Abstract
OBJECTIVES:
Cancer antigen (CA)-125 influences progression, metastasis, and outcomes in pancreatic cancer (PC). This phase I/II trial () evaluated safety, efficacy, and immunologic correlates of chemoimmunotherapy (CIT) with oregovomab (anti–CA-125), followed by stereotactic body radiotherapy (SBRT) with the radiosensitizer nelfinavir.
METHODS:
Following imaging, pathologic confirmation, and staging laparoscopy, subjects received three 3-week cycles of CIT (gemcitabine/leucovorin/fluorouracil/oregovomab). Thereafter, nelfinavir was delivered (1250mg BID) for 5 weeks, with SBRT (40Gy/5 fractions) occurring during the third week of nelfinavir. Following another cycle of CIT, pancreaticoduodenectomy was performed if resectable. Three more cycles of CIT were then delivered (total 7 cycles). In subjects with high (≥10 units/mL) CA-125, oregovomab (2mg) was administered for 7 total doses (3 pre-SBRT, 1 between SBRT and resection, and 3 postoperatively). The enzyme-linked immunospot assay evaluated development of CA-125–specific CD8 T-lymphocytes.
RESULTS:
The trial was prematurely closed because gemcitabine/leucovorin/fluorouracil was replaced by FOLFIRINOX and gemcitabine/nab-paclitaxel as standard of care. Median follow-up was 13 months. Of 11 enrolled patients, 10 had high CA-125; one patient suffered an unexpected cardiac-related death, so 9 subjects received oregovomab. Ten received SBRT and 4 underwent resection. Overall, 6/11 patients experienced any grade ≥3 event. The median survival and time to progression was 13 and 8.6 months, respectively. Five patients had samples available for immunospot testing, of whom two (40%) developed CA-125–specific CD8 T-lymphocytes.
CONCLUSIONS:
A combined PC multimodality approach using chemoimmunotherapy and radiosensitized radiotherapy is feasible and safe; immunotherapy can lead to T-cell immunity. Re-evaluation with modern systemic paradigms is recommended.
Keywords: pancreatic cancer, pancreas, stereotactic body radiation therapy, nelfinavir, oregovomab
INTRODUCTION
Neoadjuvant therapy can allow subjects with borderline resectable (BR) or unresectable (UR) pancreatic cancer (PC) disease to undergo resection, along with reducing the occurrence of positive margins [2], assessing tumor biology [3–5], and avoiding suboptimal receipt of therapy in the adjuvant setting [6]. Neoadjuvant therapies largely include chemotherapy and radiotherapy, including stereotactic body radiation therapy (SBRT), a highly conformal, safe, and efficacious technique that delivers low doses to surrounding organs-at-risk [7–9].
An emerging modality of neoadjuvant therapy for PC is immunotherapy, which is a broad term encompassing numerous compounds capable of acting on the anti-tumoral immune system, such as monoclonal antibodies, tumor vaccines, small molecules, and viral or cellular therapies. This may be of particular importance for pancreatic cancer owing to a degree of resistance to chemotherapy and/or RT, along with a high propensity for distant metastasis [10].
Although immune checkpoint inhibitors are the most commonly utilized immunotherapeutic compounds for cancer care (with many ongoing trials in pancreatic cancer [11]), other studies are targeting PC-specific biomarkers (e.g. cancer antigen (CA) 19–9, ). CA-125, also known as MUC16, is a surface glycoprotein that is elevated in a variety of cancers, including LAPC [12]. This molecule can not only assist in diagnosing PC, but may also be a co-driver of the PC oncogenic process, a marker for progression and/or metastasis, and a prognostic factor following definitive treatment [13–20]. Importantly, CA-125 may have a connection with the immune system through its upregulation of immunosuppressive Treg cells [21].
As a result, there have been multiple clinical trials aiming to block CA-125 and/or its ligands for cancer therapy [22–23]. Oregovomab is a monoclonal antibody that binds to circulating CA-125, forming antigen-antibody complexes; subsequent uptake by antigen-presenting cells may allow for more pronounced antigen presentation to T lymphocytes and hence the potential for a more brisk anti-tumoral immune response (as exemplified by multiple prospective studies in ovarian cancer) [24–27].
This investigation was a phase I/II trial of chemoimmunotherapy (CIT) using gemcitabine/leucovorin/fluorouracil/oregovomab, followed by SBRT with the radiosensitizer nelfinavir, and resection for appropriate patients. The study was discontinued prematurely owing to the strong evidence for FOLFIRINOX and gemcitabine/nab-paclitaxel as compared to the chemotherapy regimen utilized herein [28]. These results represent the final analysis of the primary and secondary endpoints of safety, efficacy, and immunologic correlates.
MATERIALS & METHODS
Study Participants
This was an Institutional Review Board approved open-label phase I/II trial of resectable, BR, and UR PC (Clinicaltrials.gov number ). Eligible patients were ≥19 years of age with newly-diagnosed, pathologically-confirmed non-metastatic pancreatic adenocarcinoma not exceeding 10 cm in maximal dimension. Additional criteria were Karnofsky performance status of ≥60% and serum laboratory values appropriate to receive chemotherapy. Patients with biliary or gastroduodenal obstruction were allowed if drainage or surgical bypass had been performed prior to starting therapy. Patients with prior malignancy were also allowed, provided treatment was completed >5 years prior to enrollment and there was no clinical evidence of prior malignancy. Exclusion criteria were patients who had received prior radiotherapy to the area (referring to disease overlap with the 20% isodose line), those with contraindications for staging laparoscopy, documented allergy to murine proteins and/or to any systemic agent in the protocol (or antiemetics), uncontrolled intercurrent illnesses (including history of significant bowel pathology including bleeding/ulcers), history of autoimmune or immunodeficiency disease(s), inability to provide informed consent, and pregnant/nursing women. Additionally, subjects taking medications known to be contraindicated with any study drug, receiving other investigational agents, and with history of hepatic insufficiency, were also considered ineligible.
Study Procedures
All patients underwent evaluation by a multidisciplinary team of surgical, medical, and radiation oncologists, with a complete history and physical examination. Blood counts and chemistries along with CA-125 and CA 19–9 level were obtained, in addition to pathologic diagnosis and imaging (contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography, and/or endoscopic/laparoscopic ultrasound). Patients then underwent staging laparoscopy (within 30 days of commencing therapy) to confirm lack of metastatic peritoneal disease and CT-guided placement of two fiducial markers (Cook Medical, platinum embolization coil, 0.45 × 5 mm) in the pancreas around the mass approximately two centimeters apart.
Both patients with high (defined per protocol as ≥10 units/mL) as well as low (<10 units/mL) CA-125 levels were eligible (subjects with CA-125 <10 units/mL would not receive oregovomab). For patients with high CA-125, baseline immunologic assessment of oregovomab was conducted, referring to the presence of CA-125–specific interferon-γ+ CD8 T-lymphocytes as measured by the enzyme-linked immunospot assay (ELISPOT) from samples of isolated peripheral blood mononuclear cells and the serum Human Anti-Mouse Antibodies (HAMA) Assay (performed at a central laboratory). Samples evaluated were taken immediately prior to 1) commencing initial chemotherapy, 2) prior to the second infusion (for the first 6 patients only), 3) prior to SBRT, 4) prior to resection or restaging, and 5) three weeks following the 7th cycle of chemotherapy (overall week 12) or the end of study.
Chemoimmunotherapy
Treatment schema of the initial portion of therapy is provided in Supplemental Figure 1. Patients initially received three cycles of 3-week CIT (weeks 1–9) with intravenous (IV) gemcitabine 750 mg/m2 (females) or 900 mg/m2 (males) and IV calcium leucovorin 50 mg/m2 over 30 minutes on days 1 and 8, 5-fluorouracil 2700 mg/m2 IV over 24 hours on days 1 and 8 and IV oregovomab (2 mg) on day 15 over 15–30 minutes. The chemotherapy regimen was chosen based on results of an institutional phase I protocol [29].
Following restaging chest CT and abdominal MRI, and provided a lack of metastasis, SBRT (details below) occurred over the 11th week, with nelfinavir (1250 mg orally twice daily) administered for 5 weeks (starting 2 weeks prior to SBRT; weeks 9–13), based on the results of a previous phase I trial [30]. A fourth cycle of the aforementioned CIT was delivered in weeks 14–16, followed by assessment of resectability.
Patients then underwent thoracoabdominal CT and/or abdominal MRI at 4–6 weeks following SBRT completion to evaluate for resectability per the surgical oncologist’s discretion. If resectable, pancreaticoduodenectomy was performed 6–8 weeks following SBRT completion.
Following surgery (or restaging scans alone, for non-operative cases), patients proceeded to the second phase of therapy following surgical oncologists’ recommendation of adequate patient recovery. Supplementary Figure 2 displays a schema of the second phase of management. In this phase, patients underwent three more cycles of 3-week CIT (same agents and doses as above). Thus, over the total course of therapy, a total of 7 cycles of CIT were to be delivered.
Radiation Therapy
Details regarding SBRT simulation, target delineation, treatment planning, and image-guided delivery are extensively described elsewhere [30]. The prescription dose for this study was 40 Gy delivered over 5 daily fractions, corresponding to the maximum tolerated dose from a prior phase I study [30]. The prescribed dose was to cover ≥95% of the PTV, but 93% was acceptable for cases not meeting bowel dose constraints. The maximum PTV dose was not to exceed 110% of the prescribed dose. Dose constraints for organs-at-risk have also been described previously [30], with the modification of a maximum point dose of 32 Gy for the stomach/small bowel.
Follow-Up
While receiving treatment, patients were seen weekly by a medical and a radiation oncologist, with toxicity evaluation per the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Following completion of all therapy, patients followed-up with interval history and examination (including toxicity assessment); contrast-enhanced CT of the chest, abdomen, and pelvis; as well as laboratory studies (e.g. CA 19–9). Follow-up was conducted every 3 months for the first year, every 4 months for the second year, and biannually thereafter. At each follow-up appointment, imaging of the primary disease was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST 1.1).
Pathologic Response
For patients who underwent surgical resection, pathological response of the tumor to neoadjuvant therapy was evaluated by an experienced gastrointestinal pathologist. The pathological response score was assigned from 0–9, which was generated from the following three categories (tumor gland dropout, tumor shrinkage, and global tumor kill). Tumor gland dropout was scored as 0 (>90% of tumor nests back-to-back (minimal dropout, densely spaced)), 1 (50–90% of tumor nests back-to-back (mild dropout)), 2 (10–49% of tumor nests back-to-back (moderate dropout)), or 3 (<10% of tumor nests back-to-back (marked dropout, hypodense)). Tumor shrinkage was quantified as 0 (>90% tumor cells with abundant cytoplasm, large multicellular glands (not shrunken)), 1 (50–90% tumor cells with abundant cytoplasm, large multicellular glands (mildly shrunken)), 2 (10–49% tumor cells with abundant cytoplasm, large multicellular glands (moderately shrunken)) or 3 (<10% tumor cells with abundant cytoplasm, large multicellular glands (markedly shrunken)). Lastly, global tumor kill was defined as 0 (no definite response (poor to no response)), 1 (minimal response (some fibrosis, moderate amount of cancer present)), 2 (moderate response (minimal residual cancer present)), or 3(complete response (no residual cancer identified)).
Statistical Analysis
The primary endpoint of this trial was disease progression at 4 months of follow-up. Based on estimates that roughly 60% of patients would progress by this time interval, this study sought to reduce this rate to 40%. As such, it was estimated that a total of 66 patients (33 patients each with low and high CA-125 levels) were required over 5 years. Secondary endpoints of the study were progression (and time to progression (TTP)), overall survival (OS), and immunologic development of CD8 T lymphocytes specific for CA-125.
This trial included a built-in safety component for the first 6 patients who received oregovomab. Interim analysis following therapy of these 6 subjects was conducted to evaluate the incidence of grade 4 toxicities not medically controlled that were possibly, probably, or definitely related to oregovomab. If there was more than 1 patient who met this criteria (of the first 6 subjects), further enrollment was to be halted.
All statistics were performed with SAS version 9.4 (Cary, NC); p<0.05 was considered statistically significant. Overall survival (OS) was computed actuarially by the Kaplan-Meier method and was defined from the day of study consent to death from any cause, or censored at last follow-up.
RESULTS
Starting from September 2013, 11 patients were enrolled; the study was terminated in February 2016 owing to the strong evidence for FOLFIRINOX and gemcitabine/nab-paclitaxel as compared to the regimen utilized herein. Of these 11 patients, 10 had high CA-125 levels. Of those 10 patients, 9 received oregovomab (four received full immunization with 7 infusions, three received 4 infusions and the remaining two received 3 infusions); one patient experienced an unexpected cardiac death following the first cycle of chemotherapy and thus did not receive oregovomab. Characteristics of the patient population can be found in Table 1.
Table 1.
Selected clinical characteristics of the study population.
| Parameter | Count (Percent) |
|---|---|
| Age (years) | |
| Median (range) | 65 (45–79) |
| Gender | |
| Male | 9 (82%) |
| Female | 2 (18%) |
| Race | |
| Caucasian | 10 (91%) |
| Other | 1 (9%) |
| Karnofsky performance status (%) | |
| 100 | 0 (0%) |
| 90 | 3 (27%) |
| 80 | 7 (64%) |
| 70 | 0 (0%) |
| 60 | 1 (9%) |
| Initial resectability status | |
| Resectable | 1 (9%) |
| Borderline resectable | 6 (55%) |
| 1) Contact with SMV > 180° | 2 |
| 2) Contact with PV > 180° | 4 |
| Unresectable | 4 (36%) |
| 1) Contact with SMA > 180° | 2 |
| 2) Contact with CA >180° | 2 |
| Clinical T classification | |
| T1 | 2 (18%) |
| T2 | 2 (18%) |
| T3 | 3 (27%) |
| T4 | 4 (36%) |
| Clinical N classification | |
| N0 | 5 (45%) |
| N1 | 6 (55%) |
| CA-125 level (units/mL) | |
| < 10 | 1 (9%) |
| ≥ 10 | 10 (91%) |
Abbreviations: SMV, superior mesenteric vein; PV, portal vein; IVC, inferior vena cava; SMA, superior mesenteric artery; CA, celiac artery
Following interim safety analysis of the first six patients (all of whom received oregovomab), one patient had four grade 4 events (sepsis, AST elevation, leukopenia, and low ANC). All events were medically controlled and resolved, and hence the study regimen was deemed safe for the trial to continue to enroll.
Adverse events possibly, probably, or definitely associated with CIT in the nine patients who received oregovomab is shown in Table 2 (toxicities attributed to the entire treatment regimen in all 11 patients is given in Table 3). In addition to the aforementioned patient with four instances of grade 4 toxicities, there were two other instances of grade 4 leukopenia and three other cases of grade 4 reduced ANC. There was also one case each of grade 4 pneumonia, acidosis, and pleural effusion. In total, 6 of 11 patients experienced any grade ≥3 adverse event.
Table 2.
Instances of toxicities possibly, probably, or definitely attributed to combined chemotherapy and oregovomab (N=9).
| Adverse Event | Grades 1– 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Hematologic | |||
| Anemia | 0 | 2 | 0 |
| Leukopenia | 0 | 3 | 3 |
| Thrombocytopenia | 0 | 4 | 0 |
| Lymphopenia | 0 | 4 | 0 |
| Reduced ANC | 0 | 2 | 4 |
| Non-hematologic | |||
| Nausea | 0 | 1 | 0 |
| Vomiting | 0 | 1 | 0 |
| Dehydration | 0 | 1 | 0 |
| Depression | 0 | 1 | 0 |
| Somnolence | 0 | 1 | 0 |
| Orthostasis | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 |
| Hypokalemia | 0 | 1 | 0 |
| Hyperkalemia | 0 | 1 | 0 |
| ALT elevation | 0 | 2 | 0 |
| AST elevation | 0 | 1 | 1 |
| Pancreatitis | 0 | 1 | 0 |
| Pneumonia | 0 | 0 | 1 |
| Acidosis | 0 | 0 | 1 |
| Urinary tract infection | 0 | 1 | 0 |
| Sepsis | 0 | 0 | 1 |
| Pleural effusion | 0 | 0 | 1 |
| Sinusitis | 0 | 1 | 0 |
| Cardiac disorders | 0 | 1 | 0 |
Table 3.
Instances of toxicities possibly, probably, or definitely attributed to any protocol therapy in all patients (N=11).
| Adverse Event | Grades 1– 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Hematologic | |||
| Anemia | 0 | 3 | 0 |
| Leukopenia | 0 | 6 | 3 |
| Thrombocytopenia | 0 | 4 | 0 |
| Lymphopenia | 1 | 4 | 0 |
| Reduced ANC | 0 | 5 | 5 |
| General | |||
| Dehydration | 0 | 1 | 0 |
| Depression | 0 | 1 | 0 |
| Somnolence | 0 | 1 | 0 |
| Orthostasis | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 |
| Hyperglycemia | 0 | 1 | 0 |
| Hypokalemia | 0 | 2 | 0 |
| Hyperkalemia | 0 | 1 | 0 |
| Hyponatremia | 0 | 1 | 0 |
| Sinusitis | 0 | 1 | 0 |
| Gastrointestinal | |||
| Nausea | 0 | 1 | 0 |
| Vomiting | 0 | 1 | 0 |
| ALT elevation | 0 | 2 | 1 |
| AST elevation | 0 | 3 | 0 |
| Pancreatitis | 0 | 1 | 0 |
| Genitourinary | |||
| Acute kidney injury | 1 | 0 | 0 |
| Urinary tract infection | 0 | 1 | 0 |
| Respiratory | |||
| Pneumonia | 0 | 0 | 1 |
| Acidosis | 0 | 1 | 1 |
| Sepsis | 0 | 0 | 1 |
| Pleural effusion | 0 | 0 | 1 |
| Acute respiratory distress syndrome | 0 | 0 | 1 |
| Acute pulmonary edema | 0 | 0 | 1 |
| Cardiac* | |||
| Myocarditis | 0 | 0 | 1 |
| Heart failure | 0 | 0 | 1 |
| Ventricular fibrillation | 0 | 0 | 1 |
| Myocardial infarction | 0 | 0 | 1 |
| Reduced ejection fraction | 0 | 1 | 0 |
One patient died of cardiac causes after the first cycle of chemotherapy; this patient had not received any other protocol therapy.
Aside from the patient who died unexpectedly, the remaining 10 patients all received SBRT. Four patients underwent resection, three of whom received all 7 doses of oregovomab (the other had low CA-125). All procedures yielded negative margins and there was no 30-day postoperative mortality. Of the four patients, 1 had been considered initially resectable, 2 were considered BR, and 1 was considered unresectable. Pathologic response scores in the three patients who received oregovomab were 3, 6 and 7; and 3 in the remaining patient who did not receive oregovomab.
All patients died at the time of data analysis (median follow-up 13 months). The best RECIST-based response of the primary disease was a complete response in three patients, stable disease in four, progressive disease in three, and not evaluable in one patient who died unexpectedly. Analysis of the primary endpoint (progression at 4 months) demonstrated that of the ten patients who remained alive, two (20%) had progressed at 4 months. The median TTP was 8.6 months (range, 3.5 months – not reached). The first site of disease progression was liver (n=4), peritoneum (n=1), retroperitoneum (n=1), and regional lymphadenopathy (n=1).
The median OS for all patients was 13 months (95% confidence interval, 7–22 months) (Figure 1A), and 13 months (7–30 months) in subjects who received oregovomab (Figure 1B). In patients who received all 7 doses of oregovomab, the median OS was 21 months (9–31 months), versus 10 months (7–22 months) in subjects who received less than 7 doses (p=0.172).
Figure 1.
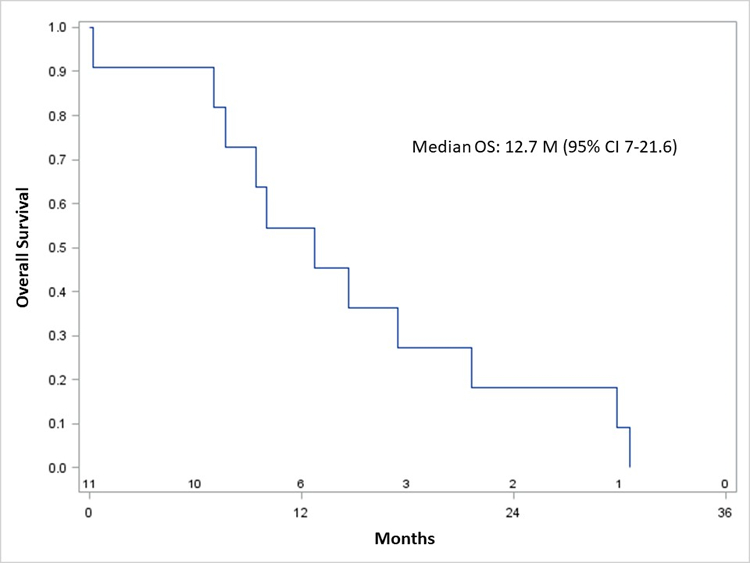
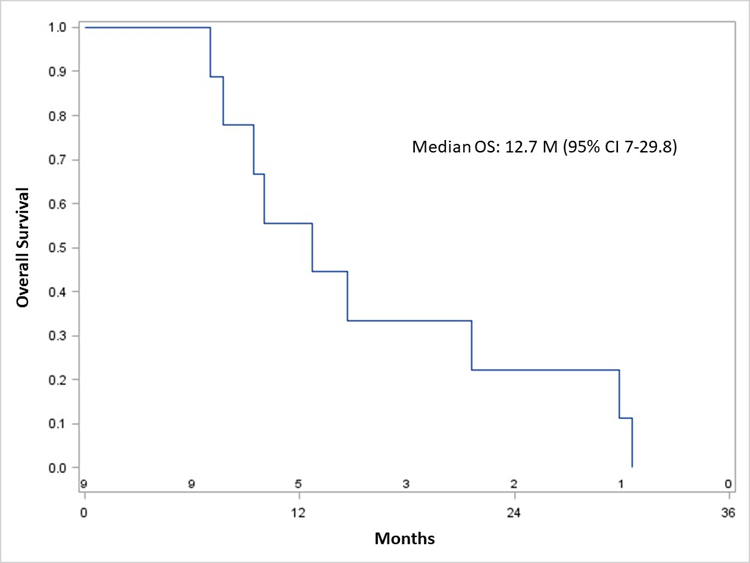
Kaplan-Meier overall survival of all patients (A) and those who received oregovomab (B).
An exploratory analysis was performed to isolate the effect of oregovomab on OS and TTP. To do this, the 9 patients who received oregovomab herein were compared to 17 patients from our previous phase I trial [30] who received the identical chemotherapy/SBRT/nelfinavir regimen as in this trial (oregovomab was not administered in that investigation). There was no significant difference in OS or TTP (p>0.5 for both).
All nine patients who received oregovomab were not highly immune reactive; only one patient had a humoral response to the administered antibody. Five patients had adequate samples in order to perform the paired ELISpot assay for CA-125–specific CD8 T-lymphocytes. Of these 5, two (40%) patients generated treatment emergent CA-125–specific T-cell signals, one of these being at the lower limit of detectability; the patient with the stronger T-cell signal had generated a humoral response. Both of these patients received 7 doses and survived for 30–31 months. The remaining three (received 3–4 doses) survived for 7, 8, and 22 months (Supplementary Table 1).
DISCUSSION
Prospective experiences of immunotherapeutic approaches for locally advanced PC are essential to provide alternative therapies for a highly aggressive neoplasm. Although this study closed prematurely, several salient conclusions remain. A combined multimodality approach using chemotherapy, immunotherapy, and radiotherapy (with or without resection) is feasible and safe. The primary endpoint of this trial was objectively met (20% progression at 4 months), although interpretation is limited given the substantially limited power for formal statistical testing. Importantly, delivery of immunotherapy in this setting yielded the development of antigen-specific CD8 T-cell immunity. Despite the premature cessation of this trial, re-evaluation with modern systemic paradigms is recommended.
CA-125 has been linked with immunosuppression [21], which may hamper the ability to mount an anti-tumoral immune response and may in part contribute to the abysmal survival observed with PC. To this extent, one of the most noteworthy findings of this study was the development of CA-125 specific CD8 T lymphocytes in 2 of 5 (40%) paired sample sets tested who received oregovomab. However, the measurement of T-cell immunity in peripheral blood may not be the most appropriate to evaluate, and the immunomodulatory effects of concomitant therapies is likely to interact in ways difficult to predict. Nevertheless, experience with oregovomab in ovarian cancer, suggests that development of T-cell responses correlates with improved prognosis [31–32] and that this effect is impacted in a CIT schedule-dependent fashion. In pancreatic cancer, if T-cell immunity develops, the tumor stroma generates numerous immunosuppressive molecules (along with other metabolic/transcriptional changes in parallel) that are inhospitable to lymphocytic infiltration [33]. Whereas T-cell activation is becoming better addressed by using immunotherapeutic compounds, addressing the hostile tumor microenvironment is more challenging and a major roadblock to immunotherapy. The potential for standard modalities including both cytotoxic treatment, surgery itself, and radiation to alter the state of immune suppression may be very significant in allowing immunotherapy to achieve therapeutic levels in this disease. The observation of T-cell immunity in this study has implications for ongoing trials evaluating adoptive T-cell therapies and viral vaccines in PC (e.g., , , , , ).
It is acknowledged that outcomes in this study were comparable to established literature, or even studies of SBRT and nelfinavir [30]. Despite the sample size issues in this trial, it is uncertain whether oregovomab may impart an independent effect on these patients. The lack of overt immunomodulatory effects herein are not uncommon, as studies of immune checkpoint inhibitors for PC have shown only modest results [34–37]. However, limitations in those studies are similar to this trial, namely the small sample sizes not designed to evaluate survival outcomes, as well as concerns that PC may not be uniformly immunogenic without other interventions. As a result, although these data are likely not enough to recommend oregovomab in this setting for randomized trials, they do not exclude its potential utility when modern systemic regimens are employed.
Although radiotherapy is not considered a cornerstone for PC, it was included in this trial owing to its ability to better release tumor antigens to the immune system and potentially modulating the tumor stroma [38–39]. Moreover, preclinical work suggests that ablative radiotherapy (rather than conventional fractionation) may better facilitate systemic effects [40], which is why it was advantageous to utilize the radiosensitizer nelfinavir in this trial. However, the optimal timing of SBRT relative to immunotherapy remains unclear, and this trial was not constructed to address such a question given the delivery of SBRT after the 3rd dose of neoadjuvant oregovomab (Supplementary Figures 1–2).
There is a notable theoretical disadvantage to delivering concurrent CIT; because immunotherapy-mediated immune galvanization occurs by means of T cells, chemotherapy-induced myelosuppression may hamper immune responses. However, the trial was designed with concurrent therapy based on the results of Braly and colleagues, who performed a randomized phase II trial of concurrent versus sequential chemotherapy-oregovomab in advanced ovarian cancer [41]. Contrary to prevailing notions, the concurrent arm developed humoral immunity quickly and also generated a cellular immune response in a higher fraction of patients. A recently reported randomized follow up study has found substantial therapeutic benefit in ovarian cancer associated with the preferred schedule of chemo-immunotherapy [32].
There are multiple limitations to the current study, although it does suggest important research pathways meriting exploration. The nonrandomized nature of this trial makes definitive assessment of the contribution of any component difficult to assess. Additionally, the effect of oregovomab and/or nelfinavir also cannot be adequately evaluated because the chemotherapy utilized is no longer the standard of care for pancreatic cancer, which led to the termination of this trial and assured that sample sizes would not permit adequate comparisons (including of the primary endpoint). Lastly, the median 13-month OS of these patients should be contextualized with the fact that nearly all patients had high levels of CA-125, which implies a “higher-risk” cohort than the general PC population [12–14, 19–20]. Despite these shortcomings, given the feasibility and safety of the treatment regimen herein, re-assessment using modern chemotherapy paradigms is recommended.
Supplementary Material
Supplementary Figure 1. Study schema of the first phase.
Supplementary Figure 2. Study schema of the second phase.
Acknowledgements:
The authors thank Sarah Radniecki and Eugene Sehi for their assistance with this trial. It is acknowledged that oregovomab is currently an investigational drug.
Funding: This study was in part supported by Spore Grant Number 2 P50 CA127297-06A1.
Footnotes
Publisher's Disclaimer: Disclaimers: A portion of this work was presented at the 2018 Annual Meeting of the American Society of Clinical Oncology.
Conflicts of Interest: All authors declare no conflicts of interest.
REFERENCES
- 1.Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol 2016;39:302–313. [DOI] [PubMed] [Google Scholar]
- 2.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raut CP, Evans DB, Crane CH, Pisters PW, Wolff RA. Neoadjuvant therapy for resectable pancreatic cancer. Surg Oncol Clin N Am 2004;13:639–661. [DOI] [PubMed] [Google Scholar]
- 4.Wayne JD, Abdalla EK, Wolff RA, et al. Localized adenocarcinoma of the pancreas: the rationale for preoperative chemoradiation. Oncologist 2002;7:34–45. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastroenterol Surg 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 6.Lim KH, Chung E, Khan A, et al. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist 2012;17:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma V, Lazenby AJ, Zheng D, et al. Dosimetric parameters correlate with duodenal histopathologic damage after stereotactic body radiotherapy for pancreatic cancer: Secondary analysis of a prospective clinical trial. Radiother Oncol 2017;122:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma V, Bhirud AR, Denniston KA, Bennion NR, Lin C. Quantification of renal function following stereotactic body radiotherapy for pancreatic cancer: secondary dosimetric analysis of a prospective clinical trial. Radiat Oncol 2017;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrelli F, Comito T, Ghidini A, et al. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int J Radiat Oncol Biol Phys 2017;97:313–322. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Berlin J, Cardin D. Harnessing the immune system in pancreatic cancer. Curr Treat Options Oncol 2018;19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushman TR, Caetano MS, Welsh JW, Verma V. Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy. Immunotherapy 2018;10:851–860. [DOI] [PubMed] [Google Scholar]
- 12.Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol 2012;43:1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Xu H, Wang W, et al. A preoperative serum signature of CEA+/CA125+/CA19–9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer 2015;136:2216–2227. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Xu HX, Wang WQ, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget 2016;7:5943–5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Xiang J, Chen R, et al. The clinical utility of CA125/MUC16 in pancreatic cancer: A consensus of diagnostic, prognostic and predictive updates by the Chinese Study Group for Pancreatic Cancer (CSPAC). Int J Oncol 2016;48:900–907. [DOI] [PubMed] [Google Scholar]
- 16.Coppin L, Benomar K, Corfiotti F, et al. CA-125, but not galectin-3, complements CA 19–9 for discriminating ductal adenocarcinoma versus non-malignant pancreatic diseases. Pancreatology 2016;16:115–120. [DOI] [PubMed] [Google Scholar]
- 17.Muniyan S, Haridas D, Chugh S, et al. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer 2016;7:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang C, Qin Y, Zhang B, et al. Oncogenic KRAS Targets MUC16/CA125 in Pancreatic Ductal Adenocarcinoma. Mol Cancer Res 2017;15:201–212. [DOI] [PubMed] [Google Scholar]
- 19.Jiang K, Tan E, Sayegh Z, et al. Cancer Antigen 125 (CA125, MUC16) Protein Expression in the Diagnosis and Progression of Pancreatic Ductal Adenocarcinoma. Appl Immunohistochem Mol Morphol 2017;25:620–623. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Cheng H, Luo G, et al. The metastasis status and tumor burden-associated CA125 level combined with the CD4/CD8 ratio predicts the prognosis of patients with advanced pancreatic cancer: A new scoring system. Eur J Surg Oncol 2017;43:2112–2118. [DOI] [PubMed] [Google Scholar]
- 21.Fan K, Yang C, Fan Z, et al. MUC16 C terminal-induced secretion of tumor-derived IL-6 contributes to tumor-associated Treg enrichment in pancreatic cancer. Cancer Lett 2018;418:167–175. [DOI] [PubMed] [Google Scholar]
- 22.Hassan R, Schweizer C, Lu KF, et al. Inhibition of mesothelin-CA-125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: Implications for cancer therapy. Lung Cancer 2010;68:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JF, Moore KN, Birrer MJ, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol 2016;27:2124–2130. [DOI] [PubMed] [Google Scholar]
- 24.Schultes BC, Baum RP, Niesen A, Noujaim AA, Madiyalakan R. Anti-idiotype induction therapy: anti-CA125 antibodies (Ab3) mediated tumor killing in patients treated with Ovarex mAb B43.13 (Ab1). Cancer Immunol Immunother 1998;46:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noujaim AA, Schultes BC, Baum RP, et al. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13—evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm 2001;16:87–203. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AN, Schultes BC, Gallion H, et al. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol 2004;94:340–351. [DOI] [PubMed] [Google Scholar]
- 27.Ehlen TG, Hoskins PJ, Miller D, et al. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. Int J Gynecol Cancer 2005;15:1023–1034. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. Pancreatic adenocarcinoma Version 12019. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf Accessed December 13, 2018.
- 29.Grem JL, Quinn MG, Keith B, et al. Phase I trial of weekly gemcitabine in combination with infusional 5-fluorodeoxyuridine and oral calcium leucovorin in adult cancer patients. Cancer Chemoth and Pharmacol 2003;53:487–496. [DOI] [PubMed] [Google Scholar]
- 30.Lin C, Verma V, Ly QP, et al. Phase I trial of concurrent stereotactic body radiotherapy and nelfinavir for locally advanced borderline or unresectable pancreatic adenocarcinoma. Radiother Oncol 2019;132:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon AN, Schultes BC, Gallion H, et al. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol 2004;94:340–351. [DOI] [PubMed] [Google Scholar]
- 32.Ferrandina G, Braly PS, Terranova C, et al. A randomized phase II study assessing an optimized schedule of oregovomab (O) anti-CA125 vaccination with carboplatin paclitaxel (CP) relative to CP alone in front-line treatment of optimally cytoreduced stage III/IV ovarian cancer (EOC). J Clin Oncol 2017;35:abstr 5536. [Google Scholar]
- 33.Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015;4:e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le DT, Crocenzi TS, Uram JN, et al. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas (with cyclophosphamide) and CRS-207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinoma (STELLAR). J Clin Oncol 2016; 34:TPS486. [Google Scholar]
- 35.Firdaus I, Waterhouse DM, Gutierrez M, et al. nab-paclitaxel (nab-P) plus nivolumab (Nivo) ± gemcitabine (Gem) in patients (pts) with advanced pancreatic cancer (PC). J Clin Oncol 2016; 34:TPS475. [Google Scholar]
- 36.Nesselhut J, Marx D, Lange H, et al. Systemic treatment with anti-PD-1 antibody nivolumab in combination with vaccine therapy in advanced pancreatic cancer. J Clin. Oncol 2016;34:3092. [Google Scholar]
- 37.Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 2018; 36:96–102. [DOI] [PubMed] [Google Scholar]
- 38.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon H, Ramapriyan R, Cushman TR, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol 2019;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braly P, Nicodemus CF, Chu C, et al. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother 2009;32:54–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study schema of the first phase.
Supplementary Figure 2. Study schema of the second phase.


