Abstract
The HIV/AIDS epidemic can be eliminated if 73% of people living with HIV take antiretroviral medications and achieve undetectable viral loads. This study assessed the effects of financial incentives in suppressing viral load. People living with HIV with detectable viral loads (N=102) were randomly assigned to Usual Care or Incentive groups. Incentive participants earned up to $10 per day for 2 years for providing blood samples that showed either reduced or undetectable viral loads. This report presents data on the first year after random assignment. Incentive participants provided more (adjusted OR=15.6, CI 4.2–58.8, p< 0.001) blood samples at 3-month assessments with undetectable viral load (72.1%) than Usual Care Control participants (39.0%). We collected most blood samples. The study showed that incentives can substantially increase undetectable viral loads in people living with HIV. Financial incentives for suppressed viral loads could contribute to the eradication of the HIV/AIDS epidemic.
Keywords: Incentives, viral suppression, antiretroviral medication adherence, HIV, ART
Abstract
La epidemia de VIH/SIDA podría ser eliminada si el 73% de las personas que viven con VIH tomaran medicamentos antirretrovirales y lograran mantener la carga viral indetectable. Este estudio evaluó el efecto de incentivos económicos sobre la supresión de la carga viral. Personas que viven con VIH cuyas cargas virales estaban detectables (N = 102) fueron asignadas aleatoriamente a recibir atención de rutina o incentivos. Los participantes en el grupo de incentivos podían recibir hasta $10 por día durante dos años si sus muestras de sangre demostaban que la carga viral se había reducido o estaba indetectable. Este informe presenta datos sobre el primer año después de la asignación al azar. Los participantes en el grupo de incentivos proporcionaron más (proporción de probablidades ajustadas = 15.6, CI 4.2–58.8, p < 0.001) muestras cada tres meses con cargas virales indetectables (72.1%) que los participantes que recibieron cuidados de rutina (39.0%). Hemos recolectado la mayoría de las muestras de sangre. El estudio demostró que los incentivos pueden aumentar sustancialmente las cargas virales indetectables en personas que viven con VIH. Los incentivos económicos por mantener la carga viral suprimida podrían contribuir a la erradicación del VIH/SIDA.
INTRODUCTION
Antiretroviral medication use by people living with human immunodeficiency virus (HIV) can suppress the amount of HIV in the blood (viral load) to an “undetectable” level and thereby improve health and reduce HIV transmission.(1,2) However, many people living with HIV do not take antiretroviral medications.(3) In Baltimore, Maryland, where this research was conducted, only 40% of people living with HIV take antiretroviral medications and achieve undetectable viral loads.(4) The Joint United Nations Programme on HIV/AIDS estimates that we can eliminate the HIV/AIDS epidemic if 73% of people living with HIV take antiretroviral medications and achieve undetectable viral loads, a percentage well above many areas.(5)
Interventions designed to improve adherence to antiretroviral medications (e.g., motivational interviewing, web-based programs, text messages, directly observed therapy) can increase adherence, but typically do not suppress viral load.(6–8) Research over the past 40 years on the use of behavioral economic incentives in the treatment of drug addiction and other health problems suggests that incentives can promote health behaviors (9–12) and could promote viral suppression. Research on incentives shows that immediate consequences exert greater influence over behavior than delayed consequences.(13) Health behaviors like medication adherence have delayed health benefits, which may explain why those health behaviors do not maintain in many people without special interventions. Incentive interventions bridge the gap between the health behaviors and the naturally occurring but delayed health benefits. Specifically, incentive interventions provide immediate incentives for health behaviors like medication adherence and thereby increase the frequency of those health behaviors.
However, two multisite studies found that incentives produced small (14) or no (15) effects on HIV viral load. These multisite studies suggest that financial incentives may be marginally effective or ineffective in promoting viral suppression in people living with HIV, but those studies used low-magnitude and delayed incentives which may have diminished effectiveness.(12,16,17) This study evaluated the effects of financial incentives in suppressing viral load using empirically-based parameters, including high-magnitude incentives with little delay.(12,16,17) We expected more participants randomly assigned to receive financial incentives for suppressed viral load would provide blood samples with undetectable viral loads than Usual Care participants.
METHODS
Setting and Study Participants
The trial was conducted at the Center for Learning and Health, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine in Baltimore, Maryland.
Inclusion/exclusion criteria
Applicants were accepted if they were ≥18 years old; had been diagnosed with HIV for at least 12 weeks; had a detectable viral load (>200 copies/mL); and were not currently receiving HIV medical care or had been in HIV medical care for at least 12 weeks. These criteria increased the chance that we enrolled people who did not take antiretroviral medications and had a detectable viral load despite ample opportunity to access and take antiretroviral medications. Including people who had been diagnosed with HIV for at least 12 weeks increased the chance that we did not enroll newly diagnosed individuals who might adhere to antiretroviral medications when prescribed. Including people who had been in HIV medical care for at least 12 weeks increased the chance that we did not enroll individuals who were new to HIV medical care and who might adhere to antiretroviral medications when prescribed. Applicants were excluded if they reported current suicidal or homicidal ideation; or had a severe psychiatric disorder. All participants provided written informed consent.
Recruitment procedures
Participants were recruited in Baltimore from settings that serve people living with HIV and through compensated referrals by participants. Interested individuals completed a brief phone interview. Potentially eligible participants were invited for a full interview. Eligible participants completed a computerized course about HIV medical care, which included instruction to take prescribed antiretroviral medication every day to decrease viral load.(18) Participants not enrolled in HIV medical care were referred for care. All participants could receive standard HIV medical care offered in their medical clinic.
Study Design
This was a two-group randomized clinical trial. Participants (N = 102) were randomly assigned (1:1) to a Usual Care or Incentive group using a computerized urn randomization procedure to balance groups on characteristics that could influence outcome: (1) opiate- or cocaine-positive urine sample (Y/N); (2) self-reported alcohol use to intoxication on ≥20 days in the past 30 based on the Addiction Severity Index (Y/N); (3) health literacy based on the Test of Functional Health Literacy in Adults (TOFHLA; score ≤ the rolling median, Y/N); (4) impulsivity as assessed by delay discounting (k value for each participant ≤ the rolling median, Y/N); and (5) depression as assessed by the Beck Depression Inventory (score ≤ the rolling median, Y/N). Various staff members involved in the protocol operated the randomization program. The participants and staff were not blind to the conditions. Participants were taught the details of their group with written instructions and quizzes with incentives for correct responses.
The Incentive Intervention
All participants in the incentive group were exposed to the incentive intervention described below to promote suppression of viral load. We used a web-based computer program to manage the incentive program.
Prescriptions for antiretroviral medications
Initially, Incentive participants were paid $70 for bringing their antiretroviral medication bottle containing at least a 2-day supply of medication to our research unit. This ensured that Incentive participants had an active prescription for antiretroviral medications before beginning the incentive program for suppressed viral load.
Blood sample collections
After bringing an antiretroviral medication bottle, participants provided a blood sample. Then, each participant provided blood samples according to the schedule described below. When scheduled to provide a blood sample, each participant reported to our research unit and a staff member drew the blood sample. We sent each blood sample to a CLIA-certified laboratory for viral load testing.
Incentives for undetectable or decreased viral load
Participants earned up to $10 per day for providing a blood sample that had an undetectable viral load (i.e., <200 copies/mL) or a viral load that had decreased by 0.15 log per week since the last viral load assessed. If viral load fell below 1,000 copies/mL, incentives were provided when viral load decreased by any amount. When a participant provided a blood sample that met the criteria for earning incentives (i.e., undetectable viral load or a viral load that had decreased by 0.15 log per week since the last viral load assessed), the participant earned $10 per day since the last viral load test. The incentive program was in effect for two years so participants could earn a maximum of up to $7,300 in total (730 days × $10 per day = $7,300).
The incentive magnitude of $10 per day was selected because it is within the range of values that have been used in prior effective incentive interventions to promote antiretroviral pill taking,(19–21) drug abstinence in injection drug users,(22–25) and medication adherence in opiate dependent adults.(26) This magnitude is substantially higher than the magnitudes that were used in prior studies that evaluated the effects of incentives for viral load suppression. In those studies, patients could earn less than $1 per day for viral suppression.(14,15) However, incentives in those studies produced little or no effect on viral suppression.
Schedule of blood sample collections and viral load tests
Blood sample collections and viral load tests were scheduled on random weeks to ensure that participants did not take antiretroviral medications selectively before viral load tests. Initially, the viral load tests occurred weekly. Once a participant provided blood samples that met the incentive criteria on 4 consecutive weeks, the inter-test interval increased to once every 2 weeks, on average. The inter-test interval then increased to once every 4, 6, 8, and 12 weeks, on average, after a participant met the incentive criteria on 2 consecutive blood samples at each inter-test interval. The inter-test interval remained at 12 weeks for the remainder of the 2-year incentive intervention as under standard care.(27)
Monday calls
To schedule blood sample collections, each Incentive participant was instructed to call the Incentive Program every Monday to determine if he/she had to provide a sample that week. Incentive participants were paid $5 for each Monday call; participants did not earn any incentive for calling after Monday. If a blood sample was scheduled for that week, the Incentive participant scheduled a collection time within that week (Monday-Friday). If a blood sample was not scheduled for that week, the staff member told the Incentive participant that a blood sample was not due that week, but the staff member told the participant what he/she would earn if a blood sample is due the next week and if they meet the viral load criterion.
Consequences for missed blood sample collections or failed viral load tests
If an Incentive participant missed a blood sample collection or provided a sample that did not meet the viral load criterion for earning an incentive, the participant did not receive an incentive, the schedule switched to weekly testing, and the incentive was decreased to $3 per day. Once the participant earned an incentive at $3 per day, the incentive increased to $6 per day. Once the participant earned an incentive at $6 per day, the incentive increased to $10 per day. The frequency of testing also decreased as described above as participants achieved progressively longer periods meeting the incentive criteria.
Feedback and adding incentives to reloadable credit cards
Each Incentive participant was given a reloadable credit card at enrollment. After receiving a viral load result, a staff member called the participant to convey the results of the viral load test and any incentives earned. If the participant met the incentive criteria, the participant was told the amount earned and that amount was added to the participant’s reloadable credit card. Participants could use the card to make purchases at most businesses.
Trial Assessments
All participants were assessed at intake to the study, every 3 months after random assignment for two years, and every 6 months during the third year. At intake we administered the Beck Depression Inventory (BDI) to screen for depression,(28) the Test of Functional Health Literacy in Adults (TOFHLA) to measure health literacy,(29) a computerized delay discounting task to assess impulsivity,(30) the Wide Range Achievement (WRAT) test to assess academic skill levels,(31) a questionnaire to assess the likely mode of exposure to HIV, and the Addiction Severity Index.(32) We conducted the following tests/instruments at all assessments: HIV-1 RNA (viral load) in blood, CD4 cell blood count, antiretroviral medications in blood,(33) a visual analog scale to assess adherence to antiretroviral medications,(34) participant reports of refilling their antiretroviral medications prescription for each month, participant reports of how many primary HIV care visits they had in the past 3 months,(35) collection and testing of urine samples for opiates and cocaine, and forms to assess the economic impacts of the interventions.(36) Almost all blood samples for both groups were collected at our research unit. A very small number of blood samples were collected at a Quest collection facility when our phlebotomist was out sick. A small number of blood samples were collected at the participant’s HIV clinic when our phlebotomist was unable to successfully draw a blood sample from a participant. Participants earned $30 for intake assessments and $100 for each 3-month assessment. Assessment payments were also paid through reloadable credit cards.
Outcomes
The primary outcome measure was the percentage of blood samples collected at the 3-month assessments that had undetectable viral loads (i.e., <200 copies/mL). Secondary outcome measures included blood tests for antiretroviral medications; participant self-report of taking >90% of all scheduled antiretroviral medications doses in the past 90 days; the percentage of months that participants refilled an antiretroviral medication prescription; and the percentage of participants that attended at least 2 HIV medical visits per year. Blood tests for antiretroviral medications; all above effects during the second year of the incentive intervention and the year after the intervention ended; and moderator, mediator and economic measures will be assessed in future reports.
Statistical Analyses
Measures assessed repeatedly over time were analyzed with a longitudinal logistic regression model. Within-person correlated outcomes were handled using generalized estimating equations.(37) Measures assessed once were analyzed using logistic regression. The magnitude of effects were expressed using odds ratios with 95% CI. Intent-to-treat analyses were adjusted for covariates used for stratification.(38) Two-sided tests with p-values <.05 were considered significant. We fit a longitudinal logistic regression model logit (Yij) = β0+ β1tx + β2–6×2–6+εij, where Yij is the presence of a detectable viral load for the ith person at the jth timepoint, β1 is the covariate of interest representing the expected decrease in log odds of a detectable viral load as a function of assignment to the treatment group, and β2–6 are the coefficients for the 5 randomization covariates. All missing values, except for blood sample and self-report collections, were imputed as the adverse outcome (e.g., detectable viral load). Model parameter estimates from this approach were compared to a method without imputation. Participants with and without missing values on the primary outcome measure (undetectable viral loads) were compared by covariates and treatment assignment. Stata Statistical Software: Release 15 (College Station, TX; StataCorp LLC) was used to perform these analyses.
We followed Liu and Liang to determine the total N required to detect a difference between groups with 80% power.(39) A sample size of 200 was expected to be sufficient to detect a difference of 15% between groups in the percentage of participants with undetectable viral loads at the eight 3-month assessments, using a within-person correlation of .69 and an AR1 correlation structure. We stopped recruitment after randomly assigning 102 participants to the study groups because the costs of incentives and viral load testing was substantially more than expected (probably because we obtained much larger effects than expected) and because we would not have sufficient funding or time to complete the trial as originally planned. Recruitment stopped on 8/30/2017, 2 years and 4 months before funding for the study ends (12/31/2019), which was just enough time for the last participant to complete the intervention (2 years) and to write up and publish the final results of the study (4 months). We planned to analyze results over two years of exposure to the incentive intervention, but this paper reports results from the first year after random assignment for the full study sample (N=102). We obtained a between-group difference in the percentage of participants with undetectable viral load of 33% (see Table 2). Thus, we observed a substantially larger effect of the incentive intervention than we anticipated, which apparently allowed us to include a smaller sample size (N=102) than planned.
Table 2.
Primary and Secondary Outcome Measures for the Two Groups at 3-Month Assessments Throughout the Year After Random Assignment
| Percentage |
||||
|---|---|---|---|---|
| Usual Care (n=50) | Incentive (n=52) | OR (95% CI) | P value | |
| Primary Outcome | ||||
| Viral Suppression | ||||
| Undetectable Viral Load (missing = detectable) | 39.0 | 72.1 | 15.6(4.2–58.8) | <0.001 |
| Undetectable Viral Load (missing = missing) | 41.5 | 76.9 | 14.3(4.3–47.7) | <0.001 |
| Secondary Outcomes | ||||
| Self-Reported Adherence to Antiretroviral Medications | ||||
| > 90 % of doses (missing = nonadherence) | 42.5 | 65.9 | 4.7(1.6–14.0) | 0.006 |
| > 90 % of doses (missing = missing) | 45.2 | 69.9 | 5.9(1.9–18.2) | 0.002 |
| Filled Perscriptons for Antiretroviral Medications | ||||
| % of Months that Prescription was Filled (missing = not filled) | 78.7 | 82.9 | 2.6(0.5–13.8) | 0.251 |
| % of Months that Prescription was Filled (missing = missing) | 80.5 | 87.9 | 5.1(1.0–25.4) | 0.046 |
| Medical Visits | ||||
| Attended 2 or More Visits in the Year (missing = not attended) | 88.0 | 94.2 | 2.0(0.4–8.7) | 0.382 |
| Attended 2 or More Visits in the Year (missing = missing) | 89.8 | 96.1 | 2.7(0.5–15.0) | 0.270 |
| Collection Rate | ||||
| Collected Blood Samples | 94.0 | 93.8 | 0.8(0.1–13.0) | 0.877 |
| Collected Self-Reports | 94.0 | 94.2 | 0.8(0.1–10.8) | 0.877 |
Abbreviations: OR, Odds Ratio; CI, Confidence Interval
RESULTS
Study Population
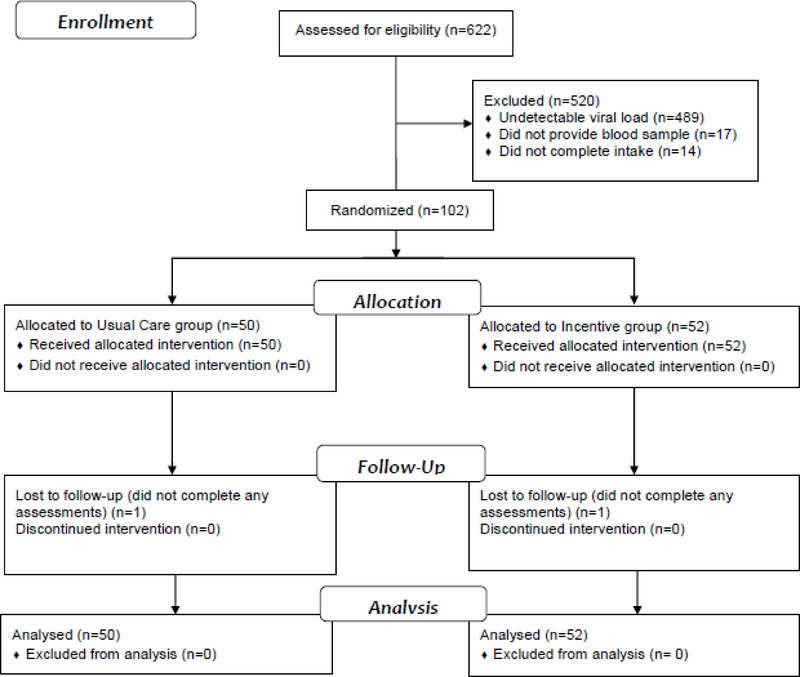
We recruited participants between 11/1/2015 and 8/30/2017 and conducted follow-up assessments until 10/23/2018. We assessed 622 participants for eligibility (see Figure 1). We excluded 489 participants (79%) who had undetectable viral loads; 17 participants (3%) who did not provide a blood sample; and 14 participants (2%) who did not complete the intake interview or the HIV education. We randomized 102 participants (50 to the Usual Care and 52 to the Incentive group). All randomized participants were included in the analyses.
Fig. 1.
Participant flow diagram through the study.
Table 1 shows characteristics of the participants who were randomized. A little over half of the participants were male; and most of the participants were black or African American, unemployed, and living in poverty. The largest percentage of participants believed that they acquired HIV through heterosexual sex, although many believed that they acquired HIV through injection drug use and men who had sex with men.
Table 1.
Participant Characteristics Before Randomization
| Usual Care (n=50) | Incentive (n=52) | |
|---|---|---|
| Age, mean (SD) | 47 (10) | 47 (9) |
| Men, n (%) | 27 (54) | 28 (54) |
| Race | ||
| White, n (%) | 5 (10) | 5 (10) |
| Black or African American, n (%) | 45 (90) | 46 (88) |
| Other, n (%) | 0 (0) | 1 (2) |
| Married, n (%) | 2 (4) | 2 (4) |
| High School Diploma or GED, n (%) | 34 (68) | 35 (67) |
| Unemployed, n (%) | 38 (76) | 45 (87) |
| Living in Poverty, n (%)a | 41 (82) | 43 (83) |
| HIV Exposure Category | ||
| Injection drug use, n (%) | 8 (16) | 10 (19) |
| Men who have sex with men, n (%) | 7 (14) | 8 (15) |
| Heterosexual sex, n (%) | 32 (64) | 25 (48) |
| Mulitple, n (%) | 1 (2) | 3 (6) |
| Other, n (%) | 2 (4) | 6 (12) |
| TOFHLA Score, mean (SD) | 79 (20) | 81 (19) |
| BDI Score, mean (SD) | 11 (10) | 9 (8) |
Living in Poverty was calculated using income, age, and family size data from the Addiction Severity Index and 2017 Poverty Thresholds from the US Census Bureau.
Study Outcomes
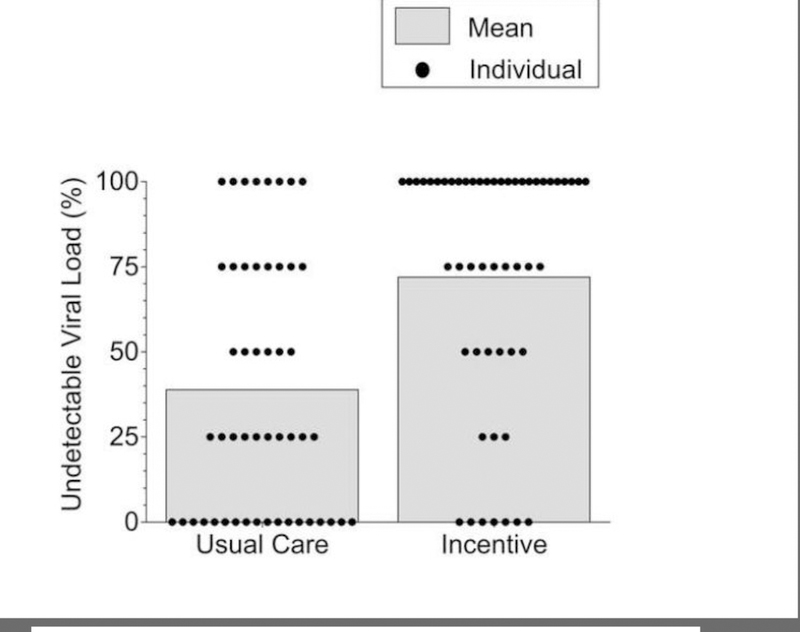
Table 2 shows the primary and secondary outcome measures based on data collected at the 3-month assessments. Incentive participants provided significantly and substantially more blood samples with undetectable viral load than Usual Care participants, independent of how missing samples were handled. Figures 2 and 3 provide detailed views of rates of undetectable viral loads for Usual Care (left panels) and Incentive (right panels) participants.
Fig. 2.
Percentage of blood samples with undetectable (<200 copies/mL) viral load. Dots represent data for individual participants and bars represent group means. Missing samples were considered detectable. Data are from blood samples collected every 3 months from all participants for the first year after random assignment (93.8% of all blood samples from the Incentive participants and 94.0% of all blood samples from Usual Care participants were collected). The difference between groups was statistically significant (Odds Ratio = 15.6; 95% CI = 4.2–58.8; P<0.001).
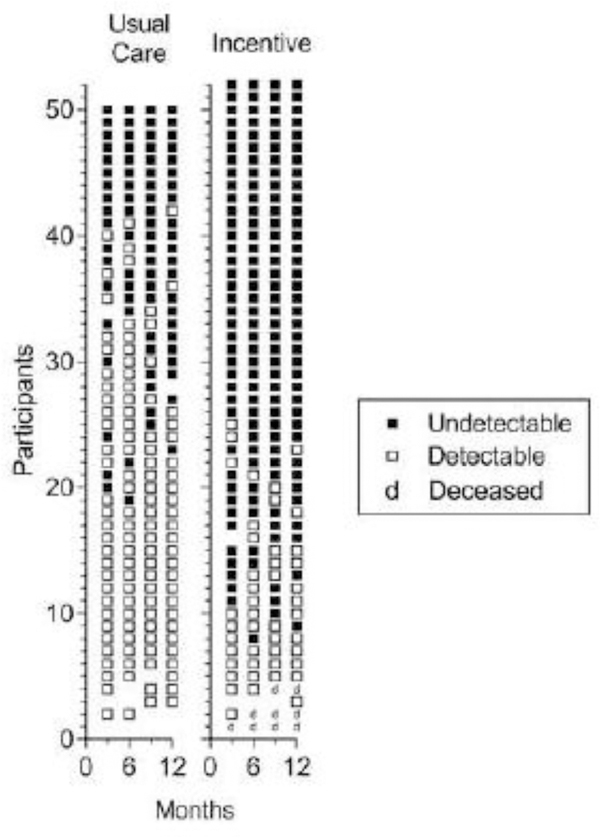
Fig. 3.
Consecutive viral load results for all blood samples collected at 3-month assessments from Usual Care (left panel) and Incentive (right panel) participants. Within each panel, each row of points represents data for the 3-, 6-, 9-, and 12-month assessment time points for a participant. Solid and open squares represent blood samples with undetectable (<200 copies/mL) and detectable viral loads, respectively. Blank spaces indicate missed samples and deceased individuals are represented by a “d.” Within each panel, participants are arranged from those showing the most blood samples with undetectable viral loads at the top to those with the fewest blood samples with undetectable viral loads at the bottom. The difference between groups was statistically significant (Odds Ratio = 15.6; 95% CI = 4.2–58.8; P<0.001).
We collected high rates of blood samples from the Usual Care (94.0%) and Incentive (93.8%) participants. Participants with missing values had higher (P = .019) scores on the Beck Depression Inventory (mean of 15.8, SD of 14.0) than participants without missing values (mean of 9.1, SD of 8.4). No further analyses were conducted due to the limited sample size.
Incentive participants reported higher rates of “taking more than 90% of the prescribed doses of antiretroviral medications” than Usual Care participants (Table 2). The two groups reported similar rates of filling antiretroviral medication prescriptions and attending medical visits.
Incentive participants earned an average (SD) of $178 ($72) for Monday calls and $2,096 ($1,210) for meeting the viral suppression criteria. Three Incentive group participants died (see Figure 3), but none appeared related to study participation. Participants reported 10 other adverse events (8 by Incentive participants) including congestive heart failure, dehydration, chest pain, infection, kidney failure, urinary problems, pneumonia (2), psychiatric treatment, and rash. None appeared related to study participation. The higher rate of adverse events reported by Incentive participants may have resulted from the fact that we had more contact with Incentive participants.
DISCUSSION
Contrary to two multisite studies,(14,15) this study shows that financial incentives can produce substantial increases in undetectable viral loads in people living with HIV. One study conducted by the Clinical Trials Network of the National Institute on Drug Abuse included 801 drug users in 11 US hospitals and failed to show that incentives increase undetectable viral load.(15) The other study conducted by the HIV Prevention Trials Network included 16,208 patients from 37 sites and showed that incentives can produce a small increase of 3.8% in percentage of participants with undetectable viral load.(14) In contrast, in this study financial incentives produced more than a 30% increase in the percentage of blood samples with undetectable viral loads. This study demonstrates conditions under which financial incentives can be effective.
Our incentive intervention differed in key respects from the incentive interventions used in previous studies. In the previous studies,(14,15) participants earned small incentive magnitudes for achieving undetectable viral loads after long delays of between 3 and 6 months, allowing participants to earn less than $1 per day. In contrast, participants in this study earned $10 per day for maintaining viral suppression and initially they could earn incentives for reduced or undetectable viral loads every week. The previous studies employed low magnitude and delayed incentives probably to enhance feasibility, but the parameters of incentive interventions can have dramatic effects on outcomes. Other studies have offered low-magnitude incentives for other health behaviors, and produced small increases in those health behaviors.(10,11) This study shows that financial incentives can have substantial effects on important health behaviors and suggests that the parameters of the incentive interventions matter.
The study populations of the prior studies and the current study also differed, which might account for the different outcomes observed across studies. All participants in the study by the Clinical Trials Network of the National Institute on Drug Abuse were drug users, whereas the current study included both drug users and non-drug users. The study by the HIV Prevention Trials Network included individuals who were taking antiretroviral medications and were virally suppressed, which may have diluted the overall impact of the incentive intervention in that study.
Most participants in both groups reported attending HIV medical care and filling antiretroviral prescriptions. The Incentive group did not receive any additional suggestions for taking medications, so it appears that the incentive intervention mainly increased motivation for taking antiretroviral medications.
Most participants lived in poverty and were black or African American. The effectiveness of financial incentives in low income, black or African American adults is particularly important since this population is disproportionately affected by the HIV/AIDS epidemic.(40)
Two major groups of people may not benefit from this incentive intervention. First, although the incentive intervention proved extremely effective in this study, many individuals exposed to the incentive intervention had a detectable viral load on one or more of the 3-month assessments (see Figure 3), showing that the incentive intervention was not sufficiently effective for these individuals. Identifying effective interventions for these treatment-refractory individuals will be an important focus of future research. Second, as shown in Figure 1, many people who were screened for this study did not qualify because they already had an undetectable viral load. Those individuals took antiretroviral medications regularly without the benefit of the incentive intervention.
This study does not show us how to apply this incentive intervention widely in society. For example, we do not know whether this incentive intervention would be appropriate or financially justifiable for all people living with HIV, including the many individuals who take antiretroviral medications consistently without an incentive intervention. However, special interventions appear required to suppress viral load in people who do not take antiretroviral medications under routine treatment conditions, which may be required to eradicate the HIV epidemic.(41) The incentive intervention examined in this study appears unusually effective and warrants further consideration. One possibility is that this intervention could be applied only to people who have detectable viral loads. We do not know whether selectively offering large incentives for viral suppression to people with detectable viral loads would encourage otherwise adherent individuals to stop taking antiretroviral medications to qualify for an incentive program. This study cannot resolve these difficult issues, but it does show clearly that financial incentives as arranged in this study can promote suppressed viral load in a difficult population and could contribute to the eradication of the HIV/AIDS epidemic. Finding reasonable ways to apply effective incentive interventions for viral suppression widely in society could be important to efforts to eradicate the HIV/AIDS epidemic.
Limitations
The study has limitations. First, it includes fewer participants than planned. We stopped recruitment early because we did not have sufficient money or time to complete the study as initially planned. Second, we reported on the results after all participants completed one year of exposure to the incentive intervention, whereas we originally planned to analyze results after two years. Third, we used a large incentive magnitude that some may view as impractical. As we have seen, small incentives may be marginally effective or ineffective,(14,15) so the larger magnitudes may be necessary to produce substantial clinical outcomes. In addition, we will conduct economic analyses to determine if the large incentive magnitudes can be justified financially. Fourth, we did not keep the amount of contact, blood sample collections, or viral load feedback equivalent between the Usual Care and Incentive groups. Incentive participants called and attended the research unit relatively often, and provided blood samples and received feedback on their viral load results more than participants in the Usual Care group. While we do not believe that those differences affected the study outcomes, we cannot rule them out.
CONCLUSIONS
This study suggests that financial incentives can produce large increases in undetectable viral loads in people living with HIV. Financial incentives for suppressed or undetectable viral loads as arranged in this study could contribute to the eradication of the HIV/AIDS epidemic.(5,41)
Acknowledgements
We are grateful to Jacqueline Hampton, who recruited participants for this study and conducted outcome assessments; Calvin Jackson, who collected blood samples for this study; and Haijuan Yan, who conducted statistical analyses. The study was supported by the National Institute of Allergy and Infectious Diseases and the National Institute on Drug Abuse of the National Institutes of Health under grants R01AI117065 and T32DA07209. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest The authors declare no conflicts of interests.
COMPLIANCE WITH ETHICAL STANDARDS
Ethical Approval The Johns Hopkins Medicine Institutional Review Board approved the study. The study was monitored by a Scientific Advisory Committee. Staff members reported all events that might be considered adverse events, including deaths, and the investigators determined if any events met the definitions of adverse events as defined by the Johns Hopkins Medicine Institutional Review Board. The investigators, the Scientific Advisory Committee and the Johns Hopkins Medicine Institutional Review Board reviewed all adverse events annually. We registered the trial on Clinicialtrials.gov before recruitment began (NCT02363387).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- (1).Montaner JS, Wood E, Kerr T et al. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55 Suppl 1:5. [DOI] [PubMed] [Google Scholar]
- (2).Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019;321(5):451–452. [DOI] [PubMed] [Google Scholar]
- (3).Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13:0 eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Baltimore Metro Annual HIV Epidemiological Profile 2016. https://phpa.health.maryland.gov/OIDEOR/CHSE/SiteAssets/Pages/statistics/Baltimore-City-HIV-Annual-Epidemiological-Profile-2016.pdf.
- (5).UNAIDS. 90–90-90: An ambitious target to help end the AIDS epidemic. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
- (6).Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence-enhancing interventions for highly active antiretroviral therapy in HIV-infected patients - a systematic review. HIV Med. 2013;14(10):583–595. [DOI] [PubMed] [Google Scholar]
- (7).Mbuagbaw L, Sivaramalingam B, Navarro T et al. Interventions for enhancing adherence to antiretroviral therapy (ART): A systematic review of high quality studies. AIDS Patient Care STDS. 2015;29(5):248–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kanters S, Park JJ, Chan K et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e40. [DOI] [PubMed] [Google Scholar]
- (9).Pilling S, Strang J, Gerada C, NICE. Psychosocial interventions and opioid detoxification for drug misuse: summary of NICE guidance. BMJ. 2007;335(7612):203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378(24):2302–2310. [DOI] [PubMed] [Google Scholar]
- (11).Halpern SD, French B, Small DS et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. [DOI] [PubMed] [Google Scholar]
- (13).Bickel WK, Johnson MW, Koffarnus MN, Mackillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).El-Sadr WM, Donnell D, Beauchamp G et al. Financial incentives for linkage to care and viral suppression among HIV-positive patients: A randomized clinical trial (HPTN 065). JAMA Intern Med. 2017;177(8):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Metsch LR, Feaster DJ, Gooden L et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: A randomized clinical trial. JAMA. 2016;316(2):156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcementof cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology (Berl). 1999;146(2):128–138. [DOI] [PubMed] [Google Scholar]
- (17).Silverman K, Kaminski BJ, Higgins ST, Brady JV. Behavior analysis and treatmentof drug addiction. In: Fisher WW, Piazza CC, Roane HS editors. New York, Guilford Press; 2011. [Google Scholar]
- (18).Subramaniam S, Getty CA, Holtyn AF et al. Evaluation of a computer-based HIV education program for adults living with HIV. AIDS Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rigsby MO, Rosen MI, Beauvais JE et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rosen MI, Dieckhaus K, McMahon TJ et al. Improved adherence with contingencymanagement. AIDS Patient Care STDS. 2007;21(1):30–40. [DOI] [PubMed] [Google Scholar]
- (21).Sorensen JL, Haug NA, Delucchi KL et al. Voucher reinforcement improvesmedication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Silverman K, Wong CJ, Higgins ST et al. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug Alcohol Depend. 1996;41(2):157–165. [DOI] [PubMed] [Google Scholar]
- (23).Silverman K, Higgins ST, Brooner RK et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53(5):409–415. [DOI] [PubMed] [Google Scholar]
- (24).Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66(5):811–824. [DOI] [PubMed] [Google Scholar]
- (25).Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol. 2004;72(5):839–854. [DOI] [PubMed] [Google Scholar]
- (26).Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54(2):127–135. [DOI] [PubMed] [Google Scholar]
- (27).Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- (28).Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–791. [DOI] [PubMed] [Google Scholar]
- (29).Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacyin adults: a new instrument for measuring patients’ literacy skills. J Gen Intern Med. 1995;10(10):537–541. [DOI] [PubMed] [Google Scholar]
- (30).Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: The concurrent presence of a substitute and an independent reinforcer. Behav Pharmacol. 2003;14(2):137–144. [DOI] [PubMed] [Google Scholar]
- (31).Wilkinson GS. WRAT-3: Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- (32).McLellan AT, Luborsky L, Cacciola J et al. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis . 1985;173(7):412–423. [DOI] [PubMed] [Google Scholar]
- (33).Marzinke MA, Breaud A, Parsons TL et al. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV Clin Trials. 2011;12(5):244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Purcell DW, Latka MH, Metsch LR et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46 Suppl 2:35. [DOI] [PubMed] [Google Scholar]
- (36).Bray JW, Zarkin GA, Miller WR et al. Measuring economic outcomes of alcoholtreatment using the Economic Form 90. J Stud Alcohol Drugs. 2007;68(2):248–255. [DOI] [PubMed] [Google Scholar]
- (37).Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- (38).Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–2930. [DOI] [PubMed] [Google Scholar]
- (39).Liu G, Liang KY. Sample size calculations for studies with correlated observations. Biometrics. 1997;53(3):937–947. [PubMed] [Google Scholar]
- (40).Oldenburg CE, Perez-Brumer AG, Reisner SL. Poverty matters: contextualizing the syndemic condition of psychological factors and newly diagnosed HIV infection in the United States. AIDS. 2014;28(18):2763–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: A plan for the United States. JAMA. 2019; 321(9):844–845. [DOI] [PubMed] [Google Scholar]