Abstract
Autophagy plays multiple roles in host cells challenged with extracellular pathogens. Here, we aimed to explore whether autophagy inhibition could prevent bacterial infections. We first confirmed widely distinct patterns of autophagy responses in host cells infected with Staphylococcus aureus, as compared with Salmonella. Only infection with Staphylococcus produced strong accumulation of lipidated autophagy-related protein LC3B (LC3B-II). Infection with virulent Staphylococcus strains induced formation of p62-positive aggregates, suggestive of accumulated ubiquitinated targets. During Salmonella infection, bacteria remain enclosed by lysosomal-associated membrane protein 2 (LAMP2)-positive lysosomes, whereas virulent Staphylococcus apparently exited from enlarged lysosomes and invaded the cytoplasm. Surprisingly, Staphylococcus appeared to escape from the lysosome without generation of membrane-damage signals as detected by galectin-3 recruitment. In contrast, Salmonella infection produced high levels of lysosomal damage, consistent with a downstream antibacterial xenophagy response. Finally, we studied the Unc-51–like autophagy-activating kinase 1 (ULK1) regulatory complex, including the essential subunit autophagy-related protein 13 (ATG13). Infection of cells with either Staphylococcus or Salmonella led to recruitment of ATG13 to sites of cytosolic bacterial cells to promote autophagosome formation. Of note, genetic targeting of ATG13 suppressed autophagy and the ability of Staphylococcus to infect and kill host cells. Two different ULK1 inhibitors also prevented Staphylococcus intracellular replication and host cell death. Interestingly, inhibition of the ULK1 pathway had the opposite effect on Salmonella, sensitizing cells to the infection. Our results suggest that ULK1 inhibitors may offer a potential strategy to impede cellular infection by S. aureus.
Keywords: autophagy, Staphylococcus aureus (S. aureus), Salmonella enterica, infection, bacterial pathogenesis, autophagy-related protein 13 (ATG13), bacterial virulence, intracellular pathogen, Unc-51-like autophagy-activating kinase 1 (ULK1), xenophagy
Introduction
Macro-autophagy (often referred to simply as autophagy) is an intracellular degradation pathway that functions as a starvation-dependent metabolic response but also as a critical housekeeping mechanism to eliminate damaged organelles (1). A third important set of pathways relates to roles of autophagy for host cell–pathogen interactions during bacterial infection. In this way, autophagy has been shown to degrade and restrict the replication of certain types of invading bacteria, leading to the initial definition of the dedicated pathway, xenophagy (foreign-eating) (2–6).
The xenophagy response following Salmonella infection has particularly been well-investigated. Following invasion of host cells, Gram-negative Salmonella enterica promote membrane remodeling that enables the bacteria to reside within specialized Salmonella-containing vacuoles (SCV).3 Bacteria can then take one of several fates depending on cell context and environmental factors. Because of SCV damage, some bacteria escape to the cytoplasm and are recognized by ubiquitination systems regulated by host E3 ligases such as leucine-rich repeat and sterile α-motif–containing 1 (LRSAM1) (7), linear ubiquitin chain assembly complex (LUBAC), and Ariadne RBR E3 ubiquitin protein ligase 1 (ARIH1) (8, 9). The concerted action of these pathways produce mixed linear and branched ubiquitin chains (10–12) that serve as primary eat-me signals to recruit ubiquitin-binding adaptor proteins (13, 14). A number of xenophagy adaptor proteins have been identified, including the proto-typical family member p62/sequestosome 1 (SQSTM1) (15), which functions during Salmonella infection together with other adaptors such as nuclear dot protein 52 kDa (NDP52, also called CALCOCO2) (16–18) and optineurin (15, 18–20). An additional atypical adaptor protein, Tax1-binding protein 1 (TAX1BP1), further supports xenophagy of Salmonella (21). Together, these adaptors form complexes that bridge ubiquitin-coated bacteria to autophagy-related protein 8 (ATG8) family members such as LC3 on autophagy elongation membranes (15, 22, 23). In this way, cytosolic Salmonella are captured into autophagosomes for transport to lysosomal compartments, where they are effectively neutralized.
In contrast to xenophagy, other types of bacteria, including Gram-positive Staphylococcus aureus, have evolved to subvert and exploit the autophagy pathway to support their replicative life cycle. Methicillin-resistant S. aureus (MRSA) now encompasses a wide collection of strains that have evolved over the last 60 years to become broadly insensitive to β-lactam antibiotics, including penicillin and amoxicillin (24). MRSA is still one of the leading causes of nosocomial infections with a wide range of targets from skin wounds to internal soft tissues. Although S. aureus was initially considered an extracellular pathogen, it is now appreciated that these bacteria can survive after internalization into professional phagocytes (e.g. macrophages and neutrophils) and nonprofessional (nonphagocytic) cells (e.g. osteoclasts and fibroblasts) (25). In vivo, the fraction of S. aureus that persists intracellularly gains protection from further antibiotics to eventually escape and spread bacteria beyond the initial site of infection (26). As such, the intracellular pool of S. aureus could be a significant underlying contributor toward chronic or recurrent infection.
Although anti-bacterial xenophagy during Salmonella infection has been extensively characterized, there are relatively fewer studies on S. aureus and the roles of autophagy. During Staphylococcus infection, bacteria internalize via phagocytosis to enter an endosomal compartment that is initially Rab5-positive and subsequently Rab7-positive (27, 28). Although still controversial, evidence indicates that staphylococci utilize a number of virulence systems to prevent full activation of the phagolysosomal degradative compartment to enable survival (25). Virulent strains of S. aureus express multiple factors, including α-hemolysin and phenol-soluble modulins, that mediate endosome remodeling, membrane disruption, and eventual bacterial escape into the cytoplasm, particularly in nonphagocytic cell types (29–31). At this stage, free cytosolic S. aureus or bacteria within damaged phagosomes are captured by autophagosomal membranes. Once within autophagosomes, Staphylococcus virulence factors are proposed to further inhibit fusion with lysosomes or acidification of the autolysosome to generate a permissive membrane-enclosed niche for bacterial replication (28, 32). The importance of this autophagy-dependent niche was highlighted by evidence of inhibited S. aureus infection in mouse embryonic fibroblasts lacking autophagy protein ATG5 (28). However, the role of autophagy during S. aureus infection across different host cell and strain contexts is not well-understood. One report has suggested that autophagosomes transport Staphylococcus to acidic lysosomal compartments for degradation (33). Other evidence has suggested that Staphylococcus replication does not require autophagy and targeting of bacteria via a ubiquitin-dependent xenophagy pathway (34).
Here, we investigated details of the autophagy–S. aureus interaction because better understanding in this area could have potential medical applications. Using nonphagocytic cell hosts, we found that MRSA infection led to strong markers of autophagy activation. Staphylococcus could be detected replicating inside lysosomal-like niche compartments but with minimal levels of membrane damage. MRSA infection also led to strong accumulation of ubiquitin-associated aggregates, but these did not localize directly around bacteria. In a parallel investigation, we found that Salmonella infection generated distinct patterns of remodeling in the host cell autophagy–lysosomal pathway. Moreover, we found that the ability of MRSA to infect and kill nonphagocytic cells was highly dependent upon autophagy. Inhibition of the canonical autophagy ULK1 regulatory kinase complex was sufficient to completely block infection and restore viability to host cells. Our results therefore identify an autophagy kinase pathway that can be targeted by small molecules to suppress cellular infection by MRSA.
Results
Activation of autophagy following infection by S. aureus
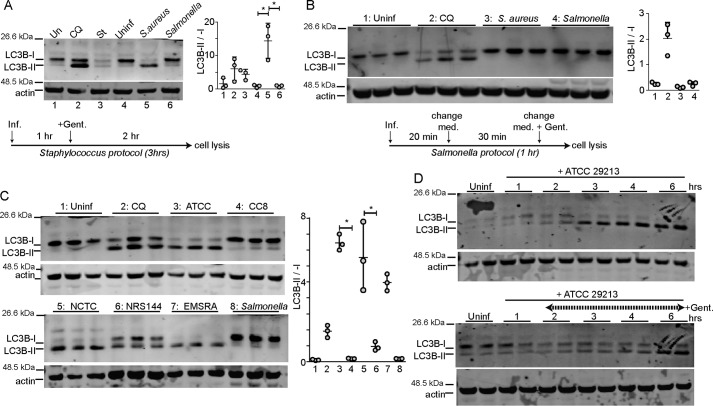
Our goal was to first study how different bacterial pathogens activate the autophagy pathway. For this test, autophagy was monitored by Western blotting for the prototypical ATG8 family member, LC3B. Normally, LC3 is synthesized and cleaved during autophagy to generate mature LC3-I, which is lipidated during autophagy activation to generate the LC3-II form that associates with autophagosomes (35). In initial experiments, we compared S. aureus (ATCC29213) and S. enterica sv. typhimurium (NCTC13347) as common reference strains used for xenophagy studies (2, 28). HeLa cells were infected with S. aureus following a previously described protocol (28). To allow direct comparison, cells were infected with S. enterica sv. typhimurium by the same method. Briefly, both pathogens were grown until OD = 0.3 and then were added to infect cells at m.o.i. 200 for 3 h (Fig. 1A). To compare xenophagy responses with standard autophagy, parallel cell samples were treated as controls with either amino acid starvation or with the autophagy/lysosome inhibitor chloroquine. Interestingly, the strongest levels of LC3B-II accumulation and the highest LC3B lipidation ratios were detected when cells were infected with ATCC29213. Incubation of HeLa cells with chloroquine to block the autophagy/lysosomal pathway also led to accumulation of LC3B-II formed under basal levels of autophagy activation. In contrast, during a typical starvation response, LC3 is activated to form LC3-II, which is then quickly degraded via the lysosome reflecting high levels of autophagic degradative flux. The high LC3-II/LC3-I ratios observed following S. aureus infection therefore suggest a combination of LC3 activation along with inhibition of degradation.
Figure 1.
Activation of autophagy in HeLa cells following invasion of S. aureus and S. enterica sv. typhimurium. HeLa cells were infected (Inf.) with S. aureus (ATCC29213) or S. enterica sv. typhimurium (NCTC13347) using two different protocols (see “Experimental procedures”). A, using the Staphylococcus protocol, bacteria were grown until OD = 0.3 and used to infect cells at 200 m.o.i. One hour post-infection, gentamicin (Gent.) (0.05 mg/ml) was added to inactivate extracellular bacteria, and the infection proceeded for a further 2 h. As control, cells were treated with EBSS (amino acid and serum starvation) or chloroquine (CQ) 25 μm and incubated for 3 h. Cell lysates were resolved by gel electrophoresis, and proteins were probed with anti-LC3B antibody. Activation of autophagy was measured via ratio of LC3B-II/LC3B-I. Average from n = 3 experiments ± S.D. is shown. *, p < 0.001 by ANOVA and Tukey's post test. Uninf., uninfected. B, using the Salmonella protocol, bacteria were grown until OD = 1.2–1.5 and then diluted 1:100. This diluted culture was used to infect cells for 20 min and then changed to fresh cell media (Med.) containing gentamycin. Infected (or control autophagy-stimulated) cells were lysed after 1 h and analyzed for LC3B lipidation. The average from n = 3 samples ± S.D. is shown. A and B, repeated three times on different days. C, HeLa cells were infected with S. aureus strains (ATCC29213, clonal complex 8 isolate (CC8), NCTC8325, NRS144 agr-mutant, or EMRSA LF78) or S. enterica sv. typhimurium (n = 3). Cells were infected at 200 m.o.i. One hour post-infection, gentamycin was added, and cells were further incubated for 3 h. Average from three samples ± S.D. is shown. Data are representative of two experiments. *, p < 0.001 by ANOVA and Tukey's post test. D, HeLa cells were infected with ATCC29213 at 100 m.o.i. Top, cells were further incubated for up to 6 h. Bottom, gentamicin (0.05 mg/ml) was added 1 h after infection. n = 2 replicates per condition.
Surprisingly, Salmonella infection did not produce similar levels of LC3B-II. To further explore this, we repeated the comparison but followed a Salmonella-optimized xenophagy protocol (see under “Experimental procedures”) (36). However, using this second method, we also could not detect LC3B-II accumulation following infection with Salmonella (Fig. 1B). Overall, xenophagy responses following S. aureus and S. enterica sv. typhimurium infection were dramatically different.
Strain-dependent autophagy induction by S. aureus
We next wanted to examine how the genotype of S. aureus affects host cell autophagy responses. Previous studies have shown that the accessory gene regulator (agr) quorum-sensing virulence system, which controls expression of factors like α-hemolysin, was critical to induce autophagy (28, 32, 33). Here, we compared the experimental S. aureus strains ATCC29213 (WT agr, methicillin-sensitive), NCTC8325 (WT agr, methicillin-resistant), and NRS144 (partial agr-deficient mutant of NCTC8325) (28). In addition, we studied a penicillin-, oxicillin-, and ciprofloxacin-resistant strain isolated from an endotracheal aspirate that we previously characterized to represent clonal complex 8 (CC8) (spa-type t008) (37) and LF78, an epidemic strain of type EMRSA-15 with wide antibiotic resistance (38). The three strains of WT virulent S. aureus (ATCC29213, NCTC8325, and LF78) all strongly induced LC3B-II accumulation following infection of HeLa cells (Fig. 1C). However, agr-mutant NRS144 only led to partial activation, whereas the CC8 strain did not induce any detectable autophagy. These results confirm that the autophagic response depends on a functional agr and factors lacking the CC8 strain.
S. aureus infection generally produced high levels of LC3B-I lipidation and LC3B-II accumulation. Upon time-course analysis, we could detect maximal levels of LCB-II in HeLa cells at 3 h of incubation with 100 m.o.i. bacteria (Fig. 1D). In this experiment, the cell population likely encounters multiple rounds of bacterial invasion, replication, and further infection from new bacterial progeny along with parallel extracellular bacterial replication. To further characterize the dynamics, an additional time course was performed to study autophagy activation due to intracellular events. In this experiment, HeLa cells were incubated with 100 m.o.i. S. aureus for 1 h after which gentamicin was added to inhibit growth of extracellular bacteria. With this protocol, LC3B-II levels peaked after 6 h of infection. Therefore, after 1 h of internalization, at least 5 additional hours are required for bacteria to transit through the initial phagosome and induce membrane damage to produce strong autophagy activation. These results highlight that levels of autophagy can increase depending on duration and extent of infection.
In light of the robust autophagy detected following Staphylococcus infection, we were puzzled by the negligible LC3-II accumulation observed upon Salmonella infection. Multiple studies have characterized the xenophagy defense mechanism for clearing Salmonella (2, 15, 18, 19, 39) We wondered whether poor autophagy activation by Salmonella might explain why LC3 lipidation was not detected. To clarify, HeLa cells were infected with GFP-expressing S. enterica sv. typhimurium (using Salmonella-optimized protocol) and then immunostained with anti-LC3B antibody. Using this imaging assay, we could confirm formation of LC3B(+) membranes elongating around or surrounding bacteria (Fig. 2). To better understand the rate of xenophagy induction, we counted the percentage of cells with autophagy membranes at different times of infection. Although no puncta were detected within 20 min of infection, nearly 90% of cells exposed to Salmonella showed LC3B(+) membranes. The percentage of LC3B(+) cells rapidly decreased between 2 and 4 h of infection suggestive of normal xenophagy flux. These results confirmed that Salmonella was able to infect cells and trigger a rapid protective xenophagy response. The findings highlight that autophagy responses following Staphylococcus versus Salmonella infections were dramatically different. Lipidation and activation of LC3 in lysates were much more pronounced from the cell population following Staphylococcus infection.
Figure 2.
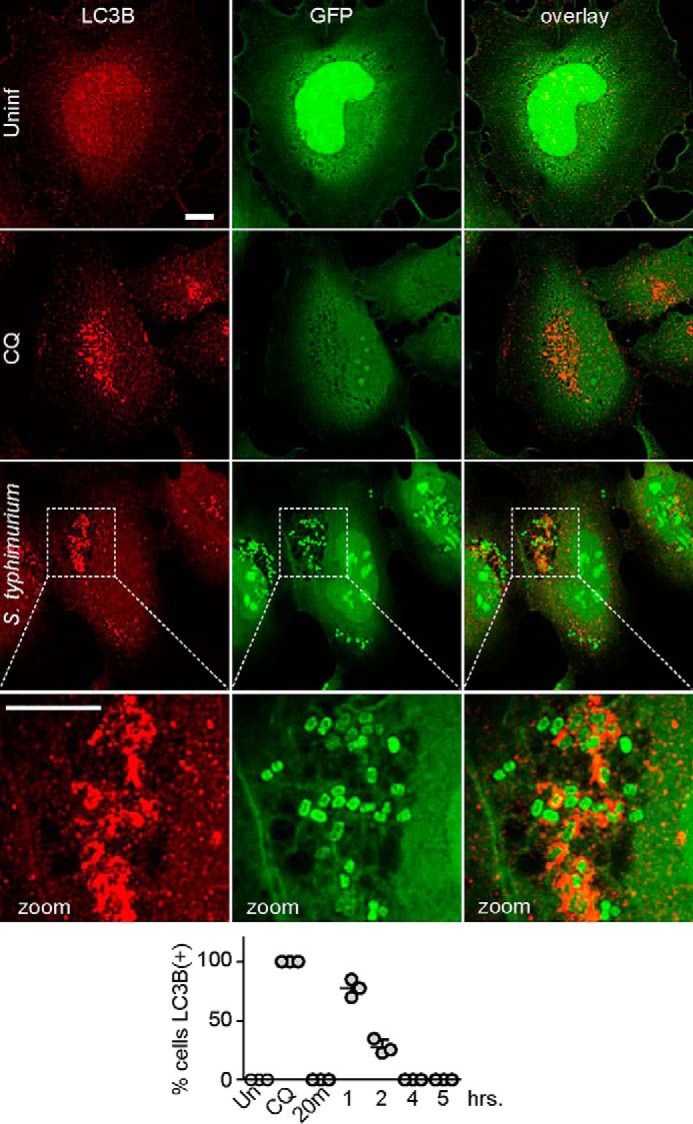
Autophagosome formation following infection by S. enterica sv. typhimurium. HeLa cells were plated on glass coverslips. As control, cells were treated with chloroquine (CQ, 25 μm) for 3 h to cause autophagosome accumulation. Alternatively, cells were infected with 1:100 m.o.i. of GFP-expressing S. enterica sv. typhimurium for 1 h using the Salmonella protocol. After treatments, cells were fixed and stained for LC3B(+) membranes. All scale bars: 10 μm. Zoom: ×3.4 magnification. Bottom, HeLa cells were infected as above with 1:100 m.o.i. GFP–Salmonella and incubated for 20 min and 1–3 or 5 h. Note: except for the 20-min time point, gentamycin was added 50 min after infection. The percentage of cells positive for LC3B puncta was counted (50–100 cells counted per sample). Average from n = 3 samples ± S.D. Uninf., uninfected.
p62/sequestosome1 recruitment during S. aureus infection
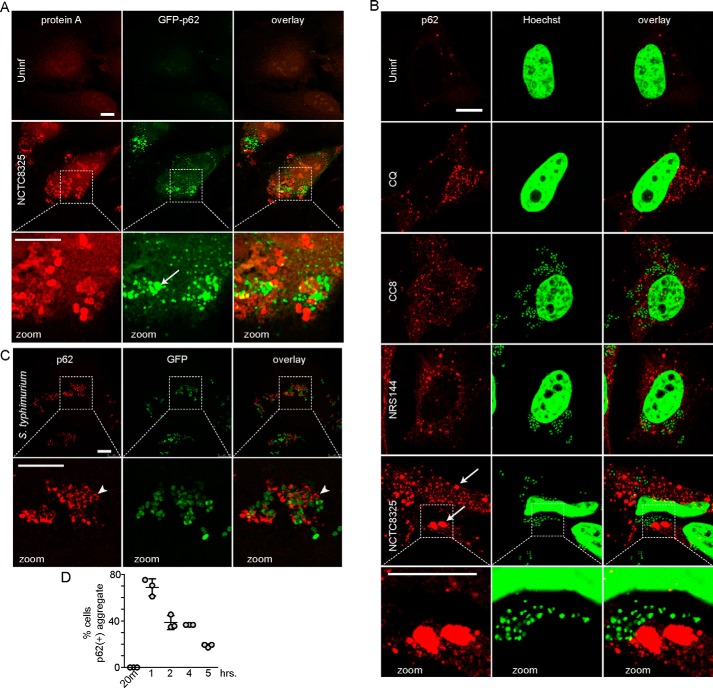
To further investigate interactions with the autophagy pathway, we studied localization of the ubiquitin-binding adaptor, p62/SQSTM1. For this, we infected HeLa cells stably expressing GFP–p62 with NCTC8325 S. aureus, which was detected by staining for protein A (Fig. 3A). One clear effect was the formation of large-sized GFP–p62 structures in cells following infection with NCTC8325. However, although increases in GFP–p62 were obvious, we could not detect clear co-localization between GFP–p62 and invading bacteria. These results suggest induction of ubiquitinated protein aggregates upon infection but raise questions regarding ubiquitin-mediated targeting of Staphylococcus. To clarify, we performed complementary experiments in which HeLa cells were infected with different Staphylococcus strains, and endogenous p62 was detected by immunostaining. In this case, staphylococci were detected using the Hoechst DNA stain (Fig. 3B). With this second approach, we again found that invasion of cells with WT MRSA NCTC8325 led to the formation of large-sized p62 puncta that intermingle, but rarely co-localize, with bacteria. Interestingly, infection with CC8 or agr-mutant NRS144 also caused formation of p62 puncta. However, these p62 puncta were generally of a smaller size range, resembling p62(+) formed after blocking the autophagy/lysosome pathway with chloroquine. Therefore, CC8 and agr-mutant Staphylococcus strains appear to lack key virulence factors required to promote strong accumulation of LC3B-II and p62 aggregates.
Figure 3.
Differential formation of p62/Sequestosome1-positive membranes following infection by Staphylococcus versus Salmonella. A, HeLa/GFP–p62 cells were infected with 100 m.o.i. NCTC8325 for 3 h (gentamicin added after 1st h). After fixation, bacteria were detected by anti-protein A staining. Arrow, p62(+) aggregate. All scale bars, 10 μm. All zoom: ×3.4 magnification. B, HeLa cells were treated with chloroquine as control (CQ, 25 μm) or infected as above with indicated strains of S. aureus. After fixation, cells were stained with antibodies for p62/SQSTM1 and Hoechst 33342 (detects bacterial and host cell DNA). Arrows: large size p62(+) aggregates. C, HeLa cells were infected with 1:100 diluted GFP–S. enterica sv. typhimurium for 1 h before fixation and staining with antibodies for p62/SQSTM1. Arrowhead, co-localization of p62 on Salmonella. D, HeLa cells were infected with GFP–Salmonella as in C for the indicated times before fixation. The percentage of cells positive for p62(+) membranes was counted (40–110 cells counted per sample). Average from n = 3 samples ± S.D. Uninf., uninfected.
For comparison, we studied responses to Salmonella invasion. In this way, we could confirm robust formation of relatively smaller-sized p62(+) structures in cells infected with Salmonella (Fig. 3C). Many of the p62 structures were arranged laterally along bacteria or occasionally completely surrounding bacteria. By 1 h after infection, p62(+) puncta could be detected in over 70% of infected cells, and this percentage reduced over time, further suggesting normal degradative flux (Fig. 3D). Collectively, these results are consistent with p62 serving as an adaptor for targeting ubiquitin-associated Salmonella to the xenophagy pathway. However, following infection with full-virulence MRSA, distinctly enlarged p62 structures were formed, which may represent aggregates of ubiquitinated cellular proteins not associated with bacteria.
Intracellular S. aureus promote noncanonical autophagosome formation
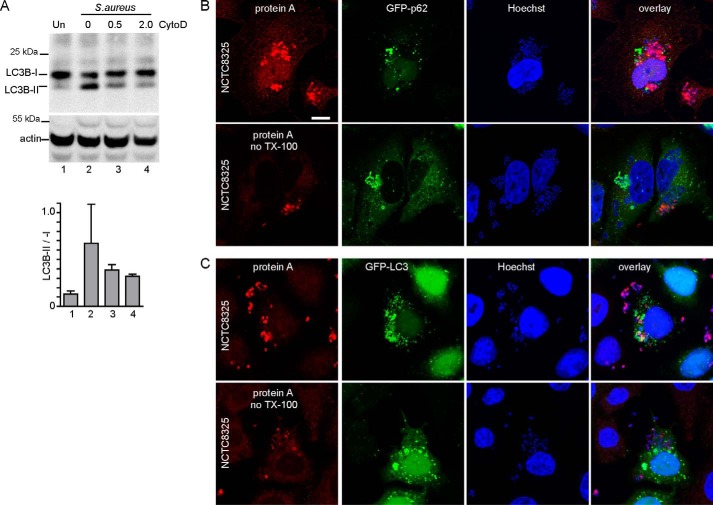
Staphylococcus has long been considered an extracellular pathogen, but the importance of the intracellular niche has become better appreciated (25). Therefore, we aimed to clarify that S. aureus bacteria were promoting autophagosome formation following entry into the cellular endocytic system. Consistent with this notion, Staphylococcus-induced LCB-II formation was reduced when cytochalasin D was included to block bacterial internalization (Fig. 4A) (40, 41). As an alternative approach, we further confirmed that robust detection of intracellular Staphylococcus required permeabilization of cell membranes before anti-protein A staining (Fig. 4, B and C).
Figure 4.
Intracellular Staphylococcus stimulates autophagosome formation. A, HeLa cells were infected with 200 m.o.i. NCTC8325 for 3 h. Where indicated, cell infections included cytochalasin D (CytoD) (micromolar). Activation of autophagy was measured via LC3B-II/LC3B-I. Average from n = 3 experiments ± S.D. B, HeLa/GFP–p62 cells were infected with 100 m.o.i. NCTC8325 for 3 h (gentamicin added after first 1 h of infection). After fixation, cells were stained for protein A and Hoechst 33342. Where indicated, cells were stained without permeabilization by Triton X-100. Scale bar, 10 μm. C, HeLa/GFP-LC3 cells were infected and stained as in B.
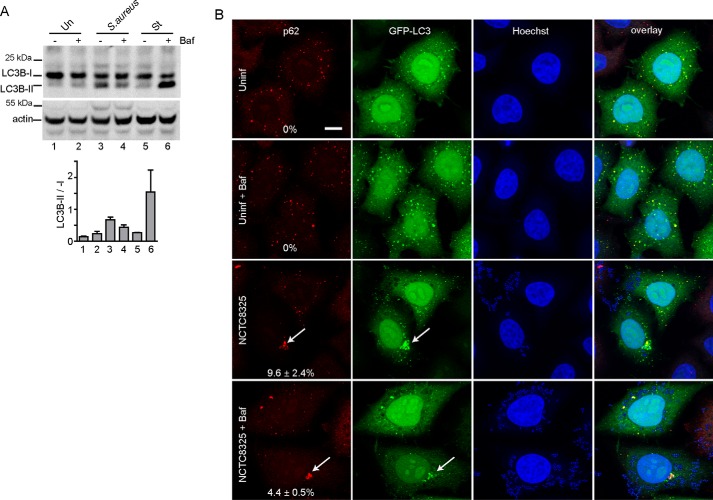
Intracellular Staphylococcus clearly promoted large-sized GFP–p62(+) and GFP–LCB(+) autophagosomes. Our other results on LC3B lipidation (Fig. 1) suggested that Staphylococcus may activate autophagy while decreasing degradative capacity. To clarify, we conducted autophagy flux tests ± the lysosomal inhibitor, bafilomycin A1 (BafA1) (Fig. 5A). We did not detect strong LC3B-II accumulation when BafA1 was added to inhibit the lysosome under basal conditions, consistent with low LC3B lipidation rates under full nutrient levels. In contrast, Staphylococcus infection promoted LC3B-II, suggestive of increased lipidation. Surprisingly, BafA1 suppressed Staphylococcus-dependent LC3B-II, which suggests that normally active lysosomes may be required to support autophagosome formation in this context. As a parallel control, we confirmed that amino acid starvation promoted rapid canonical LC3B-II formation that was highly sensitive to lysosomal inhibition by BafA1.
Figure 5.
Autophagosomes generated following Staphylococcus infection do not show rapid flux. A, HeLa cells were infected with 200 m.o.i. NCTC8325 or alternatively starved in EBSS for 3 h. Where indicated, cell treatments included bafilomycin (Baf) A1 (10 nm). Cell lysates were resolved by gel electrophoresis and probed with anti-LC3B antibody. Activation of autophagy was measured via LC3B-II/LC3B-I. Average from n = 3 experiments ± S.D. B, HeLa/GFP-LC3 cells were infected with 100 m.o.i. NCTC8325 for 3 h (gentamicin added after first 1 h of infection). Where indicated, cell treatments included bafilomycin A1 (10 nm). After fixation, cells were stained with antibodies for p62/SQSTM1 and Hoechst 33342. Scale bar, 10 μm. Arrows, large size p62(+)-GFP-LC3(+) aggregates. Shown are percentage the of cells (+) for large-size autophagosomes (n = 3 samples ± S.D.; 100–250 cells counted per sample). Uninf., uninfected.
We also investigated the relationship between autophagy flux and the formation of large p62 membrane aggregates (Fig. 5B). HeLa cells stably expressing GFP-LC3B showed small-sized membrane structures in the basal full-nutrient state. These small-sized canonical autophagosomes generally contained both GFP–LC3B and p62. Lysosomal inhibition with BafA1 did not lead to accumulation of autophagosomes, consistent with LC3B lipidation data from the parallel experiment above. In contrast, Staphylococcus infection led to large-sized LC3B(+)/p62(+) aggregate structures (in 9.6% of infected cells). Formation of Staphylococcus-dependent LC3B(+)/p62(+) aggregates still occurred when the lysosome was inhibited with BafA1 (although at lower % of cells). Altogether, the data suggest that Staphylococcus infection leads to the production of noncanonical autophagosomes, and this pathway does not reflect lysosomal inhibition.
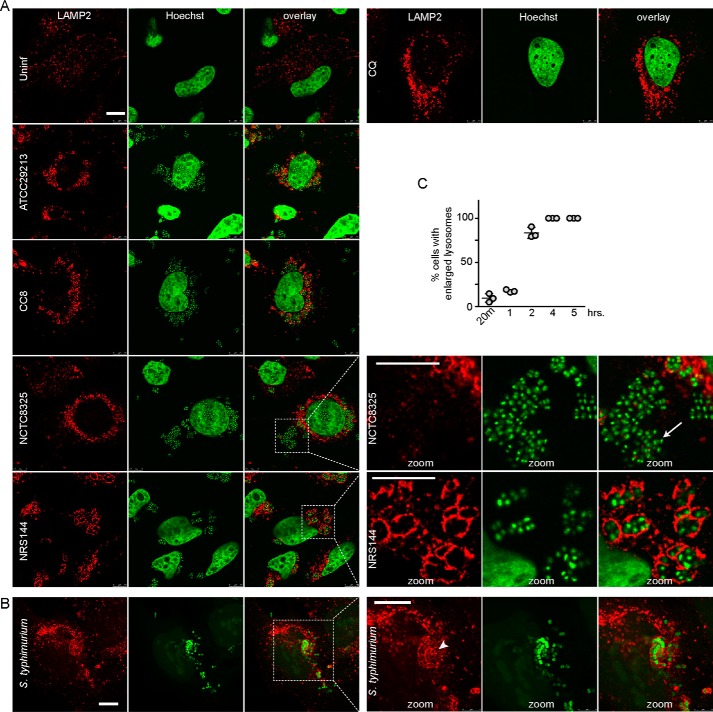
Escape of S. aureus from the lysosome-associated replication niche
Because the autophagy responses toward Staphylococcus versus Salmonella infection were markedly different by LC3B and p62 readouts, we aimed to clarify how these pathogens interacted with lysosomes. HeLa cells in the basal state have small-sized lysosomes strongly staining for LAMP-2 (Fig. 6A). Lysosome number and LAMP-2 staining greatly increased when chloroquine was added to cells to block the autophagy/lysosome pathway. We examined lysosomal localization of S. aureus strains ATCC29213, NCTC8325, NRS144, and CC8 3 h post-infection. Lysosomes became large and swollen following infection with ATCC29213 or NCTC8325 S. aureus. Infection with these WT Staphylococcus strains led to high numbers of bacteria clustered in the cytoplasm with only a small fraction of bacteria enclosed by LAMP-2(+) membranes. In contrast, agr-deficient NRS144 were predominantly enclosed in LAMP-2(+) lysosomes with very few escaping to the cytoplasm. The CC8 strain showed an intermediate result, with fewer free bacteria in the cytosol. These results are consistent with the model in which invading Staphylococcus transit via the phagosome to an enlarged late-endosome/lysosome compartment. Agr-directed virulence factors in WT strains facilitate lysosomal membrane damage and bacterial escape to the cytoplasm, which is associated with LC3B activation and p62(+)-ubiquitinated aggregates. Interestingly, the clinical CC8 MRSA isolate generates only very mild levels of LC3B accumulation, protein ubiquitination, and lysosomal disruption but was able to sustain a minimal level of human infection.
Figure 6.
Escape of virulent strains of S. aureus from swollen lysosomes. A, HeLa cells were treated with chloroquine as control for 3 h (CQ, 25 μm) or infected as in Fig. 3B with different strains of S. aureus (100 m.o.i., 3 h). After fixation, cells were stained with antibodies for LAMP-2 and Hoechst 33342 (detects bacterial and host cell DNA). All scale bars, 10 μm. Arrow, zoomed inset: S. aureus after escape from LAMP-2(+) lysosomes. Zoom: ×3.4 magnification. B, HeLa cells were infected with 1:100 diluted GFP-S. enterica sv. typhimurium. After 5 h, cells were fixed and stained for LAMP-2. Arrowhead, zoomed inset: Salmonella still confined within LAMP-2(+) lysosomes. Zoom: ×1.9 magnification. C, HeLa cells infected with GFP–Salmonella as in B for different times were quantified for percentage of cells containing swollen LAMP-2(+) lysosomes (40–85 cells counted per sample). Average from n = 3 samples ± S.D. Uninf., uninfected.
After characterizing escape of WT S. aureus from degradative lysosomes, we aimed to confirm in parallel Salmonella transit from the lysosomal compartment. In HeLa cells infected with GFP–Salmonella, we could detect intracellular bacteria predominantly enclosed (or in very close proximity) with enlarged LAMP-2(+) lysosomal membranes (Fig. 6B). Consistent with previous reports (2), at early time points after infection (20 min, 1 h post-infection), there was low frequency of distended LAMP-2(+) membranes in Salmonella-infected cells (Fig. 6C). However, by 2 h post-infection, Salmonella infection led to formation of enlarged LAMP-2(+) compartments, which remained strong even 5 h post-infection. Compared with fully virulent Staphylococcus infection, which led to bacteria mostly outside of LAMP-2 membranes, Salmonella were mostly all within LAMP(+) membranes. These observations highlight how trafficking between these two pathogens via lysosomes is markedly different. S. aureus bacteria rely on virulence factors to block transport and prevent lysosomal degradation to promote replication and eventual release into the cytoplasm. Salmonella bacteria are transported to lysosomal compartments within 2 h post-infection, via the xenophagy pathway, facilitating overall degradation of the bacteria.
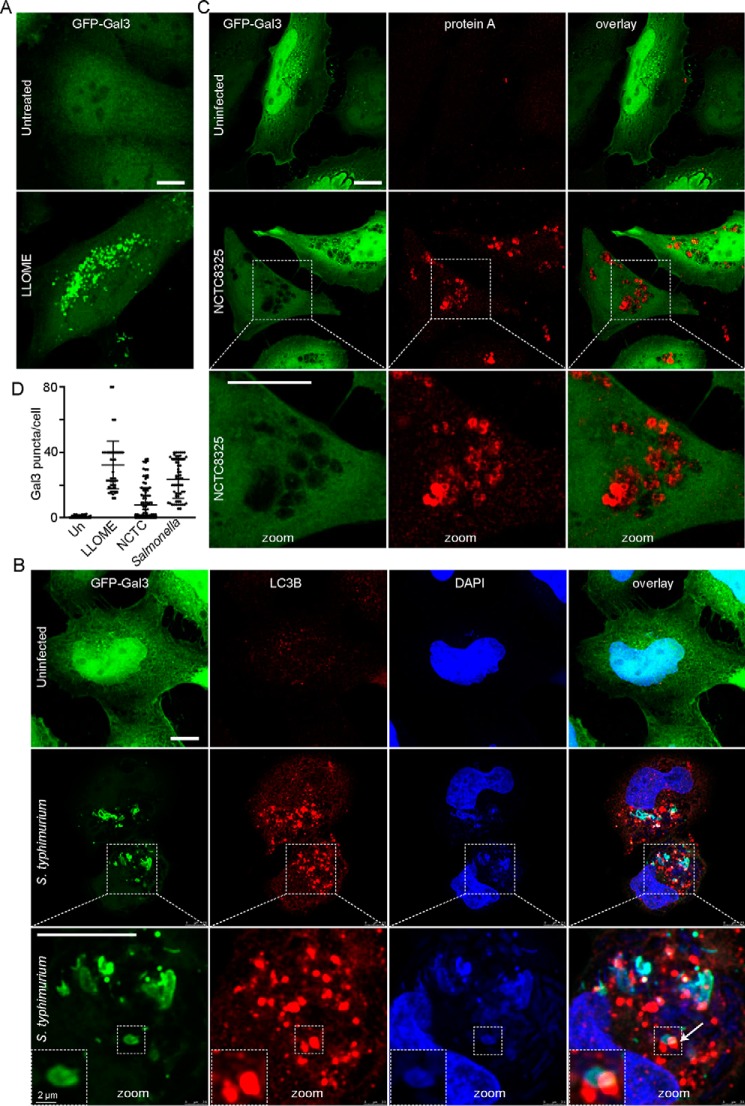
To further explore S. aureus and S. enterica sv. typhimurium interactions with the lysosome, we studied galectin-3 (Gal3) puncta formation. Members of the galectin family of β-galactoside–binding proteins have served as useful tools to detect damage of endomembranes such as lysosomes (42–45). Galectin-8 plays a critical role as a receptor for targeting damaged SCV to the xenophagy pathway (46). As a control, we first confirmed GFP–Gal3 puncta formation in HeLa cells treated with lysosomal stress compound, l-leucyl-l-leucine methyl ester (LLOME) (Fig. 7A). We next tested infection with Salmonella, because these bacteria strongly co-localized with lysosomes within 2–5 h post-infection. Extensive GFP–Gal3 puncta were found in almost all cells infected by S. enterica sv. typhimurium 3 h post-infection (Fig. 7B). Salmonella showed extensive co-localization with GFP–Gal 3. By co-staining, we found clear co-localization of endogenous LC3B(+) membranes on damaged lysosomes containing Salmonella. Therefore, our data are consistent with high levels of membrane damage on SCV to provide a targeting signal for xenophagy. Overall, the LC3B(+) membrane signal in Salmonella-infected cells was even more prominent, going beyond areas of Salmonella or lysosomal damage. Therefore, there is strong activation of autophagosome formation, likely due to reactive oxygen species produced by NADPH oxidases upon Salmonella infection (47).
Figure 7.
S. aureus infection does not generate lysosome damage signals. A and C, HeLa cells were transfected to express GFP–galectin-3 (GFP–Gal3). As control, cells were treated with LLOME (2 mm for 3 h) to induce lysosomal damage and Gal3(+) puncta. C, alternatively, cells were infected with S. aureus NCTC8325 (100 m.o.i., 5 h). All (nonlabeled) scale bars, 10 μm. Zoom: ×3.0 magnification. B, HeLa cells transfected with GFP–Gal 3 were infected with 1:100 diluted S. enterica sv. typhimurium for 3 h (gentamicin added after first 50 min of infection). Cells were fixed and co-stained with LC3B antibody (to detect autophagosomes) and DAPI (to detect Salmonella). Arrow, zoomed insets: LC3B recruitment to sites of Salmonella-induced lysosomal damage marked by Gal3. Zoom: ×3.6 magnification (with further ×2.0 magnification, inset). D, HeLa cells transfected with GFP–Gal 3 were infected with 1:100 diluted S. enterica sv. typhimurium (using Salmonella protocol) or 100 m.o.i. of NCTC8325 (using Staphylococcus protocol) for 5 h (for comparison, positive control cells were treated with LLOME for 3 h). Gal3 puncta were quantified. Average puncta/cells were from n = 50–100 cells from two independent experiments ± S.D.
In contrast, when we performed the parallel Gal3 experiment following MRSA infection, we observed entirely different trends (Fig. 7C). Cells were infected with NCTC8325 for 3 h (a time point with high levels of cytosolic bacteria). After NCTC8325 infection, we surprisingly saw low levels of GFP–Gal 3 puncta, suggesting little lysosome damage. To further evaluate this difference, we quantified GFP–Gal 3 puncta following the same 5-h time frame for Salmonella versus MRSA infection (Fig. 7D). S. enterica sv. typhimurium led to higher levels of lysosomal damage (>50 puncta/cell), similar to treatment with LLOME. These results suggest that MRSA carries virulence factors that allow bacterial replication and escape from lysosomes without high levels of membrane-damage signals. MRSA therefore appears to have mechanisms for evasion of the Gal8 xenophagy-defense pathway.
Host cell death following Staphylococcus and Salmonella infection
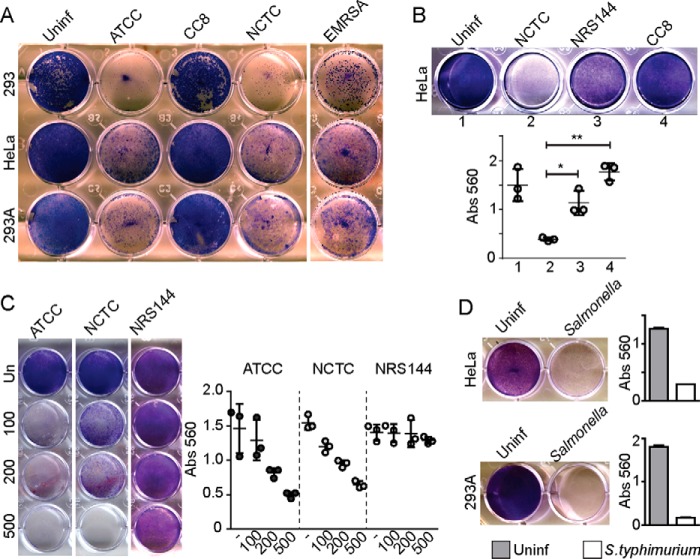
Through action of virulence factors such as α-hemolysin, S. aureus can survive within a replicative niche to then escape into the cytoplasm (28, 32) without signaling membrane damage. According to this model, we next characterized S. aureus strains for efficiency to produce host cell death. We studied two different epithelial cells lines (standard HEK293 as compared with HEK293A, a more adherent subtype) as well as HeLa cells. These three cells types represent well-characterized experimental hosts for bacterial infection and autophagy. Host cells were infected with ATCC29213, CC8, NCTC8325, and EMRSA LF78 S. aureus (Fig. 8A). The three WT-virulent S. aureus types, ATCC29213, NCTC8325, and LF78, were potently cytotoxic for HEK and HeLa cells. In contrast, CC8 S. aureus was noncytotoxic. We further measured cytotoxicity in HeLa cells following infection with MRSA NCTC8325, agr-mutant NRS144, and CC8 S. aureus (Fig. 8B). We found a gradation of cytotoxicity. Surprisingly, CC8 S. aureus was less cytotoxic than NRS144. Testing cytotoxicity at different multiplicities of infection (Fig. 8C), we found that ATCC29213 showed higher cytotoxicity than NCTC8325, whereas NRS144 did not lead to cell killing, even at 500 m.o.i., indicating that this mutant detective strain was completely nonharmful to cells. These data highlight that expression of agr-dependent virulence factors such as α-hemolysin is essential for S. aureus intracellular replication and host cell killing.
Figure 8.
Death of host cells upon infection by Staphylococcus and Salmonella. A, indicated host cell lines were infected with 100 m.o.i. of varying Staphylococcus strains (ATCC29263, CC8, NCTC8325, or EMRSA LF78). After 1 h of infection, gentamicin was added to the media, and cells were further incubated for 72 h before fixation and Giemsa staining of remaining viable cells. B, HeLa cells were infected as indicated with 200 m.o.i. S. aureus for 72 h (gentamycin added after 1st h of infection). Viable cells staining with Giemsa were quantified via absorbance. Average from n = 3 ± S.D. Data are representative of two experiments. **, p < 0.001; *, p < 0.01 by ANOVA and Tukey's post test. C, HeLa cells were infected as indicated with varying multiplicities of infection and quantified as in B. Average is from n = 3 ± S.D. D, HeLa or HEK293A cells were infected with 1:100 diluted S. enterica sv. typhimurium (using Salmonella protocol) and incubated for 72 h before fixation, staining, and quantification. Average is from n = 3 ± S.D. Uninf., uninfected.
Above, we found that Salmonella infection led to extensive lysosomal damage, p62 recruitment, and autophagosome formation consistent with xenophagy activation. Despite this anti-bacterial response, we wished to determine levels of cell death caused by Salmonella infection to complement our studies of MRSA. In macrophages, Salmonella-derived lipopolysaccharide can induce caspase- and TLR4-dependent cell death (48–50). As such, we infected cell hosts HEK and HeLa with S. enterica sv. typhimurium and tested for viable cells that remained 72 h post-infection. We found that Salmonella infection led to potent killing of both HEK and HeLa cells (Fig. 8D). Therefore, nonphagocytic cells are also susceptible to cytotoxic products produced from Salmonella infection.
Recruitment of the ULK1 complex to invading bacteria
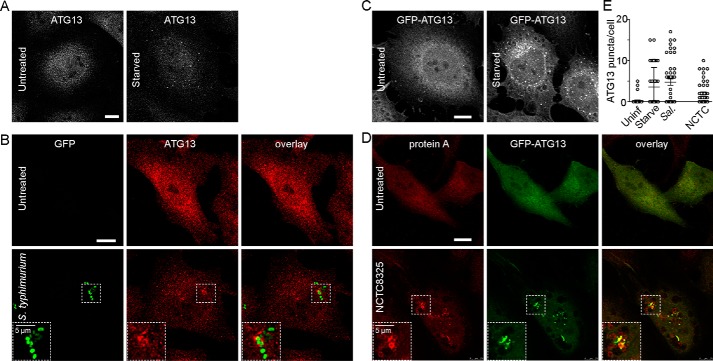
ULK1 is a serine/threonine kinase that plays an essential role during the early steps of autophagosome biogenesis, and we have previously characterized roles of this family of proteins in the regulation of canonical autophagy (51–53). In addition to standard autophagy, ULK1 complex has been shown to be important for promoting xenophagy to restrict Salmonella growth in host cells (54). Roles for ULK1-dependent autophagy have also been demonstrated during Brucella abortus infection (55). In this case, inhibition of ULK1 function showed the opposite trend leading to reduced infection. Here, we aimed to further explore the potential of targeting ULK1 for the modulation of xenophagy. To study ULK1 function during formation of the double-bilayer autophagosome, we studied puncta containing ATG13, an essential component of the ULK1 complex that we previously characterized (51). ATG13 has been proposed to play a critical role for translocation of the ULK1 complex to membrane initiation sites of autophagy and mitophagy, potentially via specific lipid-binding activity (56). As control, HeLa cells were starved of amino acids to stimulate canonical autophagy and stained for endogenous ATG13 (Fig. 9A). We could detect robust formation of ∼10–20 finely-sized ATG13(+) puncta/cell within 1 h of starvation. Next, we characterized translocation of the ULK1/ATG13 complex to invading Salmonella (Fig. 9B). A small number of larger sized ATG13(+) puncta could be observed forming around GFP–Salmonella 1 h post-infection, an early time point consistent with peak association of LC3B on bacteria.
Figure 9.
Staphylococcus and Salmonella infection both promote recruitment of ATG13 to autophagosome membranes. A, as control, HeLa cells were left untreated or starved in EBSS for 1 h before fixation and antibody staining to detect endogenous ATG13. Scale bars, 10 μm. B, HeLa cells were infected with 1:100 diluted S. enterica sv. typhimurium for 1 h and stained as in A. C, HeLa/GFP–ATG13 cells were starved as in A. D, HeLa/GFP–ATG13 cells were infected with S. aureus NCTC8325 for 3 h, fixed, and stained with anti-protein A antibody. Zoomed insets (×2.0 magnification) highlight ATG13 localizing to invading Salmonella or Staphylococcus. E, HeLa/GFP–ATG13 cells were untreated, starved in EBSS, or infected with S. enterica sv. typhimurium for 1 h. For comparison, cells were infected with NCTC8325 for 3 h. After fixation, cells were stained with DAPI, and GFP–ATG13(+) puncta were quantified. Average puncta/cell from n = 50 cells ± S.D. (representative of two independent experiments). Uninf., uninfected.
To compare, we examined ATG13(+) puncta following MRSA infection. As an alternative approach, we generated HeLa cells stably expressing GFP–ATG13 and first confirmed proper formation of starvation-induced autophagosomes using this ectopic marker (Fig. 9C), consistent with previous reports (56). To study ATG13 during MRSA-induced autophagy, we infected HeLa/GFP–ATG13 cells with NCTC8325 (Fig. 9D). We could detect robust formation of GFP–ATG13(+) membrane structures localizing around clusters of Staphylococcus 3 h post-infection (Fig. 9E). Together, these results suggest that the ULK1/ATG13 complex localizes to sites of Salmonella or Staphylococcus escape within hours of infection to drive formation of autophagosomes.
Inhibition of the ULK1–ATG13 pathway blocks S. aureus infection
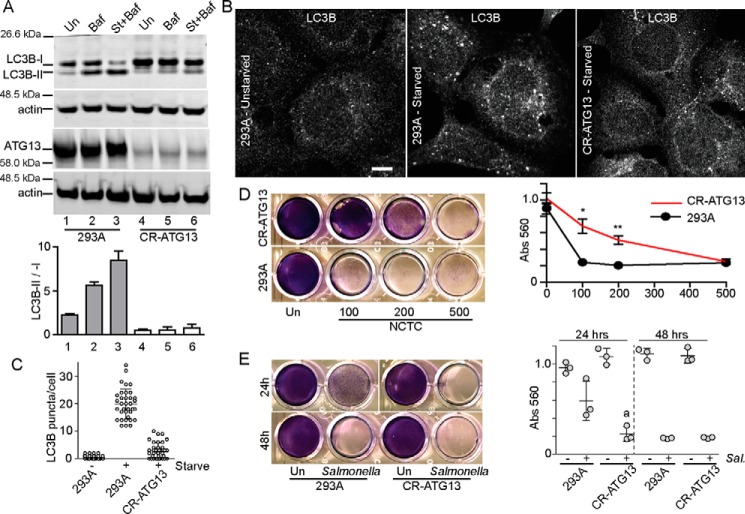
Because MRSA may subvert autophagy, we wished to determine whether the ULK1–ATG13 pathway can be targeted during S. aureus infection. We previously found that ULK1 and related family member ULK2 can both function for autophagy regulation (53). To achieve robust targeting of the ULK1/2 complex, we targeted the essential subunit ATG13 with the CRISPR–Cas9 system in HEK293A cells. As control, we confirmed that targeting of ATG13 strongly blocked starvation-induced autophagy flux as determined by lipidation of LC3B ± BafA1 (Fig. 10A). In addition, 293/ATG13–CRISPR cells were defective at LC3B(+) autophagosome formation following starvation (Fig. 10, B and C).
Figure 10.
Inhibition of ATG13-dependent autophagy suppresses Staphylococcus-induced cell death. A, ATG13 was targeted in HEK293A cells using the CRISPR–Cas9 system (CR–ATG13). WT HEK293A or CR–ATG13 cells were treated to bafilomycin A1 (Baf) (10 nm) ± starvation (St) in EBSS for 2 h. Cell lysates were analyzed for LC3B lipidation as in Fig. 1. n = 2 ± range. B and C, WT HEK293A or CR–ATG13 cells were starved in EBSS for 2 h, fixed, and stained to detect LC3B puncta. Average puncta/cell were from n = 36 cells ± S.D. Scale bar, 10 μm. D, WT HEK293A or CR–ATG13 cells were infected with varying multiplicities of infection of S. aureus NCTC8325 and assayed for cell viability as in Fig. 6. **, p < 0.001; *, p < 0.01 by unpaired t test: CR–ATG13 versus 293A (comparing equivalent multiplicities of infection). E, WT HEK293A or CR–ATG13 cells were infected with 1:100 diluted S. enterica sv. typhimurium and incubated for 24 or 48 h before staining for cell viability. Averages are from n = 3 ± S.D. a, p = 0.054 by t test: infected CR–ATG13 versus 293A at 24 h.
With strong targeting of ATG13, we next assessed effects on susceptibility to infection by S. aureus. 293/ATG13–CRISPR or control cells were infected with varying multiplicities of infection of MRSA (NCTC8325), and the remaining viable cells were measured 72 h post-infection (Fig. 10D). CRISPR targeting of ATG13 gave clear resistance to infection by NCTC8325. Therefore, inhibition of the ULK1/ATG13 autophagy complex appears to suppress the extent to which S. aureus infects and kills cells. To compare, we also tested how ATG13 targeting affected infection by Salmonella. Although WT 293A cells show clear levels of cell death following S. enterica sv. typhimurium infection, 293/ATG13–CRISPR cells were more sensitive (Fig. 10E), particularly when measuring viable cells remaining 24 h post-infection. This result further suggests that although the ULK1/ATG13 complex localizes to both invading S. aureus and Salmonella, autophagy plays opposite roles in the interaction with these pathogens.
To further explore inhibition of xenophagy, HeLa cells were generated stably expressing shRNA toward ULK1 (Fig. 11A). Strong knockdown of ULK1 was sufficient to reduce starvation-induced autophagy flux in the LC3B lipidation assay. LC3B-II generation following MRSA infection was also inhibited by ULK1 knockdown. To note, partial autophagy inhibition observed here despite strong ULK1 knockdown may reflect residual ULK2 function in HeLa cells. HeLa/shULK1 cells showed clear resistance to MRSA-induced cell death (Fig. 11B). Conversely, HeLa/shULK1 cells were more sensitive to Salmonella-induced cell death (Fig. 11C).
Figure 11.
Inhibition of ULK1 suppresses Staphylococcus intracellular replication and host cell death. A, ULK1 was targeted in HeLa cells using shRNA (shULK1). WT HeLa or shULK1 cells were treated to bafilomycin A1 (10 nm) ± starvation (St+Baf) in EBSS for 2 h. Cell lysates were analyzed and quantified for LC3B lipidation as in Fig. 1. n = 2 ± range. B, WT HeLa or shULK1 cells were infected with varying multiplicities of infection of S. aureus NCTC8325 and assayed for cell viability as in Fig. 6. Averages of n = 3 ± S.D. ***, p < 0.001; *, p < 0.05 by unpaired t test: shULK1 versus HeLa. C, WT HeLa or shULK1 cells were infected with 1:100 diluted S. enterica sv. typhimurium and incubated for 72 h before staining for cell viability. Averages of n = 3 ± S.D. **, p < 0.01 by unpaired t test. D, HEK293A cells were infected with NCTC8325 (200 m.o.i.). At the time of infection, ULK1 inhibitors MRT68921 or SBI-0206965 were added at 1 or 10 μm. Gentamycin (0.05 mg/ml) was added 1 h post-infection. Cells were incubated for 48 h and assayed for cell viability as in Fig. 6. Averages of n = 3 ± S.D. E, HEK293A cells were infected with 1:100 diluted Salmonella as in C. At the time of infection, ULK1 inhibitors were added at 10 μm. Gentamycin was added 50 min post-infection. Cells were incubated for 24 h and assayed for cell viability. Shown are averages from n = 3 ± S.D. D and E, ***, p < 0.0001; **, p < 0.001; *, p < 0.05 by unpaired t test: infected cells comparing (+) versus (−) ULK1 inhibitor. F, Staphylococcus NCTC8325 or S. enterica sv. typhimurium cultures were diluted 1:100 and grown in the presence of ULK1 inhibitors (10 μm) or gentamicin (0.05 mg/ml). Averages of n = 3 ± S.D. G and H, HeLa cells were incubated in EBSS for 2 h in the presence or absence of ULK1 inhibitors (10 μm). After fixation, cells were stained with anti-LC3B antibody. Scale bars, 10 μm. Average LC3B(+) puncta/cell from n = 40 cells per condition ± S.E. **, p < 0.001 versus starved no-drug control by ANOVA and Tukey's post test. I and J, HeLa/GFP–p62 stable cells were infected with NCTC8325 (100 m.o.i.) for 3 h in the presence or absence of ULK1 inhibitors (10 μm). After fixation, cells were stained with anti-protein A antibody. Scale bars, 10 μm. Arrow, large-sized p62 aggregates induced by NCTC8325 infection. Average large GFP–p62 aggregates/cell from n = 20 cells per condition ± S.E. Data are representative of two experiments. K, HEK293A cells were infected with NCTC8325 (100 m.o.i.) in the presence of MRT68921 (MRT) (1 μm) or SBI-0206965 (SBI) (10 μm). Gentamycin (0.05 mg/ml) was added 1 h post-infection. Cells were further incubated 3, 6 or 24 h before lysis. Bacterial titers in cell lysates were measured by growth on solid media. Average CFU from 3- and 6-h time points (n = 3 ± S.D.) are shown. Bacterial growth changes color of phenol red in bacterial plates. No host cells remained 24 h after infection in the absence of ULK1 inhibitor. Uninf., uninfected.
Several ATP pocket–binding ULK1/ULK2 inhibitors have been developed that block autophagy, for example MRT68921 and SBI-0206965, and this pathway is thus closer to becoming a therapeutic target (57–59). Here, we tested ULK1/2 inhibitors for their ability to modulate cell death following MRSA infection. Both MRT68921 and SBI-0206965 at high (10 μm) or low (1 μm) concentrations were able to improve cell viability after infection with MRSA (NCTC8325) (Fig. 11D). Incubation with 10 μm MRT68921 for the 48-h experiment led to some mild background cytotoxicity so this drug at 1 μm provided better rescue following bacterial infection. To complement this analysis, we tested effects of MRT68921 and SBI-0206965 on cell viability after S. enterica sv. typhimurium infection. Both of these ULK1/2 inhibitors led to significantly higher amounts of cell death after Salmonella infection (Fig. 11E). Therefore, pharmacological inhibition of the ULK1/2 pathway had opposite effects on cell viability after Staphylococcus versus Salmonella infection. Because ULK1/2 inhibitors appeared to block S. aureus infection of mammalian cells, we wanted to check that ULK1 inhibitors were not directly affecting bacteria. In this way, we confirmed that both ULK1/2 inhibitors (10 μm) did not have any effect on Staphylococcus or Salmonella in liquid culture growth assays (Fig. 11F). In contrast, gentamicin completely inhibited growth of both these types of bacteria.
Next, to clarify how ULK1/2 inhibitors rescue cells in MRSA experiments, we confirmed that SBI-0206965 and MRT68921 robustly inhibited autophagy in our experimental cell systems. Both compounds at 10 μm blocked formation of starvation-dependent LC3B(+) autophagosomes (Fig. 11, G and H). To further clarify the effects of ULK1/2 inhibitors on bacteria-induced autophagy, we studied p62 aggregate formation. HeLa/GFP–p62 cells treated with MRT68921 or SBI-0206965 were infected with NCTC8325. Indeed, treatment with MRT68921 reduced formation of large p62(+) aggregate structures following infection by NCTC8325 (Fig. 11, I and J). Note, we still detected smaller-sized p62 puncta without any associated NCTC8325 in the presence of MRT68921. In contrast, treatment with SBI-0206965 inhibited both large p62 aggregates and smaller p62 puncta. Interestingly, invading MRSA were still observed within infected cells treated with MRT68921 or SBI-0206965. Therefore, ULK1/2 inhibitors blocked MRSA-induced p62 aggregates and cell death but not initial stages of bacterial infection.
Finally, we clarified roles for autophagy during infection, because S. aureus still appeared to invade cells even with ULK1 inhibition. For this, we measured levels of intracellular S. aureus (colony-forming units (CFU)) within cell lysates. HEK293A cells were infected with NCTC8325 in the presence of MRT68921 (1 μm) or SBI-0206965 (10 μm). After 1 h of infection, gentamicin was added to inactivate extracellular NCTC8325, and host cells were lysed at time points thereafter to monitor replication of bacteria that invaded cells. In cells without the ULK1/2 inhibitor, S. aureus CFU increased rapidly within 3–6 h after addition of gentamicin, leading to death of HEK293A hosts within 24 h (Fig. 11K). At 3 h post-infection (without ULK inhibitors), we detected 2.7 × 106 CFU/ml in cell lysates. This titer corresponds to ∼150% of the CFU used to inoculate each well of host cells. Assuming a bacterial doubling time of 0.5 h, we estimate ∼2.3% of S. aureus (150%/26) were able to invade and proliferate within host cells within 3 h of infection. Strikingly, treatment with either MRT68921 or SBI-0206965 almost completely inhibited MRSA intracellular replication. Host cells maintained health 24–48 h (data not shown) after infection with the addition of ULK1 inhibitors. Therefore, blocking ULK1-dependent autophagy suppresses overall S. aureus infection.
Discussion
S. aureus infection remains to be a leading cause of human bacterial infections worldwide and presents a major issue for both hospital and community health care contexts (24). A further complication comes from the rise of antibiotic resistance and strains of MRSA that resist many first lines of treatment. Despite the importance of Staphylococcus as a pathogen, roles of host cell autophagy during the infection process in vitro and in vivo remain controversial (28, 60, 61) and less well-understood as compared with other bacteria such as Salmonella. Here, we aimed to clarify the role of autophagy during Staphylococcus infection of nonphagocytic cell types such as HEK293 and HeLa.
Interaction of S. aureus with the host cell autophagy pathway
We found strong indications of autophagy such as accumulation of the lipidated, activated form of the ATG8 member, LC3B, upon infection with WT, fully-virulent strains of S. aureus carrying an active agr quorum-sensing system. Our observations of robust LC3 lipidation are consistent with previous data documenting strong phenotypes on this autophagosomal marker during Staphylococcus infection (28, 32, 34, 61). Surprisingly, lipidation of LC3B after Salmonella infection was barely detectable. Moreover, we found strong accumulation of p62-positive aggregates in cells infected with S. aureus, suggestive of hyperactive protein ubiquitination or defective clearance. Interestingly, there was negligible or partial co-localization of p62 aggregates on Staphylococcus, which suggests that the ubiquitinated targets may be predominantly host cell proteins. Formation of the p62 aggregate phenotype depended on function of the agr-system, consistent with other work demonstrating the importance of Staphylococcus virulence factors such as α-hemolysin for interaction with the autophagy pathway (28, 32, 33, 61).
We were also intrigued becausea clinical Staphylococcus isolate from an endo-tracheal aspirate that we previously genotyped as CC8 (37) did not induce p62 aggregates or other markers of autophagy and infection-related cell death. There has been another report suggesting p62-mediated targeting of ubiquitinated cytosolic S. aureus using the SH1000 strain which is also CC8 (34). S. aureus SH1000 has been shown to carry less agr-dependent membrane lytic activity, resulting in lower rates of death in infected host cells (61). Although more representative strains should be investigated, our data are consistent with lower levels of p62-dependent autophagy and lower levels of proliferative cellular infection with CC8 S. aureus. Indeed, WT virulent S. aureus strains appeared to extensively escape from their lysosome-associated replicative niche into the cytoplasm, but agr-mutant or CC8 strains showed lower levels of this behavior. We had previously used Gal-3 as a cellular probe to detect lysosome membrane damage following treatment with the cancer chemotherapeutic (and anti-malarial) compound chloroquine (42). A related protein Gal-8 has been shown to detect membrane damage to mount xenophagy for the restriction of Salmonella infection (46). Members of the galectin family have multiple mechanisms to coordinate autophagy regulation (62, 63). Surprisingly, we S. aureus appeared to escape from the lysosome without strong Gal-3 recruitment, in contrast with membrane disruption events during Salmonella infection. Therefore, S. aureus may have acquired virulence factors such as α-hemolysin that can perturb membrane compartments during the infection life cycle without triggering innate anti-bacterial pathways.
ULK1 inhibition as a strategy to block S. aureus infection
We aimed to explore the potential of targeting autophagy during bacterial infection. In particular, we have had long-standing interest in the ULK1 autophagy complex which functions at an early regulatory checkpoint during autophagosome biogenesis (51–53). Several different ULK1 small molecule inhibitors have been developed that target the ATP-binding pocket of the kinase domain as a strategy to inhibit autophagy (58, 64). Here, we could show that the ULK1 complex (via ATG13) localizes to sites of either Salmonella or Staphylococcus invasion in the cytoplasm where the complex might promote autophagosome formation around bacteria. Previously, others have also shown ULK1 or members of the WIPI PI3P-binding protein family co-localizing on cytosolic Salmonella or Staphylococcus (33, 54, 65, 66). Interestingly, genetic targeting of the ULK1–ATG13 pathway blocked autophagy and prevented the ability of S. aureus infection to progress toward cell death. These data are consistent with a role for autophagy in the generation of a replicative niche to support S. aureus infection, originally described by Krut and co-workers (28). Conversely, ULK1–ATG13 targeting produced the opposite effect and sensitized cells to Salmonella infection cytotoxicity. This effect is consistent with the current model featuring anti-bacterial autophagy/xenophagy during Salmonella infection (2, 15, 19, 54, 66). Importantly, both ULK1 inhibitors that we tested (MRT68921 and SBI-0206965) (57, 58) could robustly inhibit Staphylococcus intracellular replication and host cell death. Previously, autophagy-dependent Staphylococcus infection was blocked via the phosphatidylinositol 3-kinase inhibitor wortmannin, which would widely affect other cellular pathways (28). Cells treated with ULK1 inhibitors still showed substantial numbers of Staphylococcus invading the cytoplasm. Because bacterial titers in cell lysates in these cases were dramatically low, an autophagy-dependent niche appears to be essential for intracellular replication.
Our results highlight the potential of autophagy targets like ULK1 to inhibit the formation of intracellular replication sites during bacterial infection. ULK1 inhibitors were effective at preventing Staphylococcus infection of nonprofessional phagocytic cell lines, and it will be useful to test more broadly, for example, in other physiological cell hosts like macrophages (67). The ability of S. aureus to reside within an intracellular niche provides a basis for antibiotic evasion and persistence of infection (26). Autophagy inhibition to block infection might be suitable for other bacteria dependent on formation of intracellular replication compartments such as B. abortus and Coxiella burnetii (55, 68–70). Our results further highlight that autophagy can play opposite roles depending on the pathogen because inhibition of ULK1 makes cells hypersensitive to other bacteria such as Salmonella.
Experimental procedures
Cell culture
HEK293A and HeLa cells were maintained in DMEM with 4.5 g/liter glucose (Lonza catalog no. BE12-614F) supplemented with 10% fetal bovine serum (FBS) (Labtech catalog no. FCS-SA), 4 mm l-glutamine (Lonza catalog no. BE17-605E), and 100 units/ml penicillin/streptomycin (Lonza catalog no. DE17-602E). Where indicated, HeLa cells were transduced with retrovirus carrying pMXs–IP–GFP–hATG13 (71) or pMXs–puro–GFP–p62 (72) and selected under puromycin. HEK293A cells with CRISPR–Cas9 targeting of ATG13 were generated using the lentiCRISPR version 2 (one-vector) system (73). Guide RNA sequences for ATG13 from the human GeCKO library (74) were cloned into lentiCRISPR version 2 and screened for ATG13 targeting efficiency by immunoblot. The gRNA used throughout this study was ATG13-HGLibA_03468 corresponding to GACTGTCCAAGTGATTGTCC. After transduction of cells with lentivirus, puromycin selection was performed, and isolated clones were confirmed for targeting efficiency by immunoblot. All results with ATG13 CRISPR targeting were confirmed via independent experiments using pools of CRISPR-targeted cells analyzed 1–2 passages immediately after puromycin selection. Alternatively, ULK1 knockdown was performed using pLKO.1 shRNA (TRCN0000000835, Broad Institute Genetic Perturbation Platform, obtained from Open Biosystems/Dharmacon). As autophagy control, cells were starved of amino acids (and serum) in Earle's balanced salt solution (EBSS) (52) or treated with chloroquine as described (42).
Bacterial infection
Strains of S. aureus used include the following: ATCC29213 (methicillin-sensitive, agr-WT); NCTC8325 (MRSA) (obtained from Public Health England, National Collection of Type Cultures); epidemic MRSA strain LF78 (38); NRS144 (agr-mutant); and clonal complex 8 S. aureus (37). S. enterica sv. typhimurium (NCTC 13347 (SL 1344)) was obtained from Public Health England, National Collection of Type Cultures. All S. aureus strains were maintained on mannitol salt agar (MSA) plates (OXOID 1106008) or tryptic soy broth (TSB) (Fluka Analytical, 22092) in liquid cultures. S. enterica was grown on nutrient agar plates (OXOID 1655783) and TSB liquid cultures. Where indicated, Salmonella was transformed by electroporation with GFP-expressing plasmid using standard methods followed by selection with ampicillin. We subcloned enhanced GFP as a PCR fragment via sites HindIII–EcoRI into the backbone from pGFPuv (Clontech, catalog no. 632312).
S. aureus infections were performed based on a previously reported protocol (28). Overnight cultures in TSB were diluted 1:100 on the day of the experiment and grown until mid-logarithmic phase (OD600 = 0.3 ± 0.05). Based on actual OD, bacteria pellets were adjusted to varying volumes in Pen/Strep-free DMEM to yield (typically) 100 m.o.i.
Host cells (HEK293A or HeLa) were seeded in 12-well plates for immunoblotting or on poly-d-lysine–coated coverslips in 24-well plates in Pen/Strep-free DMEM, 10% FBS. The following day, Staphylococcus/DMEM suspension was added to host cells (100 μl to each well of a 12-well plate containing 1 ml of media; 50 μl to each well of 24-well plate containing 0.5 ml media) and incubated for 1 h at 37 °C. After this hour, gentamicin (Lonza, 17–519L) was added to 0.05 mg/ml final (where indicated), and cells were further incubated at 37 °C for varying times before cell lysis or fixation.
We infected cells with Salmonella using a protocol previously reported for xenophagy experiments (36). Overnight cultures of S. enterica sv. typhimurium (NCTC 13347) in TSB were diluted 1:33 on the day of the experiment in 10 ml of TSB broth and grown for 3 h with shaking resulting in OD600 = 1.2–1.5 (corresponding to ∼108 bacteria/ml). This culture of Salmonella was further diluted 1:100 in Pen/Strep-free DMEM, 10% FBS to generate a Salmonella stock culture. For infection, medium was first removed from host cells (plated as above) and replaced with 1 ml (for a 12-well plate) or 0.5 ml (for a 24-well plate) of diluted Salmonella stock culture at time = 0. After incubation for 20 min at 37 °C, media were changed into fresh Pen/Strep-free DMEM, 10% FBS and further incubated at 37 °C for 30 min. Then the media were further exchanged with Pen/Strep-free DMEM, 10% FBS containing gentamicin (0.05 mg/ml) and incubated further for varying amounts of time.
Immunoblot analysis
Cell lysates were prepared and analyzed using 4–12% NuPAGE gels (for LC3B) or 10% hand-poured BisTris gels in MES running buffer (Thermo Fisher Scientific), as described previously (52, 53). Membranes were stained using the following antibodies: LC3B, clone 5F10 (Nanotools catalog no. 0231-100); ULK1-D8H5 (Cell Signaling catalog no. 8054); ATG13-D4P1K (Cell Signaling catalog no. 13273); actin, Ab-5 (BD Bioscience catalog no. 612656). Detection was via anti-mouse or anti-rabbit Dylight-coupled secondary antibodies and Licor Odyssey infra-red scanning.
Microscopy
After treatments, cells were fixed and stained using the following antibodies: anti-p62/SQSTM1 (BD Transduction Laboratories mouse anti-p62/lck ligand catalog no. 610832); LAMP-2 (BD Transduction Laboratories mouse anti-human CD107b); LC3B (Cell Signaling, catalog no. 2775); or ATG13-D4P1K (Cell Signaling, catalog no. 13273). In some cases, S. aureus bacteria were stained with anti-protein A (Sigma, catalog no. P2921) or Hoechst 33342. In some cases, Salmonella bacteria were stained with DAPI.
For Gal-3 puncta tests, HeLa cells plated on glass coverslips were transfected with pEGFP-hGal3 (Addgene catalog no. 73080) (45) using Lipofectamine 2000 according to standard protocols, as we have previously reported (42). After 24 h of transfection, cells were used for experiments. Cell images were captured by confocal microscopy (Figs. 2, 3, 6, 7, and 9–11: Leica, TCS SP5, HCX PL APO CS-×63–1.4NA objective and HyD GaAsP detection; Figs. 4 and 5: Zeiss LSM 710, Plan-Apochromat ×63–1.4NA objective). Puncta/cells were quantified from confocal scans (or directly by epifluorescent imaging), depending on the marker.
Cell killing by bacterial infection
At varying times (24–72 h as indicated) after infection, viable cells remaining attached to the culture dish were fixed with 10% formalin/PBS, washed in PBS/methanol (1:1), and then stained with dilute Giemsa stain (Fluka BCBK8476V). Plates were washed and then dried. To quantify Giemsa stain, dyed cellular materials were solubilized in 30% acetic acid/water and measured at A560 nm, as we and others have previously described (42, 75).
Staphylococcus intracellular replication
Infected host cells were washed with PBS once and lysed in 0.05% Triton X-100/PBS. Lysates were diluted with PBS and plated on MSA plates. Overnight colonies were counted, and normalized CFU was calculated.
ULK1 inhibitors
We obtained MRT68921 from B. Saxty, Medical Research Council UK/LifeArc (58). ULK1 inhibitor SBI-0206965 was synthesized according to the reported scheme (57). The final compound was purified by HPLC and confirmed by NMR analysis followed by tests in cells described here.
Statistics
Quantitative data were managed using GraphPad Prism and analyzed using unpaired t test (for two-way comparisons) or one-way ANOVA with Tukey post-test (multiple comparisons) as appropriate.
Author contributions
O. A. R. data curation; O. A. R. formal analysis; O. A. R., S. D., F. S., R. X. Z., and D.H.J. investigation; O. A. R. methodology; O. A. R. writing-original draft; N. C. T., J. Y., and E. Y. C. conceptualization; N. C. T. and E. Y. C. funding acquisition; J. Y. and E. Y. C. supervision; E. Y. C. project administration; E. Y. C. writing-review and editing.
Acknowledgments
We thank the EPSRC Mass Spectrometry Service, Swansea, United Kingdom, for high-resolution spectra. We thank N. Mizushima (University of Tokyo) for GFP–p62 and GFP–ATG13 constructs. LentiCRISPR version 2 was a gift from Feng Zhang (Addgene plasmid no. 52961). pEGFP-hGal3 was a gift from Tamotsu Yoshimori (Addgene plasmid no. 73080).
This work was supported by a scholarship from the Iraq Ministry of Higher Education and Scientific Research (to O. R.) and by Cancer Research UK/West of Scotland Cancer Centre/Glasgow Cancer Centre, Tenovus Scotland and Natural Sciences and Engineering Research Council (NSERC) Canada (to E. C.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The authors declare that they have no conflicts of interest with the contents of this article.
- SCV
- Salmonella-containing vacuole
- ANOVA
- analysis of variance
- m.o.i.
- multiplicity of infection
- MRSA
- methicillin-resistant S. aureus
- EBSS
- Earle's balanced salt solution
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- Pen/Strep
- penicillin/streptomycin
- BafA1
- bafilomycin A1
- CFU
- colony-forming unit
- LLOME
- l-leucyl-l-leucine methyl ester
- MSA
- mannitol salt agar
- TSB
- tryptic soy broth
- DAPI
- 4′,6-diamidino-2-phenylindole.
References
- 1. Yu L., Chen Y., and Tooze S. A. (2018) Autophagy pathway: cellular and molecular mechanisms. Autophagy 14, 207–215 10.1080/15548627.2017.1378838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birmingham C. L., Smith A. C., Bakowski M. A., Yoshimori T., and Brumell J. H. (2006) Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383 10.1074/jbc.M509157200 [DOI] [PubMed] [Google Scholar]
- 3. Gutierrez M. G., Master S. S., Singh S. B., Taylor G. A., Colombo M. I., and Deretic V. (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- 4. Zhao Z., Fux B., Goodwin M., Dunay I. R., Strong D., Miller B. C., Cadwell K., Delgado M. A., Ponpuak M., Green K. G., Schmidt R. E., Mizushima N., Deretic V., Sibley L. D., and Virgin H. W. (2008) Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4, 458–469 10.1016/j.chom.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Py B. F., Lipinski M. M., and Yuan J. (2007) Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 3, 117–125 10.4161/auto.3618 [DOI] [PubMed] [Google Scholar]
- 6. Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., Hamada S., and Yoshimori T. (2004) Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 10.1126/science.1103966 [DOI] [PubMed] [Google Scholar]
- 7. Huett A., Heath R. J., Begun J., Sassi S. O., Baxt L. A., Vyas J. M., Goldberg M. B., and Xavier R. J. (2012) The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella typhimurium. Cell Host Microbe 12, 778–790 10.1016/j.chom.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noad J., von der Malsburg A., Pathe C., Michel M. A., Komander D., and Randow F. (2017) LUBAC-synthesized linear ubiquitin chains restrict cytosol-invading bacteria by activating autophagy and NF-κB. Nat. Microbiol. 2, 17063 10.1038/nmicrobiol.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polajnar M., Dietz M. S., Heilemann M., and Behrends C. (2017) Expanding the host cell ubiquitylation machinery targeting cytosolic Salmonella. EMBO Rep. 18, 1572–1585 10.15252/embr.201643851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Wijk S. J., Fiskin E., Putyrski M., Pampaloni F., Hou J., Wild P., Kensche T., Grecco H. E., Bastiaens P., and Dikic I. (2012) Fluorescence-based sensors to monitor localization and functions of linear and K63-linked ubiquitin chains in cells. Mol. Cell 47, 797–809 10.1016/j.molcel.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujita N., Morita E., Itoh T., Tanaka A., Nakaoka M., Osada Y., Umemoto T., Saitoh T., Nakatogawa H., Kobayashi S., Haraguchi T., Guan J. L., Iwai K., Tokunaga F., Saito K., et al. (2013) Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 203, 115–128 10.1083/jcb.201304188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiskin E., Bionda T., Dikic I., and Behrends C. (2016) Global analysis of host and bacterial ubiquitinome in response to Salmonella typhimurium infection. Mol. Cell 62, 967–981 10.1016/j.molcel.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 13. Perrin A. J., Jiang X., Birmingham C. L., So N. S., and Brumell J. H. (2004) Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 14, 806–811 10.1016/j.cub.2004.04.033 [DOI] [PubMed] [Google Scholar]
- 14. Wang L., Yan J., Niu H., Huang R., and Wu S. (2018) Autophagy and ubiquitination in Salmonella infection and the related inflammatory responses. Front. Cell Infect. Microbiol. 8, 78 10.3389/fcimb.2018.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng Y. T., Shahnazari S., Brech A., Lamark T., Johansen T., and Brumell J. H. (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183, 5909–5916 10.4049/jimmunol.0900441 [DOI] [PubMed] [Google Scholar]
- 16. von Muhlinen N., Akutsu M., Ravenhill B. J., Foeglein Á., Bloor S., Rutherford T. J., Freund S. M., Komander D., and Randow F. (2012) LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell 48, 329–342 10.1016/j.molcel.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ivanov S., and Roy C. R. (2009) NDP52: the missing link between ubiquitinated bacteria and autophagy. Nat. Immunol. 10, 1137–1139 10.1038/ni1109-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thurston T. L., Ryzhakov G., Bloor S., von Muhlinen N., and Randow F. (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10, 1215–1221 10.1038/ni.1800 [DOI] [PubMed] [Google Scholar]
- 19. Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C., Dötsch V., Bumann D., and Dikic I. (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cemma M., Kim P. K., and Brumell J. H. (2011) The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 7, 341–345 10.4161/auto.7.3.14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tumbarello D. A., Manna P. T., Allen M., Bycroft M., Arden S. D., Kendrick-Jones J., and Buss F. (2015) The autophagy receptor TAX1BP1 and the molecular motor myosin VI are required for clearance of Salmonella typhimurium by autophagy. PLoS Pathog. 11, e1005174 10.1371/journal.ppat.1005174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahnazari S., and Brumell J. H. (2011) Mechanisms and consequences of bacterial targeting by the autophagy pathway. Curr. Opin. Microbiol. 14, 68–75 10.1016/j.mib.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 23. Yuk J. M., Yoshimori T., and Jo E. K. (2012) Autophagy and bacterial infectious diseases. Exp. Mol. Med. 44, 99–108 10.3858/emm.2012.44.2.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner N. A., Sharma-Kuinkel B. K., Maskarinec S. A., Eichenberger E. M., Shah P. P., Carugati M., Holland T. L., and Fowler V. G. Jr. (2019) Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218 10.1038/s41579-018-0147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moldovan A., and Fraunholz M. J. (2019) In or out: phagosomal escape of Staphylococcus aureus. Cell. Microbiol. 21, e12997 10.1111/cmi.12997 [DOI] [PubMed] [Google Scholar]
- 26. Lehar S. M., Pillow T., Xu M., Staben L., Kajihara K. K., Vandlen R., DePalatis L., Raab H., Hazenbos W. L., Morisaki J. H., Kim J., Park S., Darwish M., Lee B. C., Hernandez H., et al. (2015) Novel antibody-antibiotic conjugate eliminates intracellular Staphylococcus aureus. Nature 527, 323–328 10.1038/nature16057 [DOI] [PubMed] [Google Scholar]
- 27. Schröder A., Kland R., Peschel A., von Eiff C., and Aepfelbacher M. (2006) Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med. Microbiol. Immunol. 195, 185–194 10.1007/s00430-006-0015-0 [DOI] [PubMed] [Google Scholar]
- 28. Schnaith A., Kashkar H., Leggio S. A., Addicks K., Krönke M., and Krut O. (2007) Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 282, 2695–2706 10.1074/jbc.M609784200 [DOI] [PubMed] [Google Scholar]
- 29. López de Armentia M. M., Gauron M. C., and Colombo M. I. (2017) Staphylococcus aureus α-toxin induces the formation of dynamic tubules labeled with LC3 within host cells in a Rab7- and Rab1b-dependent manner. Front. Cell Infect. Microbiol. 7, 431 10.3389/fcimb.2017.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grosz M., Kolter J., Paprotka K., Winkler A. C., Schäfer D., Chatterjee S. S., Geiger T., Wolz C., Ohlsen K., Otto M., Rudel T., Sinha B., and Fraunholz M. (2014) Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell. Microbiol. 16, 451–465 10.1111/cmi.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blättner S., Das S., Paprotka K., Eilers U., Krischke M., Kretschmer D., Remmele C. W., Dittrich M., Müller T., Schuelein-Voelk C., Hertlein T., Mueller M. J., Huettel B., Reinhardt R., Ohlsen K., et al. (2016) Staphylococcus aureus exploits a non-ribosomal cyclic dipeptide to modulate survival within epithelial cells and phagocytes. PLoS Pathog. 12, e1005857 10.1371/journal.ppat.1005857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mestre M. B., Fader C. M., Sola C., and Colombo M. I. (2010) α-Hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus -infected cells. Autophagy 6, 110–125 10.4161/auto.6.1.10698 [DOI] [PubMed] [Google Scholar]
- 33. Mauthe M., Yu W., Krut O., Krönke M., Götz F., Robenek H., and Proikas-Cezanne T. (2012) WIPI-1 positive autophagosome-like vesicles entrap pathogenic Staphylococcus aureus for lysosomal degradation. Int. J. Cell Biol. 2012, 179207 10.1155/2012/179207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neumann Y., Bruns S. A., Rohde M., Prajsnar T. K., Foster S. J., and Schmitz I. (2016) Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy 12, 2069–2084 10.1080/15548627.2016.1226732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., and Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huett A., Ng A., Cao Z., Kuballa P., Komatsu M., Daly M. J., Podolsky D. K., and Xavier R. J. (2009) A novel hybrid yeast-human network analysis reveals an essential role for FNBP1L in antibacterial autophagy. J. Immunol. 182, 4917–4930 10.4049/jimmunol.0803050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sangal V., Girvan E. K., Jadhav S., Lawes T., Robb A., Vali L., Edwards G. F., Yu J., and Gould I. M. (2012) Impacts of a long-term programme of active surveillance and chlorhexidine baths on the clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in an Intensive Care Unit in Scotland. Int. J. Antimicrob. Agents 40, 323–331 10.1016/j.ijantimicag.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 38. Raghukumar R., Vali L., Watson D., Fearnley J., and Seidel V. (2010) Antimethicillin-resistant Staphylococcus aureus (MRSA) activity of 'pacific propolis' and isolated prenylflavanones. Phytother. Res. 24, 1181–1187 10.1002/ptr.3096 [DOI] [PubMed] [Google Scholar]
- 39. Verlhac P., Grégoire I. P., Azocar O., Petkova D. S., Baguet J., Viret C., and Faure M. (2015) Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell Host Microbe 17, 515–525 10.1016/j.chom.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 40. Agerer F., Michel A., Ohlsen K., and Hauck C. R. (2003) Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J. Biol. Chem. 278, 42524–42531 10.1074/jbc.M302096200 [DOI] [PubMed] [Google Scholar]
- 41. Wells C. L., van de Westerlo E. M., Jechorek R. P., Haines H. M., and Erlandsen S. L. (1998) Cytochalasin-induced actin disruption of polarized enterocytes can augment internalization of bacteria. Infect. Immun. 66, 2410–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gallagher L. E., Radhi O. A., Abdullah M. O., McCluskey A. G., Boyd M., and Chan E. Y. W. (2017) Lysosomotropism depends on glucose: a chloroquine resistance mechanism. Cell Death Dis. 8, e3014 10.1038/cddis.2017.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paz I., Sachse M., Dupont N., Mounier J., Cederfur C., Enninga J., Leffler H., Poirier F., Prevost M. C., Lafont F., and Sansonetti P. (2010) Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530–544 10.1111/j.1462-5822.2009.01415.x [DOI] [PubMed] [Google Scholar]
- 44. Aits S., Kricker J., Liu B., Ellegaard A. M., Hämälistö S., Tvingsholm S., Corcelle-Termeau E., Høgh S., Farkas T., Holm Jonassen A., Gromova I., Mortensen M., and Jäättelä M. (2015) Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11, 1408–1424 10.1080/15548627.2015.1063871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maejima I., Takahashi A., Omori H., Kimura T., Takabatake Y., Saitoh T., Yamamoto A., Hamasaki M., Noda T., Isaka Y., and Yoshimori T. (2013) Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 10.1038/emboj.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thurston T. L., Wandel M. P., von Muhlinen N., Foeglein A., and Randow F. (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418 10.1038/nature10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang J., Canadien V., Lam G. Y., Steinberg B. E., Dinauer M. C., Magalhaes M. A., Glogauer M., Grinstein S., and Brumell J. H. (2009) Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. U.S.A. 106, 6226–6231 10.1073/pnas.0811045106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thurston T. L., Matthews S. A., Jennings E., Alix E., Shao F., Shenoy A. R., Birrell M. A., and Holden D. W. (2016) Growth inhibition of cytosolic Salmonella by caspase-1 and caspase-11 precedes host cell death. Nat. Commun. 7, 13292 10.1038/ncomms13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hagar J. A., Powell D. A., Aachoui Y., Ernst R. K., and Miao E. A. (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 10.1126/science.1240988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu L. C., Park J. M., Zhang K., Luo J. L., Maeda S., Kaufman R. J., Eckmann L., Guiney D. G., and Karin M. (2004) The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428, 341–345 10.1038/nature02405 [DOI] [PubMed] [Google Scholar]
- 51. Chan E. Y., Longatti A., McKnight N. C., and Tooze S. A. (2009) Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell. Biol. 29, 157–171 10.1128/MCB.01082-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nwadike C., Williamson L. E., Gallagher L. E., Guan J. L., and Chan E. Y. W. (2018) AMPK inhibits ULK1-dependent autophagosome formation and lysosomal acidification via distinct mechanisms. Mol. Cell. Biol. 38, e00023–18 10.1128/MCB.00023-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McAlpine F., Williamson L. E., Tooze S. A., and Chan E. Y. (2013) Regulation of nutrient-sensitive autophagy by uncoordinated 51-like kinases 1 and 2. Autophagy 9, 361–373 10.4161/auto.23066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kageyama S., Omori H., Saitoh T., Sone T., Guan J. L., Akira S., Imamoto F., Noda T., and Yoshimori T. (2011) The LC3 recruitment mechanism is separate from Atg9L1-dependent membrane formation in the autophagic response against Salmonella. Mol. Biol. Cell 22, 2290–2300 10.1091/mbc.e10-11-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Starr T., Child R., Wehrly T. D., Hansen B., Hwang S., López-Otin C., Virgin H. W., and Celli J. (2012) Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11, 33–45 10.1016/j.chom.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karanasios E., Stapleton E., Manifava M., Kaizuka T., Mizushima N., Walker S. A., and Ktistakis N. T. (2013) Dynamic association of the ULK1 complex with omegasomes during autophagy induction. J. Cell Sci. 126, 5224–5238 10.1242/jcs.132415 [DOI] [PubMed] [Google Scholar]
- 57. Egan D. F., Chun M. G., Vamos M., Zou H., Rong J., Miller C. J., Lou H. J., Raveendra-Panickar D., Yang C. C., Sheffler D. J., Teriete P., Asara J. M., Turk B. E., Cosford N. D., and Shaw R. J. (2015) Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 59, 285–297 10.1016/j.molcel.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petherick K. J., Conway O. J., Mpamhanga C., Osborne S. A., Kamal A., Saxty B., and Ganley I. G. (2015) Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 290, 28726 10.1074/jbc.A114.627778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lazarus M. B., and Shokat K. M. (2015) Discovery and structure of a new inhibitor scaffold of the autophagy initiating kinase ULK1. Bioorg. Med. Chem. 23, 5483–5488 10.1016/j.bmc.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maurer K., Reyes-Robles T., Alonzo F. 3rd, Durbin J., Torres V. J., and Cadwell K. (2015) Autophagy mediates tolerance to Staphylococcus aureus α-toxin. Cell Host Microbe 17, 429–440 10.1016/j.chom.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O'Keeffe K. M., Wilk M. M., Leech J. M., Murphy A. G., Laabei M., Monk I. R., Massey R. C., Lindsay J. A., Foster T. J., Geoghegan J. A., and McLoughlin R. M. (2015) Manipulation of autophagy in phagocytes facilitates Staphylococcus aureus bloodstream infection. Infect. Immun. 83, 3445–3457 10.1128/IAI.00358-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chauhan S., Kumar S., Jain A., Ponpuak M., Mudd M. H., Kimura T., Choi S. W., Peters R., Mandell M., Bruun J. A., Johansen T., and Deretic V. (2016) TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev. Cell 39, 13–27 10.1016/j.devcel.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jia J., Abudu Y. P., Claude-Taupin A., Gu Y., Kumar S., Choi S. W., Peters R., Mudd M. H., Allers L., Salemi M., Phinney B., Johansen T., and Deretic V. (2018) Galectins control mTOR in response to endomembrane damage. Mol. Cell 70, 120–135.e8 10.1016/j.molcel.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., et al. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 10.1126/science.1196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dooley H. C., Razi M., Polson H. E., Girardin S. E., Wilson M. I., and Tooze S. A. (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 55, 238–252 10.1016/j.molcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thurston T. L., Boyle K. B., Allen M., Ravenhill B. J., Karpiyevich M., Bloor S., Kaul A., Noad J., Foeglein A., Matthews S. A., Komander D., Bycroft M., and Randow F. (2016) Recruitment of TBK1 to cytosol-invading Salmonella induces WIPI2-dependent antibacterial autophagy. EMBO J. 35, 1779–1792 10.15252/embj.201694491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Horn J., Stelzner K., Rudel T., and Fraunholz M. (2018) Inside job: Staphylococcus aureus host–pathogen interactions. Int. J. Med. Microbiol. 308, 607–624 10.1016/j.ijmm.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 68. Vázquez C. L., and Colombo M. I. (2010) Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ. 17, 421–438 10.1038/cdd.2009.129 [DOI] [PubMed] [Google Scholar]
- 69. Winchell C. G., Graham J. G., Kurten R. C., and Voth D. E. (2014) Coxiella burnetii type IV secretion-dependent recruitment of macrophage autophagosomes. Infect. Immun. 82, 2229–2238 10.1128/IAI.01236-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Berón W., Gutierrez M. G., Rabinovitch M., and Colombo M. I. (2002) Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect Immun. 70, 5816–5821 10.1128/IAI.70.10.5816-5821.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J. L., Oshiro N., and Mizushima N. (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 10.1091/mbc.e08-12-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Itakura E., and Mizushima N. (2011) p62 Targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 192, 17–27 10.1083/jcb.201009067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sanjana N. E., Shalem O., and Zhang F. (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Joung J., Konermann S., Gootenberg J. S., Abudayyeh O. O., Platt R. J., Brigham M. D., Sanjana N. E., and Zhang F. (2017) Genome-scale CRISPR–Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 10.1038/nprot.2017.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maycotte P., Aryal S., Cummings C. T., Thorburn J., Morgan M. J., and Thorburn A. (2012) Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8, 200–212 10.4161/auto.8.2.18554 [DOI] [PMC free article] [PubMed] [Google Scholar]