Abstract
Recent advances defining the role of the commensal gut microbiota in the development, education, induction, function and maintenance of the mammalian immune system inform our understanding of how immune responses govern the outcome of systemic virus infection. While characterization of the impact of the local oral, respiratory, dermal and genitourinary microbiota on host immune responses and systemic virus infection is in its infancy, the gut microbiota interacts with host immunity systemically and at distal non-gastrointestinal tract sites to modulate the pathogenesis of systemic viruses. Gut microbes, microbe-associated molecular patterns and microbe-derived metabolites engage receptors expressed on the cell surface, in the endosome, or in the cytoplasm to orchestrate optimal innate and adaptive immune responses important for controlling systemic virus infection.
Graphical abstract
Introduction
Experiments demonstrating enhanced susceptibility of germ-free (GF) mice to virus infection in the 1960’s first suggested a role for the microbiota, the commensal micro-organisms living on and in the host, in modulating virus infection (reviewed in [1]). In the past decade, our increased understanding of the impact of the various constituents that comprise the microbiota including bacteria, archaea, viruses, protozoa and fungi on the development, education, induction, function and maintenance of the mammalian immune system (reviewed in [2–6]) has important implications for how we think about virus infection. Indeed, using now more-readily-available sequence-based and functional characterization of the microbiota as well as antibiotic (Abx) treatment to deplete the microbiota, culture-based techniques, and gnotobiotic mice, many groups have begun to dissect how the composition of microbial communities as well as specific microbial taxa modulate immune responses during virus infection (reviewed in [7]). While characterization of the impact of the local oral, respiratory, dermal and genitourinary microbiota on host immune responses and virus infection is ongoing, it is now appreciated that the gut microbiota can impact immune responses both in the local gastrointestinal (GI) tract and at distal non-GI tract sites (reviewed in [8,9]). Accordingly, in this review, we discuss how the gut microbiota interacts with host immunity and distal non-GI tract sites to modulate the pathogenesis of systemic viruses using insights obtained from experimental mouse models.
Role of the gut microbiota in innate immune responses at non-GI sites.
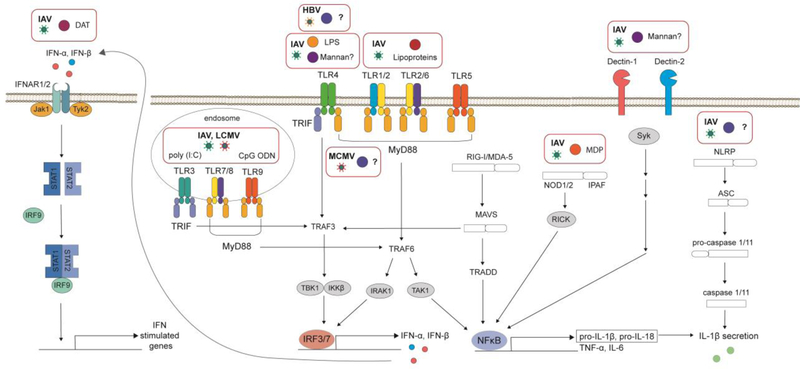
Although pattern recognition receptors (PRRs) were initially recognized for their role in sensing conserved molecular ligands called pathogen-associated molecular patterns (PAMPs) on pathogenic micro-organisms, it is now evident that signaling of commensal microbiota through PRRs via conserved ligands more generically termed microbe-associated molecular patterns (MAMPs) also shapes and modulates host immune responses (reviewed in [10]). Each PRR binds one or more conserved MAMPs such that sensing of viruses is achieved through binding of various forms of viral nucleic acids in the endosome or cytoplasm, while conserved components of bacteria, archaea, protozoa and fungi are differentially sensed by an array of PRRs on the cell surface or in the cytoplasm [reviewed in ([11–14]). Canonically, engagement of PAMPs from bacteria, protozoa and fungi results in signaling cascades that drive production of certain chemokines and pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), Interleukin (IL)-6 and IL-1β while virus recognition by endosomal and cytoplasmic PRRs results in production of interferon α/β (Type I IFN) and interferon stimulated genes (ISGs) (Fig 1). However, an emerging appreciation of the cross-talk between what classically has been thought to be separate immune programs is changing our understanding of how pathogenic and commensal micro-organisms elicit differential immune responses even though they engage the same PRRs [15–17].
Figure 1. Receptor-mediated signaling pathways that modulate host immune responses during virus infection.
The interaction of microbe-associated molecular patterns and microbe-derived metabolites with host receptors such as pathogen-recognition receptors alters the type I interferon and/or pro-inflammatory cytokine response following viral infection. IFNAR, interferon-α/β receptor; Jak, Janus Kinase; STAT, Signal transducer and activator of transcription ;IRF, IFN-regulatory factor; poly (I:C), polyinosinic:polycytidylic acid; CpG ODN, CPG oligodeoxynucleotide; IAV, Influenza Virus; LCMV, Lymphocytic choriomeningitis virus; TLR, Toll-Like Recptor; TRIF, TIR-domain-containing adaptor protein inducing IFNβ; MyD88, myeloid differentiation primary-response gene 88; IFN, interferon; MCMV, Murine Cytomegalovirus; LPS, lipopolysaccharide; PG, peptidoglycan; HBV, Hepatitis B Virus; TRAF, TNF receptor-associated factor; IRAK1, Interleukin-1 receptor-associated kinase 1; TBK1, TANK-binding kinase 1; IKKβ, inhibitor of nuclear factor kappa-B kinase subunit beta; TAK1, TGF-β-activated Kinase 1; TRADD, Tumor necrosis factor receptor type 1-associated DEATH domain; NFκB, Nuclear Factor Kappa Beta; MDA5, melanoma-differentiation-associated gene 5; MAVS, mitochondrial antiviral signaling; RIG-I, retinoic-acid-inducible gene; NOD, nucleotide-binding oligomerization domain; RICK, receptor-interacting serine/threonine kinase; IPAF, ICE-protease-activating factor; ASC, apoptosis-associated speck-like protein containing a CARD (caspase-recruitment domain; SYK, spleen tyrosine kinase; NLRP, NOD-like receptor protein; MDP, muramyl dipeptide; IL, interleukin; TNF, tumor necrosis factor; DAT, desaminotyrosine; Tyk2, tyrosine kinase.
Type I IFN-mediated anti-viral immune response.
The Type I IFN response and its induction of an innate antiviral effector program within infected and bystander cells is a central component of virus control (reviewed in [18]). While type I IFN production is canonically associated with virus infection, several studies using Abx-treatment or GF mice have demonstrated a role for the commensal gut microbiota in setting homeostatic type I IFN levels and calibrating the type I IFN response during systemic virus infection (Table 1). Abt and colleagues observed decreased expression of IFN-β and ISGs in peritoneal macrophages (MO) isolated from naïve Abx-treated mice [19]. Moreover, peritoneal MO, dendritic cells (DCs) and splenocytes from Abx-treated and GF mice have an impaired ability to respond to stimuli such as IFN-β, IFN-γ, polyinosinic:polycytidylic acid (poly (I:C)), lipopolysaccharide (LPS), Sendai virus (SeV), Influenza virus (IAV) and Lymphocytic choriomeningitis virus (LCMV) ex vivo potentially due to decreased promoter binding of Interferon Regulatory Factor 3 (IRF3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) or phosphorylation of Signal Transducer And Activator Of Transcription 1 (STAT1) [19–21]. Using IFN-β reporter mice, Ganal and colleagues observed decreased IFN-β production in multiple organs of Abx-treated mice after in vivo poly (I:C) stimulation [21]. Decreased production of type I IFN and antiviral ISGs systemically and at distal non-GI sites following IAV, LCMV, MCMV and SeV infection of Abx-treated and GF mice is associated with increased virus titers and/or enhanced susceptibility of mice to lethal infection (Table 1). In aggregate, these studies highlight a role for the commensal gut microbiota in priming type I IFN-dependent antiviral immune responses both systemically and at distal non-GI tract sites during systemic virus infection.
Table 1.
Interactions of the commensal gut microbiota with mammalian immunity during systemic virus infection.
| Mouse Model | Influence of the Gut Microbiota on Antiviral Immunity | Associated PRRs, metabolites, or microbial constituents | |||||
|---|---|---|---|---|---|---|---|
| Model of Microbiota Depletion | Outcome Measured | Cytokine-Mediated Immune Response | Innate Cellular Immune Response | Adaptive Immune Response | MAMPs, PRRs, Metabolites | Beneficial Gut Microbes | |
| Hepatitis B Virus [38] | Oral Abx-treatment | Virus titers | Virus-specific T cells: IFN-γ production of splenic T cells | TLR4 | |||
| Virus-specific antibody production: Generation of virus-specific titers in serum | |||||||
| Dengue Virus [30] | Oral Abx-treatment | Survival | |||||
| Influenza Virus [19,23,24,29,39,42, 43,45,54] | Oral Abx-treatment [19,23,39] GF mice [24,54] | Lung pathology [19] Weight- loss [19,45,54] Survival [19,39,45] Virus titers [19,23,39] |
In lung: 1. Expression of MO-associated antiviral genes [19] 2. Expression of inflammasome-associated genes [23] 3. Levels of pro-inflammatory cytokines [19,23] |
Function/recruitment of
CD103+ DCs: 1. Number of CD103+ DCs in mediastinal LN and lungs [23] 2. Expression of co-stimulatory molecules [23] 3. Presentation of viral antigen [23] |
Virus-specific T
cells: 1. Number of virus-specific CD8+ T cells in BAL, lungs, mediastinal LN and spleen[19,23,39] 2. IFN-γ production by splenic virus-specific CD8+ and CD4+ T cells [23] 3. Expression of effector molecules on CD8+ T cells in lung [19,39] 4. Regulation of activation markers on virus-specific CD8+ cells in lung [19] Tregs: Frequency of FoxP3+ CD4+ T cells in mediastinal LN and lung [23] Virus-specific antibody production: Virus-specific IgM, IgG, IgG2b, and IgA titers in serum and nasal wash [19,23] |
CpG DNA [23] LPS [23] Mannan [39] NOD2 [42,55] Peptidoglycan [23,29,42,55] Poly (I:C) [19,23] DAT [45] | L. paracasei [29] C. orbiscindens [45] C. albicans [39] S. cerevisiae [39] Wild mouse microbiota [24] Non-SPF mouse microbiota [49] |
| Lymphocytic choriomeningitis virus [19] | Oral Abx-treatment | Virus titers |
In spleen: Expression of antiviral genes In serum: Levels of IFN-β and pro-inflammatory cytokines |
Virus-specific T
cells: 1. Number of virus-specific CD8+ T cells in blood 2. Expression of effector molecules on splenic CD8+ T cells 3. Expression of inhibitory molecules on virus-specific splenic CD8+ T cells Virus-specific antibody production: Virus-specific IgG titers in serum |
|||
| Murine Cytomegalovirus [21] | Oral Abx-treatment GF mice | Virus titers |
In
spleen: IFN-α/β responses in mononuclear phagocytes In serum: Levels of IFN-α/β |
Function of splenic NK
cells: 1. Priming 2. IFN-γ production 3. Cell-mediated toxicity |
CpG DNA LPS Poly (I:C) | Altered Schaedler Flora | |
| Sendai Virus [20,48] | Oral Abx-treatment [48] GF mice [20] | Survival [48] Virus titers [48] |
In spleen: Levels of IFN-α/β In lung BAL: Levels of pro-inflammatory cytokines |
Tregs: Number of FoxP3+ CD4+ T cells in lung and distal small intestine [48] |
|||
| West Nile Virus [30] | Oral Abx-treatment | Survival Virus titers |
Virus-specific T
cells: 1. Number of virus-specific CD8+ T cells in draining LN, spleen and brain 2. Expression of effector molecules on virus-specific CD8+ T cells in draining LN, spleen and brain Tregs: Numbers of FoxP3+ CD4+ T cells in draining LN and spleen |
||||
| Zika virus [30] | Oral Abx-treatment | Survival Virus titers | |||||
MAMPs, microbe-associated molecular patterns; PRRs, pathogen recognition receptors; Abx, antibiotic; GF, germ-free; TLR, toll-like receptor; MO, macrophage; BAL, bronchoalveolar lavage; DC, dendritic cell; LN, lymph node; Tregs, T regulatory cells; LPS, lipopolysaccharide; DAT, Desaminotyrosine; SPF, specific pathogen free; NK, natural killer; poly (I:C), polyinosinic:polycytidylic acid; IFN, interferon; CpG DNA, CPG deoxynucleic acid; NOD, nucleotide-binding oligomerization domain.
Pro-inflammatory cytokine-mediated immune response
In addition to the Type I IFN response, pro-inflammatory cytokines associated with NFKB and inflammasome activation, such as TNF-α, IL-6, IL-1β and lL-18, play a critical role in limiting viral replication and modulating viral pathogenesis by orchestrating tissue-specific immune responses through their recruitment and activation of monocytes, MO, granulocytes, DCs, and natural killer (NK) cells (reviewed in [22]). Studies using Abx-treatment and GF mice uncovered a complex relationship between the gut microbiota, pro-inflammatory cytokines and systemic virus infection (Table 1). The decreased expression of Nlrp3, pro-Il-1β and pro-Il-18 mRNA as well as the reduced amount of IL-1β protein in the bronchoalveolar lavage (BAL) of IAV-infected Abx-treated mice suggests that the commensal gut microbiota may function to stimulate the inflammasome at distal sites [23]. Moreover, the reduced amount of TNF-α and IL-6 detected in the BAL and spleen of Abx-treated mice during IAV and LCMV infection, respectively, suggests an additional role for the gut microbiota in stimulating pro-inflammatory cytokines at distal sites [19,23]. However, in a recent study, Rosshart and colleagues also observed decreased amounts of pro-inflammatory cytokines including TNF-α and IL-6 following IAV infection in the BAL of GF mice reconstituted with gut microbiota from wild mice compared to GF mice reconstituted with gut microbiota from specific pathogen free-mice [24]. Since GF mice reconstituted with gut microbiota from wild mice are protected from IAV infection, these studies suggest a more nuanced role for the commensal gut microbiota in calibrating pro-inflammatory cytokine responses and regulating viral pathogenesis during systemic virus infection that may be dependent on how different constituents of the gut microbiota engage distinct components of the host immune response.
Innate cellular immune response
The gut microbiota plays a key role in hematopoiesis by regulating production of immune cell precursors in the bone marrow as well as controlling the turnover of myeloid cells such as neutrophils and inflammatory monocytes in peripheral circulation [25–28]. While a role for the gut microbiota in cell-mediated immune responses at non-GI sites during bacterial infection is well described (reviewed in [9]), studies using Abx-treatment and GF mice highlighted virus-specific roles of the commensal gut microbiota in promoting innate cell-mediated immune responses (Table 1). Belkacem and colleagues observed increased numbers of tissue resident and circulatory myeloid cells including alveolar and interstitial MO, DCs, and inflammatory and patrolling monocytes in the lungs of mice orally colonization with Lactobacillus paracasei at baseline, but not during IAV infection, which was associated with protection against lethal infection [29]. Moreover, fewer CD103+ DCs were found in the lung and mediastinal lymph nodes (LNs) of naïve Abx-treated mice [23]. While CD103+ DC from Abx-treated mice retain the ability to cross-present exogenously added antigen ex vivo, Ichinohe and colleagues found decreased numbers and impaired antigen-presenting capacity of CD103+ DC from the lungs of IAV-infected Abx-treated mice, as well as impaired migration of these cells into mediastinal LNs, a required site of antiviral CD8+ T cell priming during IAV infection [23,30]. While the development, differentiation, and maturation of splenic NK cells was maintained in naïve Abx-treated mice, Ganal and colleagues observed that NK cells from Abx-treated mice stimulated in vivo with poly (I:C), LPS, and CpG DNA were impaired in ex vivo cell-mediated cytotoxicity and IFN-γ production [21]. Moreover, the impaired NK cell priming by CD11c+ DCs or other bystander cells in Abx-treated mice resulted in increased virus titers during Murine cytomegalovirus infection (MCMV) [21]. Together, these studies demonstrate a role for the commensal gut microbiota in priming cell-mediated innate immune responses at distal non-GI sites during systemic virus infection.
Role of the gut microbiota in adaptive immune responses at non-GI sites
A role for the commensal gut microbiota in shaping the adaptive immune response is now extensively documented (reviewed in [31,32]). The commensal gut microbiota is required for the development of secondary lymphoid structures, as well as the differentiation, maturation and function of T and B cells including virus-specific effector CD4+ and CD8+ T cells, FoxP3+ CD4+ T regulatory cells (Tregs) and Th17 cells, CD4+ helper T cells, and B cells. Priming and activation of CD8+ T cells by antigen-presenting cells (APCs) results in virus-specific effector T cells armed with the ability to kill virus-infected cells through the expression of effector molecules such as perforin and granzyme B, as well as the production of cytokines such as IFN-γ and TNF-α (reviewed in [33]). Additional interactions between APCs and CD4+ T cells drive a cytokine-mediated Th1-polarized cellular program in other immune cells such as MO (reviewed in [34]). Moreover, CD4+ T cells also have a crucial role following virus infection coordinating the generation of an optimal humoral immune response through their involvement in the germinal center reaction (reviewed in [35]). In short, an adaptive immune response requires complex coordination between innate immune cells and lymphocytes to initiate an optimal antiviral immune response capable of controlling systemic virus infection.
T cell-mediated adaptive immune response
While the commensal gut microbiota is known to regulate the differentiation and function of CD8+ T cells and Tregs [36,37], its role in T cell-mediated antiviral responses during systemic virus infection is less well established. Studies using Abx-treatment and GF mice investigated the role of the commensal gut microbiota in the generation of virus-specific T cells and T regulatory cells (Tregs) during systemic virus infection (Table 1). Fewer virus-specific effector CD8+ T cells are found systemically in blood and at distal sites including the lung, spleen, and brain of Abx-treated mice following Hepatitis B virus (HBV), IAV, LCMV, and West Nile Virus (WNV) infection [19,23,30,38,39]. Increased virus burden and/or increased susceptibility to lethal HBV, IAV, LCMV and WNV infection was likely in part due to decreased virus clearance by virus-specific effector CD8+ T cells. While not examined in most studies, Ichinohe and colleagues also observed fewer virus-specific effector CD4+ T cells in the spleens of IAV-infected Abx-treated mice suggesting a role for the commensal gut microbiota in priming both antiviral effector CD4+ and CD8+ T cells during systemic virus infection [23]. In contrast, the role of the commensal gut microbiota in Treg induction appears to be more virus-specific: fewer FoxP3+ CD4+ Tregs were found in the lung and spleen of Abx-treated mice following SeV and WNV infection, respectively, while a greater frequency of FoxP3+ CD4+ Tregs was found in the lung of Abx-treated mice following IAV infection [23,30,40]. In aggregate, these studies demonstrate a role for the commensal gut microbiota in generating an optimal effector and regulatory T cell response, the balance of which determines the outcome of systemic virus infection.
B cell-mediated adaptive immune response
While a role for the gut microbiota in shaping intestinal and systemic humoral immunity has been defined (reviewed in [41]), the mechanism by which the commensal gut microbiota regulates antibody responses during systemic virus infection is not known (Table 1). Virus-specific antibody responses are diminished systemically in Abx-treated mice following HBV, IAV, and LCMV, but not WNV, infection [19,23,30,38,39]. While these studies demonstrate that the commensal gut microbiota influences the humoral response during systemic virus infection, it remains to be determined if this effect is mediated directly through the influence of the gut microbiota on B cell function or indirectly through its influence on CD4+ T helper cells that drive virus-specific antibody responses in the germinal center.
Potential mechanisms by which the commensal microbiota controls systemic virus infection
Although the molecular pathways through which the commensal gut microbial signals to generate optimal antiviral immune responses remain to be well defined, several groups have identified specific microbial constituents and signaling molecules that modulate host immunity and viral pathogenesis during systemic virus infection (Table 1). Consistent with the idea that the commensal gut microbiota modulates antiviral immune responses through the engagement of PRRs by MAMPs (Fig 1), the intrarectal administration of multiple toll-like receptor (TLR) agonists (LPS, peptidoglycan, poly (I:C), and CpG DNA) can restore inflammasome-dependent cytokines, CD103+ DC priming and migration, and effector CD8+ T responses in IAV-infected Abx-treated mice resulting in protection from lethal infection [19,23]. In addition, intravenous administration of a specific peptidoglycan component, Muramyl dipeptide (MDP), induces a NOD2-dependent type I IFN response which is protective against lethal IAV infection [42,43]. Recently, Jiang and colleagues found that oral administration of Candida albicans and Saccharomyces cerevisiae or the intrarectal administration of fungal-derived Mannan could also restore antiviral effector CD8+ T cell responses resulting in protection of IAV-infected Abx-treated mice [39]. This observation is consistent with the idea that the commensal gut microbiota acts to prime host immunity through the engagement of PRRs, since mannan can engage TLRs and C-type lectins (reviewed in Ref [13]). Signaling through TLR4 may be required for the commensal gut microbiota to clear HBV antigen, while TRIF and MyD88-dependent signaling is required for microbiota-mediated priming of NK cells and control of MCMV infection [21,38]. In summary, these studies suggest that commensal gut microbiota-derived MAMPs engage PRRs to orchestrate innate immune responses that in turn generate optimal adaptive immune responses during systemic virus infection.
Commensal gut microbial-derived metabolites play an important role in the regulation of host immunity (reviewed in Ref [44]), however, the role of microbiota-derived metabolites in systemic virus infection is not well defined (Table 1). Recently, Steed and colleagues identified a microbial-derived metabolite, desaminotyrosine (DAT), that is produced by the human-associated commensal gut bacteria Clostridium orbiscendens [45]. Oral administration of C. orbiscendens or DAT protected mice from lethal IAV infection through reduced immunopathology in the lung in a phagocyte-dependent process potentially through augmentation of the Type I IFN amplification loop (Fig 1). When coupled with the major role of commensal gut microbial-derived metabolites in the regulation of host immune responses, this study suggests that many other metabolites will modulate the outcome of systemic virus infection.
Conclusions and Future Questions
Although many investigations using Abx-treated, GF and gnotobiotic mouse models over the past 50 years demonstrate a role for the commensal gut microbiota in priming antiviral innate and adaptive immune responses at non-GI tract sites during systemic virus infection (Table 1), many details about this long-distance interaction remain to be answered. While past studies have established a role for commensal bacteria and fungi in modulating host immunity during infection with several systemic viruses (Table 1), the role of commensal viruses and protozoa is not well understood (reviewed in Ref [46,47]). Moreover, due to the pervasive influence of the commensal gut microbiota on host immunity, additional systemic viruses may also be impacted. More recent studies have identified a role for specific bacteria and fungi, as well as MAMPs, metabolites, PRRs and down-stream signaling molecules in the regulation of host immunity during systemic virus infection (Table 1). However, a more thorough understanding of the molecular mechanisms by which specific constituents of the commensal gut microbiota modulate host immunity is needed. Furthermore, despite studies which suggest that both the gut and local microbiota can independently influence host immunity in the lung during infection with several respiratory viruses [48–50] the relative contributions of the distal gut microbiota and local oral, respiratory, dermal and genitourinary microbiota during systemic virus infection are not well established. Lastly, while this review has focused on regulation of systemic and distal non-GI tract host immune responses by the commensal gut microbiota, studies showing that systemic virus infection alters the commensal gut microbiota [51–53] suggest an additional layer of complexity in the interaction between the commensal gut microbiota and systemic virus infection that should be explored.
Acknowledgments
We thank Pritesh Desai and Jim White for their helpful critiques of this review. This work was supported by the National Institutes of Health grant U19AI106772.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
special interest (*) or outstanding interest (**)
- 1.Robinson CM, Pfeiffer JK: Viruses and the Microbiota. Annu Rev Virol 2014, 1:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW: Role of the microbiota in immunity and inflammation. Cell 2014, 157:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abt MC, Artis D: The intestinal microbiota in health and disease: The influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol 2009, 25:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowan-Nash AD, Korry BJ, Mylonakis E, Belenky P: Cross-Domain and Viral Interactions in the Microbiome. Microbiol Mol Biol Rev 2019, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y, Harrison OJ: Homeostatic Immunity and the Microbiota. Immunity 2017, 46:562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D: Regulation of inflammation by microbiota interactions with the host. Nat Immunol 2017, 18:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazel-Sanchez B, Yildiz S, Schmolke M: Menage a trois: Virus, Host, and Microbiota in Experimental Infection Models. Trends Microbiol 2019, doi: 10.1016/j.tim.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Chiu L, Bazin T, Truchetet M-E, Schaeverbeke T, Delhaes L, Pradeu T: Protective Microbiota: From Localized to Long-Reaching Co-Immunity. Front Immunol 2017, 8:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RL, Clarke TB: The regulation of host defences to infection by the microbiota. Immunology 2017, 150:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu H, Mazmanian SK: Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 2013, 14:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Z, Ni G, Damania B: Innate Sensing of DNA Virus Genomes. Annu Rev Virol 2018, 5:341–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi O, Akira S: Pattern recognition receptors and inflammation. Cell 2010, 140:805–820. [DOI] [PubMed] [Google Scholar]
- 13.Plato A, Hardison SE, Brown GD: Pattern recognition receptors in antifungal immunity. Semin Immunopathol 2015, 37:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakeri A, Hansen EP, Andersen SD, Williams AR, Nejsum P: Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles. Front Immunol 2018, 9:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawashima T, Ikari N, Watanabe Y, Kubota Y, Yoshio S, Kanto T, Motohashi S, Shimojo N, Tsuji NM: Double-Stranded RNA Derived from Lactic Acid Bacteria Augments Th1 Immunity via Interferon-beta from Human Dendritic Cells. Front Immunol 2018, 9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, Kaneko D, Kumagai Y, You D-J, Carreras J, et al. : Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity 2013, 38:1187–1197. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Schorey JS: Mycobacterium tuberculosis-induced IFN-beta production requires cytosolic DNA and RNA sensing pathways. J Exp Med 2018, 215:2919–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teijaro JR: Type I interferons in viral control and immune regulation. Curr Opin Virol 2016, 16:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. : Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012, 37:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seminal paper describing a role for the commensal microbiota in calibrating innate antiviral immunity during IAV and LCMV infection.
- 20.Ito Y, Nisiyama Y, Shimokata K, Kimura Y, Nagata I: Interferon-producing capacity of germfree mice. Infect Immun 1976, 13:332 LP–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, et al. : Priming of Natural Killer Cells by Nonmucosal Mononuclear Phagocytes Requires Instructive Signals from Commensal Microbiota. Immunity 2012, 37:171–186. [DOI] [PubMed] [Google Scholar]
- 22.Chen I-Y, Ichinohe T: Response of host inflammasomes to viral infection. Trends Microbiol 2015, 23:55–63. [DOI] [PubMed] [Google Scholar]
- *23.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A: Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci 2011, 108:5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seminal paper describing a role for the commensal microbiota in priming of adaptive immunity during IAV infection.
- **24.Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, et al. : Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 2017, 171:1015–1028.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterization of a wild mouse reference microbiome that protects against lethal IAV infection.
- 25.Josefsdottir KS, Baldridge MT, Kadmon CS, King KY: Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 2017, 129:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK: Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014, 15:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hergott CB, Roche AM, Tamashiro E, Clarke TB, Bailey AG, Laughlin A, Bushman FD, Weiser JN: Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood 2016, 127:2460 LP–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, et al. : Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 2014, 193:5273–5283. [DOI] [PubMed] [Google Scholar]
- 29.Belkacem N, Serafini N, Wheeler R, Derrien M, Boucinha L, Couesnon A, Cerf-Bensussan N, Gomperts Boneca I, Di Santo JP, Taha M-K, et al. : Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS One 2017, 12:e0184976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Thackray LB, Handley SA, Gorman MJ, Poddar S, Bagadia P, Briseño CG, Theisen DJ, Tan Q, Hykes BL, Lin H, et al. : Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep 2018, 22:3440–3453.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of a role for the commensal gut microbiota in protection from lethal WNV, DENV, and ZIKV infection.
- 31.Zhao Q, Elson CO: Adaptive immune education by gut microbiota antigens. Immunology 2018, 154:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill DA, Artis D: Intestinal Bacteria and the Regulation of Immune Cell Homeostasis. Annu Rev Immunol 2010, 28:623–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weninger W, Manjunath N, von Andrian UH: Migration and differentiation of CD8+ T cells. Immunol Rev 2002, 186:221–233. [DOI] [PubMed] [Google Scholar]
- 34.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH: Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 2003, 21:713–758. [DOI] [PubMed] [Google Scholar]
- 35.Victora GD, Nussenzweig MC: Germinal centers. Annu Rev Immunol 2012, 30:429–457. [DOI] [PubMed] [Google Scholar]
- 36.Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, Visekruna A: Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep 2018, 8:14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. : Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504:446–450. [DOI] [PubMed] [Google Scholar]
- 38.Chou H-H, Chien W-H, Wu L-L, Cheng C-H, Chung C-H, Horng J-H, Ni Y-H, Tseng H-T, Wu D, Lu X, et al. : Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci 2015, 112:2175–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, Way SS: Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of commensal fungi and fungi-derived mannan which protect against lethal IAV infection.
- 40.Rohlfing M, Hunter DA, Santoro JL, Cheung DS, Grayson MH, Salzman NH, Lam V, Zemple SJ, Camarda LE, Hussain S-RA, et al. : Intestinal Microbiota Disruption Reduces Regulatory T Cells and Increases Respiratory Viral Infection Mortality Through Increased IFNγ Production. Front Immunol 2018, 9:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M, Kim CH: Regulation of humoral immunity by gut microbial products. Gut Microbes 2017, 8:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J: Muramyl dipeptide induces NOD2-dependent Ly6Chigh monocyte recruitment to the lungs and protects against Influenza virus infection. PLoS One 2012, 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egarnes B, Gosselin J: Contribution of regulatory T cells in nucleotide-binding oligomerization domain 2 response to influenza virus infection. Front Immunol 2018, 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy M, Blacher E, Elinav E: Microbiome, metabolites and host immunity. Curr Opin Microbiol 2017, 35:8–15. [DOI] [PubMed] [Google Scholar]
- **45.Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ, et al. : The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science (80- ) 2017, 357:498 LP–502. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of a microbe-derived metabolite desaminotyrosine which protects against lethal IAV infection.
- 46.Neil JA, Cadwell K: The Intestinal Virome and Immunity. J Immunol 2018, 201:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brosschot TP, Reynolds LA: The impact of a helminth-modified microbiome on host immunity. Mucosal Immunol 2018, 11:1039–1046. [DOI] [PubMed] [Google Scholar]
- 48.Grayson MH, Camarda LE, Hussain S-RA, Zemple SJ, Hayward M, Lam V, Hunter DA, Santoro JL, Rohlfing M, Cheung DS, et al. : Intestinal Microbiota Disruption Reduces Regulatory T Cells and Increases Respiratory Viral Infection Mortality Through Increased IFNgamma Production. Front Immunol 2018, 9:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Li F, Sun R, Gao X, Wei H, Li L-J, Tian Z: Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 2013, 4:2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanmani P, Clua P, Vizoso-Pinto MG, Rodriguez C, Alvarez S, Melnikov V, Takahashi H, Kitazawa H, Villena J: Respiratory Commensal Bacteria Corynebacterium pseudodiphtheriticum Improves Resistance of Infant Mice to Respiratory Syncytial Virus and Streptococcus pneumoniae Superinfection. Front Microbiol 2017, 8:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Li F, Wei H, Lian Z-X, Sun R, Tian Z: Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell–dependent inflammation. J Exp Med 2014, 211:2397 LP–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ, Tregoning JS: Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota. Front Immunol 2018, 9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M: Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018, 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DOLOWY WC, MULDOON RL: STUDIES OF GERMFREE ANIMALS. I. RESPONSE OF MICE TO INFECTION WITH INFLUENZA A VIRUS. Proc Soc Exp Biol Med 1964, 116:365–371. [DOI] [PubMed] [Google Scholar]
- 55.Egarnes B, Gosselin J: Contribution of Regulatory T Cells in Nucleotide-Binding Oligomerization Domain 2 Response to Influenza Virus Infection. Front Immunol 2018, 9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]