Abstract
Background
Primary open angle glaucoma (POAG) is a progressive optic neuropathy with an elevated intraocular pressure (IOP), where the optic nerve head becomes pathologically excavated and the visual field (VF) is characteristically altered. Ocular hypertension (OHT) is a condition with elevated IOP but without discernible pathology of the optic nerve head or the VF. It is a major risk factor for development of POAG.
Objectives
To assess and compare the effectiveness of topical pharmacological treatment for POAG or OHT to prevent progression or onset of glaucomatous optic neuropathy.
Search methods
We searched CENTRAL, MEDLINE and EMBASE in May 2007. We searched the bibliographies of identified articles and contacted experts, investigators and pharmaceutical companies for additional published and unpublished studies.
Selection criteria
Randomised controlled trials comparing topical pharmacological treatment to placebo, no treatment or other treatment for specified endpoints which included people with POAG or OHT, and with duration of treatment of at least one year.
Data collection and analysis
Two authors independently extracted data and assessed trial quality. Where appropriate, we summarised data using Peto odds ratio and mean difference after testing for heterogeneity between studies.
Main results
We included 26 trials, which randomised 4979 participants, in this review. Meta‐analysis of 10 trials clearly demonstrated reduction of onset of VF defects in treated OHT (OR 0.62, 95% CI 0.47 to 0.81). No single drug showed a significant VF protection compared to placebo or untreated controls. We did identify some border line evidence for a positive influence of treatment on VF prognosis (OR 0.67, 95% CI 0.45 to 1.00) for the beta‐blockers .
Authors' conclusions
The results of this review support the current practice of IOP lowering treatment of OHT. A visual field protective effect has been clearly demonstrated for medical IOP lowering treatment. Positive but weak evidence for a beneficial effect of the class of beta‐blockers has been shown.
Direct comparisons of prostaglandins or brimonidine to placebo are not available and the comparison of dorzolamide to placebo failed to demonstrate a protective effect. However, absence of data or failure to prove effectiveness should not be interpreted as proof of absence of any effect. The decision to treat a patient or not, as well as the decision regarding the drug with which to start treatment, should remain individualised, taking in to account the amount of damage, the level of IOP, age and other patient characteristics.
Plain language summary
Medical interventions for primary open angle glaucoma and ocular hypertension
Ocular hypertension (OHT) is a condition with raised intraocular pressure (IOP) without visual field changes or discernible pathology of the optic nerve head. Ocular hypertension with IOP above 21 mmHg untreated is a major risk factor for development of primary open angle glaucoma, which is progressive nerve fibre loss and damage to the optic disc so that the visual field develops characteristic defects. Topical medications are given to reduce the IOP as a way of preventing the onset or progression of damage and associated visual field loss. These medications may have local and systemic side effects that may be severe enough for the treatment to be stopped and include local irritation, drowsiness, shortage of breath and cardiovascular side effects, particularly in the elderly. The results of this review support the current practice of topical medication to lower IOP and clearly demonstrate a visual field protective effect. The review authors identified a total of 26 controlled trials that randomised 4979 people with OHT or open angle glaucoma to receive topical medication or a placebo, another topical medication or no treatment for at least a year. Meta‐analysis of 10 trials testing different topical medications against placebo or untreated controls showed reduced incidence of glaucomatous visual field defects with treatment for people with OHT. The odds ratio (OR) was 0.62 (range 0.5 to 0.8). The class of beta‐blockers (including timolol) had positive but weak evidence for a beneficial effect in protecting against visual field defects (OR 0.7, range 0.5 to 1.0). No single drug showed significant visual field protection in OHT with the evidence available. Medications included beta‐blockers, dorzolamide, brimonidine, pilocarpine and epinephrine. From the reports, the majority of trials were of low methodological quality. Local and systemic side effects leading to treatment being stopped were often poorly reported and did not appear to differ between treatment groups. Drop‐outs due to side effects occurred with similar frequency in people treated with beta‐blocker or placebo and appeared to be less with timolol compared to brimonidine, in three trials.
Background
Primary open angle glaucoma (POAG) is a disease in which elevated intraocular pressure (IOP) is combined with a progressive optic neuropathy, resulting in characteristic excavation of the optic nerve head and corresponding visual field defects. Ocular hypertension (OHT) is a condition in which IOP is elevated but there is no discernible glaucomatous damage to the optic nerve head and no detectable visual field changes. The distinction between OHT and POAG remains to some extent arbitrary. Optic nerve head pathology usually precedes the onset of visual field defects. Up to 50% of the optic nerve fibres are lost when the visual field shows the earliest pathologic changes (Quigley 1983). Unfortunately preperimetric damage to the optic nerve head may be missed because of the variable appearance of the normal optic nerve head.
Elevated IOP is an important risk factor for the development of glaucomatous optic neuropathy (Armaly 1980) and between 4% (Kass 2002) and 20% (Ontoso 1997) of people with OHT develop visual field defects within five years. Traditionally people with OHT have been treated with IOP lowering medication. However, the evidence for a protective effect of such treatment is questionable and has led to the recent recommendation by the European Glaucoma Society to refrain from treatment of OHT with IOP below the upper 20 millimetres mercury (mmHg) and no additional risk factor.
Normal tension glaucoma (NTG) is a clinical entity characterised by similar damage of the optic nerve head and similar visual field defects, but without an elevated IOP. Primary open angle glaucoma, which by definition is characterised by an elevated IOP, is arbitrarily distinguished from NTG using a cut‐off point of IOP of 21 mmHg.
It is widely accepted that the distinction between these two entities remains artificial, because they represent two subsets of patients along a continuum. Nevertheless people suffering from POAG and NTG may show a different clinical course concerning patterns of optic disc damage and quantity and quality of visual field loss (Caprioli 1998). In addition, there is evidence that the mechanism of damage may differ between the two chronic open angle glaucoma populations (Schulzer 1990). A recent Cochrane review resulted in only weak evidence for visual field protection by either medical or surgical treatment of NTG (Sycha 2005).
Primary open angle glaucoma has been treated over the past 100 years with various topically and systemically applied drugs to lower the IOP. The IOP lowering capacity of numerous antiglaucomatous drugs has been thoroughly studied. However, there are no well established guidelines for the treatment of POAG based on evidence of visual fields or optic nerve head protection (Rossetti 1993). Recently published evidence for OHT shows IOP lowering topical antiglaucomatous therapy effectively prevents conversion to glaucoma, defined as onset of visual field defects or morphological change of the appearance of the optic nerve head (Kass 2002). However, since this study did not specify nor restrict the medications to be used, we can neither tell which of the presently available drugs offers a beneficial effect, nor whether this protection will be achieved by all topical IOP lowering treatments.
Objectives
This review aimed to summarise the evidence for the effectiveness of different forms of topical medical treatment of POAG or OHT to prevent the progression or the onset of glaucomatous optic neuropathy. Secondly, this review aimed to answer the question, whether different drugs or classes of drugs differ in their beneficial effect? In addition, this review aimed to summarise the observed local and systemic side effects leading to the cessation of the different forms of topical medical treatment in the retrieved studies.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised trials with treatment duration of at least one year comparing different forms of topical medical therapy to placebo, no treatment or other medical treatment. We divided studies into short‐term (with a follow‐up time of less than three years) and long‐term (with a follow‐up time of three years or more) trials.
Types of participants
We included trials in which participants were people with POAG or OHT as defined by investigators using the common definitions as mentioned below or using similar definitions. We accepted trials that included people with a mean IOP above 21 mmHg.
There were no age or gender limitations.
At present the following criteria are widely used to define POAG: (1) A mean untreated IOP above 21 mmHg. (2) Open drainage angles on gonioscopy without any pathologic changes. (3) Typical optic disc damage with glaucomatous cupping and loss of neuroretinal rim. (4) Visual field defect compatible with glaucomatous cupping. (5) Absence of secondary cause for IOP elevation (e.g. pigment dispersion, uveitis).
Ocular hypertension is commonly defined according to the following criteria: (1) A mean untreated IOP above 21 mmHg. (2) Open drainage angles on gonioscopy without any pathologic changes. (3) Absence of typical optic disc damage with glaucomatous cupping and loss of neuroretinal rim. (4) Absence of visual field defects. (5) Absence of secondary cause for IOP elevation (e.g. pigment dispersion, uveitis).
Types of interventions
Interventions included topical ocular administration of pharmacological therapy with the intention to reduce the progression of signs in participants with POAG or OHT. There was no maximum limit of the duration of treatment. Minimum duration of treatment was one year.
Types of outcome measures
Primary outcomes
(1) reduction of onset or progression of visual field loss (proportion of people with visual field loss or visual field loss rate in a specified time period).
Secondary outcomes
(2) improvement of visual field; (3) reduction of nerve fibre layer loss progression (according to objective measurement); (4) reduction of optic nerve head cupping progression (according to objective assessment); (5) local and systemic side effects leading to the cessation of treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, which contains the Cochrane Eyes and Vision Group Trials Register; MEDLINE and EMBASE; and reference lists of identified trial reports. We last searched the databases on 14 May 2007. We placed no language or date restrictions in the searches for trials.
See: Appendices for details of search strategies used for each database.
Searching other resources
We searched the reference lists of identified trial reports and used the Science Citation Index to find reports that cited the identified relevant studies. We contacted experts, investigators and pharmaceutical companies in the field of glaucoma for details of further studies and unpublished studies. We placed no language restriction in the selection of trials.
Data collection and analysis
Selection of trials
Two review authors working independently assessed the titles and abstracts of all reports identified by the electronic searching. The full text copies of possibly and definitely relevant trials were obtained and assessed according to the definitions in the 'Criteria for considering studies for this review'. Only trials meeting these criteria were assessed for methodological quality. There was no restriction concerning publication date or language. The review authors were not masked to any trial detail during the assessment.
Assessment of methodological quality
Trial quality was assessed according to methods set out in section 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006a). We used four components to determine methodological quality: allocation concealment, performance bias, detection bias and attrition bias. Each component was graded A ‐ Adequate, B ‐ Unclear or C ‐ Inadequate as outlined in the Cochrane Eyes and Vision Group Review Development guidelines. Two review authors working independently assessed trial quality and disagreements were resolved by discussion, additional referees or both. The review authors were unmasked to the report authors and trial results during the assessment. We contacted trial authors for clarification concerning studies that did not report relevant details on the four components mentioned above. We excluded trials scoring C on allocation concealment.
Data collection
Two review authors working independently extracted data onto forms developed by the Cochrane Eyes and Vision Group using Section 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006b). We contacted authors of trials to obtain missing data. We compared the extracted data for differences between review authors and discrepancies were resolved by discussion.
Data synthesis
We summarised data from studies collecting similar outcomes and using similar follow‐up times after testing for heterogeneity between trial results using the I‐square test.
For dichotomous data, we expressed results as odds ratio (OR) estimates or risk ratio (RR) estimates (95% confidence interval (CI)). We also obtained the risk difference or the number needed to treat (95% CI). For continuous data, we obtained the mean and standard deviations. We converted standard errors to standard deviations. We summarised results across studies using mean differences (95% CI).
We conducted sensitivity analyses to determine the effect of excluding trials falling below a quality threshold with the following criteria (one or both of them):
(1) exclusion of trials scoring C on any aspect of methodological trial quality; (2) exclusion of trials which had assumed that eyes within an individual were independent (fellow eye used as a control group).
Results
Description of studies
Finding the trials
The electronic searches revealed 2236 abstracts of papers. We obtained the full copies of 365 potentially or definitely relevant papers. Checking reference lists revealed a further 19 papers, which were obtained in full text. Contact with the authors of identified trials and with experts and researchers in the field have not identified any further relevant papers. We screened a total of 384 papers for content and methodological quality according to their full text copies. From these articles we could easily and without any doubt exclude 334 from further assessment, as they either did not involve people with POAG or did not report randomised or controlled trials. This left a total of 50 papers describing 30 trials that addressed topical medical interventions for POAG.
We did an updated search in May 2007 which yielded a further 685 reports of studies. The Trials Search Co‐ordinator scanned the search results and removed any references which were not relevant to the scope of the review. The authors received abstracts of 80 papers for assessment for potential inclusion in the review. We have identified eight reports of studies which may be added to the review; please see 'Studies awaiting assessment' for further information.
Included/ excluded studies
We excluded four trials (eight papers) (see 'Characteristics of excluded studies' table). We excluded two trials (three papers) because they were carried out in people with POAG or NTG and data were not described separately in the results section (Araie 2003; Vainio 1999). We excluded one trial (one paper) because the protocol allowed topical therapy to be supplemented with acetazolamide, and patients on topical therapy alone were not analysed separately (Holmin 1988). We excluded one trial (four papers) because the treatment also included laser trabeculoplasty (EMGT 1999).
We included a total of 42 papers describing 26 trials in this systematic review. The trials recruited a total of 4979 participants, including 2907 Caucasian, 562 African, 59 Hispanic and 15 Asian people. Sixteen trials did not report on race data. The sample size ranged from 18 to 1636 people. Most trials compared small sample sizes; only six trials included more than 200 participants.
The included trials made three types of comparisons:
comparisons of a topical antiglaucomatous drug to placebo or no treatment;
comparisons of different topical antiglaucomatous drugs;
comparisons with unspecified topical antiglaucomatous drugs with no treatment.
Some trials had more than two arms and made more than one comparison. Twenty‐four trials were parallel arm studies, while two trials compared one treated eye to the untreated or placebo treated fellow eye (Kass 1989; Wishart 1992). See 'Characteristics of included studies'.
Sample characteristics
Inclusion and/or exclusion criteria were not uniform and not always well defined. Nine trials included only people with POAG (Collignon‐Brach 1992; Drance 1998; Fama 1996; Flammer 1992; Kaiser 1994; Sponsel 1987; Tsai 2005; Vogel 1992; Watson 2001). Eleven trials restricted entry to participants with OHT (Epstein 1989; Heijl 2000; Kamal 2003; Kass 1989; Kass 2002; Kitazawa 1990; EGPS 2005; Ravalico 1994; Schulzer 1991; Schwartz 1995; Wishart 1992). Six trials accepted both POAG and OHT participants (Alexander 1988; Collignon‐Brach 1994; Geyer 1988; Leblanc 1998; Novack 1989; Schuman 1997).
Most trials either evaluated data from one eye per patient or considered the patient as basis of evaluation. Two trials made an intra‐individual comparison of both eyes (Kass 1989; Wishart 1992).
Interventions
Eleven trials (16 papers) made comparisons to placebo (six trials) or an untreated control group (five trials). Fourteen trials made comparisons between two or three drugs, and one trial compared free topical antiglaucomatous therapy versus an untreated control group.
The following comparisons were made:
(1) comparisons versus placebo or untreated controls. (a) beta‐blockers versus placebo or untreated controls: (i) timolol versus placebo (Heijl 2000; Kass 1989; Kitazawa 1990; Schwartz 1995) or untreated controls (Epstein 1989; Schulzer 1991; Wishart 1992); (ii) levobunolol versus untreated controls (Ravalico 1994); (iii) betaxolol versus placebo (Kamal 2003); (iv) all beta‐blockers versus placebo or untreated controls; (b) dorzolamide versus placebo (EGPS 2005): (c) unspecified topical treatment versus untreated control (Kass 2002).
(2) comparisons of different topical treatments. (a) comparison of one beta‐blocker versus another: (i) timolol versus carteolol (Fama 1996; Flammer 1992; Watson 2001); (ii) timolol versus levobunolol (Geyer 1988; Novack 1989); (iii) timolol versus betaxolol (Collignon‐Brach 1992; Collignon‐Brach 1994; Drance 1998; Fama 1996; Kaiser 1994; Watson 2001); (iv) carteolol versus betaxolol (Fama 1996; Watson 2001); (b) comparison of one beta‐blocker versus other topical medication: (i) timolol versus brimonidine (Leblanc 1998; Schuman 1997; Tsai 2005); (ii) timolol versus pilocarpine (Drance 1998; Sponsel 1987; Vogel 1992); (iii) timolol versus epinephrine (Alexander 1988); (iv) betaxolol versus pilocarpine (Drance 1998).
Two studies also compared different doses of levobunolol (Geyer 1988; Novack 1989). Since it was not the goal of this review to compare different dosages of the same drug, we did not further analyse this comparison.
Outcome measures
The lack of similarity in outcome measures limited the possibility for combining data from individual trials.
(1) The primary outcome of interest in this systematic review was the reduction of onset or progression of visual field loss. There was, however, limited agreement between the trials in the presentation of visual field data. Seven trials reported on the incidences of visual field deterioration (Alexander 1988; EGPS 2005; Epstein 1989; Flammer 1992; Geyer 1988; Heijl 2000; Kamal 2003; Kass 1989; Kass 2002; Kitazawa 1990; Leblanc 1998; Novack 1989; Schwartz 1995; Schulzer 1991; Schuman 1997; Watson 2001; Wishart 1992). Nine trials reported on changes in mean sensitivity or in mean defect of visual fields. Most of the latter did not present any measure of variation for this change (Fama 1996; Kaiser 1994; Ravalico 1994; Vogel 1992; Watson 2001), while only four mentioned the standard error of change (Collignon‐Brach 1992; Collignon‐Brach 1994; Drance 1998; Sponsel 1987).
(2) Improvement of visual field: No study was focused on measuring improvement and only two trials reported cases with visual field improvement (Flammer 1992; Leblanc 1998).
(3) Reduction of nerve fibre layer loss progression (according to objective measurement): Two trials reported changes in retinal nerve fibre loss progression according to photogrammetric measurement (Schwartz 1995) or scanning laser polarimetry (Tsai 2005).
(4) Reduction of optic nerve head cupping progression (according to objective assessment): One trial reported changes in optic nerve head progression according to photogrammetric measurement (Schwartz 1995).
(5) Local and systemic side effects leading to the cessation of treatment: Not all trials presented detailed data about the reasons for drop‐outs. Some papers did not report on drop‐outs (Collignon‐Brach 1992; Wishart 1992), and some authors did not separately report numbers of patients exiting the studies due to local and systemic adverse events in the different treatment groups (Drance 1998; EGPS 2005; Geyer 1988; Heijl 2000; Kass 2002; Leblanc 1998; Ravalico 1994; Schulzer 1991; Sponsel 1987; Vogel 1992).
Risk of bias in included studies
Most studies did not describe the method of allocation concealment in detail. A double‐masked design should adequately conceal the group allocation, although this cannot be guaranteed. For the purpose of this review, we coded the five trials that stated a double‐masked design as having adequate allocation concealment (Geyer 1988; Kaiser 1994; Kitazawa 1990; Leblanc 1998; Novack 1989). In 10 trials, allocation concealment was unclear (Collignon‐Brach 1992; Collignon‐Brach 1994; Drance 1998; Fama 1996; Ravalico 1994; Schulzer 1991; Sponsel 1987; Tsai 2005; Vogel 1992; Wishart 1992).
Additional Table 7 summarises the quality aspects of the trials. Of the studies with low methodological quality, 14 had scored C in at least one category of quality assessment, and two assumed two eyes of participants to be independent. There was no relationship between low methodological quality and earlier date of publication in logistic regression analysis.
1. Methodological quality of included studies.
| Criteria | Number of trials |
| Allocation concealment adequate | 16 / 26 |
| Baseline comparability stated | 19 / 26 |
| Analysis: intention‐to‐treat | 9 / 26 |
| Withdrawals adequately reported | 17 / 26 |
| Drop‐out rate below 10% | 1 / 26 |
| Low methodological quality | 16 / 26 |
Effects of interventions
1. Comparisons versus placebo or untreated controls
(a) Beta‐blocker versus placebo or untreated controls
(i) Timolol versus placebo or untreated controls We retrieved four trials comparing timolol to placebo (Heijl 2000; Kass 1989; Kitazawa 1990; Schwartz 1995) and three trials that included an untreated control group (Epstein 1989; Schulzer 1991; Wishart 1992). All of these trials included only patients with OHT, and two of them compared one treated eye to the untreated fellow eye (Kass 1989; Wishart 1992). Five trials were long‐term studies (Epstein 1989; Heijl 2000; Kass 1989; Schulzer 1991; Wishart 1992), including 430 participants with a median follow‐up time of 63 months.
* Primary outcome measure (Analysis 1.1;Analysis 1.3) All seven trials report data on the incidence of glaucomatous visual field defects. Meta‐analysis failed to achieve clear statistical evidence for visual field protection by timolol (OR 0.66, 95% CI 0.41 to 1.05). In a sensitivity analysis there was still no significant difference (OR 0.54, 95% CI 0.19 to 1.54).
1.1. Analysis.
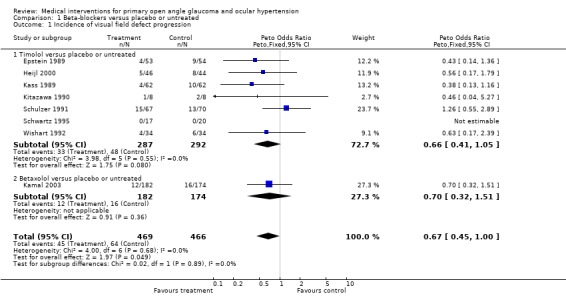
Comparison 1 Beta‐blockers versus placebo or untreated, Outcome 1 Incidence of visual field defect progression.
1.3. Analysis.
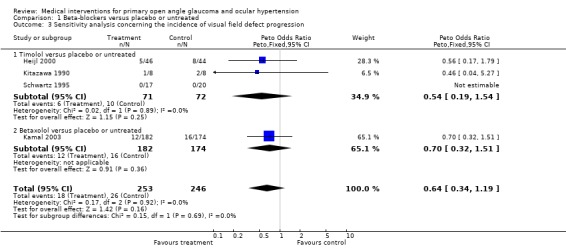
Comparison 1 Beta‐blockers versus placebo or untreated, Outcome 3 Sensitivity analysis concerning the incidence of visual field defect progression.
* Secondary outcome measures (Analysis 1.2) Schwartz 1995 analysed the morphological change of the optic nerve head and the retinal nerve fibre layer (RNFL), using a quantifying method based on fundus photographs (Takamoto 1985). In an analysis with multiple comparisons, a significant difference between groups was detected for change over time for several optic disc parameters. Considering the borderline statistical significance for most of the parameters, it remains unclear whether the significances are in part the result of multiple testing without correcting for this. However, for nearly all the significantly changed parameters, the timolol group showed an improvement, whereas the placebo group on average deteriorated. Similarly the authors observed a significant protection of the RNFL by two years of timolol treatment. On average, for both eyes there was a RNFL decrease of 0.22 μm in the placebo and an increase of 0.23 μm in the timolol group.
1.2. Analysis.
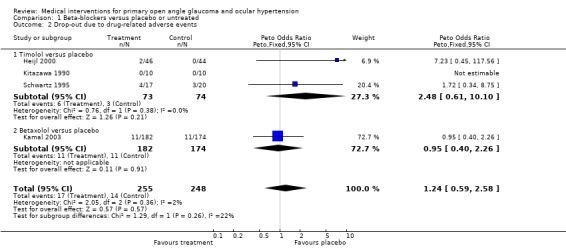
Comparison 1 Beta‐blockers versus placebo or untreated, Outcome 2 Drop‐out due to drug‐related adverse events.
Regarding local and systemic side effects leading to the cessation of treatment, only the placebo controlled studies with interindividual comparison were included in the meta‐analysis (Heijl 2000; Kitazawa 1990; Schwartz 1995). One of these studies (Heijl 2000) did not specify the side‐effect‐related drop‐outs in local and systemic adverse events. Due to the small numbers, we did not perform separate analyses regarding the local and systemic side effects. There was no statistically significant difference between groups (OR 2.48, 95% CI 0.61 to 10.10).
(ii) Levobunolol versus untreated controls Only one study compared levobunolol to an untreated control group (Ravalico 1994) in OHT patients.
* Primary outcome measure The visual field results were presented as mean deviation. At 18 months of treatment, the mean deviations of the levobunolol and the no treatment groups improved by 1.71 dB and 1.69 dB. The difference between groups was not statistically significant.
* Secondary outcome measures The authors did not detail the reasons for drop‐out of patients.
(iii) Betaxolol versus placebo We included only one placebo‐controlled trial (Kamal 2003) studying OHT patients. Follow‐up time was 60 months. * Primary outcome measure (Analysis 1.1) Incidence of visual field loss was noted in 12/182 (7.8%) in the betaxolol group, and in 16/174 (9.2%) in the placebo group. This difference was not statistically significant (RR 0.70, 95% CI 0.32 to 1.51).
* Secondary outcome measures (Analysis 1.2) Drop‐outs occurred at a very similar rate in the betaxolol and the placebo groups (OR 0.95, 95% CI 0.40 to 2.26). In the betaxolol group, eight patients had to stop treatment as a consequence of local, and three patients due to systemic adverse effects. For the placebo group, these figures were seven (local) and four (systemic).
(iv) All beta‐blockers versus placebo or untreated controls We analysed the seven trials (Epstein 1989; Heijl 2000; Kass 1989; Kitazawa 1990; Schulzer 1991; Schwartz 1995; Wishart 1992) comparing timolol to placebo or untreated control with Kamal 2003, which compared betaxolol to placebo.
* Primary outcome measure (Analysis 1.1; Analysis 1.3; Analysis 1.4) Incidence of visual field loss was registered in 45/469 (9.6%) in the beta‐blocker group, and in 64/466 (13.7%) in the placebo/untreated group. There was some borderline evidence for a positive influence of treatment on visual field prognosis (OR 0.67, 95% CI 0.45 to 1.00). In a sensitivity analysis (excluding trials scoring C or assuming both eyes are independent) which included Heijl 2000, Kitazawa 1990 and Schwartz 1995, this difference was no longer statistically significant (OR 0.64, 95% CI 0.34 to 1.19).
1.4. Analysis.
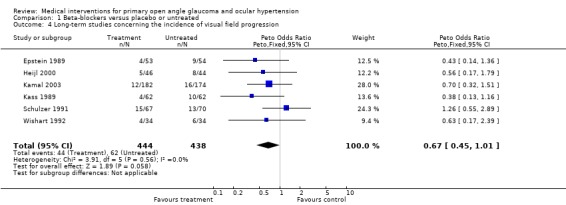
Comparison 1 Beta‐blockers versus placebo or untreated, Outcome 4 Long‐term studies concerning the incidence of visual field progression.
We performed a separate analysis which included only the long‐term studies, with a follow‐up of at least three years (Epstein 1989; Heijl 2000; Kamal 2003; Kass 1989; Schulzer 1991; Wishart 1992). This analysis failed statistical significance but again demonstrated the same trend as above (OR 0.67, 95% CI 0.45 to 1.01).
* Secondary outcome measures (Analysis 1.2)
Drop‐outs due to drug‐related side effects occurred with similar frequency in beta‐blocker treated and in placebo treated groups (OR 1.24, 95% CI 0.59 to 2.58).
(b) Dorzolamide versus placebo
EGPS 2005 reported on this comparison. Mean follow up was 55.3 months.
* Primary outcome measure (Analysis 6.1) In participants with OHT, 26/536, 4.9% dorzolamide treated and 38/541, 7.0% placebo treated developed reproducible visual field defects. Despite this trend, the difference was not statistically significant (OR 0.68, 95% CI 0.41 to 1.12). The study also failed to demonstrate a significant difference between groups when its primary endpoint (visual field defect or glaucomatous change of optic disc) was analysed (OR 0.86, 95% CI 0.58 to 1.26).
6.1. Analysis.
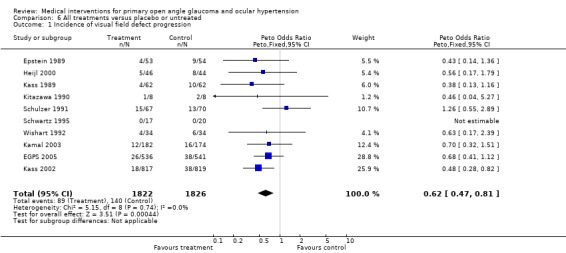
Comparison 6 All treatments versus placebo or untreated, Outcome 1 Incidence of visual field defect progression.
* Secondary outcome measures (Analysis 6.2) During the 55 months of follow‐up, 116/536 dorzolamide and 51/541 placebo treated patients had to discontinue therapy because of side effects. The nature of these side effects was not reported. Significantly more patients under dorzolamide had to discontinue due to side effects compared to placebo (OR 2.54, 95% CI 1.83 to 3.53).
6.2. Analysis.
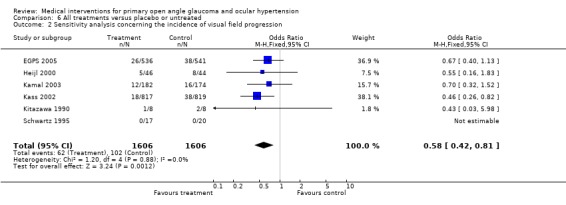
Comparison 6 All treatments versus placebo or untreated, Outcome 2 Sensitivity analysis concerning the incidence of visual field progression.
(c) Unspecified topical medication versus untreated control
The OHTS evaluated the effect of any topical medical therapy on the prevention of onset of visual field defects in OHT (Kass 2002). For meta‐analysis we included all trials comparing any topical IOP lowering medication to placebo or untreated controls (EGPS 2005; Epstein 1989; Heijl 2000; Kamal 2003; Kass 1989; Kitazawa 1990; Schulzer 1991; Schwartz 1995; Wishart 1992). With the exception of two trials (Kitazawa 1990; Schwartz 1995), all other trials were long‐term studies including 3503 participants with a follow up of 63 months.
* Primary outcome measure (Analysis 6.1; Analysis 6.2; Analysis 6.3) The OHTS (Kass 2002) found a significant reduction in the incidence of visual field defects in treated participants compared to the untreated control group (18/817, 2.2% versus 38/819, 4.6%; OR 0.48, 95% CI 0.28 to 0.82).
6.3. Analysis.
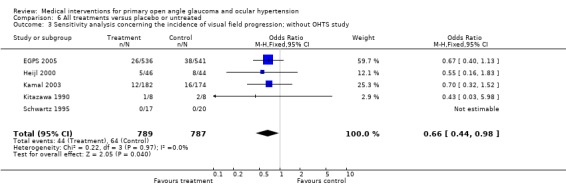
Comparison 6 All treatments versus placebo or untreated, Outcome 3 Sensitivity analysis concerning the incidence of visual field progression; without OHTS study.
Meta‐analysis of all available trials comparing any topical medical treatment to placebo or untreated controls similarly provided clear evidence of a positive treatment effect on visual field protection (OR 0.62, 95% CI 0.47 to 0.81). Sensitivity analysis (Analysis 6.2) ‐ excluding all trials scoring C on any aspect of trial quality and trials using the fellow eye as control ‐ did not change this significant protective effect of IOP lowering treatment (OR 0.58, 95% CI 0.42 to 0.81). Additional sensitivity analysis (Analysis 6.3), including only studies with an inclusion criterion of 22 mmHg (as opposed to the 24 mmHg of the OHTS) was still significant (OR 0.66, 95% CI 0.44 to 0.98). * Secondary outcome measures A comparison of drop‐outs due to unspecified topical therapy to those under placebo does not provide useful information and was thus not performed.
2. Comparisons of different topical treatments
(a) Comparison of one beta‐blocker versus another
(i) Timolol versus carteolol We identified three trials comparing timolol to carteolol that had patients with POAG (Fama 1996; Flammer 1992; Watson 2001).
* Primary outcome measure (Analysis 2.1) Two trials reported data on the progression of glaucomatous visual field defects in POAG (Flammer 1992; Watson 2001). For Flammer 1992, we extracted incidences of progression or improvement from Analysis 2.1, arbitrarily counting a slope of mean defect of plus or minus 0.3 dB or more as progression or improvement. There were fewer POAG patients with progressing visual field defects amongst those using timolol compared to carteolol treated patients (1/88, 3.4% timolol group, 9/83, 9.6% carteolol group, OR 0.18, 95% CI 0.05 to 0.62). It has to be emphasised that statistically significant heterogeneity was observed in this comparison. While the open label three‐year study of Watson 2001 described a striking difference between groups (12.5% progression with carteolol and no progression with timolol), Flammer 1992 reported in his one‐year study a much lower and more similar rate of visual field progression in both groups (1/37, 2.7% timolol group, 3/35, 8.1% carteolol group). Additionally, in the latter study, a similar proportion of patients showed improvement of visual field indices (3/37 timolol group and 2/35 carteolol group). It should be noted that this study excluded 22% of the patients after completion of the study as a result of some protocol violation or unreliable visual field exams. Together with the drop‐outs, this added to an attrition of 40%.
2.1. Analysis.
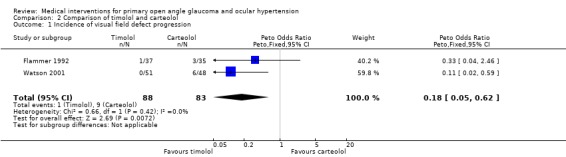
Comparison 2 Comparison of timolol and carteolol, Outcome 1 Incidence of visual field defect progression.
Two papers provided data concerning the change of visual field mean sensitivity, and data could be extracted from a figure of the third paper (Analysis 2.1) (Flammer 1992). None of the papers presented variances, which prevented a meta‐analysis. Combining the weighted means of the three studies resulted in no change of mean sensitivity for the timolol group (+0.10 dB) and an improvement of 1.44 dB for the carteolol group. This difference between groups disappeared after exclusion of the open‐label study of Watson 2001 (+0.05 dB versus +0.03 dB).
* Secondary outcome measures Only Flammer 1992 reported in detail the causes of drop‐outs. In the timolol group, seven patients had to stop treatment as a consequence of local and three patients due to systemic adverse effects, whereas in the carteolol group six patients had to discontinue due to systemic side effects.
(ii) Timolol versus levobunolol We included two trials (Geyer 1988; Novack 1989), both with a follow‐up time of four years. We included only the commercially available dose of 0.5% levobunolol in the analysis.
* Primary outcome measure (Analysis 3.1) Study sizes and incidence rates of progression were very inhomogeneous. The small study of Geyer 1988 included 50 POAG and one OHT patient, whereas the larger study of Novack 1989 (391 participants) comprised 60% OHT patients. This may in part explain why the study of Geyer 1988 reports visual field progression in 59% and 35% of the timolol and the levobunolol groups, whereas Novack 1989 detected only 18% and 9%. However, the difference between groups was similar in both studies. In a meta‐analysis there were significantly more patients with new or progressing visual field defects amongst those using timolol compared to levobunolol treated patients (32/141, 22.7% timolol group, 18/149, 12.1% levobunolol 0.5% group, OR 2.20, 95% CI 1.17 to 4.14).
3.1. Analysis.
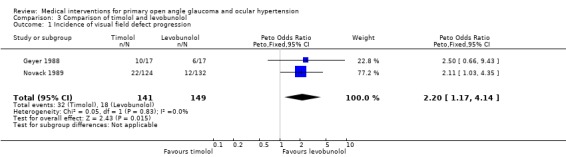
Comparison 3 Comparison of timolol and levobunolol, Outcome 1 Incidence of visual field defect progression.
* Secondary outcome measures (Analysis 3.2) There was no significant difference between treatments in the incidence of side effects leading to cessation of therapy (OR 0.80, 95% CI 0.34 to 1.87). Detailed information for drop‐outs was given only by Novack 1989. In the levobunolol group, six patients had to stop treatment as a consequence of local and four patients due to systemic adverse effects, whereas in the timolol group three patients discontinued due to local and seven patients due to systemic side effects.
3.2. Analysis.
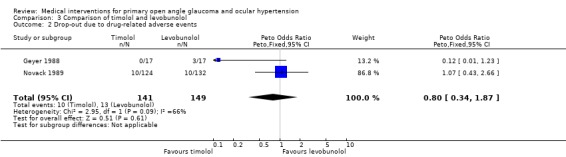
Comparison 3 Comparison of timolol and levobunolol, Outcome 2 Drop‐out due to drug‐related adverse events.
(iii) Timolol versus betaxolol Six trials were included comparing timolol and betaxolol (Collignon‐Brach 1992; Collignon‐Brach 1994; Drance 1998; Fama 1996; Kaiser 1994; Watson 2001). All trials had small samples (19 to 105 participants). Primarily OHT patients were included in Collignon‐Brach 1994, whereas the other trials comprised POAG patients only. Three trials were long‐term studies (Collignon‐Brach 1994; Kaiser 1994; Watson 2001) including 216 people with a median follow‐up time of 39 months.
* Primary outcome measure (Analysis 4.1) Watson 2001provided data on incidences of visual field progression. In this study 5/54, 9.3% betaxolol and 0/51 timolol patients developed visual field progression.
4.1. Analysis.
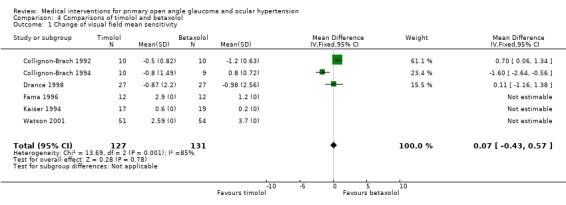
Comparison 4 Comparisons of timolol and betaxolol, Outcome 1 Change of visual field mean sensitivity.
All six trials reported data on the change of visual field mean sensitivity or mean defect (Analysis 4.1). The results of the six equivalent studies show highly variable effects, the mean differences of pre‐post changes of the primary outcome variable between the two treatments being 0.07 dB favouring timolol (CI ‐0.43 to +0.57).
A crude average of these effects (not accounting for the different sample sizes between studies) is 0.2 dB, with a standard deviation of 1.23. The standard deviations of the pre‐post differences are not available for three of the studies (Fama 1996; Kaiser 1994; Watson 2001). However, it is obvious that there are large differences in the standard deviations. In such a situation a meta‐analysis of the equivalent trials is considered not to be useful, particularly because the previous meta‐analysis of studies comparing beta‐blocker treatments with a control does not provide clear evidence for a positive treatment effect.
Combining the weighed mean differences of all six studies resulted in an improvement mean sensitivity for both groups (betaxolol group +1.43 dB, timolol group +1.11 dB). The lack of variances hindered statistically comparing the two treatments. Only three of the trials (Collignon‐Brach 1992; Collignon‐Brach 1994; Drance 1998) provided the standard error of the change. When including only these trials, mean change over time was slightly negative in both groups (betaxolol group ‐0.68 dB, timolol group ‐0.78 dB). This difference between groups was statistically not significant (WMD 0.07, 95% CI ‐0.43 to 0.57). The treatment effects showed statistically significant heterogeneity between studies.
In a sensitivity analysis, only two trials might have been included (Fama 1996; Kaiser 1994). Both did not report variances or standard errors of mean change, which prevented a meta‐analysis. The mean improvement of visual field sensitivity was 0.59 dB in the betaxolol group and 1.55 dB in the timolol group.
* Secondary outcome measures (Analysis 4.2) Collignon‐Brach 1992 did not report drop‐outs. The other five trials reported a total number of drop‐outs of 17/117 patients in the timolol group (eight local, four systemic and five unspecified side effects) and 8/121 patients in the betaxolol group (one local, five systemic and two unspecified side effects). The OR for side‐effect related drop‐out was 2.4 in favour of betaxolol (95% CI 1.04 to 5.53). In the sensitivity analysis, only two trials were includable (Fama 1996; Kaiser 1994) but only one (Fama 1996) reported a complete follow‐up of all 24 patients. Meta‐analysis was not performed on the one remaining trial.
4.2. Analysis.
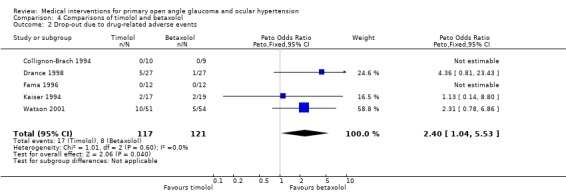
Comparison 4 Comparisons of timolol and betaxolol, Outcome 2 Drop‐out due to drug‐related adverse events.
(iv) Carteolol versus betaxolol This comparison was made by two trials (Fama 1996; Watson 2001).
* Primary outcome measure Watson 2001 reported that 6/48, 12.5% carteolol and 5/54, 9.3% betaxolol patients developed a visual field progression over three years. Patients under carteolol (+1.6 dB) or betaxolol (+1.2 dB) therapy displayed a similar change in visual field mean sensitivity over one year (Fama 1996). Meta‐analysis could not be performed due to the small number of studies and different outcome reporting.
* Secondary outcome measures In both trials, drop‐out rates due to systemic side effects were similar (no local side effect, 6/60, 10% carteolol patients and 5/66, 7.6% betaxolol patients). A statistical analysis was not possible due to the small number of trials and their heterogeneity.
(b) Comparison of one beta‐blocker versus other topical medication
(i) Timolol versus brimonidine We identified and included three trials. Leblanc 1998 and Schuman 1997 reported visual field progression and included slightly more than 50% POAG patients, the rest being OHT. Tsai 2005 included only POAG patients. This latter study did not include visual field endpoint data, but reported only on change in RNFL according to scanning laser polarimetry with fixed corneal compensation.
* Primary outcome measure (Analysis 5.1) The incidences of new visual field defects (OHT) or visual field progression (POAG) within one year (Leblanc 1998; Schuman 1997) were similar between groups (29/302, 9.6% timolol group, 22/369, 6.0% brimonidine group, OR 1.11, 95% CI 0.60 to 2.04). For Leblanc 1998, data of a three‐year extension have been published. This study comprised only 46 and 48 patients in the timolol and brimonidine groups. Compared to baseline, two patients in each group displayed a worsening of visual field, while one versus two patients appeared to improve in visual field.
5.1. Analysis.
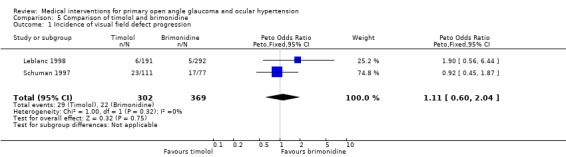
Comparison 5 Comparison of timolol and brimonidine, Outcome 1 Incidence of visual field defect progression.
Tsai 2005 included 44 patients, 39 completing the one‐year follow up. No visual field data were reported. The authors reported a significant reduction of RNFL thickness (ellipse average, superior average, temporal average, inferior average and nasal average) in the timolol group, but no change was detected in the brimonidine group. In group comparison the timolol group displayed significantly more reduction in RNFL thickness regarding the ellipse average, temporal average and inferior average.
* Secondary outcome measures (Analysis 5.2) Meta‐analysis of drop‐outs due to drug‐related adverse events showed clear evidence of better tolerance of timolol compared to brimonidine (OR 0.21, 95% CI 0.14 to 0.31).
5.2. Analysis.
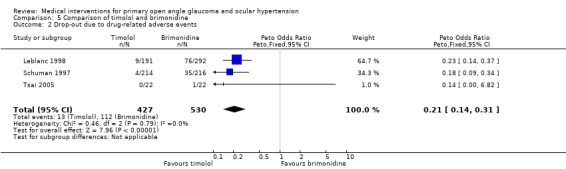
Comparison 5 Comparison of timolol and brimonidine, Outcome 2 Drop‐out due to drug‐related adverse events.
(ii) Timolol versus pilocarpine We included three trials comparing timolol versus pilocarpine (Drance 1998; Sponsel 1987; Vogel 1992). All were short‐term studies with a weighted mean follow up of 32 months.
* Primary outcome measure The change in mean sensitivity of visual field was ‐0.01 dB for the timolol group and ‐1.07 dB for the pilocarpine group (Drance 1998; Vogel 1992). Both studies were lacking the standard deviation. Sponsel 1987 analysed the progression rate of visual field score based on both Goldmann kinetic and Friedmann static suprathreshold perimetry. Patients receiving timolol lost visual field at a mean rate of 0.46 units per month (SE 0.05) and those receiving pilocarpine lost 0.92 units per month (SE 0.11). All three trials were open label and additionally it was difficult to rule out an effect of miosis on the visual field performance. A meta‐analysis was not possible for this comparison.
* Secondary outcome measures Only Drance 1998 presented the rate of participants being withdrawn due to drug‐related side effects (five timolol group, all due to unspecified side effects; six pilocarpine group, three due to local and three due to unspecified side effects).
(iii) Timolol versus epinephrine * Primary outcome measure In Alexander 1988, 0/24 timolol and 1/23 epinephrine treated OHT patients developed a visual field defect over 33 months.
* Secondary outcome measures Five patients on timolol and six patients on epinephrine were withdrawn due to adverse events. The authors did not specify the types of adverse events. (iv) Betaxolol versus pilocarpine Data for this comparison were provided from one open label trial (Drance 1998).
* Primary outcome measure The mean change of mean sensitivity of visual fields amounted to 0.98 dB in betaxolol and 0.83 dB in pilocarpine treated patients.
* Secondary outcome measures One patient on betaxolol and six patients on epinephrine were withdrawn due to adverse events. For three of these patients on pilocarpine, the reason for withdrawal was local adverse events; for all other patients the reasons were not specified.
See: Table 8 for additional information.
2. Summary table for analyses of outcomes.
| Comparison | POAG | OHT | POAG & OHT | Drop‐out due to AE |
| Beta‐blockers vs. placebo or untreated | 0.67 (0.45 to 1.00; n=935) | 1.24 (0.59 to 2.58; n=503) | ||
| Longterm trials | 0.67 (0.45 to 1.01; n=882) | |||
| Sensitivity analysis | 0.64 (0.34 to 1.19; n=499) | |||
| Timolol vs. placebo or untreated | 0.66 (0.41 to 1.05; n=579) | 2.48 (0.61 to 10.10; n=147) | ||
| Timolo vs. carteolol | 0.18 (0.05 to 0.62; n=171) + | |||
| Timolol vs. levobunolol | 2.20 (1.17 to 4.14; n=290) # | 0.80 (0.34 to 1.87; n=290) | ||
| Timolol vs. betaxolol | 0.07dB (‐0.43dB to 0.57dB; n=158) $ | 2.40 (1.04 to 5.53; n=238) § | ||
| Timolol vs. brimonidine | 1.11 (0.60 to 2.04; n=671) | 0.21 (0.14 to 0.31; n=957) ++ | ||
| All treatment vs. placebo or untreated | 0.62 (0.47 to 0.81; n=3648) | |||
| Sensitivity analysis | 0.58 (0.42 to 0.81; n=3212) | |||
| Sensitivity analysis without OHTS | 0.66 (0.44 to 0.98; n=1576) | |||
| + favours timolol; # favours levobunolol; | $ 14 participants with OHT included | § favours betaxolol | ++ favours timolol |
Discussion
The large therapeutic trials published during the past few years have helped with establishing evidence of the principle for the visual field protection in OHT and POAG by lowering IOP (EMGT 1999; Kass 2002). This represents an advance in knowledge since 1993, when Rossetti had concluded that IOP reduction had not been proven to prevent development of glaucoma (Rossetti 1993). The newer studies, however, did not support any deduction as to which (or whether possibly all) of the drugs ‐ or possibly laser treatment ‐ can be attributed to this beneficial effect. Due to its study design, the EMGT 1999 even left the question open, whether betaxolol or laser trabeculoplasty or both were responsible for the protective effect, while the OHTS (Kass 2002) did not allow any conclusion for a specific drug or class of drugs.
The fact that over 95% of all therapeutic trials dealing with glaucoma use the surrogate endpoint IOP instead of visual field progression reflects two assumptions: 1) the necessary study duration and cost of an IOP‐related trial are very much lower compared to a trial targeting progression; and 2) reduction of IOP as the main risk factor for glaucoma will uniformly reduce the risk, regardless of the specific drug used. While the first assumption is evidently correct, the second assumption should be subjected to scientific investigation. In other fields of medicine, a treatment mainly based on a surrogate parameter probably would not be widely accepted. With treatment of systemic arterial hypertension, for example, the primary outcomes of relevant trials are mortality and morbidity. A recent Cochrane review of arterial hypertension (Wiysonge 2006) has clearly demonstrated that different classes of blood pressure lowering drugs have different protective effects regarding overall mortality, as well as stroke and coronary heart disease morbidity. In this meta‐analysis, beta‐blockers had no effect on overall mortality compared to placebo, but a significant positive effect on stroke morbidity. On the other hand, calcium‐channel blockers (CCB) significantly reduced total mortality and also had a significantly stronger effect reducing cardiovascular morbidity compared to beta‐blockers, when used as treatment for arterial hypertension.
Regarding glaucoma, the basic assumption that IOP lowering of any kind will result in a similar protection of the optic nerve has never been proven. Theoretically there might be a difference in optic nerve head protection or other ocular or systemic morbidity between different classes of drugs, despite similar reduction of IOP. Our systematic review addresses three main questions: firstly, whether there is scientific evidence of visual field protection by unspecified topical medical therapy in OHT (this is in fact already known) and POAG; secondly, whether there is such evidence for any of the currently used topical IOP lowering drugs or classes of drugs; and thirdly, whether different drugs or classes of drugs differ in their beneficial effect.
The meta‐analysis of all trials testing against placebo or untreated controls ‐ irrespective of the substances used ‐ confirmed the finding of the OHTS that IOP lowering reduces the incidence of glaucomatous visual field defects in OHT. The OR was 0.62 with a 95% CI of 0.47 to 0.81, being highly significant. This result, however, is of limited practical value, due to pooling of different therapies. Of the treated participants of this meta‐analysis, 26% had received beta‐blockers (16% timolol), 29% dorzolamide and 55% unspecified therapy (including all commercially available drugs). All trials comparing medical therapy with an untreated or placebo treated control group exclusively included OHT participants. Our results also confirmed those of a recent meta‐analysis (Maier 2005), that reported a hazard ratio of 0.56 (95% CI 0.39 to 0.81) for unspecified medical therapy in OHT participants. One result of that meta‐analysis was that this effect lost significance after excluding the OHTS (Kass 2002). As a consequence of this, the authors stated that the overall beneficial effect could only be safely assumed in patients with an IOP of 24 mmHg or more, which was an inclusion criterion of the OHTS. Our meta‐analysis provides some additional evidence in remaining significant, even after exclusion of the OHTS (OR 0.66, 95% CI 0.44 to 0.98). We may thus now safely assume a protective effect in patients with an IOP of 22 mmHg or more instead of 24 mmHg or more.
There are some differences between this review and the meta‐analysis by Maier 2005. Unlike Maier 2005, we included comparative trials and had the goal of not only evaluating whether IOP lowering effectively protects from progression, but also to look for evidence that currently available drugs might be different in this respect. On the other hand, we concentrated our efforts on POAG with a baseline IOP above 21 mmHg and on topical medical therapy. There is another difference in the endpoints that have been evaluated. While Maier 2005 compared the effectiveness in prevention of visual field loss or deterioration of optic disc, we evaluated visual field loss but not change of the appearance of the optic disc. The basis of this decision has been that only loss of visual field is directly relevant to the patient, while morphologic change of the optic disc has to be deemed a (well‐accepted) surrogate parameter. We were interested in the visual field protective effect of IOP lowering treatment, which to our knowledge has never been evaluated in a meta‐analysis before. On the other hand, we included the reduction of nerve fibre loss or progressive damage to the optic disc according to objective measurement as additional outcome parameters for separate analyses. The reason for this decision was that these quantitative parameters theoretically allow for valid statistical conclusions in a shorter time with fewer participants. When designing this review, we had hoped that these advantages might have stimulated smaller placebo or active controlled trials.
For the first time, this systematic review provides at least weak evidence of visual field protection for one class of substances. At borderline significance, the class of beta‐blockers reduced the onset of glaucoma in OHT, when compared to placebo or untreated controls (OR 0.67, 95% CI 0.45 to 1.00). This evidence is based on patients on timolol (61%) or betaxolol. It should, however, be noted that in a sensitivity analysis (after exclusion of studies with questionable trial quality) the effect of beta‐blockers lost statistical significance (OR 0.64, 95% CI 0.34 to 1.19). Furthermore, we want to emphasize that the fact that this is the only class of drugs for which this evidence is available does not mean that they are more effective than the others.
We were unable to provide evidence for any single antiglaucomatous drug to prevent the onset of glaucoma in OHT. Timolol, the most frequently tested drug in this review, was lacking a significant effect (OR 0.66, 95% CI 0.41 to 1.05) although the trend was very similar to that of the whole class of beta‐blockers. While dorzolamide and betaxolol clearly failed to demonstrate evidence of a protective effect, both drugs exhibited a very similar trend. No trial comparing any of the other drugs to placebo or untreated controls could be identified. Regarding the trial investigating the effect of dorzolamide (EGPS 2005), there has been extensive discussions about possible reasons for the failure of this trial, which had shown an unusually pronounced placebo effect (Quigley 2005). This failure might have been caused in part by the commitment to dorzolamide therapy regardless of IOP lowering effect, a major regression to the mean, and selective loss to follow up of persons with higher IOP. The counter arguments in this discussion, however, should not be ignored, namely that regression to the mean would have exerted a similar IOP lowering effect on both study arms and the fact that the EGPS data show a trend of 10% risk reduction per 1 mmHg IOP lowering which is in line with other published data. It has to be emphasised that these failures to prove effectiveness should not be interpreted as proof of absence of any effect, but may as well have been a consequence of biologic variability, together with lacking power of the included trials to detect small differences.
It is noteworthy that this evidence completely arises from studies investigating OHT patients. None of the studies continued to follow the patients under their original treatment once the first visual field defects had occurred. At present there is no evidence that topical medical IOP lowering therapy reduces progression of POAG after the onset of the first visual field defects. While it may be assumed that a drug being able to avoid or postpone the onset of a disease would offer advantages for the treatment following that onset, this might not necessarily be true. The clinical impression that treatment might be more effective in the early stages has often been expressed. This is supported by some trials not the subject of this review, which indicate that IOP lowering therapy reduces progression of visual fields and optic disc appearance in patients with established POAG and NTG (CNTGS 1998; EMGT 1999).
Some drugs of other classes have been compared to timolol, such as brimonidine, pilocarpine and epinephrine. The fact that all of these comparisons failed to prove superiority in visual field protection of any of the tested substances should, however, not be interpreted as evidence of equivalence. None of these studies had been adequately powered for testing equivalence of visual field protection. Only one study indicated superiority of brimonidine over timolol (Tsai 2005) in protection from progressive RNFL damage. While this study was well conducted, it only included 39 participants in both arms and study duration was only 12 months. Study size and duration might, however, be adequate for this type of quantitative comparison of objectively measured parameters, instead of evaluating differences in incidences. A direct comparison of brimonidine to placebo or untreated controls is missing.
A number of trials have compared the different beta‐blockers timolol, levobunolol, carteolol and betaxolol. Considering the failure for each of these drugs to clearly demonstrate evidence of a positive treatment effect, these comparisons are questionable. Nevertheless levobunolol appeared to demonstrate some evidence for better visual field protection than timolol (Geyer 1988; Novack 1989). This result should be interpreted with caution, since it is based on only two trials; one being rather small (17 participants in each treatment group), it should also be noted that a direct evaluation of the treatment effect of levobunolol compared to untreated control resulted in similar change in visual field mean deviation (Ravalico 1994). Carteolol on the other hand, when compared to timolol, resulted in a significantly worse visual field outcome. Again this was the result of only two small studies, one having an open‐label design.
In the past, other studies not included in this review have provided evidence of potential harmfulness of topically applied beta‐blockers. Elderly people undergoing treatment with beta‐blockers may experience respiratory and cardiovascular side effects (Diggory 1998). In this study five of 20 patients on timolol 0.5% had to change to betaxolol because of impaired spirometry, three of them being symptomatic. Also three of 20 patients on betaxolol had to change treatment due to symptomatic decrease in respiratory function. Similarly Waldock 2000 reported a 15% reduction in spirometric parameters in five of 33 patients treated with timolol, although only one of these patients had an increase in shortness of breath. In this latter study, seven of 34 patients on brimonidine complained about drowsiness. Compared to these figures, no patient on latanoprost reported any systemic side effect. A weakness of this study is that both the observers and the participants were unmasked, and the latter were aware of the accompanying manufacturers' leaflet outlining the potential side effects associated with their treatment. Although Diggory 1998 was randomised and double masked, this avoids only a bias in the comparison between the two treatment arms: timolol and betaxolol (which was negative). The study design, with the patients aware that the medication has a potential negative effect on breathing, may still have influenced reporting of symptoms and performance in spirometry in both groups. Another unmasked study with a laser treated control group has concluded that beta‐blocker‐ induced increase in bronchial reactivity may be partially irreversible (Gandolfi 2005).
These reports contrast the relatively few reported systemic side effects out of the placebo‐controlled trials included in this review. Only one small study comparing timolol to placebo did explicitly mention the number of systemic side effects leading to cessation of therapy (2/17 versus 1/20) (Schwartz 1995). Another larger study did not find evidence for increased systemic side effects leading to cessation of therapy for betaxolol (3/182) compared to placebo (4/174) (Kamal 2003). Due to limited data reporting of most trials, a meta‐analysis of systemic side effects was not performed.
The comparisons of drop‐outs due to adverse events have to be interpreted with caution, since the primary goal of this review has been treatment efficacy. Studies not reporting data regarding treatment efficacy or with a duration below one year have not been included. As a result of this, safety data in this review represent only a smaller part of the potentially available body of evidence. Nonetheless, within the group of beta‐blockers, the frequency of side‐effect related drop‐outs was significantly larger for timolol compared to betaxolol. This was, however, mainly caused by the increased frequency of local side effects in one of three included studies (Watson 2001), while a second study did not divide the side effects into local and systemic ones (Drance 1998). Contrary to this finding, when compared to brimonidine, patients under timolol had a significantly lower risk of adverse events, necessitating cessation of treatment. This latter comparison was highly significant, with an OR of 0.21 (95% CI 0.14 to 0.31). Only one of the three studies included in this analysis divided the side effects into local and systemic (Schuman 1997). This study reported five times more systemic side effects with brimonidine compared to timolol (10 versus two).
Authors' conclusions
Implications for practice.
The results of this review support the current practice of IOP lowering treatment of OHT. For the first time we were able to demonstrate at least some evidence for a visual field protective effect of the class of beta‐blockers. We were, however, not successful in demonstrating clear evidence of a beneficial effect for any drug individually.
While direct comparisons of prostaglandins or brimonidine to placebo are missing and the published comparison of dorzolamide to placebo failed to prove a protective effect, most of these drugs have also been used by people participating in the OHTS. It is still impossible to tell which of these drugs have contributed to the positive results of that study. The decision to treat a patient or not, as well as the decision with which drug to start treatment, should remain individualised, considering the amount of damage, the level of IOP, age and other risk factors.
Implications for research.
For most of the newer IOP lowering substances, the body of evidence regarding visual field preservation is very weak if not absent. There is especially no evidence of a protective effect of prostaglandins. Further efforts should be directed to proving visual field preservation for these drugs.
Additionally, further research on the protective effect of topical medical therapy in participants with early stage POAG is warranted to provide evidence that therapy will exert a continued protective effect after the onset of glaucomatous optic neuropathy. One might argue that placebo‐controlled research in established POAG is unethical, since the larger studies and meta‐analyses like this one have proven the effectiveness of medical therapy. On the other hand, the body of evidence is based completely on trials including only participants with OHT. This evidence may not be safely extrapolated to patients suffering from POAG. Objective and quantitative measurement reduces the risks for patients in a placebo‐controlled trial, by reducing both the number of patients and duration of placebo treatment. This may warrant reconsidering the ethical aspects of placebo‐controlled shorter‐term (one to two years) trials of patients with just incipient visual field defects.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Eyes and Vision Group created and ran the electronic search strategies. We thank Marie Diener‐West, Scott Fraser and Roberta Scherer for their comments on this review, and Roger Hitchings for his comments on the protocol.
Appendices
Appendix 1. CENTRAL search strategy used for Issue 2, 2007
#1 MeSH descriptor Glaucoma, Open‐Angle #2 open near angle near glaucoma* #3 POAG or OHT #4 ocular and pressure #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Adrenergic beta‐Antagonists #7 MeSH descriptor Timolol #8 timolol* #9 MeSH descriptor Metipranolol #10 metipranolol* #11 MeSH descriptor Carteolol #12 carteolol* #13 MeSH descriptor Levobunolol #14 levobunolol* #15 MeSH descriptor Betaxolol #16 betaxolol* #17 MeSH descriptor Carbonic Anhydrase Inhibitors #18 carbonic near anhydrase near inhibitor* #19 azetazolamide* #20 brinzolamide* #21 dorzolamide* #22 MeSH descriptor Prostaglandins, Synthetic #23 latanoprost* #24 travoprost* #25 bimatoprost* #26 unoprostone* #27 brimonidine* #28 MeSH descriptor Antihypertensive Agents #29 MeSH descriptor Pilocarpine #30 pilocarpin* #31 MeSH descriptor Epinephrine #32 dipivefrin* #33 (treat* or therap* or intervention) near (drug* or medic* or pharmacologic*) #34 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33)
Appendix 2. MEDLINE search strategy used on OVID up to May 2007
1. exp clinical trial/ [publication type] 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp glaucoma open angle/ 14. exp ocular hypertension/ 15. (open adj2 angle adj2 glaucoma$).tw. 16. (POAG or OHT).tw. 17. (((increas$ or elevat$ or high$) adj3 (ocular or intra‐ocular)) and pressure).tw. 18. or/13‐17 19. exp adrenergic beta antagonists/ 20. exp timolol/ 21. timolol$.tw. 22. exp metipranolol/ 23. metipranolol$.tw. 24. exp carteolol/ 25. carteolol$.tw. 26. exp levobunolol/ 27. levobunolol$.tw. 28. exp betaxolol/ 29. betaxolol$.tw. 30. exp carbonic anhydrase inhibitors/ 31. (carbonic adj2 anhydrase adj2 inhibitor$).tw. 32. azetazolamide$.tw. 33. brinzolamide$.tw. 34. dorzolamide$.tw. 35. exp prostaglandin analogues/ 36. latanoprost$.tw. 37. travoprost$.tw. 38. bimatoprost$.tw. 39. unoprostone$.tw. 40. brimonidine$.tw. 41. exp antihypertensive agents/ 42. exp pilocarpine/ 43. pilocarpin$.tw. 44. exp epinephrine/ 45. epinephrin$.tw. 46. dipivefrin$.tw. 47. ((drug$ or medic$ or pharmacologic$) adj3 (treat$ or therap$ or intervent$)).tw. 48. or/19‐47 49. 18 and 48 50. 12 and 49
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE search strategy used on OVID up to May 2007
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp open angle glaucoma/ 34. exp ocular hypertension/ 35. (open adj2 angle adj2 glaucoma$).tw. 36. (POAG or OHT).tw. 37. (((increas$ or elevat$ or high$) adj3 (ocular or intra‐ocular)) and pressure).tw. 38. or/33‐37 39. exp beta adrenergic receptor blocking agent/ 40. exp timolol/ 41. timolol$.tw. 42. exp metipranolol/ 43. metipranolol$.tw. 44. exp carteolol/ 45. carteolol$.tw. 46. exp levobunolol/ 47. levobunolol$.tw. 48. exp betaxolol/ 49. betaxolol$.tw. 50. exp carbonate dehydratase inhibitor/ 51. (carbonic adj2 anhydrase adj2 inhibitor$).tw. 52. azetazolamide$.tw. 53. brinzolamide$.tw. 54. dorzolamide$.tw. 55. exp latanoprost/ 56. latanoprost$.tw. 57. exp travoprost/ 58. travoprost$.tw. 59. exp bimatoprost/ 60. bimatoprost$.tw. 61. exp unoprostone isopropyl ester/ 62. unoprostone$.tw. 63. exp brimonidine/ 64. brimonidine$.tw. 65. exp antihypertensive agents/ 66. exp pilocarpine/ 67. pilocarpin$.tw. 68. exp epinephrine/ 69. epinephrin$.tw. 70. dipivefrin$.tw. 71. ((drug$ or medic$ or pharmacologic$) adj3 (treat$ or therap$ or intervent$)).tw. 72. or/39‐71 73. 38 and 72 74. 32 and 73
Data and analyses
Comparison 1. Beta‐blockers versus placebo or untreated.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of visual field defect progression | 8 | 935 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.45, 1.00] |
| 1.1 Timolol versus placebo or untreated | 7 | 579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.41, 1.05] |
| 1.2 Betaxolol versus placebo or untreated | 1 | 356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.32, 1.51] |
| 2 Drop‐out due to drug‐related adverse events | 4 | 503 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.59, 2.58] |
| 2.1 Timolol versus placebo | 3 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.48 [0.61, 10.10] |
| 2.2 Betaxolol versus placebo | 1 | 356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.40, 2.26] |
| 3 Sensitivity analysis concerning the incidence of visual field defect progression | 4 | 499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.34, 1.19] |
| 3.1 Timolol versus placebo or untreated | 3 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.19, 1.54] |
| 3.2 Betaxolol versus placebo or untreated | 1 | 356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.32, 1.51] |
| 4 Long‐term studies concerning the incidence of visual field progression | 6 | 882 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.45, 1.01] |
Comparison 2. Comparison of timolol and carteolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of visual field defect progression | 2 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.05, 0.62] |
Comparison 3. Comparison of timolol and levobunolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of visual field defect progression | 2 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.20 [1.17, 4.14] |
| 2 Drop‐out due to drug‐related adverse events | 2 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.34, 1.87] |
Comparison 4. Comparisons of timolol and betaxolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of visual field mean sensitivity | 6 | 258 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.43, 0.57] |
| 2 Drop‐out due to drug‐related adverse events | 5 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.04, 5.53] |
Comparison 5. Comparison of timolol and brimonidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of visual field defect progression | 2 | 671 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.60, 2.04] |
| 2 Drop‐out due to drug‐related adverse events | 3 | 957 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.14, 0.31] |
Comparison 6. All treatments versus placebo or untreated.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of visual field defect progression | 10 | 3648 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.47, 0.81] |
| 2 Sensitivity analysis concerning the incidence of visual field progression | 6 | 3212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.81] |
| 3 Sensitivity analysis concerning the incidence of visual field progression; without OHTS study | 5 | 1576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexander 1988.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 47 participants with newly diagnosed OAG or OHT. 50% of participants included with both eyes (mean of both eyes). 6/24 timolol and 8/23 epinephrine patients were African American. Inclusion criteria: normal Goldmann visual fields and either 1) IOP between 25 and 29 mmHg and wide disc cupping or cupping asymmetry, or 2) IOP between 30 and 35 mmHg with normal optic discs. Exclusion criteria: ocular inflammation, recent ocular surgery or trauma, previous glaucoma therapy. | |
| Interventions | Timolol 0.5% twice daily. Epinephrine 1% twice daily | |
| Outcomes | Incidence of optic cup enlargement or disc haemorrhage (stereophoto). Incidence of reproducible visual field defect. Incidence of failure to achieve an IOP reduction of 20%. | |
| Notes | Mean follow up 33 months. Failures were analysed on patient basis. No drop‐outs, but 18 IOP failures: 7 timolol group, 11 epinephrine group. 5 patients originally assigned to timolol group (1 topical and 4 systemic side effects), and 4 patients of the epinephrine group (local or systemic side effects) switched the group. For failure analysis they were analysed in their original group (intent to treat). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Collignon‐Brach 1992.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 20 people with POAG. Racial constitution is not reported. Inclusion criteria: Untreated IOP of at least 20 mmHg in at least one eye. Exclusion criteria: concomitant ocular or systemic disease. | |
| Interventions | Timolol 0.5% twice daily. Betaxolol 0.5% twice daily. | |
| Outcomes | Change of visual field mean sensitivity, IOP. | |
| Notes | 2 years follow up Drop‐out rate is not reported. Authors do not state whether the visual field data are those from one of the eyes, or they did use the mean values of both eyes for analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Collignon‐Brach 1994.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 19 people with OHT or POAG (n=5). Racial constitution is not reported. Inclusion criteria: untreated IOP of at least 20 mmHg in at least one eye. Exclusion criteria: any other significant ocular pathology. | |
| Interventions | Timolol 0.5& twice daily. Betaxolol 0.5% twice daily. | |
| Outcomes | Change of visual field mean sensitivity. IOP. | |
| Notes | 4 years follow up. Drop‐out rate was 4 of 19 patients for both groups together, not specified for the groups separately. The authors state that there was no drop‐out due to drug‐related adverse events. Visual field data were averaged for both eyes of each patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Drance 1998.
| Methods | RCT. Partially masked. Active controlled. | |
| Participants | 68 people with POAG, proportion of PEX and PDS unknown. 7% African American. inclusion criteria: IOP equal or above 24 mmHg, disc and visual field abnormality, PEX and PG allowed. Exclusion criteria: history of ocular trauma, uveitis, inflammatory disease and recent ocular infection, intraocular surgery within 6 months and laser trabeculoplasty within 3 months, systemic glucocorticoids and medication that may affect IOP. | |
| Interventions | Timolol 0.5% BID. Betaxolol 0.5% BID. Pilocarpine 2% 4 times daily. | |
| Outcomes | Change in visual field mean defect. IOP. | |
| Notes | 2 years follow up. Timolol and betaxolol masked, pilocarpine open label. No difference between groups. Drop‐outs due to drug‐related adverse events: 5 timolol, 1 betaxolol, 3 pilocarpine (unspecified whether local or systemic side effects). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EGPS 2005.
| Methods | RCT. Double‐masked. Placebo controlled. Multicenter (18). | |
| Participants | 1081 people with OHT. 99.9% caucasian. Inclusion criteria: IOP between 22 and 29 mmHg, 2 normal and reliable visual fields, normal optic discs (stereophoto), PEX allowed (below 2%), normal optic discs in both eyes (stereophoto), open angle, PEX and PDS allowed. Exclusion criteria: visual acuity below 20/40, previous intraocular surgery, previous laser trabeculoplasty within 3 months, secondary causes of elevated IOP. | |
| Interventions | Dorzolamide 2% 3 times daily. Placebo. | |
| Outcomes | Incidence of reproducible visual field defects. Incidence of reproducible optic disc changes (stereophoto). | |
| Notes | Median follow up 55.3 months. 338 drop‐outs: 192 dorzolamide group (116 adverse events), 146 placebo group (51 adverse events). PEX and pigment dispersion in both groups 1 to 2 percent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Epstein 1989.
| Methods | RCT. Open label. | |
| Participants | 107 participants with OHT. 16/107 patients had only 1 eye included. Analysis was always based on patient not eye, if both eyes were included. 6/53 patients in timolol group and 15/54 untreated were African American. Inclusion criteria: IOP between 22 and 28 mmHg, normal Goldmann visual fields, normal optic disc. Exclusion criteria: previous ocular surgery, progressive retinopathy. | |
| Interventions | Timolol 0.5% twice daily. No treatment. | |
| Outcomes | Incidence of IOP above 32 mmHg on two separate occasions. Incidence of optic cup enlargement (masked stereophoto). Incidence of reproducible visual field progression on Goldmann perimeter or of progressive damage on computerized static perimetry. | |
| Notes | Mean follow up was 56 months in timolol group and 51 months in untreated group. 23 drop‐outs: 11 timolol group and 12 untreated group (timolol: 1 local and 9 systemic adverse events). Additionally 5 patients failed the IOP criterion (all untreated) and 5 patients were "escape hatched" (3 timolol and 2 untreated) because the examiner believed that the patient's vision was at risk. During the trial period computerized static perimetry was introduced and additionally used. Some of the patients might have had early POAG instead of OHT when analysed with computer perimetry. 2/4 timolol treated patients who developed visual field defects did so after discontinuation of treatment for adverse reaction or pregnancy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fama 1996.
| Methods | RCT. Active controlled. | |
| Participants | 36 people with OAG. Racial constitution is not reported. Inclusion criteria: unknown whether PEX or PDS were included, IOP above 23 mmHg without therapy, initial glaucomatous visual field defects, clinical signs of glaucomatous optic nerve damage. Exclusion criteria: active ocular infection or inflammation, acute or progressive retinal disorder, current use of systemic beta‐blocker. | |
| Interventions | Timolol 0.5% twice daily. Betaxolol 0.5% twice daily. Carteolol 2% twice daily. | |
| Outcomes | Change of visual field mean sensitivity. IOP. Corneal sensitivity. Tear production. | |
| Notes | 12 months follow up. No drop‐outs occurred. The authors do not state whether they analysed the mean sensitivities of both eyes of each patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Flammer 1992.
| Methods | RCT. Double‐masked. Active controlled. | |
| Participants | 120 patients with OAG (72 were evaluated). Both eyes of all patients included, analysis based on patient. Racial constitution is not reported. Inclusion criteria: IOP greater than 21 mmHg, visual field damage (MD greater 2 dB or CLV greater 7 dB). Exclusion criteria: visual acuity below 0.8, pupil diameter below 2.5 mm, any other type of ocular disease, diabetes mellitus, systemic hypotension, systemic drugs that might influence the IOP. | |
| Interventions | Timolol 0.5% twice daily. Carteolol 2% twice daily. | |
| Outcomes | Change of visual field mean sensitivity. Incidence of glaucomatous progression. IOP. | |
| Notes | 12 months follow up. Of the originally included 120 patients, 21 did not complete it (1 patient in carteolol group systemic adverse event). Additionally a further 27 patients were excluded after the end of follow up, because they did not match the protocol or had unreliable visual field exams. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Geyer 1988.
| Methods | RCT. Double‐masked. Active controlled. | |
| Participants | 51 participants with OAG or OHT (1 patient). Racial constitution is not reported. Inclusion criteria: IOP 23 mmHg or higher. Exclusion criteria: secondary glaucoma, angle closure glaucoma, c/d ratio of more than 0.7 in either eye, aphakia, chronic ocular inflammation, systemic beta‐blockers. | |
| Interventions | Timolol 0.5% twice daily. Levobunolol 0.5% twice daily. Levobunolol 1% twice daily. | |
| Outcomes | IOP control. Cup/disc‐ratio (direct ophthalmoscopy). Incidence of glaucomatous visual field defect (Goldmann). | |
| Notes | Allocation concealment deemed likely, based on double‐masked design. 4 years follow up. 14 drop‐outs: 6 levobunolol 0.5% (3 drug‐related adverse events), 6 levobunolol 1% (1 drug‐related adverse effect), 2 timolol (not drug‐related). Between 41% and 53% of the participants terminated their participation due to inadequate IOP control, resulting in successful completion of the study by only 23%‐24% and 35% of patients in the 3 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Heijl 2000.
| Methods | RCT. Double‐masked. Placebo controlled. | |
| Participants | 90 people with OHT, 37% (timolol group) and 23% (placebo group) had PEX or PDS. Racial constitution is not reported. Inclusion criteria: mean untreated IOP of 22 mmHg or more, normal visual fields (Competer and Goldmann), open angles by gonioscopy, one of the following risk factors: suspicious disc, positive family history, PEX or pigment dispersion syndrome, diabetes, mean IOP of at least 27 mmHg without additional risk factors. Exclusion criteria: mean untreated IOP of 35 mmHg or above, medication known to affect IOP, history of intraocular surgery, visual acuity of 0.3 or less. | |
| Interventions | Timolol 0,5% twice daily. Placebo. | |
| Outcomes | Incidence of glaucomatous visual field defect. | |
| Notes | 10 years follow up, for better comparability with the other trials data concerning 5 years follow up were used for meta‐analysis. 49 drop‐outs within 10 years (26 timolol: 2 due to adverse reaction; 23 placebo: 1 due to adverse reaction). 33 drop‐outs within 5 years (19 timolol: 2 due to adverse reaction; 14 placebo: not related to adverse reactions). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kaiser 1994.
| Methods | RCT. Double‐masked until 18 months. Active controlled. | |
| Participants | 44 participants, all Caucasian. Inclusion criteria: POAG with IOP at least 24 mmHg, early glaucomatous field defect. Exclusion criteria: diabetes mellitus, other local treatment or systemic medication with beta‐blockers, previous laser treatment or glaucoma surgery. | |
| Interventions | Timolol 0.5% twice daily. Betaxolol 0.5% twice daily. | |
| Outcomes | Change of visual field mean sensitivity and mean defect. | |
| Notes | Allocation concealment deemed likely, based on double‐masked design. 4 years follow up. Double‐masked until 18 months, thereafter open label. 15 drop‐outs (8 timolol: 1 local and 1 systemic side effect; 7 betaxolol: 2 local side effects). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kamal 2003.
| Methods | RCT. Double‐masked. Placebo controlled. | |
| Participants | 356 participants with OHT. Racial constitution is not reported. Inclusion criteria: IOP between 22 and 35 mmHg, normal visual field. Exclusion criteria: systemic beta‐blockers, diabetes mellitus. | |
| Interventions | Betaxolol 0.5% twice daily. Placebo. | |
| Outcomes | Incidence of reproducible glaucomatous visual field defect. | |
| Notes | Median follow‐up time 60 months (for those completing the trial). 102 drop‐outs: 48 betaxolol group (8 local and 3 systemic adverse effects), 53 placebo group (7 local, and 4 systemic adverse effects). Intent‐to‐treat analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kass 1989.
| Methods | RCT. Double‐masked. Placebo controlled. | |
| Participants | 62 participants with OHT (6% with PEX). Fellow eye served as control. 40% of the participants were African American. Inclusion criteria: IOP between 24 and 35 mmHg, difference in baseline IOP between right and left eyes less than or equal to 3 mmHg, age 40 years or greater, open angles. Exclusion criteria: visual acuity below 20/50, previous intraocular surgery or laser, systemic medication that alters IOP | |
| Interventions | Timolol 0.25% or 0.5% twice daily. Untreated control (contralateral eye). | |
| Outcomes | Incidence of reproducible glaucomatous visual field defect (Goldmann kinetic and static perimetry with Humphrey 30‐2 and Octopus 32). Incidence of progressive optic disc cupping (stereophoto). | |
| Notes | Mean follow up 56.1 months. 19 drop‐outs: 5 systemic adverse events caused by timolol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kass 2002.
| Methods | RCT. Observer masked. Multicenter. | |
| Participants | 1636 participants with OHT. 25% African American. Inclusion criteria: IOP between 24 and 32 mmHg in one eye and between 21 and 32 mmHg in the other eye, 2 normal and reliable visual fields, normal optic discs on stereophotographs. Exclusion criteria: visual acuity below 20/40, previous ocular surgery (uncomplicated cataract surgery allowed), diabetic retinopathy. | |
| Interventions | Any topical medical antiglaucomatous treatment. No treatment. | |
| Outcomes | Incidence of reproducible visual field defects or reproducible deterioration of optic discs attributable to POAG. IOP. | |
| Notes | Median follow up 6 years. 173 drop‐outs (89 timolol group, 84 no treatment group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kitazawa 1990.
| Methods | RCT. Double‐masked. Placebo controlled. | |
| Participants | 20 participants with OHT. Racial constitution is not reported. Inclusion criteria: moderate OHT with IOP that has never exceeded 30 mmHg, normal visual field. | |
| Interventions | Timolol 0.5% twice daily. Placebo. | |
| Outcomes | Incidence of glaucomatous visual field defect. | |
| Notes | Allocation concealment deemed likely, based on double‐masked design. Masking not stated but likely. 2 years follow up. Drop‐outs: 2 patients in each group (not adverse‐event related). Both eyes included, analysis both on basis of patient and eye. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Leblanc 1998.
| Methods | RCT. Double‐masked. Active controlled. Multicenter (7). | |
| Participants | 483 participants with POAG (55%) or OHT. 10% were African American. Inclusion criteria: IOP between 23 and 35 mmHg in each eye and both eyes within 5 mmHg of each other. Exclusion criteria: visual acuity below 20/80, active external ocular disease, severe dry eye, c/d ratio of 0.8 or greater or advanced visual field loss in either eye, history of ocular surgery or laser within 6 months, history of uncontrolled hypertension or diabetes, medication with any drug that could have a substantial effect on IOP or interact with the effects of alpha‐adrenergic agonists. | |
| Interventions | Brimonidine 0.2% twice daily. Timolol 0.5% twice daily. | |
| Outcomes | IOP. Horizontal c/d ratio. Incidence of visual field defect or defect progression. | |
| Notes | Allocation concealment deemed likely, based on double‐masked design. 1 year follow up. Uneven randomisation schedule 3:2. 40 drop‐outs: 149/292 brimonidine (76 adverse events), 48/191 timolol (9 adverse events). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Novack 1989.
| Methods | RCT. Double‐masked. Multicenter (10). | |
| Participants | 391 participants with OAG or OHT (65%). 20% African American. Inclusion criteria: bilateral IOP of 23 mmHg or higher. Exclusion criteria: use of adrenergic augmenting psychotropic drugs, topical or systemic corticosteroids, severe diabetes mellitus requiring changes in insulin dosage, aphakia, chronic ocular inflammation, severe OAG uncontrolled by concomitant administration of 2 or more drugs. | |
| Interventions | Levobunolol 0.5% twice daily. Levobunolol 1% twice daily. Timolol 0.5% twice daily. | |
| Outcomes | IOP. Horizontal c/d ratio. Incidence or progression of glaucomatous visual field defect. | |
| Notes | Allocation concealment deemed likely, based on double‐masked design. 4 years follow up. 107 drop‐outs (70 due to reasons unrelated to study medication) and 95 IOP failures: levobunolol 0.5% (4 systemic and 6 local side effects), levobunolol 1% (7 systemic and 13 local side effects), timolol (7 systemic and 3 local side effects). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ravalico 1994.
| Methods | RCT. Open label. | |
| Participants | 26 paricipants with OHT. Racial constitution is not reported. Inclusion criteria: IOP between 22 and 30 mmHg, vision 20/20, cup/disc ratio below 0.5. Exclusion criteria: glaucoma within the family, narrow angle, other ocular pathologies. | |
| Interventions | Levobunolol 0.5% twice daily. Untreated. | |
| Outcomes | IOP. Change of visual field mean defect. Horizontal c/d ratio. | |
| Notes | 24 months follow up, but considerable attrition at 24 months. Drop‐out rate by 18 and 24 months: untreated group 7 and 11 of 23 eyes; levobunolol group 9 and 19 of 26 eyes (the number of participants who dropped out is not reported). The reasons for drop‐out are not detailed. The study appears to report mean values of both eyes where both eyes were included (most cases). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schulzer 1991.
| Methods | RCT. Open label. | |
| Participants | 137 people with OHT. Racial constitution is not reported. Inclusion criteria: IOP above or equal to 22 mmHg, normal visual fields (Goldmann perimeter). Exclusion criteria: Obvious sign of glaucomatous disc changes, ocular infections, trauma or surgery within 6 months before the study, resting pulse of 50 beats or less, systemic beta‐blockers. | |
| Interventions | Timolol 0.5% twice daily. No treatment. | |
| Outcomes | Incidence of reproducible visual field defect. Incidence of disc haemorrhages. Incidence of stereophotographically documented optic nerve changes. | |
| Notes | 6 years follow up. 22 drop‐outs: 12 timolol (2 adverse drug reaction) and 10 untreated. The reasons for drop‐out are not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schuman 1997.
| Methods | RCT. Double‐masked. Active controlled. | |
| Participants | 374 people with POAG (62%) or OHT (only 188 participants examined with perimetry twice). Racial constitution is not reported. Inclusion criteria: IOP between 23 and 35 mmHg (untreated). Exclusion criteria: patients using more than 2 ocular hypotensive agents, visual acuity below 20/100, abnormally low heart rate or blood pressure, long‐term treatment with any other topical or systemic alpha‐adrenoceptor agonist or antagonist, treatment with adrenergic‐augmenting psychotropic drugs, dry eye, asymmetry of IOP of more than 5 mmHg, extensive visual field loss, ocular surgery or laser within 6 months, c/d‐ratio of 0.8 or more. | |
| Interventions | Brimonidine 0.2% twice daily. Timolol 0.5% twice daily. | |
| Outcomes | IOP. Incidence of visual field defect or defect progression. | |
| Notes | 1 year follow up. Drop‐outs due to adverse events: 35 brimonidine (25 ocular and 10 systemic adverse events), 4 timolol (2 ocular and 2 systemic adverse events). Most patients have been treated with opical beta‐blocker before washout of the study. Visual field analysis available for subgroup only, comprising 77 patients recieving brimonidine and 111 with timolol. The difference between groups in proportion of patients participating in visual field analysis subgroup poses questions about randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schwartz 1995.
| Methods | RCT. Double‐masked. Placebo controlled. | |
| Participants | 37 participants with OHT. 3 African American in timolol group, only Caucasians in placebo group. Inclusion criteria: IOP between 21 and 35 mmHg, normal visual field, PEX allowed (1 in each group). Exclusion criteria: previous ocular surgery or laser treatment. | |
| Interventions | Timolol 0,5% twice daily. Placebo. | |
| Outcomes | Change of optic disk cupping (photogrammetric measurement). Change of RNFL thickness (photogrammetric measurement). Incidence of glaucomatous visual field defects. | |
| Notes | Study duration 2 years, but effective mean duration 1.5 years. Drop‐out rate: 8 participants on timolol (2 systemic and 2 ocular adverse events), and 6 subjects on placebo (1 systemic and 2 ocular adverse events). The authors do not report visual field data in detail, but state that in both groups there was no incidence of visual field defects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sponsel 1987.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 36 people with POAG. Racial constitution is not reported. Inclusion criteria: IOP above 21 mmHg on 2 occasions, optic disc cupping supportive of a diagnosis of glaucoma, visual field loss typical of nerve fibre bundle damage. Exclusion criteria: coexisting ocular pathlogy. | |
| Interventions | Timolol 0.25% or 0.5% twice daily (adjustment according to IOP). Pilocarpine 2% or 4% twice daily (adjustment according to IOP). | |
| Outcomes | IOP control. Progression rate of visual field score (Goldmann and Friedmann static suprathreshold perimetry). | |
| Notes | Study duration 17 months. Due to early miotic intolerance, the follow‐up groups comprised 14 patients on pilocarpine, as compared to 22 patients on timolol. The number of originally included subjects is not stated. Miosis may have influenced the visual field results. The paper does not state whether data where averaged for both eyes or represent only one eye per patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tsai 2005.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 39 participants with POAG. Racial constitution is not reported. Inclusion criteria: IOP 22 mmHg or greater, visual field defect in SAP, glaucomatous appearance of optic disc, normal open angle. | |
| Interventions | Timolol 0.5% ophthalmic gel‐forming solution once daily. Brimonidine 0.2% twice daily. | |
| Outcomes | Change in RNFL thickness (ellipse average, superior average, temporal average, inferior average, nasal average). IOP. | |
| Notes | Study duration 2 years. Drop‐out rate: 2 participants on timolol (no adverse event), 3 participants on brimonidine (1 ocular adverse event). Visual field was examined at baseline only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vogel 1992.
| Methods | RCT. Observer masked. Active controlled. | |
| Participants | 189 people with POAG. Racial constitution is not reported. Inclusion criteria: IOP 22 mmHg or greater, visual field defect in SAP. Exclusion criteria: history of severe ocular trauma or intraocular surgery, ocular infection within 3 months before study start, concomitant medication known to affect IOP. | |
| Interventions | Timolol 0.25% or 0.5% twice daily (adjustment according to IOP). Pilocarpine 2% or 4% twice daily (adjustment according to IOP). | |
| Outcomes | Change of visual field mean sensitivity. IOP. | |
| Notes | Study duration 2 years. Observer masked status is questionable because of miosis. At baseline, 51 patients were excluded. Drop‐outs during follow up: 18 timolol (4 adverse event or death) and 36 pilocarpine (7 adverse event or death). The authors do not state whether the adverse events or deaths were drug‐related. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Watson 2001.
| Methods | RCT. Open label. Active controlled. | |
| Participants | 153 participants with newly diagnosed OAG. Racial constitution is not reported. Inclusion criteria: IOP of 22 mmHg, visual field changes and/or disc changes suggestive of a diagnosis of POAG. Exclusion criteria: IOP over 32 mmHg together with marked reduction of visual field, history of ocular trauma or surgery. | |
| Interventions | Timolol 0.25% twice daily. Betaxolol 0.5% twice daily. Carteolol 1% twice daily. | |
| Outcomes | Change of visual field mean deviation. Incidence of visual field deterioration. | |
| Notes | Median follow up: timolol group 42 months, betaxolol group 24 months, carteolol group 36 months. 29 drop‐outs: 12 timolol group (7 local and 3 systemic side effects), 17 betaxolol group (5 systemic side effects), 19 carteolol (6 systemic side effects). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wishart 1992.
| Methods | RCT. Open label. | |
| Participants | 34 participants with OHT and open angles and 25 participants with OHT and narrow angles. Fellow‐eye served as control. Racial constitution is not reported. Inclusion criteria: IOP above 21 mmHg in both eyes, normal visual felds (Friedmann), normal optic discs. Exclusion criteria: history of anterior segment disease. | |
| Interventions | Timolol 0.5% twice daily one eye. No treatment for the fellow eye. | |
| Outcomes | Incidence of glaucomatous visual field defects or optic disc damage. | |
| Notes | 6 years follow‐up. Data of OHT with open angles and with narrow angles were analysed separately. Only the former are included in this review. No detailed information on dropouts is given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
c/d ratio: cup to disk ratio IOP: intraocular pressure MD: mean defect mmHG: millimetres mercury OAG: open angle glaucoma OHT: ocular hypertension PDS: pigment dispersion syndrome PEX: pseudoexfoliation CLV: corrected loss variation POAG: primary open angle glaucoma RCT: randomised controlled trial RNFL: retinal nerve fibre layer SAP: standard automated perimetry
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Araie 2003 | 60% of the included patients had NTG. |
| EMGT 1999 | This RCT included patients with OAG irrespectively to their IOP. Almost 50% of the subjects were NTG patients. Additionally the trial included also patients with PEX. The study intervention also included laser treatment in one group. |
| Holmin 1988 | The study compares medical treatment with no treatment. The treatment allowed use of timolol, pilocarpine and a supplementation with acetazolamide. |
| Vainio 1999 | This trial included both NTG and POAG with elevated IOP |
IOP: intraocular pressure NTG: normal tension glaucoma OAG: open angle glaucoma POAG: primary open angle glaucoma PEX: pseudoexfoliation RCT: randomised controlled trial
Contributions of authors
Clemens Vass: protocol and review author, quality assessment, data extraction Peter Bauer: statistical analysis Oliver Findl: quality assessment, data extraction Leopold Schmetterer: protocol co‐author Thomas Sycha: protocol co‐author, search strategy, methods of the review Stefan Sacu: quality assessment, data extraction Cornelia Hirn: quality assessment, data extraction
Guarantor for review: Clemens Vass
Sources of support
Internal sources
Department of Ophthalmology, University of Vienna, Austria.
Department of Clinical Pharmacology, University of Vienna, Austria.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Alexander 1988 {published data only}
- Alexander DW, Berson FG, Epstein DL. A clinical trial of timolol and epinephrine in the treatment of primary open‐angle glaucoma. Ophthalmology 1988;95(2):247‐51. [DOI] [PubMed] [Google Scholar]
Collignon‐Brach 1992 {published data only}
- Collignon‐Brach J. Long‐term effect of ophthalmic beta‐adrenoceptor antagonists on intraocular pressure and retinal sensitivity in primary open‐angle glaucoma. Current Eye Research 1992;11(1):1‐3. [DOI] [PubMed] [Google Scholar]
Collignon‐Brach 1994 {published data only}
- Collignon‐Brach J. Longterm effect of topical beta‐blockers on intraocular pressure and visual field sensitivity in ocular hypertension and chronic open‐angle glaucoma. Survey of Ophthalmology 1994;38(Suppl 1):S149‐55. [DOI] [PubMed] [Google Scholar]
Drance 1998 {published data only}
- Drance SM. A comparison of the effects of betaxolol, timolol, and pilocarpine on visual function in patients with open‐angle glaucoma. Journal of Glaucoma 1998;7(4):247‐52. [DOI] [PubMed] [Google Scholar]
EGPS 2005 {published data only}
- The European Glaucoma Prevention Study. The European glaucoma prevention study design and baseline description of the participants. Ophthalmology 2002;109(9):1612‐21. [DOI] [PubMed] [Google Scholar]
- The European Glaucoma Prevention Study Group. Results of the European glaucoma prevention study. Ophthalmology 2005;112(3):366‐75. [DOI] [PubMed] [Google Scholar]
Epstein 1989 {published data only}
- Epstein DL, Krug JH, Hertzmark E, Remis LL, Edelstein DJ. A long‐term clinical trial of timolol versus no treatment in the management of glaucoma suspects. Ophthalmology 1989;96(10):1460‐7. [DOI] [PubMed] [Google Scholar]
Fama 1996 {published data only}
- Fama F, Santamaria S. Comparison of the ocular effects of three beta‐blockers: timolol, carteolol, and betaxolol. Annals of Ophthalmology 1996;28(5):317‐20. [Google Scholar]
Flammer 1992 {published data only}
- Flammer J, Kitazawa Y, Bonomi L, Mills B, Fsadni M, Dorigo MT, et al. Influence of carteolol and timolol on IOP and visual fields in glaucoma: a multi‐center, double‐masked, prospective study. European Journal of Ophthalmology 1992;2(4):169‐74. [DOI] [PubMed] [Google Scholar]
Geyer 1988 {published data only}
- Geyer O, Lazar M, Novack GD, Lue JC, Duzman E. Leovbunolol compared with timolol for the control of elevated intraocular pressure. Annals of Ophthalmology 1986;18(10):289‐92. [PubMed] [Google Scholar]
- Geyer O, Lazar M, Novack GD, Shen D, Eto CY. Levobunolol compared with timolol: a four‐year study. British Journal of Ophthalmology 1988;72(12):892‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heijl 2000 {published data only}
- Bengtsson B, Heijl A. A long‐term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. Journal of Glaucoma 2005;14(2):135‐8. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Heijl A. Diurnal IOP fluctuation: Not an independent risk factor for glaucomatous visual field loss in high‐risk ocular hypertension. Graefe's Archive for Clinical & Experimental Ophthalmology 2005;243(6):513‐8. [DOI] [PubMed] [Google Scholar]
- Heijl A, Bengtsson B. Long‐term effects of timolol therapy in ocular hypertension: a double‐masked, randomised trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2000;238(11):877‐83. [DOI] [PubMed] [Google Scholar]
Kaiser 1994 {published data only}
- Kaiser HJ, Flammer J, Stümpfig D, Hendrickson P. Longterm visual field follow‐up of glaucoma patients treated with beta‐blockers. Survey of Ophthalmology 1994;38(Suppl 1):S156‐60. [DOI] [PubMed] [Google Scholar]
Kamal 2003 {published data only}
- Kamal D, Garway‐Heath D, Ruben S, O'Sullivan F, Bunce C, Viswanathan A, et al. Results of the betaxolol versus placebo treatment trial in ocular hypertension. Graefes Archive for Clinical & Experimental Ophthalmology 2003;241(3):196‐203. [DOI] [PubMed] [Google Scholar]
Kass 1989 {published data only}
- Kass MA. Five‐year follow‐up study of timolol in patients at moderate risk of developing open‐angle glaucoma. Chibret International Journal of Ophthalmology 1990;7(1):5‐8. [Google Scholar]
- Kass MA. Timolol treatment prevents or delays glaucomatous visual field loss in individuals with ocular hypertension: A five‐year, randomized, double‐masked, clinical trial. Transactions of the American Ophthalmological Society 1989;87:598‐618. [PMC free article] [PubMed] [Google Scholar]
- Kass MA, Gordon MO, Hoff MR, Parkinson JM, Kolker AE, Hart WM, et al. Topical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals. A randomized, double‐masked, long‐term clinical trial. Archives of Ophthalmology 1989;107(11):1590‐8. [DOI] [PubMed] [Google Scholar]
Kass 2002 {published data only}
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatments study: a randomized trial determines that topical hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Archives of Ophthalmology 2002;120(6):701‐13. [DOI] [PubMed] [Google Scholar]
Kitazawa 1990 {published data only}
- Kitazawa Y. The effect of timolol on topographic features of the optic disk in ocular hypertension. Chibret International Journal of Ophthalmology 1990;7(1):14‐7. [Google Scholar]
Leblanc 1998 {published data only}
- Leblanc R, Brimonidine Study Group 2. Twelve‐month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Ophthalmology 1998;105(10):1960‐7. [DOI] [PubMed] [Google Scholar]
- Melamed S, David R, Brimonidine Study Group 2. Ongoing clinical assessment of the safety profile and efficacy of brimonidine compared with timolol: year‐three results. Clinical Therapeutics 2000;22(1):103‐11. [DOI] [PubMed] [Google Scholar]
Novack 1989 {published data only}
- Berson FG, Cohen HB, Foerster RJ, Lass JH, Novack GD, Duzman E. Levobunolol compared with timolol for the long‐term control of elevated intraocular pressure. Archives of Ophthalmology 1985;103(3):379‐82. [DOI] [PubMed] [Google Scholar]
- Cinotti A, Cinotti D, Grant W, Jacobs I, Galin M, Silverstone D, et al. Levobunolol vs timolol for open‐angle glaucoma and ocular hypertension. American Journal of Ophthalmology 1985;99(1):11‐7. [DOI] [PubMed] [Google Scholar]
- Novack G, The Levobunolol Study Group. Levobunolol. A beta‐adrenoceptor antagonist effective in the long‐term treatment of glaucoma. Ophthalmology 1985;92(9):1271‐6. [PubMed] [Google Scholar]
- Novack GD, The Levobunolol Study Group. Levobunolol: a four‐year study of efficacy and safety in glaucoma treatment. Ophthalmology 1989;96(5):642‐5. [PubMed] [Google Scholar]
- Ober M, Scharrer A, David R, Biedner B‐Z, Novack GD, Lue JC, et al. Long‐term ocular hypotensive effect of levobunolol: results of a one‐year study. British Journal of Ophthalmology 1985;69(8):593‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravalico 1994 {published data only}
- Ravalico G, Salvetat L, Toffoli G, Pastori G, Croce M, Parodi MB. Ocular hypertension: a follow‐up study in treated and untreated patients. New Trends in Ophthalmology 1994;9(2):97‐101. [Google Scholar]
Schulzer 1991 {published data only}
- Chauhan BC, Drance SM, Douglas GR. The effect of long‐term intraocular pressure reduction on the differential light sensitivity in glaucoma suspects. Investigative Ophthalmology and Visual Science 1988;29(10):1478‐85. [PubMed] [Google Scholar]
- Schulzer M, Drance SM, Douglas GR. A comparison of treated and untreated glaucoma suspects. Ophthalmology 1991;98(3):301‐7. [DOI] [PubMed] [Google Scholar]
Schuman 1997 {published data only}
- Schuman JS, Brimonidine Study Group. Clinical experience with brimonidine 0.2% and timolol 0.5% in glaucoma and ocular hypertension. Survey of Ophthalmology 1996;41(Suppl 1):S27‐S37. [DOI] [PubMed] [Google Scholar]
- Schuman JS, Horwitz B, Choplin NT, David R, Albracht D, Chen K, Brimonidine Study Group. A 1‐year study of brimonidine twice daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Archives of Ophthalmology 1997;115(7):847‐52. [DOI] [PubMed] [Google Scholar]
Schwartz 1995 {published data only}
- Schwartz B, Lavin P, Takamoto T, Araujo DF, Smits G. Decrease of optic disc cupping and pallor of ocular hypertensives with timolol therapy. Acta Ophthalmologica Scandinavica 1995;73(Suppl 215):5‐21. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Takamoto T, Lavin P, Smits G. Increase of retinal nerve fiber thickness in ocular hypertensives with timolol therapy. Acta Ophthalmologica Scandinavica 1995;73(Suppl 215):22‐32. [DOI] [PubMed] [Google Scholar]
Sponsel 1987 {published data only}
- Dallas NL, Sponsel WE, Hobley AJ. A comparative evaluation of timolol maleate and pilocarpine in the treatment of chronic open angle glaucoma. Eye 1988;2(Pt 3):243‐9. [DOI] [PubMed] [Google Scholar]
- Sponsel WE. Timolol vs pilocarpine in open angle glaucoma: the observation of significant differences in visual field response in patients with clinically equivalent IOP control. Chibret International Journal of Ophthalmology 1987;5(3):50‐6. [Google Scholar]
Tsai 2005 {published data only}
- Tsai JC, Chang HW. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: A prospective, unmasked study. Journal of Ocular Pharmacology and Therapeutics 2005;21(6):475‐82. [DOI] [PubMed] [Google Scholar]
Vogel 1992 {published data only}
- Vogel R, Crick RP, Mills KB, Reynolds PM, Sass W, Clineschmidt CM, et al. Effect of timolol versus pilocarpine on visual field progression in patients with primary open‐angle glaucoma. Ophthalmology 1992;99(10):1505‐11. [DOI] [PubMed] [Google Scholar]
Watson 2001 {published data only}
- Watson PG, Barnet MF, Parker V, Haybittle J. A 7‐year prospective comparative study of three topical beta‐blockers in the management of primary open angle glaucoma. British Journal of Ophthalmology 2001;85(8):962‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PG, Barnett MF, Parker V, Haybittle J, Fellman R. A 7‐year prospective comparative study of three topical beta blockers in the management of primary open angle glaucoma. Evidence Based Eye Care 2002;3(3):144‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wishart 1992 {published data only}
- Wishart P K, Batterbury M. Ocular hypertension: correlation of anterior chamber angle width and risk of progression to glaucoma. Eye 1992;6(Pt 3):248‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Araie 2003 {published data only}
- Araie M, Azuma I, Kitazawa Y. Influence of topical betaxolol and timolol on visual field in Japanese open‐angle glaucoma patients. Japanese Journal of Ophthalmology 2003;47(2):199‐207. [DOI] [PubMed] [Google Scholar]
EMGT 1999 {published data only}
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of Ophthalmology 2002;120(10):1268‐79. [DOI] [PubMed] [Google Scholar]
- Hyman LG, Komaroff E, Heijl A, Bengtsson B, Leske MC. Treatment and vision‐related quality of life in early manifest glaucoma trial. Ophthalmology 2005;112(9):1505‐13. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengsson B, Early Manifest Glaucoma Trial. Early manifest glaucoma trial. Design and baseline data. Ophthalmology 1999;106(11):2144‐53. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Komaroff E. Factors for progression and glaucoma treatment: the Early Manifest Glaucoma Trial. Current Opinion in Ophthalmology 2004;15(2):102‐6. [DOI] [PubMed] [Google Scholar]
Holmin 1988 {published data only}
- Holmin C, Thorburn W, Krakau CE. Treatment versus no treatment in chronic open angle glaucoma. Acta Ophthalmologica 1988;66(2):170‐3. [DOI] [PubMed] [Google Scholar]
Vainio 1999 {published data only}
- Vainio Jylha E, Vuori ML. The favorable effect of topical betaxolol and timolol on glaucomatous visual fields: a 2‐year follow‐up study. Graefes Archive for Clinical & Experimental Ophthalmology 1999;237(2):100‐4. [DOI] [PubMed] [Google Scholar]
- Vainio‐Jylhä E, Vuori ML, Nummelin K. Progression of retinal nerve fibre layer damage in betaxolol‐ and timolol‐treated glaucoma patients. Acta Ophthalmologica Scandinavica 2002;80(5):495‐500. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CGSG 2006 {published data only}
- Canadian Glaucoma Study Group. Canadian Glaucoma Study: 1. Study design, baseline characteristics, and preliminary analyses. Canadian Journal of Ophthalmology 2006;41(5):566‐75. [DOI] [PubMed] [Google Scholar]
Hommer 2007 {published data only}
- Hommer A, Ganfort Investigators Group I. A double‐masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03% timolol 0.5% with non‐fixed combination use in patients with glaucoma or ocular hypertension. European Journal of Ophthalmology 2007;17(1):53‐62. [DOI] [PubMed] [Google Scholar]
Kanno 2006a {published data only}
- Kanno M, Araie M, Masuda K, Takase M, Kitazawa Y, Shiose Y, et al. Phase III long‐term study and comparative clinical study of nipradilol ophthalmic solution in patients with primary open‐angle glaucoma and ocular hypertension. Arzneimittel‐Forschung 2006;56(11):729‐34. [DOI] [PubMed] [Google Scholar]
Kanno 2006b {published data only}
- Kanno M, Araie M, Masuda K, Takase M, Kitazawa Y, Shiose Y, et al. Phase III long‐term study and comparative clinical study of nipradilol ophthalmic solution in patients with primary open‐angle glaucoma and ocular hypertension. Part 2. Arzneimittel‐Forschung 2006;56(12):820‐5. [PubMed] [Google Scholar]
Noecker 2007 {published data only}
- Noecker RJ, Awadallah NS, Kahook MY. Travoprost 0.004% imolol 0.5% fixed combination. Drugs of Today 2007;43(2):77‐83. [DOI] [PubMed] [Google Scholar]
Schmier 2006 {published data only}
- Schmier JK, Halpern MT, Covert DW, Robin AL. Travoprost versus latanoprost combinations in glaucoma: economic evaluation based on visual field deficit progression. Current Medical Research & Opinion 2006;22(9):1737‐43. [DOI] [PubMed] [Google Scholar]
Sherwood 2006 {published data only}
- Sherwood MB, Craven ER, Chou C, DuBiner HB, Batoosingh AL, Schiffman RM, et al. Twice‐daily 0.2% brimonidine‐0.5% timolol fixed‐combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12‐month randomized trial. Archives of Ophthalmology 2006;124(9):1230‐8. [DOI] [PubMed] [Google Scholar]
Whitson 2006 {published data only}
- Whitson JT, Ochsner KI, Moster MR, Sullivan EK, Andrew RM, Silver LH, et al. The safety and intraocular pressure‐lowering efficacy of brimonidine tartrate 0.15% preserved with polyquaternium‐1. Ophthalmology 2006;113(8):1333‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Armaly 1980
- Armaly MF, Krueger DE, Maunder L, Becker B, Hetherington J Jr, Kolker AE, et al. Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual‐field defects. Archives of Ophthalmology 1980;98(12):2163‐71. [DOI] [PubMed] [Google Scholar]
Caprioli 1998
- Caprioli J. The treatment of normal‐tension glaucoma. American Journal of Ophthalmology 1998;126(4):578‐81. [DOI] [PubMed] [Google Scholar]
CNTGS 1998
- Collaborative Normal Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal‐tension glaucoma. American Journal of Ophthalmology 1998;126(4):498‐505. [DOI] [PubMed] [Google Scholar]
Diggory 1998
- Diggory P, Cassels‐Brown A, Vail A, Hillman JS. Randomised, controlled trial of spirometric changes in elderly people receiving timolol or betaxolol as initial treatment for glaucoma. British Journal of Ophthalmology 1998;82(2):146‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandolfi 2005
- Gandolfi SA, Ghetta A, Cimino L, Mora P, Sangermani C, Tardini MG. Bronchial reactivity in healthy individuals undergoing long‐term topical treatment with beta‐blockers. Archives of Ophthalmology 2005;123(1):135‐8. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Higgins 2006a
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006; Section 6]. In: The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2006b
- Higgins JPT, Green S, editors. Collecting data. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006; Section 7]. In: The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd.
Maier 2005
- Maier PC, Funk J, Schwarzer G, Antes G, Falck‐Ytter Y. Treatment of ocular hypertension and open angle glaucoma: meta‐analysis of randomized controlled trials. BMJ 2005;331(7509):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ontoso 1997
- Aguinaga‐Ontoso I, Guillen‐Grima F, Aguinaga‐Ontoso E, Fernandez‐Fernandez LR. Does medical treatment of mild intraocular hypertension prevent glaucoma?. European Journal of Epidemiology 1997;13(1):19‐23. [DOI] [PubMed] [Google Scholar]
Quigley 1983
- Quigley HA, Hohmann RM, Addicks EM, Massof RW, Green W. Morphologic changes in the lamina cribrosa correlated with neural loss in open‐angle glaucoma. American Journal of Ophthalmology 1983;95(5):673‐91. [DOI] [PubMed] [Google Scholar]
Quigley 2005
- Quigley HA, Miglior S, Pfeiffer N, Zeyen T, Cunha‐Vaz J, Torri V, et al. European glaucoma prevention study. Authors' reply. Ophthalmology 2005; Vol. 112, issue 9:1642‐5.
Rossetti 1993
- Rossetti L, Marchetti I, Orzalesi N, Scorpiglione N, Torri V, Liberati A. Randomized clinical trials on medical treatment of glaucoma: are they appropriate to guide clinical practice?. Archives of Ophthalmology 1993;111(1):96‐103. [DOI] [PubMed] [Google Scholar]
Schulzer 1990
- Schulzer M, Drance SM, Carter CJ, Brooks DE, Douglas DE, Lau W. Biostatistical evidence for two distinct chronic open‐angle glaucoma populations. British Journal of Ophthalmology 1990;74(4):196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sycha 2005
- Sycha T, Vass C, Findl O, Bauer P, Groke I, Schmetterer L, et al. Interventions for normal tension glaucoma. Cochrane Database of Systematic Reviews 2005, Issue 1. [Art. No.: CD002222. DOI: 10.1002/14651858.CD002222.] [DOI] [PubMed] [Google Scholar]
Takamoto 1985
- Takamoto T, Schwartz B. Reproducibility of photogrammetric optic disc cup measurements. Investigative Ophthalmology and Visual Science 1985;26(6):814‐7. [PubMed] [Google Scholar]
Waldock 2000
- Waldock A, Snape J, Graham CM. Effects of glaucoma medications on the cardiorespiratory and intraocular pressure status of newly diagnosed glaucoma patients. British Journal of Ophthalmology 2000;84(7):710‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiysonge 2006
- Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, et al. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD002003. DOI: 10.1002/14651858.CD002003.pub2] [DOI] [PubMed] [Google Scholar]


