Abstract
Introduction:
This study sought to determine if the systemic cytokine profile of rodents subjected to chronic restraint stress leads to persistent low-grade inflammation.
Methods:
Male Sprague-Dawley rats were subjected to restraint stress for a total of seven or fourteen days. Urine norepinephrine (NE), plasma interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), C-reactive protein (CRP), and stromal cell-derived factor 1 (SDF-1) were assessed with ELISA. Liver and spleen expression of IL-6 and TNF-α were assessed with real time PCR.
Results:
Chronic stress at 7 and 14 days sequentially increased plasma acute phase reactants, liver IL-6 expression, HPC mobilization, and decreased erythroid progenitor colony growth. BM cellularity was suppressed at 7 days CS, then recovered by 14 days. Weight gain was reduced by CS compared to each models’ naïve counterparts.
Conclusions:
Combining this model with trauma and sepsis models will allow evaluation of the contribution of persistent inflammation in disease progression and outcomes.
Keywords: Inflammation, Stress, cytokines
Introduction
Acute inflammation is a short-term defense response to injury and is required for healing and tissue repair.1, 2 During acute inflammation, pro-inflammatory cytokines, such tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are released. In addition, there is vasodilation, increased vascular permeability, activation of complement, and leukocyte migration. The acute inflammatory response should be terminated when it is no longer necessary, to prevent unnecessary damage to tissue. Resolution of acute inflammation occurs with the release of transforming growth factor-beta (TGF-beta), interleukin-10 (IL-10), upregulation of anti-inflammatory molecules such as interleukin-1 receptor antagonists and prostaglandin E2.2, 3 The pro-inflammatory response is balanced by an anti-inflammatory response to resume a state of homeostasis.
If there is repeated stress, neuroendocrine dysregulation is prolonged, chronic inflammation occurs which alters organ function. Chronic stress is associated with persistent activation of the hypothalamic-pituitary-adrenal axis, which leads to continuously elevated levels of stress hormones, such as glucocorticoid and catecholamines.4, 5 Several authors have previously noted that the transition from neutrophil to mononuclear cell infiltrate is a hallmark trait of acute to chronic inflammation, with IL-6 and its soluble receptor, sIL-6Ra, playing an important role.3, 6–8 During chronic stress, activated polymorphonuclear cells (PMNs) release sIL-6Rα, which combines with IL-6 to activate endothelial cells to produce monocyte chemotactic protein-1 (MCP-1), stimulating monocyte recruitment.6, 8
In-hospital mortality following infection and trauma have improved, but long-term morbidity and mortality remain high.9–12 The mechanisms linking stress, the immune system, and disease progression are not well understood. Patients with long-term critical illness frequently progress to a state of persistent inflammation, immunosuppression, and catabolism (PICS).7, 13 PICS is characterized by increased plasma concentrations of IL-6 as well as anemia.13 In addition, Gentile et al. proposed that during PICS, myeloid-derived suppressor cells (MDSCs) appear in the bone marrow and secondary lymphoid organs following the initial insult, resulting from a process known as emergency myelopoiesis.13 These cells produce TNF-α, MCP-1, stromal-derived factor 1 (SDF-1), nitric oxide and reactive oxygen species, eliciting a prolonged pro-inflammatory environment.13–15
Chronic critical illness has not been well-studied in animal models. Restraint stress to induce acute stress has been more thoroughly studied in rodents, but not in the context of chronic critical illness.16, 17 Majority of animal models focus on the acute phase of sepsis and trauma. The current study examines a rodent model of chronic stress that leads to persistent inflammation. Rodents were evaluated at seven and fourteen days following chronic stress to determine a time course for inflammation and organ dysfunction.
Methods
Animals
Male Sprague-Dawley rats age 8–9 weeks (Charles River, Wilmington, MA) were housed in pairs under barrier-sustained conditions with a 12-hour light/dark cycle, and with free access to chow and water. Female rodents were excluded from this study due to possible estrous cycle effect on systemic stress state as well as evidence of sex-related differences in leukocyte genomic response during severe injury and hormonal differences accounting for improved survival in sepsis.18 Animals were given at least a 5-day acclimation period before chronic restraint stress was initiated. Animals were randomly assigned to one of four groups: naïve weighed for 7 days (n=6), naïve weighed for 14 days (n=3), chronic stress (CS) for 7 days (n=14), or chronic stress for 14 days (n=4). After seven or fourteen days of handling or restraint stress, the rats were anesthetized using a rodent cocktail consisting of ketamine (80–100mg/kg) and (xylazine 5–10mg/kg), then underwent sacrifice through thoracotomy and exsanguination via cardiac puncture. Blood, bone marrow from the left femur, lung, spleen and liver tissue were collected at the time of sacrifice. The average percent weight change was calculated for each rodent by subtracting the starting weight from the sacrifice day weight and dividing the difference by the starting weight.
Chronic Stress
Chronic stress consisted of two hours of restraint stress daily for seven or fourteen days based on a validated model shown to be associated with bone marrow dysfunction worsened by restraint stress after traumatic injury compared to traumatic injury alone.19 Also, signs of sepsis and multiple organ failure occur in days-weeks in rodents as compared to weeks-months in humans.18 The rodents were placed in a nose cone animal cylinder (Kent Scientific Corporation, Torrington, CT) which was adjusted to restrict its movements. During the two hour period, the restraint cylinder was rotated one hundred eighty degrees every thirty minutes, and alarms were sounded for two minutes directly adjacent to the cylinders at the time of each rotation. At the end of two hours, the rats were placed back in their housing where they had free access to food and water.
Urine Catecholamines
Urine norepinephrine was measured on sacrifice day as a surrogate for catecholamine levels in the blood. Norepinephrine (NE) was analyzed rather than epinephrine because previous work has shown that persistent elevation of NE is associated with persistent injury-associated anemia.19, 20 Spontaneous urine samples were collected daily during chronic stress and stored at −20°C. Urine norepinephrine was measured by enzyme linked immunosorbent assay (LDN Immunoassay and Diagnostics, Nordhorn, Germany) according to manufacturer instructions.
Plasma Cytokines
Plasma interleukin 6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and stromal cell-derived factor 1 (SDF-1) were measured on day 7 and 14 by enzyme linked immunosorbent assay (R&D Systems, Thermo Fisher, Invitrogen, MyBioSource Inc, MyBioSource Inc, respectively) (Minneapolis, MN; Waltham, MA; Carlsbad, CA; San Diego, CA; San Diego, CA, respectively). All samples were run following the manufacturer’s protocol.
Liver, Spleen and Bone Marrow Cytokines
Liver and spleen expression of IL-6 and TNF-α was measured using real-time polymerase chain reaction (RT PCR). RT PCR was performed using SYBR Green Real-Time PCR Master Mix (Applied Biosystems, Foster City, CA). Primers were designed from specific mRNA sequences using the OligoPerfec Designer (ThermoFisher), ideally with 80–140 base pairs and a melting temperature of 60°C (Table 1). RNA isolation was performed using PureLink RNA Mini Kit (Invitrogen). RNA concentrations and purity were determined by measuring the ratio of the ultraviolet absorbance at 260nm and 280nm. RNA was reverse transcribed to cDNA using the Applied Biosystems protocol. The Mx3005P QPCR System was used for amplification (Agilent Technologies, Santa Clara, CA).
Table 1.
Polymerase chain reaction primers and conditions
| Gene | Forward Primer | Reverse Primer | Base Pair Position in mRNA sequence | qPCR Product Size (bp) | Annealing Temp (°C) |
|---|---|---|---|---|---|
| IL-6 | 5’-TGATGGATGCTTCCAAACTG -3’ | 5’-GAGCATTGGAAGTTGGGGTA -3’ | 352–581 | 229 | 56 |
| TNF-α | 5’-GAAACACACGAGACGCTGAA -3’ | 5’-GAAAGCCCATTGGAATCCTT -3’ | 1034–1115 | 82 | 56 |
| Beta-actin | 5’-AGCCATGTACGTAGCCATCC -3’ | 5’-ACCCTCATAGATGGGCACAG -3’ | 468–582 | 115 | 60 |
Hematopoietic Progenitor Cell Mobilization
Peripheral whole blood from cardiac puncture on the day of sacrifice was used to determine HPC mobilization by flow cytometry. Markers CD-71 and CD-117 (c-kit) were used to identify hematopoietic progenitor cells as they are found on early erythroid progenitors. CD-71 is a marker for BFU-E, one of the earliest erythroid progenitors.21 CD-117 is an early erythroid receptor which is expressed on progenitor cells such as CFU-GEMM, BFU-E, and CFU-E.22 100µl of whole blood was incubated in the dark with mouse anti-rat CD-71 antibody conjugated with fluorescein isothiocyanate (BD Pharmingen, San Jose, CA) and rat anti-mouse CD117 antibody conjugated with allophycocyanin (Southern BioTech, Birmingham, AL). 2ml of BD Phosflow Lyse/Fix Buffer was added then the solution was spun at 400g for 6 minutes. The fluid was decanted and 2ml 1x PBS was added to the tube. This procedure of adding phosphate-buffered saline (PBS), spinning the solution and decanting the fluid was repeated until it was clear. Once it was clear, the final fluid was drawn off and 500ml of Stain Buffer fetal bovine serum (FBS) was added. The blood sample was then run on BD LSR II flow cytometer equipped with FACSDiva software (BD Biosciences, San Jose, CA) to enumerate CD CD71+117+ cells.
Bone Marrow Cellularity and Erythroid Progenitor Colonies
Bone marrow was harvested from the left femur and BM erythroid progenitor colony growth assays were performed, including colony-forming unit granulocyte, erythrocyte, monocyte megakaryocyte (CFU-GEMM), burst-forming unit erythroid (BFU-E), and colony forming unit erythroid (CFU-E). The femur is flushed with Iscove’s Modified Dulbecco’s Medium (IMDM) + 2% Fetal Bovine Serum (FBS) (Stem Cell, Vancouver, Canada) until all bone marrow had been flushed out. A 1:100 dilution of this cell suspension was made in IMDM+2%FBS and the cells are counted in a TC-20 Automated Cell Counter (Bio Rad). 10µl of the diluted cells were mixed with 10µl of trypan blue dye and placed in the cell counter to determine the total, live and percentage of live cells. 50,000 bone marrow cells in 1ml methocult (SF M3436 for CFU-E and BFU-E from Stem Cell, and HSC012 from R&D Systems for CFU-GEMM) were added to 35mm petri dish. The cells were then incubated in a CO2 incubator maintained at 36C, 5% CO2 in air and ≥95% humidity. The colonies are enumerated after 7–14 days by a blinded observer.
Statistical Analysis
Statistical analysis and figure production were performed using GraphPad Prism Version 6.05 (GraphPad Software, La Jolla, CA) to calculate one-way analysis of variance with Bonferroni’s or Tukey’s multiple comparisons test. Significance was set at α = 0.05 and data were reported as mean ± standard deviation.
Results
Urine Catecholamines
Urine norepinephrine concentration in the CS 7-day rodents was 504% higher than that of the naïve rodents, reaching statistical significance (p=0.03) (Figure 1). Urine norepinephrine concentration in the CS 14-day rodents decreased significantly compared to the 7-day model, with a 92% decrease (p=0.04) (Figure 1).
Fig. 1.

Chronic stress increased urine epinephrine. Seven days of restraint stress significantly increased urine norepinephrine, while it decreased from 7 to 14 days of stress. *p < 0.05 vs. naïve; **p < 0.05 CS 7 Day vs. CS 14 day.
Plasma Acute Phase Reactants
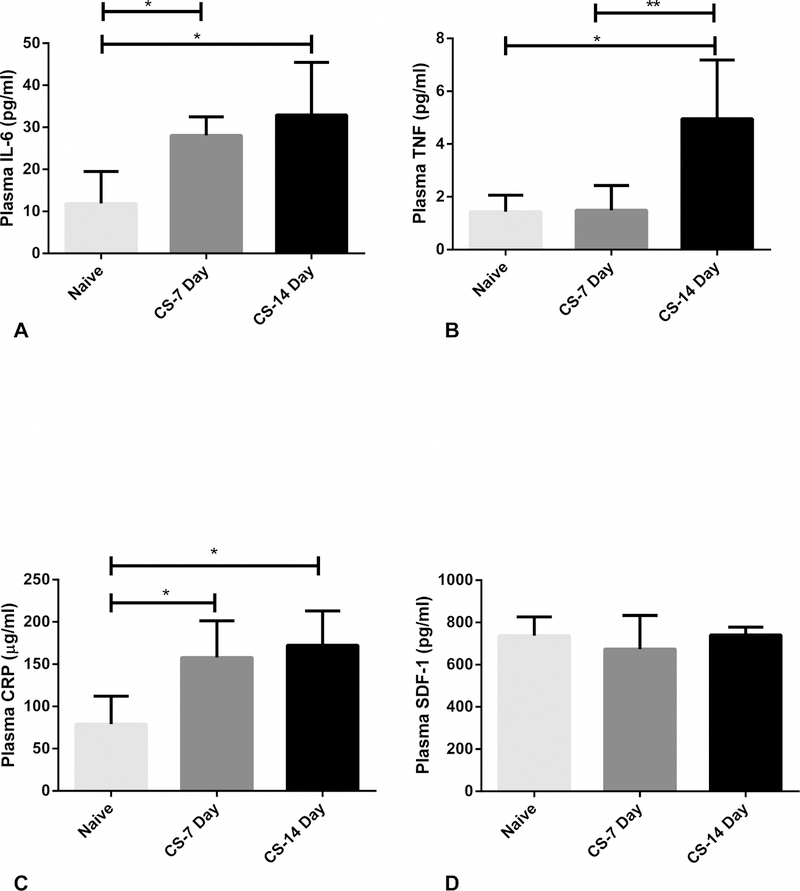
Plasma IL-6 concentration increased significantly with respect to naïve rodents in both the CS 7 and 14-day group with a 137% and 177% increase respectively (p<0.01 and p<0.01 respectively) (Figure 2a). Plasma TNF-α concentration in the CS 7 day rodents did not significantly change compared to naïve, however the CS 14 day TNF-α concentration increased 250% compared to naïve (p<0.01). The plasma concentration was also significantly increased from the CS 7 to CS 14-day rodents with a 227% increase (p<0.01) (Figure 2b). Plasma CRP concentration increased significantly from naïve to the CS 7-day rodents with a 100% increase (p<0.01), then increased again to the CS 14-day rodents with a 118% increase from naïve (p<0.01) (Figure 2c). Plasma SDF-1 concentration was not significantly altered at 7 or 14 days after chronic stress (Figure 2d).
Fig. 2.
A-D. Chronic stress increased plasma acute phase reactants. 2A. Plasma IL-6 increased significantly from naïve to 7 days of restraint stress, then again from 7 to 14 days of stress. 2B. Fourteen days of restraint stress significantly increased plasma TNF-α. 2C. Plasma CRP increased significantly from naïve to 7 days of restraint stress, then again from 7 to 14 days of stress. 2D. Plasma SDF-1 was not significantly affected by restraint stress. *p < 0.05 vs. naïve; **p < 0.05 CS 7 Day vs. CS 14 day.
Organ Dysfunction
Liver IL-6 expression increased from naïve to CS 7 day, then again from CS 7 day to CS 14 day. Expression at 14 days was significantly elevated compared to both naïve and 7-day rodents with a 180% (p=0.01) and 40% increase (p=0.03) (Figure 3a). Spleen IL-6 expression was significantly increased at 7 days compared to naïve (350% increase, p=0.01), then significantly decreased at 14 days compared to 7days (87% decrease, p=0.01) (Figure 3b).
Fig. 3.
A-B. Chronic stress increased expression of liver IL-6 and TNF-α. 3A. Fourteen days of restraint stress significantly increased liver expression of IL-6 compared to naïve and 7 days of stress. 3B. Seven days of restraint stress significantly increased spleen expression of TNF-α. *p < 0.05 vs. naïve; **p < 0.05 CS 7 Day vs. CS 14 day.
Liver and spleen TNF-α expression significantly increased from naïve to CS 7 day with a 1567% (p=0.02) and 285% (p=0.03) increase respectively (Figure 4a and 4b). Expression in both liver and spleen then significantly decreased from 7 days to 14 days with a 97% (p=0.03) and 92% (p=0.01) (Figure 4a and 4b).
Fig. 4.
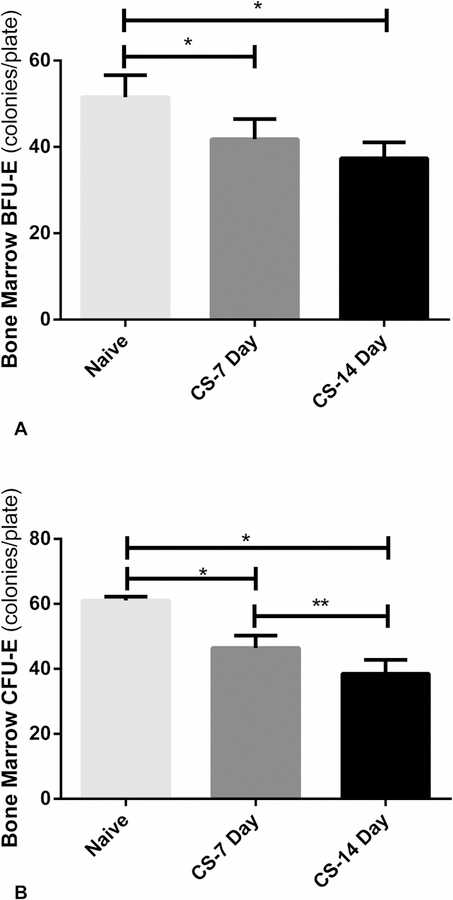
A-B. Chronic stress suppressed bone marrow erythroid progenitor colony growth. 4A. BFU-E was significantly suppressed after seven days of restraint stress, then further suppressed after 14 days of stress. 4B. CFU-E was significantly suppressed after seven days of restraint stress, then further suppressed after 14 days of stress. *p < 0.05 vs. naïve; **p < 0.05 CS 7 Day vs. CS 14 day.
Bone Marrow Cellularity and Progenitor Growth
In the CS 7-day model, bone marrow cellularity was significantly decreased compared to the naïve rodents with a 24 percent decrease in cellularity (p=0.01) (Figure 5). In the CS 14-day model, bone marrow cellularity increased from the naïve group and was significantly increased compared to the CS 7-day group with a 56% increase (p<0.01) (Figure 5).
Fig. 5.
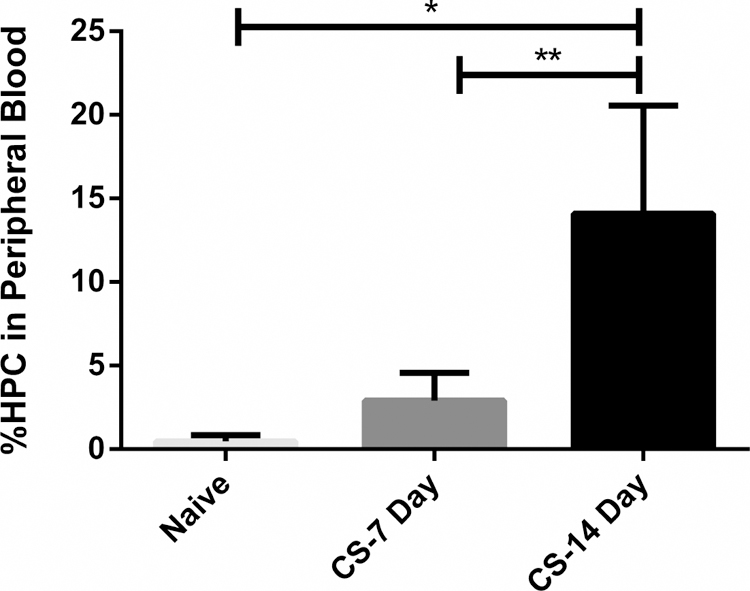
Chronic stress increased HPC mobilization. HPC mobilization to the peripheral blood was significantly increased after 7 days of restraint stress, then further increased after 14 days of stress. HPC: hematopoietic progenitor cell; *p < 0.05 vs. naïve; **p < 0.05 CS 7 Day vs. CS 14 day.
Erythroid progenitor CFU-GEMM was not significantly affected by CS in either the 7 or 14-day group (data not shown). However, both progenitor groups BFU-E and CFU-E were significantly suppressed by restraint stress. In the CS 7-day model, BFU-E was suppressed from the naïve group significantly by 19% (52 vs 42 colonies/plate, (p<0.01). After 14 days of chronic stress, BFU-E growth decreased by 27% compared to naïve (52 vs 37 colonies/plate, p<0.01) (Figure 4A). CFU-E growth decreased 24% in the 7 day chronic stress group compared to naïve (p<0.01) (Figure 4B). CFU-E growth was also significantly suppressed following 14 days of chronic stress compared to both naïve and 7 day chronic stress with a 37% (p<0.01) and 17% (p<0.01) decrease, respectively (Figure 4B).
Hematopoietic Progenitor Cell Mobilization
Hematopoietic progenitor cell mobilization to the peripheral blood was measured identifying CD71+CD117+ cells in whole blood. Hematopoietic progenitor cell mobilization to the peripheral blood was increased 511% following seven days of chronic stress when rodents compared to naïve (Figure 5). Following 14 days of chronic stress, the was a significant increase in hematopoietic progenitor cells in the peripheral blood compared to both naïve and CS 7 day with a 2780% (p<0.01) and 287% increase (p<0.01) (Figure 5).
Weight Change
All study rodents gained weight over the course of the study, but the CS 7-day rodents gained significantly less weight than their naïve counterparts by 50% (p<0.01). The CS 14-day CS rodents gained less weight than their naïve counterparts (33%), but gained significantly more weight than the 7-day CS rodents (171%) (p<0.01).
Discussion
These findings indicate that this rodent model of chronic restraint stress led to a systemic cytokine milieu associated with persistent low-grade inflammation, hypercatecholaminemia, increased acute phase reactants and evidence of organ dysfunction, similar to the persistent inflammation, immunosuppression and catabolism syndrome (PICS) described by Mira at el.23 Mira describes that trauma and/or infection leads to systemic inflammatory response syndrome (SIRS); those that do not progress to death from multiple organ failure (MOF), will either recover and reach homeostasis, or enter a stage of chronic critical illness (defined as > 14 days in intensive care unit with organ dysfunction) with ongoing immunosuppression, inflammation, and protein catabolism. Our study shows that daily restraint stress without physical trauma can also lead to elements of CCI and PICS and is an acceptable model of persistent inflammation.
Restraint stress led to a significant increase in urine norepinephrine concentration after 7 days. Urine was used as a surrogate for plasma as urine was more readily available, could be easily collected daily, and reflects systemic metabolism. Supraphysiologic norepinephrine has been shown to be associated with persistent anemia and systemic inflammation following trauma.19, 20, 24–31 Using a rodent model of lung contusion, hemorrhagic shock and chronic restraint stress, Alamo and Fonseca et al. demonstrated that increased plasma and urine norepinephrine concentrations were significantly increased on post-injury day 7, which was also associated with increased systemic acute phase reactants like IL-6.24, 26, 29
Chronic restraint stress increased the plasma acute phase reactants IL-6, TNF-α, and CRP from naïve to 7 days to 14 days of restraint stress. In a normal model of acute human healing, TNF-α reaches its peak plasma concentration within 82 minutes after traumatic injury then significantly decreases in the late trauma group.32 TNF-α from monocytes and endothelium induce IL-6 production which is first detected in the plasma of trauma patients within an hour of trauma.2 In the early hours following trauma, IL-6 induces the secretion of C-reaction protein (CRP). While our model time points were more extended than minutes to hours, the concentrations of IL-6, TNF-α, and CRP not only remained elevated at 14 days, but were increased at that time point demonstrating that chronic restraint stress was associated with a plasma cytokine milieu of chronic inflammation. These findings are in accordance with previous reports measuring acute phase reactants in rodents undergoing acute (forced swimming) and chronic stress (restraint stress).33 IL-6 and TNF-α were found to be greater in both the acute and chronic stressed groups.
As previously stated, increased levels of systemic IL-6 are seen in inflammation and stress. This lab has previously proposed that the hypercatecholamine state seen after trauma and/or stress, leads to increased IL-6 production by the liver and downstream iron metabolism dysfunction.24 Briest et al. has also explained that systemic IL-6 is an indicator to defense mechanisms like the liver, to produce acute phase reactants.34 Our study demonstrated that liver IL-6 expression was significantly increased after 7 days of CS then even further increased after 14 days of CS. Liver TNF-α expression significantly increased after 7 days of CS then decreased from 7 to 14 days. Spleen IL-6 and TNF-α expression increased after 7 days of CS, then decreased after 14 days. The spleen is an immune organ innervated by sympathetic nerves.35 Laukova et al. used Sprague-Dawley rats in a stress protocol using immobilization boards for 2 hours daily for 7 days. They found that repeated stress was associated with elevated expression of IL-6 and TNF-α in the spleen on the day of sacrifice.35 These findings reflect the increased liver and spleen expression of IL-6 and TNF-α in our model of chronic restraint stress and how this increased expression is associated with our additional findings.
Bone marrow erythroid progenitor colonies BFU-E and CFU-E were significantly suppressed following 7 days of CS and ever further suppressed following 14 days CS. Previous studies have demonstrated that the addition of chronic stress to a rodent model of trauma led to increasingly suppressed bone marrow erythroid progenitor colonies than with trauma alone.19, 24 The sympathetic nervous system terminates peripherally in organs like the bone marrow, secreting norepinephrine at times of stress.36 This model of chronic stress was associated with significantly elevated concentrations of urine norepinephrine. Fonseca et al. demonstrated that under physiologic conditions, norepinephrine accentuates BFU-E and CFU-E growth, but at severe stress levels, norepinephrine inhibits BFU-E and CFU-E growth.29 Additionally, studies using chemical sympathectomy show that erythroid progenitor colony (BFU-E and CFU-E) growth is inhibited in rodents with supraphysiologic concentration of norepinephrine.31 This relationship between norepinephrine and suppression of bone marrow erythroid progenitor growth reflect our study results.
Bone marrow cellularity was significantly decreased after 7 days of chronic stress, and HPC mobilization to the peripheral blood was significantly increased after 7 days of CS, then even further increased after 14 days. Previous studies using a rodent model of traumatic injury have shown that adding chronic restraint stress to the injury model significantly increased HPC mobilization to the peripheral blood and led to persistently increased concentration of plasma G-CSF.37 Previous work has shown that mobilization of hematopoietic stem cells (HSCs) into the peripheral blood is mediated by sympathetic innervation of the HSC bone marrow niche.38 Katayama et al. demonstrated in a mouse study that adrenergic bone marrow norepinephrine mediates G-CSF osteoblast suppression (to suppress bone formation), bone CXCL12 downregulation, and HPC mobilization.39 These findings correlate with this current study’s findings of increased norepinephrine concentration associated with significantly increased HPC mobilization to the peripheral blood following chronic restraint stress.
Chronic restraint stress was associated with decreased daily weight gain. Both 7 and 14 days of CS led to significantly less weight gain compared to their naïve counterparts. When PICS follows the SIRS response protein catabolism is associated with weight loss and decreased functional status.13, 23 Additionally, a characteristic response to a stressor is inhibition of food intake, increased heat production and increased activity with changes in body weight and hypothalamic-pituitary-adrenal (HPA) axis.16 Harris notes that chronic psychological stress is more likely to cause weight loss than weight gain in rodent models.16 In a separate study, immune activation by chronic immune challenges induced a depressive-like syndrome in rodents characterized by anhedonia, body weight loss and anorexia mediated by splenic TNF-α production.40 Additionally, TNF-α has been shown to upregulate major inflammatory mediators like nitric oxide and matrix metalloproteinases.34 When treatment attenuated adrenocortical activation and splenic TNF-α, many of the associated behavioral effects were mitigated.40 These results are similar to our current study as the naïve counterpart rodents gained more weight over the study period, while the rodents who underwent daily psychological stress gained significantly less weight.
Limitations
The major limitation of this model is that there are only two time points for sample collection and analysis when stress-induced systemic cytokine changes are known to vary throughout minutes/hours/days/weeks. A more thorough investigation would include time points starting at day 1 and extending out to day 14. Time points beyond 14 days raise concern for habituation which is more prominent in murine models.
Conclusion
Chronic restraint stress induced an overall state similar to chronic critical illness and persistent inflammation, immunosuppression, and catabolism syndrome. Stress increased urine norepinephrine, plasma IL-6, TNF-α, and CRP concentration, liver and spleen IL-6 and TNF-α expression, decreased bone marrow cellularity and erythroid progenitor growth, increased HPC mobilization, and decreased daily weight gain.
Combining this model with trauma and sepsis models will allow evaluation of the contribution of persistent inflammation in disease progression and outcomes.
CS Manuscript Highlights.
A daily restraint stress model can produce a low grade inflammatory state
Daily restraint stress increased norepinephrine and inflammatory cytokines
Daily restraint stress was associated with reduced weight gain
Adding stress to injury models can mirror the impact of the critical illness
Acknowledgements
The authors were supported in part by grants R01 GM105893–01A1 (AMM), R01 GM113945–01 (PAE), and P50 GM111152–01 (PAE and AMM) awarded by the National Institute of General Medical Sciences (NIGMS). ESM was supported by a postgraduate training grant (T32 GM-008721) in burns, trauma and perioperative injury by NIGMS. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose but do acknowledge the following grant funding.
References
- 1.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000 2013:63:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brochner AC, Toft P. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand J Trauma Resusc Emerg Med 2009:17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabay C Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006:8 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother 2018:105:1205–1222. [DOI] [PubMed] [Google Scholar]
- 5.Byrne CJ, Khurana S, Kumar A, Tai TC. Inflammatory Signaling in Hypertension: Regulation of Adrenal Catecholamine Biosynthesis. Front Endocrinol (Lausanne) 2018:9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 2003:24:25–29. [DOI] [PubMed] [Google Scholar]
- 7.Marin V, Montero-Julian FA, Gres S, Boulay V, Bongrand P, Farnarier C, Kaplanski G. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J Immunol 2001:167:3435–3442. [DOI] [PubMed] [Google Scholar]
- 8.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001:14:705–714. [DOI] [PubMed] [Google Scholar]
- 9.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg 2005:140:432–438; discussion 438–440. [DOI] [PubMed] [Google Scholar]
- 10.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma 1996:40:501–510; discussion 510–502. [DOI] [PubMed] [Google Scholar]
- 11.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O’Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, Maier RV, Inflammation, the Host Response to Injury Collaborative Research P. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med 2012:40:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA 2011:305:1001–1007. [DOI] [PubMed] [Google Scholar]
- 13.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 2012:72:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 2007:28:684–693. [DOI] [PubMed] [Google Scholar]
- 15.Noel JG, Valente JF, Ogle JD, Cornelius J, Custer DA, Li BG, Alexander JW, Ogle CK. Changes in bone marrow-derived myeloid cells from thermally injured rats reflect changes in the progenitor cell population. J Burn Care Rehabil 2002:23:75–86. [DOI] [PubMed] [Google Scholar]
- 16.Harris RB. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am J Physiol Regul Integr Comp Physiol 2015:308:R250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev 2009:33:1089–1098. [DOI] [PubMed] [Google Scholar]
- 18.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine Models of Sepsis and Trauma: Can We Bridge the Gap? ILAR J 2017:58:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg 2015:79:91–96; discussion 96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg 2014:77:54–60; discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong HY, Wilkes S, Yang H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol 2011:35:723–732. [DOI] [PubMed] [Google Scholar]
- 22.Broudy VC. Stem cell factor and hematopoiesis. Blood 1997:90:1345–1364. [PubMed] [Google Scholar]
- 23.Mira JC, Brakenridge SC, Moldawer LL, Moore FA Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit Care Clin 2017:33:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alamo IG, Kannan KB, Bible LE, Loftus TJ, Ramos H, Efron PA, Mohr AM. Daily propranolol administration reduces persistent injury-associated anemia after severe trauma and chronic stress. J Trauma Acute Care Surg 2017:82:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alamo IG, Kannan KB, Loftus TJ, Ramos H, Efron PA, Mohr AM. Severe trauma and chronic stress activates extramedullary erythropoiesis. J Trauma Acute Care Surg 2017:83:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alamo IG, Kannan KB, Ramos H, Loftus TJ, Efron PA, Mohr AM. Clonidine reduces norepinephrine and improves bone marrow function in a rodent model of lung contusion, hemorrhagic shock, and chronic stress. Surgery 2017:161:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alamo IG, Kannan KB, Smith MA, Efron PA, Mohr AM. Characterization of erythropoietin and hepcidin in the regulation of persistent injury-associated anemia. J Trauma Acute Care Surg 2016:81:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004:5:385–393. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma 2005:59:884–889; discussion 889–890. [DOI] [PubMed] [Google Scholar]
- 30.Millar JK, Kannan KB, Loftus TJ, Alamo IG, Plazas J, Efron PA, Mohr AM. Persistent injury-associated anemia: the role of the bone marrow microenvironment. J Surg Res 2017:214:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn A, Mohr AM, Shah SG, Sifri ZC, Kaiser VL, Rameshwar P, Livingston DH. Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold. J Surg Res 2010:163:e85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson KL, Taheri P, Rodriguez J, Tonapi V, Cardellio A, Dechert R. Tumor necrosis factor activity increases in the early response to trauma. Acad Emerg Med 1997:4:1035–1040. [DOI] [PubMed] [Google Scholar]
- 33.Himmerich H, Fischer J, Bauer K, Kirkby KC, Sack U, Krugel U. Stress-induced cytokine changes in rats. Eur Cytokine Netw 2013:24:97–103. [DOI] [PubMed] [Google Scholar]
- 34.Briest W, Elsner C, Hemker J, Muller-Strahl G, Zimmer HG. Norepinephrine-induced expression of cytokines in isolated biventricular working rat hearts. Mol Cell Biochem 2003:245:69–76. [DOI] [PubMed] [Google Scholar]
- 35.Laukova M, Vargovic P, Rokytova I, Manz G, Kvetnansky R. Repeated Stress Exaggerates Lipopolysaccharide-Induced Inflammatory Response in the Rat Spleen. Cell Mol Neurobiol 2018:38:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agac D, Estrada LD, Maples R, Hooper LV, Farrar JD. The beta2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun 2018. [DOI] [PMC free article] [PubMed]
- 37.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery 2015:158:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanoun M, Maryanovich M, Arnal-Estape A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015:86:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006:124:407–421. [DOI] [PubMed] [Google Scholar]
- 40.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Ann N Y Acad Sci 2000:917:478–487. [DOI] [PubMed] [Google Scholar]