Abstract
Objective:
To evaluate the performance of cervical cancer screening algorithms for women living with human immunodeficiency virus (HIV), using primary high-risk human papillomavirus (HPV) testing followed by cytology, visual inspection with acetic acid, or colposcopy.
Methods:
We conducted a prospective cohort study of women living with HIV in Botswana. All participants underwent high-risk HPV testing. Participants with positive high-risk HPV results underwent cytology, visual inspection with acetic acid, colposcopy, and biopsy. Participants with negative high-risk HPV results also underwent cytology. Histopathology was the reference standard for determination of pre-invasive cervical disease and cervical cancer. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and likelihood ratios (LR) of high-risk HPV-based two-stage screening algorithms were calculated.
Results:
Among 300 women screened, 88 (29%) had a positive high-risk HPV test, and 29 of the 88 (35%) women who were high-risk HPV-positive had CIN2+ on histopathology. High-risk HPV followed by colposcopy resulted in a sensitivity of 83%, specificity of 49%, PPV of 47%, LR+ of +1.6 and LR- of −0.4. High-risk HPV followed by visual inspection with acetic acid resulted in a reduced sensitivity of 59%, specificity of 49%, PPV of 39%, LR+ of +1.2 and LR- of −0.8. High-risk HPV testing followed by cytology also resulted in a reduced sensitivity of 62%, specificity of 77%, PPV of 60%, LR+ of +2.7 and LR- of −0.5. Stratification by HPV 16/18/45 did not improve performance of the algorithms.
Conclusion:
In a high-risk HIV population, high-risk HPV testing followed by colposcopy demonstrated the highest sensitivity and PPV in detecting high-grade cervical dysplasia. Allocating resources to colposcopy in resource-limited settings may be more effective than other screening strategies.
Precis
Colposcopy after positive high-risk human papillomavirus testing maintained sensitivity and improved positive predictive value of high-grade cervical dysplasia among women living with human immunodeficiency virus.
Introduction
Cervical cancer is the fourth leading cause of cancer death in women worldwide and the leading cause of cancer death in women in Botswana.1,2,3 The disease burden in Botswana is impacted by the high prevalence of human immunodeficiency virus (HIV), which is 22% among people aged 15–49 years and is a well-established risk factor for cervical cancer.4,5,6 Most cervical cancers are associated with infection with high-risk human papillomavirus (HPV) types.7,8,9 Globally, HPV prevalence is variable, ranging from 15–45%, with higher prevalence in women living with HIV.10,11,12 HPV 16, 18, and 45 are the high-risk types most commonly associated with cervical cancer in Africa.13,14,15 Among women living with HIV, persistent high-risk HPV positivity and infection with multiple types are strong risk factors for cervical cancer.16
Cervical cancer is largely preventable and treatable where screening and treatment programs are available.17,18,19,20 Cervical cancer screening strategies are most effective when based on local evidence and tailored to the population and resource infrastructure.21 Current National Cervical Cancer Prevention Programme guidelines in Botswana use a combination of cytology (Pap test) and visual inspection with acetic acid. However, there is mounting evidence that primary high-risk HPV testing is the most effective screening strategy because of its high sensitivity (95%).22 High-risk HPV testing is increasingly included in some national guidelines.23,24,25 High-risk HPV testing is planned for the next 5-year National Cervical Cancer Prevention Programme guidelines in Botswana, but the guidelines for managing positive high-risk HPV results remain unclear, particularly among women living with HIV.26,27,28 Appropriate triage of a positive high-risk HPV result is necessary to prevent overtreatment of high-risk HPV when it is associated with no or low-grade cervical dysplasia. The best two-stage screening strategy is unknown for women living with HIV in resource-limited settings29,30,34
In this study, we investigated the performance of primary high-risk HPV testing followed by cytology, visual inspection with acetic acid and colposcopy to predict pre-invasive cervical disease in women living with HIV in Botswana. We hypothesized that visual inspection with acetic acid, cytology and colposcopy would perform similarly as a triage test in women living with HIV who test positive for high-risk HPV. Evaluating cervical cancer screening algorithms with primary high-risk HPV testing in women living with HIV is essential for establishing an evidence-based screening strategy in this high-risk population.
Methods
We conducted a prospective cohort study of women seeking care at the infectious disease care clinic at Princess Marina Hospital, the regional tertiary referral hospital in Gaborone, Botswana. Women included in the study were HIV-positive, greater than 24 years of age, and competent to understand study procedures and give informed consent. Women were excluded if they were currently pregnant, currently menstruating heavily or with persistent vaginal discharge, had a previous hysterectomy, or had a previous diagnosis of cervical cancer.
Eligible women were provided study information by a research assistant or study nurse and offered voluntary participation while waiting for their scheduled clinical visit. After obtaining informed consent, we administered a questionnaire including demographic data, HIV treatment history, history of cervical cancer screening, and knowledge about cervical cancer. In addition to patient report, the electronic medical record was searched for results of prior cervical cancer screening. The institutional review boards of the Botswana Ministry of Health and Wellness, the University of Botswana, and the Beth Israel Deaconess Medical Center approved this study. The ethics committee of Princess Marina Hospital also approved this study. The study was registered at ClinicalTrials.gov, .
All participants underwent a speculum examination of the cervix by a trained study nurse, at which time samples were collected from the cervix for high-risk HPV testing and for cervical cytology using a Cervex-brush®. HPV specimens were placed in a PreservCyt® transport medium and testing was performed using the Xpert® HPV Assay (Cepheid, Sunnyvale, CA) at the Botswana Harvard AIDS Initiative Partnership Laboratory. The Xpert® HPV assay tests for 14 high-risk HPV types, including 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. Cytology was prepared by spreading collected cervical cells from a Cervex-brush® onto a glass slide and fixing with a spray fixative at the collection site. Cytology was sent to the National Health Laboratory for processing and pathologist evaluation and reported using the revised Bethesda classification.31 Abnormal lower genital tract cytology was evaluated at two thresholds: abnormal squamous cells of undetermined significance (ASC-US) or worse, and high-grade squamous intraepithelial lesion (HSIL) or worse.
To ensure that all participants underwent cervical cancer screening according to the standard of current guidelines in Botswana, we also collected cytology at the time of high-risk HPV sample collection. At the time of the study, there were no national clinical guidelines for management of positive high-risk HPV results. We referred participants who tested negative for high-risk HPV to colposcopy if they had a study cytology of HSIL or had a prior abnormal cytology result and study cytology result of ASC-US or worse (≥ASC-US) in accordance with current Botswana National Cervical Cancer Prevention Programme algorithms. We referred all participants who tested positive for any high-risk HPV type to visual inspection with acetic acid and colposcopy, regardless of their cytology result. The cytology result from the initial specimen collection was used for the evaluation of the two-stage screening algorithm of high-risk HPV testing followed by cytology. At the time of the colposcopy visit, participants underwent a speculum examination of the cervix with both visual inspection with acetic acid and colposcopy performed by providers who were blinded to the HPV test results and cytology results. Visual inspection with acetic acid was performed by a trained nurse midwife who had participated in the Botswana Ministry of Health and Wellness national visual inspection with acetic acid training program and was experienced in performing visual inspection with acetic acid in the clinical setting. Visual assessment was performed after applying 5% acetic acid to the cervix using a cotton swab and findings were categorized as normal, abnormal with recommendation for cryotherapy, or abnormal with recommendation for loop electrosurgical excision procedure (LEEP). In the analysis, we considered lesions recommended for cryotherapy as “low-grade” and lesions recommended for LEEP as “high-grade”. Subsequently, a gynecologist blinded to the visual inspection with acetic acid assessment performed colposcopy and normal, low-grade or high-grade impression was recorded. All participants had a biopsy collected at the time of colposcopy. If there was a visible lesion, a punch biopsy or LEEP was performed according to current best practice in Botswana. If no lesion was visible, a small endocervical excision or an endocervical curettage was performed. All women with cervical intraepithelial neoplasia ≥ CIN2 (CIN2+) on biopsy or endocervical curettage were referred for an excisional procedure. Women with histopathology showing CIN3 with microinvasion or invasive cervical cancer were referred to gynecologic providers for further assessment and treatment.
The primary outcome was performance of two-stage cervical cancer screening algorithms in detecting high grade cervical dysplasia. We defined high-grade cervical dysplasia as a colposcopy result of cervical intraepithelial neoplasia grade 2 or higher (CIN2+). Using histopathology collected at time of colposcopy as the gold standard, we calculated the sensitivity, specificity, positive predictive value (PPV) negative predictive value (NPV), and likelihood ratios (LR) to detect high-grade cervical dysplasia for 1) cytology after a positive high-risk HPV test, 2) visual inspection with acetic acid impression after a positive high-risk HPV test and 3) colposcopy impression after a positive high-risk HPV test. For each two-stage screening strategy, we evaluated test performance at two cutoffs. For cytology, we evaluated cut-offs of ASC-US and HSIL. For visual inspection with acetic acid and colposcopy, we evaluated cut-offs of low-grade and high-grade impressions. In addition, we repeated this analysis stratified by high-risk HPV type (16/18/45 and other high-risk HPV).
Data were entered into a REDCap electronic database by a designated research assistant and accuracy of data entry were verified by the study nurse and principal investigator. Descriptive statistics are presented as median with interquartile range or proportion. We compared categorical variables with the chi-square or Fisher’s exact test and continuous variables with the Wilcoxon rank sum test. We considered two-sided p values <0.05 statistically significant and used SAS 9.4 (SAS Institute, Cary, North Carolina) for analyses.
The goal of a two-stage algorithm to detect high-grade cervical dysplasia is to increase PPV while maintaining sensitivity and specificity. In a prior cervical cancer screening study among a population of women with a relatively high HIV prevalence, the PPV of a high-risk HPV positive test for high-grade cervical dysplasia was 24% (Denny, 2000). Our sample size calculation was targeted to detect an improvement in PPV from 24% for high-risk HPV testing alone to 49% for the two-stage algorithms. Assuming a two-sided alpha of 0.05, a sample size of 81 participants with high-risk HPV was needed to yield 80% power to detect the specified difference. Based on preliminary data from a recent study of women living with HIV in Botswana, we assumed high-risk HPV-positivity would be 30% (unpublished data). Thus, we needed to enroll 270 participants with HIV to yield 81 who would be high-risk HPV positive. To allow for 10% loss to follow-up between the primary high-risk HPV testing and colposcopy we aimed to enroll at least 300 participants.
Results
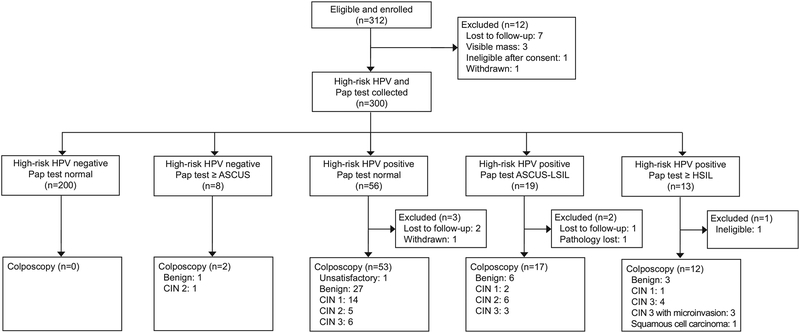
We recruited participants from April to July 2018, and all follow-up colposcopy visits were completed by August 2018. Of the 312 women living with HIV enrolled, 12 were lost to follow-up, deemed ineligible or withdrawn before cervical samples were collected at the first study visit, leaving 300 (96%) who underwent high-risk HPV testing and cytology collection. Of those participants, 88 (29%) had a positive high-risk HPV result. Among those 88 who were high-risk HPV positive, we did not have colposcopy results for 6 (3 were lost to follow-up, 1 withdrew, 1 became ineligible due to pregnancy, and 1 biopsy specimen was lost in the laboratory) and had histopathology results from colposcopy for 82 women for this analysis. Additionally, two participants who were high-risk HPV-negative underwent colposcopy for cytology of HSIL (Figure 1).
Figure 1:
Study recruitment flowchart for two-stage cervical cancer screening algorithms in women living with human immunodeficiency virus. HPV, human papillomavirus; ASCUS, atypical squamous cells of undetermined significance; LSIL; low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
Baseline characteristics were similar among women who tested positive and negative for high-risk HPV (Table 1). The majority of women reported having undergone prior cervical cancer screening (95%). There were no differences between groups in prior abnormal screening results or cervical excisional procedures. Only 5 women had a recent CD4 count of < 200/μL, and all of the participants were taking antiretroviral therapy. Only two women reported a history of smoking, and both tested negative for all high-risk HPV types.
Table 1:
Baseline characteristics of the study population
| Characteristic | All n = 300* |
hrHPV positive n = 88 |
hrHPV negative n = 212 |
p |
|---|---|---|---|---|
| Age, years [interquartile range] | 46 [42–52] | 44 [40–51] | 47 [42–52] | 0.05 |
| Education | 0.40 | |||
| ≤Primary | 94 (31) | 24 (27) | 70 (33) | |
| ≥Secondary | 206 (69) | 64 (73) | 142 (67) | |
| Employed | 197 (66) | 63 (72) | 134 (63) | 0.21 |
| Marital status | 0.85 | |||
| Single | 215 (72) | 61 (69) | 154 (72) | |
| Married | 55 (18) | 18 (20) | 37 (17) | |
| Divorced | 12 (4) | 3 (3) | 9 (4) | |
| Widowed | 18 (6) | 6 (7) | 12 (6) | |
| Parity$ | 0.15 | |||
| 0 | 11 (4) | 5 (6) | 6 (3) | |
| 1–3 | 199 (66) | 58 (66) | 141 (67) | |
| ≥4 | 75 (25) | 24 (27) | 51 (24) | |
| Sexual partners | 0.83 | |||
| 1–5 | 186 (62) | 55 (63) | 131 (62) | |
| ≥6 | 100 (33) | 28 (32) | 72 (34) | |
| Missing | 14 (5) | 5 (6) | 9 (4) | |
| Postmenopausal | 106 (35) | 27 (31) | 79 (38) | 0.38 |
| CD4 Count (per μL) | 0.63 | |||
| <200 | 5 (2) | 2 (2) | 3 (1) | |
| 200–500 | 83 (28) | 27 (31) | 56 (26) | |
| >500 | 212 (71) | 59 (67) | 153 (72) | |
| Detectable viral load | 11 (4) | 6 (7) | 5 (2) | 0.12 |
| Currently on antiretroviral therapy | 300 (100) | 88 (100) | 213 (100) | -- |
| Length of time on antiretroviral therapy, years [interquartile range] | 14 [11 – 15] | 14 [9 – 15] | 14 [12 – 15] | 0.09 |
| History of cervical cancer screening | ||||
| Yes | 285 (95) | 79 (90) | 206 (97) | 0.02 |
| Pap ≥ASC-US | 27 (9) | 11 (14) | 16 (8) | 0.44 |
| VIA positive | 3 (1) | 1 (1) | 2 (1) | 1.0 |
| History of cervical excisional procedure | 6 (2) | 3 (3) | 3 (1) | 0.18 |
All table entries are number of study subjects (%) unless otherwise noted
Data available for 285 participants
ART: antiretroviral therapy; ASC-US: abnormal squamous cells of undetermined significance; VIA: visual section with acetic acid
Of the 88 (29%) women who were positive for any high-risk HPV type, 15 of the 300 screened had HPV 16 (prevalence 5%); 21 of the 300 screened had HPV 18/45 (prevalence 7%); and 66 of the 300 screened had other high-risk HPV types (prevalence 22%). Among the 82 women with a positive high-risk HPV test who had histopathology results, 29 (35%) had CIN2+ (Table 2). The prevalence of CIN2+ by high-risk HPV type was 31%, 21%, and 43% for HPV 16, HPV 18/45, and other high-risk HPV types, respectively. Among the 11 participants co-infected with multiple high-risk HPV types, the prevalence of CIN2+ was 45%.
Table 2:
Prevalence of CIN2+ (per 100 women living with HIV) who tested positive for high-risk HPV and underwent colposcopy
| HPV type | Number undergoing colposcopy (n) |
Number with CIN2+ (n) |
Prevalence of CIN2+ (%) [95% CI] |
|---|---|---|---|
| Any high-risk HPV type | 82 | 29 | 35% [25 – 47] |
| HPV 16* | 13 | 4 | 31% [9 – 61] |
| HPV 18/45* | 19 | 4 | 21% [6 – 46] |
| Other high-risk HPV type* | 61 | 26 | 43% [30 – 56] |
| >1 high-risk HPV type | 11 | 5 | 45% [17 – 77] |
Infection with these sub-types is not mutually exclusive
CIN2+: cervical intraepithelial neoplasia grade 2 or higher
We compared the performance of the two-stage cervical cancer screening algorithms. High-risk HPV followed by colposcopy impression had a sensitivity of 83%, specificity of 49%, PPV of 47%, LR+ of +1.6 and LR- of −0.4. High-risk HPV testing followed by visual inspection with acetic acid resulted in a reduced sensitivity of 59%, specificity of 49%, PPV of 39%, LR+ of +1.2 and LR- of −0.8 at the low cut-off point of “low-grade impression”. High-risk HPV testing followed by cytology also resulted in a reduced sensitivity of 62%, specificity of 77%, PPV of 60%, LR+ of +2.7 and LR- of −0.5 at the ASC-US threshold (Table 3). Triaging high-risk HPV positive women with colposcopy impression, visual inspection with acetic acid and cytology missed CIN2+ diagnoses in 5, 12, and 11 women in our cohort, respectively. Evaluation of the two-stage algorithms stratified by HPV 16/18/45 versus other high-risk HPV types did not improve the performance of any algorithm (Table 4).
Table 3:
Performance of two-stage screening in detecting CIN2+ among women living with HIV who tested positive for high-risk HPV and underwent colposcopy
| Two-stage screen using different cut-offs | Biopsy result | Two-stage screen characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| CIN2+ (n) |
≤ CIN1 (n) |
Sensitivity (%) [95% CI] |
Specificity (%) [95% CI] |
PPV (%) [95% CI] |
NPV (%) [95% CI] |
LR +/− [95% CI] |
||
| Cytology | NILM | 11 | 41 | -- | -- | -- | -- | |
| ≥ ASC-US | 18 | 12 | 62% [42–79] |
77% [64–88] |
60% [41–77] |
79% [65–89] |
+ 2.7 [1.1 4.3] −0.5 [0.2–0.7] |
|
| ≥ HSIL | 9 | 4 | 31% [15–51] |
92% [82–98] |
69% [39 – 91] |
71% [59–81] |
||
| Visual inspection with acetic acid (VIA) | normal | 12 | 26 | -- | -- | -- | -- | |
| ≥ low-grade impression | 17 | 27 | 59% [39–76] |
49% [35–63] |
39% [24–55] |
68% [51–83] |
+ 1.2 [0.7–1.6] - 0.8 [0.4–1.3] |
|
| ≥ high-grade impression | 4 | 5 | 14% [3–32] |
91% [79–97] |
44% [14–79] |
66% [54–76] |
||
| Colposcopy impression | normal | 5 | 26 | -- | -- | -- | -- | |
| ≥ low-grade impression | 24 | 27 | 83% [64–94] |
49% [35–63] |
47% [33–62] |
84% [66–95] |
+ 1.6 [1.1–2.1] −0.4 [0.1–0.7] |
|
| ≥ high-grade impression | 4 | 5 | 14% [4–32] |
91% [79–97] |
44% [14–79] |
66% [54–76] |
||
CIN2+: cervical intraepithelial neoplasia grade 2 or higher; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; NILM: negative for intraepithelial lesion or malignancy; ASC-US: abnormal squamous cells of undetermined significance; HSIL: high-grade squamous intraepithelial lesion
Table 4:
Performance of two-stage screening in detecting CIN2+ among women living with HIV who tested positive for high-risk HPV and underwent colposcopy stratified by HPV type
| Study Arm | CIN 2+ (n) |
≤ CIN 1 (n) |
Sensitivity (%) [95% CI] |
Specificity (%) [95% CI] |
PPV (%) [95% CI] |
NPV (%) [95% CI] |
|
|---|---|---|---|---|---|---|---|
| hrHPV + Cytology | HPV 16/18/45 | ||||||
| NILM | 3 | 17 | -- | -- | -- | -- | |
| ≥ ASC-US | 5 | 7 | 63 (24 – 91) | 71 (49 – 87) | 42 (15 – 72) | 85 (62 – 97) | |
| ≥ HSIL | 3 | 2 | 38 (9 – 76) | 92 (73 – 99) | 60 (15 – 95) | 81 (62 – 94) | |
| Other hrHPV | |||||||
| NILM | 9 | 26 | -- | -- | -- | -- | |
| ≥ ASC-US | 17 | 9 | 65 (44 – 83) | 74 (57 – 88) | 65 (44 – 83) | 74 (57 – 88) | |
| ≥ HSIL | 8 | 3 | 31 (14 – 52) | 91 (77 – 98) | 73 (39 – 94) | 64 (49 – 77) | |
| hrHPV + VIA | HPV 16/18/45 | ||||||
| Normal | 4 | 15 | -- | -- | -- | -- | |
| ≥ low-grade impression | 4 | 9 | 50 (16 – 84) | 63 (41 – 81) | 31 (9 – 61) | 79 (54 – 64) | |
| ≥ high-grade impression | 1 | 1 | 13 (0 – 53) | 96 (79 – 100) | 50 (1 – 99) | 77 (58 – 90) | |
| Other hrHPV | |||||||
| Normal | 10 | 14 | -- | -- | -- | -- | |
| ≥ low-grade impression | 16 | 21 | 62 (41 – 80) | 40 (24 – 58) | 43 (27 – 61) | 58 (37 – 78) | |
| ≥ high-grade impression | 4 | 5 | 16 (5 – 36) | 86 (70 – 95) | 44 (14 – 79) | 59 (44 – 72) | |
| hrHPV + Colposcopy | HPV 16/18/45 | ||||||
| Normal | 1 | 10 | -- | -- | -- | -- | |
| ≥ low-grade impression | 7 | 14 | 88 (47 – 100) | 42 (22 – 63) | 33 (15 – 57) | 91 (59 – 100) | |
| ≥ high-grade impression | 2 | 3 | 25 (3 – 65) | 88 (68 – 97) | 78 (58 – 91) | 81 (62 – 94) | |
| Other hrHPV | |||||||
| Normal | 4 | 18 | -- | -- | -- | -- | |
| ≥ low-grade impression | 22 | 17 | 85 (65 – 96) | 51 (34 – 69) | 56 (40 – 72) | 82 (60 – 95) | |
| ≥ high-grade impression | 4 | 3 | 15 (4 – 35) | 91 (77 – 98) | 57 (18 – 90) | 59 (45 – 72) | |
Four women had histopathology results of cancer or microinvasive CIN 3. One of these women had HPV18/45 and the other three had other high-risk HPV types. All four had a cytology result of HSIL. Three had low-grade impressions on both visual inspection with acetic acid and colposcopy, while one had a high-grade impression on both visual inspection with acetic acid and colposcopy.
Discussion:
Primary high-risk HPV testing followed by colposcopy was the most sensitive two-stage algorithm for cervical cancer screening among women living with HIV in Botswana. Both visual inspection with acetic acid and cytology as second-stage screening methods had unacceptably low sensitivity, missing approximately one-third of women with high-grade cervical lesions. Triaging high-risk HPV positive results with visual inspection with acetic acid or cytology eliminated the benefit of the high sensitivity that primary high-risk HPV testing provides. Further, triaging of high-risk HPV positive results based on type did not improve the performance of any two-stage algorithm.
One third of women in our study with positive high-risk HPV primary screening had high-grade cervical disease, which is a higher proportion than found in other populations living with HIV.32 Our population also had a higher prevalence of high-grade dysplasia among women with other high-risk HPV compared to women with HPV 16 or 18/45. This is consistent with prior studies in Botswana that showed heterogeneous HPV types associated with high-grade precancerous cervical lesions among women living with HIV (16, 18, 35, 58, and 61) and a lower prevalence of HPV 16 and 18 positivity in cervical cancer specimens.33,34,35 This cross-sectional data does not support triaging strategies based on high-risk HPV type, as may be considered in other African settings.36
Primary high-risk HPV testing followed by colposcopy results in a high number of referrals for colposcopy, presenting challenges in resource-limited settings.37 Guidelines for low- and middle-income countries have presumed that scaling up colposcopy is not feasible.38,39 Recent trends in cervical cancer screening in the region have focused on visual inspection strategies as opposed to colposcopy training.40 However, consideration of available data to plan effective screening programs is vital. Our findings support concerns raised in prior studies that visual inspection with acetic acid and cytology triaging of women with high-risk HPV may have variable or low sensitivity, particularly in women living with HIV, and that referral to colposcopy may be a better alternative.41,42,43,44 Building on the infrastructure that visual inspection has developed may facilitate roll-out of colposcopy, if coupled with the training of nurses and general practice providers in the region. In Botswana, for instance, the visual inspection with acetic acid programming has equipped a number of facilities with capability to perform LEEP, and many LEEP sites have colposcopes not currently in use. If rapid high-risk HPV testing were available in the future, same-day triage with colposcopy and treatment at these sites would be feasible.
This study highlights the acute need to improve screening for cervical cancer and raises concern about the frequency of screening in women living with HIV in low- and middle-income countries. Current national strategy in Botswana recommends screening with cytology or visual inspection with acetic acid in women living with HIV every three years. While many of the participants had been screened before (over 90%), only 11% of women reported a prior abnormal result and 2–3% reported a prior excisional procedure. Our high prevalence of high-grade pre-invasive cervical disease supports the need for frequent screening to ensure diagnosis of disease prior to progression to malignancy. In addition to high rates of pre-invasive cervical disease, the rate of detection of cervical cancer in our screening cohort was relatively high at 2%. This included 3 women enrolled but immediately referred for suspicion for clinical stage IB cervical cancer on examination and 4 women with histopathology concerning for stage IA cervical cancer (cervical cancer or microinvasive CIN3). This rate was similar to another screening cohort in Zambia where 6 of 200 (3%) women living with HIV had invasive cervical cancers detected at the time of screening, but higher than other settings.45 In a large cervical cancer screening cohort of 79,506 women in India, 238 (0.3%) invasive cervical cancers were detected.25 In a cervical cancer screening cohort of 1128 women living with HIV in India, 5 (0.4%) invasive cervical cancers were detected.46
We found lower rates of high-risk HPV prevalence among women living with HIV than reported in the literature, which may highlight the improvement in HIV management over time with higher antiretroviral therapy utilization and viral suppression.47,48 Botswana has had continuous access to antiretroviral therapy in the public sector since 2002, with initiation of antiretroviral therapy at graduated CD4 counts over time (initially 200 then 350) until a test-and-treat policy was initiated in 2016. Demographic differences in study populations may also contribute to this difference. Our study had a higher median age than in studies conducted in the United States, Kenya and Brazil. Additionally, the population in New York had higher risk behaviors, as indicated by high rates of smoking and on-going intravenous drug use49 The study population in Brazil was pregnant which may have resulted in increased immunosuppression and higher high-risk HPV detection rates.50 Rates of high-risk HPV prevalence among women living with HIV in the region generally range from 47–57%, however, the prevalence is lower in women aged 40–49.51,52 In a similarly-aged cohort of women in Zambia, where 90% of participants were on antiretrovirals and only 77% virally suppressed, high-risk HPV positivity was 47%.45 On-going evaluation of high-risk HPV rates in women living with HIV in the modern antiretroviral therapy are necessary to understand if our findings are generalizable.
Our study has limitations. Our confidence intervals are wide around sensitivity, specificity, PPV and NPV as a result of our relatively small sample size. Further, research in larger populations will help to clarify if the difference in performance detected in this study is significant. The cohort was recruited from an HIV treatment center, which may represent a unique population of health-seeking individuals and may not be representative of a broader population. Ease of communication and follow-up of abnormal results may not therefore be replicated in a larger population. However, we found many women were not only reachable, but proactively followed-up their results, indicating that improved education about cervical cancer may reduce loss to follow-up and maximize dissemination of results. While the goal of this study was to evaluate screening algorithms that would be possible with pathology services currently available, external validation of cytology and histopathology specimens was not performed and thus accuracy compared to an expert gynecologic cytopathologist and pathologist was not evaluated. History of cervical cancer screening is primarily self-reported with limited ability to confirm results in the electronic medical record. In regards to study design, the effect of co-infection with multiple high-risk HPV types could not be assessed because the study sample was not sufficiently powered for this subgroup. Finally, one visual inspection with acetic acid nurse and colposcopist conducted the evaluations; therefore, performance of these tests may not be generalizable.
Follow-up of this cohort is currently underway to evaluate the best interval and modality for longitudinal screening. Further research on the performance of technology-based cervical cancer screening methods compared to current available methods in low- and middle-income countries is also being planned in a larger population. Balancing the cost of these strategies with clinical effectiveness is essential and a cost-effectiveness evaluation of these strategies in Botswana is being explored. Finally, regional adoption of a test-and-treat policy for HIV may continue to impact cervical cancer rates in the long-term as long-standing antiretroviral therapy use and initiation of treatment at higher CD4 levels may reduce incidence of cervical dysplasia, progression of dysplasia, and increase the likelihood of CIN regression.53 On-going research in our population living with HIV is essential to understand this impact.
Supplementary Material
Authors’ Data Sharing Statement.
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared: De-identified demographic and test result data.
What other documents will be available? Not applicable.
When will data be available (start and end dates)? At the termination of the follow-up study.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Proposals should be directed to rluckett@bidmc.harvard.edu. To gain access, data requestors will need to sign a data access agreement.
Acknowledgements:
The authors thank Simon Boikhutso for her work in study recruitment, Natasha Moraka and Terrence Mohammed for their support of laboratory high-risk HPV testing, Tiroyaone Lincoln Kgaswanyane for providing visual inspection with acetic acid, and Dayna Neo for her facilitation of ethics approval and support in statistical analysis design.
Financial support: This work was conducted with support from Harvard University Center for AIDS Research (NIH/NIAID 5P30AI060354–14 grant), Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The funders had no role in the conduct of the study, data analysis or manuscript preparation.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
References
- 1.World Health Organization. Botswana, Cancer Country Profile. WHO, 2014, http://who.int/cancer/country-profiles/bwa_en.pdf. Accessed 17 April 2019. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2013). GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; Available from http://globocan.iarc.fr. [Google Scholar]
- 3.Torre L, Bray F, Siegel R, Ferlay J, Lortet-Tieulent J, Ahmedin J. Global cancer statistics, 2012. CA Cancer J Clin, 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 4.Grover S, Raesima M, Bvochora-Nsingo M, Chiyapo S, Balang D, Tapela N, et al. Cervical cancer in Botswana: current state and future steps for screening and treatment programs. Front Oncol, 2015;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellerbrock T, Chiasson M, Bush T, Sun X, Sawo D, Brudney K. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA, 2000; 283(8):1031. [DOI] [PubMed] [Google Scholar]
- 6.Chin K, Sidhu J, Janssen R, Weber J. Invasive cervical cancer in human immunodeficiency virus-infected and uninfected hospital patients. Obstet Gynecol, 1998;92:83–87. [DOI] [PubMed] [Google Scholar]
- 7.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol, 2012;13: 607–615. [DOI] [PubMed] [Google Scholar]
- 8.McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol, 2008;9: 425–434. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler C Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin N Am, 2008;35:519–536. [DOI] [PubMed] [Google Scholar]
- 10.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis, 2007;7:453–9. [DOI] [PubMed] [Google Scholar]
- 11.Banura C, Franceschi S, van Doorn L, Arslan A, Wabwire-Mangen F, Mbidde E, et al. Infection with human papillomavirus and HIV among young women in Kampala, Uganda. J Inf Dis, 2008; 197: 555–62. [DOI] [PubMed] [Google Scholar]
- 12.Dunne E, Unger E, Sternberg M, McQuilan G, Swan D, Patel S, et al. Prevalence of HPV infection among females in the United States. JAMA, 2007; 297(8): 813–819. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Brit J Cancer, 2003; 88:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith J, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer, 2007: 121:621–632. [DOI] [PubMed] [Google Scholar]
- 15.de Sanjose S, Quint W, Alemany L, Geraets D, Klaustermeier J, Lloveras B et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol, 2010; 11:1048–1056. [DOI] [PubMed] [Google Scholar]
- 16.Denny LA et al. Human Papillomavirus, Human Immunodeficiency Virus and Immunosuppression. Vaccine, 2012; 30:F168–F174. [DOI] [PubMed] [Google Scholar]
- 17.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev, 2013; 2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engholm G, Ferlay J, Christensen N, et al. ; Association of the Nordic Cancer Registries; Danish Cancer Society. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries. Version 6.1 (25.04.2014). ancr.nu. Accessed June 25, 2015.
- 19.Cancer in Norway 2009 Special issue: Cancer screening in Norway (Haldorsen T, ed) Cancer Registry of Norway, Oslo, 2011. [Google Scholar]
- 20.Gibb R, Martens MG. The impact of liquid-based cytology in decreasing the incidence of cervical cancer. Rev Obstet Gynecol, 2011;4(Suppl 1): S2–S11. [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsburg O, Badwe R, Boyle P, Derricks G, Dare A, Evans T, et al. Changing global policy to deliver safe, equitable and affordable care for women’s cancers. Lancet, 2017; 389:871–880. [DOI] [PubMed] [Google Scholar]
- 22.Mayrand M, Duarte-Franco E, Rodrigues I, Walter S, Hanley J, Ferenczy A, et al. Human Papillomavirus DNA versus Papnicolaou screening tests for cervical cancer. NEJM, 2007;357(16):1579–1588. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Preventive services task force. 2017. Final recommendation statement cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening. Accessed 21 June 2018.
- 24.Ronco G, Dillner J, Elfstrom KM, et al. , for the International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014; 383: 524–32. [DOI] [PubMed] [Google Scholar]
- 25.Sankaranarayanan R, Nene B, Shastri S, Jayant K, Muwonge R, Budukh A, et al. HPV Screening for cervical cancer in rural India. N Eng J Med, 2009;360(14): 1385–94. [DOI] [PubMed] [Google Scholar]
- 26.Denny L,Sankaranarayanan R, De Vuyst H, Kim J,Adefuye P, Alemany L, et al. Recommendations for Cervical Cancer Prevention in Sub-Saharan Africa. Vaccine, 2013; 31: F73–F74. [DOI] [PubMed] [Google Scholar]
- 27.Santesso N, Mustafa R, Schunemann H, Arbyn M, Blumenthal P, Cain J, et al. World Health Organization guidelines for treatment of cervical intraepithelial neoplasia 2–3 and screen-and-treat strategies to prevent cervical cancer. Int J Obs Gyn 2016; 132:252–258. [DOI] [PubMed] [Google Scholar]
- 28.Denny L, Kuhn L, Risi L, Richart R, Pollack A, Lorincz A, et al. Two-stage cervical cancer screening: An alternative for resource-poor settings. Am J Obstet & Gynecol, 2000; 183(2): 383–88. [DOI] [PubMed] [Google Scholar]
- 29.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst, 2009; 101(2):88–99. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Hu S, Zhao S, Zhang W, Pan Q, Zhang X, et al. Accuracy of triage strategies for human papillomavirus DNA-positive women in low-resource settings: A cross-sectional study in China. Chin J Cancer Res 2017;29(6):496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayar R & Wilbur DC The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. Springer, 2015. [Google Scholar]
- 32.Wright TC Jr, Ellerbrock TV, Chiasson MA, Van Devanter N, Sun XW. Cervical intraepithelial neoplasia in women infected with human immunodeficiency virus: prevalence, risk factors, and validity of Papanicolaou smears. New York Cervical Disease Study. Obstet Gynecol 1994;84(4):591. [PubMed] [Google Scholar]
- 33.Ramogola-Masire D, McGrath C, Barnhart K, Friedman H, Zetola N. Subtype distribution of Human Papillomavirus in HIV-infected women with cervical intraepithelial neoplasia stages 2 and 3 in Botswana. Int J Gynecol Pathol, 2011;30(6):591–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLeod I, O’Donnell B, Moyo S, Lockman S, Shapiro R, Kayembe M, et al. Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV-infected women in Botswana. J Med Virol, 2011; 83(10): 1689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermel A, Ramogola-Masire D, Zetola N, Tong Y, Qadadri B, Azar M, et al. Invasive cervical cancers from women living in the United States or Botswana: differences in human papillomavirus type distribution. Infect Agent Cancer. 2014. July 8;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer 2014;134(6):1389–98. [DOI] [PubMed] [Google Scholar]
- 37.Mayrand M, Duarte-Franco E, Rodrigues I, Walter S, Hanley J, Ferenczy A, et al. Human Papillomavirus DNA versus Papnicolaou screening tests for cervical cancer. NEJM, 2007;357(16):1579–1588. [DOI] [PubMed] [Google Scholar]
- 38.Jeronimo J, Castle P, Temin S, Denny L, Gupta V, Kim J, et al. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol, 5 July 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Comprehensive cervical cancer control: A guide to essential practice, 2nd edn Geneva, Switzerland: WHO; 2014. [PubMed] [Google Scholar]
- 40.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cerical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila), 2012;5(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bigoni J, Gundar M, Tebeu P, Bongoe A, Schafer S, et al. Cervical cancer screening in sub-Saharan Africa: A randomized trial of VIA versus cytology for triage of HPV-positive women. Int J Cancer, 2015; 137:127–134. [DOI] [PubMed] [Google Scholar]
- 42.Muwonge R, Wesley RS, Nene BM, Shastri SS, Jayant K, Malvi SG, et al. Evaluation of cytology and visual triage of human papillomavirus-positive women in cervical cancer prevention in India. Int J Cancer. 2014;134:2902–2909. [DOI] [PubMed] [Google Scholar]
- 43.Basu P, Mittal S, Banerjee D, Singh P, Panda C, Dutta S, et al. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer. 2015;137:859–867. [DOI] [PubMed] [Google Scholar]
- 44.Basu P, Meheus F, Chami Y, Hariprasad R, Zhao F, Sankaranarayanan R. Management algorithms for cervical cancer screening and precancer treatment for resource-limited settings. Int J Gynecol Obstet 2017;138 (Suppl.1):26–32. [DOI] [PubMed] [Google Scholar]
- 45.Chibwesha C, Frett B, Katundu K, Bateman A, Shibemba A, Kapambwe S, et al. Clinical performance validation of four point-of-care cervical cancer screening tests in HIV-infected women in Zambia. J Low Genit Tract Dis, 2016;20(3):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi S, Sankaranarayanan R, Muwonge R, Kulkarni V, Somanathan T, Divate U. Screening of cervical neoplasia in HIV-infected women in India. AIDS 2013;27(4):607–15. [DOI] [PubMed] [Google Scholar]
- 47.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC Jr Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med (1997) 337:1343–910. [DOI] [PubMed] [Google Scholar]
- 48.Heard I, Cub ie HA, Mesher D, Sasieni P; MACH-1 Study Group. Characteristics of HPV infection over time in European women who are HIV-1 positive. BJOG, 2013;120(1):41–9. [DOI] [PubMed] [Google Scholar]
- 49.Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst, 1999;91(3):226–36. [DOI] [PubMed] [Google Scholar]
- 50.Jalil EM, Duarte G, El Beitune P, Simoes RT, Dos Santos Melli PP, Quintana SM. High Prevalence of Human Papillomavirus Infection among Brazilian Pregnant Women with and without Human Immunodeficiency Virus Type 1. Obstet Gynecol Int, 2009;485423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clifford GM, Gonçalves MA, Franceschi S; HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006. November 28;20(18):2337–44. [DOI] [PubMed] [Google Scholar]
- 52.McDonald AC, Tergas AI, Kuhn L, Denny L, Wright TC. Distribution of Human Papillomavirus Genotypes among HIV-Positive and HIV-Negative Women in Cape Town, South Africa. Front Oncol, 2014;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly H, Weiss H, Benavente Y, de Sanjose S, Mayaud P. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV, 2017; doi: 10.1016/S2352-3018(17)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.