Abstract
Mutations in the TANK binding kinase 1 gene (TBK1) are associated with amyotrophic lateral sclerosis and/or frontotemporal dementia; however, the range of clinical phenotypes and neuropathological changes associated with these mutations have not yet been completely elucidated. We present the detailed clinical, neuroimaging and neuropathological features of two brothers carrying the TBK1 p.Gly272_Thr331del mutation. Both presented with very similar and unusual clinical features including primary progressive aphasia and asymmetric-onset primary lateral sclerosis (PLS). Repeated electrophysiological studies failed to reveal any lower motor neuron involvement. Neuropathological evaluation of both cases revealed frontotemporal lobar degeneration with TDP-43 proteinopathy type B and selective involvement of upper motor neurons with TDP-43 inclusions. The stereotypical clinical presentation and neuropathological findings in these cases widen the phenotypic spectrum of TBK1 mutations and provide insights into the pathogenesis of PLS.
Keywords: TBK1, primary progressive aphasia, primary lateral sclerosis, TDP-43, neuropathology
Introduction
Frontotemporal dementia (FTD) is clinically characterized by abnormalities in personality, behaviour and/or language. Some patients with FTD also develop prominent extrapyramidal movement disorders, motor neuron disease (MND) or both. The neuropathological substrate of FTD is most often selective atrophy of the frontal and temporal lobes, termed frontotemporal lobar degeneration (FTLD). Four major subtypes of FTLD are recognized based on the identity of the protein that abnormally aggregates in neurons and glia. Of these, FTLD with inclusions of TAR DNA binding protein 43 (FTLD-TDP) has been suggested to be on a spectrum with MND, in particular amyotrophic lateral sclerosis (ALS). ALS is characterized by progressive neurodegeneration of upper and lower motor neurons (UMN and LMN, respectively) and results in spasticity, hyper-reflexia, weakness, muscle atrophy and fasciculations. A related MND, primary lateral sclerosis (PLS), affects only UMN and does not lead to muscle atrophy or fasciculations. PLS is relatively rare, representing less than 5% of all MND (1), and is usually not associated with other major neurological deficits. However, both ALS and PLS have some clinical overlap with FTD: approximately half of ALS and 22% of PLS patients develop signs of frontotemporal dysfunction and approximately 15% of ALS and 3% of PLS patients develop full-blown FTD (2-5). Although half of FTD patients develop signs of MND and approximately 15% develop full-blown ALS (6), typical PLS is uncommon.
Controversy exists as to whether PLS is simply a ‘forme fruste’ of ALS or a distinct entity with a different molecular basis. Both are characterized neuropathologically by degeneration of UMN in the primary motor cortex, corticobulbar and corticospinal tracts, but only ALS shows LMN degeneration in the brainstem and spinal cord. In addition, the vast majority of ALS cases show TDP-43 immunoreactive (-ir) inclusions in UMN, LMN and glia (7,8); whereas the molecular signature of PLS is less established. At least some cases described after the discovery of TDP-43 have been shown to display this pathology (5,9,10).
Strengthening the concept of an FTLD/ALS-TDP disease spectrum are genetic variants that may result in FTD, ALS or FTD and ALS, in members of the same family. Recently, loss-of-function (LoF) mutations in the TANK binding kinase 1 gene (TBK1) have been added to the list of ALS/FTD causing mutations (11). Initial reports estimated the prevalence of TBK1 mutations in ALS/FTD somewhere between 2 to 10% (12-14). A more recent meta-analysis reported that TBK1 mutations may be less common than originally thought (1.0% and 1.8% for TBK1 LoF and missense mutations, respectively), but that they are strong risk factors for both ALS and FTD (15). The full range of clinical and neuropathological phenotypes associated with these mutations is being elucidated.
Here we report two brothers who carried the c.992+1G>A TBK1 mutation (p.G272_T331del) and developed remarkably similar and unusual clinical features. Both presented with prominent language dysfunction and asymmetric UMN signs associated with strikingly asymmetric, left greater than right (left > right) cerebral atrophy without evidence of LMN involvement. Neuropathological evaluation of both cases demonstrated TDP-ir pathology with an anatomical distribution that correlated strongly with their primary progressive aphasia (PPA) and PLS phenotypes.
Methods
Genetics
The mutation was initially identified through whole-genome sequencing (WGS) of the proband as described elsewhere (16). Variant calling was performed according to the GATK best practice recommendations (17,18). To validate and extend these results, TBK1 sequencing of exons 2-21 was performed on three siblings. Each TBK1 exon was amplified and Sanger sequenced using Big Dye chemistry (Applied Biosystems, Foster City, CA, USA). All PCR and sequence products were purified using Mag-Bind bead technology (Omega Bio-tek, Norcross, GA, USA) and run on ABI3730 DNA Analyzer.
cDNA was prepared from frontal cortex available from the proband (Case 1) and a neuropathological normal individual (Invitrogen, Carlsbad, CA, USA). cDNA was amplified using primers located in TBK1 exons 7 and 9 and migrated on an 1% agarose gel (primers available upon request). RT-PCR products were gel extracted using the Gel extraction kit MinElute (Qiagen, Hilden, Germany) and Sanger sequenced.
Neuropathology
Formalin-fixed, paraffin-embedded brain and spinal cord tissue blocks were stained with standard histochemical, silver and Congo red stains. Immunohistochemistry (IHC) was performed for the following; alpha-synuclein (Thermo Scientific; 1:10,000), beta amyloid (DAKO; 1:100), hyperphosphorylated tau (clone AT-8; Innogenetics; 1:2000), phosphorylation-independent TDP-43 (ProteinTech; 1:1000), ubiquitin (DAKO; 1:500), FUS (Sigma-Aldrich; 1:1000) and SOD1 (monoclonal against misfolded SOD1, gift from Dr. N. Cashman; 1:500 dilution).
Results
Genetics
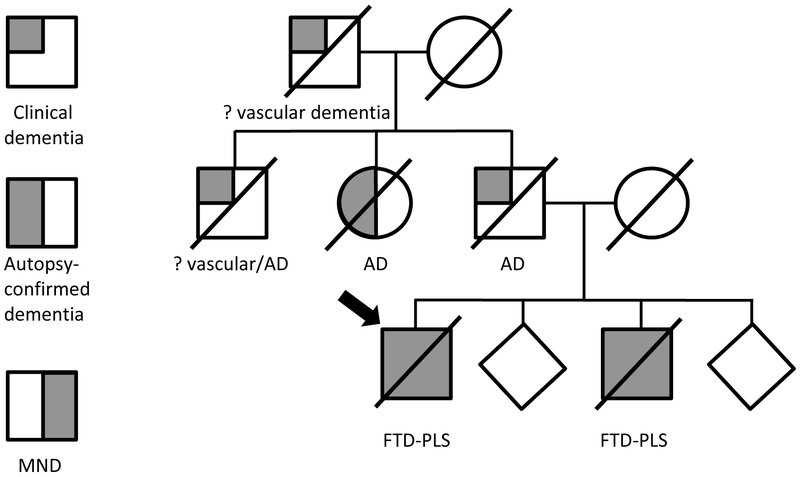
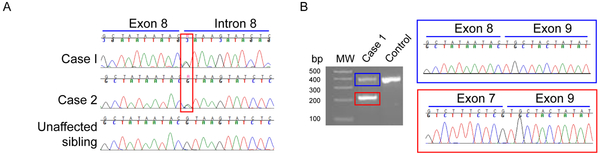
This family of European ancestry showed a suspected autosomal dominant pattern of involvement [Figure 1]. WGS revealed the presence of a c.992+1G>A TBK1 mutation in the proband (Case 1). Sanger sequencing confirmed the presence of this mutation in an affected sibling (Case 2) and its absence in an unaffected sibling [Figure 2A]. No other mutations in known neurodegenerative disease genes with a minor allele frequency of less than 5% in GnomAD non-Finnish European ancestry were observed. RT-PCR led to the identification of two products: one at the expected size (359bp) and a shorter one (179bp; Figure 2B). Sanger sequencing of the smaller product indicated the absence of exon 8, suggesting that c.992+1G>A leads to aberrant exon 8 splicing.
Figure 1.
Family pedigree. Proband (case 1) is marked with an arrow. Autopsy of the proband’s aunt confirmed only Alzheimer-type pathology.
Figure 2.
Confirmation of the TBK1 c.992+1G>A mutation and effect on TBK1 splicing. Presence of the c.992+1G>A mutation was confirmed by Sanger sequencing in Case 1 and Case 2 (red rectangle). c.992+1G>A is absent from the genomic DNA of an unaffected sibling (A). RT-PCR using primers specific for TBK1 exons 7 and 9 in Case 1 shows the presence of a product representing the wild-type cDNA (blue rectangle) and a shorter product (red rectangle). Sanger sequencing revealed that the shorter product lacks TBK1 exon 8. MW = molecular-weight size DNA marker.
Clinical presentation and imaging
Case 1
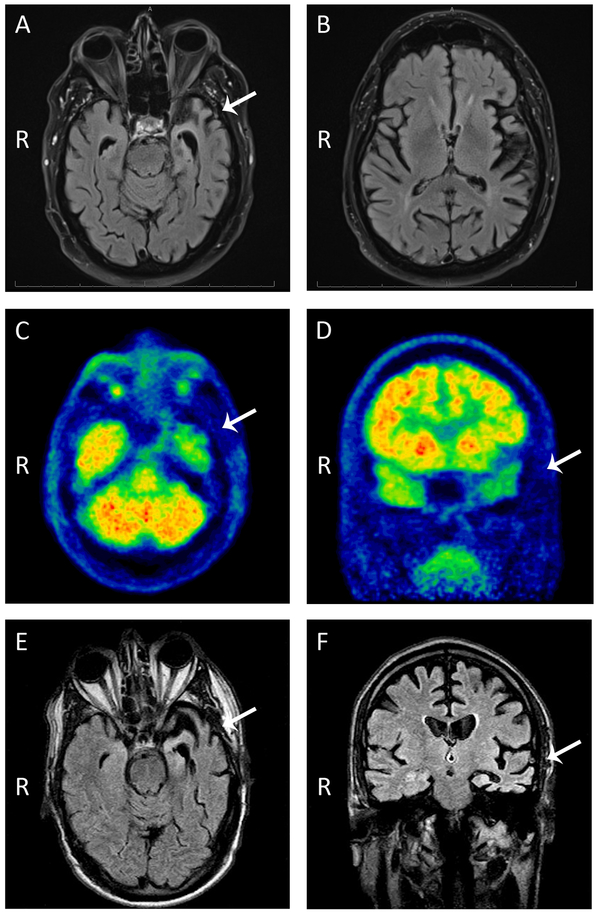
This right-handed male developed word-finding difficulties, impaired naming, and slow hesitant speech at age 68. He also showed mild difficulties in visuospatial function, depressed mood, irritability, and lack of motivation. His assessment at age 69 showed markedly reduced verbal fluency, word-finding pauses, semantic paraphasias, impaired naming, and reduced phonemic and semantic fluency with preserved repetition, comprehension, reading, writing, grammar and syntax. His memory and orientation were relatively preserved. He performed poorly on tests of abstraction and executive functioning. His initial neurological examination only showed subtle, asymmetric spasticity and hyperreflexia affecting predominantly his right upper extremity. Strength and muscle bulk were normal and there were no fasciculations. Electrophysiological studies revealed normal nerve conduction and only mildly abnormal electromyography with no sustained fibrillation potentials or positive sharp waves and normal motor unit morphology and recruitment. Magnetic resonance imaging (MRI) of his brain showed asymmetrical, left > right, temporal lobe atrophy [Figure 3]. SPECT and FDG-PET scans revealed decreased perfusion and glucose metabolism (respectively) in the left temporal and parietal lobes.
Figure 3.
MRI scan (A, B) and FDG-PET (C, D) on case 1 and MRI scan on case 2 (E, F) showed asymmetric (left greater than right) temporal lobe atrophy (white arrows) and hypometabolism, with relative preservation of frontal lobes. R= right side.
Over the next year, his language and other cognitive functions deteriorated, but his auditory comprehension and reading remained relatively preserved. His behavioural and personality changes remained stable. His right spasticity and weakness progressed to right-sided pyramidal hemiparesis with only mild involvement of the left side. He also developed bulbar symptoms. He remained free of clinical or electrophysiological signs of LMN involvement. He died at the age of 71 with clinical diagnoses of unclassifiable PPA and PLS.
Case 2
The 62 year-old, right-handed brother of the proband reported abrupt-onset slurred speech, word-finding and naming difficulties, clumsiness in his right hand, writing difficulties, and poor dexterity. His initial assessment revealed slow and effortful speech, word-finding pauses, severely impaired naming, spastic dysarthria and apraxia of speech. Other language components were normal. He also had poor delayed short-term memory and difficulty planning constructional tasks but tested normally in calculation, right-left orientation, graphesthesia, attention, and executive functioning. His initial neurological examination showed flattening of the right nasolabial fold, asymmetric (right > left) increased tone and hyperreflexia and mild right pyramidal weakness but no LMN signs. Extrapyramidal signs including decreased blink rate, prominent stare, and limited facial expression were also present. His gait was slow and narrow-based with reduced right arm swing. He had normal eye movements and no sensory or cerebellar deficits. Brain MRI showed asymmetric (left > right) atrophy of the temporal lobe, insula and inferior frontal lobe [Figure 3].
One year later he was anarthric with relative preservation of other language skills. He was perseverant and showed signs of executive dysfunction, but his judgment and recent memory were relatively intact. His motor weakness progressed to right hemiparesis and bulbar dysfunction by age 64 and later to spastic quadriplegia with generalized muscle atrophy but without fasciculations. Electrophysiological studies performed on three separate occasions showed no significant LMN involvement. He had no behavioural symptoms throughout his disease. He died at the age of 67 with clinical diagnoses of non-fluent variant PPA and PLS.
Clinical summary
Both brothers presented in their 60s with progressive speech, cognitive and motor symptoms. Their speech difficulties were due to a combination of dysarthria and aphasia, with the latter characterized by impaired fluency and anomia. The motor presentation was strikingly similar with asymmetric UMN impairment of the right upper limb which subsequently progressed to include all limbs and bulbar cranial nerves but with no significant LMN involvement. Brain imaging in both cases showed asymmetric (left > right) temporal lobe degeneration.
Minor differences included mild behavioural changes, more prominent semantic dysfunction and earlier executive dysfunction in Case 1, while Case 2 showed more problems with verbal fluency and additional extrapyramidal features.
Neuropathology
Case 1
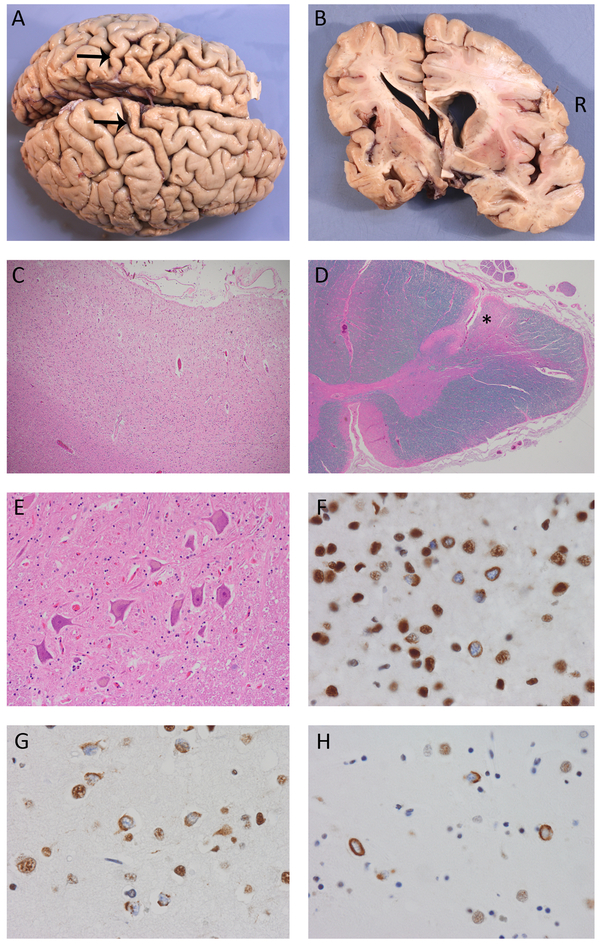
The post mortem brain showed moderate-to-severe cerebral atrophy significantly greater on the left side and preferentially involving the temporal lobe and the precentral gyrus. The ventricles were asymmetrically dilated and the left hippocampus was slightly atrophic.
Microscopic examination revealed chronic neurodegenerative changes that were most prominent in the left primary motor and temporal neocortices and both hippocampi [Figure 4, Table 1]. The basal ganglia, thalamus and substantia nigra showed mild degeneration without Lewy bodies. At all levels, the corticospinal tracts (CST) showed reduced myelin staining and numerous lipid-laden macrophages [Figure 4]. A normal complement of LMN without Bunina bodies was present in the hypoglossal nucleus and spinal cord.
Figure 4.
Pathological findings. Both cases showed similar gross and microscopic findings, including cerebral atrophy that was more severe on the left and preferentially affected the temporal lobes and primary motor cortex (arrows, Case 2) (A, B). There was moderate-to-severe chronic degeneration of the motor cortex (C) and corticospinal tracts (*, D) with a normal number of healthy-appearing lower motor neurons (E). TDP immunohistochemistry demonstrated neuronal cytoplasmic inclusions (NCI) in all cortical laminae of the primary motor (F), prefrontal (G) and temporal neocortex with relatively few dystrophic neurites and no intranuclear inclusions, consistent with FTLD-TDP type B. Many of the NCI had an unusual crescentic, annular or tangle-like morphology. NCI were also abundant in the basal ganglia (H). Magnifications: C:100x; D:20x; E:200x; F,G and H:600x.
Table 1.
Semiquantitative rating of degeneration (deg) and TDP-43 immunoreactive pathology burden (TDP) by region: − (white), none;+ (yellow), mild; ++ (orange), moderate; +++ (red), severe. CA,hippocampal pyramidal layre; cer, cerebellum; CST,=corticospinal tracts; ctx,cortex; GP,globus pallidus; HC,hippocampus; LMN, lower motor neurons; med, medulla; par,parietal; SN, substantia nigra; temp, temporal; XII, hypoglossal nucleus.
| Case 1 | Case 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| deg | TDP | deg | TDP | deg | TDP | deg | TDP | |
| motor ctx | ++ | +++ | +/++ | +++ | +++ | ++/+++ | ++/+++ | ++/+++ |
| prefrontal ctx | +/++ | ++ | + | +/++ | ++ | ++/+++ | + | ++ |
| temp ctx | ++ | +++ | + | −/+ | +++ | +++ | + | ++ |
| par ctx | + | ++ | + | −/+ | + | ++ | + | + |
| HC – dentate | + | +++ | − | ++ | ++ | +++ | − | +++ |
| HC – CA | ++ | ++ | + | + | +++ | + | ++ | + |
| deg | TDP | deg | TDP | |||||
| caudate | + | ++ | + | +++ | ||||
| putamen | + | +++ | ++ | +++ | ||||
| GP | + | + | + | +++ | ||||
| thalamus | + | + | + | + | ||||
| SN | + | + | + | + | ||||
| basis pons | − | + | − | + | ||||
| med – CST | +++ | − | +++ | − | ||||
| med – XII | − | − | − | − | ||||
| cord – CST | ++ | − | +++ | − | ||||
| cord – LMN | − | − | − | single | ||||
| cer – ctx | − | − | + | − | ||||
| cer - dentate | − | − | − | − | ||||
TDP-ir pathology was diffuse but most severe in the left hemisphere [Figure 4, Table 1]. The primary motor cortex (bilateral) and left temporal cortex were the most severely affected neocortical regions and showed TDP-ir neuronal cytoplasmic inclusions (NCI) in all cortical layers, with few neuritic profiles and no neuronal intranuclear inclusions. This pattern was most consistent with FTLD-TDP type B, with the unusual feature that the NCI were mostly compact (rather than diffuse granular) and included tangle, crescentic and annular morphologies. The hippocampi showed TDP-ir NCI and CA1 neurites, greater on the left. The striatum also showed abundant TDP-ir pathology. There were no TDP-ir NCI in LMN of the hypoglossal nucleus or spinal cord and no TDP-ir glial inclusions in the CST.
Moderate Alzheimer’s type pathology (CERAD moderate neuritic senile plaques and Braak IV/VI neurofibrillary pathology) and rare Lewy bodies restricted to the transentorhinal cortex were the only co-pathologies. Neuropathological diagnoses were (i) FTLD-TDP type B, (ii) PLS, (iii) moderate left hippocampal sclerosis with TDP proteinopathy and (iv) moderate Alzheimer-type pathology.
Case 2
The brain weighed 1235 grams. Macroscopically, there was striking asymmetric (left > right) atrophy of the frontal and temporal lobes [Figure 4]. The precentral gyri and hippocampi were asymmetrically atrophic (left > right). The deep grey nuclei appeared normal. The substantia nigra showed mild paleness.
Microscopic examination showed chronic neurodegenerative changes that were most severe in the primary motor cortex bilaterally, the left temporal neocortex and left hippocampus. There was severe degeneration of the CST at all levels, greater on the right. A normal complement of LMN was present in the hypoglossal nucleus and spinal cord with no Bunina bodies [Figure 4].
TDP-ir pathology was most abundant in the left temporal, frontal and bilateral primary motor cortices, hippocampi and basal ganglia. NCI were present in all neocortical layers without significant neuritic pathology or intranuclear inclusions; consistent with FTLD-TDP type B. Similar to case 1, most of the NCI were compact and included unusual annular, tangle and crescentic morphologies. A single filamentous TDP-ir inclusion was identified in one LMN of the lumbar spinal cord; otherwise, there was no TDP-ir pathology in the brainstem or spinal cord.
This case also showed moderate Alzheimer’s pathology (CERAD moderate senile neuritic plaques and Braak III/VI neurofibrillary pathology). Neuropathological diagnoses were (i) FTLD-TDP type B, (ii) PLS and (iii) moderate Alzheimer-type pathology.
Clinicopathologic correlations
Both cases presented with PPA. Imaging studies and neuropathological assessment showed striking left > right temporal lobar atrophy and temporal > frontal TDP-ir pathology. The asymmetric UMN signs and symptoms correlated with greater degeneration and TDP-ir pathology in the left primary motor cortex. The absence of clinical LMN features was explained by the preservation of LMN without significant TDP-ir pathology. The extrapyramidal symptoms in Case 2 may be explained by the greater involvement of the basal ganglia compared to his brother.
Discussion
TBK1 is a multifunctional kinase involved in the regulation of various cellular pathways, including immune response, inflammation, insulin signalling, cell proliferation, and autophagy (19). Mutations in TBK1 have been reported to cause ALS and FTD via a loss-of-function mechanism by either reducing mRNA or protein levels, or leading to reduced TBK1 function (13,14,20-22). The previously reported c.992+1G>A TBK1 mutation (14,23) identified in this family results in an in-frame deletion of the TBK1 transcript (p.Gly272_Thr331del) with loss of exon 8 (see results section), which forms part of the ubiquitin-like and kinase domains, likely resulting in loss of TBK1 function (20). In addition, this mutation was previously shown to reduce TBK1 protein levels by approximately 50% in in vitro and in vivo (14).
The full clinical spectrum of patients carrying TBK1 mutations has not been explored in detail. Most TBK1 mutation-associated phenotypes have been reported in the context of large genetic studies, in which clinical details are scant, and have been variably associated with bvFTD, PPA and/or ALS (bulbar and/or spinal) (13,14). More recent reports have widened that spectrum to include extrapyramidal movement disorders, such as PSP, and cerebellar manifestations (24). A report of 16 patients carrying several different TBK1 mutations described a range of phenotypes including bvFTD (N=5), PPA (N=1), bvFTD-ALS (N=1), unspecified dementia (N=2) and ALS (N=7). No definite cases of PLS were described. Imaging studies revealed varying patterns of atrophy (including none, frontotemporal and generalized) and either symmetric or asymmetric (usually right > left) involvement (23). Detailed case reports include four patients presenting with semantic dementia or non-fluent variant PPA, all of which later developed ALS, with striking asymmetric (left > right) anterior temporal atrophy (25); six affected family members carrying the p.Arg573Gly TBK1 mutation that developed either PPA or bvFTD (N=4) or bulbar or spinal PLS (N=2) (26); and a single patient carrying the p.Ala705fs* TBK1 mutation who developed bvFTD with subsequent cognitive impairment and anterior right temporal lobe atrophy (27). From these reports it is difficult to determine whether patients with TBK1 mutations have a distinct phenotype compared with other FTD/ALS-associated mutations. It is also difficult to establish (and beyond the scope of this report) a genotype/phenotype correlation for TBK1 mutations given the relative lack of detailed clinical information in most of the reported cases, the known clinical variability in familial cases with the same TBK1 mutation (26) and the fact that all TBK1 mutations result in loss of protein function at either the transcriptional, mRNA or protein levels.
Neuropathological reports of patients carrying mutations in TBK1 are relatively scant. Changes compatible with FTLD-TDP type B (p.Gly272_Thr331del, p.Val97Phefs*2, p.Ala417* and p.Thr79del) and ALS-TDP (c.1551_1552insTT) have been reported, sometimes in association with tau-ir pathology (14,21,23). A single patient with the p.Thr79del TBK1 mutation has been described as having ring-like TDP-43 ir inclusions (21) similar to the NCI found in our cases. FTLD-TDP type A with unusual neuritic structures at the cortical/white matter junction was described in the patient carrying the p.Ala705fs* TBK1 mutation (27).
To our knowledge, the only other reported case with a c.992+1G>A TBK1 mutation was a male who presented in his late 40s with a two-year duration of FTD. Neuropathological examination showed mild neuronal loss in the frontal cortex with moderate numbers of TDP-43 and p62-ir NCI and short dystrophic neurites but no neuronal intranuclear inclusions, interpreted as most compatible with FTLD-TDP type B (14,23). These features are different from those of our two cases, making a mutation-specific effect an unlikely explanation for the homogenous presentation of our brothers.
One striking clinical aspect in our cases is that their motor syndrome fulfilled criteria for PLS rather than ALS. Although PLS was anecdotally reported in two patients carrying the p.Arg573Gly TBK1 mutation, most cases with TBK1 mutations present as ALS. The cases described herein had a mutation in a gene known to cause ALS (TBK1), clinical features of FTD (commonly associated with ALS), FTLD-TDP subtype B (FTLD-TDP subtype most often present in patients with concomitant ALS) and TDP-ir inclusions in UMN. These findings support the interpretation that their pyramidal system degeneration was part of the ALS spectrum. However, the exquisitely selective UMN degeneration and nearly complete absence of TDP-ir pathology in LMN, after as much as five years of disease progression, suggests that some uncommon (likely genetic) modifying factor may act to protect LMN from degeneration. Moreover, this or other modulating factor(s) may also be responsible for the unusual and strikingly similar clinical presentation, neuroimaging and neuropathology of these brothers, which stands in contrast with the wider phenotypic and neuropathological variability described in other patients carrying the same or other TBK1 mutations. Identification of this factor would require a much larger cohort of patients with the same mutation. Candidate factors would include transcriptional regulators and interacting partners of TBK1 and relative levels of proteins with TBK1-like functions. In addition, to complete the assessment, the overall functionality of all TBK1-dependent cellular pathways would need to be compared between cases with the same mutation but different phenotypes.
Acknowledgments
This work was supported by CIHR (grant 74580), CCNA (grant 137794), the ALS Canada-Brain Canada Hudson Grant (IM, RH) and NIH/NINDS (grants R35 NS097261 and UG3 NS103870) (RR). Dr. Hsiung holds the Ralph Fisher Alzheimer Society of BC professorship.
Footnotes
Declaration of interest statement
None of the authors has conflicts of interest to declare.
References
- 1.Statland JM, Barohn RJ, Dimachkie MM, Floeter MK, Mitsumoto H. Primary Lateral Sclerosis. Neurol Clin. 2015. November;33(4):749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 2005. August 23;65(4):586–90. [DOI] [PubMed] [Google Scholar]
- 3.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 2003. April 8;60(7):1094–7. [DOI] [PubMed] [Google Scholar]
- 4.Massman PJ, Sims J, Cooke N, Haverkamp LJ, Appel V, Appel SH. Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis J Neurol Neurosurg Psychiatry. BMJ Publishing Group; 1996. November;61(5):450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries BS, Rustemeijer LMM, van der Kooi AJ, Raaphorst J, Schröder CD, Nijboer TCW, et al. A case series of PLS patients with frontotemporal dementia and overview of the literature Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. Taylor & Francis; 2017. November;18(7-8):534–48. [DOI] [PubMed] [Google Scholar]
- 6.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002. October 8;59(7):1077–9. [DOI] [PubMed] [Google Scholar]
- 7.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis Science. American Association for the Advancement of Science; 2006. October 6;314(5796):130–3. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie IRA, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007. May;61(5):427–34. [DOI] [PubMed] [Google Scholar]
- 9.Finegan E, Chipika RH, Shing SLH, Hardiman O, Bede P. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2019. January 18;100:1–13. [DOI] [PubMed] [Google Scholar]
- 10.Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve. 2007. March;35(3):291–302. [DOI] [PubMed] [Google Scholar]
- 11.Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015. March 27;347(6229):1436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Ber I, De Septenville A, Millecamps S, Camuzat A, Caroppo P, Couratier P, et al. TBK1 mutation frequencies in French frontotemporal dementia and amyotrophic lateral sclerosis cohorts. Neurobiology of Aging. 2015. November;36(11):3116.e5–3116.e8. [DOI] [PubMed] [Google Scholar]
- 13.Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Müller K, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia Nat Neurosci. Nature Publishing Group; 2015. May;18(5):631–6. [DOI] [PubMed] [Google Scholar]
- 14.Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B, et al. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort Neurology. Wolters Kluwer Health, Inc; on behalf of the American Academy of Neurology; 2015. December 15;85(24):2116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui R, Tuo M, Li P, Zhou C. Association between TBK1 mutations and risk of amyotrophic lateral sclerosis/frontotemporal dementia spectrum: a meta-analysis Neurol Sci. Springer Milan; 2018. May;39(5):811–20. [DOI] [PubMed] [Google Scholar]
- 16.Pottier C, Ren Y, Perkerson RB, Baker M, Jenkins GD, van Blitterswijk M, et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD Acta Neuropathol. Springer Berlin Heidelberg; 2019. February 9;7(1268–1283):248–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data Nat Genet. Nature Publishing Group; 2011. May;43(5):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline Curr Protoc Bioinformatics. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2013;43:11.10.1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013. April 17;587(8):1230–7. [DOI] [PubMed] [Google Scholar]
- 20.Freischmidt A, Müller K, Ludolph AC, Weishaupt JH, Andersen PM. Association of Mutations in TBK1 With Sporadic and Familial Amyotrophic Lateral Sclerosis and Frontotemporal Dementia JAMA Neurol. American Medical Association; 2017. January 1;74(1):110–3. [DOI] [PubMed] [Google Scholar]
- 21.van der Zee J, Gijselinck I, Van Mossevelde S, Perrone F, Dillen L, Heeman B, et al. TBK1 Mutation Spectrum in an Extended European Patient Cohort with Frontotemporal Dementia and Amyotrophic Lateral Sclerosis HUMAN MUTATION. John Wiley & Sons, Ltd; 2017. March;38(3):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Majo M, Topp SD, Smith BN, Nishimura AL, Chen H- J, Gkazi A- S, et al. ALS-associated missense and nonsense TBK1 mutations can both cause loss of kinase function. Neurobiology of Aging. 2018. November;71:266.e1–266.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Mossevelde S, van der Zee J, Gijselinck I, Engelborghs S, Sieben A, Van Langenhove T, et al. Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort. Brain. 2016. February;139(Pt 2):452–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilke C, Baets J, De Bleecker JL, Deconinck T, Biskup S, Hayer SN, et al. Beyond ALS and FTD: the phenotypic spectrum of TBK1 mutations includes PSP-like and cerebellar phenotypes. Neurobiology of Aging. 2018. February;62:244.e9–244.e13. [DOI] [PubMed] [Google Scholar]
- 25.Caroppo P, Camuzat A, De Septenville A, Couratier P, Lacomblez L, Auriacombe S, et al. Semantic and nonfluent aphasic variants, secondarily associated with amyotrophic lateral sclerosis, are predominant frontotemporal lobar degeneration phenotypes in TBK1 carriers. Alzheimers Dement (Amst). 2015. December;1(4):481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Tortosa E, van der Zee J, Ruggiero M, Gijselinck I, Esteban-Pérez J, García-Redondo A, et al. Familial primary lateral sclerosis or dementia associated with Arg573Gly TBK1 mutation J Neurol Neurosurg Psychiatry. BMJ Publishing Group Ltd; 2017. November;88(11):996–7. [DOI] [PubMed] [Google Scholar]
- 27.Koriath CAM, Bocchetta M, Brotherhood E, Woollacott IOC, Norsworthy P, Simón-Sánchez J, et al. The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: A longitudinal case report. Alzheimers Dement (Amst). 2017;6:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]