Abstract
Background
Delirium is now the preferred term to describe acute confusional states. It is experienced by 10 to 30% of all hospital inpatients. Delirium is potentially reversible and is related to several adverse outcomes, including increased hospital length of stay, poor functional status, persistent cognitive impairment, need for institutional care and probably mortality. Disruption of the cholinergic system has been proposed as a key mechanism of delirium. Cholinesterase inhibitors enhance the cholinergic system and there have been reports that they might be beneficial in treating delirium.
Objectives
To assess the efficacy and safety of cholinesterase inhibitors in the treatment of delirium.
Search methods
The Cochrane Dementia and Cognitive Improvement Group's Register of Clinical Trials (which includes records from MEDLINE, EMBASE, PsycINFO, CINAHL, CENTRAL, LILACS and other databases) was searched for relevant randomised controlled trials using the terms: donepezil or aricept, galantamine or reminyl, rivastigmine OR exelon and tacrine OR cognex on 19 April 2005. As this Specialised Register only contains trials relating to dementia and cognitive impairment, in addition all years of MEDLINE, EMBASE, PsycINFO and CINAHL were searched for trials of cholinesterase inhibitors for delirium in non‐demented people.
Selection criteria
Unconfounded, blinded randomised controlled trials, published or unpublished in which treatment with cholinesterase inhibitors was administered and compared with alternative interventions in patients with delirium are included.
Data collection and analysis
Two reviewers (RO, SK) independently assessed the quality of the studies according to parameters such as randomisation, blinding and how dropouts were managed. Each cholinesterase inhibitor was to be examined separately and together as a group.
The primary outcome measures of interest are length of delirium, severity of delirium and presence and severity of behavioural symptoms (e.g. agitation and hallucinations). Other outcomes of interest include: cognition, need for institutionalisation, length of hospital admission and adverse effects.
Main results
There was one included trial of donepezil compared with placebo in 15 patients. No significant difference between the treatment and placebo groups was found in the duration of delirium. The mean duration of postoperative delirium for the donepezil group was 1.0 day (Standard Error 0.0) while for the placebo group it was 1.3 days (Standard Error 0.19). No other outcomes were measured for the patients who developed delirium.
Authors' conclusions
There is currently no evidence from controlled trials that donepezil is effective in the treatment of delirium. Further trials using cholinesterase inhibitors for the treatment of delirium are needed.
Keywords: Humans, Cholinesterase Inhibitors, Cholinesterase Inhibitors/therapeutic use, Delirium, Delirium/drug therapy, Donepezil, Indans, Indans/therapeutic use, Piperidines, Piperidines/therapeutic use
No convincing evidence from one trial of the efficacy of cholinesterase inhibitors for delirium
Delirium is a confusional state that is associated with physical illness. Its characteristic features are rapid onset, altered consciousness, reduced attention and global cognitive impairment. Other symptoms are hallucinations (particularly visual hallucinations), disturbed sleep pattern and agitation. Delirium is commonly found in hospital patients and is associated with longer admissions, poor functioning level, persistent cognitive impairment and need for institutional care. Delirium is therefore an important syndrome to recognise and treat. The one included trial, of donepezil compared with placebo in 15 patients, showed no statistically significant difference in length of delirium. No other outcomes were measured.
Background
Over the years delirium has been known under many different guises including acute brain failure, acute brain syndrome, acute dementia, organic brain syndrome and toxic confusional state. The two main international classifications of disease, the ICD‐10 (WHO 1992) and DSM‐IV (APA 1994), have similar diagnostic criteria for delirium: altered consciousness and attention, global disturbance of cognition and perceptual abnormalities. Delirium's other core features are rapid onset, fluctuating course, disturbance of the sleep/wake cycle and evidence of a physical cause.
The list of physical causes of delirium is inexhaustible, with infection and medication being among the most frequent. Delirium is a common, important and serious condition. It is experienced by 10 to 30% of all hospital inpatients (Lipowski 1987; Trzepacz 1996). The incidence is higher among older people with 10 to 15% of older hospital patients having a delirium on admission (Bucht 1999) and a further 10 to 40% developing delirium during their time in hospital (Fann 2000). Increased incidence rates have been reported in certain patient groups, e.g. postoperative patients, with a rate of over 60% described in patients following surgery for fractured neck of femur (Gustafson 1988). Delirium is not exclusive to hospitals and one study estimated nursing home prevalence of delirium at 58% (Sandberg 1998). Although common, delirium is poorly identified in clinical practice with nondetection rates of 33 to 66% (Inouye 1994). It is important to detect delirium as it is potentially reversible and is related to several adverse outcomes, including increased hospital length of stay, poor functional status, persistent cognitive impairment and need for institutional care (Bross 1994; Cole 1993). There is also evidence to suggest that delirium is associated with an increase in mortality (McCusker 2002; Pompei 1994; Rabins 1982) although this is not consistent (Inouye 1998).
The pathogenesis of delirium is not clearly understood and many hypotheses have been proposed. It is most likely that delirium represents a response to diffuse cerebral dysfunction, reduced metabolism and disordered neurotransmitter synthesis. The strongest evidence is for disturbance of the cholinergic neurotransmitter system (Koponen 1999). Acetylcholine is the primary neurotransmitter that facilitates learning and attention, both of which are disturbed in delirium. Impaired cholinergic neurotransmission has been correlated with cognitive and behavioural features of delirium (Itil 1966). High serum anticholinergic activity is associated with severity of delirium (Mintzer 2000; Trzepacz 1999) while anticholinergic medications are among the most implicated in iatrogenic delirium (Tune 1993). Cholinesterases are enzymes that metabolise acetylcholine and inhibiting them reduces the inactivation of acetylcholine, which increases its activity. Cholinesterase inhibitors, which act in this way, are currently only licensed for palliative treatment of Alzheimer's disease, the most common type of dementia which, like delirium, is associated with reduced cholinergic activity. The current cholinesterase inhibitors are donepezil, rivastigmine, galantamine and tacrine, and these have been shown to effect significant improvement in cognition, global functioning, behaviour and activities of daily living in Alzheimer's disease (Birks 2003; Birks 2004; Loy 2004; Qizilbash 1998).
Treatments for delirium include the identification and correction of the underlying cause, environmental and supportive interventions and pharmacological treatment. When there is severe agitation, hostility, aggressive behaviour, or the patient poses a risk to self or others, anti‐psychotic medication such as haloperidol is the first choice of drug. Haloperidol is the most commonly used (Someya 2001) as it has fewer active metabolites and fewer anticholinergic, sedative and hypotensive effects than the other anti‐psychotic drugs. This type of medication can, however, have severe adverse effects (Flacker 1998), especially on the extra‐pyramidal system, which can cause clinical deterioration. Treatment with benzodiazepines is only recommended for delirium related to alcohol or withdrawal of benzodiazepines (Lipowski 1990) as benzodiazepines can precipitate delirium.
There is much debate regarding which medications should be used in the management of delirium. The medications most commonly used offer only symptomatic relief. Cholinesterase inhibitors, by promoting the cholinergic system may offer, in addition, actual treatment. There are several reports in the literature of cholinesterase inhibitors being of benefit in delirium (Fischer 2001; Moretti 2004; Wengel 1998). A systematic review of trials of cholinesterase inhibitors for the treatment of delirium is needed to evaluate efficacy and safety.
Objectives
The outcomes of interest include length of delirium, behavioural disturbance (including agitation, psychotic symptoms), length of stay in hospital and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Included trials have a period of treatment that exceeds one day. The management of the control group in included studies has been clearly defined as placebo or standard treatment. All included studies have utilised validated and published methods for diagnosing delirium and evaluating behavioural disturbances. Studies conducted in a hospital or community setting are included.
Types of participants
Participants included are over 16 years old, of either gender and diagnosed with delirium by a validated, operationalised, clinical criteria e.g. ICD‐10 (WHO 1992); DSM IV (APA 1994); the Confusion Assessment Method (CAM 1990); Delirium Rating Scale (DRS 1988). Participants included may be suffering from any cause of delirium, including medical illnesses, drug or alcohol withdrawal and side effects from medication.
Types of interventions
Included trials have assessed the efficacy of any of the current cholinesterase inhibitors (donepezil, rivastigmine, galantamine and tacrine), orally administered in any dosage or frequency compared with placebo or standard treatment. Standard treatment includes nursing care, environmental measures and single doses of anti‐psychotic or benzodiazepines for behavioural disturbances. Studies using regular anti‐psychotic and/or benzodiazepine medication have been excluded.
Types of outcome measures
The primary outcomes of interest are: 1. Length of delirium 2. Severity of delirium 3. Presence and severity of behavioural symptoms (e.g. agitation and hallucinations)
The choice of rating scales selected for meta‐analysis depends on which scales are used in the selected studies. Only scales which have been published and validated are included.
The secondary outcomes of interest are: 1. Cognition 2. Use of other medications (e.g. one‐off doses of anti‐psychotic) 3. Need for institutionalisation 4. Length of hospital admission 5. Adverse effects/side effects 6. Withdrawals 7. Reason for withdrawals 8. Activities of daily living 9. Death
Search methods for identification of studies
The trials were identified from a last updated search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group on 19 April 2005 using the search term delir*
The Specialized Register at that time contained records from the following databases: CENTRAL: January 2005 (issue 1); MEDLINE: 1966 to 2005/02; EMBASE: 1980 to 2005/01; PsycINFO: 1887 to 2005/01; CINAHL: 1982 to 2004/12; SIGLE (Grey Literature in Europe): 1980 to 2004/06; ISTP (Index to Scientific and Technical Proceedings): to May 2000; INSIDE (BL database of Conference Proceedings and Journals): to June 2000; Aslib Index to Theses (UK and Ireland theses): 1970 to March 2003; Dissertation Abstract (USA): 1861 to March 2003; ADEAR (Alzheimer's Disease Clinical Trials Database): to 25 March 2005; National Research Register: issue 1/2005; Current Controlled trials (last searched April 2005) which includes: Alzheimer Society GlaxoSmithKline HongKong Health Services Research Fund Medical Research Council (MRC) NHS R&D Health Technology Assessment Programme Schering Health Care Ltd South Australian Network for Research on Ageing US Dept of Veterans Affairs Cooperative Studies National Institutes of Health (NIH) ClinicalTrials.gov: last searched March 2005; LILACS (Latin American and Caribbean Health Science Literature): last searched April 2003
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the group's module (see About on T he Cochrane Library)
In addition the following databases were searched:
MEDLINE 1966‐2005/04 week 2 was searched with the following search strategy:
#1 "Delirium"/ all subheadings #2 deliri* #3 "acute confusion" #4 "acute brain failure" #5 "acute organic psychosyndrome" #6 "acute brain syndrome" #7"metabolic encephalopathy" #8 "acute psycho‐organic syndrome" #9 "clouded state" #10 "clouding of consciousness" #11 "exogenous psychosis" #12 "toxic psychosis" #13 "toxic confusion" #14 #1 or #2 #15 #3 or #4 or #5 or #6 #16 #7 or #8 or #9 or #10 #17 #11 or #12 or #13 #18 #14 or #15 or #16 or #17 #19 random* or placebo* or "standard care" or "normal care" or control* #20 "Alcohol‐Withdrawal‐Delirium"/ all subheadings #21 "delirium tremens" in TI #22 #20 or #21 #23 ((TG=animals) not (TG=humans)) and (TG =animals) #24 #18 and #19 #25 #24 not #23 #26 #25 not #22 #27 donepezil or aricept or galantamine or reminyl or tacrine or cognex or rivastigmine or exelon #26 and #27
EMBASE 1980‐2005/02 was searched with the following search strategy:
#1 explode "delirium" tree: 1/ all subheadings #2 deliri* #3 "acute psycho‐organic syndrome" or "clouded state" or "clouding of consciousness" or "exogenous psychosis" or "toxis psychosis" or "toxic confusion" #4 "acute brain confusion" or "acute brain failure" or "acute organic psychosyndrome" or "acute brain syndrome" or "metabolic encephalopathy" #5 #1 or #2 or #3 or #4 #6 "randomized‐controlled‐trial"/ all subheadings #7 random* or placebo* or control* or "normal care" or "standard care" #8 #6 or #7 #9 "delirium‐tremens"/ all subheadings #10 nonhuman* in DER #11 (nonhuman* in DER) and (human* in DER) #12 #10 or #11 #13 (#5 and #8) not #9 #14 #13 not #12 #15 donepezil or aricept or galantamine or reminyl or tacrine or cognex or rivastigmine or exelon #16 #14 and #15
PsycINFO 18??‐ 2005/02 was searched with the following search strategy:
#1 deliri* #2 "acute psycho‐organic syndrome" or "clouded state" or "clouding of consciousness" or "exogenous psychosis" or "toxis psychosis" or "toxic confusion" #3 "acute brain confusion" or "acute brain failure" or "acute organic psychosyndrome" or "acute brain syndrome" or "metabolic encephalopathy" #4 "Delirium‐" in MJ,MN #5 #1 or #2 or #3 or #4 #6 random* or placebo* or control* or "normal treatment" or "normal care" or "standard care" or "standard treatment" #7 #5 and #6 #8 donepezil or aricept or tacrine or cognex or rivastigmine or exelon or galantamine or reminyl #9 #7 and #8
Companies involved in manufacturing or marketing cholinesterase inhibitors were asked to provide information regarding ongoing trials or unpublished studies not otherwise available. Books concerning delirium and reference lists from review papers and retrieved articles were examined for additional trials.
Proceedings of relevant conferences were searched and experts in the field of delirium were contacted for further references.
Data collection and analysis
Selection of studies The titles and abstracts of citations obtained by the search were studied by two reviewers (RO and SK). Publications deemed to be irrelevant were discarded. Articles that described a relevant randomised controlled trial were retrieved for further assessment. Retrieved trials were assessed independently (RO and SK) for inclusion in the review by the criteria above. Disagreements at any stage of study selection were resolved by discussion with AB.
Quality assessment Randomised and blind assessment of outcome are inclusion criteria for the review. Quality of included trials were assessed according to the following criteria: 1. Adequate concealment of treatment allocation (e.g. opaque sealed numbered envelopes). 2. Method of randomisation (e.g. by computer randomisation, random number tables). 3. Adequate blinding (both of participants and outcome assessments). 4. Adequate documentation of how exclusions were handled after treatment allocation. 5. Losses to follow‐up (trials should have losses of less than 25%).
The assessment of quality was performed independently by RO and SK and disagreements were resolved by discussion with AB.
Data extraction To allow an intention‐to‐treat analysis, data were sought for every patient irrespective of compliance and whether or not the patient was subsequently excluded from the treatment or follow‐up. Analyses of those who completed the trial on treatment (completers) were done separately. When individual patient data were not available, data were extracted from summary statistics for each study. For continuous data the mean change from baseline, the standard deviation and the number of participants for each treatment group at each assessment were obtained. In studies that do not report changes from baseline, the mean, the standard deviation and the number of participants for each treatment group at baseline and endpoint was extracted if available. For dichotomous data the number in each treatment group and the numbers experiencing the outcome of interest was retrieved. If only treatment effects and their standard errors are reported then these were extracted.
Data analysis Statistical analysis was performed using Review Manager software. In trials that have used ordinal rating scales for measuring outcome, if the scales have a reasonably large number of categories (more than 10) the data were treated as continuous outcomes arising from a normal distribution.
Summary statistics (n, mean and standard deviation) were required for each outcome at each assessment time for each treatment group in each trial for change from baseline. When the change from baseline results are not reported, the required summary statistics were calculated from the baseline and assessment time treatment group means and standard deviations. In this case a zero correlation between the measurements at baseline and assessment time was assumed.
For trials that have used the same rating scale to assess outcome, the measure of treatment difference for the pooled trials is the weighted mean difference. Where different rating scales or tests have been used the measure of the treatment difference is the standardised mean difference, which is the absolute mean difference divided by the standard deviation. For binary outcomes, such as clinical improvement or no clinical improvement, the odds ratio is used to measure treatment effect. A weighted estimate of the typical treatment effect across trials is also calculated.
It is intended that overall estimates of the treatment difference is presented. In all cases the overall estimate from a fixed effects model is presented and a test for heterogeneity using an I2 performed. Where there is evidence of heterogeneity of the treatment effect between trials, then either only homogenous results are pooled, or a random‐effects model is used.
Sensitivity analysis was conducted, where possible, to explore the influence of high quality trials (A trials) versus moderate quality trials (B trials) versus low quality trials (C trials), as well as the effects of analysing by intention to treat, on the effect size. We intended to conduct a subgroup analysis to explore the effects of treatment in people with different causes of delirium (e.g. infection, medication) and in people of different ages (less than 65 years and more than 65 years).
Results
Description of studies
One trial was included (Liptzin 2005) comparing donepezil with placebo in the prevention and treatment of postoperative delirium in patients over the age of 50 years without dementia undergoing elective total joint‐replacement surgery. Eighty patients were randomised to receive either donepezil 5 mg once daily or a placebo tablet once daily which they commenced 14 days before the surgery and continued taking for 14 days after the surgery. The total period of randomised treatment was 28 days. The baseline demographic characteristics were similar in treatment and placebo groups. The mean age was 67 years, 36% male in the donepezil group and 49% in the placebo group. The mean Mini Mental State Examination (MMSE) (Folstein 1975) scores were 29.15 and 28.85 for the treatment and placebo groups respectively. There were also no significant differences between the groups in race, which of the two study surgeons performed the operation, which joint, or side were operated on. At baseline, both groups were cognitively intact, with no significant differences in MMSE or Clock Drawing Test (Sunderland 1989) scores.
Participants were assessed for postoperative delirium using the Delirium Symptom Interview (DSI 1992), CAM (CAM 1990), daily medical records, nurse‐observation reviews and DSM‐IV Diagnostic criteria for delirium (APA 1994). Participants who were diagnosed with delirium according to DSM‐IV (APA 1994) criteria were advised to double their dose, so they either received 10 mg of donepezil or two placebo capsules.
No rating scales were used to measure outcome. The duration of delirium in the treatment and placebo groups was recorded.
Risk of bias in included studies
The included study (Liptzin 2005) examines both the prevention of delirium and the treatment of delirium. It is only the treatment component that is relevant to this review. It is unclear whether the included trial meets all the quality assessment criteria of this review, as the details of the two components of the trial are not described separately. The participants, the investigators, research assistants and surgical staff were all blind to allocation. Subjects were randomised by a random numbers chart, with blocks of four in which subjects were randomised overall to 1:1 to donepezil or placebo. All comparisons in the study were made by an intention‐to‐treat analysis, with participants remaining in the treatment group to which they were originally assigned for analysis. The dropout rate was reported for the whole trial and not just the treated group.
Effects of interventions
There was one included trial (Liptzin 2005) which randomised 80 patients preoperatively to either receive donepezil or placebo. Fifteen patients developed delirium postoperatively: eight (20.5%) in the donepezil group and seven (17.1%) in the placebo group. Relative risk of 1.20 (95% confidence interval 0.48 to 3.00. Z = 0.39, P = 0.69). No significant difference between the treatment and placebo groups was found in the duration of delirium. It was not possible to obtain the original data from the authors of the duration of delirium for each subject. Further analysis was therefore not done. In the published paper it was reported that the mean duration of postoperative delirium for the donepezil group was 1.0 day (Standard Error 0.0) while for the placebo group it was 1.3 days (Standard Error 0.19) (difference in means ‐0.3%, 90% confidence interval ‐7.8 to 7.2, P = 0.12). It seems unlikely that the standard error for the donepezil group is 0.0 but without analysing the original data it is difficult to confirm this. No other outcomes were measured for the patients who developed delirium.
There is currently no separate data on the patients who developed a delirium in the trial for mean age, gender, baseline MMSE, type of operation and percentage who were discharged into a rehab facility. It has not been possible to obtain this data from the authors but it is hoped that it will be made available in the future.
Discussion
At the beginning of this review we highlighted the frequency and associated morbidity of delirium. It is a syndrome which can have serious consequences for patients. We also outlined the reasons why cholinesterase inhibitors might be useful in treating delirium. Our search for evidence of efficacy of cholinesterase inhibitors has produced a single randomised controlled trial. This trial investigated the effectiveness of donepezil so we are unable to include data about the other cholinesterase inhibitors.
The included trial initially examined the effectiveness of donepezil in preventing delirium. Only 15 participants developed a delirium and entered the treatment part of the trial. The report of the trial paper explored only one of the outcome measures we had identified: duration of delirium. No statistical difference was found in the length of delirium between the donepezil and placebo groups. The treatment phase of the trial lacked sufficient power to show a significant difference between the groups. The study population underwent elective surgery and were therefore relatively young, physically well and cognitively intact. They were at low risk of developing delirium and those that did had a brief and relatively benign episode. As the duration of the episodes of delirium were short, it is likely that the symptoms experienced by participants were not of a severity that treatment would be expected to be beneficial.
The included study is currently the only published randomised controlled trial in this subject. There have been, however, several published case reports which describe the effective treatment of delirium with a cholinesterase inhibitor. The causes of delirium in these case reports are varied and include vascular lesions (Kobayashi 2004; Moretti 2004), alcohol withdrawal (Hori 2003) and medication (Noyan 2003; Slatkin 2004). Rivastigmine has also been reported to be effective in treating prolonged delirium in three patients who had not responded to antipsychotic medication (Kalisvaart 2004). Despite these positive case reports there is currently only one small published randomised controlled trial and this trial does not support the efficacy of cholinesterase inhibitors.
Authors' conclusions
There is currently no evidence from controlled trials to support the efficacy of cholinesterase inhibitors in the treatment of delirium. Delirium remains a common and serious condition with no consensus on its pharmacological treatment.
Risk factors for developing delirium include older age, medical illness and pre‐existing dementia. Researching the subject is therefore fraught with difficulties. The potential adverse outcomes of delirium, however, make it an area worthy of attention. Future trials should attempt to include subjects with severe symptoms of delirium so effectiveness for the level of behavioural disturbance that is frequently seen in clinical practice can be established. Future trials should also consider including a wide range of relevant outcomes such as length of hospital admission, functional level, cognitive abilities and institutionalisation.
Acknowledgements
We gratefully acknowledge the contribution of the consumer editor, Christine Sykes. Katherine Hicks, from the Cochrane Dementia and Cognitive Improvement Group, has provided invaluable advice and support. Professor Benjamin Liptzin provided early data from the included trial.
Data and analyses
Comparison 1.
Donepezil
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Development of delirium | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.48, 3.00] |
Analysis 1.1.
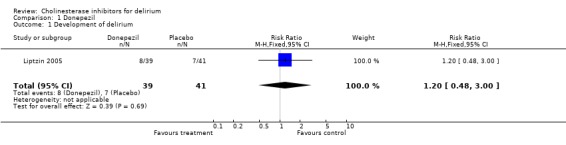
Comparison 1 Donepezil, Outcome 1 Development of delirium.
What's new
| Date | Event | Description |
|---|---|---|
| 23 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 7 October 2007 | New citation required and conclusions have changed | Substantive amendment |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: Block Randomisation. Blindness: Triple ‐ clinician, patient and assessors. Duration: 14 days. | |
| Participants | Diagnosis: Delirium. N = 15. No demographic data available. Setting: Orthopaedic hospital ward in USA. Inclusion: Clinical diagnosis of delirium following orthopaedic surgery. Over the age of 50. Exclusion: Gastroesophageal reflux disease, sick‐sinus syndrome, not already taking cholinesterase inhibitors. | |
| Interventions | Donepezil. Started at 5 mg once daily, increased to 10 mg once daily if tolerated. | |
| Outcomes | Duration of delirium | |
| Notes | ||
We were unable to include demograhic data of the patients with delirium as it was not presented in the published paper.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hori 2003 | Case report |
| Kalisvaart 2004 | Case series |
| Kobayashi 2004 | Case report |
| Moretti 2004 | Open labelled study |
| Noyan 2003 | Case report |
| Slatkin 2004 | Case report |
Contributions of authors
Ross Overshott ‐ all correspondance; drafting of protocol versions; selection of trials; extraction of data; entry of data; interpretation of data analyses; updating protocol
Salman Karim ‐ drafting of protocol versions; selection of trials; extraction of data; interpretation of data analyses
Alistair Burns ‐ arbiter in selection of trials; interpretation of data analysis
Leon Flicker ‐ Contact Editor Christine Sykes ‐ Consumer Editor
Declarations of interest
Professor Burns and Dr Overshott are currently involved in a clinical trial of rivastigmine in delirium. Professor Burns has also been involved in clinical trials on cholinesterase inhibitors for Alzheimer's disease, and has received research honoraria and hospitality from Pfizer, Eisai, Novartis, Bristol Myers Squibb, Astra Zeneca, Janssen‐Cilag. He holds no shares in any pharmaceutical company.
Edited (no change to conclusions)
References
References to studies included in this review
- Liptzin B, Laki A, Garb JL, Fingeroth R, Kushell R. Donepezil in the prevention and treatment of post‐surgical delirium. American Journal of Geriatric Psychiatry 2005;13(12):1100‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Hori K, Tominaga I, Inada T, Oda T, Hirai S, Hori I, Onaya M, Teramoto H. Donepezil‐responsive alcohol‐related prolonged delirium. Psychiatry and Clinical Neurosciences 2003;57:603‐4. [DOI] [PubMed] [Google Scholar]
- Kalisvaart CJ, Boelaarts L, Jonghe JFM, Kat MG. Successful treatment of three elderly patients suffering from prolonged delirium using the cholinesterase ihibitor rivastigmine. Ned Tijdschr Geneeskd 2004;148(30):1501‐4. [PubMed] [Google Scholar]
- Kobayashi K, Higashima M, Mutou K, Kidani T, Tachibana O, Yamashita J, Koshino Y. Severe delirium due to basal forebrain vascular lesion and efficacy of donepezil. Progess in Neuro‐Psychopharmacology and Biological Psychiatry 2004;28:1189‐94. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello R, Cattaruzza T, Cazzato G. Cholinesterase inhibition as a possible therapy for delirium in vascular dementia: a controlled, open 24‐month study of 246 patients. American Journal of Alzheimer's Disease and Other Dementias 2004;19(6):333‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2003;27:885‐7. [DOI] [PubMed] [Google Scholar]
- Slatkin N, Rhiner M. Treatment of opiod‐induced delirium with acetylcholinesterase Inhibitors. Journal of Pain and Symptom Management 2004;27(3):268‐73. [DOI] [PubMed] [Google Scholar]
Additional references
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- Birks J, Harvey R. Donepezil for dementia due to Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No.: CD001190. DOI: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley Evans J, Iakovidou V, Tsolaki M. Rivastigmine for Alzheimer's disease. Cochrane Database of Systematic Reviews 2000, Issue 4. Art. No.: CD001191. DOI: 10.1002/14651858.CD001191. [DOI] [PubMed] [Google Scholar]
- Bross MH, Tatum NO. Delirium in the elderly patient. American Family Physician 1994;50(6):1325‐32. [PubMed] [Google Scholar]
- Bucht G, Gustafson Y, Sandberg O. Epidemiology of Delirium. Dementia and Geriatric Cognitive Disorders 1999;10:315‐18. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Dyck CH, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the Confusion Assessment Method. Annals of Internal Medicine 1990;113:941‐8. [DOI] [PubMed] [Google Scholar]
- Cole M, Primeau F. Prognosis of delirium in elderly hospital patients. CMAJ 1993;149:41‐6. [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research 1988;23:89‐97. [DOI] [PubMed] [Google Scholar]
- Albert MS, Levkoff SE, Reilly C, et al. The Delirium Symptom Interview: an interview for the detection of delirium symptoms in hospitalized patients. Journal of Geriatric Psychiatry and Neurology 1992;5:14‐21. [DOI] [PubMed] [Google Scholar]
- Fann JR. The epidemiology of delirium: a review of studies and methodological issues. Seminars in Clinical Neuropsychiatry 2000;5:64‐76. [DOI] [PubMed] [Google Scholar]
- Fischer P. Successful treatment of nonanticholinergic delirium with a cholinesterase inhibitor. Journal of Clinical Psychopharmacology 2001;21(1):118. [DOI] [PubMed] [Google Scholar]
- Flacker JM, Marcantonio ER. Delirium in the elderly. Optimal management. Drugs Aging 1998;13(2):119‐30. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini‐Mental State": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- Gustafson Y, Berggren D, Brinnstrom B. Acute confusional states in elderly patients treated for femoral neck fracture. Journal of American Geriatrics Society 1988;36:525‐30. [DOI] [PubMed] [Google Scholar]
- Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalised patients. American Journal of Medicine 1994;97:278‐88. [DOI] [PubMed] [Google Scholar]
- Inouye S, Rushing J, Foreman M, Palmer R, Pompei P. Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. Journal of General Internal Medicine 1998;13:234‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itil T, Fink M. Anticholinergic‐induced delirium: experimental modification, quantitative EEG and behavioural correlations. Journal of Nervous and Mental Disorders 1966;143:492‐507. [PubMed] [Google Scholar]
- Koponen HJ. Neurochemistry and delirium. Dementia and Geriatric Cognitive Disorders 1999;10(5):339‐41. [DOI] [PubMed] [Google Scholar]
- Lipowski ZJ. Delirium (acute confusional states). JAMA 1987;258:1789‐92. [PubMed] [Google Scholar]
- Lipowski ZJ. Delirium: acute confusional states. New York: Oxford University Press, 1990. [Google Scholar]
- Loy C, Schneider L. Galantamine for Alzheimer's disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No.: CD001747. DOI: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12‐month mortality. Archives of Internal Medicine 2002;162:457‐63. [DOI] [PubMed] [Google Scholar]
- Mintzer J, Burns A. Anticholinesterase side effects of drugs in elderly people. Journal of the Royal Society of Medicine 2000;93:457‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalised older persons: outcomes and predictors. Journal of the American Geriatrics Society 1994;42:809‐15. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Whitehead A, Higgins J, Wilcock G, Schneider L, Farlow M for the Dementia Trialists' Collaboration. Cholinesterase inhibition for Alzheimer disease: a meta‐analysis of the tacrine trials. JAMA 1998;280:1777‐82. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. British Journal of Psychiatry 1982;140:149‐53. [DOI] [PubMed] [Google Scholar]
- Sandberg O, Gustafson Y, Brannstrom B, Bucht G. Prevalence of dementia, delirium and psychiatric symptoms in various care settings for the elderly. Scandinavian Journal Society of Medicine 1998;26(1):56‐62. [DOI] [PubMed] [Google Scholar]
- Someya T, Taro E, Hara T, Yagi G, Suzuki J. A survey on the drug therapy for delirium. Psychiatry and Clinical Neurosciences 2001;55(4):397‐401. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Hill JL, Mellow AM, et al. Clock‐drawing in Alzheimer's disease: a novel measure of dementia severity. Journal of the American Geriatrics Society 1989;37:725‐9. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT. Delirium, advances in diagnosis, pathophysiology and treatment. Psychiatry Clinics North America 1996;19:335‐46. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT. Update on the neuropathogenesis of delirium. Dementia and Geriatric Cognitive Disorders 1999;10:330‐4. [DOI] [PubMed] [Google Scholar]
- Tune L, Carr S, Cooper T, Klug B, Golinger RC. Association of anticholinergic activity of prescribed medications with postoperative delirium. The Journal of Neuropsychiatry and Clinical Neurosciences 1993;5(2):208‐10. [DOI] [PubMed] [Google Scholar]
- Wengel SP, Roccaforte WH, Burke WJ. Donepezil improves symptoms of delirium in dementia: implications for future research. Journal of Geriatric Psychiatry and Neurology 1998;11:59‐61. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. The ICD‐10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva: WHO, 1992. [Google Scholar]


