Abstract
OBJECTIVE:
To analyze trends in unindicated antibiotic use during vaginal delivery hospitalization.
METHODS:
This study used an administrative database to analyze antibiotic use during delivery hospitalizations from January 2006 to March 2015. Women were classified by mode of delivery and whether they had an evidence based indication for antibiotics. Indications for antibiotics included preterm premature rupture of membranes, cesarean delivery, group B streptococcus colonization, chorioamnionitis, endometritis, urinary tract infections, and other infections. The Cochran-Armitage test was used to assess trends of antibiotic administration. Unadjusted and adjusted analyses for antibiotic receipt including demographic, hospital, and obstetric and medical factors with unadjusted and adjusted risk ratios (RR, aRR respectively) with 95% confidence intervals (CI) as measures of association were performed.
RESULTS:
5,536,756 delivery hospitalizations including 2,872,286 vaginal deliveries with use of antibiotics without indication were analyzed. The most common indication for antibiotics was cesarean delivery (33.6% of the entire cohort), followed by GBS colonization (15.8%), chorioamnionitis (1.7%), PPROM (1.6%), endometritis (1.2%), urinary tract infections (0.6%), and other infections (total <0.5%). The proportion of women receiving unindicated antibiotics decreased 44.4% from 38.1% in 2006 to 21.2% in 2015. Adjusted risk for receipt of unindicated antibiotics was lower in 2015 compared to 2006 (aRR 0.56, 95% CI 0.55, 0.57).
CONCLUSION:
Use of antibiotics during vaginal delivery hospitalizations without an indication for antibiotic use declined significantly based on an analysis of a large administrative dataset.
Precis:
Analysis of an administrative database suggests that antibiotic use has decreased among women undergoing vaginal delivery who don’t have an evidence-based rationale for antibiotic administration
INTRODUCTION
In recent decades, indications for use of antibiotics during delivery hospitalizations have become increasingly specific, well defined, and supported by evidence.1–3 Benefits of antibiotic administration include both reduction of maternal infection morbidity as well as improvement of neonatal outcomes. Guideline-based administration of antibiotics to prevent perinatal group B streptococcal (GBS) disease has been associated with reduced adverse outcomes from this cause.3–6 Reduction of infection risk after cesarean with routine antibiotic prophylaxis has been established.7 Administration of antibiotics in the setting of preterm premature rupture of membranes (PPROM) is associated with reduced neonatal morbidity and prolonged latency between presentation and delivery.1,8
While appropriate antibiotic use may result in significant maternal and neonatal benefits, inappropriate use of these medications may result in unnecessary maternal and neonatal risks and contribute to antibiotic resistance.10–14 Risk for early-onset neonatal sepsis with ampicillin-resistant non-GBS organisms may be associated with broad use of antenatal ampicillin.15 Antibiotic exposure in utero may be associated with increased risk for pediatric allergic disease;16–18 antibiotics may alter the neonatal microbiome, which in turn may have consequences for long-term health outcomes.17
Currently trends in use of antibiotics during delivery hospitalizations in the United States are not well characterized. Characterizing use of antibiotic use in specific obstetric scenarios is of importance in determining whether evidence based recommendations are being adopted.19 Given this knowledge gap, the purpose of this study was to evaluate antibiotic trends during delivery hospitalizations in the US.
METHODS
The Premier Perspective database was used for this serial cross sectional analysis. It is an administrative inpatient database which reports on 100% of hospitalizations for 600 individual hospitals and ambulatory surgery centers across the United States and includes approximately 15% of hospitalizations nationally. It includes information on patient demographics, hospital characteristics, medications and devices received during hospitalizations as well as other information. Complete patient billing, hospital cost, and coding histories from are contained in the database. The Perspective database is commercially available and is commonly used across medical specialties for research that evaluates inpatient medication use.20–25 The Perspective database is maintained by Premier Incorporated (Charlotte, NC). Upon receiving data from participating hospitals, Premier undertakes an extensive 7-part data validation and correction process that includes more than 95 quality assurance checks prior to being used for research.26 After validations are complete, the data are moved to the Perspective data warehouse to populate and maintain the databases for health services research.27 This database includes data from hospitals in diverse geographic regions in the United States, both from teaching and non-teaching Institutions. The Columbia University Institutional Review Board deemed the study exempt given the data were de-identified.
For this analysis all women 18 to 54 years of age who underwent a delivery hospitalization from January 2006 through March 2015 were included. Delivery hospitalizations were identified based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) billing codes using an approach that ascertains more than 95% of deliveries (ICD-9-CM codes 650 and V27.x)28. The primary objective of this study was to estimate temporal trends in antibiotic administration without indication during vaginal delivery hospitalizations. The primary outcome was receipt of antibiotics during these hospitalizations. Evidence-based indications for antibiotic administration during vaginal deliveries included (i) PPROM, (ii) endometritis, (iii) chorioamnionitis, (iv) GBS colonization, and (v) other infectious complications such urinary tract infections, pneumonia, sepsis, and other major infections (Appendix 1). These indications were identified by ICD-9-CM diagnosis codes. For a diagnosis of PPROM, diagnoses of both PROM and preterm delivery were required. Because the development of research evidence and guidelines related to antibiotic prophylaxis for third and fourth degree vaginal lacerations, uterine tamponade, and manual extraction of the placenta developed coincident to the study period,19,29,30 women with these conditions but not another indication for antibiotics were excluded from the analysis. Because antibiotics in the setting of PPROM and GBS colonization are administered for neonatal benefit, patients with these diagnoses were excluded if a fetal demise was present and there was no other maternal indication for antibiotics. Cesarean delivery was classified based on ICD-9-CM procedure and diagnosis codes. As secondary outcomes, we evaluated temporal trends in antibiotic administration for (i) all cesarean deliveries, and (ii) vaginal deliveries with an evidence-based indication for antibiotic administration. Temporal trends were analyzed with the Cochran-Armitage test.
Perspective was queried for antibiotics that are commonly used during delivery hospitalizations. Use of penicillins, first through fourth generation cephalosporins, aminoglycosides, carbapenems, macrolides, fluoroquinolones, tetracyclines, as well as other drug classes was ascertained (Appendix 2). Both generic and trade names were queried. Hospital, patients, obstetric, and medical characteristics associated with receipt of antibiotics during (i) cesarean delivery, (ii) vaginal delivery with an evidence-based indication for antibiotic administration, and (iii) vaginal delivery without an evidence-based indication for antibiotic administration were determined (Table 1). Univariable associations are presented as unadjusted risk ratios (RR) with 95% confidence intervals (CI) as measures of association.
Table 1.
Demographics
| Mode of delivery | Vaginal delivery (no indication for antibiotics) | Vaginal delivery (indication for antibiotics) | Cesarean delivery | |||
|---|---|---|---|---|---|---|
| Antibiotics | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) |
| All patients | 879,673 (100) | 1,992,613 (100) | 616,589 (100) | 148,507 (100) | 1,688,046 (100) | 211,328 (100) |
| Year | ||||||
| 2006 | 105,787 (12.0) | 172,072 (8.6) | 62,019 (10.1) | 11,841 (8.0) | 155,495 (9.2) | 22,678 (10.7) |
| 2007 | 104,834 (11.9) | 182,234 (9.1) | 60,921 (9.9) | 12,466 (8.4) | 163,159 (9.7) | 24,544 (11.6) |
| 2008 | 96,673 (11.0) | 180,403 (9.1) | 57,407 (9.3) | 13,207 (8.9) | 159,104 (9.4) | 25,037 (11.8) |
| 2009 | 92,890 (10.6) | 188,617 (9.5) | 61,205 (9.9) | 13,826 (9.3) | 169,167 (10.0) | 24,098 (11.4) |
| 2010 | 93,605 (10.6) | 201,307 (10.1) | 63,522 (10.3) | 15,461 (10.4) | 179,146 (10.6) | 20,708 (9.8) |
| 2011 | 99,554 (11.3) | 236,282 (11.9) | 72,764 (11.8) | 17,679 (11.9) | 201,293 (11.9) | 22,157 (10.5) |
| 2012 | 103,469 (11.8) | 261,376 (13.1) | 77,453 (12.6) | 19,373 (13.0) | 218,179 (12.9) | 23,275 (11.0) |
| 2013 | 94,029 (10.7) | 266,803 (13.4) | 75,805 (12.3) | 19,221 (12.9) | 213,573 (12.7) | 22,725 (10.8) |
| 2014 | 74,615 (8.5) | 250,699 (12.6) | 69,972 (11.3) | 20,636 (13.9) | 190,557 (11.3) | 21,603 (10.2) |
| 2015 1st quarter | 14,217 (1.6) | 52,820 (2.7) | 15,521 (2.5) | 4,797 (3.2) | 38,373 (2.3) | 4,503 (2.1) |
| Age in years | ||||||
| 18-24 | 337,823 (38.4) | 680,191 (34.1) | 224,451 (36.4) | 48,348 (32.6) | 446,020 (26.4) | 42,703 (20.2) |
| 25-34 | 438,116 (49.8) | 1065901 (53.5) | 316,761 (51.4) | 79,461 (53.5) | 918,304 (54.4) | 117,499 (55.6) |
| 35-39 | 85,977 (9.8) | 205,277 (10.3) | 62,357 (10.1) | 17,135 (11.5) | 255,131 (15.1) | 39,750 (18.8) |
| ≥ 40 | 17,757 (2.0) | 41,244 (2.1) | 13,020 (2.1) | 3,563 (2.4) | 68,591 (4.1) | 11,376 (5.4) |
| Marital Status | ||||||
| Married | 379,360 (43.1) | 990,647 (49.7) | 281,395 (45.6) | 72,572 (48.9) | 860,636 (51.0) | 113,180 (53.6) |
| Single | 365,090 (41.5) | 772,161 (38.8) | 263,511 (42.7) | 58,253 (39.2) | 624,851 (37.0) | 66,379 (31.4) |
| Other/Unknown | 135,223 (15.4) | 229,805 (11.5) | 71,683 (11.6) | 17,682 (11.9) | 202,559 (12.0) | 31,769 (15.0) |
| Race | ||||||
| White | 431,168 (49.0) | 1109579 (55.7) | 318,727 (51.7) | 75,926 (51.1) | 918,988 (54.4) | 103,590 (49.0) |
| Black | 145,181 (16.5) | 215,682 (10.8) | 112,594 (18.3) | 25,033 (16.9) | 252,002 (14.9) | 25,512 (12.1) |
| Other | 303,054 (34.5) | 666,101 (33.4) | 184,939 (30.0) | 47,447 (31.9) | 516,271 (30.6) | 82,171 (38.9) |
| Unknown | 270 (0.0) | 1,251 (0.1) | 329 (0.1) | 101 (0.1) | 785 (0.0) | 55 (0.0) |
| Payer | ||||||
| Medicare | 6,812 (0.8) | 12,603 (0.6) | 4,829 (0.8) | 1,101 (0.7) | 14,991 (0.9) | 1,725 (0.8) |
| Medicaid | 422,836 (48.1) | 854,928 (42.9) | 270,912 (43.9) | 61,830 (41.6) | 682,631 (40.4) | 70,492 (33.4) |
| Commercial | 393,623 (44.7) | 1003954 (50.4) | 307,290 (49.8) | 76,250 (51.3) | 898,895 (53.3) | 128,067 (60.6) |
| Uninsured | 27,325 (3.1) | 52,362 (2.6) | 13,206 (2.1) | 3,735 (2.5) | 36,618 (2.2) | 4,082 (1.9) |
| Unknown | 29,077 (3.3) | 68,766 (3.5) | 20,352 (3.3) | 5,591 (3.8) | 54,911 (3.3) | 6,962 (3.3) |
| Obstetric comorbidity index score | ||||||
| 0 | 828,891 (94.2) | 1900207 (95.4) | 576,130 (93.4) | 139,624 (94.0) | 1500045 (88.9) | 186,980 (88.5) |
| 1 | 30,196 (3.4) | 64,154 (3.2) | 27,111 (4.4) | 5,931 (4.0) | 92,219 (5.5) | 11,703 (5.5) |
| 2 | 15,706 (1.8) | 22,032 (1.1) | 10,356 (1.7) | 2,336 (1.6) | 70,180 (4.2) | 9,488 (4.5) |
| ≥2 | 4,880 (0.6) | 6,220 (0.3) | 2,992 (0.5) | 616 (0.4) | 25,602 (1.5) | 3,157 (1.5) |
| Rurality | ||||||
| Urban | 813,235 (92.4) | 1767226 (88.7) | 564,391 (91.5) | 132,415 (89.2) | 1525197 (90.4) | 196,495 (93.0) |
| Rural | 66,438 (7.6) | 225,387 (11.3) | 52,198 (8.5) | 16,092 (10.8) | 162,849 (9.6) | 14,833 (7.0) |
| Hospital Teaching | ||||||
| No | 536,708 (61.0) | 1221453 (61.3) | 350,028 (56.8) | 68,126 (45.9) | 1047706 (62.1) | 91,771 (43.4) |
| Yes | 342,965 (39.0) | 771,160 (38.7) | 266,561 (43.2) | 80,381 (54.1) | 640,340 (37.9) | 119,557 (56.6) |
| Hospital Bed Size | ||||||
| <400 | 475,768 (54.1) | 1152025 (57.8) | 332,438 (53.9) | 75,280 (50.7) | 944,292 (55.9) | 91,957 (43.5) |
| 400-600 | 220,864 (25.1) | 521,841 (26.2) | 168,792 (27.4) | 39,571 (26.6) | 421,484 (25.0) | 63,392 (30.0) |
| >600 | 183,041 (20.8) | 318,747 (16.0) | 115,359 (18.7) | 33,656 (22.7) | 322,270 (19.1) | 55,979 (26.5) |
| Region of Hospital | ||||||
| Northeastern | 112,071 (12.7) | 329,134 (16.5) | 94,827 (15.4) | 37,176 (25.0) | 224,769 (13.3) | 87,632 (41.5) |
| Midwest | 152,704 (17.4) | 377,857 (19.0) | 129,954 (21.1) | 16,392 (11.0) | 268,580 (15.9) | 20,166 (9.5) |
| South | 456,264 (51.9) | 809,635 (40.6) | 264,178 (42.8) | 63,968 (43.1) | 875,174 (51.8) | 62,782 (29.7) |
| West | 158,634 (18.0) | 475,987 (23.9) | 127,630 (20.7) | 30,971 (20.9) | 319,523 (18.9) | 40,748 (19.3) |
| PPH | 28,424 (3.2) | 41,910 (2.1) | 22,394 (3.6) | 4,147 (2.8) | 33,616 (2.0) | 3,419 (1.6) |
| Preeclampsia | 31,153 (3.5) | 47,046 (2.4) | 18,763 (3.0) | 3,900 (2.6) | 122,889 (7.3) | 14,635 (6.9) |
| Gestational diabetes | 40,334 (4.6) | 91,979 (4.6) | 32,265 (5.2) | 7,588 (5.1) | 136,306 (8.1) | 17,553 (8.3) |
| Diabetes | 4,964 (0.6) | 7,875 (0.4) | 4,335 (0.7) | 878 (0.6) | 30,984 (1.8) | 3,282 (1.6) |
| Multiple gestation | 8,113 (0.9) | 9,651 (0.5) | 5,392 (0.9) | 1,245 (0.8) | 71,781 (4.3) | 9,953 (4.7) |
PPH, postpartum hemorrhage. Patients were categorized based vaginal versus cesarean delivery, and if vaginal delivery occurred, whether there was an evidence-based diagnosis for administering antibiotics including preterm premature rupture of membranes, endometritis, chorioamnionitis, GBS colonization, and other infectious complications.
We fit multivariable log-linear regression models with Poisson distribution and log link based on generalized estimating equations including demographic, hospital, and medical and obstetric factors accounting for hospital to determine which characteristics were associated with antibiotic administration for each of the three delivery scenarios. Multiple deliveries occurring to the same woman can be captured in Perspective if they occur at the same hospital; these models accounted for multiple deliveries to the same patient. Results are reported as adjusted risk ratios (aRR) with 95% confidence intervals (CI) as measures of association.
Three sensitivity analyses were performed for this study. First, hospitals are included in the Perspective database for varying durations of time. To account for confounding secondary to this changing sampling frame, we performed a sensitivity analysis restricting the population to only hospitals that contributed data from January 2006 to December 2014. The first quarter of 2015 was not included in this sensitivity analysis because of smaller sample sizes and less precise estimates.
Second, erythromycin is rarely indicated as a first-line medication for inpatient obstetric scenarios other than for PPROM and for women with penicillin and cephalosporin allergies. Erythromycin eye ointment is routinely administered shortly after birth, a practice supported by the United States Preventive Services Task Force.1 Given that there is a possibility for misclassification of erythromycin administration appearing in maternal as opposed to neonatal drug files when administered to newborns, we performed a sensitivity analysis evaluating temporal trends in antibiotics excluding erythromycin. For this sensitivity analysis, we excluded women with penicillin allergy (ICD-9-CM V14.0), which, aside from PPROM, is the most likely maternal indication of erythromycin. For the third sensitivity analysis, we repeated the second sensitivity analysis additionally excluding scenarios where women may have received antibiotics because their GBS status was unknown: (i) preterm labor, (ii) preterm delivery, (iii) PROM, and (iv) delayed or prolonged labor. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Of 5,657,523 delivery hospitalizations, 120,727 (2.1%) were excluded based on the above criteria leaving 5,536,756 delivery hospitalizations from 2006 through the first quarter of 2015 included in the analysis. This total included 2,872,286 vaginal deliveries without indication for antibiotics, 765,096 vaginal deliveries with an evidence-based indication for antibiotic administration, and 1,899,374 cesarean deliveries. The most common indication for antibiotics was cesarean delivery (33.6% of the entire cohort), followed by GBS colonization (15.8%), chorioamnionitis (1.7%), PPROM (1.6%), endometritis (1.2%), urinary tract infections (0.6%), and other infections (total <0.5%). Comparing patients undergoing vaginal delivery with an indication for antibiotics to those without, the former group were significantly more likely be age 35 or older, to be single, and to be black (p<0.01 for all) (Table 1).
For the primary outcome, 30.6% of women with a vaginal delivery and no indication for antibiotic administration received an antibiotic during the study period. In comparison 88.9% of women undergoing cesarean delivery and 80.6% of women with a vaginal delivery and an evidence-based indication for antibiotic administration received an antibiotic. Over the study period, the proportion of women receiving antibiotics with a vaginal delivery without indication decreased 44.4% from 38.1% in 2006 to 21.2% in 2015 (Figure 1A). Over this period, the proportion of women receiving antibiotics with a vaginal delivery and evidence-based indication for antibiotic administration decreased 9.1% from 84.0% to 76.4% (Figure 1B) and increased for cesarean delivery by 2.5% from 87.3% to 89.5% (Figure 1C). Trends for all three delivery categories were significant with the Cochran-Armitage test (p<0.01).
Figure 1:
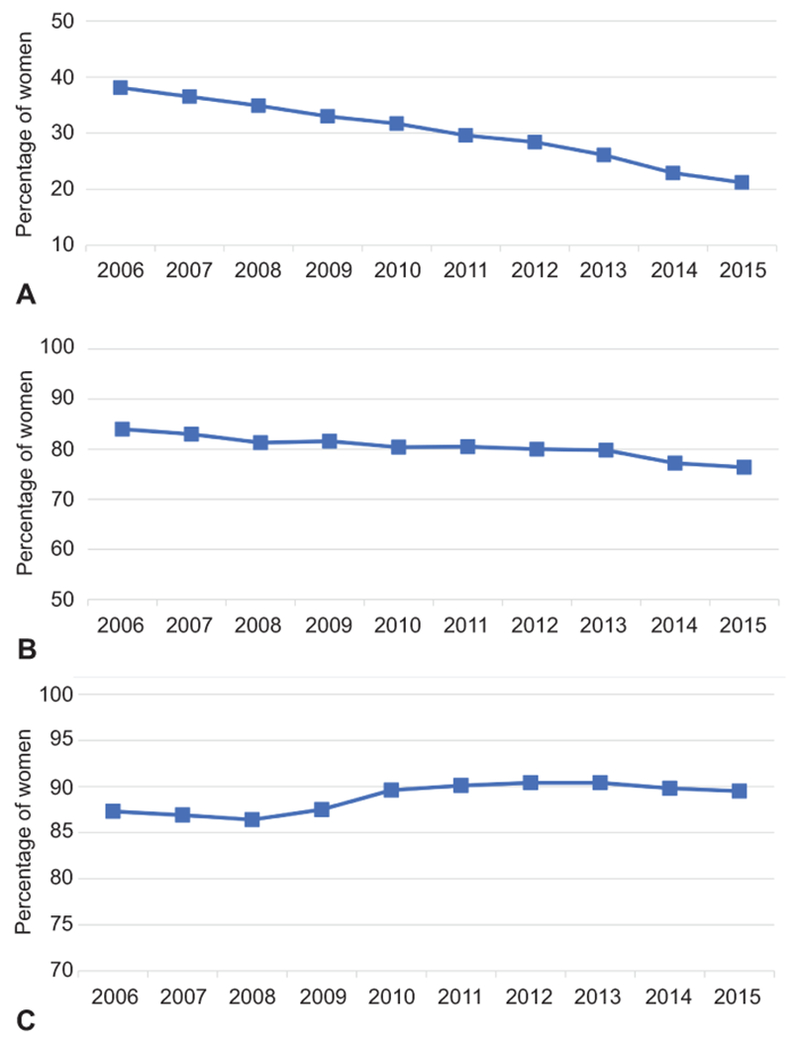
The proportion of women each year receiving antibiotics based on vaginal compared with cesarean delivery, and if vaginal delivery occurred whether there was an evidence-based diagnosis for administering antibiotics including preterm premature rupture of membranes, endometritis, chorioamnionitis, group B streptococcal colonization, and other infectious complications. Vaginal delivery without an indicated for antibiotics (A), vaginal delivery with an indication for antibiotics (B), and cesarean delivery (C).
For vaginal deliveries with no indication for antibiotics, the most commonly administered antibiotic was erythromycin (16.3% of patients), followed by ampicillin (7.8%), and penicillin (5.0%) (Appendix 3). For cesarean delivery hospitalizations the most common antibiotic administered was cefazolin (68.9% of patients), followed by erythromycin (14.9%), and ampicillin (9.3%). For vaginal delivery hospitalizations with an indication for antibiotics, the most commonly administered antibiotics included penicillin (35.0%), ampicillin (32.2%), and erythromycin (17.4%).
Temporal trends for the primary outcome were similar in the adjusted compared to the unadjusted analysis. Adjusted risk for receipt of antibiotics during vaginal delivery hospitalizations without an indication for antibiotics was lower in 2015 compared to 2006 (aRR 0.56, 95% CI 0.55, 0.57) (Table 2). Factors associated with increased antibiotic receipt during vaginal delivery hospitalizations in both unadjusted and adjusted models included black compared to white race (aRR 1.26 95% CI 1.25-1.27, respectively), urban compared to rural hospitals (aRR 1.36 95% CI 1.35-1.38, respectively), hospitals in the South compared to the Northeast (aRR 1.42 95% CI 1.41-1.43, respectively), multiple gestation (aRR 1.47 95% CI 1.44-1.51, respectively), and postpartum hemorrhage (aRR 1.33 95% 1.31-1.34, respectively).
Table 2.
Adjusted and unadjusted models for antibiotic administration during vaginal delivery hospitalizations without an indication for antibiotics
| Unadjusted model risk ratio (95% CI) | Adjusted model adjusted risk ratio (95% CI) | |
|---|---|---|
| Year | ||
| 2006 | Referent | Referent |
| 2007 | 0.96 (0.95, 0.97)** | 0.96 (0.95, 0.97)** |
| 2008 | 0.92 (0.91, 0.92)** | 0.92 (0.91, 0.93)** |
| 2009 | 0.87 (0.86, 0.87)** | 0.87 (0.86, 0.88)** |
| 2010 | 0.83 (0.83, 0.84)** | 0.83 (0.82, 0.84)** |
| 2011 | 0.78 (0.77, 0.79)** | 0.79 (0.78, 0.79)** |
| 2012 | 0.74 (0.74, 0.75)** | 0.75 (0.75, 0.76)** |
| 2013 | 0.68 (0.68, 0.69)** | 0.68 (0.68, 0.69)** |
| 2014 | 0.60 (0.60, 0.61)** | 0.60 (0.59, 0.60)** |
| 2015 1st quarter | 0.56 (0.55, 0.57)** | 0.56 (0.55, 0.57)** |
| Age in years | ||
| 18-24 | Referent | Referent |
| 25-34 | 0.88 (0.87, 0.88)** | 0.96 (0.96, 0.97)** |
| 35-39 | 0.89 (0.88, 0.90)** | 1.00 (0.99, 1.01) |
| ≥ 40 | 0.91 (0.89, 0.92)** | 1.02 (1.00, 1.03)* |
| Marital Status | ||
| Married | Referent | Referent |
| Single | 1.16 (1.15, 1.16)** | 1.06 (1.06, 1.07)** |
| Other/Unknown | 1.34 (1.33, 1.35)** | 1.23 (1.22, 1.23)** |
| Race | ||
| White | Referent | Referent |
| Black | 1.44 (1.43, 1.45)** | 1.26 (1.25, 1.27)** |
| Other | 1.12 (1.11, 1.12)** | 1.07 (1.06, 1.07)** |
| Unknown | 0.63 (0.56, 0.71)** | 0.80 (0.71, 0.90)* |
| Payer | ||
| Medicare | 1.25 (1.22, 1.28)** | 1.27 (1.24, 1.30)** |
| Medicaid | 1.17 (1.17, 1.18)** | 1.09 (1.08, 1.09)** |
| Commercial | Referent | Referent |
| Uninsured | 1.22 (1.20, 1.23)** | 1.09 (1.08, 1.10)** |
| Unknown | 1.06 (1.04, 1.07)** | 1.01 (0.99, 1.02) |
| Rurality | ||
| Urban | 1.38 (1.37, 1.40)** | 1.36 (1.35, 1.38)** |
| Rural | Referent | Referent |
| Hospital Teaching | ||
| No | Referent | Referent |
| Yes | 1.01 (1.00, 1.01)* | 0.97 (0.97, 0.98)** |
| Hospital Bed Size | ||
| <400 | Referent | Referent |
| 400-600 | 1.02 (1.01, 1.02)** | 0.99 (0.99, 1.00)* |
| >600 | 1.25 (1.24, 1.25)** | 1.14 (1.13, 1.14)** |
| Region of Hospital | ||
| Northeastern | Referent | Referent |
| Midwest | 1.13 (1.12, 1.14)** | 1.18 (1.17, 1.19)** |
| South | 1.42 (1.41, 1.43)** | 1.42 (1.41, 1.43)** |
| West | 0.98 (0.98, 0.99)** | 1.01 (1.00, 1.02)* |
| Postpartum hemorrhage | 1.33 (1.31, 1.35)** | 1.33 (1.31, 1.34)** |
| Preeclampsia | 1.31 (1.30, 1.33)** | 1.26 (1.24, 1.27)** |
| Gestational diabetes | 1.00 (0.99, 1.01) | 1.03 (1.02, 1.04)** |
| Pregestational diabetes | 1.26 (1.23, 1.30)** | 1.21 (1.18, 1.25)** |
| Multiple gestation | 1.50 (1.46, 1.53)** | 1.47 (1.44, 1.51)** |
Adjusted model includes all of the factors in the table as well as adjustment for hospital-level clustering. CI, confidence interval.
p<0.05
p<0.01.
Evidence-based diagnoses for administration of antibiotics includes preterm premature rupture of membranes, endometritis, chorioamnionitis, GBS colonization, and other infectious complications. Given the large sample and multiple comparisons, interpretation of results at p<0.01 may be more appropriate.
In the adjusted model for receipt of antibiotics during vaginal delivery hospitalizations with an indication for antibiotics, risk was lower in 2015 compared to 2006 (aRR 0.92 95% 0.91-0.92), similar to the unadjusted models (Appendix 4). For this outcome, Midwest compared to Northeast hospital location was associated with increased antibiotic receipt in both unadjusted and adjusted models (RR 1.24 95% CI 1.23-1.24, aRR 1.21 95% 1.21-1.22, respectively); other factors were not major predictors of antibiotic receipt. For the adjusted model for receipt of antibiotics during cesarean delivery hospitalizations, Northeast region was associated with decreased antibiotic administration in adjusted and unadjusted models; other factors were nor major predictors of antibiotic receipt (Appendix 5).
Results of the three sensitivity analyses aligned with those of the primary outcome analysis. When the analysis was limited hospitals that contributed data throughout the entire January 2006 to March 2015 study period similar trends in antibiotic administration were similar: antibiotic use during vaginal delivery hospitalizations without an indication for antibiotic administration decreased from 37.7% to 19.4% from 2005 to 2014, decreased from 81.6% to 75.8% for vaginal delivery hospitalizations with an indication for antibiotics, and decreased from 87.7% to 87.6% for cesarean deliveries (Appendixes 6–8). In the second sensitivity analysis restricted to women without a penicillin allergy undergoing vaginal delivery without an indication for antibiotic administration, the proportionate decrease in antibiotic administration was larger than in the primary analysis. Excluding erythromycin and women with a penicillin allergy, 20.2% of women undergoing vaginal delivery without an indication for antibiotics received antibiotics in 2006 compared to 15.1% in 2015, a 25.2% decrease (Figure 2). Additionally excluding women with premature rupture of membranes, preterm delivery, preterm labor, and delayed or prolonged labor to account for antibiotic administration to women with unknown GBS status, use of inappopriate antibiotics decreased from 17.5% in 2006 to 13.1% in 2015, a 25.4% decrease (Appendix 9).
Figure 2.
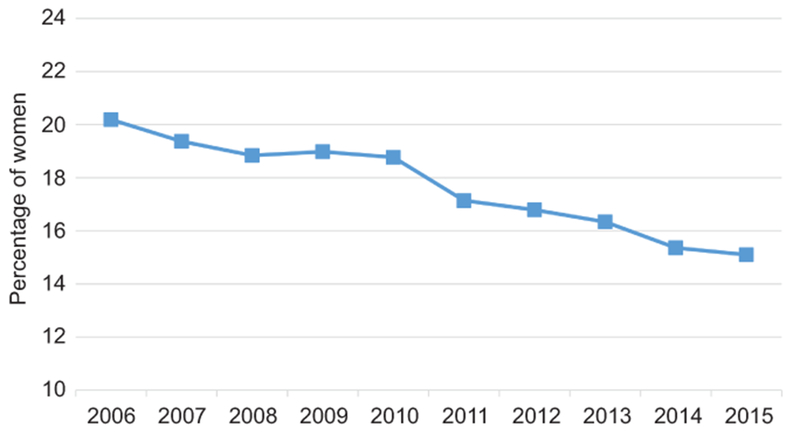
The proportion of women each year receiving antibiotics excluding erythromycin who underwent vaginal delivery without an evidence-based diagnosis for administering antibiotics. Women with penicillin allergy are excluded from this sensitivity analysis.
DISCUSSION
Our study demonstrated that for women undergoing vaginal delivery without an indication for antibiotic receipt, use of antibiotics decreased more than 40% with sensitivity analysis demonstrating significantly decreased rates of antibiotic use. These findings support that evidence based rationales for antibiotic use are becoming increasing adopted into clinical practice. This change in clinical practice may be beneficial in reducing antibiotic resistance, reducing risk for adverse for adverse reactions to unnecessary medications, and may have important downstream health effects.
According to the Centers for Disease Control and Prevention, 20% to 50% of all antibiotics prescribed during acute care hospitalizations in the United States are unnecessary or inappropriate;2 this antibiotic use has led to increased antibiotic resistance. Evidence based guidelines have been established to characterize specific indications, timing, duration and type of antibiotic use in labor and delivery.31 Child birth represents one of the most common indications for acute care hospitalizations and less frequent use of inappropriate antibiotics would represent an important public health achievement. Further research regarding care quality related to antibiotic management is indicated based upon these findings. Specifically, informatics data from hospital-system electronic medical records may be able to demonstrate more granular detail in demonstrating appropriate and timely administration of antibiotics based on individual diagnoses. While the overall trends in our analysis demonstrated improvements in indication-based antibiotic indication, it is possible that there may be important variation in care in specific obstetric scenarios.
Our study has several strengths. First, it includes a large sample size including over 5 million delivery hospitalizations and it is reflective of the practice patterns of a large proportion of deliveries within the United States. The database provides information on a wide range of demographics and different hospital settings and is representative of a geographically and clinically diverse sample. Our analysis was strengthened by a sensitivity analysis restricted to hospitals contributing data over the entire period, demonstrating that trends were not a result of the changing sampling frame of hospitals in the database.
In interpreting study results there are several important limitations. First, with administrative data, which is used mainly for billing purposes, there is concern for misclassification and under-ascertainment of both medications and diagnoses. Specifically, in our analysis there was a larger than anticipated proportion of women receiving erythromycin. We thus performed a sensitivity analysis excluding erythromycin among women undergoing vaginal delivery with no other indication for antibiotics; this analysis found that excluding erythromycin the decrease in the proportion of women receiving antibiotics without an indication was attenuated. While the high rate of erythromycin use is likely due to neonatal administration assigned to the maternal drug file, we are not able to evaluate more granular, chart-level data to validate actual medication receipt. It is possible that because of erythromycin misclassification the true decreased likelihood of unindicated antibiotic use may be of lesser magnitude than in the primary analysis.
Second, a significant proportion of women undergoing cesarean or vaginal delivery with an indication for antibiotics did not receive an antibiotic; further research with more granular data is indicated to determine to what degree our findings may be due to suboptimal care versus under-ascertainment of diagnoses in administrative data. To some degree trends noted could have been due to improved ascertainment and documentation over the study period with indications being more commonly captured in administrative data; likewise, some women who received antibiotics may have had an indication for receipt not captured in coding. However, that there was a relatively large decrease in unindicated antibiotic administration over the study period supports that these findings are in fact representative of change in clinical practice.
A third limitation is that our results reflect practices up to 2015; there could have been subsequent improvement of obstetric antibiotic administration since that time which is not examined in our study. Last, given that the data derives from an administrative database, we could not perform chart review to analyze the causes for non-compliance with antibiotic guidelines. Finally, we are not able to evaluate a number of important characteristics related to appropriate use of antibiotics including timing of administration relative to diagnosis of individual conditions, whether the antibiotics were administered antepartum, intrapartum or postpartum, whether individual drug dosages and duration of therapy were appropriate, and whether medications were continued on an outpatient basis when indicated
Over the study period, use of antibiotics during vaginal delivery hospitalizations without an indication for antibiotic use declined significantly. This trend represents a meaningful improvement in obstetric care. Hospital-system level clinical studies further characterizing antibiotic use with granular informatics data are indicated to further characterize care quality and optimization with regards to use of antibiotics.
Supplementary Material
Acknowledgments
Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Presented at the 39th Annual Meeting for the Society for Maternal Fetal Medicine, February 11–16, 2019, Las Vegas, Nevada.
Financial Disclosure
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. D’Alton has a leadership role in ACOG II’s Safe Motherhood Initiative, which has received funding from Merck for Mothers. The other authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
REFERENCES
- 1.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003;101:178–93. [DOI] [PubMed] [Google Scholar]
- 2.Tuuli MG, Liu J, Stout MJ, et al. A Randomized Trial Comparing Skin Antiseptic Agents at Cesarean Delivery. N Engl J Med 2016;374:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion No. 782. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019;134:e19–40.31241599 [Google Scholar]
- 4.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases NCfI, Respiratory Diseases CfDC, Prevention. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59:1–36. [PubMed] [Google Scholar]
- 5.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009;360:2626–36. [DOI] [PubMed] [Google Scholar]
- 6.Lukacs SL, Schrag SJ. Clinical sepsis in neonates and young infants, United States, 1988-2006. J Pediatr 2012;160:960–5 e1. [DOI] [PubMed] [Google Scholar]
- 7.Smaill FM, Gyte GM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2010:CD007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA 1997;278:989–95. [PubMed] [Google Scholar]
- 9.Chapman E, Reveiz L, Illanes E, Bonfill Cosp X. Antibiotic regimens for management of intra-amniotic infection. Cochrane Database Syst Rev 2014:CD010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland ML, Vermillion ST, Soper DE, Austin M. Antibiotic resistance patterns of group B streptococci in late third-trimester rectovaginal cultures. Am J Obstet Gynecol 2001;184:1125–6. [DOI] [PubMed] [Google Scholar]
- 11.Mercer BM, Carr TL, Beazley DD, Crouse DT, Sibai BM. Antibiotic use in pregnancy and drug-resistant infant sepsis. Am J Obstet Gynecol 1999;181:816–21. [DOI] [PubMed] [Google Scholar]
- 12.Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med 2014;12:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez de Tejada B Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health 2014;11:7993–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan S, Holliman R, Russell AR. Hazards of widespread use of erythromycin for preterm prelabour rupture of membranes. Lancet 2003;361:437. [DOI] [PubMed] [Google Scholar]
- 15.Towers CV, Carr MH, Padilla G, Asrat T. Potential consequences of widespread antepartal use of ampicillin. Am J Obstet Gynecol 1998;179:879–83. [DOI] [PubMed] [Google Scholar]
- 16.Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1,401 US children. Am J Epidemiol 2011;173:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med 2016;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr 2013;162:832–8 e3. [DOI] [PubMed] [Google Scholar]
- 19.Coleman JMA, Silverman NS. ACOG Practice Bulletin No. 199: Use of Prophylactic Antibiotics in Labor and Delivery. Obstetrics and gynecology 2018;132:e103–3119. [DOI] [PubMed] [Google Scholar]
- 20.Fang MC, Maselli J, Lurie JD, Lindenauer PK, Pekow PS, Auerbach AD. Use and outcomes of venous thromboembolism prophylaxis after spinal fusion surgery. Journal of thrombosis and haemostasis : JTH 2011;9:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulik A, Rassen JA, Myers J, et al. Comparative effectiveness of preventative therapy for venous thromboembolism after coronary artery bypass graft surgery. Circulation Cardiovascular interventions 2012;5:590–6. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakaran S, Herbers P, Khoury J, et al. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage? Stroke 2015;46:369–75. [DOI] [PubMed] [Google Scholar]
- 23.Ritch JM, Kim JH, Lewin SN, et al. Venous thromboembolism and use of prophylaxis among women undergoing laparoscopic hysterectomy. Obstet Gynecol 2011;117:1367–74. [DOI] [PubMed] [Google Scholar]
- 24.Wright JD, Lewin SN, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Annals of surgery 2011;253:1140–6. [DOI] [PubMed] [Google Scholar]
- 25.Zacharia BE, Youngerman BE, Bruce SS, et al. Quality of Postoperative Venous Thromboembolism Prophylaxis in Neuro-oncologic Surgery. Neurosurgery 2017;80:73–81. [DOI] [PubMed] [Google Scholar]
- 26.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. Jama 2010;303:2479–85. [DOI] [PubMed] [Google Scholar]
- 27.Swanson SJMB, Gunnarsson CL, Moore M, Howington JA, Maddaus MA, McKenna RJ, Miller DL. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93(4):1027–32. [DOI] [PubMed] [Google Scholar]
- 28.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Maternal and child health journal 2008;12:469–77. [DOI] [PubMed] [Google Scholar]
- 29.WHO guidelines for the management of postpartum haemorrhage and retained placenta. 2009:19–20. [PubMed]
- 30.Duggal NMC, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstetrics and gynecology 2008;111:1268–73. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan SA, Soper D. Antibiotic prophylaxis in obstetrics. Am J Obstet Gynecol 2015;212:559–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


