Abstract
Purpose:
To quantify the association between daily physical activity measured by accelerometer and 1-year changes in symptoms among people with knee osteoarthritis.
Methods:
Participants from the Osteoarthritis Initiative (OAI) had knee radiographs and physical activity assessed using GT1M ActiGraph uniaxial accelerometers at the 48 months visit. Physical activity was calculated and categorized as tertiles of average daily minutes in light and moderate-to-vigorous activity. Outcomes were 1-year change in symptoms measured by Western Ontario and McMaster Universities (WOMAC) scales including pain, stiffness, and physical function. Adjusted multivariable linear models estimated the relationship between tertiles of light or moderate-to-vigorous physical activity and changes in knee symptoms.
Results:
Among 1,059 participants (55% women, mean age 66±9 years), greater time in light activity was associated with a trend towards declined physical function (P = 0.01). Greater time in daily moderate-to-vigorous activity was also associated with declined physical function (P = 0.01) and increased pain (P = 0.08). None of these average changes in symptoms reached minimally important clinical differences. However, greater daily time in both activities was associated with higher probability of worsening symptoms among persons with K-L grade 4 osteoarthritis.
Conclusion:
Objectively measured daily activity was not associated with 1-year symptom improvements among community dwelling adults with knee osteoarthritis. In those with advanced disease (K-L grade 4), greater daily minutes in physical activity were associated with worsening symptoms. How best to implement exercise regimens in persons with advanced knee osteoarthritis to reduce the deleterious impact on symptoms needs to be explored.
Keywords: Physical activity, Patient-reported outcomes, Knee osteoarthritis
Introduction
Knee osteoarthritis is a leading cause of pain and functional limitations among older adults.1,2 Although no effective remedy for osteoarthritis exists, the American College of Rheumatology (ACR) and the US federal government recommend self-management strategies such as regular physical activity.3,4 The beneficial effects of physical activity among patients with osteoarthritis are generally supported through randomized clinical trials.5 These trials vary in duration, intensity, and type of interventions, have selective populations such as milder conditions that received intense encouragement and monitoring, and assess outcomes over short periods of time, all of which reduce generalizability to activities of normal daily living.5,6
The details related to different levels of intensity, type, and duration for daily physical activity needed to improve symptoms and function among osteoarthritis populations are limited and contradictory.7-9 Non-experimental studies examining the association between physical activity and symptoms have been cross-sectional or have used self-reported questionnaires to quantify activity.8,10-12 Longitudinal studies to quantify the impact of varying levels of intensity with regard to activities of daily living on pain, stiffness, and function in patients with knee osteoarthritis using objectively measured physical activity are needed to understand what intensity of activities should be recommended in what types of patients with osteoarthritis to improve symptoms.
The Osteoarthritis Initiative (OAI) data provide an opportunity to investigate the association between physical activity on one-year changes in symptoms because it offers a subcohort with objectively measured physical activity and comprehensive examinations of knee symptoms. The current study will quantify the association of objectively measured physical activity of daily living on changes in symptoms in persons with knee osteoarthritis and evaluate the extent to which the observed association is similar across levels of disease severity. We hypothesized that: 1) greater daily minutes of physical activity at baseline would be associated with improved symptoms over a one-year period among knee osteoarthritis patients; and 2) beneficial effects may not be observed across all levels of disease severity.
METHODS
This study used publicly available data from the OAI.13 The University of Massachusetts Institutional Review Board considered this study exempt.
Design and Setting Overview
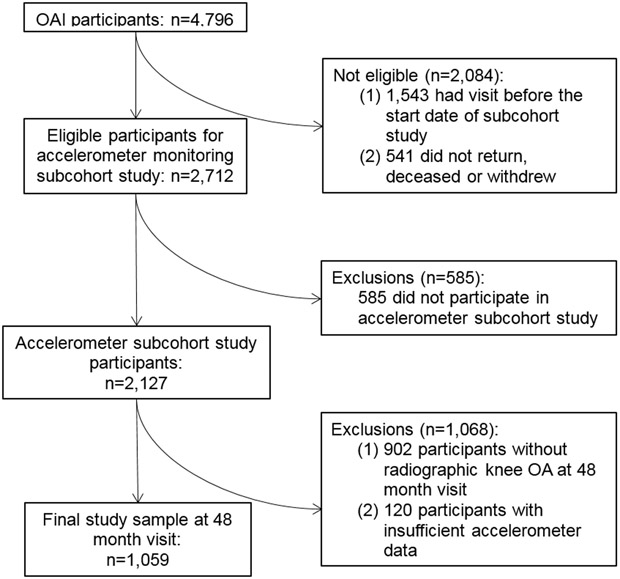
The OAI was a prospective study examining the development and progression of knee osteoarthritis in adults aged 45-79 years at enrollment. The OAI enrolled 4,796 adults (2004 - 2006) with symptomatic osteoarthritis in at least one knee or at least one established risk factors for knee osteoarthritis. Participants had annual follow-up examinations. Accelerometer monitoring data were collected on a subcohort of participants at the 48 month follow-up visit, considered the baseline assessment for this study (n=2,127; 78.4% of eligible; Figure 1).
Figure 1.
Flow of analytic sample of participants with accelerometer data through study follow-up.
Study Participants
We identified 1,225 participants with radiographic knee osteoarthritis at baseline in at least one knee (Kellgren-Lawrence (K-L) grade ≥2).14 To provide a sufficient estimation of physical activity based on the accelerometer, 1,105 participants with 4-7 valid days (≥10 wear hours per day) of physical activity monitoring data were included.15 After excluding participants with missing outcome data (n=46), the final sample included 1,059 participants.
Patient-reported OsteoarthritisSymptoms
Participants completed the knee-specific Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index (Version LK 3.1) at annual evaluations. The WOMAC measured Pain (5 items), Stiffness (2 items), and Physical Function (17 items).16 Five Likert responses, ranging from ‘0=none’ to ‘4=extreme’ were available for each item. Reponses to items of dimensions in Pain, Stiffness, and Physical Function were summed to establish subscale scores, with higher scores indicating worse symptoms of the knee or knee-related function. If participants had radiographic osteoarthritis in both knees, the worst WOMAC measurements were used. The primary outcome was the difference between 60 months follow-up visit scores and 48 month visit scores for each subscale.
Measurements of Physical Activity
Physical activity was objectively measured using GT1M ActiGraph uniaxial accelerometers at the 48 month visit. The accelerometer captures measurements of vertical acceleration and deceleration and provides information on frequency, intensity, and duration of physical activity.17 Detailed study protocol and measurements of physical activity are available elsewhere. Except during water activities, participants wore accelerometers after arising in the morning and until retiring at night for seven consecutive days.
The output of accelerometer data was generated analytically and categorized using validated methods in patients with arthritis conditions.15,18 The accelerometer data measures physical activity duration and intensity by capturing activity counts. Activity counts measured using one-minute intervals were then used to differentiate overall physical activity levels: 1) light physical activity (100 to 2,019 counts/min); and 2) moderate-to-vigorous activity (≥2020 counts/min).15 Minutes attributed as “light physical activity” were summed up for each dayand the daily mean was generated across all valid days. Then, an average daily minutes was computed for light activity and participants were categorized into light activity tertiles. This process was repeated for activity counts classified as moderate-to-vigourous activity. For each type of activity count (light or moderate-to-vigorous), twho other metrics were used: 1) the weekly and daily duration of time in each activity level (minutes); 2) the proportion of daily minutes of total wear time. Participants were considered to have met 2008 U.S. Department of Health and Human Services (DHHS) physical activity guidelines if they had minutes in moderate-to-vigorous activity level for ≥ 150 minutes per week.19
Confounders
Sociodemographics, clinical characteristics of knee osteoarthritis, body mass index (BMI), general health status, and health behaviors were considered as confounders.20-25 Sociodemographics including age at time of the 48 month visit and ethnicity, sex, education, and income recorded at enrollment. Clinical characteristics included history of having a knee injury or surgery, Kellgren and Lawrence (K-L) grade, and multi-joint symptoms of pain.26 BMI was calculated as weight (kg) divided by height (m2) and classified as normal weight (18.5-24.9 kg/m2), overweight ( 25.0-29.9 kg/m2), or obese ( ≥ 30 kg/m2).27 The 12-item Short-Form Health Survey (SF-12) was used to evaluate general overall health.28 The Charlson index was used for comorbid conditions. Depressive symptoms were present if scores for Centers for Epidemiologic Studies Depression Scale (CES-D) were ≥ 16. Health behaviors included the report of smoking and drinking status.
Statistical Analyses
Descriptive characteristics by tertiles of average daily minutes in light physical activity were calculated. The distributions of changes in WOMAC subscales (pain, stiffness, and function) were visually inspected to rule out departures from normality. To examine the relationship between physical activity and changes in osteoarthritis symptoms, separate models were developed: 1) based on tertiles of in light activity; and 2) based on tertiles of average daily minutes in moderate-to-vigorous physical activity. Linear trends across tertiles were evaluated.
For each physical activity type (e.g. light or moderate-to-vigorous activity), we developed 3 models to examine the relationship between tertiles of physical activity and one-year change in osteoarthritis symptoms (e.g. pain, stiffness, and physical function). Multivariable linear models estimated the relationship between tertiles of light or moderate-to-vigorous physical activity and changes in knee symptoms. Age and gender were included in all adjusted models. Variables whose inclusion changed the physical activity regression coefficients by ≥10% were retained.29 The beta coefficients and 95% confidence intervals (CIs) were derived corresponding to each tertile compared to the tertile with lowest minutes of light or moderate-to-vigorous activities. Positive beta coefficients corresponded to the absolute increases (worsening) of pain, stiffness, and physical function. Negative beta coefficients corresponded to the absolute decreases (improvement) of symptoms. We a priori defined minimum clinically important differences (MCID) for WOMAC scores:30,31 a change in score ranging from 1.2 to 4.6 for pain, 0.5 to 1.5 for stiffness, and 4.1 to 9.9 for physical function. The minimum cutpoints were used (e.g. 1.2 for pain, o.5 for stiffness, and 4.1 for function).
To further examine the nature of the relationship, stratified analyses were performed using nonparametric logistic models. While we originally sought to focus on improved symptoms according to MCID thresholds, after evaluating the results from the aforementioned models, we also developed models that estimated the predicted probabilities of worsened symptoms according to MCID thresholds. For light activity, three models (pain, stiffness, and function) were developed and stratified by the disease severity measured by K-L grades with a binary outcome variables defined as one-year MCID for improving (yes/no). Three models were also run with a binary outcome defined as one-year MCID for worsening symptoms. This process was repeated using moderate-to-vigorous activity as the primary determinant of interest. Parametric assumptions about the trend of physical activity were not applied.32 In total, 12 models using the generalized additive models (PROC GAM) (SAS Institute, Cary, NC) were created. Graphs depicting these associations were created for each symptom by the level of physical activity with a 95% confidence band.
RESULTS
Table 1 shows the characteristics of participants with radiographic knee osteoarthritis according to tertiles of daily minutes in light physical activity level based on the physical activity level at the 48 months visit (low: ≤243 minutes/day; medium: 244– 305 minutes/day; high: >305 minutes/day). Compared to the participants who spent the lowest tertile of daily time in light activity, participants with higher daily minutes were more likely to be younger and women and to have depression and higher SF-12 physical component summary scores.
Table 1.
Characteristics of participants according to tertiles of average daily minutes in light physical activity (n=1,059).
| Characteristics | Tertiles of daily light physical activity | ||
|---|---|---|---|
| Low (≤243 minutes) |
Medium (244 – 305 minutes) |
High (>305 minutes) |
|
| Sociodemographics | |||
| Mean (SD) age in years | 69.3 (9.3) | 64.8 (8.4) | 63.7 (8.2) |
| Women (%) | 45.9 | 54.6 | 63.0 |
| Race/ethnicity (%) | |||
| Non-Hispanic White | 83.4 | 82.0 | 77.1 |
| Non-Hispanic Black | 14.0 | 15.8 | 20.3 |
| Other race/ethnicity | 2.6 | 2.2 | 2.6 |
| Education (%)¶ | |||
| High school or less | 13.6 | 13.9 | 15.2 |
| Some or college graduate | 43.5 | 41.7 | 48.0 |
| At least some graduate school | 42.9 | 44.4 | 36.8 |
| Income (%)¶ | |||
| <$25,000 | 12.4 | 10.8 | 10.1 |
| $25,000 - $50,000 | 25.4 | 18.6 | 28.5 |
| >$50,000 | 62.1 | 70.6 | 61.5 |
| Clinical characteristics | |||
| Knee injury (%) | 5.7 | 4.7 | 4.0 |
| Knee surgery (%) | 4.9 | 2.5 | 3.4 |
| Kellgren-Lawrence grade (%) | |||
| 2 | 47.9 | 57.1 | 50.7 |
| 3 | 32.7 | 30.2 | 37.0 |
| 4 | 19.5 | 12.7 | 12.3 |
| Multi-joint symptoms (%) | 45.3 | 46.5 | 51.9 |
| General health | |||
| Body mass index (%)¶ | |||
| Normal | 19.5 | 20.6 | 20.6 |
| Overweight | 35.5 | 42.6 | 39.8 |
| Obese | 45.0 | 36.8 | 39.5 |
| PCS, mean (SD)*¶ | 46.6 (9.7) | 48.3 (8.7) | 48.4 (8.3) |
| MCS, mean (SD)*¶ | 54.5 (8.2) | 55.2 (7.9) | 53.6 (8.5) |
| Charlson Comorbidity Index¶ | |||
| 0 | 62.8 | 73.2 | 70.7 |
| 1 | 18.0 | 16.1 | 19.3 |
| ≥2 | 19.2 | 10.7 | 10.1 |
| CES-D (>16) (%)**¶ | 8.7 | 8.4 | 10.7 |
| Smoking¶ | |||
| Current | 3.8 | 3.7 | 4.6 |
| Past | 39.7 | 38.4 | 38.3 |
| Never | 56.5 | 58.0 | 57.1 |
| At-risk alcohol use (%)¶ | 6.9 | 7.3 | 9.5 |
PCS: SF-12 Physical Component Summary scores; MCS: Mental Component Summary scores.
CES-D: Centers for Epidemiologic Studies Depression Scale;
Number of participants with missing information: education (6), annual household income (5), body mass index (2), SF-12 Physical Component Summary Scores (11), SF-12 Mental Component Summary Scores (11), Charlson Comorbidity Index (12), elevated depressive symptoms (12), smoking status (15), at-risk alcohol use (8). We imputed missing annual income with conditional means of income given age, sex, race/ethnicity, and education for 79 participants.
Table 2 shows characteristics of objectively measured physical activity and WOMAC scores according to tertiles of light activity. Only 11.8% of all participants met the guidelines (data not shown) and 9.2% in the lowest tertile of light activity and 13.2% in the highest met guidelines. The tertiles of daily minutes in moderate-to-vigorous physical activity were: lowest tertile: ≤5 minutes/day; middle tertile: 6 – 19 minutes/day; highest tertile: >19 minutes/day. Compared with those who spent the least daily time in light physical activity, participants with higher daily minutes were more likely to have higher average daily and weekly minutes in light or moderate-to-vigorous activity. On average, participants in the highest tertile of light activity had nearly 10 minutes more per day in moderate-to-vigorous activities and 170 minutes per day in light activities compare to the lowest tertile.
Table 2.
Characteristics of physical activity and symptoms according to tertiles of daily light physical activity minutes (n=1,059).
| Characteristics | Tertiles of daily light physical activity | ||
|---|---|---|---|
| Low (≤243 minutes) |
Medium (244 – 305 minutes) |
High (>305 minutes) |
|
| Meeting Physical Activity Guidelines (%) | 9.2 | 13.0 | 13.2 |
| Tertiles of moderate-to-vigorous physical activity (%) | |||
| Low (≤5 minutes) |
53.3 | 29.1 | 18.1 |
| Medium (6 – 19 minutes) |
27.5 | 36.8 | 37.0 |
| High (>19 minutes) |
19.2 | 34.1 | 45.0 |
| Mean daily duration (mins/day (SD)) | |||
| Light | 196 (34.4) | 273 (18.1) | 366 (54.0) |
| Moderate-to-vigorous | 11 (16.8) | 17 (16.9) | 21 (18.8) |
| Mean weekly duration (mins/day (SD)) | |||
| Light | 1,276 (297.9) | 1,837 (213.3) | 2,466 (424.5) |
| Moderate-to-vigorous | 72 (112.5) | 116 (114.8) | 142 (127.5) |
| Proportion of daily duration of wear time (%) | |||
| Light | 23.3 | 30.9 | 39.8 |
| Moderate-to-vigorous | 1.3 | 1.9 | 2.3 |
| Mean WOMAC scores (SD) | |||
| Pain | 3.6 (3.7) | 2.9 (3.5) | 3.2 (3.6) |
| Stiffness | 2.0 (1.8) | 1.7 (1.7) | 1.8 (1.8) |
| Physical function | 11.0 (12.1) | 9.0 (11.6) | 9.5 (11.3) |
Using multivariable analyses (Table 3), participants reporting ≥305 minutes per day at baseline assessment had 1-year declined physical function compared to those reporting the least light activity (≤243 minutes per day). The average worsening physical function scores were 1.90 (adjusted β in tertile high; 95% CI: 0.42 to 3.38). Increasing tertile categories of time spent was associated with worsening WOMAC physical function scores (P for trend = 0.01). For 1-year change in symptoms using tertiles of average daily minutes in moderate-to-vigorous activity (Table 4), increasing tertile categories of time was associated with worsening WOMAC pain (P for trend = 0.01) and physical function scores (p trend =0.02). Compared to the lowest tertile of average daily minutes in moderate-to-vigorous physical activity, the average worsening pain scores were 0.51 (adjusted β in tertile medium; 95% CI: 0.02 to 0.99) and 0.71 (adjusted β in tertile high; 95% CI: 0.18 to 1.23). Compared to the lowest tertile of average daily minutes in moderate-to-vigorous physical activity, the average worsening physical function scores were 1.83 (adjusted β in tertile medium; 95% CI: 0.32 to 3.34) and 2.07 (adjusted β in tertile high; 95% CI: 0.38 to 3.76).
Table 3.
Associations Between Change in Symptoms and Tertiles of Averaged Daily Light Physical Activity Minutes.
| Terrtiles of daily light physical activity | ||||
|---|---|---|---|---|
| Low (lowest mins:≤243) |
Medium (244 – 305) |
High (highest mins:>305) |
P-value for linear trend |
|
| Pain (Minimum clinically important difference: 1.2-4.6)** | ||||
| Crude* | (Referent) | 0.60 (0.16 to 1.05) |
0.53 (0.08 to 0.98) |
|
| Adjusted*§ | (Referent) | 0.52 (0.06 to 0.98) |
0.43 (−0.04 to 0.91) |
0.08§ |
| Stiffness (Minimum clinically important difference: 0.5-1.5)** | ||||
| Crude* | (Referent) | 0.05 (−0.17 to 0.28) |
0.09 (−0.13 to 0.32) |
|
| Adjusted*¶ | (Referent) | 0.06 (−0.17 to 0.29) |
0.10 (−0.14 to 0.35) |
0.39¶ |
| Physical Function (Minimum clinically important difference: 4.1-9.9)** | ||||
| Crude* | (Referent) | 1.01 (−0.38 to 2.39) |
1.99 (0.59 to 3.38) |
|
| Adjusted*┼ | (Referent) | 0.92 (−0.52 to 2.36) |
1.90 (0.42 to 3.38) |
0.01┼ |
β coefficient (95% Confidence Interval (CI)).
Adjusted for age (linear term), gender, race/ethnicity, Kellgren-Lawrence grade, history of knee surgery, multi-joint symptoms, and SF-12 Physical Component Summary scores (estimates did not change if adjusted for individual items of PCS scores without pain).
Adjusted for age (linear term), gender, education, Kellgren-Lawrence grade, history of knee surgery, and Charlson index.
Adjusted for age (linear term), gender, history of knee injury, history of knee surgery, Body Mass Index, Charlson index, and SF-12 Physical Component Summary scores (estimates did not change if adjusted for individual items of PCS scores without physical functioning).
Positive beta coefficients corresponded to worsening symptoms and negative beta coefficients correspond to improved knee symptoms.
Table 4.
Associations Between Change in Symptoms and Tertiles of Averaged Daily Moderate-to-Vigorous Physical Activity Minutes.
| Tertiles of daily moderate-to-vigorous physical activity | ||||
|---|---|---|---|---|
| Low (≤5 minutes) |
Medium (6 – 19 minutes) |
High (>19 minutes) |
P-value for linear trend |
|
| Pain (Minimum clinically important difference: 1.2-4.6)** | ||||
| Crude* | (Referent) | 0.39 (−0.06 to 0.84) |
0.56 (0.11 to 1.01) |
|
| Adjusted*§ | (Referent) | 0.51 (0.02 to 0.99) |
0.71 (0.18 to 1.23) |
0.01§ |
| Stiffness (Minimum clinically important difference: 0.5-1.5)** | ||||
| Crude* | (Referent) | 0.04 (−0.19 to 0.26) |
0.21 (−0.02 to 0.44) |
|
| Adjusted*¶ | (Referent) | 0.08 (−0.17 to 0.32) |
0.22 (−0.05 to 0.49) |
0.11¶ |
| Physical Function (Minimum clinically important difference: 4.1-9.9)** | ||||
| Crude* | (Referent) | 1.41 (0.03 to 2.79) |
1.65 (0.25 to 3.04) |
|
| Adjusted*┼ | (Referent) | 1.83 (0.32 to 3.34) |
2.07 (0.38 to 3.76) |
0.02┼ |
β coefficient (95% Confidence Interval (CI)).
Adjusted for age (linear term), gender, and SF-12 Physical Component Summary scores (estimates did not change if adjusted for individual items of PCS scores without physical functioning).
Adjusted for age (linear term), gender, Kellgren-Lawrence grade, Charlson index, and SF-12 Physical Component Summary scores.
Adjusted for age (linear term), gender, race/ethnicity, education, Body Mass Index, and SF-12 Physical Component Summary scores (estimates did not change if adjusted for individual items of PCS scores without physical functioning).
Positive beta coefficients corresponded to worsening symptoms and negative beta coefficients correspond to improved knee symptoms.
Figure 2 shows the effect of physical activity was different according to the severity of disease. Despite some fluctuations, a stronger trend relationship was found among persons with K-L grade 4. The dose-response relationship suggested that increased daily minutes in light or moderate-to-vigorous activities were associated with increased probabilities of worsening symptoms, especially for participants with K-L grade 4. The relationship between predicted probabilities of improved symptoms and daily activities was also explored (Appendix Figure 1). The dose-response relationship was reverse which suggested that increased daily minutes in light or moderate-to-vigorous activities were less likely to be associated with the probabilities of improving symptoms among participants with K-L grade 4.
Figure 2.
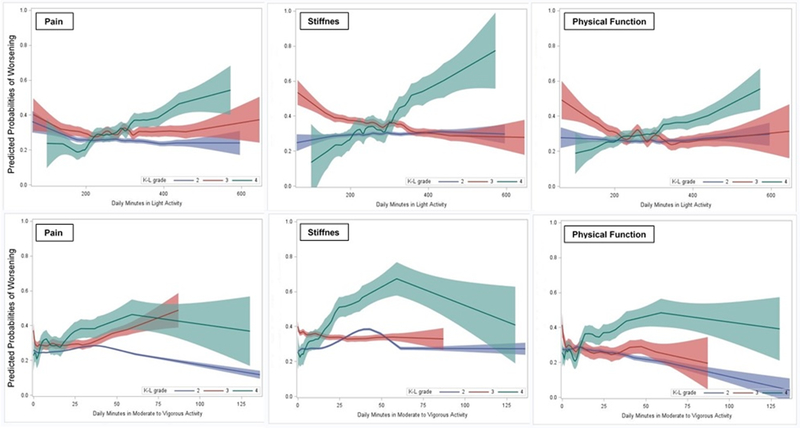
Associations between predicted probabilities of one-year worsening symptoms and daily physical activity by Kellgren-Lawrence grades.
Predicted probabilities of one-year worsening symptoms were estimated using nonparametric logistic models adjusted for covariates listed in Table 3 and Table 4. Solid line represents estimated probabilities from nonparametric logistic models; shade areas indicate 95% confidence bands for the estimates.
Discussion
This 1-year longitudinal study used accelerometer data to examine the relationship between objectively measured physical activity and knee symptoms among adults with radiographically confirmed knee osteoarthritis. Our findings did not support our hypothesis and the prevailing wisdom that greater time in daily physical activity would improve symptoms in patients with osteoarthritis. We found a significant linear positive trend between daily minutes spent in moderate-to-vigorous physical activity and increased knee pain and declined physical function, but no change in stiffness. This is an important finding since engaging in moderate-to-vigorous physical activity of ≥150 minutes a week is generally recommended by the 2008 DHHS physical activity guidelines to reduce pain and improve function for people with osteoarthritis. Our results suggest that daily minutes in light or moderate-to-vigorous physical activity may not be beneficial to improve the knee symptoms over a year for individuals with advanced knee osteoarthritis. In those with K-L grade 4, daily activity may be associated with worsening symptoms.
Our findings are consistent with a previous study using self-reported physical activity data from OAI using advanced analytical methods,9 but inconsistent with previous systematic reviews and randomized controlled trials which reported that physical activities such as strengthening and aerobic exercise help reduce pain and improve function among patients with hip and knee osteoarthritis.5,33 The discrepancy might be because the improvement of knee pain and function may result from the accumulation of regularly frequent exercise rather than a sudden increase of intensity.34,35 Clinical trials usually include highly selective populations (e.g. K-L grades 2-35) and tend to use exercise programs that are more structured, gradually progressive, under supervised, and focused on improving aerobic capacity, quadriceps muscle strength, or lower extremity performance rather than activities of normal daily living.5
In our study, participants spent over half the time in light activities (or less) for most of the week and did not appear to meet guidelines under a regular pattern of higher level of activities. Participants were from multiple geographic sites so that a broad spectrum of radiographically confirmed knee osteoarthritis patients was included. Physical activity may be helpful in reducing osteoarthritis symptoms, but only in those with habitually active or milder disease severity under supervised and structured exercise programs.33 Understanding the relationship between objectively measured physical activity and changes in knee symptoms among knee osteoarthritis patients for varying forms of physical activity as well as for varying severity of osteoarthritis is warranted.
Meeting guidelines or regular exercise is considered to have many health benefits among people with osteoarthritis,11,33,36 especially in reducing weight which is associated with joint contact force and symptoms relief.37,38 Determining the optimal duration, types, and adherence of physical activity in daily lifestyle activities among osteoarthritis populations is important. Pain may act as a barrier for individuals with knee osteoarthritis to engage in physical activity.39 A combination of treatment intervention and pain management may be needed to help people with osteoarthritis better meet physical activity guidelines. Studies of physical activity have primarily focused on time spent in moderate-to-vigorous activity,24 with few studies examining the potential health benefits of light activities compared to sedentary lifestyles. Given that only 12% of participants in our study met the 2008 DHHS physical activity guidelines, research may focus on other formats and levels of light activities or effects of disruption and reducing time in sedentary lifestyle among knee osteoarthritis populations.
The study strengths include the prospective study design, a large number of community-dwelling adults with radiographically confirmed knee osteoarthritis, and the objectively measured physical activity by accelerometer monitoring. Several limitations are acknowledged. Estimates of effects from observational data cannot be interpreted as causal. The OAI offers the ability to adjust for many potential confounders; however, residual confounding cannot be ruled out. Misclassification of physical activity is possible, despite the use of accelerometers. Physical activity was assessed at the 48 month visit and we acknowledge that it may have changed over time. Accelerometers are not sensitive to detect all activities (e.g. bicycling) and they cannot be used during water activities. Although swimming is the fourth most common exercise activity in the U.S. general population and aquatic exercise is encouraged in patients with osteoarthritis,40,41 the extent to which study participants engaged in these activities (and thus had their activity underestimated) is unknown. Physical activity measured by accelerometers cannot tell if participants engage in types of activities that are recommended by guidelines for persons with osteoarthritis, such as low-impact activities and muscle strengthening exercises.
CONCLUSIONS
Greater time of daily physical activities was not associated with improving symptoms among adults with radiographically confirmed knee osteoarthritis over one year. Among persons with K-L grade 4, greater time in daily activities was associated with worsening symptoms over 1 year. Given the overall health benefits of exercise to improve general health and findings from the current study, recommendations for physical activity in osteoarthritis33,36 should take stages of disease into account. How best to incorporate greater intensity physical activity into daily practice without exacerbating knee osteoarthritis symptoms is needed, especially for those with severe knee osteoarthritis. Our findings do not support the adage “the motion is the lotion”.
Supplementary Material
Appendix Figure 1. Associations between predicted probabilities of one-year improving symptoms and daily physical activity by Kellgren-Lawrence grades.
Acknowledgments
Funding: This study was supported by National Heart, Lung and Blood Institute (Contract number: HHSN268201000020C, Reference Number: BAA-NHLBI-AR1006). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. Dr. Eaton is a principal investigator of an OAI site, and Dr. McAlindon a co–principal investigator of the same site.
Footnotes
Conflict of Interest: Dr. Eaton has received grants and has served as a consultant to Pfizer. Dr. Lapane has served as a consultant to Janssen and GlaxoSmithKline. We do not have other conflict of interests to disclose.
REFERECNES
- 1.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am. J. Public Health 1994;84:351–358. doi: 10.2105/AJPH.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J. Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 3.Westby Marie. Exercise and Arthritis. Am. Collge Rheumatol. 2012. Available at: https://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Exercise_and_Arthritis/.
- 4.United States Department of Health & Human Services, Office of the Secretary, Office of the Assistant Secretary for Health O of DP and HP. Advisory Committee Report - G5. Musculoskeletal Health. Available at: http://www.health.gov/paguidelines/Report/G5_musculo.aspx#_Toc199945420. Accessed December 21, 2015.
- 5.Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. (Hoboken, N.J.) 2014;66:622–636. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 6.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M BK. Exercise for osteoarthritis of the kneeNo Title. Cochrane Database Syst. Rev. 2015;(1):Art. No.: CD004376. doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng NTM, Heesch KC, Brown WJ. Efficacy of a progressive walking program and glucosamine sulphate supplementation on osteoarthritic symptoms of the hip and knee: a feasibility trial. Arthritis Res. Ther. 2010;12(1):R25. doi: 10.1186/ar2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop DD, Song J, Semanik PA, Sharma L, Chang RW. Physical activity levels and functional performance in the osteoarthritis initiative: A graded relationship. Arthritis Rheum. 2011;63(1):127–136. doi: 10.1002/art.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansournia MA, Danaei G, Forouzanfar MH, et al. Effect of physical activity on functional performance and knee pain in patients with osteoarthritis : analysis with marginal structural models. Epidemiology 2012;23:631–40. doi: 10.1097/EDE.0b013e31824cc1c3. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop DD, Semanik P, Song J, et al. Moving to Maintain Function in Knee Osteoarthritis: Evidence From the Osteoarthritis Initiative. Arch. Phys. Med. Rehabil. 2010;91(5):714–721. doi: 10.1016/j.apmr.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S-H, Waring ME, Eaton CB, Lapane KL, Eaton CB. Association of objectively measured physical activity and metabolic syndrome among U.S. adults with osteoarthritis. Arthritis Care Res. (Hoboken). 2015;67(10):1371–1378. doi: 10.1002/acr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlop DD, Song J, Semanik P a, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ 2014;348(April):g2472. doi: 10.1136/bmj.g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Osteoarthritis Initiative (OAI). Available at: https://oai.epi-ucsf.org/datarelease/default.asp. Accessed December 24, 2015.
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 16.Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand. J. Rheumatol. 1999;28(4):210–215. doi: 10.1016/S0161-4754(00)90101-5. [DOI] [PubMed] [Google Scholar]
- 17.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Semanik P, Sharma L, et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res. (Hoboken). 2010;62:1724–1732. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washingt. DC Dep. Heal. Hum. Serv. USA 2008. Available at: http://www.health.gov/paguidelines/guidelines/. Accessed December 21, 2015.
- 20.Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis National Health Interview Survey, 2002. Am J Prev Med 2006;30:385–393. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Rosemann T, Kuehlein T, Laux G, Szecsenyi J. Factors associated with physical activity of patients with osteoarthritis of the lower limb. J. Eval. Clin. Pract. 2008;14:288–293. doi: 10.1111/j.1365-2753.2007.00852.x. [DOI] [PubMed] [Google Scholar]
- 22.Dekker J, van Dijk GM, Veenhof C. Risk factors for functional decline in osteoarthritis of the hip or knee. Curr. Opin. Rheumatol. 2009;21:520–524. doi: 10.1097/BOR.0b013e32832e6eaa. [DOI] [PubMed] [Google Scholar]
- 23.Knittle K, De Gucht V, Maes S. Lifestyle- and behaviour-change interventions in musculoskeletal conditions. Best Pract. Res. Clin. Rheumatol. 2012;26:293–304. doi: 10.1016/j.berh.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Song J, Hootman JM, et al. Obesity and other modifiable factors for physical inactivity measured by accelerometer in adults with knee osteoarthritis. Arthritis Care Res. (Hoboken). 2013;65:53–61. doi: 10.1002/acr.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pate RR, Heath GW, Dowda M, Trost SG. Associations between physical activity and other health behaviors in a representative sample of US adolescents. Am. J. Public Health 1996;86:1577–1581. doi: 10.2105/AJPH.86.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okma-Keulen P, Hopman-Rock M. The onset of generalized osteoarthritis in older women: a qualitative approach. Arthritis Rheum. 2001;45:183–190. [DOI] [PubMed] [Google Scholar]
- 27.Michael D Jensen MD, Donna H Ryan MD, Caroline M Apovian MDF, et al. 2013. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2013:1–70. [Google Scholar]
- 28.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care 1996;34(3):220–233. doi: 10.2307/3766749. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJGS. Modern Epidemiology. 1st ed.; 1998.
- 30.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower ex. Arthritis Rheum. 2001;45(4):384–91. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal art. Am. J. Sports Med. 2010;38(5):891–902. doi: 10.1177/0363546509354163. [DOI] [PubMed] [Google Scholar]
- 32.Hastie TJ, Tibshirani R. Generalized Additive Models.; 1990. doi: 10.1016/j.csda.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Semanik PA, Chang RW, Dunlop DD. Aerobic Activity in Prevention and Symptom Control of Osteoarthritis. PM R 2012;4. doi: 10.1016/j.pmrj.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992;13:25–36. [DOI] [PubMed] [Google Scholar]
- 35.Mangione KK, McCully K, Gloviak A, Lefebvre I, Hofmann M, Craik R. The Effects of High-Intensity and Low-Intensity Cycle Ergometry in Older Adults with Knee Osteoarthritis.; 1999. [DOI] [PubMed]
- 36.Bennell KL, Dobson F, Hinman RS. Exercise in osteoarthritis: Moving from prescription to adherence. Best Pract. Res. Clin. Rheumatol. 2014;28(1):93–117. doi: 10.1016/j.berh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Knarr BA, Higginson JS, Zeni JA. Change in knee contact force with simulated change in body weight. Comput. Methods Biomech. Biomed. Engin. 2015:1–4. doi: 10.1080/10255842.2015.1018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen R, Henriksen M, Leeds AR, et al. Effect of Weight Maintenance on Symptoms of Knee Osteoarthritis in Obese Patients: A Twelve-Month Randomized Controlled Trial. Arthritis Care Res. (Hoboken). 2015;67(5):640–650. doi: 10.1002/acr.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holden M a, Nicholls EE, Young J, Hay EM, Foster NE. Role of exercise for knee pain: what do older adults in the community think? Arthritis Care Res. (Hoboken). 2012;64:1554–64. doi: 10.1002/acr.21700. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 41.United States Department Labor. Bureau of Labor Statistics. Sports and Exercise: BLS Spotlight on Statistics. Available at: http://www.bls.gov/spotlight/2008/sports/. Accessed December 21, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Associations between predicted probabilities of one-year improving symptoms and daily physical activity by Kellgren-Lawrence grades.