Summary Statement:
Patients with pulmonary arterial hypertension have exceptionally high perioperative risk. This review summarizes the clinical presentation and therapies for PAH, and highlights evidence for inflammation as a driver of disease pathogenesis and a therapeutic target.
Introduction
Pulmonary hypertension, defined as a resting mean pulmonary artery pressure (mPAP) greater than or equal to 25 mmHg, results from a myriad of conditions. The Fifth World Symposium on Pulmonary Hypertension proposed a classification system that organizes pulmonary hypertension into five groups based on common hemodynamic, pathophysiologic and therapeutic parameters.1 This review focuses on pulmonary arterial hypertension (PAH; Group 1 pulmonary hypertension), a disease in which progressive pulmonary vascular obstruction, remodeling and destruction lead to increased right ventricular afterload and hypertrophy, right heart failure and death. On a cellular level, the dysregulated proliferation of endothelial, smooth muscle, and immune cells predominates within the diseased vessels.2 Hemodynamically, PAH can be defined as a mPAP > 25 mmHg, a pulmonary capillary wedge pressure < 15 mmHg, and a pulmonary vascular resistance (PVR) > 3 Wood units in the absence of a more common etiology such as left heart disease, chronic venothromboembolic disease, or lung disease. Like pulmonary hypertension in general, PAH can result from multiple etiologies, including infectious and autoimmune pathologies. In this review, our focus will be on PAH specifically (WHO Group 1), comprised predominantly of idiopathic, and heritable PAH, as well as PAH associated with connective tissue disease, congenital heart disease or other systemic conditions.
Major pulmonary hypertension clinical registries report PAH incidence rates between 1.1 to 7.6 cases of PAH per million adults per year with a prevalence ranging from 11 to 26 cases per million adults.2 The disease demonstrates a gender bias, occurring 4 times more frequently in women, but with worse survival in male patients.3 While PAH is a relatively rare disease, it has a grim prognosis with mortality 5 years after diagnosis as high as 60%.1 However, the preceding decades have seen significant improvement in the survival of patients with PAH,4 likely due to a combination of increased awareness, improved management of right heart failure and access to PAH therapies and anticoagulation. As a result, patients with PAH are increasingly presenting for non-cardiac surgery.
PAH remains an indolent, progressive disease without a cure. Current therapies delay clinical worsening and improve quality of life, but generally have not been shown to decrease mortality. Therapeutic approaches include both supportive and disease-specific measures. Supportive measures include salt and fluid restriction, supplemental oxygen to maintain systemic arterial oxygen saturation greater than 90%,5 exercise training, and consideration of diuretics for venous congestion due to right heart failure. In some patients, anticoagulation with warfarin is prescribed to mitigate thrombosis in resistance pulmonary arteries; however, its benefit appears contingent on the underlying type and cause of PAH.6 The role of new oral anticoagulants remains unknown.
Current PAH therapies work through dilating the pulmonary vasculature, and thereby offset the elevated right ventricular afterload. These temporizing measures may neglect the underlying mechanism of disease pathogenesis and new approaches are wanting. To that end, PAH has more recently been associated with dysregulated and aberrant inflammation independent of the underlying etiology.7,8 In this narrative review, we delineate the immunological basis of PAH, highlighting biomedical and clinical evidence, and review ongoing clinical trials targeting the immune system in PAH, as well as potential immunomodulatory therapeutic strategies for future study. Throughout, we emphasize the implications for perioperative and critical care specialists, beginning with a discussion of the perioperative management of patients with PAH.
Perioperative management of patients with PAH
Patients with PAH represent some of the greatest management challenges for anesthesiologists and critical care physicians alike.9 Their perioperative risk has primarily been evaluated through retrospective studies,10–14 partly limited by differences in methodology and consideration of combined WHO pulmonary hypertension groups. Yet, together they uniformly speak to the considerable risk of morbidity and mortality in patients with PAH across the perioperative period,10–15 above that seen in other types of pulmonary hypertension.16 Rates of serious perioperative complications, including respiratory failure, heart failure, cardiovascular instability and myocardial infarction approach 40%,10 and non-cardiac surgical mortality occurs in 3.5–8% of patients.10,11,15 Death occurs most commonly within the first 48 hours of surgery and typically results from right heart failure.12,16 The risk of death remains elevated following both minor and major surgeries, with a further increase in the context of emergency procedures.13,15 No differential risk of complication or death has been demonstrated between anesthetic techniques.16 Not surprisingly, patients with PAH experience longer intensive care unit and hospital lengths of stay with higher 30-day readmission rates.11
Given the high risk of morbidity and mortality in the perioperative period for patients with PAH, delaying elective procedures in order to undertake a thorough preoperative evaluation by a multidisciplinary team with specific consultation with a pulmonary hypertension specialist is warranted.9 PAH is itself an independent predictor of perioperative complications.17 Patient-risk factors of morbidity and mortality include a right atrial pressure >7 mmHg and a 6-minute walk test <399 m.15 The preoperative period is thus an opportunity to optimize medical therapy and exercise capacity. Pulmonary vasodilators are generally routinely continued perioperatively.18,19
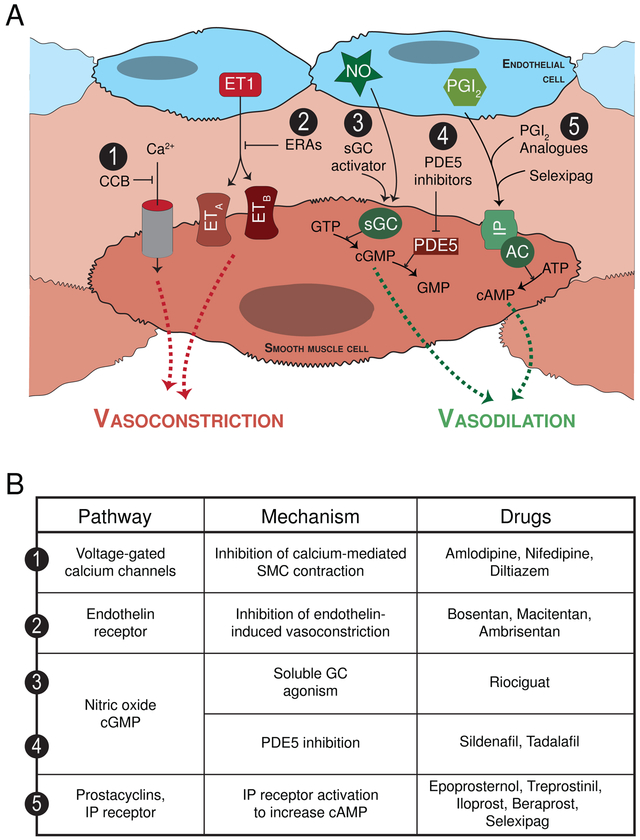
At present, the FDA has approved fourteen PAH-specific therapies that target four major molecular pathways all related to pulmonary vascular tone (Figure 1). Notably, none directly address pulmonary vascular remodeling or target the right ventricle. Approaches to PAH treatment and the PAH-specific therapies have been thoroughly reviewed2 and are summarized in Figure 1. The management of severe pulmonary hypertension in the operative setting has similarly been comprehensively reviewed.9
Figure 1.
(A) Current PAH-specific therapies target four molecular pathways all of which are directed at vasodilating the pulmonary vasculature. (1) Calcium channel blockers: The calcium channel blockers amlodipine, nifedipine and diltiazem all block calcium entry into vascular smooth muscle cells (SMC) thereby preventing SMC contraction and pulmonary vasoconstriction. Unfortunately, this class of medications is only effective in <10% of PAH patients, and long-term responses often limited. (2) Endothelin pathway: Endothelin acts through its endothelin A and B receptors (ETA and ETB, respectively) to cause vasoconstriction as well as SMC proliferation. Bosentan and macitentan are both dual endothelin receptor anatagonists (ERA), while ambrisentan is a selective endothelin receptor A antagonist. (3) Nitric oxide pathway: Nitric oxide (NO) is a vasodilator that signals through soluble guanylate cyclase (sGC), which generates cyclic guanosine monophosphate (cGMP) and causes pulmonary artery SMC relaxation. The cGMP is subsequently degraded by phosphodiesterases (PDE). Accordingly, the (3) direct sGC stimulator riociguat and the (4) PDE5 inhibitors sildenafil and tadalafil drive the NO pathway to enhance vasodilation. The use of inhaled nitric oxide is currently reserved for the intraoperative and intensive care unit acute management of pulmonary hypertension treatment. (5) Prostacyclin pathway: Prostacyclin and prostanoids bind the IP receptor to stimulate cyclic adenosine monophosphate, which in turn causes pulmonary vasodilation. Various preparations of prostanoids are available and can be administered via different routes including continuous infusion intravenously or subcutaneously, by inhalation, and orally. The prostanoids include epoprosternol, treprostinil, iloprost and beraprost. Selexipag, in contrast, is the first non-prostanoid IP receptor activator. (B) Currently approved PAH-specific medications with their respective pathway target and mechanism of action.
In this review, we deal separately with the chronic management of PAH (the main focus herein) and the rescue of acute, decompensated right-sided heart failure or an acute PVR crisis. In the acute setting, the importance of pulmonary vasodilators and inotropic support cannot be over-emphasized: reduction in PVR and support of the right ventricle represent the anesthesiologists’ most pressing priorities. Pulmonary vasodilator drugs target one of 4 major pathways using several routes of administration. The cellular pathways in question involve L-type calcium channels, endothelin-1, nitric oxide, and prostacyclin.9,20 Calcium channel blockers, specifically indicated for the subset of patients demonstrating positive vasoactive testing in the cardiac catheterization laboratory, can be used at high doses and should be terminated or supplemented by other drugs if no effect is noted.6 Of note, significant systemic hypotension may arise as a result of calcium channel blockade in this population. Careful multi-disciplinary consultation is advised for potential bridging to other drugs in the perioperative setting.
Endothelin receptor antagonists (bosentan, macitentan, ambrisentan) are among the best studied drugs for the chronic management of PAH. The landmark SERAPHIN trial demonstrated the efficacy of macitentan in PAH patients for decreasing risk of a primary outcome of clinical worsening, lung transplantation, atrial septostomy, initiation of prostanoids, or death.21 Importantly, patients taking macitentan may develop both elevated liver enzymes and anemia, so testing liver function and hemoglobin concentration prior to major surgery would be prudent.21 While macitentan has a clinical effect shortly after initiation of therapy, patients should ideally be at a “steady-state” with respect to their disease prior to non-emergent surgery.
Nitric oxide is a potent pulmonary vasodilator, endogenously produced in the lung endothelium, acting through the production of cyclic guanosine monophosphate (cGMP) by the enzyme soluble guanylyl cyclase (sGC).22 Patients with PAH often produce less NO, as part of an overall endothelial dysfunction and imbalance of vasoconstrictor versus vasodilator substances.23 Pharmacologically, NO can be targeted in 3 ways: by delivering inhaled NO acutely,24 using oral phosphodiesterase-5 inhibitors (sildenafil, tadalafil) to decrease cGMP breakdown,25 or more recently, using an activator of sGC (riociguat).26 For acute use, inhaled NO can be attached to an anesthesia circuit for rapid addition, and has a long history of successful use in the acute intra-operative setting.9 The on-going INOvation-1 trial is a placebo-controlled, randomized controlled trial designed to evaluate the long-term safety and efficacy of inhaled nitric oxide in PAH (clinicaltrials.gov NCT02725372). The chronic vasodilatory effects of sildenafil and other drugs discussed herein should give pause to anesthesiologists, as they can cause hypotension, have anti-platelet effects, and may also inhibit hypoxic pulmonary vasoconstriction during thoracic surgery and one-lung ventilation.9,27 Stable patients are unlikely to be successfully transitioned away from these drugs before elective surgery, so continuing them through the perioperative period, with knowledge of their potential adverse effects under anesthesia, is of paramount importance.
Finally, another mainstay drug class in the therapy of PAH are the prostanoids. These drugs can be inhaled for acute use, delivered by continuous intravenous infusion, or given orally in a new formulation.6 The inhaled formulations demonstrate a high degree of specificity for the pulmonary vasculature, and can synergize with inhaled or intravenous milrinone as well.28 These drugs are direct vasodilators of the pulmonary circulation, while also providing some anti-platelet and anti-inflammatory benefit.23 In the commonest form for chronic use, intravenous epoprostenol is delivered centrally via continuous infusion. In the operating room, such a system requires the utmost vigilance, as access to the line, as well as its patency, must be maintained or a critical PVR crisis may arise upon discontinuation.6 Furthermore, meticulous sterile technique must be maintained, since line infection and loss of central access is a devastating complication for this group.29
In summary, great care and planning must go into the surgical care of PAH patients. Multidisciplinary involvement is critical, and major surgery should be carried out in centers with appropriate post-operative intensive care available. The specific interactions of PAH therapies with anesthesia and surgical insults (including systemic hypotension, anti-platelet effects, interaction with other vasodilators, the need for special access and delivery equipment, and other considerations) necessitate holistic planning on the part of the perioperative team for the safe management of a high-risk population.29
Immunological basis of pulmonary arterial hypertension
A large body of evidence from both human trials and pre-clinical models currently supports the fundamental immunological underpinnings of PAH, implicating both soluble and cellular immune mediators across the innate and adaptive immune systems.30
Cytokines such as tumor necrosis factor (TNF) and the interleukins (IL)-1β and IL-6 are all found in increased concentrations in the blood of PAH patients as compared to controls,8,31,32 and in some cases, these levels correlate with disease severity. For instance, circulating IL-6 serves as a high-fidelity marker of right ventricular dysfunction in PAH33 and is elevated in almost all human and animal studies of the disease. Other non-canonical immune mediators have similarly been implicated. In particular, the pro-inflammatory protein high mobility group box-1 (HMGB1) is elevated in the lungs and plasma of patients with idiopathic and congenital heart disease-related PAH.34,35 HMGB1 is notable in that it is able to activate both innate and adaptive immunity pathways.36 As such, it represents an active area of research in PAH pathogenesis, and its modulation may form the basis for novel therapeutics. To that end, encouraging preclinical work in rodent models of PH has demonstrated that HMGB1 neutralization protects against disease development.37
Immune cells are also important drivers of PAH. A broad histopathological survey of lungs from patients with PAH demonstrated significant perivascular and interstitial inflammation with immune cell infiltration, regardless of the etiology of PAH.38 The immune cell types that circulate and infiltrate the lungs of patients with PAH include B and T lymphocytes,39–43 macrophages,43 neutrophils,44–46 dendritic cells,7,43 and mast cells.7,43 Interestingly, patients with PAH have a different immune cellular landscape in their lungs as compared with those of control patients that suggests a shift toward the adaptive immune system. Recruited immune cells not only represent a potential source of local and circulating cytokines, but also directly contribute to pulmonary vascular remodeling. The abnormal pulmonary adventitia and vessels can also secrete pro-inflammatory molecules that activate immune cells,47 further amplifying this process. Taken together, positive feedback loops propagate inflammation within the vessel wall and perpetuate the vasculopathy of PAH.
The immunological basis of PAH development continues to evolve as the disease itself progresses. Later in the course of the disease, the adaptive immune system grows in influence and a more complex network of immune reactions develops within the vessel wall. The importance of adaptive immunity in PAH has been further highlighted in several recent preclinical studies.48,49 Yet, the importance of adaptive immunity in clinical disease is not a new concept. More than two decades ago, idiopathic PAH (iPAH) was speculated to represent an autoimmune disease.50 This notion has since gained increasing traction. The lungs of patients with PAH contain bronchus-associated lymphoid tissue (BALT)51 and tertiary lymphoid tissue52 capable of producing autoantibodies in a self-sustained fashion. While the importance of autoimmunity in PAH may have been anticipated in connective tissue disease and other rheumatological illnesses, it is unexpected to find self-reactive antibodies in patients with PAH resulting from non-immunological disease states. Yet, as many as 60% of patients with iPAH had circulating autoantibodies,50 as do even a proportion of patients with congenital heart disease-related PAH.53 The anti-vascular autoantibodies that are produced in this tissue will be made in a self-sustained fashion in a germinal center.52 Therefore, tissue destruction and inflammation can continue in the absence of the initial immune stimulus. This, along with epigenetic changes in parenchymal cells,54 may well form the basis for the chronic, progressive nature of PAH.
PAH clinical trials targeting the immune system
Given the lack of meaningful long-term improvement seen with vasodilator therapy, clinicians and scientists are attempting to test new management paradigms clinically. To that end, the predominance of clinical and pre-clinical evidence pointing to the importance of the immune system in PAH has spawned a host of clinical trials. The guiding principles for such trials are outlined above with both cellular and soluble immune targets now informing clinical inquiry.
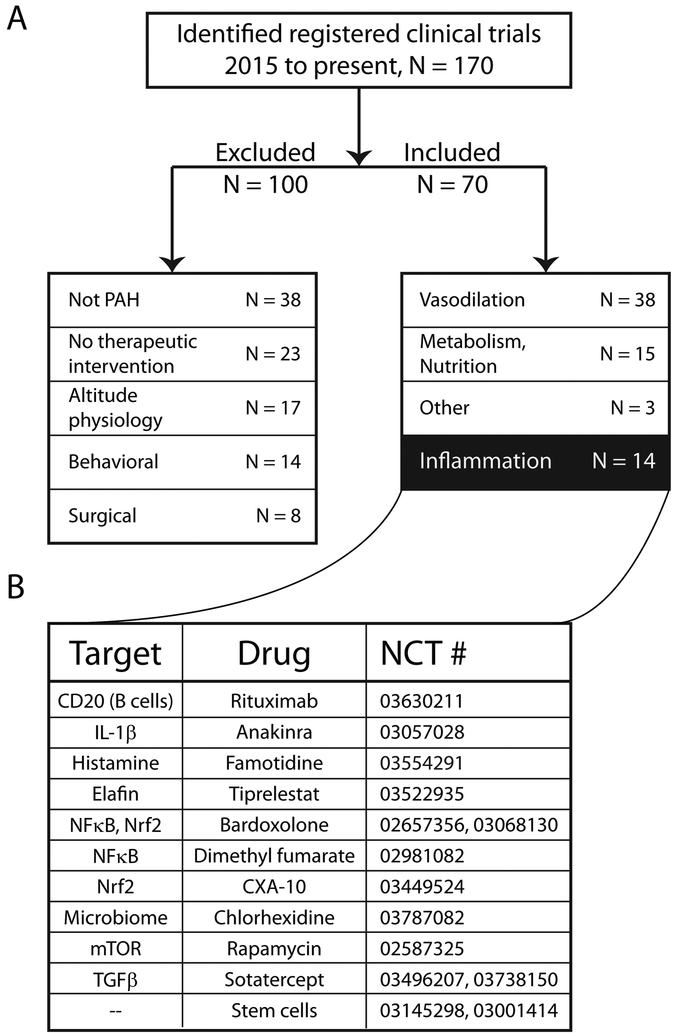
To investigate the current clinical interest in immunotherapy for PAH, we conducted a search of active clinical trials in PAH using the United States National Library of Medicine’s clinical trials registry (Figure 2; see legend for search details). From January 1, 2015, to the present, 70 active trials met our inclusion criteria from a total of 170. Of the included trials, the largest group continues to investigate the use of vasodilator therapy (54%), which is in line with the current role of these drugs in standard of care. Notably, therapies targeting metabolism and nutrition (21%) represented the next largest group, followed closely by immunotherapies (20%). Of course, many of the tested therapies cross between these overall categories: metabolic treatments may have anti-inflammatory roles, as do several traditional vasodilators. However, entirely immunological therapeutics, such as inhibition of neutrophil elastase by elafin, the use of stem and progenitor cells, IL-1β antagonism with anakinra, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibition by bardoxolone, are the subject of large-scale human trials.
Figure 2.
The immune system is being investigated as a potential therapeutic target in PAH. (A) Current clinical trials investigating PAH therapies. A search was performed on clinicaltrials.gov for “pulmonary hypertension” from January 1, 2015, to the January 1, 2019. Limits were set to “interventional study”, and adult patients only. Trial status classifications included were not yet recruiting, recruiting, enrolling by invitation, active not recruiting, and unknown status. A total of 170 studies were identified, and inclusion and exclusion categories are depicted in the flow chart. Of the 70 interventional trials included, half (54%) are investigating vasodilator therapy, 20% focus on immunological targets, while 21% are targeting metabolism and nutrition. (B) Specific examples of immune targets and therapies currently under clinical study in PAH. NCT refers to the registered Clinical Trial number, with study information available at clinicaltrials.gov.
One patient group of particular interest is systemic sclerosis-associated PAH (SSc-PAH), which is one of the deadliest forms of the disease.1 Etiologically, SSc-PAH has a strong autoimmune component, driven in part by B lymphocytes. As such, a prospective, double-blind, placebo-controlled trial is currently underway testing B cell depletion with the anti-CD20 monoclonal antibody, rituximab, (NCT01086540) in patients with SSc-PAH.
Soluble mediators are also being targeted. In the recently reported CANTOS trial55 of 10061 patients with prior myocardial infarction, elevated C-reactive protein and on optimal medical therapy, IL-1β inhibition with the monoclonal antibody canakinumab reduced the incidence of repetitive atherothrombotic events, albeit with a small effect size. This study provides evidence that an anti-inflammatory therapy can modify an inflammatory vascular disease in humans. Moreover, in a case report, IL-1β antagonism with the IL-1 receptor inhibitor, anakinra, was found to reverse PAH in a single patient with Still’s Disease.56 A clinical trial of anakinra is currently underway to assess exercise tolerance and safety in adults with PAH (NCT03057028). These data will provide important insights into the potential feasibility of immunomodulation as a treatment paradigm in PAH.
Similarly, there are case reports of PAH improvement in patients with pulmonary hypertension secondary to inflammatory conditions treated with the IL-6 receptor antagonist tocilizumab.57 A small Phase 2 trial of tocilizumab in PAH has since been completed (NCT02676947).58 While the full data have not yet been reported, the drug was administered to 23 patients with a good safety profile. A larger, therapeutic study is expected.
Many of the inflammatory and immune pathways discussed in this review are under the control of the transcription factor NF-κB. NF-κB activation results in the upregulation of genes encoding IL-6, IL-1β, TNF, and other important immunological signals.59 These pathways promote the recruitment and activation of immune cells in target tissues, including the lung. Multiple lines of evidence from rodent models have shown that NF-κB blockade can prevent the development of PH.60 Furthermore, patients with iPAH have activated NF-κB in their lung tissue,61 indicating a potential role for this pathway in human disease. It is therefore no surprise that neutralization of NF-κB signaling is an active field of clinical investigation in PAH. A trial is currently assessing the drug bardoxolone for the treatment of connective tissue disease associated PAH (CTD-PAH; NCT02657356). Bardoxolone blocks NF-κB by stimulating the regulatory transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf-2).62 This trial will assess the effect of bardoxolone on 6-minute walk test distance in patients with CTD-PAH. Another trial is currently completing a long-term follow-up in CTD-PAH patients who received bardoxolone previously (NCT03068130). These studies will assess the safety profile of NF-κB manipulation in PAH and will likely stimulate further trials looking at transcription regulators in inflammation.
A final area of current clinical therapeutic inquiry involving inflammation and PAH centers on the inhibition of neutrophil elastase. Elastase, which can also be secreted by pulmonary vascular smooth muscle cells, plays a fundamental role in the adverse remodeling of the lung vasculature in PAH. While the clinical use of elastase inhibitors has been limited by hepatotoxicity, the endogenous elastase inhibitor, elafin, remains a promising therapeutic possibility. Elafin, in addition to blocking the action of elastase, enhances bone morphogenic protein receptor (BMPR)-mediated signaling, inhibits NF-κB, and dampens the innate immune system.63 Administration of elafin in animal models reverses PAH.64 As such, clinical exploration of elafin as a PAH therapy is underway, beginning with a safety and tolerance trial in healthy volunteers (NCT03522935). The position of elafin at the crossroads of so many disease-relevant pathways makes this line of investigation particularly exciting.
Inflammation in PAH: Implications for the anesthesiologist
Our evolving understanding of the pathobiology of PAH and the development of new treatment paradigms will have implications for anesthesiologists and critical care physicians. Hopefully such treatments will result in improved pre-operative optimization of these high-risk patients. However, as with any new therapies, their interactions with anesthetic drugs and the operative environment will have to be studied and accounted for. It stands to reason that some of these experimental agents will result in some degree of immunosuppression, which will necessitate vigilance and aggressive treatment of infection and sepsis. The overall inflammatory milieu in this population may predispose them to further insults. For instance, PAH may serve as a “first hit”, increasing susceptibility to ventilator-induced lung injury and acute respiratory distress syndrome (ARDS). In fact, several of the biomarkers and inflammatory mediators seen in PAH are also found in ARDS and correlate with poor outcome.65
There is a broad base of evidence showing that the perioperative period is a time of immune dysregulation and inflammation, and that anesthesia can have a meaningful impact on this state.66 One could speculate that anesthetic techniques that serve to minimize these insults – including regional anesthesia, and pre-emptive analgesia – may benefit patients with PAH coming for surgery. As new treatments emerge for PAH patients, and our understanding of disease pathogenesis deepens, this will represent an important field of study for perioperative and critical care physicians. Until such agents are approved for use, it is unlikely that specific perioperative data will be available to guide anesthetic care. For a discussion of the interactions of pulmonary vasodilators with the operative setting, please see the “perioperative management of patients with PAH” section above, and other publications.9 As an example of the potential for immunotherapy for PAH in the critical care setting, an instructional case series using tacrolimus has been published.67 Having identified tacrolimus as an agent that modulates pathways important in PAH,68 a series of 3 patients with end-stage, treatment-refractory PAH were given low-dose tacrolimus. One patient was subsequently de-listed from the lung transplant registry due to her improvement to low-risk status within 2 months of treatment initiation. A second patient had substantial recovery of RV function within 3 months, with concomitant improvements in her functional status. A third patient improved, voluntarily discontinued tacrolimus and worsened, only to again improve following re-institution of therapy.67 A randomized trial of tacrolimus for PAH is currently underway to systematically assess its utility in PAH (NCT01647945), but this small series of patients may offer a glimpse into the future of PAH management in the clinic and the intensive care unit. The fact that palliative disease was improved suggests that this strategy may become useful in the acute setting.
Future immune targets and therapeutic strategies in PAH
While much debate continues on the topic of the primary insult that leads to PAH, it is clear that the disease is characterized by “smoldering inflammation”.69 This inflammation involves the totality of the immune system, affecting almost all known cell types and branches of immunity. It follows that therapeutic immune targeting necessitates restricted modulation of specific immunologic responses in order to mitigate off-target effects and global immunosuppression. For example, preclinical work is investigating the use of biased agonists and antagonists that preferentially propagate or inhibit signaling down one of several downstream pathways, respectively.70
Our better understanding of PAH as an immunological disease will certainly usher in a new era of targeted therapies for this fatal condition and shift management away from vasodilation toward immunomodulation. This would parallel the history of asthma, another inflammatory lung disease. Prior to the 1900’s, the mainstays of therapy were bronchodilators, ironically in the form of “anticholinergic cigarettes”.71 These treatments were relatively effective for acute exacerbations, but failed to address the root cause in the way that modern “controller” inhalers do. With the advent of newer drugs, but more importantly, with an understanding of the inflammatory nature of the disease, asthma management shifted toward immunotherapy, with steroids coming to the fore in the 1950’s.71 We see PAH following a similar trajectory, with vasodilators maintaining a role, but immunomodulators taking over as mainstay therapy. Like asthma, PAH is a clinical syndrome, encompassing a host of causative etiologies. As such, it is likely that a variety of treatments will be required to address the various causes of PAH. New clinical trials should shed light on the feasibility of such treatments in the coming years, and active translational research will continue to feed this pipeline. New targeted therapies will hopefully begin to have substantive impacts upon patients suffering from this terrible disease.
Funding Statement:
“Support was provided from institutional and/or departmental sources, along with a grant from the International Anesthesia Research Society to NMG.”
Footnotes
Clinical trial number and registry URL, Not applicable
Prior Presentations: Not applicable
Conflicts of Interest: “The authors declare no competing interests.”
Contributor Information
Neil M Goldenberg, Department of Anesthesia and Pain Medicine, The Hospital for Sick Children, Toronto, Ontario Sick Kids Research Institute, Program in Cell Biology, Toronto, Ontario.
Marlene Rabinovitch, Department of Pediatrics and Vera Moulton Wall Center for Pulmonary Vascular Disease, Stanford University School of Medicine, Stanford, California
Benjamin E Steinberg, Department of Anesthesia and Pain Medicine, The Hospital for Sick Children, Toronto, Ontario Sick Kids Research Institute, Program in Neuroscience and Mental Health, Toronto, Ontario.
References:
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R: Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62:D34–41 [DOI] [PubMed] [Google Scholar]
- 2.Thenappan T, Ormiston ML, Ryan JJ, Archer SL: Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018; 360:j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs W, van de Veerdonk MC Trip P, de Man F Heymans MW, Marcus JT, Kawut SM, Bogaard H-J, Boonstra A, Vonk Noordegraaf A: The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 2014; 145:1230–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD: Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137:376–87 [DOI] [PubMed] [Google Scholar]
- 5.Ulrich S, Hasler ED, Saxer S, Furian M, Müller-Mottet S, Keusch S, Bloch KE: Effect of breathing oxygen-enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham-controlled cross-over trial. Eur Heart J 2017; 38:1159–68 [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group: 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endor. Eur Heart J 2016; 37:67–119 [DOI] [PubMed] [Google Scholar]
- 7.Marsh LM, Jandl K, Grünig G, Foris V, Bashir M, Ghanim B, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G: The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2018; 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR: Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115:165–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couture EJ, Provencher S, Denault AY: Management of Severe Pulmonary Hypertensive Disease for Surgical and Nonsurgical Procedures. Int Anesthesiol Clin 2018; 56:e28–55 [DOI] [PubMed] [Google Scholar]
- 10.Price LC, Montani D, Jaïs X, Dick JR, Simonneau G, Sitbon O, Mercier FJ, Humbert M: Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J 2010; 35:1294–302 [DOI] [PubMed] [Google Scholar]
- 11.Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA: Pulmonary hypertension: an important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med 2011; 105:619–24 [DOI] [PubMed] [Google Scholar]
- 12.Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD: Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 2005; 45:1691–9 [DOI] [PubMed] [Google Scholar]
- 13.Lai H-C, Lai H-C, Wang K-Y, Lee W-L, Ting C-T, Liu T-J: Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth 2007; 99:184–90 [DOI] [PubMed] [Google Scholar]
- 14.Memtsoudis SG, Ma Y, Chiu YL, Walz JM, Voswinckel R, Mazumdar M: Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg 2010; 111:1110–6 [DOI] [PubMed] [Google Scholar]
- 15.Meyer S, McLaughlin VV, Seyfarth H-J, Bull TM, Vizza CD, Gomberg-Maitland M, Preston IR, Barberà JA, Hassoun PM, Halank M, Jaïs X, Nickel N, Hoeper MM, Humbert M: Outcomes of noncardiac, nonobstetric surgery in patients with PAH: an international prospective survey. Eur Respir J 2013; 41:1302–7 [DOI] [PubMed] [Google Scholar]
- 16.Steppan J, Diaz-Rodriguez N, Barodka VM, Nyhan D, Pullins E, Housten T, Damico RL, Mathai SC, Hassoun PM, Berkowitz DE, Maxwell BG, Kolb TM: Focused Review of Perioperative Care of Patients with Pulmonary Hypertension and Proposal of a Perioperative Pathway. Cureus 2018; 10:e2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ: Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010; 14:R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox DL, Stream AR, Bull T: Perioperative management of the patient with pulmonary hypertension. Semin Cardiothorac Vasc Anesth 2014; 18:310–8 [DOI] [PubMed] [Google Scholar]
- 19.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN, American College of Cardiology, American Heart Association: 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–137 [DOI] [PubMed] [Google Scholar]
- 20.Fox CJ, Cornett EM, Hart BM, Kaye AJ, Patil SS, Turpin MC, Valdez A, Urman RD, Kaye AD: Pulmonary vasodilators: Latest evidence and outcomes in the perioperative setting. Best Pract Res Clin Anaesthesiol 2018; 32:237–50 [DOI] [PubMed] [Google Scholar]
- 21.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani H-A, Jansa P, Jing Z-C, Brun F-O Le, Mehta S, Mittelholzer CM, Perchenet L, Sastry BKS, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G, SERAPHIN Investigators: Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369:809–18 [DOI] [PubMed] [Google Scholar]
- 22.Félétou M, Köhler R, Vanhoutte PM: Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med 2012; 44:694–716 [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg NM, Kuebler WM: Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Compr Physiol 2015; 5:531–59 [DOI] [PubMed] [Google Scholar]
- 24.Ng J, Finney SJ, Shulman R, Bellingan GJ, Singer M, Glynne PA: Treatment of pulmonary hypertension in the general adult intensive care unit: a role for oral sildenafil? Br J Anaesth 2005; 94:774–7 [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G, Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group: Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353:2148–57 [DOI] [PubMed] [Google Scholar]
- 26.Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, CHEST-1 Study Group: Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369:319–29 [DOI] [PubMed] [Google Scholar]
- 27.Ross AF, Ueda K: Pulmonary hypertension in thoracic surgical patients. Curr Opin Anaesthesiol 2010; 23:25–33 [DOI] [PubMed] [Google Scholar]
- 28.Laflamme M, Perrault LP, Carrier M, Elmi-Sarabi M, Fortier A, Denault AY: Preliminary Experience With Combined Inhaled Milrinone and Prostacyclin in Cardiac Surgical Patients With Pulmonary Hypertension. J Cardiothorac Vasc Anesth 2015; 29:38–45 [DOI] [PubMed] [Google Scholar]
- 29.Pritts CD, Pearl RG: Anesthesia for patients with pulmonary hypertension. Curr Opin Anaesthesiol 2010; 23:411–6 [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg NM, Steinberg BE: Inflammation Drives Pulmonary Arterial Hypertension. Anesthesiology 2018 [DOI] [PubMed] [Google Scholar]
- 31.Selimovic N, Bergh C-H, Andersson B, Sakiniene E, Carlsten H, Rundqvist B: Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009; 34:662–8 [DOI] [PubMed] [Google Scholar]
- 32.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW: Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122:920–7 [DOI] [PubMed] [Google Scholar]
- 33.Prins KW, Archer SL, Pritzker M, Rose L, Weir EK, Sharma A, Thenappan T: Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37:376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM: High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 2012; 18:1509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y-Y, Su W, Zhu Z-W, Tang L, Hu X-Q, Zhou S-H, Fang Z-F, Li J: Elevated serum HMGB1 in pulmonary arterial hypertension secondary to congenital heart disease. Vascul Pharmacol 2016; 85:66–72 [DOI] [PubMed] [Google Scholar]
- 36.Andersson U, Tracey KJ: HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 2011; 29:139–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K-I, Inoue H, Maruyama I: HMGB1 Promotes the Development of Pulmonary Arterial Hypertension in Rats. PLoS One 2014; 9:e102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM: Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186:261–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blum LK, Cao RRL, Sweatt AJ, Bill M, Lahey LJ, Hsi AC, Lee CS, Kongpachith S, Ju C-H, Mao R, Wong HH, Nicolls MR, Zamanian RT, Robinson WH: Circulating plasmablasts are elevated and produce pathogenic anti-endothelial cell autoantibodies in idiopathic pulmonary arterial hypertension. Eur J Immunol 2018; 48:874–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bourcy CFA Dekker CL, Davis MM, Nicolls MR, Quake SR: Dynamics of the human antibody repertoire after B cell depletion in systemic sclerosis. Sci Immunol 2017; 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huertas A, Phan C, Bordenave J, Tu L, Thuillet R, Hiress M Le, Avouac J, Tamura Y, Allanore Y, Jovan R, Sitbon O, Guignabert C, Humbert M: Regulatory T Cell Dysfunction in Idiopathic, Heritable and Connective Tissue-Associated Pulmonary Arterial Hypertension. Chest 2016; 149:1482–93 [DOI] [PubMed] [Google Scholar]
- 42.Hautefort A, Girerd B, Montani D, Cohen-Kaminsky S, Price L, Lambrecht BN, Humbert M, Perros F: T-helper 17 cell polarization in pulmonary arterial hypertension. Chest 2015; 147:1610–20 [DOI] [PubMed] [Google Scholar]
- 43.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT: Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186:897–908 [DOI] [PubMed] [Google Scholar]
- 44.Klinke A, Berghausen E, Friedrichs K, Molz S, Lau D, Remane L, Berlin M, Kaltwasser C, Adam M, Mehrkens D, Mollenhauer M, Manchanda K, Ravekes T, Heresi GA, Aytekin M, Dweik RA, Hennigs JK, Kubala L, Michaëlsson E, Rosenkranz S, Rudolph TK, Hazen SL, Klose H, Schermuly RT, Rudolph V, Baldus S: Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI insight 2018; 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbaum L, Baaske KM, Simon M, Oqueka T, Sinning C, Glatzel A, Lüneburg N, Sydow K, Bokemeyer C, Klose H: Exploratory analysis of the neutrophil to lymphocyte ratio in patients with pulmonary arterial hypertension. BMC Pulm Med 2017; 17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor S, Dirir O, Zamanian RT, Rabinovitch M, Thompson AAR: The Role of Neutrophils and Neutrophil Elastase in Pulmonary Arterial Hypertension. Front Med 2018; 5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasmi KCEl, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR: Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 2014; 193:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR: Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 2011; 109:867–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamosiuniene R, Manouvakhova O, Mesange P, Saito T, Qian J, Sanyal M, Lin Y-C, Nguyen LP, Luria A, Tu AB, Sante JM, Rabinovitch M, Fitzgerald DJ, Graham BB, Habtezion A, Voelkel NF, Aurelian L, Nicolls MR: Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ Res 2018; 122:1689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanai-Landau H, Amital H, Bar-Dayan Y, Levy Y, Gur H, Lin HC, Alosachie IJ, Peter JB, Shoenfeld Y: Autoimmune aspects of primary pulmonary hypertension. Pathobiology 1995; 63:71–5 [DOI] [PubMed] [Google Scholar]
- 51.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME: Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 2013; 188:1126–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN: Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185:311–21 [DOI] [PubMed] [Google Scholar]
- 53.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, Schermuly RT, Nickel N, Hoeper MM, Lukitsch I, Gollasch M, Kuebler WM, Bock S, Burmester GR, Dragun D, Riemekasten G: Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med 2014; 190:808–17 [DOI] [PubMed] [Google Scholar]
- 54.Hu C-J, Zhang H, Laux A, Pullamsetti SS, Stenmark KR: Mechanisms contributing to persistently activated cell phenotypes in pulmonary hypertension. J Physiol 2018; 521:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group: Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377:1119–31 [DOI] [PubMed] [Google Scholar]
- 56.Campos M, Schiopu E: Pulmonary Arterial Hypertension in Adult-Onset Still’s Disease: Rapid Response to Anakinra. Case Rep Rheumatol 2012; 2012:537613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuya Y, Satoh T, Kuwana M: Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheumatol 2010; 2010:720305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernández-Sánchez J, Harlow L, Church C, Gaine S, Knightbridge E, Bunclark K, Gor D, Bedding A, Morrell N, Corris P, Toshner M: Clinical trial protocol for TRANSFORM-UK: A therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulm Circ 2018; 8:2045893217735820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Lenardo MJ, Baltimore D: 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017; 168:37–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, Komada Y: A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 2007; 132:1265–74 [DOI] [PubMed] [Google Scholar]
- 61.Price LC, Caramori G, Perros F, Meng C, Gambaryan N, Dorfmuller P, Montani D, Casolari P, Zhu J, Dimopoulos K, Shao D, Girerd B, Mumby S, Proudfoot A, Griffiths M, Papi A, Humbert M, Adcock IM, Wort SJ: Nuclear factor κ-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS One 2013; 8:e75415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chin MP, Bakris GL, Block GA, Chertow GM, Goldsberry A, Inker LA, Heerspink HJL, O’Grady M, Pergola PE, Wanner C, Warnock DG, Meyer CJ: Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am J Nephrol 2018; 47:40–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, Diebold I, Hennigs JK, Kim K-Y, Miyagawa K, Wang L, Cao A, Sa S, Jiang X, Stockstill RW, Nicolls MR, Zamanian RT, Bland RD, Rabinovitch M: Elafin Reverses Pulmonary Hypertension via Caveolin-1-Dependent Bone Morphogenetic Protein Signaling. Am J Respir Crit Care Med 2015; 191:1273–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M: Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 2000; 6:698–702 [DOI] [PubMed] [Google Scholar]
- 65.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K: Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995; 107:1062–73 [DOI] [PubMed] [Google Scholar]
- 66.Rossaint J, Zarbock A: Perioperative Inflammation and Its Modulation by Anesthetics. Anesth Analg 2018; 126:1058–67 [DOI] [PubMed] [Google Scholar]
- 67.Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, Veerdonk MC van de Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, Lawrie A, Swift AJ, Rabinovitch M, Zamanian RT: Low-Dose FK506 (Tacrolimus) in End-Stage Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2015; 192:254–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Dijke P Ten, Rabinovitch: FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013; 123:3600–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomez-Arroyo J, Abbate A, Voelkel NF: Pulmonary arterial hypertension and the Enigma code of smouldering inflammation. Eur Respir J 2016; 48:305–7 [DOI] [PubMed] [Google Scholar]
- 70.Smith JS, Nicholson LT, Suwanpradid J, Glenn RA, Knape NM, Alagesan P, Gundry JN, Wehrman TS, Atwater AR, Gunn MD, MacLeod AS, Rajagopal S: Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation. Sci Signal 2018; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu EK, Drazen JM: Asthma: one hundred years of treatment and onward. Am J Respir Crit Care Med 2005; 171:1202–8 [DOI] [PubMed] [Google Scholar]