Abstract
Objectives
To assess the 3-month impact on physical function of a program for community-dwelling frail older adults, based on the integration of primary care, geriatric medicine, and community resources, implemented in “real life”.
Design
Interventional cohort study.
Setting
Primary care in Barcelona, Spain.
Participants
Individuals aged ≥80 years (n=134), presenting at least one sign of frailty (i.e., slow gait speed, weakness, memory complaints, involuntary weight loss, poor social support). Intervention: After frailty screening by the primary care team, candidates were referred to a geriatric team (geriatrician + physical therapist), who performed a comprehensive geriatric assessment and designed a tailored multidisciplinary intervention in the community, including a) multi-modal physical activity (PA) sessions, b) promotion of adherence to a Mediterranean diet c) health education and d) medication review.
Measurements
Participants were assessed based on a comprehensive geriatric assessment including physical performance (Short Physical Performance Battery -SPPB- and gait speed), at baseline and at a three month follow-up.
Results
A total of 112 (83.6%) participants (mean age=80.8 years, 67.9% women) were included in this research. Despite being independent in daily life, participants’ physical performance was impaired (SPPB=7.5, SD=2.1, gait speed=0.71, SD=0.20 m/sec). After three months, 90.2% of participants completed ≥7.5 physical activity sessions. The mean improvements were +1.47 (SD 1.64) points (p<0.001) for SPPB, +0.08 (SD 0.13) m/sec (p<0.001) for gait speed, −5.5 (SD 12.10) sec (p<0.001) for chair stand test, and 53% (p<0.001) improved their balance. Results remained substantially unchanged after stratifying the analyses according to the severity of frailty.
Conclusions
Our results suggested that a “real-world” multidisciplinary intervention, integrating primary care, geriatric care, and community services may improve physical function, a marker of frailty, within 3 months. Further studies are needed to address the long-term impact and scalability of this implementation program.
Key words: Frailty, integrated care, physical activity, primary care
Introduction
Frailty, a dynamic state of decreased biological reserves and vulnerability to stressors, increases the risk of negative health events, such as a rapid progression towards disability, falls, fractures, institutionalization, and death (1). Frailty is potentially reversible (2, 3). Therefore, its early assessment, recognition and management represent an important component of preventive strategies within healthcare systems as well as a challenge (4).
Various studies have shown that interventions aimed to detect at an early stage and manage frailty are effective (2, 5–11). These interventions incorporate individualized actions aimed to reduce or slow down the progression of frailty towards disability and, consequently, to reduce and/ or eliminate the potential negative consequences on health. Due to the multifactorial nature of frailty, interventions targeting frailty in the community should also be multifactorial, and designed according to the results of a comprehensive geriatric assessment (CGA) when possible [5–9, 12, 13]. Among other interventions, the promotion of physical activity is crucial (14). Such programs may find an ideal place in primary care settings, integrating the expertise of a geriatric team (15). Different studies have approached frailty in the primary care or community setting (5–9, 13), but most of them had rigidly controlled experimental conditions and schemes and their submission to specific funds make the subsequent sustainability, implementation and scaling-up of the same activities in real life, difficult (5, 7–9). Under these circumstances, we recently proposed different recommendations to design and develop complex pragmatic interventions aimed at comprehensively managing frailty (16). In agreement with these recommendations, we have designed a multifactorial person-centered intervention against frailty (16).
The aim of the present study is to assess a 3-month impact on physical function of a “real life” program for frail older adults in the community, based on the integration of primary care, geriatric medicine, and community services.
Methods
The methodology of the +AGIL Barcelona program (“Atenció primària i Geriatria Integrades amb visió Longitudinal” in Catalan) is described elsewhere (16). Shortly, it stems from a strategic alliance between the principal public provider of primary care in Barcelona (Institut Català de la Salut, Àmbit d’Atenció Primària de Barcelona) and the major public provider of intermediate care, geriatrics and palliative care in Catalonia (Parc Sanitari Pere Virgili). The program is conducted in the primary care center of Bordeta-Magòria (Barcelona, Spain), which provides healthcare services to 32,340 citizens (20% aged ≥65 years, 6.9%≥80 years). The protocol was approved by the Clinical Research Ethics Committee of the Institut Universitari d’Investigació en Atención Primaria, Jordi Gol.
+AGIL Barcelona began in July 2016 as an additional service and as a result, as part of the geriatric outpatient clinic which was transferred to the community. The geriatric team, including a geriatrician and a physical therapist, attends the primary care center once a week in order to assess the patients referred by the primary care team (i.e., family physicians, nurses, social workers). The program also fosters the subsequent maintenance of physical activity through the existing community resources. Together with the nearby civic center we co-designed a continuation activity which initially is performed under the supervision of a trainer and progressively shifts towards self-practice. The implementation includes refinement with end-users (both professional and participants) (16).
Study population
Primary care is in charge of identifying the potential beneficiaries of the intervention. The screening strategy is generally directed to older adults without physical disability and/or acute clinical disease, aged ≥80 years and presenting at least one sign of frailty (i.e., slow gait speed, weakness, memory complaints, involuntary weight loss, or poor social support). In particular, the Gerontopôle Frailty Screening Tool (GFST), an 8-item questionnaire validated for frailty screening in primary care (17), was selected by a primary care team to support the identification of possible candidates. Being this is a “real life” program, different from a rigid clinical trial, the referral is flexible depending on the judgment of the primary care team and eventually include individuals with a higher degree of frailty (i.e. with some already established disability) or “less than frail” (mostly sedentary), as previously detailed (16).
Assessment
The intervention follows the principles of the Frailty Intervention Trial (9), being tailored according to the problems detected through a CGA conducted by the geriatrician and the physical therapist. Socio-demographic data (age, sex, marital status, living alone), clinical characteristics (past medical history, Charlson comorbidity index (18), and current treatment), functional status (Barthel index for basic [0–100 points] (19) and Lawton index for instrumental activities for daily living [0–8 points] (20)), nutrition (Mini Nutritional Assessment-Short Form [MNA-SF®] (21) plus a standardized assessment of adherence to a Mediterranean diet (22)), cognition (Mini-cog® (23)), depressive symptoms (Geriatric Depression Scale (24)), and physical function (Short Physical Performance Battery [SPPB, 0–12 points] (25)) were collected. Frailty status was measured using the Clinical Frailty Scale (CFS) (26).
Intervention
The participant and their family or caregiver received the results of the CGA the same day of the evaluation. Through a shared decision-making process, carried out on the basis of motivational interviewing techniques, the geriatrician proposed a tailored strategy to achieve shared goals. The participants retained a copy of the agreements, set goals, and recommendations in plain language. Primary care staff could join geriatric visits and had access to the complete report through the shared electronic health records (EHR).
The multifactorial intervention, as previously described (16) included:
Physical activity. After a first individual assessment, an expert physical therapist recommended an adapted exercise program to be performed at home, which included strength, resistance, balance, and coordination exercises. The therapist ran a supervised program including up to 10 sessions (1 h/ week) of multi-modal group exercises, where group socialization was also promoted. The therapist modulated the exercises according to each participant’s capacity and needs, in terms of intensity, time and type (including dual-tasking if needed). The exercises had a functional and significant character in order to increase the therapeutic adherence and make the exercises similar to the daily activity of the participant. Different strategies were directed to empower the person and to foster adherence to physical activity over time, including the delegation of the participant to lead their last group session for a short time, the recommendation of personalized exercises as “homework” and the indication of existing community resources to maintain physical activity after the 10 sessions. The Vivifrail® app or a printed chart of tailored exercises derived from it, is given to each participant as complementary material (27), and with positive reinforcement based on motivational interviewing.
Nutrition. The intervention aimed at promoting adherence to a Mediterranean diet, following the Prevention with Mediterranean diet (PREDIMED) intervention paradigm (28), and took into account the caloric and protein intake needs.
Health education. This included the promotion of healthy habits, reinforcing ongoing activities in the primary care center (e.g., smoke or alcohol control) or others (e.g., sleep hygiene, fall prevention recommendations).
Adequacy of pharmacological treatment. In agreement with the family physician, the geriatrician reviewed the patients’ medication to increase value-based prescriptions. No standardized tool to test the medication adequacy was used owing to the presence of a geriatrician with specific expertise on medication review and de-prescribing. The geriatrician was also remotely supported, in case of need, by the clinical pharmacist working at the intermediate care hospital.
Both the therapist (on a weekly basis, when running the group activity) and the geriatrician (in one single follow-up visit at 3 months) monitored the intervention plan over time. Subsequently, the patient kept the usual follow-up with the referent primary care professional, who continued promoting the achievement of the initially shared goals. The geriatrician’s intervention remained on demand. The coordination/interaction of the primary care and geriatric teams was facilitated by a shared EHR and formal meetings on regular basis.
Outcomes
The primary outcome of interest was the 3-month, pre/post intervention modification of physical performance (measured by either SPPB and/or its sub-item gait speed). As a secondary outcome, the change in the CFS was explored.
Statistical analyses
Baseline characteristics of the sample are presented as mean values and standard deviation (SD) for continuous variables, median values and interquartile range (IQR) for ordinal variables and percentages for categorical variables. Differences among participants included in the intervention and those who were missing, were analyzed using the Student’s t-test or the Mann-Whitney U-test and Chi-square test, as appropriated.
The pre/post intervention analysis was done by a paired sample t-test for repeated samples or Wilcoxon signed-rank for continuous variables and Chi-square for categorical variables. For those participants who were unable to perform the chair stand test (n=9), the value of 61 seconds was imputed according to the reference categories previously published (25). The Analysis of variance (ANOVA) and Jonckheere-Terpstra trend test were used for continuous variables when the analyses were stratified by the frailty degree according to 3 CFS categories (“Managing well or fit”, “vulnerable”, “any degree of frailty”).
A sensitivity analysis was also performed considering the participants who did not undergo the follow-up assessment because of incident events, or for other reasons that could not be ascertained. In these cases, the worst change of the assessed group for either SPPB (3 points loss), gait speed (0.32 m/sec loss), chair stand test (45.6 sec increase) or balance impairment (an impairment at follow-up) were imputed.
Finally, changes in the CFS between baseline and three-month follow-ups were analyzed by the Chi-square test.
In all analyses, p-values <0.05 were regarded as statistically significant. The 95% confidence intervals (95%CI) were calculated. Analyses were performed using Stata version 14.
Results
From July 2016 to July 2018, 134 individuals out of 180 screened (74.4 %) accepted to participate in the program (Fig 1). Twenty-two (16.4%) missed the follow-up (5 refused to undergo the follow-up visit after correctly completing ≥75% of physical activity sessions and without any incident event, 14 because of an intercurrent medical event, and 3 did not attend the follow-up visit despite the absence of medical complications). Finally, 112 individuals (mean [SD] age=80.8 [SD 5.8] years; 67.9% women) were included in the analysis. Baseline characteristics were comparable between the 112 included participants and the 17 excluded because of events or unknown reasons (Table 1).
Figure 1.
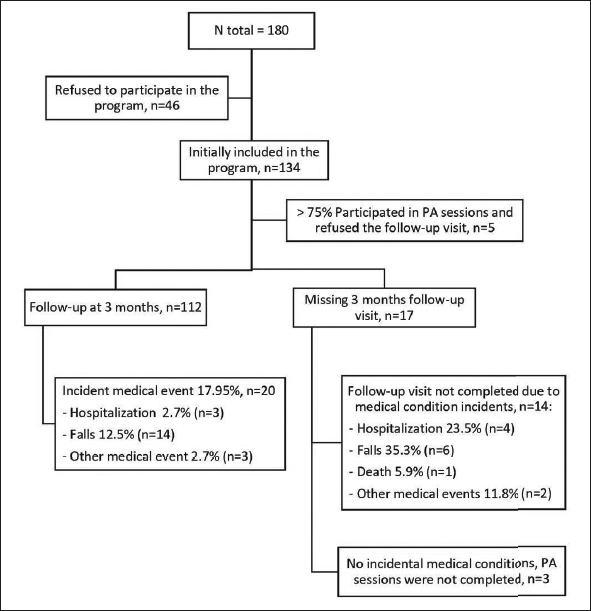
Population follow chart
PA: Physical activity.
Table 1.
Baseline characteristics of the sample
| Baseline characteristics | Included n= 112 | Missing n=17 | p |
|---|---|---|---|
| Age, mean (SD) | 80.9 (5.8) | 82.4 (4.1) | 0.210 |
| Woman, % (n) | 67.9 (76) | 88.2 (15) | 0.086 |
| Barthel index a, median (IQR) | 95 (90–100) | 95 (85–100) | 0.494 |
| Lawton index b, median (IQR) | 6 (4–8) | 7 (4–8) | 0.617 |
| Lives alone, % (n) | 42.0 (47) | 64.7 (11) | 0.079 |
| Education, % (n) | |||
| Illiterate | 4.5 (5) | 12.5 (2) | 0.131 |
| Primary school | 32.1 (36) | 50.0 (8) | |
| Secondary school | 49.1 (55) | 37.5 (6) | |
| University degree | 14.3 (16) | 0 (0) | |
| Adequate physical activity c, % (n) | 38.4 (43) | 23.5 (4) | 0.235 |
| Falls in the last year, % (n) | 34.8 (39) | 41.2 (7) | 0.610 |
| Malnutrition risk d, % (n) | |||
| Normal nutrition status | 61.6 (69) | 76.5 (13) | 0.209 |
| At risk of malnutrition | 36.6 (41) | 17.7 (3) | |
| Malnourished | 1.8 (2) | 5.8 (1) | |
| Charlson Comorbidity, median (IQR) | 1 (0–2) | 2 (1–4) | 0.052 |
| Previous diagnosis of cognitive impairment, % (n) | 18.8 (21) | 25.0 (4) | 0.483 |
| Number of drugs, median (IQR) | 7 (5–9) | 9 (6–10) | 0.171 |
| Gerontopôle FST positive, % (n) | 94.6 (106) | 100 (17) | 0.328 |
| Clinical Frail Scale - vulnerable or any frailty degree, % (n) | 63.4 (71) | 64.7 (11) | 0.917 |
| SPPB e, mean (SD) | 7.48 (2.12) | 7.18 (2.60) | 0.650 |
| Gait speed, mean (SD) | 0.71 (0.20) | 0.67 (0.20) | 0.486 |
| Chair stand test, mean (SD) | 22.40 (13.67) | 24.52 (18.20) | 0.898 |
| Balance impairment, % (n) | 43.8 (49) | 58.8 (10) | 0.245 |
| Physical activity sessions, mean (SD) | 9.2 (1.9) | 5.4 (3.1) | <0.001 |
IQR: interquartile range, SD: standard deviation. Student’s t-test or the Mann-Whitney U-test were used for continuous variables as appropriated and Chi-square test for categorical. a. Independence for activities of daily living, range from 0–100. b. Independence for instrumental activities of daily living, range from 0–8. c. At least 30 min of physical activity 3 times/ week (based on WHO recommendation). d. Mini-Nutritional Assessment Short form score: 8–11 points. e. Short Physical Performance Battery, range from 0–14
The 112 included participants were generally independent for basic Activities of Daily Living (ADL, median Barthel Index=95, IQR=90–100) and instrumental ADL (IADL, median Lawton’s score=6, IQR=4–8), had a relatively low comorbidity (median Charlson index=1, IQR=0–2), but presented polypharmacy (median number of drugs=7, IQR=5–9) and low physical performance (SPPB mean=7.5 [SD 2.1] and gait speed mean=0.7 [SD 0.2]).
Regarding the intervention, physical activity was performed in the whole sample, with a high adherence (90.2% attended ≥75% session, mean [SD]=9.2 [SD 1.9] out of 10 planned sessions), as well as health education interventions and nutritional recommendations, whereas pharmacological treatment was modified in 76 (67.9%) participants.
Regarding the primary outcomes, after three months, the SPPB and gait speed improvements were +1.47 [SD 1.64] points (p<0.001) and +0.08 [SD 0.13] m/sec (p<0.001) respective, as is shown in Table 2. Improvement in chair stand test (−5.5 [SD 12.1], p<0.001), and balance (53%, p<0.001) were also analyzed. Results remained substantially unchanged after stratifying the analyses by frailty degree (Table 3). However, no linear association between improvement and frailty degrees were found. Additionally, after imputing the worst observed changes in the 17 participants without the 3 month visit because of events or for unknown reasons, we confirmed statistically significant improvement in SPPB (+0.88 [SD 2.16] points, p<0.001), chair stand (−10.83 [SD 17.7] sec, p<0.001), balance (44.1% improved, p<0.001), and a nonsignificant improvement in gait speed (+0.02 [SD 0.20] sec, p=0.301).
Table 2.
Effect of the Multifactorial Intervention on physical function capacity
| Before | After | Difference (95%CI) | p | |
|---|---|---|---|---|
| SPPB a, mean (SD) | 7.48 (2.12) | 8.94 (2.03) | 1.47 (1.16 – 1.78) | <0.001 |
| Gait speed (m/sec), mean (SD) | 0.71 (0.20) | 0.79 (0.18) | 0.08 (0.05 – 0.10) | <0.001 |
| Balance impairment, % (n) | 43.8 (49) | 20.5 (23) | 53.1 (26) | <0.001 |
| Chair stand test (sec), mean (SD) | 22.40 (13.63) | 17.00 (10.62) | -5.50 (−7.78 – −3.22) | <0.001 |
SD: standard deviation; a. SPPB: Short Physical Performance Batteiy, range from 0 to 12; 90.2% of participants attended ≥75% session, mean (SD)=9.2 (1.9) sessions; For those participants unable to perform the chair stand test (n=9), we imputed a value of 61 seconds according to the reference categories previously published (25); Paired sample t-test for repeated samples or Wilcoxon signed-rank were used for continuous variables as appropriated and chi square for categorical variables.
Table 3.
Effect of the Multifactorial Intervention on physical function capacity stratified by a frailty degree according to the Clinical Frailty Scale
| Fit, Stable, managing well (CFS 1–3), n=41 | Vulnerable (CFS 4–6), n=48 | Any grade of Frailty (CFS 7–9), n=23 | p for trend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Difference (95% IC) | p | Before | After | Difference (95% IC) | p | Before | After | Difference (95% IC) | p | ||
| SPPB a, mean (SD) | 8.43 | 9.68 | 1.25 | <0.001 | 7.13 | 8.67 | 1.54 | <0.001 | 6.52 | 8.21 | 1.70 | <0.001 | 0.279 |
| (2.05) | (2.08) | (0.81 – 1.69) | (2.02) | (1.95) | (.99 – 2.09) | (1.90) | (1.76) | (1.04 – 2.35) | |||||
| Gait speed (m/sec), mean (SD) | 0.77 | 0.86 | 0.08 | <0.001 | 0.71 | 0.78 | 0.07 | 0.005 | 0.60 | 0.69 | 0.09 | <0.001 | 0.940 |
| (0.18) | (0.17) | (0.05 – 0.12) | (0.21) | (0.17) | (0.02 – 0.11) | (0.14) | (0.16) | (0.04 – 0.13) | |||||
| Balance impairment, % (n) | 31.7 | 14.6 | 53.8 | 0.001 | 52.1 | 25.0 | 52.0 | 0.009 | 47.8 | 21.7 | 54.5 | 0.043 | 0.540 |
| (13) | (6) | (7) | (25) | (12) | (13) | (11) | (5) | (6) | |||||
| Chair stand test (sec), mean (SD) | 18.28 | 13.97 | −4.32 | 0.002 | 25.58 | 18.16 | −7.43 | <0.001 | 23.37 | 19.83 | −3.55 | 0.235 | 0.352 |
| (9.99) | (4.85) | (−6.89 – −1.75) | (15.91) | (11.91) | (−11.45 – −3.41) | (13.92) | (13.84) | (−9.56 – −2.47) | |||||
SD: standard deviation; a. SPPB: Short Physical Performance Battery, range from 0 to 12; The Analysis of variance (ANOVA) and Jonckheere-Terpstra trend test were used for continuous variables as appropriated and Chi-Square for categorical variables.
Analyzing the change of frailty degree at 3 months, according to the CFS, 14.3% of the participants experienced progression towards a higher level of frailty, 64.3% remained stable, and 21.43% improved of some degree (p<0.001).
Discussion
Our results show that +AGIL Barcelona, a multifactorial intervention program for older community-dwellers, based on the integration of primary care, geriatric medicine and community services, has a positive impact on improving frailty. The reported improvement of physical function was statistically and clinically significant, as meeting well-established criteria for meaningful changes in SPPB (0.3–0.8 points) (29) and gait speed (at least 0.05 m/s) [30]. The benefits were consistent across different initial frailty degrees, from milder to more advanced.
Strong clinical evidence from intervention studies support the positive impact of multifactorial and multidisciplinary program interventions on frailty in order to improve physical function (5, 7–9, 11) and revert frailty (10). In this sense, our results are in line with the available literature. Specifically, +AGIL Barcelona effect size at 3 months is similar to a local study that offered higher physical activity intensity (7) and greater than those reported in a systematic review that analyzes the effect of physical activity on SPPB and gait speed in older adults (14).
Despite the similarities in the multidisciplinary and multifactorial approach, with a common component such as the promotion of physical activity, there are differences and novelties worth noting in +AGIL Barcelona. First, it was and is still implemented in real life and did not depend on specific research funding. In other words, it broke the rigid structure of traditional research methodology concerning the inclusion/ exclusion criteria. For example, dementia or severe frailty did not constitute reasons for being excluded from participation in +AGIL Barcelona (5, 7–11). In fact, the screening and referral of +AGIL Barcelona relied much on the clinical judgment of primary care teams, as requested by the GFST (17). It is believed that the complementary approach between the continuum of care offered by primary care and the time-limited specialized geriatric intervention represents an added value for the program. Second, in line with the principles of the Frailty Intervention Trial described by Cameron et.al., (9) +AGIL Barcelona proposed a flexible and adaptable intervention within a definite range of possibilities, based on shared decision-making, and far from a “one-fits-all” approach or rigid standard intervention. This might have promoted the adequacy of the intervention and might very well reduce overtreatment (12). The contribution of specialized professionals such as the geriatrician and the physical therapist favors the person-centered adaptation. Third, the promotion of physical activity mainly aimed at fostering user’s empowerment, resulted in the intervention becoming sustainable over the long term. We speculate this might be the justification for the similar 3-month impact of +AGIL Barcelona compared to existing literature (5, 7, 9). Despite the resemblance with the other intervention components, +AGIL Barcelona offers a relatively lower frequency and duration of supervised physical activity (60-minute sessions, once a week for 10 weeks). In the aim of the project, the different empowerment strategies (including the motivational approach, the recommendations for self-practice through a validated existing tool such as ViviFrail® (27), the delegation to the participant during the sessions) are meant to compensate the reduced frequency and duration of directed physical activity.
It is important to remark that +AGIL Barcelona is sustainable through a reorganization of existing resources. During the refinement phase of the program, we also requested feedback from participants about the possible lack of adherence to the different interventions, including a higher weekly frequency of the physical activity program. The relatively reduced frequency, together with other factors (such as the “prescription” by reference primary care and the motivational approach) might explain the high adherence to the proposed activities compared to other programs (5, 7–9).
According to the realistic theoretical framework of the reasons why frailty interventions may or may not work, recently proposed by Gwyther et al. (12), interventions need to be co-designed, multicomponent, include physical activity in a group setting that promotes social interaction and psychological techniques in order to perform a person-centered care intervention which promotes lifestyle changes and patient empowerment. We had highlighted very similar requirements in our previous methodological paper (16), and +AGIL Barcelona seems in line with most of it.
Among the strengths of our study, +AGIL Barcelona is a “real world” intervention program with a continuative implementation, and we have already highlighted the positive differential elements and the potential advantages compared to previous intervention studies. The relatively low number of missing data at the follow-up is also remarkable. We also acknowledge different limitations in our study: the absence of a control group and the lack of randomization do not allow conclusive evidence on the impact of this intervention. However, building on existing evidence, the study stems from a spontaneous change occurred in our clinical practice, shifting from the classical approach towards a new integrated care model. Second, the follow-up period is relatively short. However, potential maintenance or improvement in physical function even at 3 months, in such an older and frail population, is already a relevant achievement for the individual and for society.
In conclusion, +AGIL Barcelona shows the implementation and evaluation of a multifactorial, interdisciplinary, and integrated care program for frail older adults, which is based on the reorganization of existing resources. This data suggests potential for scaling-up and replicating similar initiatives in other areas, after contextualization with local specificities. From a general perspective, our results reinforce the urgent need of shifting towards a change in paradigms for the management of frailty before the establishment of the disabling process.
Acknowledgements
We would like to thank all the +AGIL Barcelona participants and the contribution of Ana Pomares, primary care social worker, Aracelli Abilla, primary care physician, Lydia Fumado, primary care nurse of the Bordeta-Magòria center, Paola Mulero, head of Civic Center Casal Cívic Magòria and Heidi Schmidt for review and edit the manuscript.
Funding: This implementation program has been developed using internal funding from the participating institutions, Parc Sanitari Pere Virgili and Institut Català de la Salut.
Ethical Standards: The protocol was approved by the Clinical Research Ethics Committee of the Institut Universitari d’Investigació en Atención Primaria, Jordi Gol and comply with the current laws of Spain.
Conflicts of interest: The authors have no financial or personal conflicts to disclose.
References
- 1.Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, et al. Frailty: An Emerging Public Health Priority. J Am Med Dir Assoc. 2016;17:188–92. doi: 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions Between Frailty States Among Community-Living Older Persons. Arch Intern Med. 2006;166:418. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet. 2015;385:e7–e9. doi: 10.1016/S0140-6736(14)61595-6. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty Consensus: A Call to Action. J Am Med Dir Assoc. Elsevier Ltd. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, et al. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J Am Med Dir Assoc. Elsevier Inc. 2016;17:426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Tavassoli N, Guyonnet S, Abellan Van Kan G, Sourdet S, Krams T, Soto M-E, et al. Description of 1,108 older patients referred by their physician to the “Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability” at the gerontopole. J Nutr Health Aging. 2014;18:457–64. doi: 10.1007/s12603-014-0462-z. [DOI] [PubMed] [Google Scholar]
- 7.Romera-Liebana Laura, Orfila Francesc, Segura Josep Maria, Real Jordi, Fabra Maria Lluïsa, Möller Mercedes, Lancho Santiago, Ramirez Anna, Marti Nuria, Cullell Montserrat, Bastida Nuria, Martinez Dolors, Giné Maria, Cendrós Patricia, Bistuer Anna, Perez Elena, Fabregat Maria Assumpta, Foz Gonçal. Effects of a Primary Care-Based Multifactorial Intervention on Physical and Cognitive Function in Frail, Elderly Individuals: A Randomized Controlled Trial. The Journals of Gerontology: Series A. 2018;73(12):1668–1674. doi: 10.1093/gerona/glx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gené Huguet L., Navarro González M., Kostov B., Ortega Carmona M., Colungo Francia C., Carpallo Nieto M., Hervás Docón A., Vilarrasa Sauquet R., García Prado R., Sisó-Almirall A. Pre Frail 80: Multifactorial Intervention to Prevent Progression of Pre-Frailty to Frailty in the Elderly. The journal of nutrition, health & aging. 2018;22(10):1266–1274. doi: 10.1007/s12603-018-1089-2. [DOI] [PubMed] [Google Scholar]
- 9.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theou O, Park GH, Garm A, Song X, Clarke B, Rockwood K. Reversing Frailty Levels in Primary Care Using the CARES Model. Can Geriatr J. 2017;20:105–111. doi: 10.5770/cgj.20.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–96. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwyther H, Bobrowicz-Campos E, Apóstolo JLA, Marcucci M, Cano A, Holland C. A realist review to understand the efficacy and outcomes of interventions designed to minimise, reverse or prevent the progression of frailty. Health Psychol Rev. 2018;7199:1–23. doi: 10.1080/17437199.2018.1488601. [DOI] [PubMed] [Google Scholar]
- 13.Cesari M, Demougeot L, Boccalon H, Guyonnet S, Vellas B, Andrieu S. The Multidomain Intervention to preveNt disability in ElDers (MINDED) project: rationale and study design of a pilot study. Contemp Clin Trials. 2014;38:145–54. doi: 10.1016/j.cct.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:753–769.e3. doi: 10.1016/j.apmr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 15.WHO ~ WHO Guidelines on Integrated Care for Older People (ICOPE). WHO. World Health Organization; 2018
- 16.Inzitari Marco, Pérez Laura Mónica, Enfedaque M. Belén, Soto Luís, Díaz Francisco, Gual Neus, Martín Elisabeth, Orfila Francesc, Mulero Paola, Ruiz Rafael, Cesari Matteo. Integrated primary and geriatric care for frail older adults in the community: Implementation of a complex intervention into real life. European Journal of Internal Medicine. 2018;56:57–63. doi: 10.1016/j.ejim.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Vellas B, Balardy L, Gillette-Guyonnet S, Abellan Van Kan G, Ghisolfi-Marque A, Subra J, et al. Looking for frailty in community-dwelling older persons: the Gérontopôle Frailty Screening Tool (GFST) J Nutr Health Aging. 2013;17:629–31. doi: 10.1007/s12603-013-0363-6. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney FI, Barthel DW. Funtional evaluation: The Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782–8. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 22.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 23.Carnero-Pardo C, Cruz-Orduña I, Espejo-Martínez B, Martos-Aparicio C, López-Alcalde S, Olazarán J. Utility of the Mini-Cog for Detection of Cognitive Impairment in Primary Care: Data from Two Spanish Studies. Int J Alzheimers Dis. 2013;2013:1–7. doi: 10.1155/2013/285462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez J, Onís MC, Dueñas R, Albert C, Aguado C, Luque R. Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Medifam. 2002;12:620–630. [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: associa-tion with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izquierdo M, Rodriguez-Mañas L, Sinclair AJ. What is new in exercise regimes for frail older people — How does the Erasmus Vivifrail Project take us forward? J Nutr Health Aging. 2016;20:736–737. doi: 10.1007/s12603-016-0702-5. [DOI] [PubMed] [Google Scholar]
- 28.Zazpe I, Sanchez-Tainta A, Estruch R, Lamuela-Raventos RM, Schröder H, Salas-Salvado J, et al. A Large Randomized Individual and Group Intervention Conducted by Registered Dietitians Increased Adherence to Mediterranean-Type Diets: The PREDIMED Study. J Am Diet Assoc. 2008;108:1134–1144. doi: 10.1016/j.jada.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–44. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]


