Abstract
The United States lags far behind other industrialized countries on major markers of population health. Population health experts identify unhealthy behavior patterns (e.g., cigarette smoking, other substance use disorders, physical inactivity and poor food choices, nonadherence with recommended medical regimens) as the largest contributor to the status quo. Because these risk behaviors are overrepresented in socioeconomically disadvantaged and other vulnerable populations, they also increase health disparities. Hence, identifying evidence-based strategies to promote and sustain health-related behavior change is critical to improving U.S. population health. In this report, we review research demonstrating the efficacy of voucher-based contingency management delivered alone or in combination with other interventions for treating substance use disorders and other health-related behavior problems. The efficacy supporting these interventions is robust and discernible at the level of controlled randomized clinical trials and meta-analyses. Unfortunately, these evidence-based interventions are being underutilized in routine clinical care for substance use disorders, although they are used broadly in private-sector wellness programs and international programs to reduce chronic poverty. This report reviews the evidence supporting the efficacy of voucher-based contingency management using projects developed at the University of Vermont as exemplars and discusses dissemination of the model to public and private sector efforts to improve individual and population health.
Keywords: Cigarette smoking, Nicotine, Substance use disorders, Reinforcement, Financial incentives, Vulnerable populations, Contingency management, Community reinforcement
Introduction
The focus of this report is on reducing drug use and promoting other health-related behavior changes, but it is helpful to understand this effort in a larger context. U.S. population health is poor relative to other industrialized nations (Higgins, 2014; Schroeder, 2016) despite spending orders of magnitude more on health care than any other country (Woolf & Aron, 2013). The United States ranks near the bottom among industrialized nations on key measures of population health including infant mortality, longevity from birth, and years of life lost before age 50 (Higgins, 2014; Kaplan, 2014; Schroeder, 2007, 2016). Among the multiple contributors to population health, the domain of personal behavior offers the greatest potential for improvements (Schroeder, 2007, 2016). Substance use is the largest but by no means the only contributor to this relation between personal behavior patterns (lifestyle) and poor health outcomes (Avendano & Kawachi, 2014; Centers for Disease control and Prevention, 2019; Higgins, 2014; Schroeder, 2016; Woolf & Aron, 2013). The current opioid addiction epidemic is a striking and highly lethal example (Compton, Boyle, & Wargo, 2015; Sigmon et al., 2016; Volkow & Collins, 2017). Unhealthy personal behavior patterns, especially cigarette smoking and other substance abuse, and physical inactivity and unhealthy food choices (obesity), being responsible for approximately 40% of annual U.S. premature deaths (Avendano & Kawachi, 2014; Danaei et al., 2009; Higgins, 2014; Schroeder, 2016; U.S. Department of Health & Human Services, 2014; Woolf & Aron, 2013). The development of more effective strategies to promote behavior change is critical to resolving this problem.
Considerable scientific effort has been devoted to understanding the motivational processes underpinning substance use and addiction and that work leaves no question the reinforcement process plays a fundamental role (Bouton & Trask, 2016; Catania, 2013). It is clear is that cocaine, nicotine, opioids, and other abused drugs all stimulate dopamine-based mesolimbic brain reward centers, which directly increases the likelihood that these same activities will be repeated in the future (Kelley & Berridge, 2002). These reward pathways evolved to assure that humans and other organisms would learn to (a) consume basic nutrients necessary to sustain themselves, (b) engage in reproductive behavior to sustain the species, and (c) engage in other similarly adaptive behavior patterns. When considered in the context of reinforcement’s connection to individual and species survival, the powerful control that abused drugs can exert is less perplexing. It is also important to underscore that the control that these reinforcers exert is enhanced considerably when they are contacted under conditions with few competing sources of healthier reinforcement due to poverty or mental illness or other disabilities that limit one’s opportunity or ability to engage effectively in complex environments (e.g., Higgins, Bickel, & Hughes, 1994; Higgins, 1997; Higgins, Heil, & Lussier, 2004).
The overarching aim of the research reviewed below is to demonstrate how that same reinforcement process can be leveraged to reduce drug use and promote other health-related behavior change. We review research illustrating how systematically offering vouchers exchangeable for retail items, or similar monetary reinforcers, contingent on objectively verified behavior change has been successfully used for treating cocaine dependence (Higgins, Heil, & Lussier, 2004), reducing cigarette smoking during pregnancy (Higgins et al., 2012), and reducing other types of health-related behavior problems including unplanned pregnancies (Heil et al., 2016) and poor adherence with medical regimens (Gaalema et al., 2016). We review evidence from meta-analyses and randomized clinical trials (RCTs), with research conducted by investigators at the University of Vermont to illustrate the latter. We focus on research conducted at the University of Vermont where this practice of offering vouchers contingent on objective evidence of health-related behavior change was developed and as detailed below applied effectively to a variety of different clinical conditions.
Intervention for Cocaine-Dependent Outpatients
The U.S. cocaine epidemic in the 1980s and 1990s (Higgins & Katz, 1998) was comparable to the current opioid epidemic in terms of having an unanticipated adverse national impact on individual and population health, although it was not associated with the high levels of lethal overdose common with opioids corresponding to their effect of suppressing respiration rate. Nationally representative surveys administered during this time indicated that cocaine use peaked around 1985 with approximately 5.8 million users, but persisted at epidemic levels well into the 1990s, with current levels at 1.9 million and 4.3 million of misusers of other psychomotor stimulants are included (Substance Abuse & Mental Health Services Administration, 2017). Concerns are being raised in the scientific literature (Shiels, Freedman, Thomas, & Berrington de Gonzalez, 2018) and mainstream media (Frakt, 2018) about the possibility of another U.S. cocaine epidemic.
Turning back to the earlier epidemic, clinical and scientific communities were unprepared to deal with the adverse consequences of such rampant cocaine use and with the resulting cocaine-related death and disability. Indeed, the program of research described below was conducted in a context in which behavioral and pharmacological treatments targeting cocaine addiction were failing miserably. Moreover, the neuropharmacology of cocaine (i.e., mechanisms of action including brain dopamine systems) suggested substantial obstacles to developing an efficacious medication that reduced cocaine use while leaving the dopamine systems sufficiently intact to support basic everyday functioning. Those obstacles to treating cocaine use disorders pharmacologically remain today. There is currently no efficacious pharmacotherapy for cocaine use disorders. It was in this context that the use of voucher-based CM, or financial incentives in general, gained a toehold in the domain of health promotion when it was used as part of a multicomponent intervention for outpatient treatment of cocaine dependence, known as the Community Reinforcement Approach (CRA) plus Vouchers intervention (Higgins et al., 1993). This intervention offered 24 weeks of structured individual counseling based on an adaptation of the CRA intervention for alcohol use disorders (Hunt & Azrin, 1973) in combination with a 12-week CM intervention that provided vouchers exchangeable for retail items contingent on objective evidence of recent abstinence from cocaine use.
CRA + Vouchers Treatment
The overarching aim of the CRA component is to assist patients in engaging with resources in their community that will reinforce healthy, drug-free lifestyle choices that over time can come to compete with the allure of obtaining reinforcement through drug use. CRA integrates several treatment components, including building motivation to abstain from drug use, reengaging with earlier sources or identifying new sources of nondrug reinforcement (e.g., healthy forms of recreation and entertainment), and developing new coping skills (e.g., drug refusal, managing negative affect, sleep hygiene), all of which are designed to teach an individual how to recruit nondrug sources of reinforcement for healthy living and decrease relapse risk in his or her surrounding community.
In brief, cocaine-dependent individuals received a 24-week treatment intervention and a subsequent 24 weeks of aftercare. The initial 12 weeks consisted of twice weekly counseling with thrice weekly urine toxicology testing to detect any cocaine use during the initial 12 weeks. This schedule of counseling and urine toxicology testing was subsequently thinned to once weekly counseling and twice weekly urinalysis during weeks 13–24, and weeks 25–52 consisted of an aftercare program wherein participants completed once-monthly check-ins with a counselor and random urinalysis. During those initial 12 weeks, patients earned vouchers for providing objective evidence of cocaine abstinence. The rationale for including the incentive program in the initial 12 weeks was in recognition that assisting patients with establishing naturalistic sources of reinforcement for a healthy lifestyle (e.g., establishing healthy social relationships, securing a meaningful job, learning how to recreate without drugs involved) takes time, and during that period a lack of reinforcement relative to what patients were used to in the cocaine-abusing lifestyle could precipitate treatment dropout and relapse. Thus, these contrived sources of relatively immediate reinforcement in the form of vouchers exchangeable for retail items were provided to bridge that temporal gap between entering treatment and initiating cocaine abstinence and establishing natural sources of nondrug reinforcement in one’s community that would be necessary to sustain longer-term abstinence.
In this voucher system, participants earned points (1 point = $0.25) for providing cocaine-negative urine specimens at their thrice-weekly visits. The first negative urine sample was worth 10 points (the equivalent of $2.50), and points earned per sample (i.e., magnitude of reinforcement) increased across consecutive negative samples to reinforce sustained cocaine abstinence. In addition, points equivalent to a $10.00 bonus were provided for every three consecutive negative tests with the goal of further reinforcing sustained abstinence. If participants tested positive for cocaine, they earned zero points for that sample and the value of the voucher available for the next cocaine-negative test result was reset back to the initial 10 points ($2.50). However, to encourage patients to continue efforts to abstain from cocaine use following a reset, five consecutive negative urine toxicology tests following a reset restored voucher value back to where it was prior to the reset. The goal behind the escalating schedule with the reset contingency was to differentially reinforce periods of continuous cocaine abstinence. Vouchers could be redeemed through study staff for retail items in the community, but had to approved by their therapist as being aligned with the overarching CRA aim of recruiting natural sources of reinforcement for a cocaine-free lifestyle. Maximum total vouchers a patient could earn was $997.50 across the 12-week period. Of course, not all patients successfully abstained and thus the average earnings in vouchers were typically about one-half of that maximum.
Efficacy Supported by Meta-Analysis
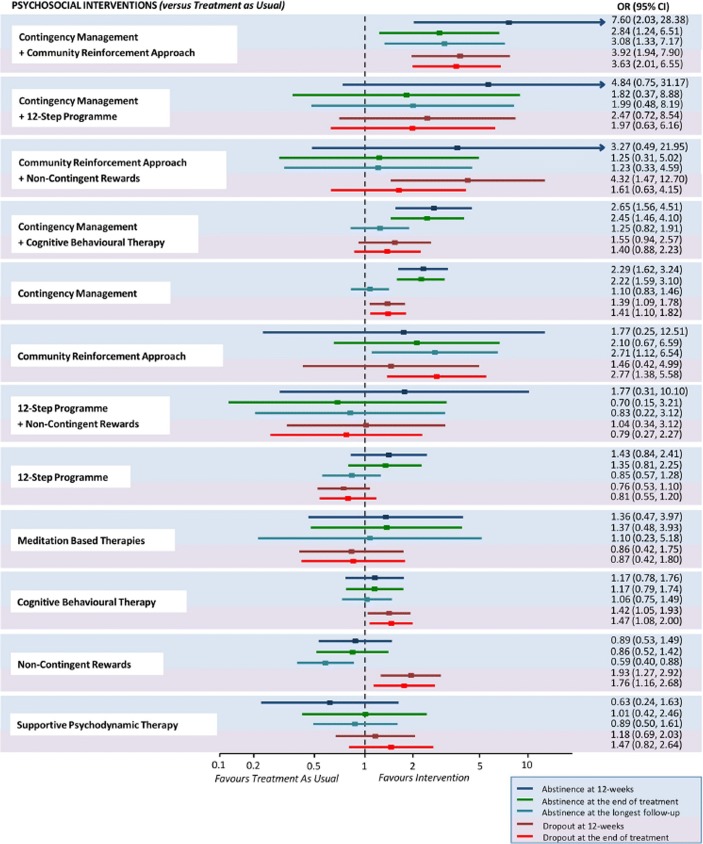
The vouchers component of this intervention for treating substance use disorders in general has been supported by several meta-analyses (e.g., Davis et al., 2016; Lussier, Heil, Mongeon, Badger, & Higgins, 2006). However, a recent meta-analysis examining psychosocial interventions for cocaine and amphetamine use disorders is most relevant to assessing the efficacy of the CRA + Vouchers treatment (De Crescenzo et al., 2018). That review included 50 RCTs involving 6,942 participants with cocaine or amphetamine use disorders and compared CRA + vouchers and 11 other structured interventions against treatment as usual (TAU) or in head-to-head comparisons. TAU can vary considerably but often involves drug-abuse counseling based on a disease model of addiction encouraging regular and lifetime participation in self-help groups such as alcoholics or narcotics anonymous. Across trials, 5,158 participants were treated with one of the structured interventions and 1,784 with TAU. Five primary outcomes were used: (1) percent of participants biochemically verified to be abstinent from drug cocaine/amphetamine use at 12 weeks after treatment entry, (2) percent abstinent at end of the recommended course of treatment, (3) percent abstinent at the longest posttreatment follow-up, (4) percent of patients retained in treatment at 12 weeks, and (5) percent retained at end of recommended course of treatment. The review provided robust evidence supporting the efficacy of the CRA + Vouchers intervention. Shown in Figure 1, for example, is a forest plot of odds ratios and 95% CIs for each of the 12 structured interventions compared to TAU. Only the CRA + Vouchers treatment differed significantly from TAU (i.e., no overlap of CIs with 1 on the X axis) across each of the primary outcomes, with increases in odds ranging from 2.84 (95% CI: 1.24, 6.51) to 7.60 (95% CI: 2.03, 28.38) across the five outcomes. As another example of evidence supporting its efficacy, CRA + Vouchers had the greatest number of significant results in head-to-head comparisons with the other structured interventions.
Fig. 1.
Abstinence and dropout at different time points for each psychosocial intervention versus treatment as usual. Estimates are reported as ORs, where an OR above 1 favors the psychosocial intervention indicated on the left side over treatment as usual. For each intervention, efficacy outcomes are reported in the blue-shaded area, whereas acceptability outcomes are reported in the pink-shaded area. OR = odds ratio. (Reprinted from De Crescenzo et al., 2018)
Efficacy Trials Conducted at the University of Vermont
The efficacy of the CRA plus Vouchers intervention in treating cocaine dependence was demonstrated empirically in seven consecutive randomized controlled clinical trials conducted at the University of Vermont (Higgins et al., 1991; Higgins et al., 1993; Higgins, Budney et al., 1994; Higgins et al., 1995; Higgins, Badger, & Budney, 2000; Higgins et al., 2003; Higgins et al., 2007). We briefly review those trials below.
CRA plus vouchers was first compared to TAU in two trials to assess whether the intervention was more effective than treatment that was already available in the community (Higgins et al., 1991; Higgins et al., 1993). Next, four trials were completed experimentally isolating the initial and longer-term effects of the voucher-based CM component (Higgins, Budney et al., 1994; Higgins et al., 1995; Higgins et al., 2000; Higgins et al., 2007). A seventh trial experimentally isolated the contributions of the CRA therapy component of the treatment on cocaine abstinence and other outcomes (Higgins et al., 2003).
Results were highly consistent across the two trials comparing this intervention to TAU (Higgins et al., 1991; Higgins et al., 1993). We report only those from the second trial. Participants were 38 cocaine-dependent adults who were randomly assigned to one of the two treatment conditions (19 participants per condition). Separate therapist teams trained in the respective treatment approaches delivered the interventions. Those assigned to the CRA plus Vouchers condition received the 24-week intervention outlined above. Those assigned to the TAU control condition received counseling based on the disease model of addiction and recovery often referred to as the “12-step model.” Outcomes were strikingly different across the two conditions (Figure 2). The two conditions were initially comparable in terms of cocaine use, but those assigned to TAU quickly resumed cocaine use or dropped out of treatment consistent with the literature on outpatient treatment of cocaine dependence at that time. In contrast to that pattern, those assigned to the CRA plus Vouchers condition largely remained in treatment and sustained relatively high levels of cocaine abstinence throughout, which was an unprecedented observation in this clinical population. These promising results provided strong support for the efficacy of the multicomponent behavioral treatment for retaining patients in outpatient care and promoting cocaine abstinence.
Fig. 2.
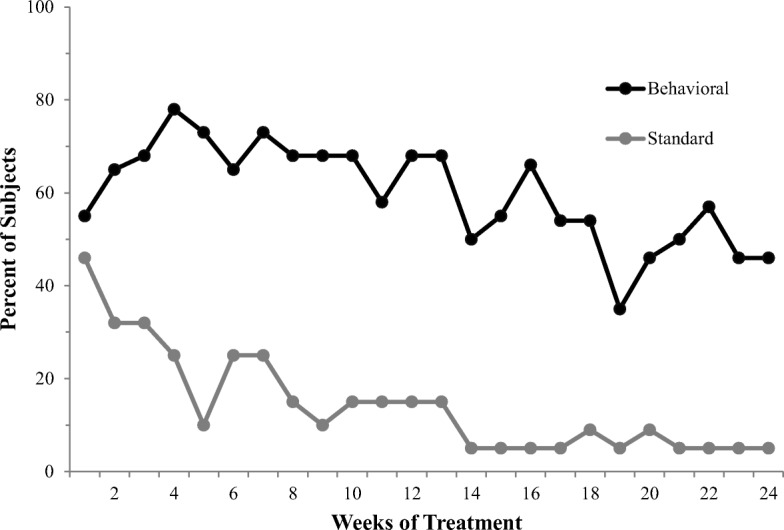
Percentage of participants cocaine abstinent during consecutive treatment weeks. Behavioral treatment indicates CRA + vouchers condition and Standard treatment indicates TAU control condition. (From Higgins et al., 1993)
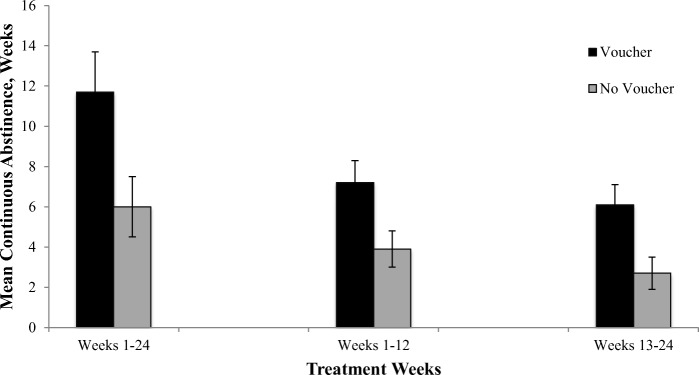
In the initial trial isolating the effects of the incentives component of this treatment (Higgins, Budney et al., 1994), 40 cocaine-dependent adults were randomly assigned to 24-weeks of either the CRA plus Vouchers intervention outlined above or to a CRA-therapy-only condition. Again, results were clear: 75% of participants assigned to the CRA plus Vouchers group completed 24 weeks of treatment versus 40% of those assigned to the CRA-only condition, and average durations of continuous cocaine abstinence during weeks 1 through 24 of treatment were approximately doubled in the CRA plus Vouchers condition compared to the CRA-only condition (Figure 3). These approximately two-fold differences were also observed when duration of abstinence during weeks 1–12 and weeks 13–24 were examined separately.
Fig. 3.
Mean duration of continuous cocaine abstinence over the 24-week duration of treatment and in the first and second halfs. “Voucher” indicates CRA + Voucher condition and “No Voucher” indicates CRA-only condition. (From Higgins et al., 1994)
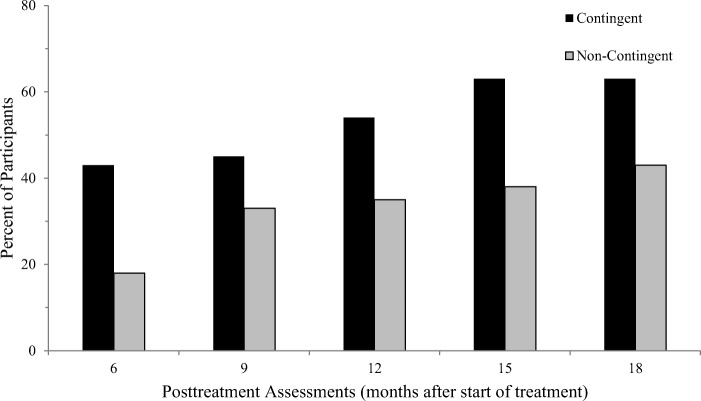
Next, Higgins et al. (2000) examined longer-term outcomes (18 months following treatment entry). Seventy cocaine-dependent outpatients were randomly assigned to one of two study conditions. In both conditions, participants received 24 weeks of treatment with CRA plus Vouchers. However, vouchers were provided contingent on cocaine-negative urine toxicology results in one condition, as they were in the earlier trials, whereas in the other condition they were provided independent of urine toxicology results (i.e., noncontingent incentives control condition). The noncontingent incentives control condition equates access to material resources across study conditions and experimentally isolates the effect of the contingency of requiring cocaine abstinence to obtain the incentives on treatment outcomes. There were no differences in treatment retention as participants in both conditions had to come to the clinic to receive vouchers. However, the proportion of participants abstinent from cocaine use was greater in the condition with contingencies on abstinence compared to the noncontingent condition during treatment and throughout one year of posttreatment follow-up assessments (Figure 4). These results provided strong evidence that the effects of the voucher-based incentives were a result of the reinforcement contingency (i.e., participants had to abstain to obtain them), and that those effects remained discernible through 15 months after the incentive program was discontinued.
Fig. 4.
Percentage of participants who were cocaine abstinent at posttreatment assessments. “Contingent” refers to CRA + Vouchers received contingent on abstinence. “Noncontingent” refers to CRA + Vouchers provided independent of abstinence. All points along the X-axis were following discontinuation of treatment and represent months after treatment started. (From Higgins et al., 2000)
A secondary analysis of predictors of who achieves longer-term abstinence indicated that those who were able to achieve a sustained period of abstinence during the initial treatment period had the best odds of sustaining longer-term abstinence (Higgins, Badger, & Budney, 2000). To test that observation experimentally, a randomized controlled clinical trial was conducted in which 100 cocaine-dependent outpatients were randomly assigned to one of two CRA plus Contingent Voucher treatment conditions (Higgins et al., 2007). In one condition, vouchers were set at twice the usual monetary value (maximum of $1,995 during the 12-week intervention) whereas in the other treatment condition they were set at half the usual value (maximum of $499 during the 12-week intervention). All else in the treatment conditions remained consistent with the usual CRA plus Vouchers treatment. Increasing the value of the vouchers increased the duration of continuous cocaine abstinence achieved during the 24-week treatment period twofold, and as hypothesized, point-prevalence cocaine abstinence was consistently greater among those treated in the high-magnitude voucher condition compared to the low-magnitude condition in assessments conducted every 3 months throughout an 18-month follow-up period.
The seventh randomized clinical trial in this series experimentally isolated the effects of the CRA intervention to outcomes (Higgins et al., 2003). One hundred cocaine-dependent adults were randomly assigned to one of two treatment conditions: CRA plus Vouchers or Vouchers Only. All patients earned vouchers exchangeable for retail items contingent on cocaine-free urinalysis results. Incentives were combined with the 24-week course of CRA therapy in the CRA plus Vouchers condition as described above but represented the primary treatment in the Vouchers Only condition. Patients assigned to the CRA plus Vouchers condition were retained better in treatment and used cocaine at a lower frequency during the treatment period, but did not differ from those assigned to Vouchers Only on measures of continuous cocaine abstinence or cocaine abstinence at posttreatment follow-up. It is important to note that those assigned to the full CRA plus Vouchers condition reported a lower frequency of drinking to intoxication during treatment and posttreatment follow-up compared with patients treated with Vouchers Only condition. They also reported a higher frequency of days of paid employment during treatment and the initial 6 months of follow-up, decreased depressive symptoms during the treatment period, and fewer hospitalizations and legal problems during posttreatment follow-up. These results demonstrated that the CRA component produced a broad array of important therapeutic gains during treatment and posttreatment follow-up that should not be underestimated in terms of the overarching goal of improving health in this clinical population, the vast majority of whom have multiple comorbid problems. The results also indicated that the effects on posttreatment cocaine use observed in the earlier trials were likely attributable to the voucher component of the intervention, which was shared across the two treatment conditions in this trial and did not differ between them.
Although the series of trials detailed above were underway, a trial demonstrating the efficacy of the CRA plus Vouchers in combination with buprenorphine for treating opioid dependence was conducted as well (Bickel, Amass, Higgins, Badger, & Esch, 1997). Thirty-nine opioid-dependent individuals were randomly assigned receive CRA plus Vouchers or TAU while undergoing a 26-week buprenorphine detoxification. The results were clear: the CRA plus Vouchers intervention retained 53% of patients in treatment through the recommended duration compared to only 20% of those treated with TAU, and 47% of those treated with CRA plus Vouchers achieved 8 or more weeks of continuous abstinence from opioid use compared to only 15% of those treated with TAU.
Alongside the clinical trials described above, numerous complementary clinical laboratory experimental studies were also conducted by investigators at the University of Vermont, often substituting cigarette smoking for cocaine use as the target substance (e.g., Alessi, Badger, & Higgins, 2004; Higgins, Bickel, & Hughes, 1994; Roll, Higgins, & Badger, 1996; Roll & Higgins, 2000). Those laboratory studies were used to experimentally examine questions in a more time-efficient manner than is feasible in clinical trials. For example, they demonstrated that including the reset contingency in the voucher delivery schedule increased the duration of continuous abstinence achieved compared to when the same value vouchers were available but without that contingency (Roll et al., 1996; Roll & Higgins, 2000). The studies also provided experience with using financial incentives to promote abstinence from cigarette smoking, which was the start of what eventually became the intervention used in the research on smoking cessation among pregnant women described below.
Conclusions
This series of RCTs provide robust empirical evidence that this CRA plus Vouchers treatment produces meaningful and sustained behavior change among cocaine-dependent outpatients. The studies experimentally isolated the contribution of the voucher-based CM intervention to increasing cocaine abstinence and the CRA therapy in decreasing drinking to intoxication, depressive symptoms, legal problems, and hospitalizations, and increasing employment (i.e., increasing social stability and health). The trial demonstrating that the efficacy of the CRA plus Vouchers intervention extends to opioid use disorders is highly relevant to efforts to curtail the current U.S. opioid epidemic.
The vouchers-based CM intervention garnered a great deal of interest among researchers in the area of addictions. For example, Silverman and colleagues went on to demonstrate the efficacy of the voucher program for treating cocaine addiction among individuals enrolled in methadone maintenance treatment for opioid dependence and developing a highly efficacious treatment model based on an adaptation of this vouchers model that is known as the Therapeutic Workplace (e.g., Silverman et al., 2001; Silverman et al., 2002; Silverman et al., 2007). A report focused on that important work is included in this Special Issue (Silverman, this issue) as are others (Dallery, this issue; Volpp, this issue). Indeed, a literature of controlled studies examining the efficacy and effectiveness of vouchers and related financial incentives interventions across a wide range of different types of substance use disorders, populations, and settings grew rapidly. That literature has been reviewed in a three-report series that encompassed all controlled studies on treating substance use disorders using vouchers and related monetary incentives from 1991 when the first report on CRA plus Vouchers was reported through 2014 (Lussier et al., 2006; Higgins, Sigmon, & Heil, 2011; Davis et al., 2016). Across the 24 years encompassed by those three reviews, 176 controlled studies were reported with 151 (86%) reporting statistically significant treatment effects. One of those extensions was pursued at the University of Vermont focusing on smoking cessation among pregnant women. That work is reviewed briefly below.
Intervention for Pregnant Cigarette Smokers
Cigarette smoking during pregnancy is a longstanding public health problem in the United States and other developed countries for which investigators have been trying to develop effective treatments since the mid-1980s (Higgins, Washio et al., 2012a; Higgins & Solomon, 2016). Smoking during pregnancy is the leading preventable cause of poor pregnancy outcomes, increasing risk for pregnancy complications, preterm birth, stillbirth, infant death, impaired lung development, childhood illness and developmental/behavioral problems, and later-in-life increased risk for cardiovascular disease, obesity, and metabolic syndrome (Barker, 2004; Dietz et al., 2010; Leslie, 2013). About 22% of women of childbearing age are regular cigarette smokers, including about 13% of pregnant women (Alshaarawy & Anthony, 2015; Kurti et al., 2017; Lopez et al., 2017). Smoking during pregnancy is highly associated with socioeconomic disadvantage (Higgins et al., 2009; Kandel, Griesler, & Schaffran, 2009). Although approximately 20% of pregnant smokers quit smoking soon after learning of pregnancy, the vast majority will smoke through pregnancy in the absence of a formal intervention (Heil et al., 2014; Solomon & Quinn, 2004).
A direct extension of the research described above on psychomotor stimulants was an intervention using voucher-based CM to decrease smoking during pregnancy (Higgins et al., 2012a; Higgins & Solomon, 2016). As with the discussion of treatments for psychomotor stimulants above, we begin by reviewing meta-analyses examining the use of financial incentives to increase smoking cessation among pregnant women.
Efficacy Supported by Meta-Analysis
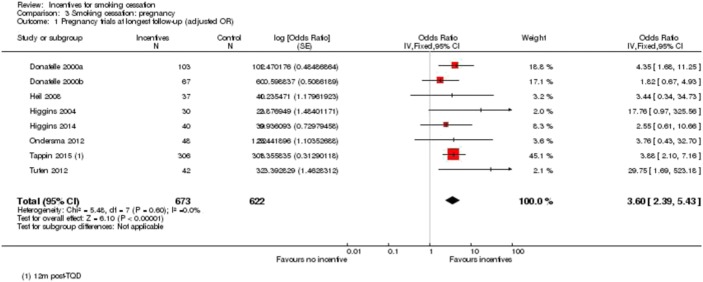
In a series of meta-analyses examining psychosocial and pharmacological treatments for smoking cessation during pregnancy that involved more than 77 controlled clinical trials and 77,000 women, financial incentives (i.e., voucher-based CM) were identified as producing the largest treatment effect size, yielding an average increase in antepartum cessation rates of 24% above control levels whereas all other treatments produced an average increase of 6% (Chamberlain et al., 2013; Lumley et al., 2009). Shown in Figure 5 is a plot of individual trial and overall effect sizes and 95% CIs for achieving late-pregnancy abstinence in eight randomized clinical trials involving 1297 women treated with financial incentives contingent on biochemically verified abstinence or a control condition (Cahill, Hartmann-Boyce, & Perera, 2015). Financial incentives increased the odds of antepartum abstinence by an average of 3.79 fold above control levels (95% CIs: 2.74–5.25). Odds for abstaining at the longest follow-up assessment were also increased by 3.60 (95% CIs: 2.39, 5.43; not shown).
Fig. 5.
Odds of smoking cessation at late-pregnancy assessment for individual clinical trials that met criteria for inclusion in the meta-analysis and for all trials considered together. (From Cahill et al., 2015)
Efficacy Trials Conducted at the University of Vermont
We use results from three controlled clinical trials conducted at the University of Vermont to briefly illustrate the basic incentives treatment approach used to incentivize smoking cessation with pregnant women (for more detailed reviews, see Higgins, Washio et al., 2012a; Higgins & Solomon, 2016). Trial participants were recruited from obstetric practices and WIC programs in the greater Burlington, Vermont, community. The majority were Caucasian, < 25 years old, unemployed, without health insurance, and have < 12 years of education. After enrolling in the study, women were assigned to an abstinence-contingent vouchers condition or a noncontingent incentives control condition. In addition to vouchers, women in both conditions received usual care from their providers plus pregnancy-specific smoking cessation educational materials provided by research staff. Women in both conditions also received serial ultrasounds at 30- and 34-weeks gestation to examine fetal growth trajectories, and medical records were examined to evaluate birth outcomes.
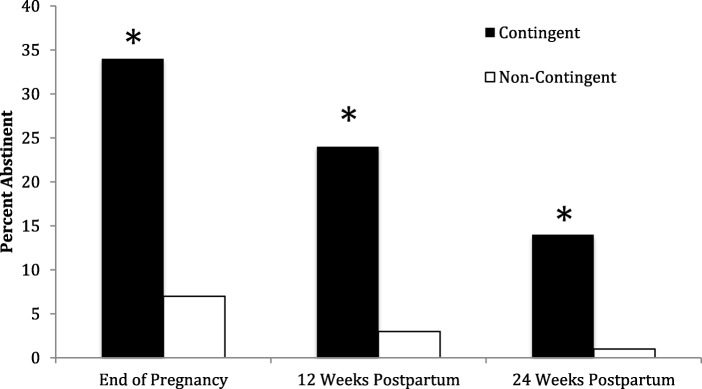
Across trials, end of pregnancy point-prevalence abstinence rates have been almost five-fold greater in the abstinence-contingent incentives condition compared to controls (34.1% vs. 7.4%), and treatment effects have remained discernible through 24-weeks postpartum (14.1% vs. 1.2%), 12 weeks after discontinuation of the incentives (Figure 6).
Fig. 6.
Percent of women abstinent at antepartum and postpartum assessments during University of Vermont smoking-cessation trials. Contingent bars represent women assigned to vouchers contingent on verification of abstinence and Noncontingent bars represent women assigned to vouchers not contingent on verification of abstinence. (Data from Higgins et al. 2012)
Other research questions examined by University of Vermont investigators included which baseline participant characteristics predicted quit success. Nicotine dependence indicators including cigarettes smoked per day and time to first cigarette (Kurti, Davis, Skelly, Redner, & Higgins, 2016), as well as educational attainment (Higgins et al., 2016), independently predict late-pregnancy smoking status. Also investigated was whether the relationships between early and longer-term abstinence occurred among pregnant smokers as it does for cocaine-dependent outpatients and smokers from the general population. Consistent with those prior results, a woman’s smoking status in the initial two weeks of a quit attempt was a remarkably strong predictor of late-pregnancy smoking status (Higgins et al., 2006). Among women who abstained during the first two weeks of the cessation effort, 88% were abstinent at their late-pregnancy assessment compared to only 15% among those who smoked even just a few puffs during the first two weeks.
Conclusions
Consistent with the evidence for treatment of psychomotor stimulants, there is robust evidence supporting the efficacy of voucher-based CM for increasing smoking cessation among pregnant women that is evident at the level of RCTs and across three Cochrane meta-analyses (Cahill et al., 2015; Chamberlain et al., 2013; Lumley et al., 2009), which represent the gold standard for evaluating interventions for medical conditions. The importance of the antepartum treatment effects is underscored by accumulating evidence that in utero smoke exposure causes a broad range of adverse effects on the developing fetus, some of which remain evident across the life span. Treatment effects of the incentives are not as strong postpartum as they are antepartum, but have been reported at the level of individual trials (Heil et al., 2008; Higgins, Heil et al., 2004) and in meta-analysis (Cahill et al., 2015). Where there is more to be learned is regarding efficacy for sustaining longer-term maternal cessation rates after the incentives have been discontinued. We know of no alternative intervention in this population with comparable support for efficacy. Ierfino et al. (2015) demonstrated the effectiveness of the incentives intervention developed at the University of Vermont when delivered by clinicians in a clinical rather than research setting in a large urban community hospital in Chesterfield, England, where incentives produced cessation rates of 20% above control levels, a difference that is consistent with the Cahill et al. (2015) meta-analysis. At least one study has been reported supporting the cost-effectiveness of this treatment approach (Boyd, Briggs, Bauld, Sinclair, & Tappin, 2016).
Interventions to Promote Adherence with Medical Regimens among Vulnerable Populations
The greatest amount of research on voucher-based CM has been conducted on decreasing drug use, but this has also been demonstrated to have generality across a wide range of behavioral health problems (Higgins, Silverman et al., 2012b). Below we provide two illustrative examples.
Efficacy Trials Conducted at the University of Vermont
The current U.S. opioid dependence epidemic has produced a high rate of in utero drug exposure among infants born to opioid-dependent women. Most of those pregnancies are unplanned and result from no or ineffective contraceptive use. To experimentally pilot test an intervention to increase use of prescription contraceptives, 31 opioid-dependent women were randomly assigned to TAU or an experimental intervention (Heil et al., 2016). TAU involved women receiving an informational booklet about birth control methods, a list of local providers, and an offer of free condoms and a dose of emergency contraception. The experimental intervention included TAU combined with the World Health Organization's (WHO) contraception initiation protocol, including free contraceptives, and vouchers contingent upon attending 13 follow-up visits over 6 months to help manage common side effects and other issues that can lead women to discontinue contraceptives. It is important to note that vouchers were not contingent on using a contraceptive. Maximal potential voucher earnings for attending all visits was $390.
Women assigned to the experimental versus usual care control conditions initiated prescription contraceptive use (100% vs. 29%) and reported prescription contraceptive use at the 1-month (63% vs.13%), 3-month (88% vs. 20%), and 6-month (94% vs. 13%) assessments. None of the experimental condition participants became pregnant during the 6-month protocol versus three women (20%) in the control condition. In addition, 38% of women in the experimental condition chose the most effective prescription contraceptive methods (long-acting reversible contraceptives) versus 7% of women in the control condition.
To our knowledge, this is the first experimental study demonstrating the efficacy of an intervention for increasing prescription contraceptive use among opioid-maintained women, an especially high-risk group for unplanned pregnancies. This model has considerable potential for increasing contraceptive use and decreasing unplanned pregnancies among opioid dependent women and other vulnerable populations. Preliminary results from an ongoing trial examining the relative efficacy of the WHO and CM components of this multi-element intervention suggest that both contribute to the efficacy of the intervention.
Another behavioral health problems examined by University of Vermont investigators is underutilization of cardiac rehabilitation (CR) among socioeconomically disadvantaged patients. CR is a structured, progressive exercise program (often 36 sessions) that also includes components designed to reduce coronary risk factors (e.g., introducing patients to heart healthy diets, addressing medication adherence). CR is highly effective at reducing morbidity and mortality rates following a myocardial infarction (MI) or coronary revascularization (Lawler, Filion, & Eisenberg, 2011) reducing cardiac rehospitalizations over 12 months and mortality rates over 2–3 years (Lawler et al., 2011). A longstanding problem is that socioeconomically disadvantaged patients are less likely to participate in or complete CR compared to more affluent patients even though Medicaid (public insurance for the socioeconomically disadvantaged) covers the cost of doing so. In a study of 322 CR eligible Medicaid patients in the state of Washington, for example, only two (< 1%) attended CR within the year following their MI (Oberg, Fitzpatrick, Lafferty, & LoGerfo, 2009). In a study of CR adherence, 19% of patients without socioeconomic disadvantage (insured only by Medicare) attended CR as recommended compared to only 3-5% of those with socioeconomic disadvantage (insured by Medicare and Medicaid; Suaya et al., 2007).
In an initial pilot study (Gaalema et al., 2016) and subsequent RCT (Gaalema et al., 2019), we demonstrated that financial incentives increase CR adherence among socioeconomically disadvantaged patients. In the RCT, 130 patients with Medicaid insurance coverage and a CR qualifying cardiac event were randomly assigned to a TAU condition or a condition that included TAU plus financial incentives contingent upon completion of each of the 36 CR sessions with a maximal potential earnings of $1,238. Patients assigned to the financial incentives condition were almost twice as likely (55.4% vs. 29.2%) to complete the recommended 36 sessions of CR. These results provided strong experimental evidence supporting the efficacy of financial incentives for increasing CR participation among Medicaid patients.
Conclusions
The studies on the efficacy of financial incentives in promoting use of effective contraception and adherence to critical medical services like cardiac rehabilitation illustrate the breadth and potential of this behavior change model for addressing medically important but often ignored challenges to improving health and reducing disparities in vulnerable populations. Financial incentives clearly have a potentially substantive contribution to make to promoting health in vulnerable populations and reducing the problem of health disparities.
Dissemination of CM
The record of successful dissemination of Voucher-based CM for treating substance use disorders is mixed. The most notable instance of broad adoption is in the U.S. Veterans Administration (VA) hospital system where the intervention has been implemented nationwide (see Petry, DePhilippis, Rash, Drapkin, & McKay, 2014). In 2011, VA leadership issued a notice that despite the robust record supporting the efficacy of CM, only 1% of patients treated in the VA system in 2010 received that form of care. They called for nationwide implementation of CM in all VA intensive outpatient treatment programs or the equivalent defined as programs that provided at least 3 hours per day of care at least thrice weekly. The directive underscored the need to use the approach with disorders for which no efficacious pharmacotherapy is available using stimulant use disorders as an example. The directive also recognized the need for training and funding and provided initial funding to move forward on both. A 5-year follow-up on that directive is positive showing that CM had been implemented at 94 sites, fidelity measures of adherence to treatment guidelines were good, and that treatment retention (> 50% attendance at CM sessions) and urine toxicology test results (> 90% negative for targeted substance) among those treated with CM were at levels consistent with the efficacy shown in controlled trials.
A major factor that allowed for this thoughtful and apparently successful rollout of CM is the VA system could readily surmount the obstacle of funding. Most VA hospital sites offer Veterans Canteen Services where veterans are offered merchandise and services at reduced prices. Hence, the CM programs in VA hospitals can offer vouchers exchangeable for retail items at the local Canteen, which is what most do (DePhilippis, Petry, Bonn-Miller, Rosenbach, & McKay, 2018). In brief, the VA system is almost ideally suited for adoption and implementation of Voucher-based CM. The vast majority of community addiction treatment services in the United States are not similarly situated, meaning that there is no simple answer to the question of how to fund CM and what type of exchange system can be established for providing financial incentives in a scalable manner.
One notable example of a community program that has surmounted the challenge of a scalable and sustainable Voucher-based CM program is the Positive Reinforcement Opportunity Project (PROP) that was initiated by the San Francisco Public Health Department in 2004 to decrease methamphetamine-associated sexual risk behavior and associated infectious disease (Shoptaw et al., 2006), and is currently supported by the San Francisco AIDS Foundation, a public nonprofit organization. PROP is a 12-week CM program where gay/bisexual men and transgender women who want to stop using methamphetamine can earn up to $330 in gift cards contingent on methamphetamine-negative urine toxicology results.
Medicaid has come to play a sizeable and growing role in funding of treatment for substance use disorders, which disproportionately affect economically disadvantaged populations (Medicaid.gov, 2019, Boozang; Bachrach, & Detty, 2014). If Voucher-based CM is going to be broadly implemented in U.S. community substance abuse treatment settings, Medicaid funding will be essential. It is interesting that Section 4108 of the Affordable Care Act mandated that $100 million dollars be spent investigating the use of Medicaid funds as financial incentives to promote health-related behavior change for the purpose of preventing chronic disease. At a reduced but nevertheless sizable investment ($80 million), 10 States (California, Connecticut, Hawaii, Minnesota, Montana, Nevada, New Hampshire, New York, Texas, and Wisconsin) were awarded grants to carry out this mandate. Included in this effort was a contract to Research Triangle Institute (RTI) to evaluate this effort (see Hoerger et al., 2017). The projects focused on a broad range of health conditions and preventive services, including smoking cessation, weight loss, and diabetes prevention. None involved treatment of illicit drug use disorders, which is unfortunate as that is the problem for which there is the greatest amount of experimental evidence for efficacy. Nevertheless, the evidence demonstrated that incentives were effective at increasing use of preventive services generally including use of tobacco cessation quit lines and other services, although results on improving health outcomes or decreasing health-care utilization were negligible. That was perhaps not surprising given that the projects were only funded for 5 years. In terms of achieving objectively verified behavior change, the best outcomes were obtained for smoking cessation. Again, that is probably not so surprising as drug use was the area where the greatest amount of controlled experimental research had been conducted on how to effectively incentivize behavior change.
Wisconsin implemented two smoking-cessation programs, one for nonpregnant adults and the other for pregnant and recently postpartum women. Financial incentives significantly increased biochemically verified cessation rates among nonpregnant adults by 7.8% (21.6% vs. 13.8%) (maximal earnings ~$190 for combination of attending cessation counseling sessions and verified abstinence) and among pregnant/recently postpartum women by 16 % (39% vs. 23%) (maximal earnings of ~$460 for combination of attending cessation counseling sessions and verified abstinence; Baker et al., 2018; Fraser et al., 2017), with evidence supporting cost-effectiveness in one of the reports (Fraser et al., 2017; Mundt et al., 2019). The smoking cessation project in New Hampshire among smokers with serious mental illness did not produce significant treatment effects as reported by RTI, but a subsequent report in a peer-reviewed journal indicated that biochemically verified abstinence rates among those who received financial incentives were consistently and significantly greater than controls at periodic assessments through one-year of follow-up (adjusted odds = 1.77; 95% CI: 1.15-2.72; Brunette et al., 2018). A smoking-cessation study in Connecticut also focused on smokers with serious mental illness but did not demonstrate that financial incentives significantly increased abstinence levels in the RTI report; we know of no follow-up reports on that project in a peer-reviewed journal.
It is important to underscore the scale of these projects and the relatively treatment-recalcitrant populations involved. The projects in Wisconsin, New Hampshire, and Connecticut involved 2,928, 2,031, and 4,052 smokers, respectively. Those numbers exceeded any prior controlled study on CM for smoking-cessation, especially in exclusively economically disadvantaged populations, many of whom were selected because they had comorbid serious mental illness or were continuing to smoke during pregnancy. Economically disadvantaged populations in general and those with serious mental illness or who are continuing to smoke during pregnancy in particular are well-known to be relatively treatment recalcitrant in the smoking-cessation literature (e.g., Higgins, 2014; Schroeder, 2016). In our opinion, achieving statistically significant treatment effects across three of these four smoking-cessation projects at that scale and with lead investigators not previously associated with incentive-based interventions is a testament to the robust efficacy of CM.
The challenge now is to try to build on these efforts. As one example, we are in discussions with Medicaid officials in Vermont to move the vouchers-based CM program for smoking cessation among pregnant and newly postpartum women developed at the University of Vermont into routine care. We are also planning discussions with those same officials about moving forward with voucher-based CM programs for treating stimulant use disorders among patients enrolled in Medication Assisted Treatment for opioid use disorder, a population in which use of cocaine and methamphetamine is increasing.
When discussing dissemination of CM it is important not to overlook its implementation in the private sector. The Affordable Care Act also included provisions supporting use of financial incentives to promote healthy behavior change and the use of preventive services among employees. They also provided funds for the RAND Corporation to evaluate use of incentives as part of employee wellness programs offered by major employers in the United States (i.e., those with > 50 employees). At least two reports have been published (Huang et al., 2016; Mattke et al. 2014). In brief, among 589 employers who responded to a RAND survey, 69% (406) indicated that they offered an employee wellness program. Among those offering a wellness program, 75% (305) provided financial incentives as part of their program. Financial incentives vary considerably and include discounts on gym memberships, cash payments, and reductions in health insurance costs (Huang et al., 2016). Based on employer-provided details, use of financial incentives doubled median participation rates in preventive services including smoking cessation (20% to 40%).
Worth noting is that independent of this RAND assessment several large RCTs demonstrating the efficacy of incentive-based smoking cessation interventions in large corporate settings have been reported in high-impact medical journals (Halpern et al., 2018; Volpp et al., 2009). How well the practices from these RCTs are being implemented in the large number of employee wellness programs offered by U.S. employers is unclear. However, the rate at which the private sector has attempted to implement the scientific advances made in the use of financial incentives to promote health-related behavior change into their routine affairs is impressive and far exceeds efforts in the public sector. Again, the differential is almost surely funding. Major employers fund their own wellness programs rendering moot the major obstacle to their use in the public sector. The incentive for the employers is that healthy employees are more productive and can be expected to have lower healthcare expenses, keeping in mind that the Affordable Care Act mandates that major employers in the United States provide health insurance for full-time employees.
Lastly, an international application of the CM model that is striking in scope but not often discussed in direct connection to dissemination of CM is what is known as a conditional cash transfer (CCT) program, which is designed to decrease chronic poverty in low- and middle-income countries (Lagarde, Haines, & Palmer, 2007; Owusu-Addo, Renzaho, & Smith, 2018; Ranganathan & Lagarde, 2012). These programs offer to transfer cash to impoverished families or individuals conditional on them engaging in certain health-related behaviors (economist terminology for CM). For example, mothers in impoverished families can receive cash and nutritional supplements contingent on vaccinating their infants, attending well-baby checkups, and enrolling older children in school. These programs, largely funded by the World Bank, began in Latin America and have now been extended across multiple continents and many millions of families. As one might expect considering the scale of such an effort, results are complex but, in many instances, impressive (Lagarde et al., 2007; Owsusu-Addo et al., 2018; Ranganathan & Lagarde, 2012). As just one example, population level studies have attributed improvements in the national mortality rates for infants and children in Brazil to CCT programs (Rasella et al., 2013; Shei, 2013). Although the CCT model surely has multiple precipitants, the evidence of CM for substance use disorders provided important empirical support for the concept (Ranganathan & Lagarde, 2012). As Higgins has noted previously (Higgins & Davis, 2016), he and other CM researchers received requests from the World Bank in the mid- to late 1990s requesting copies of their reports on the efficacy of incentives to reduce drug use. To our knowledge, there was no further communication between World Bank representatives and CM researchers beyond that initial inquiry, but studies on CCT programs began to appear in the literature several years after that initial request.
Summary and Conclusions
The evidence is clear from meta-analyses and rigorous RCTs that CM delivered alone or in combination with intensive lifestyle counseling such as CRA is highly efficacious at increasing abstinence from drug use among populations addicted to a wide range of substances. Extrapolating from the extensive research literature on cocaine and other psychomotor stimulants, combining CM with intensive counseling appears to provide the greatest opportunity for sustaining behavior change once an incentive program is discontinued. It also merits mention that there may be circumstances where longer-term treatment with incentives, or what is referred to in the pharmacotherapy literature as maintenance treatment, may be the recommended course of treatment as is illustrated in the research by Silverman and colleagues on the therapeutic workplace (Silverman et al., 2001). The early admonition from Stokes and Baer (1977) encouraging behavior-change interventionists to develop explicit plans for sustaining treatment effects after an intervention is discontinued as opposed to treating and hoping for sustained outcomes certainly has relevance for CM researchers.
It is worth emphasizing that there are several conditions where the evidence from meta-analyses and RCTs indicates that CM interventions are uniquely efficacious. After several decades involving more than 50 controlled trials on psychosocial interventions alone and even more on pharmacotherapies, we know of no other intervention other than voucher-based CM that can reliably increase cocaine abstinence in RCTs (De Crescenzo et al., 2018). Likewise, several decades of research involving more than 75 RCTs and 25,000 participants on smoking cessation among pregnant women demonstrates that voucher-based CM increases effect sizes approximately four-fold over what is achieved with the many other psychosocial and pharmacological interventions examined (Cahill et al., 2015; Chamberlain et al., 2013; Higgins & Solomon, 2016; Lumley et al., 2009).
The impressive outcomes achieved with CM under conditions where so many other psychosocial and pharmacological interventions have been ineffective has garnered considerable attention. As reviewed above, the majority of U.S. major corporations are now using CM in their employee wellness programs. In addition, the World Bank has made CM a cornerstone of its international efforts to curtail chronic poverty in low- and middle-income countries in the form of CCT programs. These programs are far from perfect in terms of attending to some of the basics of CM best practices, but their adoption of this general approach shows that their policy makers are attuned to empirical evidence. That is strikingly not the case in the public sector, especially community substance abuse treatment facilities in the United States. That is ironic in that these are the programs that have responsibility for the very conditions where there is the greatest amount of empirical evidence for the efficacy of CM. Indeed, if decisions around treatment practices were based on empirical evidence alone, community substance abuse treatment facilities would be the settings where CM would have the greatest presence. The failure of community substance abuse treatment programs to offer evidence-based interventions is a longstanding matter that is not unique to CM. Many still fail to offer medication assisted therapy for opioid use disorders despite overwhelming evidence for its efficacy, or offer it only in the form of detoxification protocols or at lower-than-recommended doses (e.g., National Center on Substance Abuse and Child Welfare, n.d.). The challenge of funding mechanisms for CM mentioned above may have some unique features, but it certainly is not an insurmountable issue, as the PROP project in San Francisco clearly demonstrates. In our opinion, there needs to be greater efforts to understand and assist community treatment programs in surmounting barriers to delivering evidence-based treatments, along with greater effort to regularly evaluate the treatment outcomes achieved in these programs. Given that substance use disorders disproportionately affect economically disadvantaged populations who often lack the resources to advocate for better care, this responsibility may fall to professionals in the addictions field. There is broad recognition that substance use disorders result in chronic disease and premature death (Higgins, 2015; Schroeder, 2016). Indeed, the current rate of drug overdoses in the United States is occurring at a sufficiently high rate to alter U.S. population life expectancy (Centers for Disease Control & Prevention, 2018; Murphy, Xu, Kochanek, & Arias, 2018). Undertreating this problem has serious individual and population health impacts. More needs to be done to promote use of evidence-based treatments including but certainly not limited to CM. As noted above, efforts have been initiated advocating for broader use of CM in Vermont as well as greater access to Medication Assisted Treatment for opioid use disorders. We hope that other professionals trained in substance use disorders will similarly advocate for evidence-based treatments in their communities.
Compliance with ethical standards
Declaration of competing interests
The authors have no conflict of interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental & Clinical Psychopharmacology. 2004;12:276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- Alshaarawy O, Anthony JC. Month-wise estimates of tobacco smoking during pregnancy for the United States, 2002–2009. Maternity & Child Health Journal. 2015;19(5):1010–1015. doi: 10.1007/s10995-014-1599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano M, Kawachi I. Why do Americans have shorter life expectancy and worse health than do people in other high-income countries? Annual Review of Public Health. 2014;35:307–325. doi: 10.1146/annurev-publhealth-032013-182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, T. B., Fraser, D., Kobinsky, K., Adsit, R., Smith, S. S., Khalil, L., Alaniz, K. M., et al. (2018). A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: effects on post-birth abstinence. Journal of Consulting & Clinical Psychology, 86(5), 464–473. 10.1037/ccp0000278. [DOI] [PubMed]
- Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting & Clinical Psychology. 1997;65:803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Boozang, P., Bachrach, D., & Detty, A. (2014). Coverage and delivery of adult substance abuse services in Medicaid managed care. Center for Medicare & Medicaid Services, Technical Assistance Brief 2 (2).
- Bouton ME, Trask S. Role of the discriminative properties of the reinforcer in resurgence. Learning & Behavior. 2016;44:137–150. doi: 10.3758/s13420-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KA, Briggs AH, Bauld L, Sinclair L, Tappin D. Are financial incentives cost-effective to support smoking cessation during pregnancy? Addiction. 2016;111(2):360–370. doi: 10.1111/add.13160. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Pratt SI, Bartel SJ, Scherer EA, Sigmon SC, Ferron NC, Santos M, et al. Randomized trial of interventions for smoking cessation among Medicaid beneficiaries with mental illness. Psychiatric Services. 2018;69(3):274–280. doi: 10.1176/appi.ps.201700245. [DOI] [PubMed] [Google Scholar]
- Cahill, K., Hartmann-Boyce, J., & Perera, R. (2015). Incentives for smoking cessation. Cochrane Database Syst Rev, 5, CD004307. 10.1002/14651858.CD004307.pub5, Last accessed July 8, 2019. [DOI] [PubMed]
- Catania AC. A natural science of behavior. Review of General Psychology. 2013;17(2):133–139. [Google Scholar]
- Centers for Disease Control & Prevention (2019). CDC director’s statement on U.S. life expectancy. Retrieved from https://www.cdc.gov/media/releases/2018/s1129-US-life-expectancy.html, Last accessed July 8, 2019.
- Chamberlain C, O'Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, Thomas J. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews. 2013;10:CD001055. doi: 10.1002/14651858.CD001055.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Boyle M, Wargo E. Prescription opioid abuse: Problems and responses. Preventive Medicine. 2015;80:5–9. doi: 10.1016/j.ypmed.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Medicine. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive Medicine. 2016;92:36–46. doi: 10.1016/j.ypmed.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crescenzo F, Ciabattini M, Loreto D’Alo G, De Giorgi R, Del Giovane C, Cassar C, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: A systematic review and network meta-analysis. PLoS Medicine. 2018;15(12):e1002715. doi: 10.1371/journal.pmed.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the department of veterans affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend. 2018;185:367–373. doi: 10.1016/j.drugalcdep.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventive Medicine. 2010;39(1):45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Fraser DL, Fiore MC, Kobinsky K, Adsit R, Smith SE, Johnson ML, Baker TB. A randomized trial of incentives for smoking treatment in Medicaid members. American Journal of Preventive Medicine. 2017;53(6):754–763. doi: 10.1016/j.amepre.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakt, A. (2018). Overshadowed by the opioid crisis: A comeback by cocaine. The New York Times. Retrieved from https://www.nytimes.com/2018/03/05/upshot/overshadowed-by-the-opioid-crisis-a-comeback-by-cocaine.html, Last accessed July 8, 2019.
- Gaalema, D. E., Elliott, R. J., Savage, P. D., Rengo, J. L., Cutler, A. Y., Pericot-Valverde, I., et al. (2019). Financial incentives to increase cardiac rehabilitation participation among low-socioeconomic status patients: a randomized clinical trial. JACC: Heart Failure. 10.1016/j.jchf.2018.12.008. [DOI] [PMC free article] [PubMed]
- Gaalema DE, Savage PD, Rengo JL, Cutler AY, Higgins ST, Ades PA. Financial incentives to promote cardiac rehabilitation participation and adherence among Medicaid patients. Preventive Medicine. 2016;92:47–50. doi: 10.1016/j.ypmed.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. New England Journal of Medicine. 2018;378(24):2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- Heil SH, Hand DJ, Sigmon SC, Badger GJ, Meyer MC, Higgins ST. Using behavioral economic theory to increase use of effective contraceptives among opioid-maintained women at risk of unintended pregnancy. Preventive Medicine. 2016;92:62–67. doi: 10.1016/j.ypmed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST. Examining the timing of changes in cigarette smoking upon learning of pregnancy. Preventive Medicine. 2014;68:58–61. doi: 10.1016/j.ypmed.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacology, Biochemistry & Behavior. 1997;57(3):419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST. Behavior change, health, and health disparities: An introduction. Preventive Medicine. 2014;68:1–4. doi: 10.1016/j.ypmed.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental & Clinical Psychopharmacology. 2000;8(3):377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Science. 1994;55(3):179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Badger GJ, Foerg FE, Ogden D. Outpatient behavioral treatment for cocaine dependence: One-year outcome. Experimental & Clinical Psychopharmacology. 1995;3(2):205–212. [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150(5):763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins, S. T., & Davis, D. R. (2016). Division 28 and the development of contingency management interventions for substance use disorders. Paper presented at the annual meeting of the American Psychological Association, Denver, CO.
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug & Alcohol Dependence. 2009;104(suppl. 1):S100–S105. doi: 10.1016/j.drugalcdep.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Katz JL, editors. Cocaine abuse: Behavior, pharmacology, and clinical application. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Higgins ST, Solomon LJ. Some recent developments on financial incentives for smoking cessation among pregnant and newly postpartum women. Current Addiction Reports. 2016;3(1):9–18. doi: 10.1007/s40429-016-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug & Alcohol Dependence. 2006;85(2):138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review of Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research. 2004;6(6):1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Kurti AN, Redner R, White TJ, Keith DR, Gaalema DE, et al. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Preventive Medicine. 2016;92:110–117. doi: 10.1016/j.ypmed.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Heil SH. Contingency management in the treatment of substance use disorders. Trends in the literature. In: Ruiz P, Strain E, editors. Lowinson and Ruiz’s Substance Abuse: A comprehensive textbook. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 603–621. [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine dependent outpatients. Archives of General Psychiatry. 2003;60(10):1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins, S.T., Silverman, K., Sigmon, S.C., & Naito, N.A. (2012a). Incentives and health: An introduction. Preventive Medicine. 55, S2–S6. PMCID: PMC4107351. [DOI] [PMC free article] [PubMed]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, Bernstein IM. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine. 2012;55(suppl):S33–S40. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hoerger T, Boland E, Acquah JK, Alva M, Doto JK, Farrell K, et al. Medicaid incentives for prevention of chronic diseases. (RTI Project No. 0212790.004.000.005) Baltimore, MD: RTI International; 2017. [Google Scholar]
- Huang H, Mattke S, Batorsky B, Miles J, Liu H, Taylor E. Incentives, program configuration, and employee uptake of workplace wellness programs. Journal of Occupational & Environmental Medicine. 2016;58(1):30–34. doi: 10.1097/JOM.0000000000000613. [DOI] [PubMed] [Google Scholar]
- Hunt GM, Azrin NH. A community-reinforcement approach to alcoholism. Behaviour Research Therapy. 1973;11(1):91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau TM. Financial incentives for smoking cessation in pregnancy: A single-arm intervention study assessing cessation and gaming. Addiction. 2015;110(4):680–688. doi: 10.1111/add.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug & Alcohol Dependency. 2009;104(suppl. 1):S24–S33. doi: 10.1016/j.drugalcdep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RM. Behavior change and reducing health disparities. Preventive Medicine. 2014;68:5–10. doi: 10.1016/j.ypmed.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Kelley, A. E., & Berridge, K. C. (2002). The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience, 22(9), 3306–3311. 20026361 [DOI] [PMC free article] [PubMed]
- Kurti AN, Davis DR, Skelly JM, Redner R, Higgins ST. Comparison of nicotine dependence indicators in predicting quitting among pregnant smokers. Experimental & Clinical Psychopharmacology. 2016;24(1):12–17. doi: 10.1037/pha0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti, A. N., Redner, R., Lopez, A. A., Keith, D. R., Villanti, A. C., Stanton, C. A., et al. (2017). Tobacco and nicotine delivery product use in a national sample of pregnant women. Preventive Medicine. 117, 52–60. 10.1016/j.ypmed.2017.07.030. [DOI] [PMC free article] [PubMed]
- Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health intervention in low- and middle-income countries a systematic review. Journal of the American Medical Association. 2007;298(16):1900–1910. doi: 10.1001/jama.298.16.1900. [DOI] [PubMed] [Google Scholar]
- Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. American Heart Journal. 2011;162(4):571–584. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Medicine. 2013;11:27. doi: 10.1186/1741-7015-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Redner R, Kurti AN, Keith DR, Villanti AC, Stanton CA, et al. Tobacco and nicotine delivery product use in a U.S. national sample of women of reproductive age. Preventive Medicine. 2017;117:61–68. doi: 10.1016/j.ypmed.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley, J., Chamberlain, C., Dowswell, T., Oliver, S., Oakley, L., & Watson, L. (2009). Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews, Jul 8; (3), CD001055. 10.1002/14651858.CD001055.pub3 [DOI] [PMC free article] [PubMed]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Mattke S, Kapinos K, Caloyeras JP, Taylor EA, Batorsky B, Liu H, et al. Workplace wellness programs services offered, participation, and incentives. Santa Monica, CA: RAND; 2014. [PMC free article] [PubMed] [Google Scholar]
- Medicaid.gov (2019). Keeping America healthy: substance use disorders. https://www.medicaid.gov/medicaid/benefits/bhs/substance-use-disorders/index.html. Last accessed July 8, 2019.
- Mundt MP, Baker TB, Fraser DL, Smith SS, Piper ME, Fiore MC. Paying low-income smokers to quit? The cost-effectiveness of incentivizing tobacco quit line engagement for Medicaid recipients who smoke. Value Health. 2019;22:177–184. doi: 10.1016/j.jval.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S. L., Xu, J. Q., Kochanek, K. D., & Arias, E. (2018). Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. [PubMed]
- National Center on Substance Abuse & Child Welfare. (n.d.) Access to medication assisted treatment. Retrieved from https://ncsacw.samhsa.gov/resources/opioid-use-disorders-and-medication-assisted-treatment/access-to-medication-assisted-treatment.aspx
- Oberg EB, Fitzpatrick AL, Lafferty WE, LoGerfo JP. Secondary prevention of myocardial infarction with nonpharmacologic strategies in a Medicaid cohort. Preventing Chronic Disease. 2009;6:A52. [PMC free article] [PubMed] [Google Scholar]
- Owusu-Addo E, Renzaho AMN, Smith BJ. The impact of cash transfers on social determinants of health and health inequalities in sub-Saharan Africa: A systematic review. Health Policy & Planning. 2018;33:675–696. doi: 10.1093/heapol/czy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, McKay JR. Nationwide dissemination of contingency management: The Veterans Administration initiative. American Journal of Addiction. 2014;23(3):205–210. doi: 10.1111/j.1521-0391.2014.12092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Lagarde M. Promoting healthy behaviours and improving health outcomes in low and middle income countries: A review of the impact of conditional cash transfer programmes. Preventive Medicine. 2012;55(suppl):S95–S105. doi: 10.1016/j.ypmed.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Rasella D, Aquino R, Santos CAT, Paes-Sousa R, Barreto M. Effect of a conditional cash transfer program on childhood mortality: a nationwide analysis of Brazilian municipalities. Lancet. 2013;382:57–64. doi: 10.1016/S0140-6736(13)60715-1. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug & Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare BB, et al. A public health response to the methamphetamine epidemic: The implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SA. Shattuck Lecture. We can do better: Improving the health of the American people. New England Journal of Medicine. 2007;357(12):1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- Schroeder SA. American health improvement depends upon addressing class disparities. Preventive Medicine. 2016;92:6–15. doi: 10.1016/j.ypmed.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Shei A. Brazil’s conditional cash transfer program associated with declines in infant mortality rates. Health Affairs. 2013;32(7):1274–1281. doi: 10.1377/hlthaff.2012.0827. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A. Trends in U.S. drug overdose deaths in non-Hispanic black, Hispanic, and non-Hispanic white persons, 2000–2015. Annals of Internal Medicine. 2018;168(6):453–455. doi: 10.7326/M17-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Ochalek TA, Meyer AC, Hruska B, Heil SH, Badger GJ, et al. Interim Buprenorphine vs. Waiting List for Opioid Dependence. New England Journal of Medicine. 2016;375(25):2504–2505. doi: 10.1056/NEJMc1610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Experimental & Clinical Psychopharmacology. 2001;9(1):14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: Three-year abstinence outcomes. Experimental & Clinical Psychopharmacology. 2002;10(3):228–240. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, et al. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. Journal of Applied Behavior Analysis. 2007;40(3):387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L, Quinn V. Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine & Tobacco Research. 2004;6(suppl. 2):S203–S216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- Stokes TF, Baer DM. An implicit technology of generalization. Journal of Applied Behavior Analysis. 1977;10(2):349–367. doi: 10.1901/jaba.1977.10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaya JA, Shepard DS, Normand ST, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- Substance Abuse & Mental Health Services Administration . Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52) Rockville, MD: Author; 2017. [Google Scholar]
- Substance Use Disorders (n.d.). Retrieved from https://www.medicaid.gov/medicaid/benefits/bhs/substance-use-disorders/index.html
- U.S. Department of Health & Human Services . The health consequences of smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: Author; 2014. [Google Scholar]
- Volkow ND, Collins FS. The role of science in addressing the opioid crisis. New England Journal of Medicine. 2017;377(4):391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine. 2009;60(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: Findings from a National Research Council/Institute of Medicine report. Journal of the American Medical Association. 2013;309(8):771–772. doi: 10.1001/jama.2013.91. [DOI] [PubMed] [Google Scholar]