Abstract
Genetic predisposition might affect neurodevelopmental outcomes of prenatal methylmercury exposure. We examined suspected heterogeneities for modification of exposure-related neurodevelopment in children from the Avon Longitudinal Study of Parents and Children (1991–2000), Bristol, United Kingdom. A subgroup (n = 1,127 from a pilot study and 1,045 from the present study) was identified based on the availability of the mercury concentration of cord tissue as a measure of prenatal methylmercury exposure, data on 247 single-nucleotide polymorphisms (SNPs), and Wechsler Intelligence Scale for Children intelligence quotient (IQ) scores. Log10-transformed mercury concentration was positively associated with IQ, but adjustment for confounding cofactors attenuated this association. A finding of enhanced interaction with methylmercury was replicated in this study for the minor allele of rs1042838 (progesterone receptor) (β = −11.8, 95% confidence interval: −23.0, −0.6; P for interaction = 0.004) and weakly for rs662 (paraoxonase 1) (β = −3.6, 95% confidence interval: −11.4, 4.3; P = 0.117). In the joint sample, new interacting single-nucleotide polymorphisms were discovered in relation to superoxide dismutase 2, ATP binding cassette subfamily A member 1, and metallothionein 1M genes. While the low-level prenatal exposure to methylmercury was not associated with child cognition, progesterone receptor rs1042838 minor alleles revealed a negative association of mercury exposure with IQ.
Keywords: ALSPAC, cognitive functions, genes, mercury, neuropsychological development, population-based birth cohort, pregnancy, SNPs
Methylmercury exposure can impair neurodevelopment, mainly during prenatal stages, potentially leading to permanent cognitive deficits (1–3). Prevention of developmental exposure to methylmercury aims at protecting against doses that are associated with declines in average cognitive performance (4), but recent studies suggest that common genetic heterogeneities could affect neurotoxic responses (5–7). In a pilot study of 1,127 members of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, Bristol, United Kingdom, we identified 4 single-nucleotide polymorphisms (SNPs) in genes for brain-derived neurotrophic factor (BDNF), paraoxonase 1 (PON1), transferrin (TF), and progesterone receptor (PGR), where presence of the minor allele was associated with greater methylmercury-linked cognitive deficits at the low background exposure of this population (8). The study considered 247 candidate SNPs involved in the potential biological pathways of prenatal methylmercury neurotoxicity, including those implicated in brain development, neurotransmitter metabolism, cholesterol metabolism, iron regulation, and peroxidative defense (8).
In order to determine the overall impact of genetic predisposition on methylmercury neurotoxicity, we have now obtained data from 1,045 additional cohort members. We first sought replication of the potential modifying effect of the 4 SNPs previously identified and then explored the possible impact of other candidate SNPs in the overall sample (n = 2,172).
Seafood intake is the main source of methylmercury exposure (9, 10), but particularly fatty fish is rich in essential nutrients, such as long chain fatty acids (PUFAs), selenium, and vitamin D, that are necessary for neurodevelopment (11). These potentially beneficial factors are related to the frequency of seafood intake and thus tend to correlate with methylmercury exposure, thereby potentially exerting a confounding role on the methylmercury–cognitive function association (12). Thus, when statistically controlling for these confounding factors (13), adverse methylmercury-outcome associations might become stronger (9, 14).
In the present study, we analyzed the impact of relevant SNPs on the association between prenatal methylmercury exposure and child cognitive deficits at age 8 years while considering the potential confounding role of maternal socioeconomic position and seafood diets during pregnancy.
METHODS
Participants
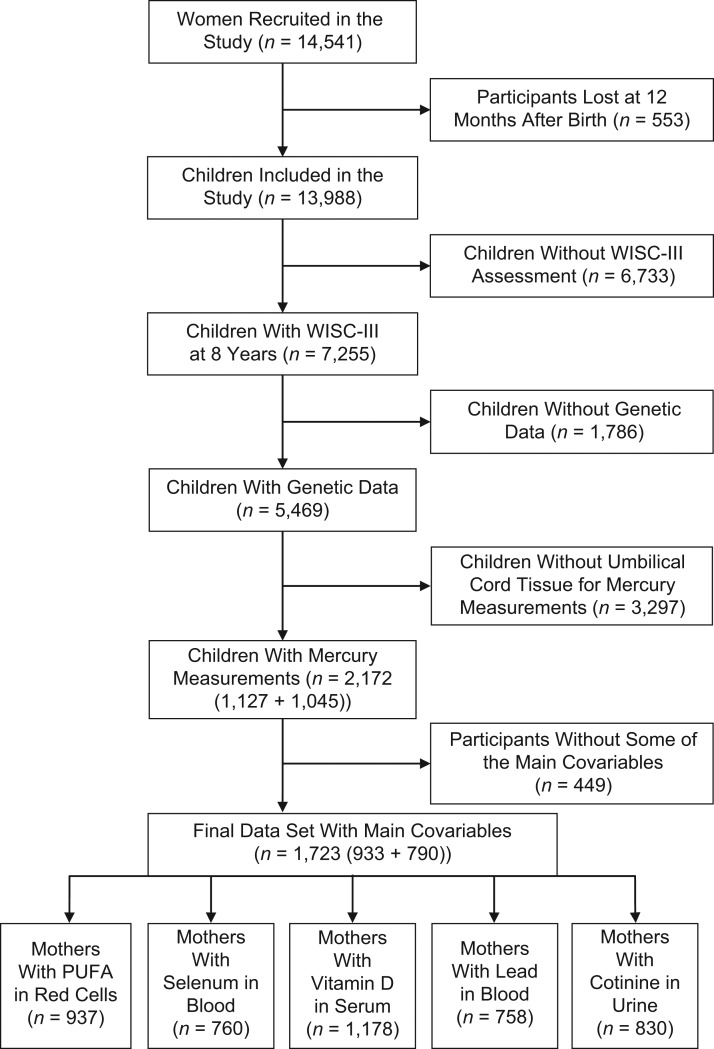
ALSPAC is an ongoing longitudinal cohort study designed to investigate the determinants of development, health, and disease during childhood and beyond (15–17). Pregnant women, residing in the former Avon health authority area in Southwest England, with an expected delivery date between April 1, 1991, and December 31, 1992, were eligible to participate in the study. A cohort of 14,541 pregnant women was established, resulting in 13,988 children who were alive at 12 months of age. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees. The study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (18). Based on the availability of SNPs and an intelligence quotient (IQ) score, biobanked slices of umbilical cords were analyzed for the total mercury concentration as a measure of the prenatal methylmercury concentration (19). A total of 2,172 cohort members were included, of whom 1,127 belonged to the pilot study (8), while an additional 1,045 became available for the present study. All subjects had available data on SNPs within relevant genes (8), as well as IQ scores at age 8 years. Final models with the covariate variables included 1,723 complete cases, of which 933 originated from the pilot study and 790 were newly identified. See Figure 1 for a flow chart of participants in this study. Inverse-probability weighting, to control for potential selection bias induced by restricting the analysis to complete cases (i.e., individuals with no missing values) (20), was applied as sensitivity analyses that are shown in Web Tables 1–5 (available at https://academic.oup.com/aje).
Figure 1.
Main phases of the study and flowchart of the population, Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000. PUFA, polyunsaturated fatty acid; WISC-III, Wechsler Intelligence Scale for Children, Third Edition.
Mercury measurement
Cord samples were taken by the midwife at birth and frozen at −20°C. Samples were defrosted briefly to divide them into several 1-cm slices, which were then stored at −20°C. After freeze-drying the cord tissue slices, the total mercury dry-weight concentration was determined using a direct mercury analyzer (DMA-80; Milestone, Inc., Shelton, Connecticut) at the University of Southern Denmark (8). The dry-weight measure is known to have an appropriate precision (21).
SNP genotyping
We chose candidate genes belonging to pathways considered of possible relevance after systematically reviewing the literature. Gene candidates were selected based on their possible role in pathways associated with methylmercury toxicity (8). They relate to 4 major biological pathways considered important to neurodevelopment or metal neurotoxicity: 1) brain development and neurotransmitter metabolism, 2) cholesterol metabolism, 3) iron regulation, and 4) peroxidative defense. We selected polymorphisms with a minor allele frequency of at least 10%, and the SNPs chosen have been previously described (8).
ALSPAC children were genotyped using the Illumina HumanHap550 (Illumina, Inc., San Diego, California) quadchip genotyping platform. Standard quality-control methods were performed and have been previously described in the scientific literature (22). Genotypic data were subsequently imputed using MACH (23) and phased haplotype data from HapMap CEU (r22) (24). Data from genotyped and imputed SNPs (using the most likely genotype) were extracted for 247 SNPs.
Neuropsychological data
When the children were 8 years of age, a short form of the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) (25), was used to assess IQ. Using lookup tables provided in the WISC-III manual, age-scaled scores were obtained from the raw scores for each subtest, and total, performance, and verbal IQ scores were calculated. The mean age at assessment was 8.5 years (standard deviation, 0.3 years). The testers were trained psychologists who were overseen by a senior psychologist with long experience of psychometric testing within the study. She observed each tester, met with the group regularly to discuss the precise administration of each subtest, and checked their scoring (26, 27)
Selected covariate variables
We used the same covariates as in our pilot study (8). Briefly, the following variables were taken into account in the associative models between the exposure and outcome: sex, age at WISC-III assessment, and WISC-III examiner. Several covariates were retained in the final model because of prior knowledge that they were related to the exposure. Omega-3 fatty acid intake estimates were derived from a food-frequency questionnaire on seafood consumption during pregnancy (26, 28). Maternal age, maternal smoking during pregnancy, parity, house ownership status, parental education, and social class were recorded during pregnancy. The final models previously published (8) also adjusted for the healthy component of the diet during pregnancy and estimated the children’s processed component of the diet at age 8 years. For this study, these 2 covariate variables were excluded due to incomplete observations (lacking data: pilot study n = 90; extended study n = 137) and marginal impact on the final models (shown as secondary analyses). Allowing inclusion of these subjects caused only slight changes in the findings, compared with pilot sample analyses previously published (8). The extended set of samples showed higher prevalence of lower maternal social class than the pilot subsample (data not shown), and adjustment for maternal social class was retained in all models. Furthermore, in sensitivity analyses, we included biomarkers related to seafood intake and other pollutants: maternal whole blood selenium concentration during pregnancy (29), maternal blood lead concentration during pregnancy (30), maternal serum 25(OH)D concentration (reflecting both vitamin D2 and vitamin D3) during pregnancy (31), and maternal urine cotinine concentration during pregnancy (32). Maternal long-chain fatty acid concentrations in red-cell membranes from blood samples during pregnancy (33), docosahexaenoic acid and arachidonic acid, were measured in a subgroup and included in sensitivity analyses. Figure 1 shows numbers of participants with these variables available.
Statistical analysis
Except when attempting to replicate the findings for the 4 SNPs identified in the pilot study (8), all quantitative analyses were based on complete data sets comprising both subsamples. Mercury concentrations were normally distributed after log10 transformation, and crude and adjusted linear regressions were therefore used to assess the relationship between methylmercury exposure and child WISC-III outcomes.
Eleven SNPs were removed due to (unexpected) low minor allele frequency (<10%) or poor imputation quality (R2 < 0.8) so that the genetic analyses ultimately included 236 SNPs. The 4 SNPs identified in the previous study, TF rs3811647, PON1 rs662, BDNF rs2049046, and PGR rs1042838, were first examined for possible replication in the extended sample. As a heuristic, in the total sample, the SNPs were scanned for “main effects” using a nominal selection level for further investigation of a P value ≤ 0.05 for association with child IQ outcomes and methylmercury exposure. The “main effects” were assessed using crude linear regression models assuming an additive mode of inheritance (e.g., genotypes coded as 0, 1, 2). A total of 32 of the 236 SNPs passing this threshold were then further analyzed in an interaction model adjusting for the covariate variables. When testing interactions between SNPs and methylmercury, multiple comparisons were addressed by correcting nominal P values using Bonferroni criteria (0.05/(32 SNPs) = 0.0016). ALSPAC genetic ancestry data was introduced in the final models to adjust for any ancestry confounding (34). Finally, inverse-probability weighting was applied in sensitivity analyses (20). All analyses were performed using Stata, release 12 (StataCorp LLC, College Station, Texas).
RESULTS
The overall mercury mean concentration in umbilical cord was 25.2 (standard deviation, 12.7) ng/g dry weight. This corresponds to a cord-blood mercury concentration of 2.70 μg/L (19). The mercury concentration showed associations with the study covariate variables similar to the previous study, and adding 1,045 cases did not change the previous descriptive results. Higher strata of maternal social class and higher levels of omega-3 fatty acid intake during pregnancy remained reliably associated with cord-mercury concentrations (Web Table 1). The Spearman correlation coefficient between the mercury concentration and the calculated omega-3 fatty acid intake from seafood consumption during pregnancy was ρ = 0.45; it was ρ = 0.26 between the mercury concentration and the ratio between docosahexaenoic acid and arachidonic acid concentrations in maternal pregnancy red-cell membranes.
Crude mercury associations with WISC-III outcomes showed positive coefficients that were very substantially attenuated by adjustment for sociodemographic and omega-3 fatty acid intake variables. This association pattern was similar in the extended and joint sample analyses (Table 1). No changes were observed if we included maternal red-cell membrane docosahexaenoic acid–arachidonic acid concentration ratio in the models (data not shown). The final models for the joint sample stratified by maternal social class showed an interaction in which higher social-class strata showed the expected negative coefficient for the mercury association with the child’s performance IQ (Web Table 2).
Table 1.
Adjusted Regression Coefficients for Cord-Mercury Concentration as a Predictor of 8-Year Psychometric Outcomes in the New and Combined Subgroups, Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000
| WISC-III Score | Model 1a | Model 2b | Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | βd | 95% CId | No. | βd | 95% CId | No. | βd | 95% CId | |
| Extended sample (new group) | 1,051 | 795 | 792 | ||||||
| Total IQ | 11.6 | 6.6, 16.4 | 3.5 | −2.0, 9.0 | 2.6 | −3.4, 8.6 | |||
| Verbal IQ | 12.8 | 7.9, 17.8 | 3.2 | −2.3, 8.8 | 1.9 | −4.1, 7.9 | |||
| Performance IQ | 7.2 | 2.1, 12.3 | 2.8 | −3.3, 8.8 | 2.6 | −4.0, 9.1 | |||
| Joint sample (pilot group + new group) | 2,281 | 1,813 | 1,808 | ||||||
| Total IQ | 12.1 | 8.9, 15.2 | 3.0 | −0.4, 6.4 | 2.6 | −1.1, 6.4 | |||
| Verbal IQ | 13.3 | 10.1, 16.6 | 3.4 | −0.1, 6.9 | 2.9 | −0.9, 6.8 | |||
| Performance IQ | 7.5 | 4.2, 10.8 | 1.8 | −2.0, 5.5 | 1.6 | −2.5, 5.7 | |||
| Joint sample complete cases | 1,808 | 1,808 | 1,808 | ||||||
| Total IQ | 12.1 | 8.6, 15.6 | 3.0 | −0.4, 6.5 | 2.6 | −1.1, 6.4 | |||
| Verbal IQ | 12.9 | 9.2, 16.5 | 3.3 | −0.2, 6.8 | 2.9 | −0.9, 6.8 | |||
| Performance IQ | 8.0 | 4.3, 11.8 | 1.8 | −1.9, 5.6 | 1.6 | −2.6, 5.7 | |||
Abbreviations: CI, confidence interval; IQ, intelligence quotient; WISC-III, Wechsler Intelligence Scale for Children, Third Edition.
a Adjusted for sex, age, and examiner. These analyses included subjects without genetic data, resulting in a slightly larger sample size than the genetic data analyses.
b Additionally adjusted for parental education level, maternal age, smoking during pregnancy, social class, parity, and house ownership status.
c Additionally adjusted for maternal estimated omega-3 intake (omega-3 intake estimated from seafood intake).
d β and 95% CI for log10(cord-mercury (ng/g)).
Sensitivity analyses of the mercury and WISC-III outcome associations are shown in Table 2. The full models in Table 1 additionally and separately adjusted for several biomarkers: pregnancy blood selenium, blood lead, serum vitamin D, and urine cotinine concentrations. No material changes were observed in the results after inclusion of these covariate variables. Due to a high degree of missingness of these new covariables, we applied corrections by inverse-probability weighting, and the results were unchanged (Web Table 3).
Table 2.
Adjusted Regression Coefficients for Cord-Mercury Concentration as a Predictor of the 8-Year Psychometric Outcomes in the Combined Group in Sensitivity Analyses for Maternal Selenium, Lead, Vitamin D, and Cotinine Concentration During Pregnancy, Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000
| Measure | WISC-III Score | ||||||
|---|---|---|---|---|---|---|---|
| Total IQ | Verbal IQ | Performance IQ | |||||
| No. | βa | 95% CIa | βa | 95% CIa | βa | 95% CIa | |
| Selenuimb | |||||||
| Model 1c | 760 | −0.7 | −6.5, 5.1 | −0.8 | −6.8, 5.2 | −0.7 | −7.2, 5.8 |
| Model 2d | 760 | −0.3 | −6.2, 5.6 | 0.1 | −6.1, 6.1 | −1.1 | −7.7, 5.5 |
| Leadb | |||||||
| Model 1c | 758 | −0.7 | −6.6, 5.1 | −0.8 | −6.8, 5.2 | −0.8 | −7.3, 5.7 |
| Model 3e | 758 | −1.0 | −6.8, 4.9 | −1.1 | −7.1, 4.9 | −0.9 | −7.4, 5.6 |
| Vitamin D totalb | |||||||
| Model 1c | 1,178 | 0.9 | −3.7, 5.5 | 1.4 | −3.3, 6.1 | 0.3 | −4.8, 5.4 |
| Model 4f | 1,178 | 1.0 | −3.7, 5.6 | 1.4 | −3.3, 6.1 | 0.5 | −4.6, 5.6 |
| Cotinineb | |||||||
| Model 1c | 830 | −1.5 | −7.0, 4.0 | −1.9 | −7.6, 3.8 | −0.7 | −7.0, 5.5 |
| Model 5g | 830 | −1.5 | −7.0, 4.0 | −1.9 | −7.6, 3.8 | −0.7 | −7.0, 5.6 |
Abbreviations: CI, confidence interval; IQ, intelligence quotient; WISC-III, Wechsler Intelligence Scale for Children, Third Edition.
a β and 95% CI for log10(cord-mercury (ng/g)).
b Selenium (median, 111 ug/l); lead (median, 3 ug/L); vitamin D total (median, 64 nmol/L); cotinine (median, 27 ng/mL).
c Basic model adjusted for sex, age, examiner, parental education level, maternal age, smoking during pregnancy (except for cotinine models), social class, parity, house ownership status, and estimated omega-3 intake (omega-3 intake estimated from seafood intake).
d Additionally adjusted for maternal selenium blood concentration during pregnancy.
e Additionally adjusted for maternal lead blood concentration during pregnancy.
f Additionally adjusted for maternal total vitamin D blood concentration during pregnancy.
g Additionally adjusted for maternal cotinine urine concentration during pregnancy.
Mercury concentrations for selected child genotypes are shown in Table 3. The first 4 SNPs were previously selected in the pilot study on gene-mercury interactions and child IQ, while the additional SNPs emerged after examination of the combined sample. No SNP showed a clear association with mercury concentrations. Likewise, no SNP was associated with omega-3 fatty acid intake from seafood consumption during pregnancy (data not shown).
Table 3.
Cord-Mercury Concentrations According to Selected Child Genotypes in the Pilot and the Combined Groups, Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000
| Gene | SNP | Major/Minor Allele | Cord-Mercury Concentration, ng/g | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Homozygous for the Major Allele | Heterozygous | Homozygous for the Minor Allele | |||||||
| No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | ||||
| Pilot SNPs (Discovery From Pilot Sample Analyses) | |||||||||
| TF | rs3811647 | G/A | 1,004 | 26 (18) | 1,010 | 25 (13) | 256 | 24 (11) | 0.17 |
| PON1 | rs662 | T/C | 1,180 | 25 (12) | 929 | 25 (19) | 161 | 24 (13) | 0.73 |
| BDNF | rs2049046 | T/A | 679 | 26 (18) | 1,121 | 25 (12) | 470 | 25 (18) | 0.47 |
| PGR | rs1042838 | G/T | 1,598 | 25 (16) | 612 | 25 (12) | 60 | 27 (12) | 0.70 |
| New SNPs (Discovery From Joint Sample Analyses) | |||||||||
| SOD2 | rs5746136 | G/A | 1,081 | 25 (18) | 993 | 25 (13) | 196 | 25 (12) | 0.82 |
| ABCA1 | rs4149268 | C/T | 931 | 25 (12) | 1,016 | 26 (18) | 323 | 24 (12) | 0.41 |
| ABCA1 | rs3890182 | G/A | 1,659 | 26 (16) | 568 | 25 (13) | 43 | 24 (11) | 0.33 |
| MT1M | rs2270836 | C/T | 842 | 26 (19) | 1,029 | 25 (13) | 370 | 25 (11) | 0.50 |
Abbreviations: ABCA1, ATP binding cassette subfamily A member 1; BDNF, brain-derived neurotrophic factor; Hg, mercury; MT1M, metallothionein 1M; PGR, progesterone receptor; PON1, paraoxonase 1; SD, standard deviation; SNP, single nucleotide polymorphism; SOD2, superoxide dismutase 2; TF, transferrin.
a One-way analysis of variance (ANOVA) P value between cord-slice Hg concentration and the SNP variants.
The results in Table 4 show gene-environment interaction analyses—within the pilot, extended, and joint sample materials—of mercury associations with the WISC-III outcomes for the 4 genotypes previously identified (8). PGR rs1042838 presented a similar interaction pattern to that previously reported. PON1 rs662 showed a similar but weaker interaction pattern. TF rs3811647 and BDNF rs2049046 associations were not replicated in this extended sample. The P-for-interaction value for PGR rs1042838 even passed a Bonferroni correction in the joint sample. Furthermore, as shown in Table 5, apparently novel findings were identified in the joint sample: superoxide dismutase 2 (SOD2) rs5746136, ATP binding cassette subfamily A member 1 (ABCA1) rs4149268, ABCA1 rs3890182, and metallothionein 1M (MT1M) rs2270836 tended to show major allelic variants with lower or negative mercury coefficients in the stratification analyses, in contrast to the minor alleles showing negative coefficients in the heterogeneities first identified.
Table 4.
Adjusted Regression Coefficientsa for the Cord-Mercury Concentration (ng/g) as a Predictor of Intelligence Quotient According to Selected Genotypes (Pilot, Extended, and Combined Groups), Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000
| Gene | Log10(Hg), Pilot Sampleb | Log10(Hg), Extended Sample | Log10(Hg), Joint Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | β | 95% CI | P for Interaction | No. | β | 95% CI | P for Interaction | No. | β | 95% CI | P for Interaction | |
| rs3811647 (TF) 11 | 402 | 4.8 | −2.8, 12.3 | 0.142 | 358 | 6.7 | −2.4, 15.8 | 0.288 | 760 | 6.2 | 0.4, 11.9 | 0.672 |
| rs3811647 (TF) 12 | 427 | 2.8 | −5.3, 10.9 | 341 | −4.7 | −14.1, 4.7 | 768 | 0.9 | −5.1, 7.0 | |||
| rs3811647 (TF) 22 | 104 | −7.1 | −24.5, 10.3 | 91 | 5.9 | −12.4, 24.2 | 195 | 0.9 | −11.3, 13.1 | |||
| rs662 (PON1) 11 | 494 | 7.5 | 0.5, 14.5 | 0.156 | 404 | 8.6 | −1.0, 18.2 | 0.117 | 898 | 7.8 | 2.2, 13.3 | 0.054 |
| rs662 (PON1) 12+22c | 439 | −2.3 | −10.0, 5.5 | 386 | −3.6 | −11.4, 4.3 | 825 | −2.2 | −7.7, 3.3 | |||
| rs1042838 (PGR) 11 | 648 | 6.7 | 0.4, 12.9 | 0.049 | 549 | 7.0 | −0.3, 14.3 | 0.004 | 1,197 | 7.0 | 2.3, 11.7 | 0.001 |
| rs1042838(PGR) 12+22c | 285 | −5.3 | −14.9, 4.3 | 241 | −11.8 | −23.0, −0.6 | 526 | −7.0 | −14.1, 0.0 | |||
| rs2049046 (BDNF) 11 | 274 | 8.2 | −1.9, 18.3 | 0.446 | 240 | 0.8 | −9.5, 11.1 | 0.653 | 514 | 4.8 | −2.3, 11.9 | 0.986 |
| rs2049046 (BDNF) 12 | 473 | 3.6 | −3.8, 10.9 | 381 | −0.1 | −9.7, 9.6 | 854 | 3.1 | −2.7, 8.9 | |||
| rs2049046 (BDNF) 22 | 186 | −6.2 | −17.7, 5.2 | 169 | 7.8 | −4.5, 20.1 | 355 | 1.4 | −6.7, 9.5 | |||
Abbreviations: BDNF, brain-derived neurotrophic factor; CI, confidence interval; Hg, mercury; PGR, progesterone receptor; PON1, paraoxonase 1; TF, transferrin.
a All multivariate linear regression models adjusted for sex, age, examiner, parental education level, maternal age, smoking during pregnancy, social class, parity, house ownership status, and estimated omega-3 intake (omega-3 intake estimated from seafood intake).
b Results from the pilot sample were slightly different from previous published results (8), due to adding more observations here but not due to adding a different confounding structure (data not shown).
c The alleles 12 and 22 were combined into a unique category due to low number of observations (22 alleles <10% of the total sample).
Table 5.
Adjusted Regression Coefficientsa for the Cord-Mercury Concentration (ng/g) as a Predictor of Total Intelligence Quotient According to Selected Genotypes, From a Joint Sample, Avon Longitudinal Study of Parents and Children, United Kingdom, 1991–2000
| Genotype | Log10(Cord-Slice Hg, ng/g) | |||
|---|---|---|---|---|
| No. | β | 95% CI | P for Interaction | |
| Cases with WISC-III IQ score available | 1,723 | |||
| New SNPs (discovery from joint sample analyses) | ||||
| rs5746136 (SOD2) 11 | 797 | 0.9 | −4.5, 6.3 | 0.082 |
| rs5746136 (SOD2) 12+22b | 926 | 5.5 | −0.2, 11.2 | |
| rs4149268 (ABCA1) 11 | 695 | −4.4 | −10.7, 1.8 | 0.049 |
| rs4149268 (ABCA1) 12 | 764 | 6.2 | 0.4, 12.0 | |
| rs4149268 (ABCA1) 22 | 264 | 9.2 | −1.6, 19.9 | |
| rs3890182 (ABCA1) 11 | 1,257 | 1.2 | −3.4, 5.8 | 0.038 |
| rs3890182 (ABCA1) 12+22b | 466 | 8.4 | 0.8, 16.1 | |
| rs2270836 (MT1M) 11 | 645 | −1.2 | −7.4, 5.1 | 0.054 |
| rs2270836 (MT1M) 12 | 777 | 5.1 | −0.8, 11.0 | |
| rs2270836 (MT1M) 22 | 281 | 10.3 | −0.1, 20.8 | |
Abbreviations: ABCA1, ATP binding cassette subfamily A member 1; CI, confidence interval; Hg, mercury; IQ, intelligence quotient; MT1M, metallothionein 1M; SOD2, superoxide dismutase 2; WISC-III, Wechsler Intelligence Scale for Children, Third Edition.
a All multivariable linear regression models adjusted for sex, age, examiner, parental education level, maternal age, smoking during pregnancy, social class, parity, house ownership status, and estimated omega-3 intake (omega-3 intake estimated from seafood intake).
b The alleles 12 and 22 were combined into a unique category due to low number of observations (22 alleles <10% of the total sample).
All the findings presented in Tables 4 and 5 were unchanged after adjusting for ALSPAC genetic ancestry (data not shown). The same models were repeated for the joint samples with inverse-probability weighting corrections, and again the results were unchanged (see Web Table 4). We further observed a linear and monotonic dose-response pattern with the mercury–total IQ association from PGR variants in generalized additive models (see Web Figure 1). Furthermore, no changes were observed with the inclusion of additional covariates, such as maternal healthy diet score during pregnancy and child processed diet score (Web Table 5).
DISCUSSION
In this extended subgroup within the ALSPAC prospective cohort study, the mercury concentration in cord tissue was not associated with 8-year cognitive development. Substantial attenuation of the positive coefficient was observed after adjustment for maternal social class and estimated omega-3 fatty acid intake from seafood during pregnancy, and the small, residual positive association could well be null or inverse if plausible levels of measurement error in the confounders and exposure were assumed (35). No substantial change in the association was observed after adjustment for maternal pregnancy selenium and vitamin D concentrations. Because fish intake is the main human source of methylmercury exposure (36), the low exposure levels might be a result of low-frequency fish intake in the ALSPAC cohort, in accord with the food habits of England’s general population (27). Studies on prenatal exposure to methylmercury have observed stronger adverse associations with mercury after statistically controlling for the opposing associations with fish intake and omega-3 fatty acids (8, 9, 13, 37). Other nutrients linked to fish intake, such as selenium and vitamin D, might confer healthy neurodevelopment (11, 38–40), but they did not appear to affect mercury-related cognitive deficits, as previously reported for selenium (13, 41, 42). Likewise, no change in the methylmercury coefficient was observed after adding biomarkers of other neurotoxicant exposures, such as lead in blood and urine-cotinine during pregnancy. The low seafood intake and the smaller sample size for these additional biomarker measurements might have prevented us from detecting any potential inverse confounding. Indeed, the biomarker analyses are not fully comparable, because samples with available biomarkers overlapped only little.
In relation to the genetic predisposition analyses, PGR rs1042838 SNPs findings were clearly replicated in the extended data set, and PON1 rs662 received modest additional supportive evidence. New SNP results suggested additional interactions with methylmercury exposure and cognitive development in the joint sample (i.e., children with wild-type genetic variants of SOD2 rs5746136, ABCA1 rs4149268 and rs3890182, and MT1M rs2270836 showed greater vulnerability to methylmercury neurotoxicity). Thus, children with these variants showed evidence of methylmercury neurotoxicity that was not detectable in the cohort sample as a whole (i.e., without taking genetic predisposition into account).
The PROGINS-haplotype variant PGR rs1042838 is plausible as an indicator of greater vulnerability to methylmercury exposure. A biological explanation might be that progesterone appears to act as a neuroprotector. Thus, PROGINS-variant carriers tend to have more neurological problems along with lower progesterone levels (43). Specifically, rs1042838 minor variant carriers might impair PGR protein functions by reducing the transcription or signaling of the PGR gene, thereby decreasing the effect of progesterone. Moreover, progesterone acts to oppose the effect of estrogen on glutamate homeostasis disruption (43, 44). Indeed, methylmercury is considered a metalloestrogen (45); in this sense, the rs1042838 minor variant carriers might be more vulnerable to mercury toxicity by increasing estrogen levels, on the one hand, and showing a reduced protective effect of progesterone on the glutamate homeostasis on the other. Glutamate is an important neurotransmitter involved in high-order cognitive functions, such as learning and memory, in the brain (46). However, there is a need to further understand the effects of the PGR rs1042838 modification with methylmercury and other environmental exposures. Of note, some recent studies detected an increased risk of breast cancer and endometrial cancer among rs1042838 minor variant carriers (44, 47), but they did not assess any potential effect modification by environmental factors.
The PON1 rs662 association was weakly replicated with a dominant model in the joint sample analyses. Other PON1 SNPs conferring predisposition to methylmercury neurotoxicity have also been reported (48). PON1 codes for an enzyme that inhibits oxidation of lipoproteins. Such oxidative damage might be induced by methylmercury (8, 48). A recent study found prenatal methylation changes to be associated with prenatal mercury and cognitive performance with PON1 (49). Because BDNF SNP (rs2049046) and TF SNP (rs3811647) associations could not be replicated in the joint sample, we conclude that these variants are not important modulators of methylmercury toxicity in the present cohort, although we cannot rule out a potential role at higher exposure levels.
New SNPs were identified in the joint sample analyses of SOD2, ABCA1 and MT1M, all previously considered in studies on genetic influence on mercury toxicokinetics (50). In an experimental study, mice were fed methylmercury-contaminated food, and the authors reported an exposure-stimulated expression in the brain of wild-type variants for mitochondrially encoded cytochrome b, cytochrome c oxidase subunit I, superoxide dismutase 1, SOD2, ATP-binding cassette subfamily B member 1A, and BCL2-associated X protein (51). Interestingly, we found more methylmercury vulnerability in the wild-type SOD2 rs5746136 genotype. The SOD2 protein binds to the superoxide byproducts of oxidative phosphorylation; it encodes a mitochondrial protein and binds 1 manganese ion per subunit. SOD2 might also bind methylmercury molecules and thereby increase oxidative stress inside neuronal cells by disrupting mitochondrial functions (51). The ABCA1 gene belongs to the superfamily of ABC transporters responsible for the active transport of various compounds across biological membranes (50). A recent population study found correlations between the hair-mercury concentration and alleles of this gene (52), and another study relying on cord-blood mercury measurements in 2 Mediterranean cohorts found similar results (7). Furthermore, ABCA1 rs4149268 and rs3890182 were found to be related to lipid and obesity profiles (53–55). The increased expression of ABCA1 SNPs wild-type variants might augment the passage of methylmercury molecules into neuronal cells and thereby further enhance the neurotoxic effect (50). Genes encoding metallothionein proteins have been explored because this protein actively binds metals via thiol groups in cysteine residues and protects against metal toxicity and oxidative stress in kidney, liver, and brain (50). A recent publication on 515 dental professionals found that MT1M polymorphisms were associated with mercury concentrations (56). Another study reported that male children’s verbal learning scores showed a greater reduction at elevated urinary mercury concentrations if they were carriers of MT1M rs2270836 wild-type variant (5); our findings for the same SNP are in the same direction. The tentative novel findings we report here require replication if reliable evidence is to be generated to support their role in methylmercury toxicity.
The exposure measurement was based on freeze-dried cord tissue, which has shown good correlation to mercury concentrations in cord blood, considered to be the best indicator of prenatal methylmercury neurotoxicity (8, 19). The estimation of omega-3 fatty acid intake from seafood was based on food frequency questionnaires only. Although valid, such methods are probably less precise than the biomarker used for methylmercury exposure in this study. When a variable is measured with imprecision, some of the variance might be erroneously attributed to other associated variables that are assessed more precisely (8, 13, 57). In the regression analyses, positive associations of fish intake might therefore be attributed to the more precisely assessed methylmercury variable rather than the confounding positive factors themselves, with apparently absent methylmercury toxicity being artefactually observed (8, 14). Furthermore, the results showed a tendency of mercury adverse associations with child IQ scores in higher social class strata (i.e., the group with higher exposure levels). In less-advantaged groups, risks of adverse exposures might vary much more, thereby contributing unexplained variance (8, 58).
In our study, we replicated findings of a genetic predisposition to methylmercury neurotoxicity, using the same interaction models previously applied in a separate subgroup from the same cohort. We validated the predisposition from PGR rs1042838 and, with less certainty, PON1 rs662. None of the selected SNPs for interaction models presented any association with the exposure, and this finding suggests that the negative IQ association with the exposure occurred only among subjects carrying the particular alleles. Such interaction could increase the association of exposure with adverse changes of the outcome, although not observed due to confounding in the overall population (59).
Assessment of methylmercury neurotoxicity carried out by regulatory agencies relies on average association coefficients in population studies. Thus, in the Faroese birth cohort study that was used as a basis for policy making in the United States, each doubling of the prenatal methylmercury exposure was associated with a loss of about 1.5 IQ points at age 7 years (13). In that cohort, with almost 10-fold higher cord-mercury concentrations than in the present study (19), the IQ loss for a 10-fold increased exposure would then correspond to most of the estimated 5 points. At the much lower exposures in the ALSPAC cohort, no overall adverse association with IQ was detected, and the rs1042838 wild type even showed an association of an approximate IQ increase of 7 points with a 10-fold mercury increase, likely due to residual confounding from beneficial factors associated with seafood intake and perhaps advantageous social factors related to higher fish intake. Conversely, the PROGINS genotype was associated with a loss of 7 IQ points for a 10-fold increased exposure (i.e., a total difference of 14 points) as compared to the wild type. Assuming a similar frequency of the PROGINS variant at about 30% in the Faroes, most of the estimated 5-IQ-point average loss in the Faroese cohort could potentially be explained by disproportionate IQ losses in PROGINS carriers.
Overall, these findings emphasize that evaluation of methylmercury neurotoxicity must take into consideration the impact of genetic predisposition. While the genes identified relate to functions that are probably not directly associated with methylmercury neurotoxicity, the PGR heterogeneity could be of wider relevance to developmental neurotoxicity and therefore deserves further exploration.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Jordi Julvez, Philippe Grandjean); ISGlobal, Barcelona Institute for Global Health—Campus MAR, Barcelona Biomedical Research Park, Barcelona, Catalonia, Spain (Jordi Julvez); Medical Research Council Integrative Epidemiology Unit, University of Bristol, Bristol, United Kingdom (George Davey Smith, Susan Ring); Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom (George Davey Smith, Susan Ring); and Department of Environmental Medicine, University of Southern Denmark, Odense, Denmark (Philippe Grandjean).
The UK Medical Research Council, the Wellcome Trust (grant 102215/2/13/2) and the University of Bristol currently provide core support for Avon Longitudinal Study of Parents and Children. Avon Longitudinal Study of Parents and Children genome-wide association data were generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. The present work was supported by a Miguel Servet fellowship (grant MS14/00108) awarded to J.J. from the Spanish Institute of Health Carlos III (Ministry of Economy and Competitiveness) as well as the Instituto de Salud Carlos III (grants CP14/00108, PI16/00261. It was cofunded by European Regional Development Fund (“A way to make Europe”). G.D.S. and S.R. work in a unit that receives funding from the UK Medical Research Council (grant MC_UU_00011/1/2/5) and the University of Bristol. P.G. is supported by the National Institute of Environmental Health Sciences (grants ES09797 and ES021447). J.J. serves as guarantor for the data analyses.
We thank the families who took part in this study, the midwives for their help in recruiting them, and the whole Avon Longitudinal Study of Parents and Children team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We also thank Flemming Nielsen and Ranja Bjerring, who were responsible for the mercury analyses in Denmark, and Colleen Bouzan for giving English grammar and style support.
The contents of this paper are solely the responsibility of the authors and do not represent the views of the Avon Longitudinal Study of Parents and Children executive or the official views of the National Institute of Environmental Health Sciences, National Institutes of Health, or any other funding agency.
Conflict of interest: none declared.
Abbreviations
- ABCA1
ATP binding cassette subfamily A member 1
- ALSPAC
Avon Longitudinal Study of Parents and Children
- BDNF
brain-derived neurotrophic factor
- IQ
intelligence quotient
- MT1M
metallothionein 1M
- PGR
progesterone receptor
- PON1
paraoxonase 1
- SNP
single-nucleotide polymorphism
- SOD2
superoxide dismutase 2
- TF
transferrin
- WISC-III
Wechsler Intelligence Scale for Children, Third Edition
REFERENCES
- 1. Karagas MR, Choi AL, Oken E, et al. . Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120(6):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex. 2016;74:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Research Council Toxicological Effects of Methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 5. Woods JS, Heyer NJ, Russo JE, et al. . Modification of neurobehavioral effects of mercury by genetic polymorphisms of metallothionein in children. Neurotoxicol Teratol. 2013;39:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Julvez J, Grandjean P. Genetic susceptibility to methylmercury developmental neurotoxicity matters. Front Genet. 2013;4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Llop S, Engström K, Ballester F, et al. . Polymorphisms in ABC transporter genes and concentrations of mercury in newborns—evidence from two Mediterranean birth cohorts. PLoS One. 2014;9(5):e97172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Julvez J, Smith GD, Golding J, et al. . Prenatal methylmercury exposure and genetic predisposition to cognitive deficit at age 8 years. Epidemiology. 2013;24(5):643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llop S, Ballester F, Murcia-Hinarejos M, et al. . Prenatal exposure to mercury and neuropsychological development in young children: the role of fish consumption. Int J Epidemiol. 2017;46(3):827–838. [DOI] [PubMed] [Google Scholar]
- 10. Mahaffey KR, Sunderland EM, Chan HM, et al. . Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69(9):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Julvez J, Méndez M, Fernandez-Barres S, et al. . Maternal consumption of seafood in pregnancy and child neuropsychological development: a longitudinal study based on a population with high consumption levels. Am J Epidemiol. 2016;183(3):169–182. [DOI] [PubMed] [Google Scholar]
- 12. Choi AL, Cordier S, Weihe P, et al. . Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38(10):877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandjean P, Herz KT. Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. Mt Sinai J Med. 2011;78(1):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budtz-Jørgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115(3):323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golding J, Pembrey M, Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. [DOI] [PubMed] [Google Scholar]
- 16. Fraser A, Macdonald-Wallis C, Tilling K, et al. . Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyd A, Golding J, Macleod J, et al. . Cohort Profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, Bristol, UK. http://www.bristol.ac.uk/alspac/researchers/our-data/. Accessed June 7, 2019.
- 19. Grandjean P, Budtz-Jørgensen E, Jørgensen PJ, et al. . Umbilical cord mercury concentration as biomarker of prenatal exposure to methylmercury. Environ Health Perspect. 2005;113(7):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. [DOI] [PubMed] [Google Scholar]
- 21. Grandjean P, Jørgensen PJ. Measuring mercury concentration. Epidemiology. 2005;16(1):133. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Willer C, Sanna S, et al. . Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paternoster L, Zhurov AI, Toma AM, et al. . Genome-wide association study of three-dimensional facial morphology identifies a variant in PAX3 associated with nasion position. Am J Hum Genet. 2012;90(3):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HapMap 3. Wellcome Sanger Institute. http://www.bristol.ac.uk/alspac/researchers/our-data/. Accessed July 19, 2019.
- 25. Wechsler D, Golombok S, Rust J. Wechsler Intelligence Scale for Children. 3rd ed London, UK: The Psychological Corporation; 1992. [Google Scholar]
- 26. Northstone K, Joinson C, Emmett P, et al. . Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. J Epidemiol Community Health. 2012;66(7):624–628. [DOI] [PubMed] [Google Scholar]
- 27. Taylor CM, Kordas K, Golding J, et al. . Data relating to prenatal lead exposure and child IQ at 4 and 8 years old in the Avon Longitudinal Study of Parents and Children. Neurotoxicology. 2017;62:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hibbeln JR, Davis JM, Steer C, et al. . Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–585. [DOI] [PubMed] [Google Scholar]
- 29. Golding J, Hibbeln JR, Gregory SM, et al. . Maternal prenatal blood mercury is not adversely associated with offspring IQ at 8 years provided the mother eats fish: a British prebirth cohort study. Int J Hyg Environ Health. 2017;220(7):1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor CM, Emond AM, Lingam R, et al. . Prenatal lead, cadmium and mercury exposure and associations with motor skills at age 7 years in a UK observational birth cohort. Environ Int. 2018;117:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lawlor DA, Wills AK, Fraser A, et al. . Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381(9884):2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor AE, Davey Smith G, Bares CB, et al. . Partner smoking and maternal cotinine during pregnancy: implications for negative control methods. Drug Alcohol Depend. 2014;139:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sallis H, Steer C, Paternoster L, et al. . Perinatal depression and omega-3 fatty acids: a Mendelian randomisation study. J Affect Disord. 2014;166:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor AE, Jones HJ, Sallis H, et al. . Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2018;47(4):1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–655. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization Preventing Disease through Healthy Environments/Exposure to Mercury: A Major Public Health Concern 2007https://www.who.int/ipcs/features/mercury.pdf. Accessed June 7, 2019.
- 37. Strain JJ, Yeates AJ, van Wijngaarden E, et al. . Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101(3):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morales E, Julvez J, Torrent M, et al. . Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology. 2015;26(4):458–465. [DOI] [PubMed] [Google Scholar]
- 39. Amorós R, Murcia M, González L, et al. . Maternal selenium status and neuropsychological development in Spanish preschool children. Environ Res. 2018;166:215–222. [DOI] [PubMed] [Google Scholar]
- 40. Polanska K, Krol A, Sobala W, et al. . Selenium status during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Pediatr Res. 2016;79(6):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oken E, Rifas-Shiman SL, Amarasiriwardena C, et al. . Maternal prenatal fish consumption and cognition in mid childhood: mercury, fatty acids, and selenium. Neurotoxicol Teratol. 2016;57:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choi AL, Budtz-Jørgensen E, Jørgensen PJ, et al. . Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res. 2008;107(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palmirotta R, Barbanti P, Ialongo C, et al. . Progesterone receptor gene (PROGINS) polymorphism correlates with late onset of migraine. DNA Cell Biol. 2015;34(3):208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Z, Wang SH, Zhou J, et al. . Contribution of PGR genetic polymorphisms to the pathogenesis of endometrial cancer: a meta-analysis. J Cancer Res Ther. 2015;11(4):810–817. [DOI] [PubMed] [Google Scholar]
- 45. Gaudet HM, Christensen E, Conn B, et al. . Methylmercury promotes breast cancer cell proliferation. Toxicol Rep. 2018;5:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen Kadosh K, Krause B, King AJ, et al. . Linking GABA and glutamate levels to cognitive skill acquisition during development. Hum Brain Mapp.2015;36(11):4334–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghali RM, Al-Mutawa MA, Ebrahim BH, et al. . Progesterone receptor (PGR) gene variants associated with breast cancer and associated features: a case-control study [published online ahead of print January 4, 2018]. Pathol Oncol Res. 10.1007/s12253-017-0379-z. [DOI] [PubMed] [Google Scholar]
- 48. Ayotte P, Carrier A, Ouellet N, et al. . Relation between methylmercury exposure and plasma paraoxonase activity in Inuit adults from Nunavik. Environ Health Perspect. 2011;119(8):1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cardenas A, Rifas-Shiman SL, Agha G, et al. . Persistent DNA methylation changes associated with prenatal mercury exposure and cognitive performance during childhood. Sci Rep. 2017;7(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Llop S, Ballester F, Broberg K. Effect of gene-mercury interactions on mercury toxicokinetics and neurotoxicity. Curr Environ Health Rep. 2015;2(2):179–194. [DOI] [PubMed] [Google Scholar]
- 51. Bourdineaud JP, Laclau M, Maury-Brachet R, et al. . Effects of methylmercury contained in a diet mimicking the Wayana Amerindians contamination through fish consumption: mercury accumulation, metallothionein induction, gene expression variations, and role of the chemokine CCL2. Int J Mol Sci. 2012;13(6):7710–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Croes K, De Coster S, De Galan S, et al. . Health effects in the flemish population in relation to low levels of mercury exposure: from organ to transcriptome level. Int J Hyg Environ Health. 2014;217(2–3):239–247. [DOI] [PubMed] [Google Scholar]
- 53. Kong X, Zhao Q, Xing X, et al. . Genetic variants associated with lipid profiles in chinese patients with type 2 diabetes. PLoS One. 2015;10(8):e0135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao MH, Guo H, He J, et al. . Interactions of six SNPs in ABCA1 gene and obesity in low HDL-C disease in Kazakh of China. Int J Environ Res Public Health. 2016;13(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dumitrescu L, Carty CL, Taylor K, et al. . Genetic determinants of lipid traits in diverse populations from the Population Architecture Using Genomics and Epidemiology (PAGE) study. PLoS Genet. 2011;7(6):e1002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Goodrich JM, Gillespie B, et al. . An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels. Environ Health Perspect. 2012;120(4):530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Phillips AN, Smith GD. How independent are “independent” effects? relative risk estimation when correlated exposures are measured imprecisely. J Clin Epidemiol. 1991;44(11):1223–1231. [DOI] [PubMed] [Google Scholar]
- 58. Hanscombe KB, trzaskowski M, Haworth CM, et al. . Socioeconomic status (SES) and children’s intelligence (IQ): in a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. PLoS One. 2012;7(2):e30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 2011;6(1):27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.