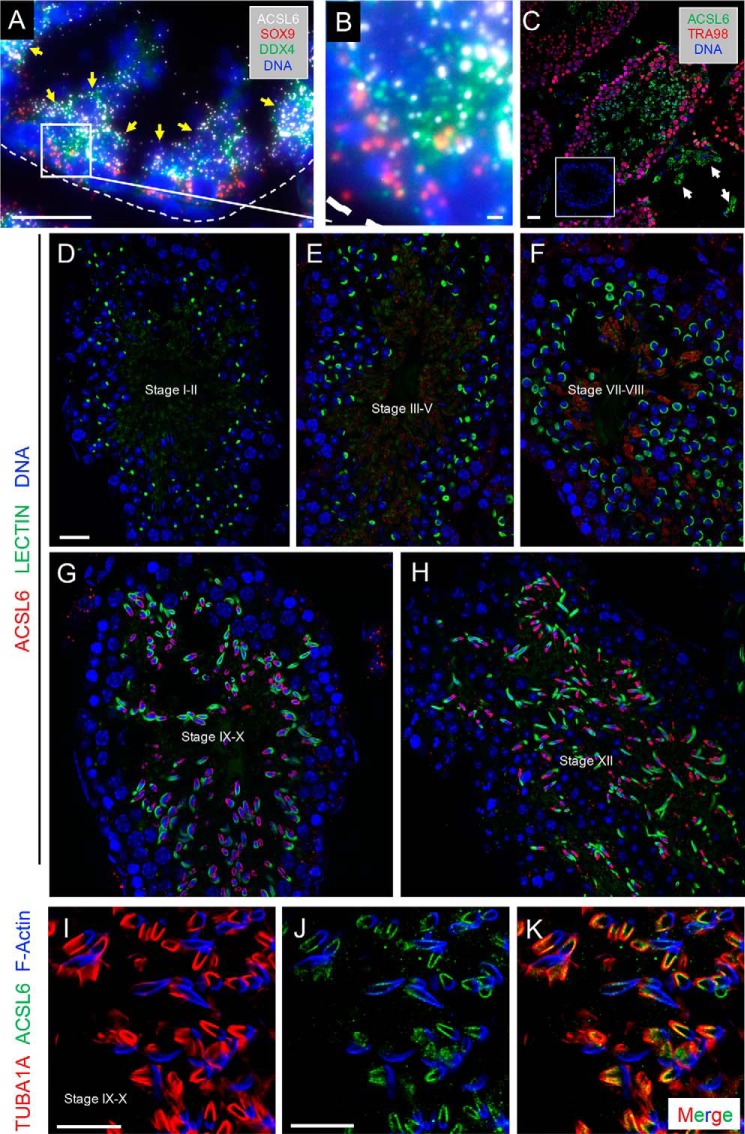
Outside of the central nervous system, DHA levels and Acsl6 expression are highest in the testis (16). During testis development, Acsl6 mRNA levels significantly increased (12-fold) from postnatal day (P)1 to P8 (Fig. S1iA), coincident with the appearance of the first meiotic preleptotene spermatocytes during the first wave of spermatogenesis. From P8 Acsl6 increased by 33-fold in the adult testis (Fig. S1A). To identify the specific cell type(s) within the testis expressing Acsl6, we queried recently published single-cell transcriptomes of the developing and adult testis from our lab and others (18–21). Data from these reports indicated predominant expression of Acsl6 in spermatocytes and round spermatids (Fig. S1B). To confirm this, we performed single-molecule RNA fluorescence in situ hybridization (smFISH) and found that Acsl6 mRNA was most highly abundant in pachytene spermatocytes and round spermatids, which are Ddx4+/Sox9− and located in the second and third layers (away from the basement membrane) of the seminiferous epithelium (Fig. 1, A and B). We detected ACSL6 immunostaining in interstitial Leydig cells (Fig. 1C, white arrows) and at higher levels in adluminal TRA98− germ cells (Fig. 1C). Within germ cells, ACSL6 protein was faintly detectable in spermatocytes but became much more prominent in spermatids (Fig. 1C). ACSL6 staining varied between seminiferous tubule cross-sections, suggestive of stage-specific ACSL6 expression across the cycle of the seminiferous epithelium. To define the stages of spermatogenesis when ACSL6 is expressed, we co-labeled with fluorescent lectin to identify the glycoprotein-rich acrosome (22–25). In addition to faint staining in spermatocytes (Fig. 1C), ACSL6 was detectable at low levels in step 1–8 spermatids (epithelial stages I-VII), became readily detectable in step 9–12 elongating spermatids (stages IX-XII), and then was found adjacent to later spermatids in “residual bodies” (step 13–16, stages I-VIII), which become phagocytosed by Sertoli cells (26) (Fig. 1, D–G, and negative controls in Fig. S2).
Figure 1.
ACSL6 is expressed in meiotic spermatocytes and postmeiotic haploid spermatids. A, smFISH was performed to detect Acsl6 (white), Sox9 (red, Sertoli-expressed), and Ddx4 (green, germ cell-expressed) mRNAs, and nuclei are labeled with DNA (blue). B, enlarged image of inset in A. Immunostaining was done to detect ACSL6 protein in WT testes. C, ACSL6 (green), TRA98 (red), and DNA (blue). Arrows indicate ACSL6+ Leydig cells. D–H, immunostaining was done to detect ACSL6 protein in WT testes, ACSL6 (red), Lectin to detect acrosomes (green), and DNA (blue). The stage(s) of each tubule segment are indicated in the lumina. I–K, immunostaining was done to detect ACSL6 (green) protein in control testes, with TUB1A1 to detect manchette (red), and F-Actin to represent the acrosome region (blue). Scale bars, 50 μm in A and C–H and 10 μm in B and I–K.
Within elongating spermatids, ACSL6 was discretely localized to the caudal postacrosomal region of the nucleus (Fig. 1, G and H), the site of the transient microtubule-containing structure in elongating spermatids termed the manchette. The manchette has proposed roles in nuclear elongation and shaping of the spermatid head, as well as in nucleocytoplasmic transport during flagella formation (27, 28). ACSL6 was localized to the manchette as indicated by colocalization with TUBA1A, but not at the acrosome (Lectin or F-actin+; Fig. 1, I–K).
Acsl6 deletion causes near-complete male infertility and disrupted spermatogenesis
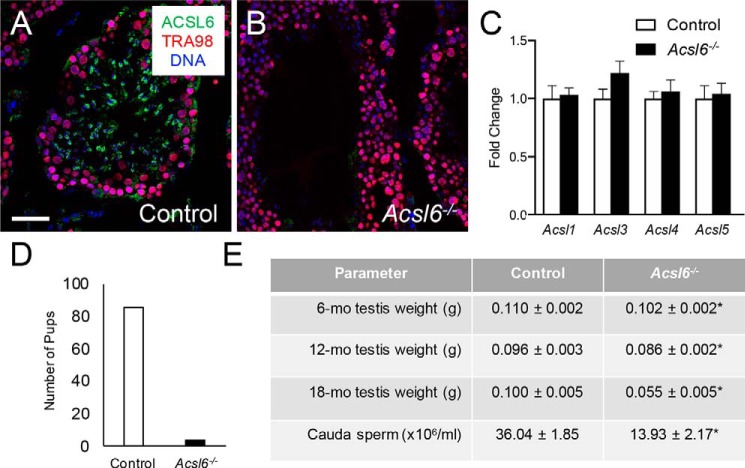
The requirement for ACSL6 in male fertility was determined using Acsl6−/− mice (16). Loss of ACSL6 protein in Acsl6−/− testes was confirmed by immunostaining (Fig. 2, A and B), and no changes were evident in the mRNA levels for paralogs Acsl1,3–5 in Acsl6−/− testes (Fig. 2C). In a 2-month breeding trial, 6 WT males generated a total of 86 pups, and although the 6 Acsl6−/− males generated copulatory plugs, only 5 pups were born (Fig. 2D). Underlying this severe subfertility were significantly reduced numbers of cauda epididymal sperm in 6-month-old Acsl6−/− males and a 10% reduction in testis weights in 6- and 12-month-old Acsl6−/− mice and 45% smaller testes by 18 months (Fig. 2E). We also considered whether Acsl6−/− males had reduced action of the hypothalamic–pituitary–gonadal axis. We found no significant differences in serum testosterone between Acsl6−/− (184.86 ± 74.12 pg/ml) and control (127.86 ± 44.87 pg/ml) males.
Figure 2.
ACSL6 deficiency causes severe male subfertility. A and B, immunostaining was done on testes from control (A) and Acsl6−/− (B) mice. ACSL6 is in green, TRA98 labels all germ cells red, and DNA is in blue. C, levels of mRNAs for the Acsl6 paralogs Acsl1, Acsl3, Acsl4 and Acsl5 as assessed by qRT-PCR. D, the numbers of pups from a 2-month breeding trial of six control and six Acsl6−/− males. E, there were significant reductions in Acsl6−/− testis weights at each age examined (6, 12, and 18 months), as well as cauda epididymal sperm counts (at 6 months; n = 7–9). The data represent averages ± S.E., and an asterisk indicates significance at p ≤ 0.05 by Student's t test. Scale bar in A, 50 μm.
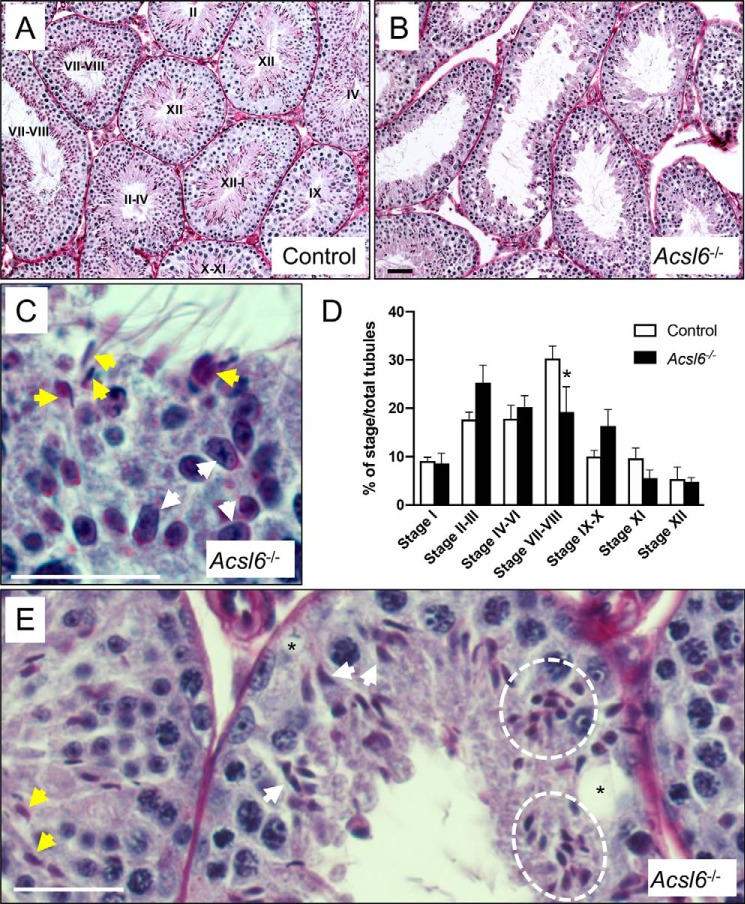
Because spermatogenic cells make up the vast majority of the testicular cell complement, decreased testis weights typically reflect loss of germ cells. Compared with controls (Fig. 3A), seminiferous epithelia of Acsl6−/− tubules were disorganized and contained multiple defects, including missing germ cell layers and vacuolization (Fig. 3, B and D). An obvious example of this disorganization was seen around stage VIII. This is a particularly important stage, at which the following retinoic acid-dependent processes occur: 1) differentiation of spermatogonia; 2) initiation of meiosis; and 3) release of condensed spermatids into the tubule lumina at spermiation (29–31). By stage IX, these critical processes are completed, and no condensed spermatids normally remain in the epithelium. However, Acsl6−/− tubule segments contained both elongated (step 9) and condensed (step 16) spermatids (Fig. 3C), suggesting significant defects in the normal progression of spermatogenesis and delayed spermiation. Acsl6−/− testes also contained ∼10% fewer stage VII–VIII tubules as compared with controls (Fig. 3D). Although some spermatids appeared normal, many had apparent head-shape defects (Fig. 3E).
Figure 3.
ACSL6 deficiency disrupts spermatogenesis. A and B, testis sections from control (A) and Acsl6−/− (B) mice were PAS-stained. Stages are indicated in the lumina of control tubules. C and D, sections from PAS-stained Acsl6−/− testes are enlarged to show detail. C, a tubule at stage IX (because of early elongating spermatids, white arrows) also erroneously contains condensed spermatids that should have already been released at spermiation (yellow arrows). D, seminiferous epithelial stages were quantified in control and Acsl6−/− testes, and there was a significant decline in the numbers of stages VII–VIII. E, tubules contain normal-appearing condensing spermatids (yellow arrows), as well as individual (white arrows), and clusters (white dashed circle) of misshapen spermatid heads, as well as vacuoles (asterisks). The data represent averages ± S.E., and an asterisk indicates significance at p ≤ 0.05 by Student's t test. Scale bars in B–D, 60 μm.
Because Acsl6−/− mice had smaller testes with apparent loss of germ cells, reduced sperm counts, and fewer stage VII–VIII tubules, we quantified cell types to assess when defects originated by immunostaining control and Acsl6−/− testes using antibodies against established markers of somatic and germ cell types. Although there were no differences in Sertoli cells (GATA4+; Fig. 4, A, B, and I), there was a significant decrease in undifferentiated spermatogonia (ZBTB16+; Fig. 4, C, D, and I). There was no significant difference in differentiating spermatogonia (KIT+; Fig. 4, E, F, and I) between genotypes, but Acsl6−/− testes contained significantly fewer preleptotene spermatocytes (RHOX13+ and in stages VII–VIII, Fig. 4G–H, I) and round spermatids (readily identified by morphology; Fig. 4I). Taken together, these results reveal that Acsl6−/− testes exhibited multiple spermatogenesis defects, from reduced numbers of premeiotic, meiotic, and postmeiotic germ cells to stage disruption, abnormal spermatid head shape, and delayed spermiation.
Figure 4.
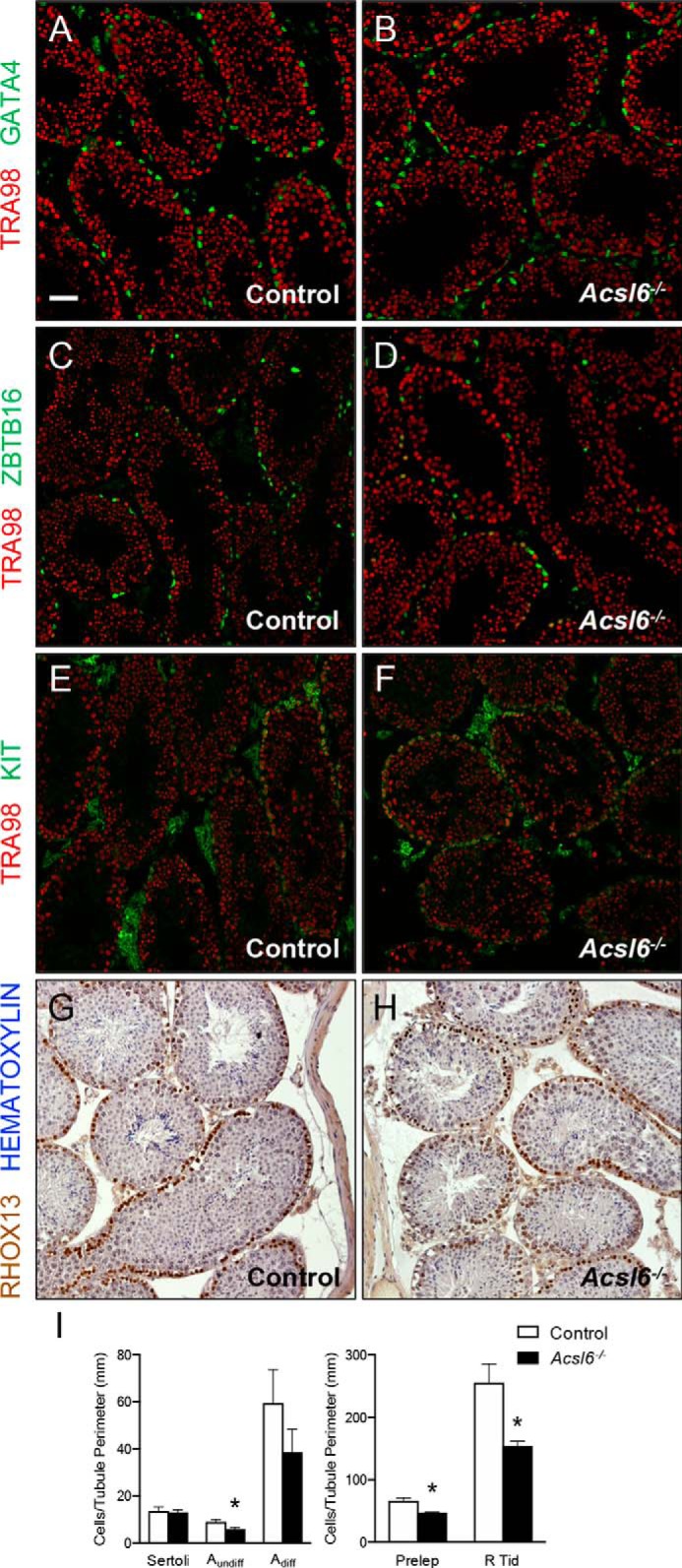
Adult Acsl6−/− testes have significantly reduced numbers of germ cells at multiple stages of spermatogenesis. A–H, immunostaining was done to detect protein markers of specific somatic and germ cell types, and all germ cells are TRA98+ (red in cryosections, A–F). A and B, Sertoli cells are GATA4+ (green). C and D, undifferentiated type A spermatogonia are ZBTB16+ (green). E and F, differentiating spermatogonia are KIT+ (green). G and H, immunohistochemistry was done on Bouin's fixed paraffin sections, and preleptotene spermatocytes are RHOX13++ (brown) and in stages VII–VIII; sections are counterstained with hematoxylin (blue). I, quantification of germ cell numbers from six control and six Acsl6−/− mice. The data represent averages ± S.E., and asterisks indicate significance at p ≤ 0.05 by Student's t test. Scale bar in A, 60 μm.
ACSL6 deficiency in the testis reduces membrane DHA and increases AA
The Acsl6 locus encodes several characterized splice variants that differ by alternative transcriptional start site usage (1a, 1b, or 1c) and alternative splicing-mediated exon inclusion, encoding distinct gate domains (Y or F) containing the catalytic site for fatty acid substrate binding (Fig. S3). These two gate domains have distinct substrate preferences, with the Y-gate domain strongly preferring DHA as substrate (32, 33). To identify which variant was predominantly expressed in the germ line, we amplified the alternative exons from RNAs isolated from brain, whole testis, and pachytene spermatocytes. Although all variants were present in brain and both the Y- and F-gate domains were detected in whole testis, spermatocytes only expressed exon 1a and the DHA-preferring Y-gate domain, which was sequence-verified (Fig. S3).
To determine how the loss of ACSL6 altered the fatty acid profile of testis, we performed targeted total fatty acid profiling by GC-MS/MS. Compared with controls, testes from Acsl6−/− mice fed a DHA-free diet had significant differences in several high-abundance highly unsaturated fatty acids: 20% increase in AA, 18% reduction in DPAn6, and 25% reduction in DHA (Table 1). In addition, several low-abundance fatty acid species were significantly different: 8% increase in 18:1n7, 23% increase in 20:3n6, and a 38% decrease in 26:5n6 (Table S1). These data confirm the requirement for ACSL6 in enrichment of the C22 highly unsaturated fatty acids, DHA, and DPA, in the testes and revealed that C20:4n6 AA and its precursor 20:3n6 were increased.
Table 1.
ACSL6 deficiency decreases total DHA and DPAn6 and increase AA
The percentage of total testis fatty acids (%, w:w) in control and Acsl6−/− 6-month-old testes (n = 6). Only fatty acids above 2% of total fatty acids are shown. The data represent averages ± S.E.
| Fatty acid | Common name | Control | Acsl6−/− |
|---|---|---|---|
| 16:0 | Palmitic acid | 25.8 ± 0.86 | 27.9 ± 0.28a |
| 18:0 | Stearic acid | 7.4 ± 0.21 | 8.1 ± 0.38 |
| 18:1n9 | Oleic acid | 8.3 ± 0.37 | 9.3 ± 0.22 |
| 18:1n7 | Vaccenic acid | 2.1 ± 0.03 | 2.2 ± 0.04a |
| 18:2n6 | Linoleic acid | 2.7 ± 0.58 | 3.0 ± 0.29 |
| 20:4n6 | AA | 13.3 ± 0.20 | 15.8 ± 0.66a |
| 22:5n6 | DPA | 16.7 ± 0.27 | 13.7 ± 0.82a |
| 22:6n3 | DHA | 8.6 ± 0.19 | 6.5 ± 0.40a |
| 24:5n6 | Tetracosapentaenoic acid | 2.8 ± 0.15 | 2.6 ± 0.06 |
a p ≤ 0.05 by Student's t test.
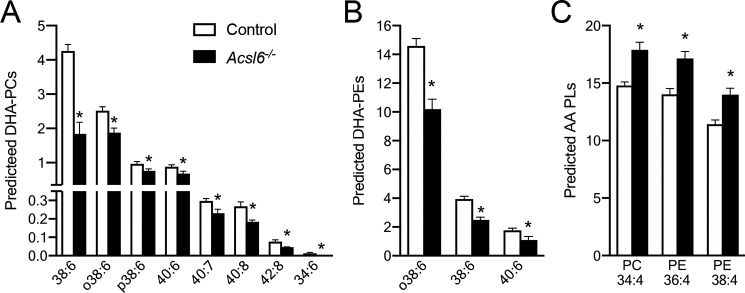
To determine which complex lipid species were affected by these changes in fatty acid content, we performed unbiased lipidomic profiling by direct inject multiple reaction monitoring. Levels of DHA-containing species of the two most abundant phospholipids in testes (phosphatidylcholine (PC) and phosphatidylethanolamine (PE)) exhibited ∼40% reductions in Acsl6−/− testes (Fig. 5, A and B). Reciprocally, the most abundant predicted AA-containing PC and PE species were increased ∼20% (Fig. 5C). The highly saturated phospholipid species, which are generally low in overall abundance, were either unchanged or slightly decreased in Acsl6−/− testes (Fig. S4, A and B). Between control and Acsl6−/− mice, the lipid profiles were broadly unchanged of phosphatidylserine, cholesteryl esters, and the predicted DHA-containing phosphatidylglycerol (PG) species (Fig. S4, C and D). However, the two most abundant PG species were significantly altered by loss of ACSL6, with a 40% increase in PG 34:1 and a 33% decrease in PG 32:0 (Fig. S4E). The two most abundant phosphatidylinositol (PI) species were unchanged between genotypes, although several predicted DHA-containing PI species were reduced in Acsl6−/− testes (Fig. S4F). Together, these data support the concept that ACSL6 preferentially activates DHA in testes for incorporation into phospholipids, particularly PC, PE, and PI.
Figure 5.
Acsl6 deficiency decreases DHA phospholipids in testes. A–C, lipid profile of predicted DHA-containing PCs (A) and PEs (B) and AA-containing PC and PEs (C) in 6-month-old control and Acsl6−/− testes, expressed as percentages of ion intensity distribution within the phospholipid class (n = 6). The data represent averages ± S.E., and asterisks indicate statistical significance at p ≤ 0.05, as assessed by Student's t test.
ACSL6 deficiency disrupts lipid distribution across the testes
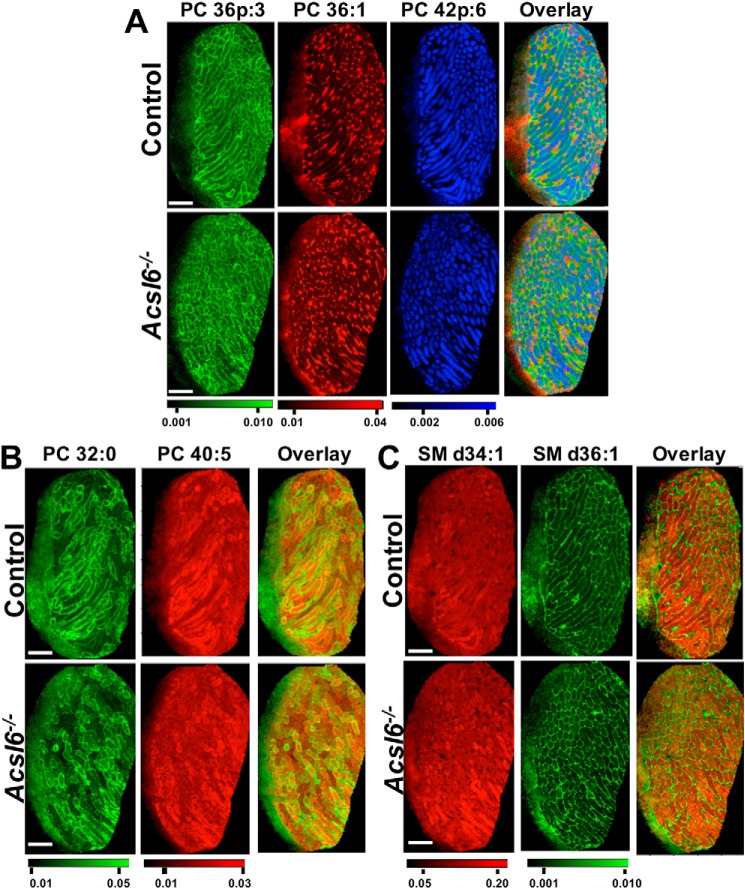
Spermatogenesis proceeds asynchronously in the mammalian testis, and as a result, individual tubule segments contain somatic cells along with a complex and dynamic cohort of germ cells at various points in development. This high level of heterogeneity has prevented the use of traditional methods to assign specific lipid species to specific compartments within the context of normal spermatogenesis. To overcome this bottleneck, we performed MALDI lipid imaging to visualize lipid species-specific patterns across the intact testis using freshly frozen slices. In both control and Acsl6−/− testes, results clearly depicted several specific and consistent patterns of lipid distribution across the testes. For example, PC 36p:3 was concentrated near the basal aspect of the tubules, where spermatogonia and early primary spermatocytes as well as somatic peritubular myoid cells reside, along with the base of somatic Sertoli cells (Fig. 6A). PC 36:1 was exclusively found in the interstitium, which contains the vasculature, as well as somatic Leydig cells and macrophages (Fig. 6A). In contrast, PC 42p:6 was restricted within seminiferous tubules, which contain Sertoli and mitotic spermatogonia, meiotic spermatids, and haploid spermatids (Fig. 6A). Unique lipid distribution was also observed between individual tubules, as demonstrated by PC 32:0 and PC 40:5 (Fig. 6B). Sphingomyelin (SM) d34:1 was enriched within seminiferous tubules, whereas d36:1 and all other SM imaged (d40:1, d42:2, and d42:1, not shown) were enriched in the interstitium (Fig. 6C). Overall, these data demonstrate localization of distinct lipid species to distinct testis compartments, suggesting selective regulation of lipid activation and incorporation into complex lipids in the testis.
Figure 6.
Distinct lipid species are asymmetrically distributed in the testis. Lipid imaging by MALDI on control or Acsl6−/− testes reveal distinct lipid species distribution patterns for PC 36p:3 m/z = 806.546 [M+K]+; PC 36:1 m/z = 826.5723 [M+K]+; and PC 42p:6 m/z = 884.593 [M+K]+ (A); PC 32:0 m/z = 772.5253, [M+K]+; PC40:5 m/z = 874.5723, [M+K]+ (B); and SM d34:1 m/z = 741.5307, [M+K]+; SM d36:1 m/z = 769.562, [M+K]+ (C) in control and Acsl6−/− testes. Scale bars, 1 mm.
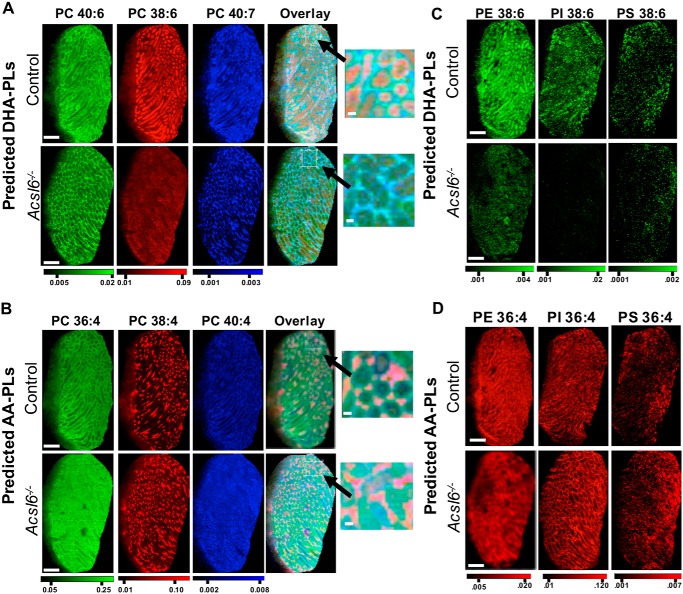
When comparing MALDI images of control and Acsl6−/− testes, several strikingly distinct lipid distribution patterns emerged. Specifically, in Acsl6−/− testes, DHA-containing phospholipid species PC 40:7 (18:1/22:6), PC 40:6 (18:0/22:6), and PC 38:6 (16:0/22:6) were reduced in abundance, as expected. However, these lipids were also redistributed from tubules to the interstitium (Fig. 7A). The predicted AA-containing phospholipid species PC 40:4 (20:0/20:4), PC 36:4 (16:0/20:4), and PC 38:4 (18:0/20:4) were increased in abundance, as expected; however, these lipids were redistributed and seen as diffuse signals across the testes, with abnormally high enrichment in tubules (Fig. 7B). The altered signal intensity and seminiferous tubule exclusion or inclusion of predicted DHA and AA phospholipids, respectively, were also observed for PE (36:4 and 38:6), PI (36:4 and 38:6), and PS (36:4 and 38:6) (Fig. 7, C and D). In agreement with reduced levels of DPAn6 (Table 1), the predicted DPA-containing PC 40:5 (Fig. 6B) (and PC 38:5, PE 40:5; data not shown) had similar distribution patterns but lower signals in Acsl6−/− testes. These data demonstrate that although quantitative analyses revealed reduced DHA and increased AA in Acsl6−/− tubules, lipid imaging enabled the differential localization of these lipids. Overall, testes lacking ACSL6 had a shift in lipid organization, with an increase in AA species but a decrease in DHA species within the germ cell–containing seminiferous tubules.
Figure 7.
ACSL6-deficient testes exhibit altered DHA and AA abundance and localization. Redistribution of predicted DHA-containing PCs (PC 40:6 m/z = 872.5566, [M+K]+; PC 38:6 m/z = 844.5253 [M+K]+; PC 40:7 m/z = 870.541 [M+K]+; overlay and inset, A), and predicted AA-containing PCs (PC 36:4 m/z = 820.5253 [M+K]+; PC 38:4 m/z = 848.5566 [M+K]+; PC 40:4 m/z = 876.5879 [M+K]+; overlay and inset, B), and predicted DHA-(38:6) (C) or AA-(36:4)–containing PE, PI, and PS (PE, m/z = 816.43425, [M-H+2K]+ and m/z = 840.43425, [M-H+2K]+; PI m/z = 857.5186, [M-H]−; and m/z = 881.5186, [M-H]−; and PS m/z = 782.4978, [M-H]− and m/z = 806.4978, [M-H]− respectively) (D), because of ACSL6 deficiency. Scale bars, 1 mm; zoom scale bars, 100 μm.
Discussion
Mechanisms enriching DHA in testes and its effects on mammalian spermatogenesis and male fertility (3, 8, 10) remain unresolved. To begin to define mechanisms by which testes is enriched with DHA, we developed a novel and diet-independent genetic mouse model of DHA deficiency by whole-body deletion of Acsl6 (acyl-CoA synthetase 6), which encodes an enzyme essential for cellular DHA metabolism. Here, we defined the requirement for ACSL6 in normal spermatogenesis and male fertility, characterized the pleiotropic defects in the severely subfertile Acsl6−/− male mice, and performed high resolution lipidomics and lipid mapping to define the changes in testis lipid composition. Our results reveal DHA conjugation to CoA by ACSL6 is critical for enriching germ cells with DHA and that ACSL6 is required for male fertility.
Based on single-cell transcriptome and smFISH results, Acsl6 mRNAs were predominantly in spermatocytes and round spermatids. However, ACSL6 protein did not become readily detectable until spermatid elongation, suggesting post-transcriptional control regulates protein levels. There are numerous examples of such delays in protein accumulation during spermatogenesis, especially affecting transcripts expressed early during meiosis whose encoded proteins are required later in spermatids during spermiogenesis. RNA-binding proteins such as TSN/TBRBP (translin), YBX2/MSY2 (Y box protein 2), and PTBP2 (polypyrimidine tract-binding protein) are active in spermatocytes and regulate transcript stability, alternative splicing, and translational efficiency (34–39).
We detected abundant ACSL6 protein in step 9–11 elongating spermatids, localized specifically to the caudal postacrosomal region of the nucleus. This region is the site of the tubulin-rich manchette, a transient structure proposed to play significant roles in nuclear reshaping and transport of essential cargoes down the length of the developing flagellum (27, 28). The mechanisms underlying the requirement of ACSL6-mediated lipid metabolism for this process are unknown, but possible roles include increasing membrane fluidity during spermatid head shaping, increasing membrane flexibility of the beating flagellum, serving as a docking/anchor site for membrane–protein interactions, and/or driving membrane compartment formation, budding, and transport of cargo-carrying vesicles. Alternative mechanisms may occur via the bioactive signaling regulated by ω-6 and ω-3 fatty acid derivatives (40, 41). For instance, AA is released from membrane phospholipids and enzymatically converted into pro-inflammatory signaling molecules such as prostaglandins, leukotrienes, and thromboxanes. Conversely, DHA is released from membrane phospholipids and converted into anti-inflammatory signaling molecules such as maresins, resolvinsDs, and protectins. The role of these mediators in testes biology has remained unexplored. An additional class of lipid signaling molecules, lipoamines (42, 43), are known to impact both Sertoli cell biology and sperm function (44, 45). In the germ cell, the role of lipoamines remains unclear, despite the identification of all of the endocannabinoid system components in isolated germ cells (46). In summary, the role of fatty acids as bioactive molecules in mediating spermatogenesis is unexplored and requires further investigation.
Previous studies have clearly demonstrated that low DHA levels are strongly correlated with male infertility (8–10, 47). Knockout (KO) mice have been created for other genes encoding enzymes involved in DHA synthesis (FADS2, ELOVL2) or for inserting DHA into phospholipids (AGPAT3/LPAAT3). These KO mice exhibited a wide range of significant fertility defects, including 1) Elovl2 KO = absence of haploid spermatids; 2) Fads2 KO = inability of spermatids to elongate (48, 49); and 3) Agpat3/Lpaat3 KO = reduced sperm counts, abnormal sperm with retained cytoplasm (50). Of interest, infertility in Fads2 KO mice was completely rescued with dietary DHA supplementation (3).
We recently reported that ACSL6 is a critical enzyme in the central nervous system (CNS) for FA activation and DHA enrichment (16, 17). Interestingly, although the testis and CNS are particularly enriched for DHA, the DHA-containing phospholipid species in the testis and CNS are strikingly distinct, suggesting unique lipid metabolic demands and regulation. From the compilation of lipidomics data presented in this report, we have additional predictions about ACSL6 action. For example, the loss of ACSL6 had the greatest impact on the abundance of predicted DHA- and AA-containing phospholipids with saturated fatty acids (palmitate C16:0 and stearate C18:0) in the sn-1 position. These data suggest ACSL6 works in concert with acyltransferases (AGPATs/LPAATs) that have chiral preferences for lysophosphatidic acids containing long-chain saturated fatty acids in the sn-1 position. Additionally, a clear majority of the predicted DHA-plasmalogen phospholipids were unchanged in abundance and distribution between control and Acsl6−/− testes, suggesting that ACSL6 does not interact with plasmalogen-forming acyltransferases. The identity of acyltransferases that ACSL6 provides substrate for, or potentially interacts with, and during which phospholipid metabolic pathway (e.g. de novo synthesis or remodeling) remain unknown. However, the preferential enrichment of DHA into membrane phospholipids has been demonstrated by AGPAT3 activity, which is highly expressed in testes and preferentially ligates DHA-CoA as its substrate onto the glycerol backbone during phospholipid synthesis (50). The generation of a DHA-CoA by the action of ACS is a prerequisite for AGPAT3 activity, perhaps suggesting coordinate action of AGPAT3 and ACSL6 in the testis. Our lipid imaging results revealed differential distribution of DHA-containing lipids within individual tubules in control mice, where several stages of spermatogenesis would be represented. Thus, it appears that DHA incorporation into germ cells, as well as ACSL6 expression, occurs in a stage-dependent manner. When and for what reason ACSL6-mediated DHA regulation varies during the progression of spermatogenesis remain to be resolved. The loss of the DHA and DPAn6 in Acsl6−/−germ cell membranes appears to be largely replaced by AA. Although DPAn6 is reduced in Acsl6−/− testes, DPAn6 has been shown to be dispensable for spermatogenesis, suggesting that the shift in non-DPA fatty acids is related to infertility in Acsl6−/−mice.
Our lipid mapping using testis cryosections from Acsl6−/− and control mice provide the first spatial data demonstrating phospholipid localization across the mouse testis. In addition to our discovery that ACSL6 activates DHA/DPA for incorporation and/or retention in the testis, we provide evidence of differential distribution of DHA within the testes in an ACSL6-dependent manner. Specifically, our lipid imaging data demonstrate a lack of several predicted DHA-containing species within the seminiferous tubules of Acsl6−/− mice, which contain meiotic and postmeiotic male germ cells. Our data also reveal several DHA species that are highly abundant in the interstitium of Acsl6−/− testes. Thus, we predict that the ACSL6 variant expressed in Leydig cells contains the non–DHA-preferring F-gate domain, and therefore ACSL6 deficiency does not limit DHA metabolism in the interstitium.
Our results demonstrate, for the first time, that ACSL6 is essential for activation of DHA for incorporation into testis phospholipids and for normal spermatogenesis and male fertility. Specifically, ACSL6 produces DHA-containing phospholipids within the seminiferous tubules, which may play roles in regulating membrane formation, fluidity, and/or signaling. The expression of ACSL6 protein in elongating spermatids suggests that the critical morphogenetic transitions during spermiogenesis have the highest requirements for ACSL6-mediated fatty acid metabolism.
Experimental procedures
Animals
All animal procedures were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the Purdue Animal Care and Use Committee (approval A3231-01), East Carolina University (approval A3469-01), and the University of Texas at San Antonio (approval A3592-01). The animals were maintained under conditions of ad libitum food and water with constant light–dark cycles. Female Acsl6−/− mice were normal and fertile, similar to controls. Control and Acsl6−/− mice were maintained on chow diet with soy oil as essential fatty acid source (Teklad Global 18% protein rodent diet; Envigo). The Acsl6flox/flox mice were bred to CMV-Cre (Jackson Laboratories catalog no. 006054) transgenic mice to generate ubiquitous Cre-mediated whole-body Acsl6−/− mice, as recently described (16). Male WT or floxed littermates lacking Cre were used as controls.
Tissue preparation, histological staining, cellular isolations, and serum testosterone quantitation
Collected testes were fixed overnight in either Bouin's fixative or 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm using standard methods, as described previously (51). Slides from Bouin's-fixed testes stained with hematoxylin and eosin or periodic acid–Schiff (PAS), using standard methods. Brightfield microscopic images were captured using an Axio Observer A1 microscope equipped with an Axiocam 503 color digital camera (Carl Zeiss Microscopy). Pachytene spermatocytes were isolated from testis single-cell suspensions by gravity sedimentation through a BSA gradient (Sta-Put) to fractionate based on their large cell size, as described previously (52), and purity was determined to be >90% for each preparation. Cauda epididymides were minced and passed through a pipette tip in 1× PBS at room temperature, and cauda epididymal sperm counts were determined by counting on a hemocytometer after dilution in water. Serum testosterone levels were quantified from a 1:20 dilution of plasma from Acsl6−/− and control mice using a testosterone ELISA kit (catalog no. ADI-900-065, Enzo Life Sciences) based on the manufacturer's instructions.
RNA isolation, transcript variant RT-PCR, and qRT-PCR
Total RNA was isolated from whole testes or isolated pachytene spermatocytes using TRIzol reagent (Thermo Fisher Scientific) following the manufacturer's protocol, and cDNA was synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Acsl6 variant-specific primers were designed for RT-PCR based on NCBI reference sequences for Acsl6 mRNA variant 1 (NM_144823.4), variant 2 (NM_001033597.1), variant 3 (NM_001033598.1), and variant 4 (NM_001033599.1). Product amplification was performed using 94 °C for 1 min followed by 30 cycles of 98 °C for 30 s and 68 °C for 3 min. Amplicons were separated and visualized by agarose gel electrophoresis; DNA from excised bands was purified using the NucleoSpin PCR Clean-up kit (Takara). Amplicons were sequenced by Sanger dideoxy sequencing (Eurofin Genomics) to identify the amplified products. For qRT-PCR, total tissue RNA was extracted using TRIzol (Life Technologies), purified (PureLink RNA mini kit; Life Technologies), quantified by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and used to synthesize cDNA with high-capacity cDNA reverse trancriptase (Applied Biosystems). Real-time PCR was performed using SYBR Green Master Mix (Bio-Rad) and primers for the target genes and analyzed using a StepOnePlus real-time PCR system thermocycler (Applied Biosystems). Gene expression was normalized to the average Ct values of the housekeeping gene Rpl22 and expressed as 2−ΔCt.
Immunostaining and smFISH
Indirect immunofluorescence was done as described previously (53). Briefly, testes were fixed overnight in 4% paraformaldehyde, washed in 1× PBS, and then incubated overnight in 30% sucrose, all at 4 °C. Testes were embedded in OCT, frozen, and cut into 5-μm sections. The sections were incubated with primary antibodies for 1 h at room temperature. Primary antibody was omitted in negative controls. Following stringency washes, the sections were incubated with secondary antibodies for 1 h at room temperature. Nonspecific serum was used in lieu of primary antibody as an additional negative control (Fig. S2), and images from secondary antibody-only staining were used to set exposure threshold for every technical replication. See Table 1 for antibody sources and dilutions. Coverslips were mounted with Vectastain containing 4′,6′-diamino-2-phenylindole (Vector Laboratories), and images were captured using a Fluoview FV1000 confocal laser-scanning microscope (Olympus America). Photomicrographs were analyzed using ImageJ software (54).
Germ cell populations were identified using stage-specific markers of spermatogenesis. Sections of adult testes were stained with antibodies against ZBTB16 (R&D Systems AF2944, 1:1000) to detect undifferentiated spermatogonia, KIT (Cell Signaling Technology 3074, 1:1000) to detect differentiated spermatogonia, RHOX13 (55) (1:500) to detect preleptotene spermatocytes, and TRA98 (Abcam ab82427, 1:1000) to label all germ cells. Round spermatids were identified based on position within seminiferous tubules and chromatin appearance. Apoptotic germ cells were identified as cells positive for both the pan-germ cell marker, TRA98, and apoptotic marker, cleaved poly(ADP-ribose) polymerase 1 (Cell Signaling Technology, 9544). Male germ cells were counted from photomicrographs using ImageJ software to manually outline seminiferous tubules and count individual germ cells. The number of marker-positive germ cells per tubule perimeter was quantified to determine each germ cell population per seminiferous tubule. Immunostaining using an antibody against ACSL6 (Sigma HPA040470, 1:200) was conducted on adult testis sections.
smFISH was performed using the RNAscope Multiplex fluorescent version assay (Advanced Cell Diagnostics to detect Acsl6 (Advanced Cell Diagnostics catalog no. 584161), Ddx4 (Advanced Cell Diagnostics catalog no. 572461-c3), and Sox9 (Advanced Cell Diagnostics catalog no. 401051-c2) transcripts on fixed frozen tissue sections from adult C57BL/6 mice. Testes were fixed in 4% paraformaldehyde at 4 °C, washed in a graded sucrose series (10%, 20%, 30%), embedded in OCT, and sectioned (8 μm). In situ hybridization detection was performed essentially according to manufacturer recommendations using Opal dyes and 4′,6′-diamino-2-phenylindole counterstain. Probes targeting Polr2a (polymerase RNA II DNA-directed polypeptide A), Ppib (peptidylprolyl isomerase B/cyclophilin B), and Ubc (ubiquitin C) mRNA were used as positive controls (Advanced Cell Diagnostics catalog no. 320881), whereas a probe targeting Escherichia coli 4-hydroxy-tetrahydrodipicolinate reductase (dapB) mRNA was used as a negative control (Advanced Cell Diagnostics catalog no. 320871). Fluorescently labeled sections were imaged with 20×/0.8NA and 63×/1.4NA objectives using an AxioImager M1 and an AxioCam MRm (Carl Zeiss Microscopy).
Lipidomics
Total fatty acid composition was determined on extracted and fatty acid methyl esters (FAMEs) prepared using a one-step extraction/methylation method as described in detail elsewhere (56). Testes samples of ∼10 mg were treated with an aqueous solution (CH3OH:2,2-dimethoxypropane:H2SO4, 8.5:1.1:0.4, v:v:v) and an organic solution (heptane:toluene, 6.3:3.7, v:v) at 80 °C for 2 h. FAMEs were reconstituted in heptane and stored at 20 °C until analysis. FAMEs were positively identified by high resolution capillary GC covalent adduct chemical ionization tandem MS with GCMS TQ8050 triple quadrupole mass spectrometer equipped with a prototype solvent assisted chemical ionization device interfaced to a GC QP2010Plus gas chromatograph (Shimadzu Scientific Instruments). FAME were quantified using a separate QP2010 GC with a flame ionization detector. Response factors were measured daily using an external standard and applied to peak areas to yield calibrated weight percentages (57).
Broad multiple reaction monitoring–based lipid profiling was performed with Purdue's Bindley Metabolite Profiling Facility, as described (16). Briefly, lipids were extracted from tissues using the Bligh and Dyer method (58). The lipid phase was dried, resuspended, and injected through a microautosampler (G1377A) into a QQQ6410 triple quadrupole mass spectrometer (Agilent Technologies) operated in the positive ion mode and equipped with Jet stream electrospray ionization ion source. The data were analyzed by calculating the percent distribution for each ion (ion peak of m/z intensity/total ion intensity) and identified based on LipidMaps database.
MALDI lipid imaging of flash frozen testes sliced 10-μm thick was performed by the Structural Biology Core of National Institute on Drug Abuse Intramural Research Program using Thermo Scientific MALDI LTQ-XL-Orbitrap (Thermo Fisher Scientific) and Xcalibur software in positive and negative ion mode with a mass resolution of 60,000 in the mass range of 600–1000 Da. The raster step size of 40 μm for both the X and Y directions. DHB matrix (at 40 mg/ml concentration in 70% methanol) is sprayed using a Tissue MALDI sprayer (HTX Technologies). Assignment of lipid species identity is based upon accurate mass with mass error of ≤2 ppm in positive ion mode and ≤3.5 ppm in negative ion mode.
Statistics
Statistical analyses were performed using Student's t test, and the level of significance was set at p < 0.05.
Author contributions
B. J. H., R. F. F., S. Q. K., V. D. D., S. N. J., L. L., J. T. B., and B. P. H. data curation; B. J. H., R. F. F., J. T. B., B. P. H., C. B. G., and J. M. E. formal analysis; B. J. H., C. B. G., and J. M. E. writing-original draft; J. T. B. visualization; B. P. H. investigation; C. B. G. and J. M. E. conceptualization; C. B. G. and J. M. E. supervision; C. B. G. and J. M. E. project administration; C. B. G. and J. M. E. writing-review and editing.
Supplementary Material
Acknowledgments
We thank Joani Zary-Oswald for technical assistance and Dr. Shinnosuke Suzuki for assistance visualizing existing single-cell transcriptome data.
This work was supported in part by NICHD, National Institutes of Health Grant R01-HD090083 (to C. B. G.) and R01-HD090007 (to B. P. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Table S1 and Figs. S1–S4.
- AA
- arachidonic acid
- DHA
- docosahexaenoic acid
- DPA
- docosapentaenoic acid
- ACS
- acyl-CoA synthetase
- Pn
- postnatal day n
- smFISH
- single-molecule RNA fluorescence in situ hybridization
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- SM
- sphingomyelin
- KO
- knockout
- CNS
- central nervous system
- PAS
- periodic acid–Schiff
- qRT-PCR
- quantitative RT-PCR
- FAME
- fatty acid methyl ester.
References
- 1. Crawford M. A., Casperd N. M., and Sinclair A. J. (1976) The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comp. Biochem. Physiol. B 54, 395–401 10.1016/0305-0491(76)90264-9 [DOI] [PubMed] [Google Scholar]
- 2. Crawford M. A., Broadhurst C. L., Guest M., Nagar A., Wang Y., Ghebremeskel K., and Schmidt W. F. (2013) A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot. Essent. Fatty Acids 88, 5–13 10.1016/j.plefa.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 3. Roqueta-Rivera M., Stroud C. K., Haschek W. M., Akare S. J., Segre M., Brush R. S., Agbaga M.-P., Anderson R. E., Hess R. A., and Nakamura M. T. (2010) Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 51, 360–367 10.1194/jlr.M001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simopoulos A. P. (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 60, 502–507 10.1016/j.biopha.2006.07.080 [DOI] [PubMed] [Google Scholar]
- 5. Calder P. C. (2016) Docosahexaenoic Acid. Ann. Nutr. Metab. 69, 7–21 [DOI] [PubMed] [Google Scholar]
- 6. Papanikolaou Y., Brooks J., Reider C., Fulgoni V. L. 3rd (2014) Adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 13, 31 10.1186/1475-2891-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castellano C. A., Audet I., Laforest J. P., Matte J. J., and Suh M. (2011) Fish oil diets alter the phospholipid balance, fatty acid composition, and steroid hormone concentrations in testes of adult pigs. Theriogenology 76, 1134–1145 10.1016/j.theriogenology.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 8. Esmaeili V., Shahverdi A. H., Moghadasian M. H., and Alizadeh A. R. (2015) Dietary fatty acids affect semen quality: a review. Andrology 3, 450–461 10.1111/andr.12024 [DOI] [PubMed] [Google Scholar]
- 9. Lorenzetti A., Marotta F., Yadav H., Celep G., Minelli E., Carrera-Bastos P., Jain S., Polimeni A., and Solimene U. (2012) Improving sperm quality and spermatogenesis through a bioactive marine compound: an experimental study. Acta Biomed. 83, 108–113 [PubMed] [Google Scholar]
- 10. Suh M., Merrells K. J., Dick A., and Taylor C. G. (2011) Testes of obese rats are highly responsive to n-3 long-chain fatty acids. Br. J. Nutr. 106, 1005–1012 10.1017/S0007114511001292 [DOI] [PubMed] [Google Scholar]
- 11. Wang D. D., Wu F., Wen M., Ding L., Du L., Xue C. H., Xu J., and Wang Y. M. (2018) Replenishment of docosahexaenoic acid (DHA) in dietary n-3-deficient mice fed DHA in triglycerides or phosphatidylcholines after weaning. J. Food Sci. 83, 481–488 10.1111/1750-3841.14017 [DOI] [PubMed] [Google Scholar]
- 12. Watkins P. A., Maiguel D., Jia Z., and Pevsner J. (2007) Evidence for 26 distinct acyl-CoA synthetase genes in the human genome. J. Lipid Res. 48, 2736–2750 10.1194/jlr.M700378-JLR200 [DOI] [PubMed] [Google Scholar]
- 13. Ellis J. M., Frahm J. L., Li L. O., and Coleman R. A. (2010) Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 21, 212–217 10.1097/MOL.0b013e32833884bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis J. M., Bowman C. E., and Wolfgang M. J. (2015) Metabolic and tissue-specific regulation of acyl-CoA metabolism. PLoS One 10, e0116587 10.1371/journal.pone.0116587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coleman R. A. (2019) It takes a village: channeling fatty acid metabolism and triacylglycerol formation via protein interactomes. J. Lipid Res. 60, 490–497 10.1194/jlr.S091843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez R. F., Kim S. Q., Zhao Y., Foguth R. M., Weera M. M., Counihan J. L., Nomura D. K., Chester J. A., Cannon J. R., and Ellis J. M. (2018) Acyl-CoA synthetase 6 enriches the neuroprotective omega-3 fatty acid DHA in the brain. Proc. Natl. Acad. Sci. U.S.A. 115, 12525–12530 10.1073/pnas.1807958115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chouinard-Watkins R., and Bazinet R. P. (2018) ACSL6 is critical for maintaining brain DHA levels. Proc. Natl. Acad. Sci. U.S.A. 115, 12343–12345 10.1073/pnas.1817557115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grive K. J., Hu Y., Shu E., Grimson A., Elemento O., Grenier J. K., and Cohen P. E. (2019) Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLOS Genet. 15, e1007810 10.1371/journal.pgen.1007810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Green C. D., Ma Q., Manske G. L., Shami A. N., Zheng X., Marini S., Moritz L., Sultan C., Gurczynski S. J., Moore B. B., Tallquist M. D., Li J. Z., and Hammoud S. S. (2018) A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-Seq. Dev. Cell 46, 651–667.e10 10.1016/j.devcel.2018.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sohni A., Tan K., Song H. W., Burow D., de Rooij D. G., Laurent L., Hsieh T. C., Rabah R., Hammoud S. S., Vicini E., and Wilkinson M. F. (2019) The neonatal and adult human testis defined at the single-cell level. Cell Rep. 26, 1501–1517.e4 10.1016/j.celrep.2019.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hermann B. P., Cheng K., Singh A., Roa-De La Cruz L., Mutoji K. N., Chen I. C., Gildersleeve H., Lehle J. D., Mayo M., Westernströer B., Law N. C., Oatley M. J., Velte E. K., Niedenberger B. A., Fritze D., et al. (2018) The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep. 25, 1650–1667.e8 10.1016/j.celrep.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meistrich M. L., and Hess R. A. (2013) Assessment of spermatogenesis through staging of seminiferous tubules. In Spermatogenesis: Methods and Protocols (Carrell D. T., and Aston K. I., eds) pp. 299–307, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 23. Ahmed E. A., and de Rooij D. G. (2009) Staging of mouse seminiferous tubule cross-sections. In Meiosis: Cytological Methods (Keeney S., ed) Vol. 2, pp. 263–277, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 24. Muciaccia B., Boitani C., Berloco B. P., Nudo F., Spadetta G., Stefanini M., de Rooij D. G., and Vicini E. (2013) Novel stage classification of human spermatogenesis based on acrosome development. Biol. Reprod. 89, 60 [DOI] [PubMed] [Google Scholar]
- 25. Clermont Y., and Leblond C. P. (1955) Spermiogenesis of man, monkey, ram and other mammals as shown by the periodic acid–Schiff technique. Am. J. Anat. 96, 229–253 10.1002/aja.1000960203 [DOI] [PubMed] [Google Scholar]
- 26. Sakai Y., and Yamashina S. (1989) Mechanism for the removal of residual cytoplasm from spermatids during mouse spermiogenesis. Anat. Rec. 223, 43–48 10.1002/ar.1092230107 [DOI] [PubMed] [Google Scholar]
- 27. Lehti M. S., and Sironen A. (2016) Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151, R43–R54 10.1530/REP-15-0310 [DOI] [PubMed] [Google Scholar]
- 28. Kierszenbaum A. L. (2001) Spermatid manchette: plugging proteins to zero into the sperm tail. Mol. Reprod. Dev. 59, 347–349 10.1002/mrd.1040 [DOI] [PubMed] [Google Scholar]
- 29. Endo T., Freinkman E., de Rooij D. G., and Page D. C. (2017) Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. U.S.A. 114, E10132–E10141 10.1073/pnas.1710837114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griswold M. D. (2016) Spermatogenesis: the commitment to meiosis. Physiol. Rev. 96, 1–17 10.1152/physrev.00013.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teletin M., Vernet N., Yu J., Klopfenstein M., Jones J. W., Féret B., Kane M. A., Ghyselinck N. B., and Mark M. (2019) Two functionally redundant sources of retinoic acid secure spermatogonia differentiation in the seminiferous epithelium. Development 146, dev170225 10.1242/dev.170225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klett E. L., Chen S., Yechoor A., Lih F. B., and Coleman R. A. (2017) Long-chain acyl-CoA synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids. J. Lipid Res. 58, 884–894 10.1194/jlr.M072512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Horn C. G., Caviglia J. M., Li L. O., Wang S., Granger D. A., and Coleman R. A. (2005) Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry 44, 1635–1642 10.1021/bi047721l [DOI] [PubMed] [Google Scholar]
- 34. Yang J., Morales C. R., Medvedev S., Schultz R. M., and Hecht N. B. (2007) In the absence of the mouse DNA/RNA-binding protein MSY2, messenger RNA instability leads to spermatogenic arrest1. Biol. Reprod. 76, 48–54 10.1095/biolreprod.106.055095 [DOI] [PubMed] [Google Scholar]
- 35. Yang S., and Hecht N. B. (2004) Translin associated protein X is essential for cellular proliferation. FEBS Lett. 576, 221–225 10.1016/j.febslet.2004.08.082 [DOI] [PubMed] [Google Scholar]
- 36. Yang J., Medvedev S., Yu J., Tang L. C., Agno J. E., Matzuk M. M., Schultz R. M., and Hecht N. B. (2005) Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc. Natl. Acad. Sci. U.S.A. 102, 5755–5760 10.1073/pnas.0408718102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang J., Medvedev S., Reddi P. P., Schultz R. M., and Hecht N. B. (2005) The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc. Natl. Acad. Sci. U.S.A. 102, 1513–1518 10.1073/pnas.0404685102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu M., and Hecht N. B. (2007) Polypyrimidine tract binding protein 2 stabilizes phosphoglycerate kinase 2 mRNA in murine male germ cells by binding to its 3′UTR1. Biol. Reprod. 76, 1025–1033 10.1095/biolreprod.107.060079 [DOI] [PubMed] [Google Scholar]
- 39. Xu M., and Hecht N. B. (2011) Polypyrimidine tract-binding protein 2 binds to selective, intronic messenger RNA and microRNA targets in the mouse testis. Biol. Reprod. 84, 435–439 10.1095/biolreprod.110.087114 [DOI] [PubMed] [Google Scholar]
- 40. Serhan C. N. (2017) Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 31, 1273–1288 10.1096/fj.201601222R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 10.1038/nature13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toguri J. T., Leishman E., Szczesniak A. M., Laprairie R. B., Oehler O., Straiker A. J., Kelly M. E. M., and Bradshaw H. B. (2018) Inflammation and CB2 signaling drive novel changes in the ocular lipidome and regulate immune cell activity in the eye. Prostaglandins Other Lipid Mediat. 139, 54–62 10.1016/j.prostaglandins.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 43. Piscitelli F., and Bradshaw H. B. (2017) Endocannabinoid analytical methodologies: techniques that drive discoveries that drive techniques. Adv. Pharmacol. 80, 1–30 10.1016/bs.apha.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 44. Miller M. R., Mannowetz N., Iavarone A. T., Safavi R., Gracheva E. O., Smith J. F., Hill R. Z., Bautista D. M., Kirichok Y., and Lishko P. V. (2016) Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555–559 10.1126/science.aad6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Battista N., Meccariello R., Cobellis G., Fasano S., Di Tommaso M., Pirazzi V., Konje J. C., Pierantoni R., and Maccarrone M. (2012) The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 355, 1–14 10.1016/j.mce.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 46. Grimaldi P., Orlando P., Di Siena S., Lolicato F., Petrosino S., Bisogno T., Geremia R., De Petrocellis L., and Di Marzo V. (2009) The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 11131–11136 10.1073/pnas.0812789106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zalata A. A., Christophe A. B., Depuydt C. E., Schoonjans F., and Comhaire F. H. (1998) The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol. Hum. Reprod. 4, 111–118 10.1093/molehr/4.2.111 [DOI] [PubMed] [Google Scholar]
- 48. Zadravec D., Tvrdik P., Guillou H., Haslam R., Kobayashi T., Napier J. A., Capecchi M. R., and Jacobsson A. (2011) ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 52, 245–255 10.1194/jlr.M011346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stroud C. K., Nara T. Y., Roqueta-Rivera M., Radlowski E. C., Lawrence P., Zhang Y., Cho B. H., Segre M., Hess R. A., Brenna J. T., Haschek W. M., and Nakamura M. T. (2009) Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J. Lipid Res. 50, 1870–1880 10.1194/jlr.M900039-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iizuka-Hishikawa Y., Hishikawa D., Sasaki J., Takubo K., Goto M., Nagata K., Nakanishi H., Shindou H., Okamura T., Ito C., Toshimori K., Sasaki T., and Shimizu T. (2017) Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 292, 12065–12076 10.1074/jbc.M117.791277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niedenberger B. A., Busada J. T., and Geyer C. B. (2015) Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction 149, 329–338 10.1530/REP-14-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bryant J. M., Meyer-Ficca M. L., Dang V. M., Berger S. L., and Meyer R. G. (2013) Separation of spermatogenic cell types using STA-PUT velocity sedimentation. J Vis Exp. e50648 10.3791/50648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niedenberger B. A., and Geyer C. B. (2018) Advanced immunostaining approaches to study early male germ cell development. Stem Cell Res. 27, 162–168 10.1016/j.scr.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abramoff M., Magalhães P., and Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 55. Geyer C. B., and Eddy E. M. (2008) Identification and characterization of Rhox13, a novel X-linked mouse homeobox gene. Gene 423, 194–200 10.1016/j.gene.2008.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou Y., Nijland M., Miller M., Ford S., Nathanielsz P. W., and Brenna J. T. (2008) The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids 43, 525–531 10.1007/s11745-008-3186-1 [DOI] [PubMed] [Google Scholar]
- 57. Brenna J. T. (2013) Fatty acid analysis by high resolution gas chromatography and mass spectrometry for clinical and experimental applications. Curr. Opin. Clin. Nutr. Metab. Care 16, 548–554 10.1097/MCO.0b013e328363bc0a [DOI] [PubMed] [Google Scholar]
- 58. Bligh E. G., and Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37, 911–917 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.