Abstract
Background
A range of treatments have been proposed to improve pregnancy outcome in recurrent pregnancy loss associated with antiphospholipid antibody (APL). Small studies have not resolved uncertainty about benefits and risks.
Objectives
To examine outcomes of all treatments given to maintain pregnancy in women with prior miscarriage and APL.
Search methods
We searched the Pregnancy and Childbirth Group's Trials Register (30 May 2004), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2003, Issue 2), MEDLINE (1966 to June 2003), EMBASE (1988 to June 2003), handsearched Lupus (volume one to eight, 1991 to 1999) and conference proceedings from the International Symposium on APL up to 1999. We also scanned bibliographies of all located articles and contacted experts in the field.
We updated the search of the Pregnancy and Childbirth Group's Trials Register on 10 September 2009 and added the results to the awaiting classification section.
Selection criteria
Randomised or quasi‐randomised, controlled trials of interventions in pregnant women with a history of pregnancy loss and APL.
Data collection and analysis
Two review authors independently assessed quality and extracted data for studies up to December 1999. One review author performed this for studies after 1999.
Main results
Thirteen studies were found (849 participants). The quality was not high; 50% had clear evidence of allocation concealment. Participant characteristics varied between trials.
Unfractionated heparin combined with aspirin (two trials; n = 140) significantly reduced pregnancy loss compared to aspirin alone (relative risk (RR) 0.46, 95% confidence interval (CI) 0.29 to 0.71). Low molecular weight heparin (LMWH) combined with aspirin compared to aspirin (one trial; n = 98) did not significantly reduce pregnancy loss (RR 0.78, 95% CI 0.39 to 1.57). There was no advantage in high‐dose, over low‐dose, unfractionated heparin (one trial; n = 50). Three trials of aspirin alone (n = 135) showed no significant reduction in pregnancy loss (RR 1.05, 95% CI 0.66 to 1.68). Prednisone and aspirin (three trials; n = 286) resulted in a significant increase in prematurity when compared to placebo, aspirin, and heparin combined with aspirin, and an increase in gestational diabetes, but no significant benefit. Intravenous immunoglobulin +/‐ unfractionated heparin and aspirin (two trials; n = 58) was associated with an increased risk of pregnancy loss or premature birth when compared to unfractionated heparin or LMWH combined with aspirin (RR 2.51, 95% CI 1.27 to 4.95). When compared to prednisone and aspirin, intravenous immunoglobulin (one trial; n = 82) was not significantly different in outcomes.
Authors' conclusions
Combined unfractionated heparin and aspirin may reduce pregnancy loss by 54%. Large, randomised controlled trials with adequate allocation concealment are needed to explore potential differences between unfractionated heparin and LMWH.
[Note: The 15 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Plain language summary
Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant
Treatments for recurrent miscarriage when there are antibodies in the mothers blood.
Miscarriage can be very distressing for parents and their families. Miscarriage is sometimes associated with substances in the mother blood called 'antiphospholipid antibodies' or 'lupus anticoagulant'. These antibodies are associated with clotting and so it is suggested that anticlotting drugs may be helpful. The review found the quality of the included trials was quite variable, and that prednisone appears to have adverse effects so it has no role in the treatment of recurrent miscarriage. However, a combination of unfractionated heparin with aspirin may be helpful but there are potential side‐effects for mothers. More research is needed.
Background
The association between antiphospholipid antibodies or lupus anticoagulant and recurrent fetal loss has been acknowledged for many years, and various interventions have been recommended to assist in the maintenance of the pregnancy until delivery of a live infant.
Historically, the association between recurrent fetal loss and antiphospholipid antibodies predated the anticardiolipin antibody assay and the diagnosis was reliant on the presence of the lupus anticoagulant and/or a 'false positive' VDRL (a non‐specific serological assay for syphilis) test for syphilis (Laurell 1957; Lubbe 1985; Nilsson 1975). With advancing technology, it became possible to detect anticardiolipin antibodies. Other antiphospholipid antibodies and beta‐2‐glycoprotein I antibodies can now be detected, but their role in recurrent miscarriage remains controversial (Forastiero 1997; Higashino 1998; Lynch 1999; Yetman 1996). Consequently, detection of either lupus anticoagulant or anticardiolipin antibodies in women with recurrent miscarriage remains the main diagnostic indicator for intervention.
The prevalence of anticardiolipin antibodies in general obstetric clinics has been reported to be between 2.7% and 7% (Lockwood 1989; Lynch 1994; Yasuda 1995). Prospective studies of low‐risk pregnancies have found their presence carried a three to nine times greater risk of fetal loss (Lockwood 1989; Lynch 1994; Lynch 1999; Yasuda 1995). Women with a history of at least three prior miscarriages and no abnormality other than the presence of antiphospholipid antibodies are highly likely to have a future miscarriage. In a prospective study of 20 women who declined treatment, 90% miscarried and 94% of the fetal losses occurred in the first trimester (Rai 1995). This finding is controversial as is the reported association between anticardiolipin antibodies and maternal complications or low birthweight infants (Lockwood 1989; Lynch 1994; Lynch 1999).
Antiphospholipid antibodies are associated with venous and arterial thrombosis. In pregnancy, thrombosis of placental vessels may result in placental insufficiency, which can lead to fetal death. Placental pathology is variable but can include infarction with utero‐placental thrombus, perivillous fibrin deposits, and even chronic inflammatory lesions (Nilsson 1975; Salafia 1997). Annexin‐V, an anticoagulant phospholipid‐binding protein found on normal placental villi, appears to be reduced in the presence of antiphospholipid antibodies and it has been postulated that this may play a role in the placental insufficiency and consequent fetal loss (Rand 1994; Rand 1997). There is also 'in vitro' evidence that these antibodies may inhibit proliferation of trophoblasts which could result in impaired implantation (Chamley 1998).
The first successful treatment in 1975 involved preterm caesarean section in a woman who had experienced three prior fetal losses (Nilsson 1975). Subsequently, the combination of prednisone and aspirin was reported, in 1983, to be successful in a case‐series of five out of six participants (Lubbe 1983). Concerns with respect to the effect of prednisone on both the mother and the child resulted in exploration of alternative therapy. In 1988, low‐dose aspirin alone was reported to have a dramatic effect on pregnancy outcome in women with a poor obstetric history, which included some with anticardiolipin antibody (Elder 1988). In the same year, three case reports of the successful use of intravenous immunoglobulin therapy were published (Carreras 1988; Francois 1988; Scott 1988). Unfractionated heparin therapy was promoted in 1990 (Rosove 1990) and, in 1992, the use of low molecular weight heparin was described (Many 1992). In the same year, the successful use of plasmapheresis in one participant was reported (Kobayashi 1992).
In considering treatment both efficacy and adverse outcomes need to be considered. There is potential for morbidity in both mother and fetus with these treatments, especially prednisone with its effect on blood sugar, blood pressure and bone density. In addition heparin carries potential risks of haemorrhage, thrombocytopenia and osteoporosis. Although there is extensive experience in the use of low‐dose aspirin in the treatment and prevention of pre‐eclampsia without excessive adverse outcomes in mother or neonate, its safety when used in this setting can not be assumed. Plasmapheresis is invasive and increases the risk of infection while thrombosis in particular is a potential risk with high‐dose intravenous immunoglobulin. The best way to assess the balance of benefit and risk is via a systematic review of randomised controlled trials.
A number of relatively small randomised controlled studies have been performed looking at some, but not all, of the proposed treatments. Findings have not always been consistent. Current management generally includes heparin combined with aspirin. There has been a move towards using low molecular weight heparin because of the advantage of once daily dosing and a perception that it may have less effect on bone mineral density (Nelson‐Piercy 1994; Shefras 1996). This systematic review, which looks at all potential therapies, is necessary to highlight the benefits and in particular, the risks, of the different regimens, and to explore the many areas where the evidence is not yet available, and further research is required.
Objectives
To examine the effects of treatment used during pregnancy to prevent fetal loss in women with prior miscarriage associated with the presence of the antiphospholipid antibody.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials.
Types of participants
Pregnant women with at least one fetal loss and evidence of antiphospholipid antibodies. Antiphospholipid antibody presence determined by either a positive anticardiolipin antibody (IgG or IgM), a positive lupus anticoagulant or a falsely positive VDRL test.
Types of interventions
Any form of therapy including aspirin, unfractionated heparin, low molecular weight heparin, prednisone, intravenous immunoglobulin and plasmapheresis. Treatments compared with another or with placebo. Combinations of treatment included.
Types of outcome measures
Pregnancy loss
Preterm delivery (< 37 weeks)
Fetal loss in the first trimester (<= 14 weeks)
Fetal loss after the first trimester (> 14 weeks)
Maternal antepartum haemorrhage
Maternal postpartum haemorrhage requiring transfusion
Pregnancy associated hypertension (diastolic blood pressure (BP) >= 90 mm Hg or a rise in systolic BP >= 30 mm Hg or a rise in diastolic BP >= 15 mm Hg)
Caesarean section
Small‐for‐gestational age (birthweight < 10th percentile for gestational age)
Neonatal bleeding/bruising
Neonatal intensive care unit admission
Birthweight
Maternal fracture during pregnancy or up to one month postdelivery
Maternal bone mineral densitometry
Maternal death
Maternal side‐effects
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group Trials Register by contacting the Trials Search Co‐ordinator (30 May 2004).
We updated this search on 10 September 2009 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library 2003, Issue 2), MEDLINE (1996 to June 2003) and EMBASE (1988 to June 2003) using the search strategies detailed in Appendix 1.
Searching other resources
Handsearching
Lupus, volume one to volume eight (1991 to 1999 inclusive)
Conference proceedings from the International Symposium on Antiphospholipid Antibodies up to 1999.
We also scanned bibliographies of all located articles and contacted experts in the field.
We did not apply any language restrictions.
Data collection and analysis
From the initial search, two review authors independently reviewed the titles and abstracts from the database searches to determine whether the inclusion criteria were satisfied, and agreement was assessed by the kappa statistic. The full text of identified articles, including those where there was disagreement in the initial title/abstract scanning, were then reviewed independently by two review authors to ensure inclusion criteria were met. Where necessary the author was contacted for additional information. Agreement was assessed by the kappa statistic and disagreements were dealt with by consensus and, where necessary, involvement of a third review author. One review author reviewed contents pages of all issues of Lupus. Two review authors independently reviewed abstracts and, as necessary, full articles of the selected titles for fulfillment of the inclusion criteria. One review author scanned conference proceedings and included if adequate information was obtained either from the abstract or from personal communication. One review author identified articles from other sources (experts or reference lists) as possible and then two review authors assessed them independentlay against the inclusion criteria as above. Blinding to authors, journal of origin or institutions did not occur. Two review authors independently assessed abstracts of non‐English articles for fulfillment of the inclusion criteria; full article translation was not required as none fulfilled the criteria.
Two independent review authors extracted study characteristics and data from included studies including assessments of quality. Disagreements were resolved by involvement of a third review author and consensus. We contacted trial authors where information was lacking or data insufficient.
We assessed several aspects of study quality in the studies fulfilling the inclusion criteria. These included generation of randomisation sequence, allocation concealment, blinding of participant, investigator, and outcome assessor, less than 20% loss to follow up, and analysis by intention to treat. Each criterion was graded according to the Cochrane recommendations: A ‐ criterion met, B ‐ partially met or unclear and C ‐ not met. Agreement between the two review authors was assessed with the kappa statistic. Despite this quality assessment, no study was excluded on the basis of quality.
One review author performed a subsequent database search from December 1999 to June 2003. The same author applied the inclusion criteria, quality assessment and data extraction in an identical manner to that performed previously but without the second review author.
We reported outcome variables using the random‐effects model as a more conservative estimate taking into account between‐study variability. All estimates of effect for dichotomous variables are summarised as relative risks, except where there was evidence of heterogeneity (Q statistic exceeding the degrees of freedom). The measure of effect for the continuous variable, birthweight, is a weighted mean difference. We were unable to assess bone mineral densitometry in this way as it was not measured consistently in any study. We assessed heterogeneity of individual studies by visualisation of the summary graphs and assessment of the Q statistic. Due to the low sensitivity of this statistic, heterogeneity was assumed to be present where Q exceeded the degrees of freedom, rather than relying upon statistical significance. Where heterogeneity was present results were not pooled. Exploration of reasons for heterogeneity by subgroup analysis was not possible due to the paucity of studies. Similarly, subgroup analysis to assess the effect of poorer quality studies on the estimate of effect was not possible. Hypothesis‐generating subgroup analysis using the following criteria was not possible due to insufficient data: (1) women with three or more embryonic losses compared to those with less; (2) women with moderate or high‐positive anticardiolipin antibody (at levels greater than 15 G phospholipid units (GPL) or greater than 6 M phospholipid units (MPL)) compared to those with low level (less than 15 GPL or less than 6 MPL) anticardiolipin antibody and negative lupus inhibitor; (3) women with moderate or high‐positive anticardiolipin antibody (at levels greater than 15 GPL or greater than 6 MPL) compared to those with negative anticardiolipin antibody but positive lupus inhibitor; (4) unfractionated heparin compared to low molecular weight heparin; (5) fixed heparin doses compared to doses which vary according to laboratory monitoring and (6) different fixed heparin doses. We were not able to explore meta‐regression to explore the effect of baseline risk due to insufficient trials. Publication bias assessment via a funnel plot was also not possible with so few trials.
Results
Description of studies
For details of included studies, see 'Characteristics of included studies' table and Table 1. In the initial computerized database search (up to 1999), 551 studies were identified as potentially relevant (k = 0.62) and a further 24 studies were identified by bibliography checks. Ten studies from this initial search were included (k = 0.92). In the subsequent database search (up to June 2003) an additional 400 studies were identified as potentially relevant; however, a number of these were duplicates. Three additional studies published since 1999 were identified.
1. Summary of participants in the studies.
| Individual studies | No. of subjects | Mean age (years) | Ave fetal loss/woman | No. 1st T. loss only | No. prior live birth | Mean ACL level | LA alone | LA and ACL | IgM ACL alone |
| Branch 2000 | 16 | 29 | 60.2 (G) | 0/16 | |||||
| Cowchock 1992 | 45 | 3 | 22/45 | 12/45 | |||||
| Cowchock 1997 | 19 | 0.6 | 8/19 | ||||||
| Farquharson 2002 | 98 | 33 | 3 | 41/98 | 40/98 (G or M) | 8/98 | |||
| Kutteh 1996a | 50 | 33 | 3.8 | 29/50 | 15/50 | 46.6 (G and M) | 0/50 | 0/50 | 11/50 |
| Kutteh 1996b | 50 | 33 | 3.8 | 27/50 | 13/50 | 42 (G and M) | 0/50 | 0/50 | |
| Laskin 1997 | 202 | 33 | 3.5 | 139/202 | 68/88 | 6/88 (G) | 0/88 | ||
| Pattison 2000 | 50 | 31 | 3/40 | 6/40 (G or M) | |||||
| Rai 1997 | 90 | 32 AH, 34 A (median) | 4 | 60/90 | 33/90 | 12.5 (median) | 74/90 | 8/90 (G or M) | 1/90 |
| Silver 1993 | 39 | 31 | 0/34 | 2/34 (G) | 4/34 | ||||
| Triolo 2003 | 42 | 31 | 3.7 | 53.3 (G) | 0/40 | 27/40 (G) | 0/40 | ||
| Tulppala 1997 | 66 | 0/6 | 0/6 | 0/6 | |||||
| Vaquero 2001 | 82 | 31 | 2.7 | 89% > 20 GPL/MPL | 46/82 | 25/82 (G or M) |
(Fifteen reports from an updated search in September 2009 have been added to Studies awaiting classification.)
The study designs, inclusion and exclusion criteria and interventions are shown in the 'Characteristics of included studies' table. A total of 849 participants were enrolled in the 13 trials. Three trials compared aspirin with placebo or standard care (n = 135) (Cowchock 1997; Pattison 2000; Tulppala 1997). Six explored the efficacy of heparin combined with aspirin; two of these used low molecular weight (LWM) heparin combined with aspirin (n = 140) and compared this to aspirin alone (Farquharson 2002) or intravenous immunoglobulin (IVIG) (Triolo 2003). The others used unfractionated heparin combined with aspirin; two compared the combination to aspirin alone (n = 140) (Kutteh 1996a; Rai 1997), one compared low‐dose with high‐dose heparin both combined with aspirin (n = 50) (Kutteh 1996b), and one compared the combination with prednisone and aspirin (n = 45) (Cowchock 1992). Two trials compared prednisone and aspirin with placebo or aspirin (n = 241) (Laskin 1997; Silver 1993). Three trials used IVIG; in one study all participants received aspirin and heparin with the addition of either IVIG or placebo (n = 16) (Branch 2000). Another study included above compared IVIG to LMW heparin and aspirin (n = 42) (Triolo 2003). The third study compared IVIG to prednisone and aspirin (n = 82) (Vaquero 2001). No trials of plasma exchange were identified.
Two trials that were included had some participants who were antiphospholipid antibody (APL) negative (Laskin 1997; Tulppala 1997). For the primary outcome, pregnancy loss, subgroup data from the APL positive participants were used (n = 12/66 (Tulppala 1997) and 88/202 (Laskin 1997)); for all other outcomes including the composite ones, the complete study data were used. Two other trials included some participants who had not experienced a fetal loss, (n = 10/19 (Cowchock 1997) and 1/16 (Branch 2000)). Subgroup data were not available in these studies and therefore data from all participants were used.
Characteristics of the trial participants, summarized in Table 1, were not available from all studies. The mean number of pregnancy losses per woman ranged from 0.6 to 4 (Cowchock 1992; Cowchock 1997; Farquharson 2002; Kutteh 1996a; Kutteh 1996b; Laskin 1997; Rai 1997; Triolo 2003; Vaquero 2001). The proportion of women with only first trimester pregnancy losses ranged from 49% to 67% in the four studies that described this (Cowchock 1992; Kutteh 1996a; Kutteh 1996b; Rai 1997). A previous successful pregnancy had occurred in between 26% and 69% in the five studies that described this (Cowchock 1997; Kutteh 1996a; Kutteh 1996b; Laskin 1997; Rai 1997). Anticardiolipin (ACL) antibody levels ranged from a median of 12.5 to a mean of 60.2 G phospholipid units (GPL) units in five trials reporting this (Branch 2000; Kutteh 1996a; Kutteh 1996b; Rai 1997; Triolo 2003), reflecting the various definitions of positive ACL antibody used in the inclusion criteria of individual trials. One study reported that 89% of participants had at least moderate level ACL antibodies (greater than 20 GPL/M phospholipid units) (Vaquero 2001). Ten studies reported the frequency of an isolated lupus anticoagulant; this ranged from 0% (criteria for exclusion in two studies) (Kutteh 1996a; Kutteh 1996b) to 82% (Farquharson 2002; Laskin 1997; Pattison 2000; Rai 1997; Silver 1993; Triolo 2003; Tulppala 1997; Vaquero 2001).
Risk of bias in included studies
The quality of the included trials was variable as shown in Table 2. Three quasi‐randomised studies did not conceal allocation of therapy (Kutteh 1996a; Kutteh 1996b; Vaquero 2001). Only one study had any loss to follow up (Triolo 2003). Four studies did not analyse by intent to treat (Cowchock 1992; Pattison 2000; Silver 1993; Triolo 2003); two stated the analysis was performed both with and without excluded participants but did not publish the data (Pattison 2000; Silver 1993), and one provided outcome data on all participants according to their allocation group so that the data entered for the meta‐analysis was by intent to treat (Cowchock 1992). It was not clear from the information provided in the quasi‐randomised studies whether there was loss to follow up or an analysis by intent to treat was performed as the total number of participants presenting during the recruitment phase (the denominator) was not published (Kutteh 1996a; Kutteh 1996b; Vaquero 2001).
2. Quality assessment of methodology of included studies.
| Individual Studies | Randomisation method | Allocation concealed | Blinding of subject | Blinding of provider | Blinding of assessor | Loss to follow up | Intention to treat |
| Branch 2000 | Computer generated random number table. The key was available only to the pharmacist. | Adequate. | Yes. | Yes. | Yes. | No. | Yes. |
| Cowchock 1992 | Central randomisation using a computer generated sequence of random numbers. | Adequate. | No. | No. | Unclear. | No. | No, but outcome data for excluded subjects published and allowed inclusion of all subjects in the meta‐analysis. |
| Cowchock 1997 | Not described. | Not described. | No. | No. | Unclear. | No. | Yes. |
| Farquharson 2002 | Central randomisation using a computer generated sequence of random numbers. | Adequate. | No. | No. | Yes. | No. | Yes. |
| Kutteh 1996a | Alternative assignment. | No concealment. | No. | No. | No. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. |
| Kutteh 1996b | Sequential block of 25 allocated to one treatment group and a second sequential block of 25 allocated to another treatment group. | No concealment. | Unclear. | Unclear. | Unclear. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. |
| Laskin 1997 | Central randomisation. Stratified by age and week of gestation of previous fetal losses using a balanced four‐block procedure. | Adequate. | Yes. | Yes. | Yes. | No. | Yes. |
| Pattison 2000 | Sealed envelopes according to a computer generated list of study numbers. | Possibly adequate. | Yes. | Yes. | Yes. | No. | No. Five subjects excluded from each arm. Paper states that analyses were performed with and without these subjects but results from included subjects only published. |
| Rai 1997 | Computer generated random number list kept by an independent member of the staff. | Adequate. | No. | No. | No. | No. | Yes. |
| Silver 1993 | Computer generated random number table with sequential opaque envelopes. | Adequate. | No. | No. | Unclear. | No. | No. Five subjects excluded from the combined treatment arm. Paper states that analyses were performed with and without these subjects but results from included subjects only published. |
| Triolo 2003 | Central randomisation using a computer generated sequence of random numbers. | Adequate. | No. | No. | Unclear. | Yes (2/21 subjects). | No. Two non‐compliant subjects from the heparin arm withdrew and were not included in the analysis. |
| Tulppala 1997 | Not described. | Not described. | Yes. | Unclear. | Unclear. | No. | Yes. |
| Vaquero 2001 | Two centres each using a single treatment modality. | No concealment. | No. | No. | No. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. | Unclear as the number who refused treatment or were treated with an alternative therapy during the recruitment phase is not known. |
Effects of interventions
Thirteen studies, involving 849 participants, were included. The first set of analyses graphs summarises the effects of the different comparisons on the primary outcome (pregnancy loss).
Heparin
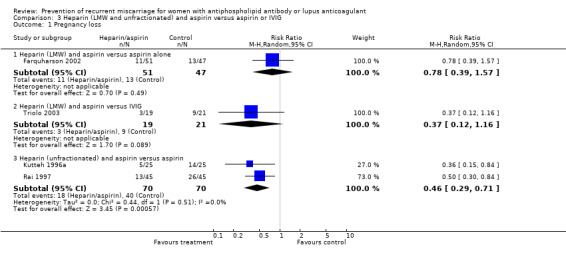
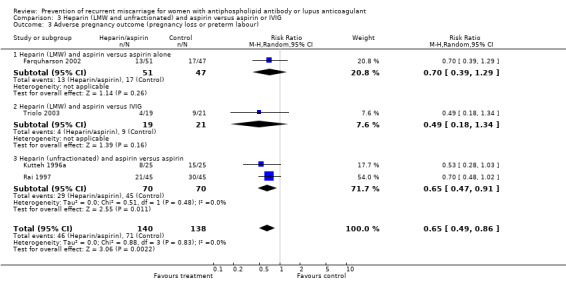
Of the interventions examined, only unfractionated heparin combined with aspirin was shown to reduce the incidence of pregnancy loss (relative risk (RR) 0.46, 95% confidence interval (CI) 0.29 to 0.71) when compared with aspirin alone. Low molecular weight (LMW) heparin combined with aspirin had no statistically significant effect when compared to aspirin alone (RR 0.78, 95% CI 0.39 to 1.57) or intravenous immunoglobulin (IVIG) (RR 0.37, 95% CI 0.12 to 1.16); however, the point estimates are in the direction of benefit, although the confidence intervals are wide. No head‐to‐head study comparing LMW and unfractionated heparin met our inclusion criteria and, therefore, the relative effects of unfractionated versus LMW heparin are unknown. The treatment advantage of unfractionated heparin was maintained with the composite adverse pregnancy outcomes of 'pregnancy loss or intrauterine growth restriction' (IUGR) (RR 0.57, 95% CI 0.39 to 0.83) and 'pregnancy loss or premature delivery' (RR 0.65, 95% CI 0.47 to 0.91). The LMW studies did not provide IUGR data but they did include premature delivery data. The risk of 'pregnancy loss or premature delivery' when LMW heparin combined with aspirin is compared to aspirin or IVIG is very similar to the unfractionated heparin studies although they do not reach statistical significance (RR 0.70, 95% CI 0.39 to 1.29 and RR 0.49, 95% CI 0.18 to 1.34 respectively). When the LMW and unfractionated heparin studies are pooled there is a 35% reduction in pregnancy loss or premature delivery (RR 0.65, 95% CI 0.49 to 0.86). High‐dose unfractionated heparin did not differ from low‐dose unfractionated heparin in its effects. Thrombocytopenia was either not reported or did not occur except for in one study where it was described as mild in two participants receiving LMW heparin (Triolo 2003).
Aspirin alone
Aspirin, when compared to placebo or standard care, had no significant effect on any of the outcomes examined even after exclusion of the study that had participants without antiphospholipid antibodies (Tulppala 1997).
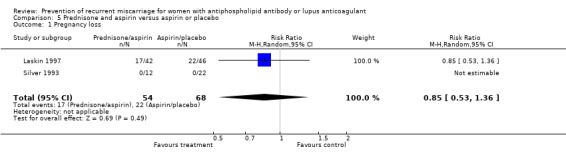
Prednisone
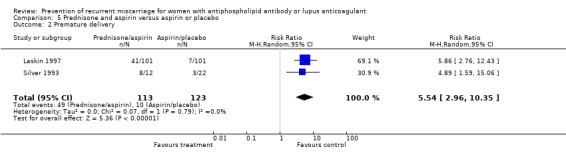
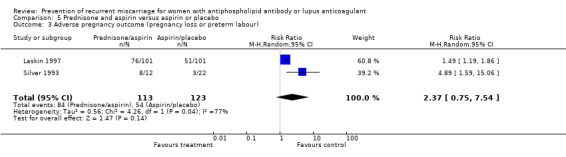
Prednisone and aspirin compared to placebo or aspirin alone did not have a significant effect on the risk of pregnancy loss (RR 0.85, 95% CI 0.53 to 1.36). A similar lack of effect was found when compared to heparin and aspirin (RR 1.17, 95% CI 0.47 to 2.93). However, there was significant increase in premature delivery in all prednisone groups and when this adverse pregnancy outcome was combined with pregnancy loss the control treatment (aspirin RR 4.89, 95% CI 1.59 to 15.06; placebo RR 1.49, 95% CI 1.19 to 1.86; heparin and aspirin RR 1.99, 95% CI 1.22 to 3.25) was favoured. A summary estimate was not appropriate though because of significant heterogeneity between the aspirin or placebo groups (Q 4.26, df 1) and a clear difference between these and the study using heparin/aspirin in the control group.
Other adverse outcomes were increased in prednisone treated participants. The neonatal intensive care unit admission in one study was nine times more likely in the prednisone treated group than the placebo group (95% CI 2.14 to 37.78) (Laskin 1997). The rate of pre‐eclampsia and hypertension was higher in the prednisone treated participants compared to others as documented below. Prednisone was also associated with a 3.3 times (95% CI 1.53 to 6.98) greater risk of gestational diabetes when compared with placebo, aspirin alone, heparin and aspirin, or IVIG (Cowchock 1992; Laskin 1997; Silver 1993; Vaquero 2001). Birthweight was significantly less in the prednisone and aspirin‐treated groups compared to aspirin (weighted mean difference (WMD) ‐552.00, 95% CI ‐1064.79 to ‐39.21) (Silver 1993) or IVIG (WMD ‐351.00, 95% CI ‐587.94 to ‐114.06) (Vaquero 2001).
Intravenous immunoglobulin (IVIG)
IVIG studies used a range of treatments in the control groups and it is therefore not appropriate to combine any of these studies together. There was no reduction in pregnancy loss in any of the studies; however, one study had no pregnancy loss in either the treatment group or the control group (Branch 2000). This was a small study (n = 16) and all participants received heparin and aspirin in addition to the study/control medication. This study demonstrated a significant increase in premature delivery (RR 3.00, 95% CI 1.19 to 7.56). There was no significant heterogeneity between this study and the study comparing IVIG with LMW heparin and aspirin (Triolo 2003) when the composite outcome pregnancy loss or premature delivery was explored (Q .33, df 1). In these two studies IVIG increased the risk of pregnancy loss or premature delivery two and a half times (95% CI 1.27 to 4.95). In contrast IVIG did not significantly differ from prednisone and aspirin in outcomes (Vaquero 2001).
Other adverse outcomes
No participants died in any of the studies and significant hemorrhage did not occur in mother or neonate. Maternal fracture was not reported and this was generally not analyzed. Only two studies performed bone mineral densitometry, and this was restricted to the heparin‐receiving participants only (Rai 1997; Triolo 2003). A median decrease of 5.4% of lumbar spine bone mineral densitometry was documented in one study using unfractionated heparin (Rai 1997) and no change was noted in the other which used LMW heparin (Triolo 2003). Our definition used for hypertension was not adopted in any trial, but pre‐eclampsia, variously defined or undefined, was reported in some trials. Three heparin trials when pooled reported seven cases of pre‐eclampsia in 190 women (Kutteh 1996a; Kutteh 1996b; Rai 1997). The rates were a little higher in two aspirin‐only trials with three of twenty in each of the placebo and aspirin groups in one trial (Pattison 2000) and one of 33 compared to three of 33 in the aspirin and placebo groups respectively of another trial (Tulppala 1997). The rate of pre‐eclampsia was higher in the prednisone and aspirin treated participants compared to those receiving heparin and aspirin (32% versus 4%) (Cowchock 1992). Hypertension was higher in the prednisone and aspirin treated participants compared to placebo (13% versus 5%) (Laskin 1997) and to IVIG (14% versus 5%) (Vaquero 2001). In the other IVIG studies there were eight cases of pre‐eclampsia; 3/7 IVIG participants compared to 1/9 placebo (Branch 2000) and 1/21 IVIG compared to 0/19 LMW heparin (Triolo 2003).
Subgroup analyses
It was not possible to establish whether interventions were of similar efficacy in preventing early (before 14 weeks) compared with later pregnancy losses as there were insufficient losses after 14 weeks in the trials. Likewise, there were insufficient trials per therapeutic group to explore possible effect modification by study quality, varying heparin doses, and participant characteristics such as the number of prior pregnancy losses, or antibody type and level.
Discussion
The major finding in this systematic review is that the combination of unfractionated heparin and aspirin reduced pregnancy loss by 54%. However, this is based on only two small trials and one of these lacked adequate allocation concealment. There is a suggestion that low molecular weight (LMW) heparin also has a beneficial effect; however, this finding was not statistically significant and uncertainty remains.
A head‐to‐head trial comparing LMW and unfractionated heparin for prophylaxis in pregnancy has been published in abstract form only (De Veciana 2001). Insufficient information was available to determine whether this study fulfilled our criteria (especially what proportion of women had antiphospholipid antibody syndrome) and an attempt to contact the author for additional information failed. Consequently this trial could not be included; however, the abstract suggests there may be clinical differences between these agents when used prophylactically in thrombophilia associated with pregnancy. The absence of a good head‐to‐head trial comparing LMW and unfractionated heparin results in uncertainty in the relative effects of these two treatment modalities. There are biological differences in pharmacologic effect of these forms of heparin for example their ability to bind to thrombin and other proteins; however, clinical trials show them to be at least of equivalence as antithrombotic agents in non‐pregnant women (Hirsh 1998). During pregnancy the effects of differences in protein binding may be greater; studies here have not been adequate to prove equivalence as antithrombotic agents in this group (Ensom 1999). In addition, in recurrent miscarriage due to antiphospholipid (APL) syndrome, the antithrombotic effect of the heparins may not be the main mode of action. There is in vitro evidence that APL antibodies affect trophoblast differentiation, proliferation and invasion all of which may adversely affect the early pregnancy (Chamley 2002). In vitro studies have shown that LMW heparin can restore trophoblast function but no comparison with unfractionated heparin has been made (Di Simone 1997; Di Simone 1999). Currently, therefore, one can not assume that the LMW heparin and unfractionated heparin have equivalent biological effects.
In addition, there are differences between studies in the diagnostic criteria for lupus anticoagulant (LA) and anticardiolipin (ACL), which determined participant populations. These may have influenced the baseline risk; the control rate of pregnancy loss is lower in the Farquharson 2002 study (28%) in which the majority of participants had low positive ACL antibodies compared to Kutteh 1996a and Triolo 2003 and the ratio used to define the LA was lower than that used by Rai 1997. The control rates of pregnancy loss in the other three studies were 43% (Triolo 2003), 56% (Kutteh 1996a) and 57% (Rai 1997) . These differences may have influenced the size of the effect if they are effect modifiers. Unfortunately, there were insufficient studies to address this possibility and individual participant data meta‐analysis is required. Thus population differences, rather than biological differences between the drugs, may have influenced the differences in estimates of effect. In addition, one LMW heparin study used intravenous immunoglobulin (IVIG) instead of aspirin in the control arm, (Triolo 2003) and it may not be valid to combine this study with the other heparin studies.
The improvement in pregnancy outcome with unfractionated heparin is associated with a non‐significant increase in risk of prematurity and intrauterine growth retardation (relative risk (RR) 2.2 and 3.0 respectively) but this may be a result of prolongation of pregnancies, which if untreated would have been lost and this could therefore bias the adverse outcomes such as prematurity, intrauterine growth restriction (IUGR), etc, to appear more common with the drug most successful in preventing pregnancy loss. An alternative way of assessing these outcomes could be to assess the risk in the subgroup with a live birth. However, baseline risk is unlikely to be similar in the two comparative subgroups with live births. The control subgroup may only contain those with a low baseline risk compared to the effective treatment group where there may be participants with high baseline risk also. Consequently, the effect of randomisation on confounders is lost and the comparison is prone to bias. Therefore we considered it more appropriate to use composite outcomes. All pregnancy related adverse outcomes could not be combined due to overlap in outcomes for example premature babies may also be included in the IUGR and caesarean outcomes etc. Therefore two composite outcomes were used; pregnancy loss or premature delivery, and pregnancy loss or IUGR.
The risk of pregnancy loss or premature delivery is reduced by 35% in those treated with either form of heparin combined with aspirin. The effect of LMW heparin on IUGR could not be assessed as neither study supplied these data; however, unfractionated heparin combined with aspirin reduced pregnancy loss or IUGR by 43%.
Potential heparin related hazards, including significant maternal thrombocytopenia and haemorrhage, did not occur. The possibility of osteoporosis developing while on long‐term heparin is of concern. Fractures were not reported but may have been missed. Only one unfractionated heparin trial measured the bone mineral density (Rai 1997); controls were not assessed but the finding of a 5.4% decrease in lumbar spine bone mineral density in those treated with heparin is concordant with a prospective study demonstrating a 5% decrease in lumbar bone mineral density in LMW heparin treated pregnant participants, compared to 3% in the pregnant controls (Shefras 1996). One LMW heparin study assessed bone mineral density in 12/19 participants receiving heparin but once more did not assess this in the controls (Triolo 2003). Instead the bone mineral density at 14 weeks' gestation was compared to a postnatal assessment and no change was documented.
In determining the potential benefit versus hazard to an individual, baseline risk is important (Glasziou 1995). A prospective cohort of women attending a general antenatal outpatient clinic who were found to be ACL positive, (20% of whom had a previous pregnancy loss) had a subsequent rate of pregnancy loss of 28% (low‐risk) (Yasuda 1995). Treatment of 100 women of such risk with combined unfractionated heparin and aspirin would benefit 15. In contrast a high‐risk cohort (20 women who refused treatment and were positive for either ACL, LA or both with at least three previous pregnancy losses) had a subsequent pregnancy loss rate of 90% (Rai 1995). Treatment of 100 would benefit 49. If the baseline risk is taken as a more conservative number, 52% (the mean of the three highest control rates in the heparin trials), treatment would benefit 28 of the 100 treated. Hazards associated with treatment occur infrequently and the risk cannot be assessed in this manner.
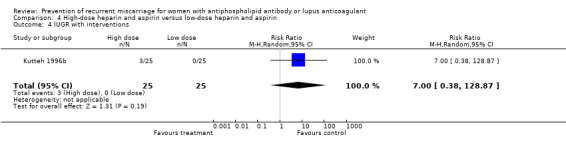
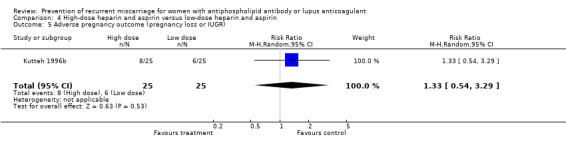
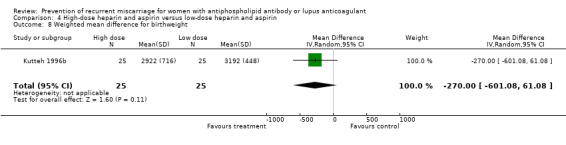
The optimal dose of heparin to maximise benefit and minimise harm is unknown. Various regimens were used in these studies but this did not alter the outcomes significantly as demonstrated by the absence of significant heterogeneity. The study which compared high‐dose to low‐dose heparin had methodological problems (quasi‐randomised with lack of allocation concealment) but also lacked the power to detect a significant difference (Kutteh 1996b). Similarly it remains unknown whether LMW heparin can be substituted for unfractionated heparin.
A small benefit with aspirin alone or IVIG cannot be excluded on the basis of the available studies. However, what is available suggests that IVIG with or without heparin and aspirin is inferior to LMW heparin combined with aspirin or unfractionated heparin and aspirin alone (pregnancy loss or premature delivery RR 2.5, confidence interval 1.27 to 4.95). The two studies from which this is derived are small and further studies are required. However, given the uncertainty, IVIG treatment for APL antibody associated pregnancy loss should only occur as part of a randomised controlled trial.
In the trials of prednisone and aspirin no benefit was shown irrespective of whether the control group received aspirin, placebo, heparin and aspirin, or IVIG. Any small benefit that may have been missed in this systematic review is likely to be negated by the increase in adverse neonatal and maternal outcomes. When the outcome pregnancy loss or premature delivery was considered all control treatments (aspirin, placebo and heparin and aspirin) with the exception of IVIG, significantly reduced the risk compared to prednisone and aspirin. Gestational diabetes and other adverse outcomes were increased even at doses of prednisone as low as 10 mg/day. Based on these results prednisone appears to have no role in the treatment of recurrent pregnancy loss associated with APL antibodies. However, when other indications are present such as active systemic lupus erythematosus, the potential benefits will need to be weighed against the potential harms.
The terminology and inclusion criteria for this review were broad. One of the aims was to explore various participant characteristics, as subgroups, to look for evidence of effect modification, and as indicators of baseline risk. This was not possible due to the small number of studies retrieved and the aggregate data used. Efficacy studies which focus on the current proposed classification of antiphospholipid antibody syndrome (Wilson 1999) which requires at least three consecutive early (less than 10 weeks) fetal losses or at least one late (greater than 10 weeks) fetal loss may limit applicability assessments. Similarly the ACl cut‐offs for defining the syndrome are much higher than used in most of these studies. It is not known whether the antibody levels have a modifying effect on the treatment. Women who do not fall into the 'syndrome' classification may still benefit from treatment and this needs to be explored. There is currently no evidence that efficacy is limited to certain subsets of participants only, and the level of baseline risk below which potential harms outweigh potential benefits is unknown.
This systematic review has several potential limitations. The number of trials and enrolled participants were small limiting the precision of all estimates. The partial failure to identify significant effects may be due to a type 2 errors. The quality of trials was variable; three included trials were quasi‐randomised only and allocation concealment, a potent source of bias if not incorporated (Schulz 1995), was adequate in only 50% of all trials. Despite this, the studies within their therapeutic groups were generally consistent but there was evidence of heterogeneity in outcomes particularly in the heparin trials. Two trials included some participants without a history of pregnancy loss but their effect was likely only to reduce the baseline risk rather than introduce bias into the relative effect measure. On the other hand, two studies included participants who were APL antibody negative. Effect‐modification by the APL antibody may result in bias from inclusion of these studies but this would not affect the primary outcome, pregnancy loss, where it was possible to extract data for those who were antibody positive. With respect to the other outcome measures, if the effect is purely related to the treatment then no bias is likely. Alternatively if the APL antibody itself has an effect on prematurity and intrauterine growth retardation, then the inclusion of these studies may result in a bias towards the null reducing the apparent efficacy of treatment on their occurrence. Lastly, it is unlikely that selection bias occurred as the search strategy was quite intensive and non‐English language studies were not excluded on the basis of language. Formal assessment with a funnel plot was not possible due to the small number of trials.
Authors' conclusions
Implications for practice.
The combination of twice‐daily unfractionated heparin and low‐dose aspirin appears to be of benefit in pregnant women with antiphospholipid antibodies and recurrent pregnancy loss not related to other causes. The benefits in low‐risk participants may not be sufficient to warrant its use. LMW may be of benefit but there is no evidence that it has similar efficacy to heparin and its use as a substitute for unfractionated heparin can not be justified based on present data. There is no evidence that other therapies may provide benefit but there is some evidence of harm with prednisone and intravenous immunoglobulin.
Implications for research.
Further large trials of heparin (both unfractionated and LMW) combined with aspirin are needed to reduce clinically important uncertainty about the benefits and harms. A large multicentre study comparing unfractionated heparin and aspirin with LMW heparin and aspirin, and aspirin alone is well overdue. Until this is done, debate about the efficacy of LMW heparin, unfractionated heparin and their interchangability will continue.
[Note: The 15 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Feedback
Cundiff, 19 July 2007
Summary
Since three randomised trials of aspirin versus placebo showed no benefit, aspirin should be avoided and excluded from future trials. The conclusion that heparin added to aspirin increases live births and should become standard care for women with recurrent miscarriages associated with antiphospholipid antibodies is premature. The two small trials included only 140 women. No long term follow up was reported or anticipated. The lack of efficacy of low molecular weight heparin in the only randomised trial suggests that the efficacy of unfractionated heparin may have been a statistical aberration. Safety of heparin (which can lead to osteopenia, bleeding, heparin induced thrombocytopenia, and fetal abnormalities) cannot be determined from these small trials. Even if adding heparin to aspirin does increase the chances of live birth in a single pregnancy, in this situation from 30/70 (43%) to 52/70 (74%), it is not significantly better that the chance of a live birth in two pregnancies with aspirin (or placebo) alone (i.e., 1 x [0.57 x 0.57] = 68%). For women with recurrent miscarriages, anticoagulants should not be used outside of randomised trials. Given the risks of anticoagulants, the endpoint of any future trials should be a healthy baby in two or three pregnancies, rather than one pregnancy.
[Summary of comment received from David K Cundiff, July 2007]
Reply
The three trials of aspirin versus placebo were small (total n = 135) and so, as stated in discussion in the review “A small benefit with aspirin alone ... cannot be excluded on the basis of the available studies”. Also, the intervention arm of the heparin trials used a combination of heparin (unfractionated or LMW) with aspirin, and compared this to aspirin in the control group. It is not known whether there is an interaction between heparin and aspirin that might contribute to the reported benefit, and the only way to assess this would be to compare heparin plus aspirin with heparin alone. No such studies have been performed, and therefore current evidence is that there appears to be some benefit with the combination of aspirin and heparin. There is insufficient evidence to state that aspirin has no benefit. Further studies are needed. Trials of aspirin for the prevention of pre‐eclampsia suggests that low dose aspirin is safe for the mother and child (Duley 2007).
We completely agree it is premature to claim heparin plus aspirin increases live births and should become standard care for women, and have not stated this. What we do state is: “The combination of twice‐daily unfractionated heparin and low‐dose aspirin appears to be of benefit in pregnant women with antiphospholipid antibodies and recurrent pregnancy loss not related to other causes. The benefits in low‐risk participants may not be sufficient to warrant its use”. We agree the trials were small, and hence conclude “Further large trials of heparin (both unfractionated and LMW) combined with aspirin are needed to reduce clinically important uncertainty about the benefits and harms.” Potential harms from heparin, including long‐term outcome, and the importance of considering baseline risk are discussed in paragraph six and seven respectively of the discussion.
The data for LMW heparin should not be interpreted as ‘lack of efficacy’, as this trial was small and the confidence intervals are wide. Lack of evidence of an effect (as here) is not the same as evidence of lack of an effect. In addition, head to head trials comparing LMW heparin and unfractionated heparin for prophylaxis in pregnancy are inadequate and, as discussed in paragraph two of the discussion, they may not have the same biological effects. Therefore, the results of a LMW study do not refute the results of unfractionated heparin studies.
The calculation of the chance of a live birth in a second pregnancy is based on assuming the same risk for both pregnancies, but it is unclear whether this is a valid assumption.
We agree there is a need for further studies, and the next update of this review which is in preparation will include new trials. Nevertheless, the data in the current review should be discussed with women who are considering these interventions, to help them make an informed decision. The benefits of performing a trial over more than one pregnancy are unclear. This would not be a trial attractive to women, who are concerned about their current pregnancy. It would certainly increase the cost, complexity and attrition. Information about cumulative safety over more than one pregnancy might come from a prospective cohort, rather than an RCT.
Contributors
Reply to feedback from Marianne Empson, February 2011.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 14 February 2011 | Feedback has been incorporated | Feedback added from David K Cundiff with a reply from Marianne Empson. |
| 10 September 2009 | Amended | Search updated. Fifteen reports added to Studies awaiting classification. |
| 20 September 2008 | Amended | Converted to new review format. |
Notes
The first set of analyses graphs summarises the effects of the different comparisons on the primary outcome (pregnancy loss).
Acknowledgements
The authors wish to thank Doctors DW Branch, R Farquharson, J Kwak, C Laskin, CG Mueller‐Eckhardt, and K Stern, and Professors W Kutteh, and N Pattison who supplied additional information or unpublished studies.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search strategies
CENTRAL (The Cochrane Library 2003, Issue 2), and MEDLINE (1996 to June 2003): (lupus coagulation inhibitor (MeSH) OR antibodies, anticardiolipin (MeSH) OR antibodies, antiphospholipid (MeSH) OR antiphospholipid syndrome (MeSH) OR lupus inhibitor (tw) OR lupus anticoagulant (tw) OR anticardiolipin (tw) OR antiphospholipid (tw) OR cardiolipin antibod$ (tw) OR phospholipid antibod$ (tw)) AND (fetal death (MeSH) OR abortion, spontaneous (MeSH) OR abortion, habitual (MeSH) OR fetal loss (tw) OR miscarriage$ (tw) OR recurrent abortion$ (tw) OR recurrent miscarriage$ (tw)).
The term MeSH refers to medical subject headings and tw to text word in the title or abstract. The $ is a truncation character which allows all possible suffix variations of the root word.
The result of the MEDLINE search was combined with the phase one and phase two search strategy developed by Carol Lefebvre of the UK Cochrane Centre (Alderson 2004).
EMBASE (1988 to June 2003) Authors combined the sensitive strategy developed by the Cochrane Stroke Group (Sandercock 2004) with the following terms: (lupus anticoagulant (sh) OR phospholipid antibody (sh) OR antiphospholipid syndrome (sh) OR cardiolipin antibody (sh) OR anticardiolipin (tw) OR antiphospholipid (tw) OR lupus inhibitor (tw)) AND (spontaneous abortion (sh) OR recurrent abortion (sh) OR fetus wastage (sh) OR fetus death (sh) OR miscarriage$ (tw) OR recurrent miscarriage$).
Data and analyses
Comparison 1. All interventions ‐ pregnancy loss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aspirin versus placebo or usual care | 3 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.66, 1.68] |
| 2 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Heparin (LMW) and aspirin versus aspirin alone | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.39, 1.57] |
| 2.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.16] |
| 2.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.71] |
| 3 High‐dose heparin and aspirin versus low‐dose heparin and aspirin | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.29, 2.38] |
| 4 Prednisone and aspirin versus aspirin or placebo | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 5 Prednisone and aspirin versus heparin and aspirin | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.47, 2.93] |
| 6 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.86, 8.57] |
| 6.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.12] |
1.1. Analysis.
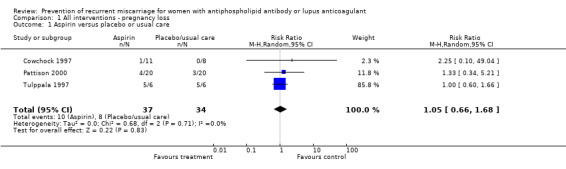
Comparison 1 All interventions ‐ pregnancy loss, Outcome 1 Aspirin versus placebo or usual care.
1.2. Analysis.
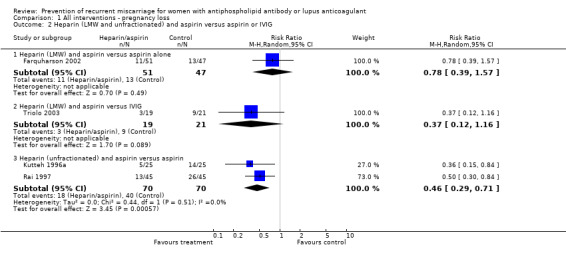
Comparison 1 All interventions ‐ pregnancy loss, Outcome 2 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG.
1.3. Analysis.
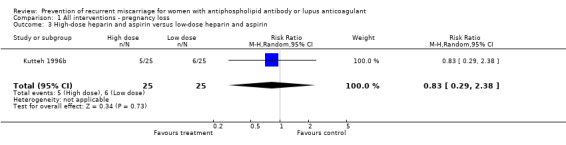
Comparison 1 All interventions ‐ pregnancy loss, Outcome 3 High‐dose heparin and aspirin versus low‐dose heparin and aspirin.
1.4. Analysis.
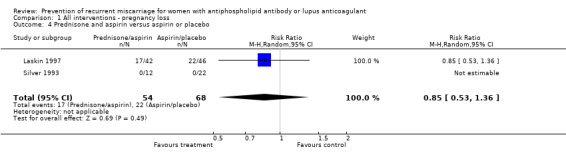
Comparison 1 All interventions ‐ pregnancy loss, Outcome 4 Prednisone and aspirin versus aspirin or placebo.
1.5. Analysis.
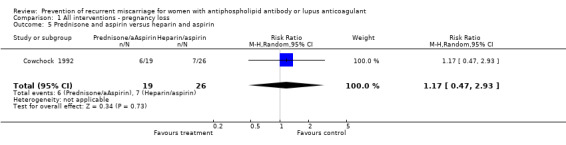
Comparison 1 All interventions ‐ pregnancy loss, Outcome 5 Prednisone and aspirin versus heparin and aspirin.
1.6. Analysis.

Comparison 1 All interventions ‐ pregnancy loss, Outcome 6 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin.
Comparison 2. Aspirin versus placebo or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 3 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.66, 1.68] |
| 2 Premature delivery | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.26, 98.00] |
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.58, 6.91] |
| 4 IUGR with interventions | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.17, 1.72] |
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 3 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.55, 1.49] |
| 6 Neonatal intensive care admission | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.16, 6.42] |
| 7 Caesarean section | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.47, 2.61] |
| 8 Weighted mean difference for birthweight | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 177.39 [‐66.59, 421.36] |
2.1. Analysis.

Comparison 2 Aspirin versus placebo or usual care, Outcome 1 Pregnancy loss.
2.2. Analysis.

Comparison 2 Aspirin versus placebo or usual care, Outcome 2 Premature delivery.
2.3. Analysis.

Comparison 2 Aspirin versus placebo or usual care, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
2.4. Analysis.
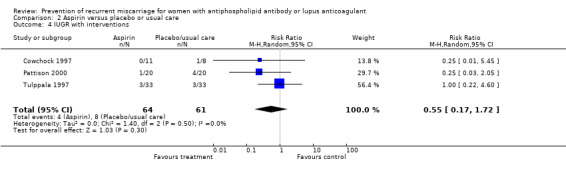
Comparison 2 Aspirin versus placebo or usual care, Outcome 4 IUGR with interventions.
2.5. Analysis.

Comparison 2 Aspirin versus placebo or usual care, Outcome 5 Adverse pregnancy outcome (pregnancy loss or IUGR).
2.6. Analysis.
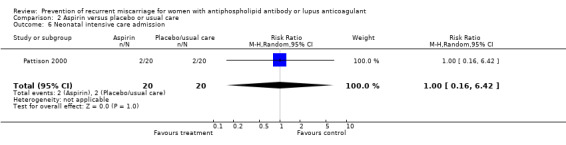
Comparison 2 Aspirin versus placebo or usual care, Outcome 6 Neonatal intensive care admission.
2.7. Analysis.
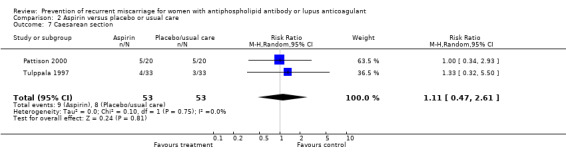
Comparison 2 Aspirin versus placebo or usual care, Outcome 7 Caesarean section.
2.8. Analysis.

Comparison 2 Aspirin versus placebo or usual care, Outcome 8 Weighted mean difference for birthweight.
Comparison 3. Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Heparin (LMW) and aspirin versus aspirin alone | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.39, 1.57] |
| 1.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.16] |
| 1.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.71] |
| 2 Premature delivery | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Heparin (LMW) and aspirin versus aspirin alone | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.09, 2.40] |
| 2.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.3 [0.14, 76.46] |
| 2.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.80, 5.93] |
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.86] |
| 3.1 Heparin (LMW) and aspirin versus aspirin alone | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.39, 1.29] |
| 3.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.34] |
| 3.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.91] |
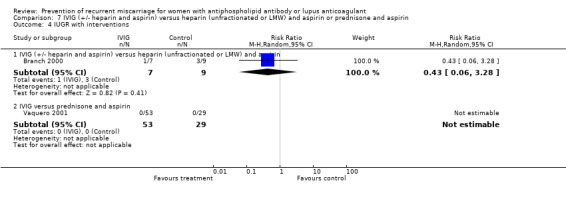
| 4 IUGR with interventions | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.63, 14.31] |
| 4.1 Heparin (LMW) and aspirin versus aspirin alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Heparin (LMW) and aspirin versus IVIG | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.63, 14.31] |
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.83] |
| 5.1 Heparin (LMW) and aspirin versus aspirin alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Heparin (LMW) and aspirin versus aspirin alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Heparin (unfractionated) and aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.83] |
| 6 Neonatal intensive care admission | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.50] |
| 6.1 Heparin (LMW) and aspirin versus aspirin alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.50] |
| 6.3 Heparin (unfractionated) and aspirin versus aspirin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Heparin (LMW) and aspirin versus aspirin alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.50] |
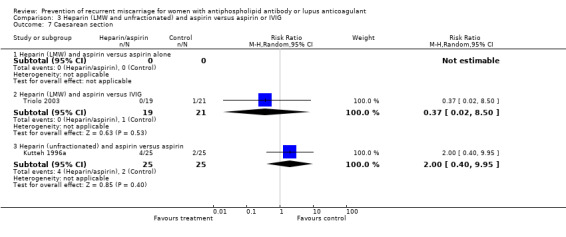
| 7.3 Heparin (unfractionated) and aspirin versus aspirin | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.40, 9.95] |
| 8 Weighted mean difference for birthweight | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Heparin (LMW) and aspirin versus aspirin alone | 1 | 98 | Mean Difference (IV, Random, 95% CI) | ‐92.00 [‐379.00, 193.00] |
| 8.2 Heparin (LMW) and aspirin versus IVIG | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 52.0 [‐89.26, 193.26] |
| 8.3 Heparin (unfractionated) and aspirin versus aspirin | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐142.0 [‐515.33, 231.33] |
3.1. Analysis.
Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 1 Pregnancy loss.
3.2. Analysis.

Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 2 Premature delivery.
3.3. Analysis.
Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
3.4. Analysis.

Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 4 IUGR with interventions.
3.5. Analysis.

Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 5 Adverse pregnancy outcome (pregnancy loss or IUGR).
3.6. Analysis.

Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 6 Neonatal intensive care admission.
3.7. Analysis.
Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 7 Caesarean section.
3.8. Analysis.

Comparison 3 Heparin (LMW and unfractionated) and aspirin versus aspirin or IVIG, Outcome 8 Weighted mean difference for birthweight.
Comparison 4. High‐dose heparin and aspirin versus low‐dose heparin and aspirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.29, 2.38] |
| 2 Premature delivery | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.33, 26.92] |
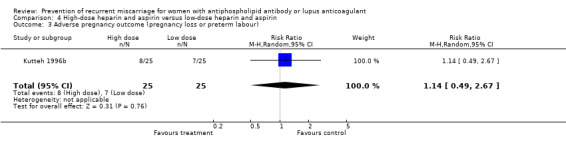
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.49, 2.67] |
| 4 IUGR with interventions | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 128.87] |
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.54, 3.29] |
| 6 Neonatal intensive care admission | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.33, 5.36] |
| 8 Weighted mean difference for birthweight | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐270.0 [‐601.08, 61.08] |
4.1. Analysis.

Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 1 Pregnancy loss.
4.2. Analysis.

Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 2 Premature delivery.
4.3. Analysis.
Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
4.4. Analysis.
Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 4 IUGR with interventions.
4.5. Analysis.
Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 5 Adverse pregnancy outcome (pregnancy loss or IUGR).
4.7. Analysis.

Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 7 Caesarean section.
4.8. Analysis.
Comparison 4 High‐dose heparin and aspirin versus low‐dose heparin and aspirin, Outcome 8 Weighted mean difference for birthweight.
Comparison 5. Prednisone and aspirin versus aspirin or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 2 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 2 Premature delivery | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 5.54 [2.96, 10.35] |
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.75, 7.54] |
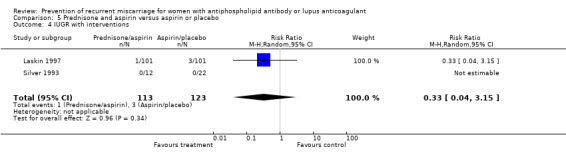
| 4 IUGR with interventions | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.15] |
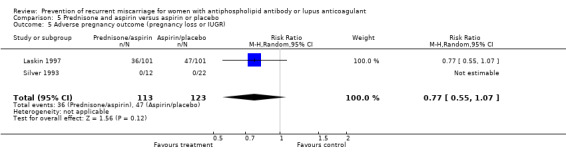
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.07] |
| 6 Neonatal intensive care admission | 1 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [2.14, 37.78] |
| 7 Caesarean section | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.40, 2.79] |
| 8 Weighted mean difference for birthweight | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐552.0 [‐1064.78, ‐39.22] |
5.1. Analysis.
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 1 Pregnancy loss.
5.2. Analysis.
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 2 Premature delivery.
5.3. Analysis.
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
5.4. Analysis.
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 4 IUGR with interventions.
5.5. Analysis.
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 5 Adverse pregnancy outcome (pregnancy loss or IUGR).
5.6. Analysis.

Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 6 Neonatal intensive care admission.
5.7. Analysis.

Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 7 Caesarean section.
5.8. Analysis.
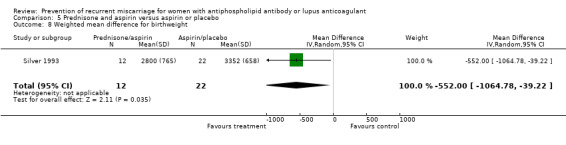
Comparison 5 Prednisone and aspirin versus aspirin or placebo, Outcome 8 Weighted mean difference for birthweight.
Comparison 6. Prednisone and aspirin versus heparin and aspirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.47, 2.93] |
| 2 Premature delivery | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [1.26, 9.27] |
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.22, 3.25] |
| 4 IUGR with interventions | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Neonatal intensive care admission | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Weighted mean difference for birthweight | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 Prednisone and aspirin versus heparin and aspirin, Outcome 1 Pregnancy loss.
6.2. Analysis.
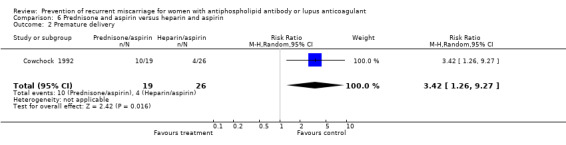
Comparison 6 Prednisone and aspirin versus heparin and aspirin, Outcome 2 Premature delivery.
6.3. Analysis.
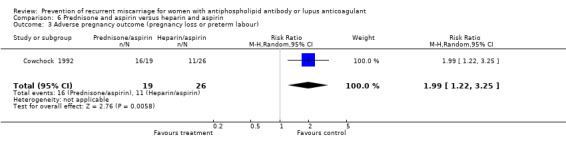
Comparison 6 Prednisone and aspirin versus heparin and aspirin, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
Comparison 7. IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy loss | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.86, 8.57] |
| 1.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.12] |
| 2 Premature delivery | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.19, 11.17] |
| 2.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.08, 3.68] |
| 3 Adverse pregnancy outcome (pregnancy loss or preterm labour) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [1.24, 4.58] |
| 3.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.42, 1.72] |
| 4 IUGR with interventions | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.28] |
| 4.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse pregnancy outcome (pregnancy loss or IUGR) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.28] |
| 5.2 IVIG versus prednisone and aspirin | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.12] |
| 6 Neonatal intensive care admission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.09, 4.69] |
| 6.2 IVIG versus prednisone and aspirin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Caesarean section | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.12, 63.19] |
| 7.2 IVIG versus prednisone and aspirin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Weighted mean difference for birthweight | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐56.34 [‐195.01, 82.33] |
| 8.2 IVIG versus prednisone and aspirin | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 351.0 [114.07, 587.93] |
7.1. Analysis.

Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 1 Pregnancy loss.
7.2. Analysis.

Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 2 Premature delivery.
7.3. Analysis.
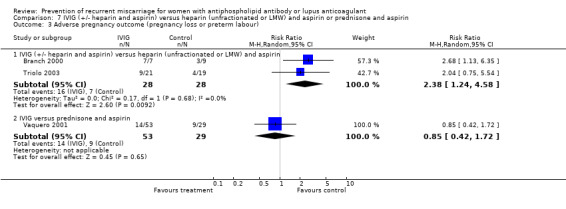
Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 3 Adverse pregnancy outcome (pregnancy loss or preterm labour).
7.4. Analysis.
Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 4 IUGR with interventions.
7.5. Analysis.
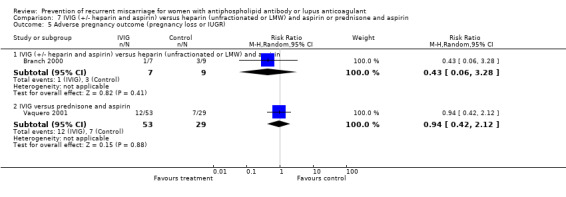
Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 5 Adverse pregnancy outcome (pregnancy loss or IUGR).
7.6. Analysis.
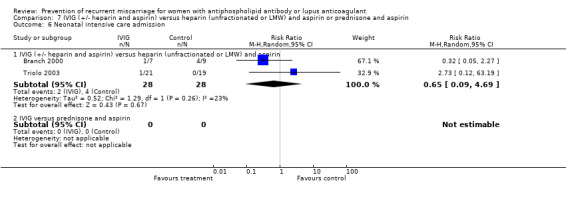
Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 6 Neonatal intensive care admission.
7.7. Analysis.

Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 7 Caesarean section.
7.8. Analysis.

Comparison 7 IVIG (+/‐ heparin and aspirin) versus heparin (unfractionated or LMW) and aspirin or prednisone and aspirin, Outcome 8 Weighted mean difference for birthweight.
Comparison 8. Prednisone and aspirin ‐ diabetes as an outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes | 4 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [1.53, 6.98] |
8.1. Analysis.
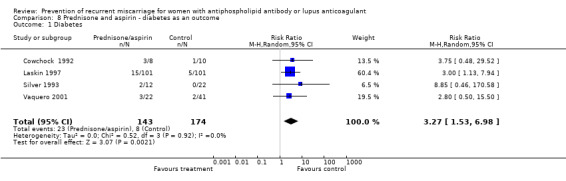
Comparison 8 Prednisone and aspirin ‐ diabetes as an outcome, Outcome 1 Diabetes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Branch 2000.
| Methods | Multicentre, double‐blind, placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) A single live fetus of </= 12 weeks' gestation. 2) Either IgG ACL >/= 20 GPL units and a history of fetal death and/or venous/arterial thromboembolism or IgG ACL >/=40 GPL units or LA, but no history of fetal death or thromboembolism. Exclusion criteria: 1) Thrombocytopenia. 2) Bleeding disorder. 3) Osteoporosis. 4) Allergy to IVIG or heparin or aspirin. 5) Active renal disease, SLE, insulin dependant diabetes mellitus or hypertension. | |
| Interventions | Intravenous immunoglobulin (10%) 1 g/kg versus placebo (albumin 5%), on 2 days every 4 weeks. All participants also received aspirin 81 mg/day and heparin 7500 units twice daily sc. | |
| Outcomes | Multiple obstetric and neonatal outcomes. | |
| Notes | 1/16 subjects had no prior fetal loss. Randomisation and treatment commenced once a single live conceptus </= 12 weeks identified. Gestational age in IVIG and placebo groups respectively for: randomisation 9, 9.7 weeks; start of aspirin 5.5, 4.2 weeks; start of heparin 5.1, 5.5 weeks; start of IVIG/placebo 11, 11.3 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cowchock 1992.
| Methods | Multicentre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) >/= 2 unexplained fetal losses. 2) Exclusion of other causes of recurrent miscarriage or fetal death. 3) >/= 2 +ve APL tests over at least a 6 week period determined by IgG ACL > 30 GPL units, IgM ACL > 11 MPL units, or presence of LA (APTT or dRVVT at least 2 SDs greater than the mean and lack of correction with 1:1 fresh frozen plasma). Exclusion criteria: 1) A contraindication or indication for use of one of the therapeutic agents. (This was enforced in a number of subjects postrandomisation). | |
| Interventions | Heparin 10,000 units twice daily sc plus aspirin 80 mg/day versus prednisone 20 mg twice daily plus aspirin 80 mg/day. Heparin dose decreased by 2000 units to maintain mid interval APTT within normal range or at the prolonged baseline value. |
|
| Outcomes | Medical and obstetric complications, eg fetal distress, preterm delivery (< 37 weeks), low birthweight (< 10th percentile gestational age), and maternal morbidity. | |
| Notes | This study was an interim analysis. The study was designed to recruit 50 subjects.
56% of subjects were excluded due to being ineligible or refusing to take study medication but data provided to allow intention‐to‐treat analysis. Randomisation, aspirin/prednisone commenced at confirmation of pregnancy; heparin commenced when viable pregnancy shown by ultrasound (6.5 to 8 weeks). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cowchock 1997.
| Methods | Multicentre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) Low‐risk pregnancy with 0‐2 unexplained fetal losses, only one of which could have occurred after 12 weeks of pregnancy. 2) No history of antiphospholipid antibody related complications eg thrombosis, thrombocytopenia or early onset pre‐eclampsia. 3) Persistently positive IgG or IgM ACL antibody or LA. | |
| Interventions | Aspirin 81 mg/day versus usual care. | |
| Outcomes | Fetal death or distress at term and birthweight < 5th percentile. | |
| Notes | Brief report of low‐risk pregnancies identified at the time of a larger trial of high‐risk pregnancies. About 50% of subjects had not experienced fetal loss. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Farquharson 2002.
| Methods | Single centre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) 18‐41 years. 2) > 2 consecutive pregnancy losses or 2 consecutive losses with proven fetal death after 10 weeks. 3) 2 +ve APL antibodies > 6 weeks apart determined by LA (dRVVT > 1.09 with > 20% correction with platelets) or IgG ACL > 9 GPL units or IgM ACL > 5 MPL units. | |
| Interventions | Asprin 75 mg/day versus aspirin 75 mg/day and LMW heparin 5000 units sc/day. | |
| Outcomes | Embryo loss (no visible crown rump length or fetal heart activity) and fetal loss (loss of fetal heart activity). | |
| Notes | 11/47 in the aspirin group also took LMW heparin and 13/51 in the aspirin/heparin group took aspirin alone. Randomisation occurred < 12 weeks' gestation, mean 6.3 weeks for aspirin group and 7.1 weeks for aspirin and heparin group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kutteh 1996a.
| Methods | Single centre, quasi‐randomised (alternatively assigned to treatment) non‐blinded, non‐placebo controlled. | |
| Participants | Inclusion criteria: 1) Desire to become pregnant. 2) Agreement to be completely evaluated. 3) >/= 3 consecutive pregnancy losses. 4) Consent to alternative treatment assignment. 5) +ve APL antibody on at least 2 occasions determined by IgG ACL or antiphosphotidylserine > 27 GPL units or IgM ACL or antiphosphotidylserine > 23 MPL units. Exclusion criteria: 1) SLE. 2) Positive LA. 3) Presence of another abnormal test result. 4) Aspirin allergy. 5) Other reason for anticoagulation. 6) Refused treatment or allocation. | |
| Interventions | Heparin 5000 units twice daily sc plus aspirin 81 mg/day versus aspirin 81 mg/day. Heparin dose increased by 1000 units/dose weekly until PTT 1.2 ‐ 1.5 times baseline. |
|
| Outcomes | Multiple obstetric and neonatal outcomes. | |
| Notes | Aspirin commenced before conception. Heparin commenced at the first confirmed pregnancy test (5.3 weeks postgestation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kutteh 1996b.
| Methods | Single centre, quasi‐randomised (sequentially assigned to treatment) non‐blinded, non‐placebo controlled. | |
| Participants | Inclusion criteria: 1) Desire to become pregnant. 2) Agreement to be completely evaluated. 3) >/= 3 consecutive pregnancy losses. 4) Consent to treatment protocol. 5) +ve APL antibody on at least 2 occasions determined by IgG > 27 GPL units (> 2.5 multiples of the median). Exclusion criteria: 1) SLE. 2) Positive LA. 3) Presence of another abnormal test result. 4) Aspirin allergy. 5) Documented bone disorder. 6) Refused treatment. | |
| Interventions | Heparin 5000 units twice daily sc adjusted to maintain the PTT at 1.2 to 1.5 times the baseline (high‐dose) plus aspirin 81 mg/day versus heparin 5000 units twice daily adjusted to maintain the PTT at the upper limit of normal (low‐dose) plus aspirin 81 mg/day. Mean daily dose: high dose 13,300 units BD; low dose 8127 units BD. |
|
| Outcomes | Multiple obstetric and neonatal outcomes. | |
| Notes | Aspirin commenced before conception. Heparin commenced at the first confirmed pregnancy test (5.3 and 5.2 weeks postgestation for high dose and low dose respectively). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Laskin 1997.
| Methods | Single centre, fully blinded, placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) Age 18‐39 years. 2) >/= 2 consecutive fetal losses < 32 weeks' gestation. 3) +ve antibody on at least 2 occasions including at least one of the following: antinuclear, antiDNA (single or double stranded), antilymphocyte IgM, or IgG ACL (> 15 GPL units) antibodies, or LA (APTT, dRVVT, KCT or tissue thromboplastin‐inhibition time). Exclusion criteria: 1) Chromosomal abnormality. 2) Anatomical abnormality. 3) Luteal phase defect (determined by a timed endometrial biopsy). 4) Previously untreated tuberculosis. 5) Previous prednisone therapy. 6) Confirmed peptic ulcer disease within the past three years. 7) SLE fulfilling 4 or more of the American College of Rheumatologists criteria. 8) Diabetes, aspirin sensitivity, or diastolic BP > 90 on at least 2 occasions. | |
| Interventions | Prednisone 0.8 mg/kg (maximum 60 mg) for the first four weeks and then 0.5 mg/kg (maximum 40 mg) plus aspirin 100 mg/day versus placebo. | |
| Outcomes | Live infant, maternal side‐effects, infant birthweight, Apgar score and admission to neonatal ICU. | |
| Notes | 44% of all subjects in the study had APL antibodies. Randomisation and drug treatment commenced after a confirmed pregnancy test (confirmation via a rise in BHCG or ultrasound demonstration of fetal heart beat and appropriate fetal size). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pattison 2000.
| Methods | Single centre, fully blinded, placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) >/= 3 miscarriages. 2) +ve APL antibody either pre‐pregnancy or early in pregnancy determined by a IgG ACL > 5 GPL units or IgM ACL > 5 MPL units or presence of LA (APTT, dRVVT or KCT). Exclusion criteria: 1) History of thrombosis. 2) SLE. 3) Current or planned corticosteroids, NSAIDs, heparin or marine lipids. | |
| Interventions | Aspirin 75 mg/day versus placebo. | |
| Outcomes | Live birth, antenatal outcomes and neonatal outcomes. | |
| Notes | 20% subjects excluded from each treatment arm on the basis of ineligibility. Randomisation occured when pregnancy diagnosed if APL antibodies +ve before pregnancy or when detected during pregnancy. Aspirin/placebo commenced 50/44 days respectively after last menstrual period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rai 1997.
| Methods | Single centre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) >/= 3 consecutive miscarriages. 2) +ve APL antibody on at least 2 occasions > 8 weeks apart determined by ACL IgG > 5 GPL units or ACL IGM > 3 MPL units or a positive LA (APTT, dRVVT ratio>/= 1.1 confirmed by platelet neutralisation ‐ decrease of >/= 10% of ratio). Exclusion criteria: 1) Previous thromboembolism. 2) SLE. 3) Uterine abnormality on ultrasound. 4) Hypersecretion of luteinising hormone. 5) Multiple pregnancy. 6) Abnormal karyotype of either partner. | |
| Interventions | Calcium heparin 5000 units twice daily sc plus aspirin 75 mg/day versus aspirin 75 mg/day alone. | |
| Outcomes | Live birth, gestational age and weight, congenital abnormality, admission to neonatal ICU, bone mineral densitometry and maternal morbidity. | |
| Notes | Aspirin commenced in all when +ve pregnancy test. Randomisation occurred when fetal heart activity noted on ultrasound (6.6 weeks in aspirin group and 6.7 weeks in aspirin/heparin group). Heparin commenced in heparin only group after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Silver 1993.
| Methods | Single centre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) >/= 1 unexpected fetal death > 12 weeks' gestation OR >/= 2 unexplained first trimester losses. 2) Anatomical, genetic, and hormonal abnormalities were excluded. 3) +ve APL antibody before and during the index pregnancy determined by IgG ACL > 8 GPL units or IgM ACL > 5 MPL units or LA (dRVVT and mixing study with normal plasma). Exclusion criteria: 1) Therapy with heparin, immunosuppressives or cytotoxic therapy. 2) Multiple pregnancies. 3) Uterine malformation. 4) Cervical incompetence. | |
| Interventions | Prednisone 20 mg/day plus aspirin 81 mg/day versus aspirin 81 mg/day. Prednisone dose modified according to ACL level stability or decrease, by 10 mg increments or decrements respectively, within the range of 10‐40 mg. 10/12 on prednisone were on 10 mg by 2nd or 3rd trimester; 1/12 each on 20 and 40 mg. |
|
| Outcomes | Live birth, preterm (< 37 weeks) birth, low birthweight (< 10th percentile), birthweight, gestational age at delivery and maternal morbidity. | |
| Notes | 29% of subjects were excluded from the combined treatment arm due to withdrawal of consent or ineligibility. Mean gestational age at commencement of: aspirin, 6.7 and 8.4 weeks in the aspirin only versus aspirin/prednisone groups; prednisone, 11.8 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Triolo 2003.
| Methods | Single centre, non‐blinded, non‐placebo controlled RCT. | |
| Participants | Inclusion criteria: 1) 18‐39 years. 2) >/= 3 consecutive fetal losses < 10 weeks' gestation. 3) >/= 2 +ve results for ACL (intervals >/= 3 months) determined by IgG ACL > 40 GPL units. Exclusion criteria: 1) Chromosomal or anatomical abnormality or luteal phase defect. 2) Confirmed peptic ulcer. 3) SLE. 4) Diabetes mellitus or abnormal glucose tolerance test. 5) Previous thromboembolism. 6) Aspirin sensitivity. 7) Hypertension or current treatment with antihypertensives. 9) Previous prednisone. 10) Abnormal chest X‐ray. 11) Positive tuberculin test. | |
| Interventions | IVIG 400 mg/kg/day for 2 consecutive days then single monthly dose versus LMW heparin (Seleparina) 5700 IU/day and aspirin 75 mg/day. | |
| Outcomes | Pregnancy loss, maternal side‐effects, preterm delivery (< 37 weeks), neonatal ICU admission, low birthweight and neonatal bleeding or bruising. | |
| Notes | 9.5% of the LMW heparin group withdrew because of poor compliance and were excluded from the analysis. All treatment commenced as soon as a +ve pregnancy test. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tulppala 1997.
| Methods | Single centre, placebo controlled RCT. Blinding unclear. | |
| Participants | Inclusion criteria: 1) Recurrent miscarriage. 2) Thorough investigation of subject and partner and no obvious cause for miscarriage found. 3) Pregnancy. | |
| Interventions | Aspirin 50 mg/day versus placebo. | |
| Outcomes | Multiple obstetric and neonatal outcomes. | |
| Notes | Only 18% of the subjects were IgG ACL antibody +ve. Treatment commenced when home urinary pregnancy test +ve; mean time from missed period 6 and 6.9 days in the aspirin and placebo groups respectively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vaquero 2001.
| Methods | Two centre, quasi‐randomised (each centre providing one treatment), non‐blinded, non‐placebo controlled. | |
| Participants | Inclusion criteria: 1) </= 2 unexplained 1st trimester miscarriages. 2) >/= 2 +ve tests for APL antibody > 6 weeks apart before and during pregnancy. APL antibodies = LA (APTT, dRVVT, dAPTT, KCT > 2 SD above the mean and lack of correction with fresh frozen plasma) or ACL (IgG > 11 GPL units or IgM > 20 MPL units). 3) Other causes of recurrent spontaneous abortion excluded. | |
| Interventions | IVIG 0.5 g/kg 2 days per month versus aspirin 100 mg/day and prednisone 15‐20 mg/day decreasing to 10‐15 mg/day after week 28. | |
| Outcomes | Live birth rate, obstetric complications and evidence of viral transmission. | |
| Notes | IVIg commenced in the 5th week of pregnancy. Prednisone/aspirin commenced from the diagnosis of pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
ACL: anticardiolipin APL: antiphospholipid APTT: Activated partial thromboplastin time BD: twice daily BHCG: Beta human chorionic gonadotrophin BP: blood pressure dRVVT: dilute Russell's viper venom test GPL: G phospholipid units ICU: intensive care unit IgG: immunoglobulin G IgM: immunoglobulin M IU: internationall unit IVIG: intravenous immunoglobulin KCT: Kaolin clotting time LA: Lupus anticoagulant MPL: M phospholipid units NSAIDS: Non‐steroidal anti‐inflammatory drugs PTT: Partial thromboplastin time RCT: randomised controlled trial sc: subcutaneous SDs: standard deviations SLE: systemic lupus erythematosus
LA and ACL measurement methods/criteria for positivity included where documented in the study. Timing of randomisation included where documented in the study. Mean gestational ages at randomisation and commencement of therapy included where documented in the study.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Momen 1993 | Non‐randomised. |
| Backos 1999 | Observational study with no control group. |
| Balasch 1993 | Non‐randomised. |
| Blumenfeld 1991 | Comparison between antiphospholipid antibody positive and negative groups. All positives received treatment. |
| Boda 1999 | Non‐randomised and study participants did not fulfil criteria ie not all antiphospholipid positive and recurrent miscarriage. |
| Branch 1992 | Non‐randomised. |
| Caruso 1997 | Case‐series with no control group. |
| Christiansen 1995 | Antiphospholipid antibody negative. |
| Corosu 1998 | Non‐randomised. |
| Costa 1999 | Non‐randomised. |
| Cowchock 1988 | Non‐randomised. |
| Cowchock 1996 | Review paper. |
| De Veciana 2001 | Abstract containing insufficient information to determine whether satisfies criteria, and to contact author for additional details. |
| Diejomaoh 2002 | Non‐randomised. |
| Erkan 2001 | Non‐randomised. |
| Franklin 2002 | Non‐randomised. |
| Geva 1998 | IVF embryo transfer failure endpoint rather than live birth. |
| Gordon 1998 | Review paper. |
| Granger 1997 | Non‐randomised. |
| Gris 1995 | Antiphospholipid antibody positive excluded. |
| Gris 2002 | Non‐randomised. |
| Hasegawa 1992 | Non‐randomised. |
| Kaaja 1993 | Recurrent fetal loss excluded. |
| Kutteh 1997 | Assessment of treatment on IVF implantation rates; not all antiphospholipid antibody positive. |
| Kwak 1992 | Non‐randomised. |
| Lima 1996 | Non‐randomised. |
| Lockshin 1989 | Non‐randomised. |
| Mankuta 1999 | Outcomes differed. |
| Many A 1992 | Retrospective case series. |
| Martin 1997 | Review paper. |
| Mazzucconi 1996 | Case series. |
| McParland 1993 | Review paper. |
| Mueller‐Eckhardt 199 | Outcome data given in separate paper but only 1/3 of patients had antiphospholipid antibodies and it was not possible to determine the primary outcome for this group only. |
| Ogasawara 1998 | Non‐randomised. |
| Passaleva 1993 | Insufficient information to determine whether randomised and additional information unavailable. |
| Perino 1997 | Antiphospholipid antibody positive excluded. |
| Quenby 1992 | Abstract only, quasi‐randomised and missing information re history of fetal loss not available. |
| Rai 1997 b | Case report. |
| Rai 2000 | Non‐randomised. |
| Reece 1997 | Case series. |
| Reznikoff‐Etievant | Non‐randomised. |
| Ruffatti 1997 | Observational study with no control group. |
| Sammaritano 2001 | Review paper. |
| Scopelitis 1994 | Letter on screening for antiphospholipid antibodies in recurrent miscarriage. |
| Semprini 1989 | Observational study with no control group. |
| Shefras 1995 | Non‐randomised. |
| Sher 1994 | Unclear whether randomised. Participants have infertility rather than recurrent miscarriage. |
| Sher 1998 | Non‐randomised. Participants have infertility rather than recurrent miscarriage. |
| Spinnato 1995 | Case series. |
| Stern 2003 | Conference abstract with insufficient information. |
| Takakuwa 1997 | Observational study with no control group. |
| Vahid 1999 | Conference abstract with insufficient information. |
| Yamamoto 1994 | Non‐randomised. |
IVF: in vitro fertilisation
Contributions of authors
Conceiving the review: Marianne Empson, James Scott Designing the review: Marianne Empson, Marissa Lassere, Jonathan Craig Co‐ordinating the review: Marianne Empson Data extraction: Marianne Empson, Marissa Lassere Statistical analysis: Marianne Empson Writing review: Marianne Empson Editing review: Marianne Empson, Marissa Lassere, Jonathan Craig, James Scott
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Branch 2000 {published and unpublished data}
- Branch DW, Peaceman AM, Druzin M, Silver RK, El‐Sayed Y, Silver RM, et al. A multicentre, placebo‐controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The pregnancy loss study group. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 1):122‐7. [DOI] [PubMed] [Google Scholar]
Cowchock 1992 {published data only}
- Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low‐dose heparin treatment. American Journal of Obstetrics and Gynecology 1992;166(5):1318‐23. [DOI] [PubMed] [Google Scholar]
Cowchock 1997 {published data only}
- Cowchock S, Reece EA. Do low‐risk pregnant women with antiphospholipid antibodies need to be treated? Organizing group of the antiphospholipid antibody treatment trial. American Journal of Obstetrics and Gynecology 1997;176(5):1099‐100. [DOI] [PubMed] [Google Scholar]
Farquharson 2002 {published data only}
- Farquharson RG. Antiphospholipid syndrome in pregnancy: a controlled treatment trial. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S22. [Google Scholar]
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics & Gynecology 2002;100(3):408‐13. [DOI] [PubMed] [Google Scholar]
- Dadelszen P, Kent N. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics & Gynecology 2003;101(3):618. [PubMed] [Google Scholar]
Kutteh 1996a {published data only}
- Kutteh WH. Antiphospholipid antibody‐associated recurrent pregnancy loss: treatment with heparin and low‐dose aspirin is superior to low‐dose aspirin alone. American Journal of Obstetrics and Gynecology 1996;174(5):1584‐9. [DOI] [PubMed] [Google Scholar]
Kutteh 1996b {published data only}
- Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody‐associated recurrent pregnancy loss with lower dose heparin and aspirin. American Journal of Reproductive Immunology 1996;35(4):402‐7. [DOI] [PubMed] [Google Scholar]
Laskin 1997 {published data only}
- Laskin CA, Bombardier C, Hannah ME, Mandel FP, Ritchie JW, Farewell V, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. New England Journal of Medicine 1997;337(3):148‐53. [DOI] [PubMed] [Google Scholar]
Pattison 2000 {published data only}
- Merrill JT. Appropriate management of antiphospholipid‐related pregnancy in women without lupus who have low titer autoantibodies. Current Rheumatology Reports 2001;3:269‐70. [DOI] [PubMed] [Google Scholar]
- Pattison NS, Chamley LW, Birdsall M, Zanderigo AM, Liddell HS, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2000;183(4):1008‐12. [DOI] [PubMed] [Google Scholar]
Rai 1997 {published data only}
- Cohen H. Randomised trial of aspirin versus aspirin and heparin in pregnant women with the antiphospholipid syndrome. Annales de Medecine Interne 1996;147:44. [PubMed] [Google Scholar]
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997;314(7076):253‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Regan L. Antiphospholipid antibodies and recurrent miscarriage. Acta Obstetricia et Gynecologica Scandinavica 1997;76 Suppl(167:4):6. [Google Scholar]
- Rai RS, Cohen H, Regan L. Prospective randomized trial of aspirin versus aspirin + heparin in pregnant women with a history of recurrent miscarriage in association with antiphospholipid antibodies. Human Reproduction 1996;11:25. [Google Scholar]
Silver 1993 {published data only}
- Silver RK, MacGregor SN, Sholl JS, Hobart JM, Neerhof MG, Ragin A. Comparative trial of prednisone plus aspirin versus aspirin alone in the treatment of anticardiolipin antibody‐positive obstetric patients. American Journal of Obstetrics and Gynecology 1993;169(6):1411‐7. [DOI] [PubMed] [Google Scholar]
- Silver RK, Sholl JS, MacGregor SN, Hobart JH, Neerhof MG, Hickman AH. Prospective evaluation of single (low‐dose aspirin) vs combined (aspirin plus prednisone) therapy in the treatment of the antiphospholipid syndrome. Proceedings of 39th Annual Meeting of the Society for Gynecologic Investigation; 1992; San Antonio, Texas, USA. 1992:125.
Triolo 2003 {published data only}
- Triolo G, Ferrante A, Ciccia F, Accardo‐Palumbo A, Perino A, Castelli A, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis & Rheumatism 2003;48(3):728‐31. [DOI] [PubMed] [Google Scholar]
Tulppala 1997 {published data only}
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Ailus K, Palosuo T, Ylikorkala O. Low dose aspirin in the prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(1):191. [DOI] [PubMed] [Google Scholar]
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Foudila T, Ailus K, Palosuo T, et al. Low‐dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(7):1567‐72. [DOI] [PubMed] [Google Scholar]
Vaquero 2001 {published data only}
- Vaquero E, Lazzarin N, Valensise H, Menghini S, Pierro G, Cesa F, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low‐dose aspirin. American Journal of Reproductive Immunology (Copenhagen) 2001;45(3):174‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Momen 1993 {published data only}
- Al‐Momen AKM, Moghraby SA, El‐Rab MOG, Gader AMA, al‐Balla SR, Al, Meshari AA, al‐Nuaim L. Pregnancy outcome in women with antiphospholipid antibodies. Clinical Rheumatology 1993;12(3):381‐6. [DOI] [PubMed] [Google Scholar]
Backos 1999 {published data only}
- Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. British Journal of Obstetrics and Gynaecology 1999;106(2):102‐7. [DOI] [PubMed] [Google Scholar]
Balasch 1993 {published data only}
- Balasch J, Carmona F, Lopez‐Soto A, Font J, Creus M, Fabregues F, et al. Low‐dose aspirin for prevention of pregnancy losses in women with primary antiphospholipid syndrome. Human Reproduction 1993;8(12):2234‐9. [DOI] [PubMed] [Google Scholar]
Blumenfeld 1991 {published data only}
- Blumenfeld Z, Weiner Z, Lorber M, Sujov P, Thaler I. Anticardiolipin antibodies in patients with recurrent pregnancy wastage: treatment and uterine blood flow. Obstetrics & Gynecology 1991;78(4):584‐9. [PubMed] [Google Scholar]
Boda 1999 {published data only}
- Boda Z, Laszlo P, Pfliegler G, Tornai I, Rejto L, Schlammadinger A. Low molecular weight heparin as thromboprophylaxis throughout pregnancy in heritable thrombophilic women. Clinical and applied thrombosis/hemostasis 1999;5(3):198‐9. [PubMed] [Google Scholar]
Branch 1992 {published data only}
- Branch DW, Silver RM, Blackwell JL, Reading JC, Scott JR. Outcome of treated pregnancies in women with antiphospholipid syndrome: an update of the Utah experience. Obstetrics & Gynecology 1992;80(4):614‐20. [PubMed] [Google Scholar]
Caruso 1997 {published data only}
- Caruso A, Carolis S, Ferrazzani S, Santis L, Carducci B, Trivellini C, et al. Antiphospholipid antibodies in pregnant patients. International Journal of Immunopathology & Pharmacology 1997;10(2 Suppl):137‐8. [Google Scholar]
Christiansen 1995 {published data only}
- Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, et al. Placebo‐controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with i.v. immunoglobulin. Human Reproduction 1995;10(10):2690‐5. [DOI] [PubMed] [Google Scholar]
Corosu 1998 {published data only}
- Corosu R, Roma B, Cocola M, Marziali M. Antiphospholipid syndrome in obstetrics. Minerva Ginecologica 1998;50(1‐2):9‐13. [PubMed] [Google Scholar]
Costa 1999 {published data only}
- Costa M, Rossi E. Antiphospholipid antibodies and pregnancy. Annals of the New York Academy of Sciences 1999;876:383‐6. [DOI] [PubMed] [Google Scholar]
Cowchock 1988 {published data only}
- Cowchock FS, Wapner RJ, Needleman L, Filer R. A comparison of pregnancy outcome after two treatments for antibodies to cardiolipin. Clinical and Experimental Rheumatology 1988;6(2):200. [Google Scholar]
Cowchock 1996 {published data only}
- Cowchock S. Prevention of fetal death in the antiphospholipid antibody syndrome. Lupus 1996;5(5):467‐72. [DOI] [PubMed] [Google Scholar]
De Veciana 2001 {published data only}
- Veciana M TP, Dattel B, Slotnick R, Abuhamad A. Dalteparin versus unfractionated heparin for prophylactic anticoagulation during pregnancy (abstract). American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S182. [Google Scholar]
Diejomaoh 2002 {published data only}
- Diejomaoh MF, Al‐Azemi MM, Bandar A, Egbase PE, Jirous J, Al‐Othman S, et al. A favorable outcome of pregnancies in women with primary and secondary recurrent pregnancy loss associated with antiphospholipid syndrome. Archives of Gynecology and Obstetrics 2002;266(2):61‐6. [DOI] [PubMed] [Google Scholar]
Erkan 2001 {published data only}
- Erkan D, Merrill JT, Yazici Y, Sammaritano L, Buyon JP, Lockshin MD. High thrombosis rate after fetal loss in antiphospholipid syndrome: effective prophylaxis with aspirin.[comment]. Arthritis & Rheumatism 2001;44(6):1466‐7. [DOI] [PubMed] [Google Scholar]
Franklin 2002 {published data only}
- Franklin RD, Kutteh WH. Antiphospholipid antibodies (APA) and recurrent pregnancy loss: treating a unique APA positive population. Human Reproduction 2002;17(11):2981‐5. [DOI] [PubMed] [Google Scholar]
Geva 1998 {published data only}
- Geva E, Amit A, Lerner‐Geva L, Lessing J.B. Prevention of early pregnancy loss in autoantibody seropositive women (letter). Lancet 1998;351(9095):34‐5. [DOI] [PubMed] [Google Scholar]
Gordon 1998 {published data only}
- Gordon C, Kilby MD. Use of intravenous immunoglobulin therapy in pregnancy in systemic lupus erythematosus and antiphospholipid antibody syndrome. Lupus 1998;7:429‐33. [DOI] [PubMed] [Google Scholar]
Granger 1997 {published data only}
- Granger KA, Farquharson RG. Obstetric outcome in antiphospholipid syndrome. Lupus 1997;6(6):509‐13. [DOI] [PubMed] [Google Scholar]
Gris 1995 {published data only}
- Gris JC, Neveu S, Tailland ML, Courtieu C, Mares P, Schved JF. Use of a low‐molecular weight heparin (enoxaparin) or of a phenformin‐like substance (moroxydine chloride) in primary early recurrent aborters with an impaired fibrinolytic capacity. Thrombosis and Haemostasis 1995;73(3):362‐7. [PubMed] [Google Scholar]
Gris 2002 {published data only}
- Gris JC, Balducchi JP, Quere I, Hoffet M, Mares P. Enoxaparin sodium improves pregnancy outcome in aspirin‐resistant antiphospholipid/antiprotein antibody syndromes. Thrombosis and Haemostasis 2002;87(3):536‐7. [PubMed] [Google Scholar]
Hasegawa 1992 {published data only}
- Hasegawa I, Takakuwa K, Goto S, Yamada K, Sekizuka N, Kanazawa K, et al. Effectiveness of prednisolone/aspirin therapy for recurrent aborters with antiphospholipid antibody. Human Reproduction 1992;7(2):203‐7. [DOI] [PubMed] [Google Scholar]
Kaaja 1993 {published data only}
- Kaaja R, Julkunen H, Viinikka L, Ylikorkala O. Production of prostacyclin and thromboxane in lupus pregnancies: effect of small dose of aspirin. Obstetrics & Gynecology 1993;81:327‐31. [PubMed] [Google Scholar]
Kutteh 1997 {published data only}
- Kutteh WH, Yetman DL, Chantilis SJ, Crain J. Effect of antiphospholipid antibodies in women undergoing in‐vitro fertilization: Role of heparin and aspirin. Human Reproduction 1997;12(6):1171‐5. [DOI] [PubMed] [Google Scholar]
Kwak 1992 {published data only}
- Kwak JYH, Gilman‐Sachs A, Beaman KD, Beer AE. Reproductive outcome in women with recurrent spontaneous abortions of alloimmune and autoimmune causes: preconception versus postconception treatment. American Journal of Obstetrics and Gynecology 1992;166(6 I):1787‐98. [DOI] [PubMed] [Google Scholar]
Lima 1996 {published data only}
- Lima F, Khamashta MA, Buchanan NMM, Kerslake S, Hunt BJ, Hughes GRV. A study of sixty pregnancies in patients with the antiphospholipid syndrome. Clinical and Experimental Rheumatology 1996;14(2):131‐6. [PubMed] [Google Scholar]
Lockshin 1989 {published data only}
- Lockshin MD, Druzin ML, Qamar T. Prednisone does not prevent recurrent fetal death in women with antiphospholipid antibody. American Journal of Obstetrics and Gynecology 1989;160:439‐43. [DOI] [PubMed] [Google Scholar]
Mankuta 1999 {published data only}
- Mankuta D, Spitzer KA, Seaward G, Farine D, Ryan G, Clark‐Soloninka C, et al. Prednisone does not affect the biophysical score in pregnant women. American Journal of Obstetrics and Gynecology 1999;180:S163. [Google Scholar]
Many A 1992 {published data only}
- Many A, Pauzner R, Carp H, Langevitz P, Martinowitz U. Treatment of patients with antiphospholipid antibodies during pregnancy. American Journal of Reproductive Immunology 1992;28(3‐4):216‐8. [DOI] [PubMed] [Google Scholar]
Martin 1997 {published data only}
- Martin ME, Fernandez CJ. Prophylaxis of the spontaneous interruption of pregnancy (fetal miscarriage) in the antiphospholipid syndrome. Revista Espanola de Reumatologia 1997;24(3):95‐100. [Google Scholar]
Mazzucconi 1996 {published data only}
- Mazzucconi MG, Dragoni F, Chistolini A, Peraino M, Paesano R, Di P, et al. Efficacy of low dose prednisone plus aspirin in preventing spontaneous abortions and/or fetal deaths due to antiphospholipid antibodies: results of a pilot study. Autoimmunity 1996;24(2):123‐5. [DOI] [PubMed] [Google Scholar]
McParland 1993 {published data only}
- McParland P. Low‐dose aspirin in pregnancy. Contemporary Reviews in Obstetrics and Gynaecology 1993;5(1):30‐8. [Google Scholar]
Mueller‐Eckhardt 199 {published data only}
- Mueller‐Eckhardt G, Mallmann P, Neppert J, Lattermann A, Melk A, Heine, et al. Immunogenetic and serological investigations in nonpregnant and in pregnant women with a history of recurrent spontaneous abortions. Journal of Reproductive Immunology 1994;27(2):95‐109. [DOI] [PubMed] [Google Scholar]
Ogasawara 1998 {published data only}
- Ogasawara M, Sasa H, Katano K, Aoyama T, Aoki K, Suzumori K. Recurrent abortion and moderate or strong antiphospholipid antibody production. International Journal of Gynecology & Obstetrics 1998;62(2):183‐8. [DOI] [PubMed] [Google Scholar]
Passaleva 1993 {published data only}
- Passaleva A, Massai G, D'Elios MM, Livi C, Abbate R. Prevention of miscarriage in antiphospholipid syndrome. Autoimmunity 1993;14(2):121‐5. [DOI] [PubMed] [Google Scholar]
Perino 1997 {published data only}
- Perino A, Vassiliadis A, Vucetich A, Colacurci N, Menato G, Cignitti M, et al. Short‐term therapy for recurrent abortion using intravenous immunoglobulins: results of a double‐blind placebo‐controlled Italian study. Human Reproduction 1997;12(11):2388‐92. [DOI] [PubMed] [Google Scholar]
Quenby 1992 {published data only}
- Quenby S, Farquharson R, Ramsden G. The obstetric outcome of patients with positive anticardiolipin antibodies: aspirin versus no treatment. 26 British Congress of Obstetrics and Gynaecology; 1992; Manchester, UK. 1992:443. [443]
Rai 1997 b {published data only}
- Rai R, Regan L. Antiphospholipid antibodies, infertility and recurrent miscarriage. Current Opinion in Obstetrics and Gynecology 1997;9:279‐82. [PubMed] [Google Scholar]
Rai 2000 {published data only}
- Rai R, Backos M, Baxter N, Chilcott I, Regan L. Recurrent miscarriage‐‐an aspirin a day?. Human Reproduction 2000;15(10):2220‐3. [DOI] [PubMed] [Google Scholar]
Reece 1997 {published data only}
- Reece EA, Garofalo J, Zheng X‐Z, Assimakopoulos E. Pregnancy outcome: influence of antiphospholipid antibody titer, prior pregnancy losses and treatment. Journal of Reproductive Medicine 1997;42(1):49‐55. [PubMed] [Google Scholar]
Reznikoff‐Etievant {published data only}
- Reznikoff‐Etievant MF, Cayol V, Zou GM, Abuaf N, Robert A, Johanet C, et al. Habitual abortions in 678 healthy patients: investigation and prevention. Human Reproduction 1999;14(8):2106‐9. [DOI] [PubMed] [Google Scholar]
Ruffatti 1997 {published data only}
- Ruffatti A, Orsini A, Lenardo L, Nardelli GB, Patrassi GM, Truscia D, et al. A prospective study of fifty‐three consecutive calcium heparin treated pregnancies in patients with antiphospholipid antibody‐related fetal loss. Clinical and Experimental Rheumatology 1997;15(5):499‐505. [PubMed] [Google Scholar]
Sammaritano 2001 {published data only}
- Sammaritano LR. Update on the management of the pregnant patient with antiphospholipid antibody. Current Rheumatology Reports 2001;3(3):213‐21. [DOI] [PubMed] [Google Scholar]
Scopelitis 1994 {published data only}
- Scopelitis E, Wilson WA, Perez MC, Lynch A, Emlen W. Antiphospholipid antibodies in pregnancy. Annals of Internal Medicine 1994;121(7):547‐8. [DOI] [PubMed] [Google Scholar]
Semprini 1989 {published data only}
- Semprini AE, Vucetich A, Garbo S, Agostoni G, Pardi G. Effect of prednisone and heparin treatment in 14 patients with poor reproductive efficiency related to lupus anticoagulant. Fetal Therapy 1989;4(Suppl 1):73‐6. [DOI] [PubMed] [Google Scholar]
Shefras 1995 {published data only}
- Shefras J, Farquharson. Heparin therapy, bone density and pregnancy. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin. 1995:93. [93]
Sher 1994 {published data only}
- Sher G, Feinman M, Zouves C, Kuttner G, Maassarani G, Salem R, et al. High fecundity rates following in‐vitro fertilization and embryo transfer in antiphospholipid antibody seropositive women treated with heparin and aspirin. Human Reproduction 1994;9(12):2278‐83. [DOI] [PubMed] [Google Scholar]
Sher 1998 {published data only}
- Sher G, Matzner W, Feinman M, Maassarani G, Zouves C, Chong P, et al. The selective use of heparin/aspirin therapy, alone or in combination with intravenous immunoglobulin G, in the management of antiphospholipid antibody‐positive women undergoing in vitro fertilization. American Journal of Reproductive Immunology (Copenhagen) 1998;40(2):74‐82. [DOI] [PubMed] [Google Scholar]
Spinnato 1995 {published data only}
- Spinnato J, Clark A, Pierangeli S, Harris N. Intravenous immunoglobulin therapy for the antiphospholipid syndrome in pregnancy. American Journal of Obstetrics and Gynecology 1995;172:690‐4. [DOI] [PubMed] [Google Scholar]
Stern 2003 {published data only (unpublished sought but not used)}
- Stern, K, Kornman L, Norris H, Hale L, Baker HWG. Heparin / aspirin do not help antiphospholipid‐positive women with recurrent spontaneous abortion. Human Reproduction 2003;18(Suppl 1):55 xviii. [Google Scholar]
Takakuwa 1997 {published data only}
- Takakuwa K, Arakawa M, Honda K, Imai T, Tamura M, Kurabayashi T, et al. Immunosuppressive therapy for recurrent aborters with positive antiphospholipid antibodies and alteration of 6ketoPGF1 alpha/TXB2 ratio. Journal of Perinatal Medicine 1997;25(6):509‐11. [DOI] [PubMed] [Google Scholar]
Vahid 1999 {published data only}
- Vahid Dastjerdi M, Aleyasin A, Kashaf H, Marsoosi V, Aghahosscini A. The effect of acetyl salicylic acid and prednisolone before and during pregnancy in reducing unexplained recurrent abortions. Fertility and Sterility 1999;72:S203. [Google Scholar]
Yamamoto 1994 {published data only}
- Yamamoto H, Kanaya M, Mori S, Inoue Y, Kiya T, Goto T, et al. The prevalence and treatment of recurred spontaneous abortions caused by autoimmunity. Japanese Journal of Fertility and Sterility 1994;39(4):25‐30. [Google Scholar]
References to studies awaiting assessment
Carta 2005 {published data only}
- Carta G, Iovenitti P, Falciglia K. Recurrent miscarriage associated with antiphospholipid antibodies: prophylactic treatment with low‐dose aspirin and fish oil derivates. Clinical & Experimental Obstetrics & Gynecology 2005;32(1):49‐51. [PubMed] [Google Scholar]
Dendrinos 2007 {published data only}
- Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou‐Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clinical and Experimental Obstetrics and Gynecology 2007;34(3):143‐5. [PubMed] [Google Scholar]
Dendrinos 2009 {published data only}
- Dendrinos S, Sakkas E, Makrakis E. Low‐molecular‐weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. International Journal of Gynecology & Obstetrics 2009;104(3):223‐5. [DOI] [PubMed] [Google Scholar]
Ensom 2004 {published data only}
- Ensom MH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. Journal of the Society for Gynecologic Investigation 2004;11(6):377‐83. [DOI] [PubMed] [Google Scholar]
Goel 2006 {published data only}
- Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Medical Science Monitor 2006;12(3):CR132‐6. [PubMed] [Google Scholar]
Laskin 2009 {published data only}
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. Journal of Rheumatology 2009;36(2):279‐87. [DOI] [PubMed] [Google Scholar]
Mahmoud 2004 {published data only}
- Mahmoud F, Diejomaoh M, Omu A, Abul H, Haines D. Effect of IgG therapy on lymphocyte subpopulations in the peripheral blood of Kuwaiti women experiencing recurrent pregnancy loss. Gynecologic and Obstetric Investigation 2004;58(2):77‐83. [DOI] [PubMed] [Google Scholar]
Malinowski 2003 {published data only}
- Malinowski A, Dynski MA, Maciolek‐Blewniewska G, Glowacka E, Pawlowski T, Babula G. Treatment outcome in women suffering from recurrent miscarriages and antiphospholipid syndrome [Wyniki leczenia kobiet z nawracajacymi samoistnymi poronieniami i zespolem antyfosfolipidowym.]. Ginekologia Polska 2003;74(10):1213‐22. [PubMed] [Google Scholar]
Merrill 2001 {published data only}
- Merrill JT. Appropriate management of antiphospholipid‐related pregnancy in women without lupus who have low titer autoantibodies. Current Rheumatology Reports 2001;3:269‐70. [DOI] [PubMed] [Google Scholar]
Noble 2005 {published data only}
- Noble LS, Kutteh WH, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low‐molecular‐weight heparin versus unfractionated heparin. Fertility & Sterility 2005;83(3):684‐90. [DOI] [PubMed] [Google Scholar]
Rai 1996 {published data only}
- Rai RS, Regan L, Dave M, Cohen H. Prospective randomised trial of aspirin versus aspirin plus heparin in pregnant women with the antiphospholipid syndrome [abstract]. British Journal of Haematology 1996;93(Suppl 1):5. [Google Scholar]
Regan 2006 {published data only}
- Regan L. Steroids and antiphospholipid syndrome‐related pregnancy loss (planned trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Shu 2002 {published data only}
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi//Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. [PubMed] [Google Scholar]
Stephenson 2004 {published data only}
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (aps) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(8):729‐34. [DOI] [PubMed] [Google Scholar]
Tulppala 1997a {published data only}
- Tulppala M, Marttunen M, Soderstrom‐Anttila V, Ailus K, Palosuo T, Ylikorkala O. Low dose aspirin in the prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(1):191. [DOI] [PubMed] [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Medline search strategies for optimal sensitivity in identifying randomised clinical trials. Cochrane Reviewers’ Handbook 4.2.2 [updated December 2003]; 11a. Appendix B. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Carreras 1988
- Carreras LO, Perez G, Vega H, Casavilla F. Lupus anticoagulant and recurrent fetal loss: successful treatment with gammaglobulin. Lancet 1988;ii:393‐4. [DOI] [PubMed] [Google Scholar]
Chamley 1998
- Chamley L, Duncalf A, Mitchell M, Johnson P. Action of anticardiolipin and antibodies to beta2‐glycoprotein‐1 on trophoblast proliferation as a mechanism for fetal death. Lancet 1998;352:1037‐8. [DOI] [PubMed] [Google Scholar]
Chamley 2002
- Chamley L. Antiphospholipid antibodies: biological basis and prospects for treatment. Journal of Reproductive Immunology 2002;57(1‐2):185‐202. [DOI] [PubMed] [Google Scholar]
Di Simone 1997
- Simone N, Ferrazzani S, Castellani R, Carolis S, Mancuso S, Caruso A. Heparin and low‐dose aspirin restore placental human chorionic gonadotrophin secretion abolished by antiphospholipid antibody‐containing sera. Human Reproduction 1997;12(9):2061‐5. [DOI] [PubMed] [Google Scholar]
Di Simone 1999
- Simone N, Caliandro D, Castellani R, Ferrazzani S, Carolis S, Caruso A. Low‐molecular weight heparin restores in‐vitro trophoblast invasiveness and differentiation in presence of immunoglobulin G fractions obtained from patients with antiphospholipid syndrome. Human Reproduction 1999;14(2):489‐95. [DOI] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Elder 1988
- Elder M, Swiet M, Robertson A, Flloyd E, Hawkins D. Low‐dose aspirin in pregnancy. Lancet 1988;i:410. [DOI] [PubMed] [Google Scholar]
Ensom 1999
- Ensom MH, Stephenson MD. Low‐molecular‐weight heparins in pregnancy. Pharmacotherapy 1999;19(9):1013‐25. [DOI] [PubMed] [Google Scholar]
Forastiero 1997
- Forastiero RR, Martinuzzo ME, Cerrato GS, Kordich LC, Carreras LO. Relationship of anti beta2‐glycoprotein I and anti prothrombin antibodies to thrombosis and pregnancy loss in patients with antiphospholipid antibodies. Thrombosis and Haemostasis 1997;78(3):1008‐14. [PubMed] [Google Scholar]
Francois 1988
Glasziou 1995
- Gasziou P, Irwig L. An evidence based approach to indivdualising treatment. BMJ 1995;311:1356‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higashino 1998
- Higashino M, Takakuwa K, Arakawa M, Tamura M, Yasuda M, Tanaka K. Anti‐cardiolipin antibody and anti‐cardiolipin beta‐2‐glycoprotein I antibody in patients with recurrent fetal miscarriage. Journal of Perinatal Medicine 1998;26(5):384‐9. [DOI] [PubMed] [Google Scholar]
Hirsh 1998
- Hirsh J, Warkentin T, Raschke R, Granger C, Ohman E, Dalen J. Heparin and low‐molecular‐weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 1998;114(5):489S‐510. [DOI] [PubMed] [Google Scholar]
Kobayashi 1992
- Kobayashi S, Tamura N, Tsuda H, Mokuno C, Hashimoto H, Hirose S. Immunoadsorbent plasmapheresis for a patient with antiphospholipid syndrome during pregnancy. Annals of the Rheumatic Diseases 1992;51(3):399‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laurell 1957
- Laurell A, Nilsson I. Hypergammaglobulinaemia, circulating anticoagulant, and biologic false positive Wassermann Reaction. Journal of Laboratory and Clinical Medicine 1957;49(5):694‐707. [PubMed] [Google Scholar]
Lockwood 1989
- Lockwood C, Romero R, Feinberg R, Clyne L, Coster B, Hobbins J. The prevalence and biologic significance of lupus anticoagulant and anticardiolipin antibodies in a general obstetric population. American Journal of Obstetrics and Gynecology 1989;161:369‐73. [DOI] [PubMed] [Google Scholar]
Lubbe 1983
- Lubbe W, Butler W, Palmer S, Liggins G. Fetal survival after prednisone suppression of maternal lupus‐anticoagulant. Lancet 1983;i:1361‐3. [DOI] [PubMed] [Google Scholar]
Lubbe 1985
- Lubbe W, Liggins G. Lupus anticoagulant and pregnancy. American Journal of Obstetrics and Gynecology 1985;153:322‐7. [DOI] [PubMed] [Google Scholar]
Lynch 1994
- Lynch A, Marlar R, Murphy J, Davila G, Santos M, Rutledge J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Annals of Internal Medicine 1994;120(6):470‐5. [DOI] [PubMed] [Google Scholar]
Lynch 1999
- Lynch A, Byers T, Emlen W, Rynes D, Shetterly SM, Hamman RF. Association of antibodies to beta2‐glycoprotein 1 with pregnancy loss and pregnancy‐induced hypertension: a prospective study in low‐risk pregnancy. Obstetrics & Gynecology 1999;93(2):193‐8. [DOI] [PubMed] [Google Scholar]
Many 1992
- Many A, Pauzner R, Carp H, Langevitz P, Martinowitz U. Treatment of patients with antiphospholipid antibodies during pregnancy. American Journal of Reproductive Immunology 1992;28:216‐8. [DOI] [PubMed] [Google Scholar]
Nelson‐Piercy 1994
- Nelson‐Piercy C. Low molecular weight heparin for obstetric thromboprophylaxis. British Journal of Obstetrics and Gynaecology 1994;101:9‐17. [DOI] [PubMed] [Google Scholar]
Nilsson 1975
- Nilsson I, Astedt B, Hedner U, Berezin D. Intrauterine death and circulating anticoagulant ("antithromboplastin"). Acta Medicine Scandinavia 1975;197:153‐9. [DOI] [PubMed] [Google Scholar]
Rai 1995
- Rai RS, Clifford K, Cohen H, Regan L. High prospective fetal loss rate in untreated pregnancies of women with recurrent miscarriage and antiphospholipid antibodies. Human Reproduction 1995;10(12):3301‐4. [DOI] [PubMed] [Google Scholar]
Rand 1994
- Rand JH, Wu XX, Guller S, Gil J, Guha A, Scher J, et al. Reduction of annexin‐V (placental anticoagulant protein‐I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. American Journal of Obstetrics and Gynecology 1994;171(6):1566‐72. [DOI] [PubMed] [Google Scholar]
Rand 1997
- Rand JH, Wu XX, Andree H, Lockwood C, Guller S, Scher J, et al. Pregnancy loss in the antiphospholipid‐antibody syndrome ‐ a possible thrombogenic mechanism. New England Journal of Medicine 1997;337:154‐60. [DOI] [PubMed] [Google Scholar]
Rosove 1990
- Rosove M, Tabsh B, Wasserstrum N, Howard P, Hahn B, Kalunian K. Heparin therapy for pregnant women with lupus anticoagulant or anticardiolipin antibodies. Obstetrics & Gynecology 1990;75(4):630‐4. [PubMed] [Google Scholar]
Salafia 1997
- Salafia CM, Cowchock FS. Placental pathology and antiphospholipid antibodies: a descriptive study. American Journal of Perinatology 1997;14(8):435‐41. [DOI] [PubMed] [Google Scholar]
Sandercock 2004
- Sandercock P, Algra A, Anderson C, Bath P, Bereczki D, Berge E, et al. Stroke Group. About the Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2004, Issue 4. Art. No.: STROKE.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Scott 1988
- Scott J, Branch D, Kochenour N, Ward K. Intravenous immunoglobulin treatment of pregnant patients with recurrent pregnancy loss caused by antiphospholipid antibodies and Rh immunization. American Journal of Obstetrics and Gynaecology 1988;159:1055‐6. [DOI] [PubMed] [Google Scholar]
Shefras 1996
- Shefras J, Farquharson R. Bone density studies in pregnant women receiving heparin. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;65:171‐4. [DOI] [PubMed] [Google Scholar]
Wilson 1999
- Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop.[see comment]. Arthritis & Rheumatism 1999;42(7):1309‐11. [DOI] [PubMed] [Google Scholar]
Yasuda 1995
- Yasuda M, Takakuwa K, Tokunaga A, Tanaka K. Prospective studies of the association between anticardiolipin antibody and outcome of pregnancy. Obstetrics & Gynecology 1995;86(4 Pt 1):555‐9. [DOI] [PubMed] [Google Scholar]
Yetman 1996
- Yetman DL, Kutteh WH. Antiphospholipid antibody panels and recurrent pregnancy loss: prevalence of anticardiolipin antibodies compared with other antiphospholipid antibodies. Fertility and Sterility 1996;66(4):540‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Empson 2002
- Empson M, Lassere M, Craig J, Scott J. Recurrent pregnancy loss with antiphospholipid antibody: A systematic review of therapeutic trials. Obstetrics & Gynecology 2002;99(1):135‐44. [DOI] [PubMed] [Google Scholar]


