Abstract
Background
Biologic evidence suggests that angiotensin II may play a role in tumor progression or growth. We compared the short-term colorectal cancer (CRC) risk among initiators of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) versus guideline-recommended clinical alternatives (beta blockers, calcium channel blockers [CCB], and thiazides).
Methods
We conducted a new-user cohort study on U.S. Medicare beneficiaries aged over 65 years, who initiated antihypertensive monotherapy during 2007–2013 and were free of cancer diagnosis prior to drug initiation. Follow-up began 6 months post-initiation to allow time for the diagnostic delay. We estimated hazard ratios (HR) with 95% confidence intervals (CI) using propensity score weighted Cox regression, overall and stratified by time since drug initiation, and 5-year cumulative risk differences (RD) using Kaplan–Meier estimator. We assessed the potential for unmeasured confounding using supplemental data from Medicare Current Beneficiary Survey.
Results
For analyses without censoring for treatment changes, we observed 532 CRC events among 111,533 ACEI/ARB initiators. After a median follow-up of 2.2 years (interquartile range: 1.0–3.7), CRC risk was similar between ACEI/ARB and active comparators, with adjusted HRs of 1.0 (95% CI: 0.85, 1.1) for ACEI/ARB versus beta blockers, 1.2 (95% CI: 0.97, 1.4) for ACEI/ARB versus CCB and 1.0 (95% CI: 0.80, 1.3) for ACEI/ARB versus thiazide. Five-year RDs and as-treated analyses, which censored follow-up at medication changes, produced similar findings.
Conclusions
Based on real-world antihypertensive utilization patterns in Medicare beneficiaries, our study suggests no association between ACEI/ARB initiation and the short-term CRC risk.
Keywords: Angiotensin Converting Enzyme Inhibitors, Angiotensin Receptor Blockers, Antihypertensive agents, Colorectal Neoplasms, Cohort Study, Medicare
Introduction
Angiotensin II is the endogenous peptide hormone which is involved in blood pressure regulation as a part of the renin–angiotensin–aldosterone system.1 In vitro and in vivo studies have shown that angiotensin II stimulates angiogenesis, neovascularization, and smooth muscle cell proliferation in blood vessels and induces a pathway that leads to DNA synthesis and cell proliferation in intestinal epithelial cells, thus suggesting its potential role in the growth and progression of tumors.2,3,4,5 A colon cancer cell line study suggested that medications that inhibit angiotensin II, such as angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB), play a role in reducing colon cancer growth and invasion, thus highlighting their potential role in cancer prevention.6
A meta-analysis of randomized clinical trials found that ARBs increase the relative risk of cancer by approximately 10% even when limited to studies with cancer as the pre-specified endpoint (risk ratio 1.1, 95% CI: 1.0–1.2).7 Subsequent meta-analyses of clinical trials, however, reported contradictory findings of no association of ARB use with the overall cancer incidence and mortality.8,9 Non-experimental studies are also conflicting on colorectal cancer (CRC) incidence: some reported inverse associations between ACEI/ARB use and the risk of CRC,10–12, one study a slightly increased CRC risk with ACEI/ARB usage13, and some an inconclusive association.14–18 A systematic review and meta-analysis of non-experimental studies in 2015 reported a reduced risk of CRC with ACEI/ARB use.19 Most of these studies could, however, suffer from healthy user bias due to the inclusion of prevalent drug users, outcome detection bias (differential outcome ascertainment by treatment group) due to the non-user comparison group, or time-related bias (immortal time bias).20–22
Given the common use of ACEI/ARB as first/second line antihypertensives23 and the conflicted literature of their effects on CRC outcomes, more robust epidemiologic evidence based on real-world data in the US healthcare settings is needed. We therefore aimed to compare ACEI/ARB use versus guideline recommended clinical alternatives in terms of their potential effects on CRC incidence.24–32
Methods
We conducted an active-comparator, new user cohort study to estimate the effects of ACEI and/or ARB versus alternative antihypertensive agents on CRC incidence.32 Our study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Study Population
We identified antihypertensive drug initiators of interest (ACEI, ARB, thiazide, CCB, and beta blockers; less commonly used alpha blockers were excluded) using a 20% random sample of Medicare fee-for-service data including hospital care (part A), physician office and outpatient services (part B), and pharmacy drug claims (part D) from 2007– 2013. Medicare is the largest public insurance in the U.S. and covers over 98% of adults aged 65+ years.33 The actual proportion of Medicare enrollees with continuous fee-for-service coverage ranges from 58%−65% according to 2007–2013 data.33,34
Individuals were required to have continuous coverage for hospital and outpatient plans for 12 months (6 months for prescription drug plans) prior to the initial prescription. We excluded any evidence of cancer diagnoses or cancer-related procedures (except non-melanoma skin cancer) during this 12-month baseline (through to the second prescription date) using a highly sensitive algorithm (eTable 1 shows codes used in identifying such prevalent cancer cases).35
Study treatment
We defined medication initiators as individuals having claims for an antihypertensive drug prescription of interest (ACEI, ARB, thiazide, CCB, beta blocker) after at least 6 months of drug-free period, during which any antihypertensive drug use was excluded.32 In additional analyses, we allowed the baseline use of antihypertensive classes other than the ones under comparison (e.g., in ACEI/ARB versus beta blocker comparison, thiazide, and CCB users during this 6-month baseline were eligible for inclusion). To reduce misclassification of prescription drug data due to patients not taking their dispensed medications, we required at least one refill with the same drug class within 30 days after the end of the initial prescription’s days of supply. We limited our study population to monotherapy initiators, who initiated only a single drug class between the initial and second prescription dates, to reduce potential confounding by factors related to disease severity or frailty.22,23 Flowchart of the study population is presented in eFigure 1.
Individuals were considered to be on treatment until they were censored for discontinuation of the initial drug class or switch to or augmentation with a comparator drug class. Treatment switch or augmentation with drugs other than the specific active comparator was allowed (e.g., in the ACEI/ARB versus beta blockers comparison, augmentation with CCB or thiazide in either cohort was allowed).
Outcome
The main outcome, incident CRC, was defined using a claims-based algorithm which has been shown to have high specificity (99%) and sensitivity (80%).36 This definition required the presence of at least two ICD-9-CM diagnosis codes (153.xx, 154.0–154.2, 230.3, 230.4 excluding anal canal cancers) over a 2-month period.
Follow-up
Follow-up started 6 months after the second prescription date to allow for an empirical induction period (i.e., induction period for cancer pathogenesis and diagnostic delay) and also to reduce detection bias.37 For the intention-to-treat (first treatment carried forward) analyses, individuals were followed until the earliest of: the date of any incident cancer diagnosis (except for non-melanoma skin cancer), death, end of the study (31 December 2013), or enrollment gap in parts A or B insurance plans for more than 1 month. We censored the development of any incident cancer other than the CRC so that diagnosis or treatment of these cancers would not lead to differential detection of the main CRC outcome. As treated analyses were also performed in which we additionally censored follow-up for treatment discontinuation, switching or augmentation (as defined above) plus a 6-month lag allowing for the delayed diagnosis/detection of cancer.
Measured and unmeasured confounding – external validation study
All covariates were measured during a year prior to the initial prescription date (6 months for comedications). To assess the potential for unmeasured confounding we conducted an external validation study using the supplemental data source, the Medicare Current Beneficiary Survey (MCBS), a multipurpose survey of a nationally representative population of Medicare beneficiaries. It reports information on health status and physical functioning, that are not available in the Medicare claims. From this data (2007–2011), we identified a cohort of individuals using similar eligibility criteria as the main Medicare study cohort.
Potential confounders assessed included demographics, selected comorbidities, comedications, and overall measures of healthcare utilization as a proxy for the general health status and frailty (similar to the main Medicare cohort, as in Table 1). We then compared distributions of CRC risk factors such as body mass index (BMI) and smoking,38,39 that are unavailable in claims data, between initiators of ACEI/ARB and comparator drugs. We assume that these variables are risk factors for the outcome and could lead to confounding if they are also associated with the choice of antihypertensive drug class.40
Table 1.
Baseline characteristics of initiators of antihypertensive monotherapy among Medicare fee-for-service beneficiaries over 65 years of agea
| BEFORE WEIGHTINGb | AFTER WEIGHTINGb | ||||||
|---|---|---|---|---|---|---|---|
| ACEI/ARB, % | THZ, % | BB, % | CCB, % | THZ, % | BB, % | CCB, % | |
| N 111,533 | N 29,043 | N 78,746 | N 39,905 | N 29,560 | N 78,166 | N 39,687 | |
| Demographics | |||||||
| Age | |||||||
| 66–75 | 60 | 60 | 53 | 49 | 58 | 60 | 59 |
| 76–85 | 29 | 29 | 33 | 33 | 30 | 30 | 30 |
| 85+ | 10 | 11 | 15 | 17 | 11 | 11 | 11 |
| Sex (male) | 37 | 25 | 36 | 32 | 38 | 37 | 36 |
| Race | |||||||
| Whites | 83 | 85 | 87 | 79 | 83 | 83 | 83 |
| Blacks | 6.4 | 8.4 | 5.4 | 11 | 6.1 | 6.5 | 6.4 |
| Others | 10 | 6.5 | 7.5 | 10 | 10 | 11 | 10 |
| Year of drug initiation | |||||||
| 2007 | 7.4 | 8.5 | 7.8 | 7.1 | 8.2 | 7.8 | 7.1 |
| 2008 | 18 | 20 | 18 | 17 | 20 | 18 | 17 |
| 2009 | 17 | 18 | 16 | 17 | 19 | 16 | 17 |
| 2010 | 17 | 16 | 16 | 17 | 17 | 16 | 17 |
| 2011 | 17 | 16 | 16 | 17 | 16 | 16 | 17 |
| 2012 | 17 | 15 | 17 | 17 | 15 | 17 | 17 |
| 2013 | 7.5 | 6.4 | 7.6 | 8.2 | 6.0 | 7.7 | 8.4 |
| Comorbidities | |||||||
| Diabetes mellitus | 39 | 17 | 25 | 26 | 40 | 38 | 38 |
| Diabetes nephropathy | 2.0 | 0.4 | 1.5 | 2.1 | 2.5 | 2.0 | 1.9 |
| Diabetes neuropathy | 6.1 | 2.3 | 4.0 | 3.9 | 7.2 | 6.3 | 6.4 |
| Diabetes retinopathy | 4.9 | 1.6 | 2.9 | 2.8 | 5.9 | 5.0 | 5.0 |
| Heart failure | 11 | 6.9 | 16 | 15 | 13 | 12 | 12 |
| Myocardial infarction | 0.5 | 0.2 | 1.9 | 0.5 | 0.6 | 0.5 | 0.6 |
| Emphysema | 3.0 | 3.0 | 3.7 | 5.7 | 3.3 | 3.0 | 3.2 |
| Chronic bronchitis | 16 | 14 | 17 | 23 | 17 | 16 | 16 |
| Gastrointestinal diseases | 0.8 | 0.9 | 1.2 | 1.0 | 0.9 | 0.8 | 0.8 |
| Comedications | |||||||
| Metformin | 17 | 4.5 | 6.3 | 5.0 | 18 | 16 | 17 |
| Sulphonylureas | 10 | 3.0 | 4.6 | 4.2 | 12 | 10 | 10 |
| Thiazolidinediones | 4.8 | 1.4 | 1.8 | 1.5 | 6.1 | 4.8 | 5.1 |
| Other oral anti-hyperglycemic drugs | 3.2 | 0.9 | 1.4 | 1.2 | 4.2 | 3.4 | 3.4 |
| Insulin | 6.9 | 2.0 | 4.1 | 4.5 | 8.7 | 7.0 | 7.3 |
| Statins | 40 | 30 | 37 | 31 | 40 | 39 | 40 |
| NSAIDsc | 18 | 20 | 16 | 17 | 19 | 19 | 19 |
| Aspirinc | 1.5 | 1.4 | 1.9 | 1.9 | 1.6 | 1.6 | 1.6 |
| Healthcare utilization | |||||||
| Colonoscopy | 8.7 | 9.3 | 9.3 | 8.3 | 8.6 | 8.9 | 8.7 |
| Fecal occult blood test | 9.3 | 10 | 9.2 | 8.4 | 9.4 | 9.3 | 9.4 |
| Hospital admissions (≥1) | 21 | 16 | 35 | 35 | 24 | 22 | 22 |
| Physician office visits (≥1) | 92 | 93 | 91 | 89 | 93 | 92 | 92 |
ACEI – Angiotensin Converting Enzyme Inhibitors, ARB – Angiotensin Receptor Blockers, BB – beta blockers, THZ – thiazide Diuretics, BB – Beta Blockers, CCB – Calcium Channel Blockers, SD – standard deviations
Baseline was 12 months before the first prescription date. All potential confounders were assessed during this 12-months (6 months for comedications) before the first prescription.
Propensity score weighting was implemented by the stabilized morbidity ratio weighting where patients were weighted to reflect the covariate distributions in the ACEI/ARB population. ACEI/ARB cohort was given a weight of 1 and each comparator PS/(1-PS) * (1-prev)/prev, in which PS is the propensity score and prev is the marginal prevalence (proportion) of ACEI/ARB users in the study population.
NSAIDs and Aspirin use might not be captured well in the claims data since most of these agents are available over the counter.
Statistical analysis
To achieve confounding control we estimated three separate propensity scores (PS), the probability of initiating an ACEI/ARB versus each of the other antihypertensive classes based on measured covariates, using multivariable logistic regression.41 We then balanced the distribution of these potential confounders between our comparison cohorts by assigning weights of one to the ACEI/ARB cohort and the propensity odds (PS/(1-PS)) times the stabilizing factor, the inverse of the odds of the marginal prevalence of the ACE/ARB cohort ((1-prevalence)/prevalence), to each comparator.42 This stabilized standardized morbidity ratio (SMR) weighting allowed us to standardize the covariate distribution in the comparator cohorts (non-angiotensin antihypertensive initiators) to that in the ACEI/ARB initiator cohort. Thus, assuming no unmeasured confounding, our treatment comparisons would be unconfounded with respect to their effect on the outcome. Covariate balance was assessed using absolute standardized mean differences, of which <0.1 was considered adequate.43
We estimated treatment effects with Cox proportional hazards regression after PS weighting and checking the proportional hazards assumption by assessing an interaction between (log) time and treatment. We used robust variance estimators to estimate 95% confidence intervals (CI) to account for the weighting. We also used Kaplan–Meier methods to estimate the PS weighted cumulative risk of CRC over time and weighted risk differences (RD) at one, three and five years of follow-up to provide absolute risks in addition to the traditional HR.44
Sensitivity analyses
For primary analyses, we assumed the empirical induction (lag period) of 6 months, both following drug initiation and discontinuation. We varied this period to 0, 12, and 24 months to assess the robustness of our findings. Initiators of ACEI and ARB were analyzed separately to estimate their independent effects on the outcome. To evaluate the proportional hazards assumption and also to explore the potential time varying effects with sustained drug use, we conducted analyses stratified by time since drug initiation.45 We also varied the maximum lengths of assumed risk periods (i.e., maximum follow-up durations) for the intention-to-treat analyses, to assess the varying cumulative effects of the drugs under study. Since diabetes mellitus is a strong potential confounder, we restricted analyses to patients without baseline diabetes diagnoses to reduce residual confounding by diabetes severity.20,46,47 We also conducted analyses excluding patients with a history of congestive heart failure and myocardial infarction diagnoses, since they are major indications for ACEI/ARB, and could potentially bias our findings via informative censoring (i.e., if patients censored for these events are at higher risk of CRC than those who are not).
Results
We identified a total of 111,533 ACEI/ARB initiators, 78,746 beta blocker initiators, 39,905 CCB initiators and 29,043 thiazide initiators. Patient characteristics before and after PS weighting are reported in Table 1. ACEI/ARB initiators were the youngest with a mean age at first prescription of 75 (standard deviation 7.3), while CCB initiators were the oldest with mean age 77.0 (standard deviation 8.1). ACEI/ARB and beta blocker initiators were less likely to be African American (6.4% and 5.4% respectively) than thiazide and CCB initiators (8.4% and 11% respectively). ACEI/ARB initiators were more likely to be diagnosed with diabetes mellitus (39%) compared to thiazide (17%), beta blockers (25%), and CCB (26%). After stabilized SMR weighting, all measured patient characteristics were balanced between all treatment contrast groups (absolute standardized mean differences are presented in eFigure 2). In our external validation study, the distributions of potential unmeasured confounders, BMI and smoking, were similar between ACEI/ARB and comparator drugs (eTables 4–6 show the baseline characteristics of antihypertensive initiators among MCBS respondents).
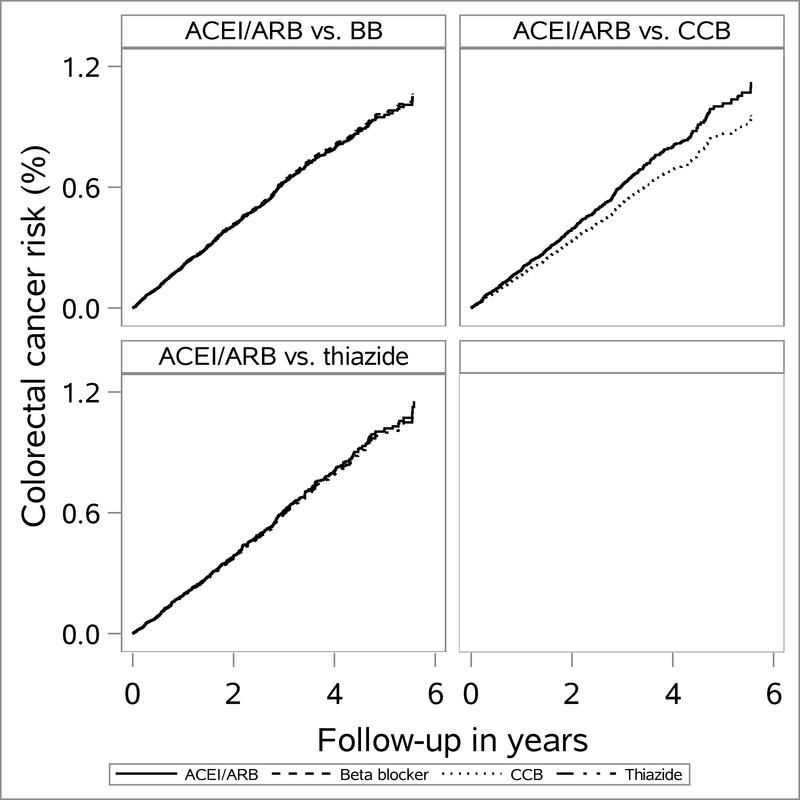
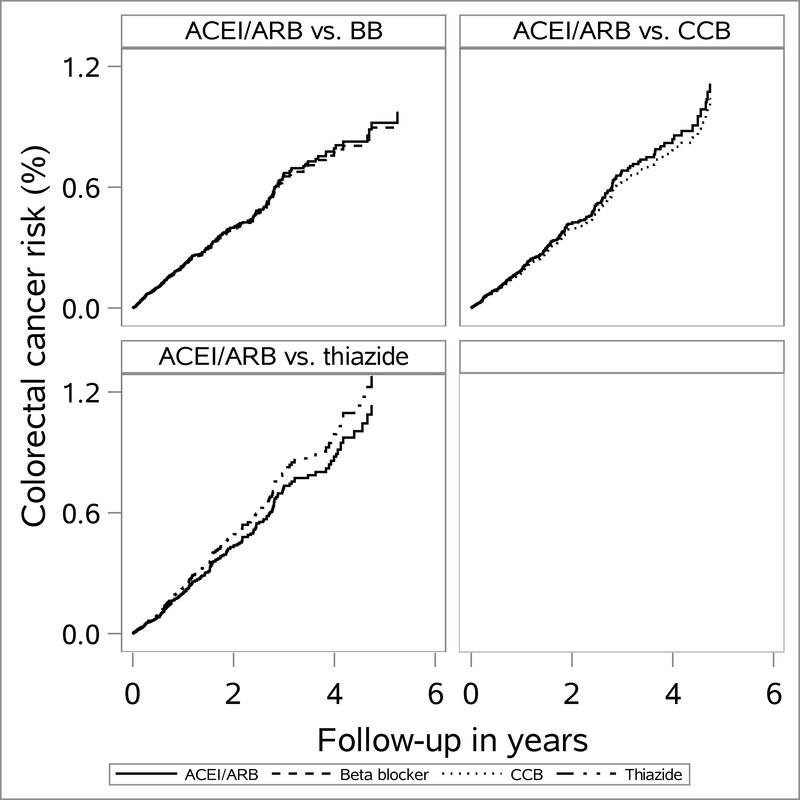
Intention-to-treat and as treated follow-up showed near-null associations between ACEI/ARB versus comparator drugs and CRC outcomes (Table 2). For the intention-to-treat analyses with 6-month lag, PS weighted HRs (95% CI) were 1.0 (0.85, 1.1) for ACEI/ARB versus beta blocker, 1.2 (0.97, 1.4) for ACEI/ARB versus CCB and 1.0 (0.80, 1.3) for ACEI/ARB versus thiazide. Weighted cumulative incidence (Kaplan–Meier) curves are presented in Fig. 1 and 2 and show similar incidence patterns between our comparator drugs over the course of follow-up. For the intention-to-treat analyses, 5-year RD per 1,000 were 1.4 (95% CI: −0.26, 2.9) for ACEI/ARB versus beta blocker, 1.7 (95% CI: −0.4, 3.8) for ACEI/ARB versus CCB and 0.4 (95% CI: −2.5, 3.2) for ACEI/ARB versus thiazide. We present cumulative risks of CRC for various follow-up periods by each drug class in eTable 7.
Table 2.
Colorectal cancer incidence by cohorts of antihypertensive drug initiatorsa
| Drug initiators and lag period | No. of events | Total sample size | Follow-up years (median, IQR) | Incidence rates (per 100,000) | Crude HR (95% CI) | Weighted HR (95% CI)c |
|---|---|---|---|---|---|---|
| Intention-to-treat analysesb | ||||||
| 6-month lag periodd | ||||||
| ACEI/ARB | 532 | 111,533 | 2.2 (1.0, 3.7) | 200 | 1.0 (0.89, 1.2) | 1.0 (0.85, 1.1) |
| BB | 364 | 78,746 | 2.1 (0.9, 3.6) | 197 | 1.0 | 1.0 |
| ACEI/ARB | 532 | 111, 533 | 2.2 (1.0, 3.7) | 200 | 1.0 (0.84, 1.2) | 1.2 (0.97, 1.4) |
| CCB | 177 | 39,905 | 1.9 (0.8, 3.4) | 200 | 1.0 | 1.0 |
| ACEI/ARB | 532 | 111,533 | 2.2 (1.0, 3.7) | 200 | 1.1 (0.89, 1.3) | 1.0 (0.80, 1.3) |
| THZ | 138 | 29,043 | 2.4 (1.1, 4.0) | 186 | 1.0 | 1.0 |
| As treated analysesb | ||||||
| 6-month lag periodd | ||||||
| ACEI/ARB | 231e | 110,930 | 0.6 (0.3, 1.3) | 205 | 1.1 (0.85, 1.3) | 1.0 (0.82, 1.3) |
| BB | 149 | 78,292 | 0.5 (0.2, 1.3) | 195 | 1.0 | 1.0 |
| ACEI/ARB | 235e | 110,930 | 0.6 (0.3, 1.4) | 204 | 1.0 (0.78, 1.3) | 1.1 (0.80, 1.4) |
| CCB | 81 | 39,705 | 0.6 (0.2, 1.3) | 204 | 1.0 | 1.0 |
| ACEI/ARB | 238e | 110,930 | 0.6 (0.3, 1.4) | 210 | 1.1 (0.82, 1.5) | 0.9 (0.59, 1.3) |
| THZ | 52 | 28,855 | 0.5 (0.2, 1.2) | 191 | 1.0 | 1.0 |
ACEI – Angiotensin Converting Enzyme Inhibitors, ARB – Angiotensin Receptor Blockers, BB – beta blockers, THZ – thiazide, CCB – calcium channel blockers, HR – hazard ratios, IQR – interquartile range, CI – confidence intervals
Drug initiators were identified after a 6-month washout period and prevalent cancers during the 12-month period prior to the drug initiation were excluded
Intention-to-treat analysis was based on follow-up until the end of study/enrollment or death or other incident cancer outcomes. As treated (AT) analysis was based on follow-up until the events in ITT or switching/discontinuation/ augmentation of drugs.
Adjusted for covariates in Table 1. Weighting was implemented by stabilized morbidity ratio weighting, where patients were weighted to reflect the covariate distributions in the ACEI/ARB population. ACEI/ARB cohort was given a weight of 1 and each comparator PS/(1-PS) * (1-prev)/prev, in which PS is the propensity score and prev is the marginal prevalence (proportion) of ACEI/ARB users in the study population.
Between second prescription date and the start of follow-up.
The number of CRC among ACEI/ARB is different for each comparison because of differing patterns of censoring for switching/augmenting across treatment comparisons.
Figure 1.
Propensity score weighted cumulative incidence (from Kaplan–Meier estimator) of colorectal cancer among initiators of angiotensin converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB) versus beta blocker (BB), calcium channel blockers (CCB) and thiazide initiators according to intention-to-treat analyses
Figure 2.
Propensity score weighted cumulative incidence (from Kaplan–Meier estimator) of colorectal cancer among initiators of angiotensin converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB) versus beta blocker (BB), calcium channel blockers (CCB) and thiazide initiators according to as treated analyses
After excluding patients with a history of diabetes mellitus and major cardiovascular diseases, hazard ratios revealed consistent near-null findings (eTables 10 and 11). Results of other sensitivity analyses were consistent with primary findings. These included varying induction/lag period following drug initiation and discontinuation (eTables 15 and 16), imposing varying limits of maximum follow-up for intention-to-treat analyses (Table 3), separating ACEI and ARB groups (eTable 12 shows CRC risks for ACEI or ARB alone versus other antihypertensives), analyses stratified over the follow-up duration (eTables 8 and 9), and allowing prevalent users of other antihypertensive drug classes in treatment comparisons (eTable 13).
Table 3.
Colorectal cancer incidence by cohorts of antihypertensive drug initiators after varying the maximum follow-up duration (intention-to-treat analyses)a
| Maximum follow-up duration | Drugs | No. of events | Total sample size | Follow-up years (median, IQR) | Incidence ratesb | Crude HR (95% CI) | Weighted HR (95% CI)c |
|---|---|---|---|---|---|---|---|
| ACEI/ARB versus beta blocker | |||||||
| 2 years | ACEI/ARB | 258 | 111,551 | 1.5 (1.0, 1.5) | 192 | 0.84 (0.70, 1.0) | 0.80 (0.68, 1.0) |
| BB | 214 | 78,753 | 1.5 (0.9, 1.5) | 228 | 1.0 | 1.0 | |
| 3 years | ACEI/ARB | 375 | 111,551 | 2.2 (1.0, 2.5) | 194 | 0.90 (0.78, 1.0) | 0.90 (0.75, 1.1) |
| BB | 287 | 78,753 | 2.1 (0.9, 2.5) | 213 | 1.0 | 1.0 | |
| 4 years | ACEI/ARB | 470 | 111,551 | 2.2 (1.0, 3.5) | 202 | 0.90 (0.83, 1.1) | 0.90 (0.80, 1.1) |
| BB | 339 | 78,753 | 2.1 (0.9, 3.4) | 210 | 1.0 | 1.0 | |
| ACEI/ARB versus CCB | |||||||
| 2 years | ACEI/ARB | 258 | 111,551 | 1.5 (1.0, 1.5) | 192 | 1.0 (0.81, 1.3) | 1.1 (0.84, 1.5) |
| CCB | 87 | 39,909 | 1.5 (0.8, 1.5) | 187 | 1.0 | 1.0 | |
| 3 years | ACEI/ARB | 375 | 111,551 | 2.2 (1.0, 2.5) | 194 | 1.0 (0.82, 1.2) | 1.1 (0.90, 1.4) |
| CCB | 127 | 39,909 | 1.9 (0.8, 2.5) | 193 | 1.0 | 1.0 | |
| 4 years | ACEI/ARB | 470 | 111,551 | 2.2 (1.0, 3.5) | 202 | 1.0 (0.84, 1.2) | 1.2 (0.96, 0.4) |
| CCB | 156 | 39,909 | 1.9 (0.8, 3.4) | 199 | 1.0 | 1.0 | |
| ACEI/ARB versus thiazide | |||||||
| 2 years | ACEI/ARB | 258 | 111,551 | 1.5 (1.0, 1.5) | 192 | 1.1 (0.82, 1.4) | 1.1 (0.75, 1.6) |
| THZ | 64 | 29,044 | 1.5 (1.1, 1.5) | 178 | 1.0 | 1.0 | |
| 3 years | ACEI/ARB | 375 | 111,551 | 2.2 (1.0, 2.5) | 193 | 1.0 (0.83, 1.3) | 1.0 (0.75, 1.3) |
| THZ | 99 | 29,044 | 2.4 (1.1, 2.5) | 188 | 1.0 | 1.0 | |
| 4 years | ACEI/ARB | 470 | 111,551 | 2.2 (1.0, 3.5) | 202 | 1.1 (0.89, 1.3) | 1.0 (0.81, 1.4) |
| THZ | 119 | 29,044 | 2.4 (1.1, 3.5) | 186 | 1.0 | 1.0 | |
ACEI – Angiotensin Converting Enzyme Inhibitors, ARB – Angiotensin Receptor Blockers, BB – beta blockers, THZ – thiazide diuretics, CCB – Calcium Channel Blockers, HR – hazard ratios, CI – confidence intervals, IQR – interquartile range
Drug initiators were identified after excluding prevalent users of any antihypertensive drugs, i.e., no prevalent use was allowed for any antihypertensive drug class.
Incidence rates are per 1,000 population per year.
Adjusted for baseline covariates in Table 1. Weighting was implemented by standardized morbidity ratio weighting, where every patient was weighted to reflect covariate distributions in the exposed population (ACEI/ARB).
Discussion
In our new user cohort study estimating the comparative effect of ACEI/ARB initiators versus other antihypertensive initiators on CRC risk, we observed no evidence of short-term effect. A majority of our population did not stay on the same medication class for an extended time period before switching to or augmenting with other antihypertensive agents. Such treatment dynamics in real world data limit our ability to isolate long-term treatment effects. Using prevalent users instead of drug initiators does not solve this problem since it would restrict the study population to a selected group of patients who could tolerate prior antihypertensive treatment and therefore are different in health status from those who are newly starting therapy (healthy user bias).20 Nonetheless, all of our time varying analyses over a maximum follow-up of 5–7 years showed consistent near-null findings.
Meta-analyses of clinical trials reported conflicting findings on the effects of ARB or ACEI/ARB on the overall cancer incidence.7–9 Interpretations of these analyses require caution because of variations in diagnostic criteria used to identify incident cancers (which were also not limited to CRC) and the prevalence of missing data. Our findings are consistent with most observational studies in the literature. Studies from Canada and UK utilized the new user designs similar to ours and reported near-null associations between ACEI or ARB versus thiazide diuretics and CRC risk.15,16 Other studies also reported null or near-null findings but none reflect a broader hypertensive population treated within the US healthcare system and most included prevalent medication users, or employed non-user comparison groups, which could introduce healthy user bias and differential outcome detection bias respectively.14,17–19,22,32 Some studies suggested contradictory evidence of protective effects of ACEI or ARB on the risk of CRC.10–12 While these findings could reflect genuine differences in study settings or populations, study design differences such as the exposure definition (incident versus prevalent users), the choice of the comparison group, or the potential for immortal time bias might explain some of the contradictory findings.20–22,32
A major strength of our study is the active comparator, new user design. Exclusion of prevalent drug users from our study reduces the sample size and follow-up time; nonetheless, it allows us to synchronize the beginning of follow-up between the treatment groups, reducing the potential for time-related (immortal time) and healthy user biases.20,21,32 The choice of non-angiotensin based antihypertensive alternatives also implicitly improves confounding control since we are limiting our study cohorts to individuals likely at a similar state of hypertension progression and related comorbid conditions.22,32 While hypertension is a minor risk factor for CRC, such guideline-recommended antihypertensive alternatives nonetheless reduces the potential for differential ascertainment of outcomes that often occurs with non-user comparison group, since antihypertensive users are more likely to be in contact with healthcare system than non-users.22,46 Most measured confounders were indeed balanced across treatment groups, even prior to PS weighting. We were able to remove the remaining small differences using SMR weighting.
We evaluated the robustness of various assumptions concerning our study design. We varied the empirical induction for cancer up to 2 years and, while this time period appears short, prior in vivo and in vitro studies showed that angiotensin II might play a role in both cancer initiation and progression, thus supporting our assumption of short induction.2–5 We assessed cancer incidence in short incremental intervals over the course of follow-up to assess time-varying effects, potentially due to residual confounding or detection bias associated with time varying treatment patterns: estimates were consistent throughout.45 Our findings were generally robust regardless of censoring for medications stopping, switching, or augmenting, which indicates that these censoring events were not differentially associated with CRC risk.
Although we did not allow prevalent users of other antihypertensive drug classes in each new-user and active-comparator cohort, we allowed them in additional analyses (e.g., in the comparison against beta blocker, prevalent CCB and thiazide users were included). Such analyses substantially increased our sample size by allowing the inclusion of chronic hypertension patients, who might be more prone to cancer outcomes and findings were consistent nonetheless (eTable 13).
As with most observational studies, our results are subject to unmeasured confounding; especially by BMI, smoking, alcohol, diet, and functional limitations (frailty) in old adults.38, 39, 47, 48 In our external validation study using the MCBS survey data, we found similar distributions of BMI and smoking between treatment groups defined similarly to ours. Since MCBS survey participants are a random sample of the Medicare population and cohort eligibility criteria were similar between both cohorts, it implies that the potential for unmeasured confounding by BMI and smoking in our study is small. There is also no empirical evidence to expect that such unmeasured characteristics would be systematically different between our comparison cohorts to the extent of materially changing our estimates.
The interpretation of our study is also limited by the validity of exposure and outcome definitions. Antihypertensive therapy could be misclassified if patients are dispensed medications from the pharmacy, but do not actually take them. We therefore required our study population to refill the same medication class at least once following the initial prescription claim and the time between first and second prescriptions is relatively short (median time and interquartile range, IQR, ranges from 29 days (IQR: 26, 38) up to 33 days (IQR: 28, 52) across drug classes). Claims data have an advantage in identifying medications in that they are recorded when prescriptions are dispensed from the pharmacy (even when prescribed by physicians from different healthcare systems), whereas electronic medical records generally provide only physician orders of medications in a single healthcare setting. A prior study showed that antihypertensive drugs are less likely to be given as sample drugs compared to other chronic medications.49 Therefore, misclassification of prescription data due to the sample use from physician offices is less likely.
The claims-based measure of incident CRC also has a high reported specificity (99%)36 with a reasonable sensitivity (80%) and very specific outcome definitions have been shown to produce less biased ratio measures of association.50 In addition, we reported CRC incidence rates (per 100,000) of approximately 200–210 among ACEI/ARB and 186–204 among initiators of comparator drugs, which is comparable to incidence rates of CRC among U.S. adults over age 65 (201 per 100,000 person-years in 2009–2013).51
We only examined the most common antihypertensive medication classes; thus, effects of newer and less commonly prescribed classes such as direct renin inhibitors (available in the US since 2007) could not be discerned. We also did not conduct dose–response analyses but analyses stratified by duration of use (a good proxy for cumulative dose) showed that CRC risk did not change, implying that the chronicity of drug use is not associated with increased cancer risk. We did not address death as a competing risk, since mortality among our cohorts was low: approximately 3% among ACEI/ARB, 4% among beta blocker, 7% among CCB,and 2% among thiazide diuretics initiators.
In our active comparator, new user study based on large US healthcare claims data, we found no evidence of a short-term effect of ACEI/ARB use on CRC risk relative to alternative antihypertensives. Given that our follow-up duration was relatively short, a function of treatment dynamics in the hypertensive population in real world data, we could not exclude a potential long-term effect. However, the absolute risk of CRC in this population was small, and thus the influence of CRC is unlikely to be an important consideration for physicians and patients when selecting among available therapeutic alternatives for the management of hypertension.
Supplementary Material
Acknowledgments
P.T.H, J.L.L, M.J.F and T.S were involved in study design and conception. P.T.H and V.P had access to the data and performed data cleaning and statistical programming. P.T.H was responsible for literature searches, statistical analyses, data interpretation and preparation of the initial manuscript. All coauthors were involved in reviewing, providing feedback and revising the manuscript. All authors have given permission to be named in the manuscript.
Sources of funding
The results reported herein correspond to specific aims of grant R01 AG056479 to investigator Til Stürmer from National Institute of Aging.
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the population-based evaluation of drug benefits and harms in older U.S. adults (GIL200811.0010); the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the Comparative Effectiveness Research Strategic Initiative of UNC’s Clinical and Translational Science Award (UL1TR002489); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine.
M.J.F reports salary support from NIH National Center for Advancing Translational Sciences (NCATS, 1UL1TR001111), consulting fee paid to the UNC via GlaxoSmithKline, Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health and other research support from AstraZeneca.
Footnotes
Conflicts of interest:
P.T.H has no conflicts of interest. T.S. reports salary support from Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck), research support from AstraZeneca, Amgen, and ownership interest in Novartis, Roche, BASF, AstraZeneca, Johnson & Johnson, and Novo Nordisk.
R.J.S. is a paid consultant to Amgen, Pfizer and Merck.
Computing code required to reproduce our findings
The code is available upon request. We cannot provide Medicare data due to data user agreement but the data could be requested from the Centers for Medicare and Medicaid Services.
References
- 1.Nelson DL, Lehninger AL, Cox MM. Lehninger principles of biochemistry. New York: W.H. Freeman; 2005. [Google Scholar]
- 2.Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985;105(2):141–145. [PubMed] [Google Scholar]
- 3.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. [DOI] [PubMed] [Google Scholar]
- 4.Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991;68(2):450–456. [DOI] [PubMed] [Google Scholar]
- 5.Chiu T, Santiskulvong C, Rozengurt E. ANG II stimulates PKC-dependent ERK activation, DNA synthesis, and cell division in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G1–11. [DOI] [PubMed] [Google Scholar]
- 6.Yasumaru M, Tsuji S, Tsujii M, et al. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 2003; 63: 6726–6734. [PubMed] [Google Scholar]
- 7.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010; 11: 627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARB Trialists Collaboration. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens. 2011;29:623–35. [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Huang YM, Wang M, et al. Renin-angiotensin system blockade for the risk of cancer and death. J Renin Angiotensin Aldosterone Syst. 2016; 17: 10.1177/1470320316656679. Print 2016 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One. 2012;7(12):e50893. doi: 10.1371/journal.pone.0050893 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KL, Liu CJ, Chao TF, et al. Long-term use of angiotensin II receptor blockers and risk of cancer: a population-based cohort analysis. Int J Cardiol. 2013; 167: 2162–2166. [DOI] [PubMed] [Google Scholar]
- 12.Makar GA, Holmes JH, Yang YX. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst. 2014;106(2):djt374. doi: 10.1093/jnci/djt374 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: A population-based case-control study. Br J Clin Pharmacol. 2012;74(1):180–188. doi: 10.1111/j.1365-2125.2012.04170.x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R, Mandelson MT. Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3076–3080. doi: 10.1158/1055-9965.EPI-08-0095 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assimes TL, Elstein E, Langleben A, Suissa S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf. 2008;17(11):1039–1049. doi: 10.1002/pds.1656 [doi]. [DOI] [PubMed] [Google Scholar]
- 16.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK general practice research database. BMJ. 2012;344 : e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A, Stricker BH. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: The rotterdam study. Cancer. 2008;112(4):748–757. doi: 10.1002/cncr.23215 [doi]. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg L, Rao RS, Palmer JR, et al. Calcium channel blockers and the risk of cancer. JAMA. 1998; 279: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 19.Dai YN, Wang JH, Zhu JZ, et al. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: a systematic review and meta-analysis. Cancer Causes Control. 2015; 26: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 20.Shrank WH, Patrick AR, Alan Brookhart M. Healthy User and Related Biases in Observational Studies of Preventive Interventions: A Primer for Physicians. Journal of General Internal Medicine. 2010; 26: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suissa S Immortal Time Bias in Pharmacoepidemiology. American Journal of Epidemiology. 2008; 167: 492–499. [DOI] [PubMed] [Google Scholar]
- 22.D’arcy M, Stürmer T, Lund JL. The importance and implications of comparator selection in pharmacoepidemiologic research. Current Epidemiology Reports. 2018; 5:272–283. 10.1007/s40471-018-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 24.Jick H, Jick S, Derby LE, Vasilakis C, Myers MW, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349(9051):525–528. doi: S0140–6736(97)80084–0 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Pahor M, Guralnik JM, Ferrucci L, et al. Calcium-channel blockade and incidence of cancer in aged populations. The Lancet. 1996; 348: 493–497. [DOI] [PubMed] [Google Scholar]
- 26.Grimaldi-Bensouda L, Klungel O, Kurz X, et al. Calcium channel blockers and cancer: a risk analysis using the UK Clinical Practice Research Datalink (CPRD). BMJ Open. 2016; 6: e009147–2015-009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes S, Griffith EJ, Musto G, Minuk GY. Antihypertensive medications and survival in patients with cancer: A population-based retrospective cohort study. Cancer Epidemiology. 2013;37(6):881–5. doi: 10.1016/j.canep.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 28.Zacharski LR, Moritz TE, Haakenson CM, et al. Chronic calcium antagonist use in carcinoma of the lung and colon: a retrospective cohort observational study. Cancer Invest.1990; 8: 451–458. [DOI] [PubMed] [Google Scholar]
- 29.Chang PY, Huang WY, Lin CL, et al. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine (Baltimore). 2015; 94: e1097. doi: 10.1097/MD.0000000000001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen L, Below J, Chang-Claude J, Brenner H, Hoffmeister M. Beta blocker use and colorectal cancer risk: Population-based case-control study. Cancer. 2012;118(16):3911–3919. doi: 10.1002/cncr.26727 [doi]. [DOI] [PubMed] [Google Scholar]
- 31.Numbere B, Fleming KM, Walker A, Card TR. Adrenergic blockers and the risk for common solid cancers: A case-control study. Eur J Cancer Prev. 2015. doi: 10.1097/CEJ.0000000000000218 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: Historical foundations and contemporary application. Current Epidemiology Reports. 2015;2(4):221–228. 10.1007/s40471-015-0053-5. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virnig B, Madeira AD. Strengths and limitations of CMS administrative data in research. http://www.resdac.org/resconnect/articles/156. Published 2012. Accessed 20 Aug 2016
- 34.Centers for Medicare and Medicaid Services. Chronic Conditions Data Warehouse. https://www.ccwdata.org/web/guest/medicare-charts/medicare-enrollment-charts. Accessed 10 Aug 2016.
- 35.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol. 2012; 65:343–349.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007; 18: 561–569. [DOI] [PubMed] [Google Scholar]
- 37.Rothman KJ. Induction and latent periods. Am J Epidemiol. 114(2): 253–259. [DOI] [PubMed] [Google Scholar]
- 38.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016; 375: 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008; 122: 155–164. [DOI] [PubMed] [Google Scholar]
- 40.Stürmer T, Schneeweiss S, Avorn J, Glynn RJ. Adjusting Effect Estimates for Unmeasured Confounding with Validation Data using Propensity Score Calibration. Am J Epidemiol. 2005; 162: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70: 41–55. [Google Scholar]
- 42.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003; 14: 680–686. [DOI] [PubMed] [Google Scholar]
- 43.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011; 46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole SR, Lau B, Eron JJ, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol. 2015; 181: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011; 54: 2263–2271. [DOI] [PubMed] [Google Scholar]
- 46.Stürmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev. 2006; 15:2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015; 112: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014; 349: g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hampp C, Greene P, Pinheiro SP. Use of Prescription Drug Samples in the USA: A Descriptive Study with Considerations for Pharmacoepidemiology. Drug Saf. 2016; 39: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 51.Surveillance, Epidemiology, and End Results (SEER) Program Populations (2009–2013) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; https://seer.cancer.gov/csr/1975_2015/browse_csr.php?sectionSEL=6&pageSEL=sect_06_table.10. Accessed March 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.