ABSTRACT
Background
The phenylalanine requirement of the elderly is not known. Current recommendations are based on studies in young adults and are derived from a combined estimate of the total aromatic amino acids, phenylalanine, and tyrosine.
Objectives
The purpose of this study was to determine the dietary phenylalanine requirement of adults aged >65 y, using the direct amino acid oxidation method, by measuring the oxidation of l-[1-13C]phenylalanine to 13CO2 in response to graded phenylalanine intakes in the presence of excess tyrosine.
Methods
Twelve subjects (6 men, 6 women), aged 73.8 ± 6.7 y (mean ± SD) and with a BMI (in kg/m2) of 26.4 ± 4.8 and 25.2 ± 4.4 for men and women, respectively, were randomized to phenylalanine intakes ranging from 7.20 to 40.0 mg .kg−1 .d−1 for a total of 66 studies. Study diets were isocaloric and isonitrogenous, providing protein and energy at 1.0 g .kg−1 .d−1 and 1.5 × resting energy expenditure (REE), respectively. Protein was provided as an amino acid mixture patterned after egg protein, with an excess of tyrosine and alanine to balance the nitrogen as phenylalanine intakes were varied. Two days prior to the study day, subjects were adapted to a milkshake diet providing protein at 1.0 g.kg−1 .d−1 and energy at 1.7 × REE. The mean phenylalanine requirement was determined using biphase linear regression analysis, which identified a breakpoint in the F13CO2 in response to graded phenylalanine intakes.
Results
The mean and upper 95% CIs (approximating the recommended dietary allowance) of phenylalanine requirements were estimated to be 9.03 and 15.9 mg.kg−1 .d−1, respectively.
Conclusion
These results are similar to previously derived estimates of 9.1 and 13.6 mg.kg−1 .d−1 in young adult men and suggest that higher protein needs of the elderly to stimulate similar muscle protein synthesis rates as young adults are not driven by an increased requirement for phenylalanine. This trial was registered at clinicaltrials.gov as NCT02971059.
Keywords: phenylalanine requirement, amino acid requirement, older adults, direct amino acid oxidation, aromatic amino acids
Introduction
The current DRI recommendation for total aromatic amino acids (AAAs; phenylalanine and tyrosine) for adults aged 19 y or older is 27 mg.kg−1 .d−1 (1). This recommendation is based on estimates derived from 3 studies in young adults (2–4). Phenylalanine is an indispensable amino acid (IAA), whereas tyrosine can be synthesized from phenylalanine via the process of hydroxylation (5, 6). For healthy individuals without defects in the hydroxylation pathway, the total AAA requirement can be met by phenylalanine alone; this is termed maximal phenylalanine requirement (7). If, however, the requirement for phenylalanine is determined in the presence of excess tyrosine, the phenylalanine requirement so derived is the minimum obligatory phenylalanine requirement, which is the portion of the phenylalanine requirement that cannot be replaced by tyrosine (7). The minimum phenylalanine requirement, estimated in the presence of excess tyrosine, provides a robust and sensitive estimate of the phenylalanine requirement, which can then be applied as an indicator in the derivation of the requirement for other IAAs.
Using the direct amino acid oxidation (DAAO) method, we previously estimated the minimum requirement for phenylalanine in the presence of excess tyrosine (40 mg .kg−1 .d−1) in young men (2). We subsequently applied this estimate of phenylalanine requirement with excess tyrosine to successfully determine all IAA requirements in young adults. Those estimates, along with those of other researchers in the field, formed the basis of the current DRI recommendation for amino acids (AAs) in adults (1). Because there are no data on AA requirements in adults aged >65 y, the DRI committee used the same data derived in young adults to set recommendations for older adults (1).
Aging is associated with structural and functional changes, including loss of lean body mass and changes in protein and AA metabolism (8–10), that are not fully understood. Aging cells are less sensitive to IAAs for muscle protein synthesis (11), and current evidence suggests that increased availability of AAs (12–14), particularly IAAs (15), is required to stimulate muscle protein synthesis in the elderly. On the other hand, aging is associated with increased splanchnic uptake of IAAs (16, 17), which could decrease IAA supply to peripheral tissue, thus providing a potential mechanism for sarcopenia in the elderly. Evidence from physiological studies suggests a higher AA requirement for muscle protein synthesis in older compared with younger adults (18), but reports are conflicting (16, 19). Despite their importance, physiological studies do not quantify the dietary requirement for the IAAs. Clearly there is an urgent need to determine the requirement for all IAAs in the elderly. Because phenylalanine is the main indicator AA used by our group to determine IAA requirements with the indicator amino acid oxidation (IAAO) method, we need to begin with phenylalanine. Therefore, the purpose of this study was to determine the dietary requirement for phenylalanine in the presence of excess tyrosine (the minimum phenylalanine requirement) in adults aged >65 y using the DAAO method.
Methods
Subjects
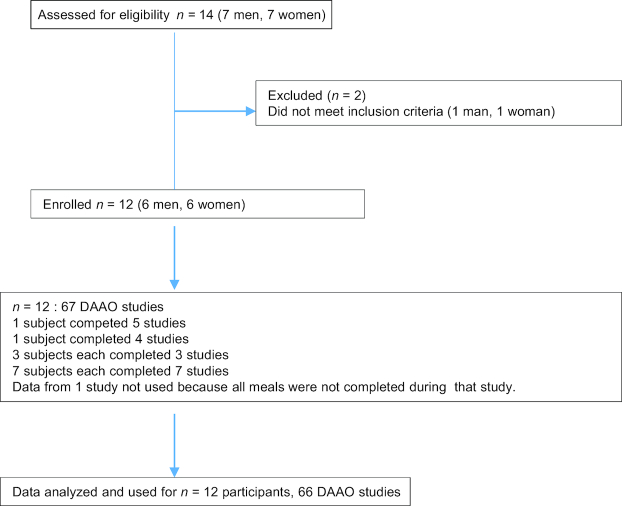
Adults aged >65 y were invited to participate in the study via postings in specified areas at the Hospital for Sick Children and through “word of mouth” from staff members at the hospital to their family members. Subjects were considered eligible if on initial screening they were community dwelling, with no physical disabilities (did not use any assistive device for walking) and able to participate in at least 2 experiments. Subjects were excluded if they had a recent history of weight loss, diabetes, kidney disease, or other illness known to affect protein and AA metabolism (e.g., HIV and inflammatory disease such as Crohn's and colitis), or were on medications known to affect protein and AA metabolism (e.g., steroids). Subjects with hypertension were not excluded if their blood pressure was well controlled and their antihypertensive medications were taken as prescribed by their physician. The Research Ethics Board at The Hospital for Sick Children approved all procedures. Informed written consent was obtained from all participating subjects. Subjects received financial compensation for their participation. The study was conducted at the Clinical Research Center (CRC), The Hospital for Sick Children, Toronto, Canada, from August 2016 to June 2018 (Figure 1).
FIGURE 1.
CONSORT flowchart of the study determining phenylalanine requirement in adults aged >65 y. CONSORT, Consolidated Standards of Reporting Trials; DAAO; direct amino acid oxidation.
Experimental design
The study design was adapted from the DAAO protocol (2). The DAAO method is a stable isotope method developed by Basile-Filho et al. (4) and previously used by our group to determine phenylalanine requirement in young adult men (2). The technique is based on the concept that AAs in excess of the amounts needed for protein synthesis are oxidized. When the intake of an IAA is deficient in the diet, oxidation is low and constant because most of the AA is used for protein synthesis. As the supply increases above the requirement for protein synthesis, catabolism increases (20). This increased catabolism can be measured with the use of a stable isotope, usually 13C, which can then be measured in breath (2, 21).
Each subject was initially invited to the CRC for a prestudy assessment on the morning following a 12-h overnight fast. The prestudy assessment consisted of a 10-mL blood sample taken for measurements of glucose, creatinine, and urea to assess for diabetes and renal function. Resting energy expenditure (REE) was measured by continuous, open-circuit indirect calorimetry (Vmax Encore Metabolic Cart; VIASYS), and body composition was measured by 4 skinfold thicknesses (triceps, biceps, subscapular, and super iliac) which were measured to the nearest 1 mm with a Harpenden caliper (Habdirect) to obtain estimates of fat mass (22). Bioelectrical impedance analysis (23) was performed using a fixed frequency analyzer (50 kHz) (BIA model 101A; RJL Systems). Resistance (R) and reactance (Xc) measurements were taken using a 4-terminal bioelectrical impedance analyzer. Three readings for both R and Xc (in Ω) were taken for each subject, and equations (24) were used to determine lean body mass as previously described (25, 26).
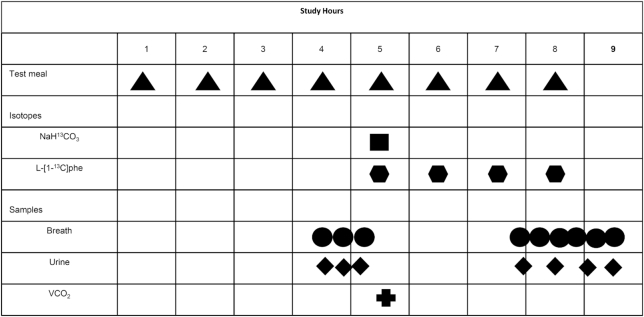
Each level of phenylalanine intake was studied over 3 d (2 adaptation days followed by a DAAO study day) (2, 27) (Figure 2). During the adaptation days, subjects received a lactose-free milkshake maintenance diet (Scandishake: Scandipharm) supplying 1.0 g protein.kg−1 .d−1 and 1.7 × REE (25, 26). On the third study day, after a 12-h overnight fast, subjects were randomized to receive test phenylalanine intakes ranging from 7.2 to 40.0 mg .kg−1 .d−1 (25, 26). Each level of phenylalanine intake was different, and no subject received the same levels of phenylalanine intake. The design of the study was for each subject to receive 7 levels of phenylalanine intake; however, because of dropouts, only 7 subjects received all 7 intakes, 1 subject received 5 intakes, 1 received 4 intakes, and 3 received 3 intakes each (Figure 1). For the 9 levels of intakes from 3 subjects, 4 were below and 5 above the breakpoint. The lowest phenylalanine intake consisted of the tracer only. Each 3-d study period was separated by 1–2 wk.
FIGURE 2.
Typical study day protocol for each DAAO study in which graded intakes of phenylalanine were provided in random order. The experimental diet was given as 8 isocaloric, isonitrogenous meals representing 1/10th of each subject's daily requirement. Priming doses of the isotopes NaH13CO3 and l-[1–13C]phenylalanine were given at the fifth meal; an hourly dose of l-[1–13C]phenylalanine was given simultaneously and continued throughout the remaining 4 h. Three baseline breath samples and 3 baseline urine samples were collected 45, 30, and 15 min before the tracer protocol began. Four plateau urines and 6 plateau breath samples were collected at isotopic steady state beginning 2.5 h after the tracer protocol began. The carbon dioxide production rate (VCO2) was measured by indirect calorimetry after the fifth hourly meal. DAAO; direct amino acid oxidation.
Study diets
All study diets were prepared in our Metabolic Kitchen at The Hospital for Sick Children. The adaptation diet was prepared as 4 equal meals daily for each subject as previously described (26). Briefly, lactase-treated homogenized milk was supplemented with additional protein (Beneprotein; Nestle Clinical Nutrition) and carbohydrate (Polycose; Abbott Nutrition) to provide individualized calorie requirement (1.7 × REE). During the 2 adaptation days, meals were consumed at home and subjects were instructed to consume all 4 meals 3 h apart. Subjects consumed a daily multivitamin supplement (Centrum Cardio; Wyeth Consumer Health Care) for the duration of all studies. The diet provided all the subjects’ micro nutrient needs based on the current DRI. On the adaptation days, subjects were not allowed to consume anything but water, in addition to 1 cup of clear tea or coffee.
On day 3 of each study period (DAAO study day), subjects presented to the CRC on the morning following a 12-h overnight fast (Figure 2). Subjects consumed 8 hourly isocaloric, isonitrogenous meals, with each meal representing 1/10th of the daily energy requirement and protein needs of each subject (28). Because phenylalanine was the AA being studied as well as the isotope used in this study, the isotope dose represented the lowest possible level of phenylalanine intake that can be studied. The consumption of 1/10th compared with 1/12th of daily energy and protein needs allowed us to study lower amounts of phenylalanine intake below the predicted breakpoint, allowing a more balanced design. The experimental diet consisted of a protein-free liquid formula made with protein-free powder (PFD1; Mead Johnson), flavored drink crystals (Tang and Kool-Aid; Kraft Foods), grape seed oil, and protein-free cookies. A crystalline AA mixture patterned after egg protein provided the protein component of the diet except for phenylalanine, which was the test AA being varied in the study; tyrosine (40 mg .kg−1 .d−1), which was maintained constant across phenylalanine intakes; and alanine, which was used to balance the nitrogen as phenylalanine intake was varied in the diet. On the DAAO study day, energy was provided at 1.5 × REE (25, 26). The macronutrient composition of the diet averaged 51% carbohydrate, 36% fat, and 13% protein. The factors 1.7 and 1.5 × REE (measured by indirect calorimetry) used to calculate total energy requirement for adaptation and study days, respectively, were based on FAO recommendation for moderate and sedentary levels of physical activity (29). The FAO expert committee on energy requirements reported that both young and old adults burn similar amounts of calories when subjected to standardized activities in a controlled environment (29). Because subjects in the current study were independently living and moderately active (based on self-report), we used similar activity factors for moderate (adaptation days) and sedentary lifestyle (study days) as proposed by the FAO report (29). On the study days, subjects were sedentary—sitting on a chair and watching television for most of the study's duration except when samples were being collected.
Isotope protocol
The oral tracer protocol started on day 3 of each study period (DAAO study day) at the fifth meal by administering a prime of 2.07 µmol .kg−1 of NaH13CO3 and 3.99 µmol .kg−1 of l-[1–13C]phenylalanine (99 atom percent excess; Cambridge Isotope Laboratories) and an hourly dose of 7.22 µmol .kg−1 .h−1 of l-[1-13C]phenylalanine given until the eighth meal (Figure 2). The quantity of phenylalanine supplied as l-[1-13C]phenylalanine during the last 4 h of the study was subtracted from the test phenylalanine intake so that phenylalanine intake for each study was based on the level of intake being tested for each study. Tyrosine was provided at 40 mg .kg−1 .d−1 divided equally across all meals to provide an excess of tyrosine, ensuring that the tracer not be delayed in the tyrosine pool (30) and to allow an estimation of the minimum requirement for phenylalanine as was previously done in young adults (2), which must be accomplished in the presence of excess tyrosine (7).
Sample collection and analysis
On each DAAO study day, breath and urine samples were collected based on our minimally invasive protocol (28) (Figure 2). Three baseline breath and urine samples were collected 45, 30, and 15 min before the tracer protocol began. Six plateau breath and 4 urine samples were collected at isotopic steady state every 30 min beginning at 2.5 h after the start of the tracer protocol. Breath samples were collected in disposable Exetainer tubes (Labco) with a collection mechanism (Easy-Sampler; Quintron) that permitted the removal of dead-space air. Breath samples were stored at room temperature, and urine samples were stored at −20°C until analysis. During each study day, the rate of carbon dioxide production (VCO2) was measured immediately after the fifth meal for a period of 20 min with an indirect calorimeter (Vmax Encore Metabolic Cart).
Expired 13CO2 enrichment was measured with a continuous-flow isotope ratio mass spectrometer (CF-IRMS 20/20 isotope analyzer; PDZ Europa). Enrichments were expressed as atom percent excess compared with a reference standard of compressed CO2 gas. Urinary l-[1-13C] phenylalanine enrichment was analyzed by an API 4000 triple quadrupole mass spectrometer (Applied Biosystems-MDS Sciex) in positive electrospray ionization mode as previously described. Isotopic enrichment was expressed as mole percent excess and calculated from peak area ratios at isotopic steady state at baseline and plateau.
Blood samples were sent to Clinical Chemistry at The Hospital for Sick Children for analysis of serum glucose, urea, and creatinine. Samples were analyzed on an automated analyzer (Roche Cobas 6000). Precision and accuracy of the instrument are both <5%.
Estimation of isotope kinetics
The whole-body phenylalanine flux was calculated according to the stochastic model of Matthews et al. and as previously described (31–33). Isotopic steady state in the tracer enrichment at baseline and plateau was represented as unchanging values of l-[1-13C]phenylalanine in urine (28) and 13CO2 in breath.
Phenylalanine flux (µmol. kg−1 .h−1) was calculated from the dilution of orally administered l-[1-13C]phenylalanine into the metabolic pool (at steady state) using enrichment of l-[1-13C]phenylalanine in urine (28, 32). The rate of appearance of 13CO2 in breath (F13CO2, µmol .kg−1 .h−1) after the oxidation of ingested l-[1-13C]phenylalanine was calculated according to the model of Matthews et al. (32) using a factor of 0.82 to account for CO2 retained in the body's bicarbonate pool (34). The rate of phenylalanine oxidation (µmol.kg−1 .h−1) was calculated from F13CO2 and urinary l-[1-13C]phenylalanine enrichment (25, 26).
Statistical analysis
Statistical analysis was performed on primary and derived variables. Data are expressed as means ± SDs. Differences in subject characteristics between men and women were assessed using 2-sample t test. Linear regression was used to assess the effect of phenylalanine intake on flux. The mean phenylalanine requirement was estimated by breakpoint analysis of the F13CO2 using a mixed model 2-phase linear regression crossover analysis (35). A stepwise partitioning of F13CO2 data between 2 separate regression lines allowed the creation of multiple breakpoint estimates. The model with the lowest standard error and highest coefficient of determination (R2) was chosen as the mean requirement (2). The 95% CI of the requirement estimate was determined using Fieller's theorem (2, 35). The requirement estimate was performed separately for men and women but was not different, so data were pooled for estimation of 1 group requirement. Once phenylalanine requirement was estimated per kilogram body weight, the derived estimate was converted to a total daily requirement by multiplying by each subject's weight and then dividing by fat-free mass to obtain the estimated requirement per kilogram of fat-free mass. A 2-sample t test was used to assess for differences between men and women. All statistical analyses were performed with SAS (SAS/STAT version 9.4; SAS Institute) for Windows. Statistical significance was established at P ≤ 0.05.
Estimated glomerular filtration rate (eGFR; a marker of kidney function) was calculated using the standard equation eGFR = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.209 × 0.993age × 1.018 [if woman] × 1.159 [if black] from the National Kidney Association website (https://www.kidney.org/professionals/KDOQI/gfr_calculator) (36).
Results
Subjects
Fourteen adults aged >65 y were recruited; 12 met the inclusion criteria, participated in the study (Figure 1, Table 1), and completed a total of 66 DAAO studies. All subjects had normal kidney function and fasting blood glucose concentrations (Table 1). Two subjects were on anti-hypertensive medications; 2 were on cholesterol-lowering medications; and 1 was taking a blood thinner, a medication to help with smoking cessation and hypothyroidism. One was taking strontium for improvement of bone density, and 2 women were on low-dose Premarin for management of postmenopausal symptoms. Eight subjects were on daily multivitamin and/or vitamin D, 3 were taking fish oil, and 2 were taking calcium and magnesium supplements. Each subject participated in at least 3 and at most 7 studies for a total of 31 DAAO studies in men, 35 in women, and a combined total of 66 DAAO studies (Figure 1). Subjects remained weight-stable during the course of the study, with no significant gain or loss of weight (P = 0.3; data not shown).
TABLE 1.
Characteristics of the older men and women who participated in the phenylalanine requirement study1
| Characteristics | Men | Women | P value |
|---|---|---|---|
| Participants, n | 6 | 6 | |
| Age, y | 70.8 ± 5.4 | 76.7 ± 7.0 | 0.14 |
| Weight, kg | 82.9 ± 17.4 | 63.6 ± 14.5 | 0.06 |
| Height, cm | 176.6 ± 5.9 | 158.3 ± 6.4 | <0.01 |
| BMI, kg/m2 | 26.4 ± 4.9 | 25.3 ± 4.4 | 0.67 |
| Fat-free mass, kg | 62.8 ± 9.9 | 44.8 ± 8.8 | <0.01 |
| Fat mass, kg | 20.1 ± 9.0 | 18.8 ± 6.8 | 0.78 |
| Fat mass, % | 23.5 ± 5.96 | 29.1 ± 5.8 | 0.13 |
| Fasting serum glucose, mmol/L | 5.2 ± 0.7 | 4.9 ± 0.3 | 0.18 |
| Serum creatinine, µmol/L | 72.5 ± 12.0 | 66.2 ± 7.7 | 0.15 |
| Serum urea, mmol/L | 5.4 ± 1.3 | 7.9 ± 2.4 | 0.02 |
| Estimated glomerular filtration rate, mL/min/1.732 | 91.2 ± 10.4 | 74.8 ± 12.8 | 0.08 |
| Resting energy expenditure, kcal/d | 1482 ± 137 | 1223 ± 285 | 0.08 |
Values are means ± SDs unless otherwise noted.
Phenylalanine flux
Phenylalanine flux (Table 2) for all subjects was 80.0 ± 49.9 µmol.kg−1 .h−1 (mean ± SD) and ranged from 21 to 221 µmol .kg−1 .h−1. There was no effect of phenylalanine intake on phenylalanine flux (P = 0.38). Men had higher flux compared with women (P = 0.02), and the effect of subject on flux was significant (P < 0.01) (Table 2).
TABLE 2.
Phenylalanine intake used in individual subjects and the effect of phenylalanine intake on phenylalanine flux in men and women aged >65 y1
| Subjects | Test phenylalanine intakes, mg .kg−1 .d−1 | Phenylalanine flux, µmol .kg−1 .h−1 |
|---|---|---|
| Men | ||
| 1 | 7.2, 8.5, 10, 25, 40 | 39.6 ± 14.6a |
| 2 | 9, 15.5, 8 | 23.4 ± 5.22a |
| 3 | 16, 37, 7.3 | 66.0 ± 27.2a |
| 4 | 7.3, 30, 12, 13, 9.3, 24 | 67.4 ± 40.18a |
| 5 | 38, 7.4, 25, 18.5, 15.4, 14, 32 | 138 ± 21.4b |
| 6 | 17, 8, 22, 36, 26, 15.2, 10.5 | 156 ± 32.4b |
| All | — | 94.5 ± 56.5 |
| Women | ||
| 7 | 39, 7.5, 15.6 | N/A |
| 8 | 9.4, 11.4, 25.4, 8.4, 7.4, 10.4, 38.4 | 43.2 ± 10.0a |
| 9 | 27, 19, 16, 7.6, 9.9, 36, 37.5 | 50.3 ± 14.5a |
| 10 | 7.2, 40, 17, 16.2 | 56.3 ± 13.1a |
| 11 | 7.5, 11, 9.5, 20, 37, 15.9, 28 | 44.7 ± 15.5a |
| 12 | 8.2, 18, 14.5, 13.5, 22, 21, 12 | 132 ± 21.7b |
| All | — | 66.1 ± 38.5* |
| Combined | — | 80.0 ± 49.9 |
Values are means ± SDs unless otherwise noted. Subjects participated in a range of phenylalanine intakes (7.2–40 mg .kg−1 .d−1); each subject participated in a minimum of 3 test intakes for a total of n = 66 studies. *Different from men, P = 0.02. Values with different superscript letters within each sex group were significantly different, P < 0.05; a < b. Post hoc analysis was performed using Bonferroni multiple comparison tests. N/A, not available—urine for participant 7 was not able to be collected because the subject had difficulty providing samples.
l-[1-13C]Phenylalanine oxidation
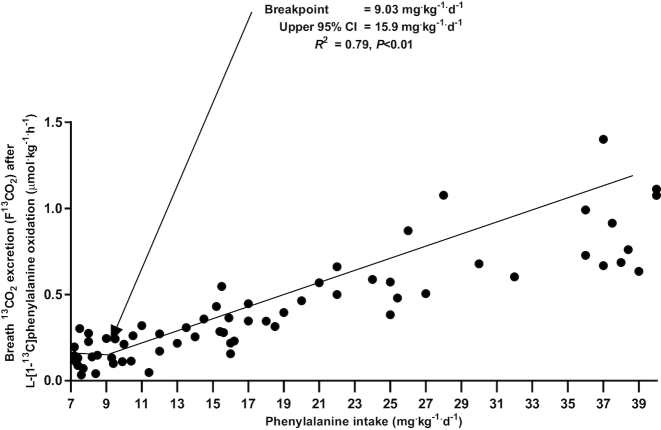
The rate of 13CO2 released from the oxidation of l-[1-13C]phenylalanine (F13CO2), which provided an estimate of the phenylalanine requirement, was analyzed separately for men and women. Breakpoints of 9.3 and 8.4 mg. kg−1 .d−1, which did not differ (P = 0.98), were calculated for men and women, respectively. Therefore, all data were combined for estimation of the phenylalanine requirement (Figure 3). The rate of 13CO2 released from the oxidation of l-[1-13C]phenylalanine (F13CO2) remained unchanged in response to phenylalanine intake up to 9.03 mg.kg−1.d−1 (Figure 3). Thereafter, additional increases in phenylalanine intakes resulted in a linear increase in F13CO2 values. This indicates that once the requirement of 9.03 mg .kg−1 .d−1 was met, additional increase in phenylalanine intake did not produce a further increase in phenylalanine uptake for protein synthesis. Biphase linear regression crossover analysis of the F13CO2 data resulted in the identification of a breakpoint for the mean phenylalanine requirement of 9.03 mg.kg−1 .d−1 (R2 = 0.79). The safe population intake estimated by determining the upper 95% CIs of the breakpoint was at 15.9 mg .kg−1 .d−1.
FIGURE 3.
Effect of phenylalanine intake on production of 13CO2 from phenylalanine oxidation (F13CO2) by DAAO in adults aged >65 y; n = 12 subjects, 66 DAAO studies. Biphase linear regression analysis of the F13CO2 data identified a breakpoint of 9.03 mg .kg−1 .d−1, which represents the estimated mean phenylalanine requirement (R2 = 0.79). The safe population intake estimated by determining the upper 95% CIs of the breakpoint was 15.9 mg .kg−1 .d−1. DAAO, direct amino acid oxidation.
Given that 3 subjects only completed 3 levels of intake, we also analyzed the data with the removal of each subject in a stepwise manner. There were 3 possible combinations for analysis. The 3 breakpoints generated were 9.6, 9.2, and 8.9 mg.kg−1 .d−1. When all 3 subjects were removed, the breakpoint was 10.3 mg .kg−1 .d−1. Given that all possible combinations fell within the 95% CI of the estimate when all data were included, the data were all used in the final analysis in Figure 3.
When phenylalanine requirement was calculated on the basis of fat-free mass, the mean requirement estimate was 11.9 ± 0.94 and 12.8 ± 0.94 mg.kg fat-free mass−1 .d−1 for men and women, respectively, but the results were not different (P = 0.11).
Discussion
The results of this study provide estimates of the mean and 95% CI (approximating the RDA) of phenylalanine requirement in adults aged >65 y of 9.03 and 15.9 mg .kg−1 .d−1, respectively. These estimates are very similar to the mean and 95% CI of 9.1 and 13.6 mg .kg−1 . d−1 previously derived in young adult men using the DAAO method (2). This is the first study to directly determine the requirement for an IAA in the elderly. Data on AA requirements of adults aged >65 y are lacking; hence, current recommendations (1, 37) are based on data obtained from studies conducted in young adults. These recommendations are predominantly based on studies conducted by our group and others using the IAAO and DAAO methods (1, 37). Differences exists in AA metabolism between older and young adults (16, 17), and higher splanchnic uptake of phenylalanine in elderly compared with young adults (16) has been taken as evidence of a potential higher requirement. The results from the current study demonstrate that at least for phenylalanine, this is not the case.
Results from physiological studies demonstrate that more protein is required by older adults to stimulate similar rates of muscle protein synthesis as those of young adults (18, 38). Because only dietary IAAs stimulate muscle protein synthesis (15), this observation provides evidence for potentially higher IAA requirements in elderly compared with young adults. Indeed, evidence from physiological studies points to a potentially higher requirement for leucine in the elderly compared with young adults (39, 40). In addition, evidence from Volpi et al. (16) of a higher splanchnic uptake of phenylalanine in the elderly begs the question as to whether the dietary requirement for this AA is increased. The results from the current study provide evidence that the dietary requirement for phenylalanine is not different between young and elderly humans.
An important observation from the current study is the large variation in the flux data and the lack of associations between phenylalanine intake and flux. This is in contrast to a previous study of phenylalanine requirement in young men by Zello et al. (2), in which the variation in phenylalanine flux was lower and phenylalanine intake had a significant effect on flux. There are several differences between the study by Zello et al. (2) and the current study. First, in the study by Zello et al. (2), each subject was studied at each of the same 7 levels of phenylalanine intake, whereas in the current study each subject received a different level of phenylalanine intake and no 2 subjects received the same level of phenylalanine intake. Second, Zello et al. (2) infused the isotope intravenously, whereas it was given orally in the current study. Oral administration of isotope produces more variation in the flux data (28) but does not affect the requirement estimate (31). Finally, Zello et al. (2) measured isotopic enrichment in plasma, whereas urine was used in the current study. Urinary enrichments are more variable than plasma enrichments (28). In addition, subjects in the current study had difficulty providing urine samples on demand. This might be 1 of the disadvantages of using urinary enrichments to calculate flux in elderly subjects and might suggest a need to take blood samples for plasma phenylalanine enrichments. The variability in flux, however, had no effect on the estimation of phenylalanine requirement, which was derived from the F13CO2 data (Figure 3), calculated from 13C breath enrichments and VCO2 (32).
The IAA phenylalanine (41, 42) is predominantly metabolized in the liver, and its main function is protein synthesis. Through its irreversible conversion to tyrosine (43), phenylalanine can provide the dietary needs for tyrosine and hence the total AAA requirement can be supplied as phenylalanine only, termed “maximum phenylalanine requirement” (7). Tyrosine in turn can spare a portion of the dietary phenylalanine requirement (2, 6, 44); therefore, providing an excess of tyrosine in studies of phenylalanine requirement (as was done in the current study) will provide the “minimum obligatory requirement” for phenylalanine (2). In the presence of excess tyrosine, phenylalanine intake in excess of that required for protein synthesis is preferentially oxidized without significant equilibration within the body's tyrosine pool (2, 30, 45). Thus, phenylalanine is a well-regulated AA with a relatively small pool size, which makes it an ideal AA for use as an indicator in studies of amino requirements using the IAAO method (46). An important criterion of the IAAO method is that the indicator should be fed close to its requirement, at which point it is most sensitive (46). Because no data exist on phenylalanine requirement in older adults, use of phenylalanine as an indicator AA in studies of AA requirements in older adults is limited. Given its significance as a sensitive indicator AA, knowledge of its requirement in older adults is a crucial starting point toward the understanding of IAA requirements in this population. Furthermore, the total AAA requirements (maximal phenylalanine requirement) in older adults will need to be determined as we have earlier reported in young adults (47, 48). These studies were conducted by IAAO using either a lysine tracer (49) or a leucine tracer (48), and similar results were obtained.
Aging is associated with a decrease is skeletal muscle mass (50) along with a decrease in its contribution to whole-body protein metabolism (10, 51, 52). At the same time, this is accompanied by an increased contribution of nonmuscle lean tissue to whole-body protein turnover (10, 52). In addition, older adults demonstrate anabolic resistance to IAAs (11), which potentially decreases the efficiency of utilization and hence increases the obligatory requirement. However, anabolic resistance and increased splanchnic uptake observed by Volpi et al. (16) do not translate into a higher requirement for phenylalanine in older (current study) compared with young adults (2). It is possible that the decrease in the contribution of skeletal muscle to whole-body AA metabolism (10) and the relative increase in the contribution of visceral tissue result in a different partitioning of AAs between those various tissues. Despite higher splanchnic extraction of phenylalanine in the study by Volpi et al. (16), muscle protein synthesis was not different between the young and the elderly. The AA dose ingested by subjects in the Volpi et al. (16) study was similar to doses used to stimulate maximal rates of muscle protein synthesis in the elderly (18). Therefore, another possibility should be considered—that a different AA rather than phenylalanine could be driving protein synthesis. Indeed, it is well documented that leucine is the most important stimulator of muscle protein synthesis (40, 53, 54). Because studies on AA requirements in older adults are lacking, there is currently no point of comparison, but the results of the current study open an important door for the use of phenylalanine for determination of the requirement of all other IAAs in the elderly.
The current recommendation for phenylalanine provides an estimate of total AAAs (phenylalanine and tyrosine) (1, 37). Because phenylalanine is the dietary IAA of the 2 AAAs and tyrosine is also provided in mixed diets, knowledge of the minimal phenylalanine requirement is arguably of greater nutritional importance in individuals with a functional pathway for tyrosine synthesis. In addition, because current evidence suggests that the IAAs (rather than the dispensable AAs) are the primary stimulus of muscle protein synthesis (15), knowledge of the phenylalanine requirement in the presence of preformed tyrosine is of greater physiological relevance.
Two of the women in the current study were on hormone replacement therapy (Premarin). Hormone replacement therapy is associated with decreased skeletal muscle protein breakdown and a more positive muscle protein balance (55), as well as increased bone density (56). However, it is not clear whether it affects AA requirements. The low dose (0.3–0.625 mg.d−1) taken by these women was to help alleviate rather than remove all menopausal symptoms. When both women were removed from the statistical analysis, the phenylalanine requirement remained exactly the same whether for women only or for men and women combined. Therefore, they were included in all further analysis. Further work is needed to assess the impact of hormone replacement therapy on protein and AA requirements in the elderly.
In conclusion, based on the results of the current study, the dietary requirements for phenylalanine (in the presence of excess tyrosine) of adults aged >65 y are 9.03 and 15.9 mg. kg−1 .d−1 (mean and RDA). These estimates are similar to previously derived estimates of 9.1 and 13.6 mg.kg−1 .d−1 in young adult men (2). The similarity in the whole-body phenylalanine requirement between the elderly in the current study and young men in the previous study (2) suggests that previously observed higher AA and/or protein (18, 57) needs of the elderly to stimulate similar rates of muscle protein synthesis as young adults are not driven by an increased requirement for phenylalanine.
Acknowledgments
The authors’ responsibilities were as follows—PBP, ROB, RE, and GC-M: designed the study; KEM and MR: conducted the study; MR and SS: analyzed the samples; MR and GC-M: performed the statistical analysis; KEM, PBP, ROB, RE, and GC-M: wrote the manuscript; GC-M: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by Canadian Institute of Health Research grant MOP-10321. Pfizer Consumer Healthcare donated the seniors' multivitamins. Mead Johnson Nutritionals donated the protein-free powder for experimental diets.
Author disclosures: KEM, PBP, MR, ROB, SS, RE, and GC-M, no conflicts of interest.
Abbreviations used: AA, amino acid; AAA, aromatic amino acid; CRC, Clinical Research Center; DAAO, direct amino acid oxidation; eGFR, estimated glomerular filtration rate; IAA, indispensable amino acid; IAAO, indicator amino acid oxidation; REE, resting energy expenditure.
References
- 1. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes: energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 2. Zello GA, Pencharz PB, Ball RO. Phenylalanine flux, oxidation, and conversion to tyrosine in humans studied with l-[1-13C]phenylalanine. Am J Physiol. 1990;259(6 Pt 1):E835–43. [DOI] [PubMed] [Google Scholar]
- 3. Roberts SA, Thorpe JM, Ball RO, Pencharz PB. Tyrosine requirement of healthy men receiving a fixed phenylalanine intake determined by using indicator amino acid oxidation. Am J Clin Nutr. 2001;73(2):276–82. [DOI] [PubMed] [Google Scholar]
- 4. Basile-Filho A, el-Khoury AE, Beaumier L, Wang SY, Young VR. Continuous 24-h l-[1-13C]phenylalanine and l-[3,3-2H2]tyrosine oral-tracer studies at an intermediate phenylalanine intake to estimate requirements in adults. Am J Clin Nutr. 1997;65(2):473–88. [DOI] [PubMed] [Google Scholar]
- 5. Rose WC, Leach BE, Coon MJ, Lambert GF. The amino acid requirements of man: IX. The phenylalanine requirement. J Biol Chem. 1955;213(2):913–22. [PubMed] [Google Scholar]
- 6. Rose WC, Wixom RL.. The amino acid requirements of man: XIV. The sparing effect of tyrosine on the phenylalanine requirement. J Biol Chem. 1955;217(1):95–101. [PubMed] [Google Scholar]
- 7. Pencharz PB, Hsu JW, Ball RO. Aromatic amino acid requirements in healthy human subjects. J Nutr. 2007;137(6 Suppl 1):1576S–8S.; discussion 97S-98S. [DOI] [PubMed] [Google Scholar]
- 8. Ramachandran R, Gravenstein KS, Metter EJ, Egan JM, Ferrucci L, Chia CW. Selective contribution of regional adiposity, skeletal muscle, and adipokines to glucose disposal in older adults. J Am Geriatr Soc. 2012;60(4):707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. [DOI] [PubMed] [Google Scholar]
- 10. Uauy R, Winterer JC, Bilmazes C, Haverberg LN, Scrimshaw NS, Munro HN, Young VR. The changing pattern of whole body protein metabolism in aging humans. J Gerontol. 1978;33(5):663–71. [DOI] [PubMed] [Google Scholar]
- 11. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. [DOI] [PubMed] [Google Scholar]
- 12. Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose–response study. J Physiol. 2003;552(Pt 1):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101(9):2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94(5):1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–20. [DOI] [PubMed] [Google Scholar]
- 17. Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65(2):489–95. [DOI] [PubMed] [Google Scholar]
- 18. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 19. Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86(2):451–6. [DOI] [PubMed] [Google Scholar]
- 20. Brookes IM, Owens FN, Garrigus US. Influence of amino acid level in the diet upon amino acid oxidation by the rat. J Nutr. 1972;102(1):27–35. [DOI] [PubMed] [Google Scholar]
- 21. el-Khoury AE, Fukagawa NK, Sanchez M, Tsay RH, Gleason RE, Chapman TE, Young VR. The 24-h pattern and rate of leucine oxidation, with particular reference to tracer estimates of leucine requirements in healthy adults. Am J Clin Nutr. 1994;59(5):1012–20. [DOI] [PubMed] [Google Scholar]
- 22. Durnin JV, Rahaman MM.. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21(3):681–9. [DOI] [PubMed] [Google Scholar]
- 23. Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985). 1986;60(4):1327–32. [DOI] [PubMed] [Google Scholar]
- 24. Roubenoff R, Baumgartner RN, Harris TB, Dallal GE, Hannan MT, Economos CD, Stauber PM, Wilson PW, Kiel DP. Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci. 1997;52(3):M129–36. [DOI] [PubMed] [Google Scholar]
- 25. Rafii M, Chapman K, Elango R, Campbell WW, Ball RO, Pencharz PB, Courtney-Martin G. Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J Nutr. 2016;146(4):681–7. [DOI] [PubMed] [Google Scholar]
- 26. Rafii M, Chapman K, Owens J, Elango R, Campbell WW, Ball RO, Pencharz PB, Courtney-Martin G. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J Nutr. 2015;145(1):18–24. [DOI] [PubMed] [Google Scholar]
- 27. Elango R, Humayun MA, Ball RO, Pencharz PB. Indicator amino acid oxidation is not affected by period of adaptation to a wide range of lysine intake in healthy young men. J Nutr. 2009;139(6):1082–7. [DOI] [PubMed] [Google Scholar]
- 28. Bross R, Ball RO, Pencharz PB. Development of a minimally invasive protocol for the determination of phenylalanine and lysine kinetics in humans during the fed state. J Nutr. 1998;128(11):1913–9. [DOI] [PubMed] [Google Scholar]
- 29. Food and Agriculture Organization/World Health Organization/United Nations University. Human energy requirements. Rome (Italy):Food and Agriculture Organization; 2001. [Google Scholar]
- 30. Shiman R, Gray DW.. Formation and fate of tyrosine: intracellular partitioning of newly synthesized tyrosine in mammalian liver. J Biol Chem. 1998;273(52):34760–9. [DOI] [PubMed] [Google Scholar]
- 31. Kriengsinyos W, Wykes LJ, Ball RO, Pencharz PB. Oral and intravenous tracer protocols of the indicator amino acid oxidation method provide the same estimate of the lysine requirement in healthy men. J Nutr. 2002;132(8):2251–7. [DOI] [PubMed] [Google Scholar]
- 32. Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of l-[1-3C]leucine. Am J Physiol. 1980;238(5):E473–9. [DOI] [PubMed] [Google Scholar]
- 33. Elango R, Humayun MA, Ball RO, Pencharz PB. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr. 2011;94(6):1545–52. [DOI] [PubMed] [Google Scholar]
- 34. Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol. 1989;257(3 Pt 1):E426–38. [DOI] [PubMed] [Google Scholar]
- 35. Seber G. Linear regression analysis. New York (NY): Wiley; 1977. [Google Scholar]
- 36. National Kidney Foundation. GFR calculator. New York (NY): National Kidney Foundation; 2019. [Google Scholar]
- 37. World Health Organization/ Food and Agriculture Organization/United Nations University. Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. WHO Technical Report Series. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 38. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302(8):E992–9. [DOI] [PubMed] [Google Scholar]
- 39. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. [DOI] [PubMed] [Google Scholar]
- 40. Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104(6):1594–606. [DOI] [PubMed] [Google Scholar]
- 41. Womack M, Rose WC.. The partial replacement of dietary phenylalanine by tyrosine for purposes of growth. J Biol Chem. 1946;166(2):429–34. [PubMed] [Google Scholar]
- 42. Rose WC, Womack M.. The utilization of the optical isomers of phenylalanine, and the phenylalanine requirement for growth. J Biol Chem. 1946;166(1):103–10. [PubMed] [Google Scholar]
- 43. Tessari P, Deferrari G, Robaudo C, Vettore M, Pastorino N, De Biasi L, Garibotto G. Phenylalanine hydroxylation across the kidney in humans rapid communication. Kidney Int. 1999;56(6):2168–72. [DOI] [PubMed] [Google Scholar]
- 44. Tolbert B, Watts JH.. Phenylalanine requirement of women consuming a minimal tyrosine diet and the sparing effect of tyrosine on the phenylalanine requirement. J Nutr. 1963;80(1):111–16. [DOI] [PubMed] [Google Scholar]
- 45. Moldawer LL, Kawamura I, Bistrian BR, Blackburn GL. The contribution of phenylalanine to tyrosine metabolism in vivo: studies in the post-absorptive and phenylalanine-loaded rat. Biochem J. 1983;210(3):811–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zello GA, Wykes LJ, Ball RO, Pencharz PB. Recent advances in methods of assessing dietary amino acid requirements for adult humans. J Nutr. 1995;125(12):2907–15. [DOI] [PubMed] [Google Scholar]
- 47. Arentson-Lantz E, Clairmont S, Paddon-Jones D, Tremblay A, Elango R. Protein: a nutrient in focus. Appl Physiol Nutr Metab. 2015;40(8):755–61. [DOI] [PubMed] [Google Scholar]
- 48. Nosworthy MG, Neufeld J, Frohlich P, Young G, Malcolmson L, House JD. Determination of the protein quality of cooked Canadian pulses. Food Sci Nutr. 2017;5(4):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsu JW, Goonewardene LA, Rafii M, Ball RO, Pencharz PB. Aromatic amino acid requirements in healthy men measured by indicator amino acid oxidation. Am J Clin Nutr. 2006;83(1):82–8. [DOI] [PubMed] [Google Scholar]
- 50. Bales CW, Ritchie CS.. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–23. [DOI] [PubMed] [Google Scholar]
- 51. Morais JA, Gougeon R, Pencharz PB, Jones PJ, Ross R, Marliss EB. Whole-body protein turnover in the healthy elderly. Am J Clin Nutr. 1997;66(4):880–9. [DOI] [PubMed] [Google Scholar]
- 52. Morais JA, Ross R, Gougeon R, Pencharz PB, Jones PJ, Marliss EB. Distribution of protein turnover changes with age in humans as assessed by whole-body magnetic resonance image analysis to quantify tissue volumes. J Nutr. 2000;130(4):784–91. [DOI] [PubMed] [Google Scholar]
- 53. Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575(Pt 1):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. 2018;148(7):1088–95. [DOI] [PubMed] [Google Scholar]
- 55. Hansen M. Female hormones: do they influence muscle and tendon protein metabolism?. Proc Nutr Soc. 2018;77(1):32–41. [DOI] [PubMed] [Google Scholar]
- 56. Shea KL, Gavin KM, Melanson EL, Gibbons E, Stavros A, Wolfe P, Kittelson JM, Vondracek SF, Schwartz RS, Wierman ME et al.. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18–23. [DOI] [PubMed] [Google Scholar]