Abstract
Background
Currently hydergine is used almost exclusively for treating patients with either dementia, or 'age‐related' cognitive symptoms. Since the early 1980s there have been over a dozen more clinical trials, yet hydergine's efficacy remains uncertain. Although previous reviews offer generally favourable support for hydergine's efficacy, they were, however, limited by a bias with respect to the particular clinical studies chosen (e.g., the inclusion of case reports, and uncontrolled trials), and by authors' impressionistic assessments of results. Not surprisingly, there has been a lack of consensus among reviewers with regard to the efficacy of hydergine.
In 1994, a meta‐analysis was published by the present reviewers who reported that overall, hydergine was more effective than placebo. However they also observed that the statistical evidence for efficacy in 'possible or probable Alzheimer's disease' patients was so modest that one additional statistically non‐significant trial would have reduced the results to non‐significance.
Objectives
Because of uncertainty surrounding the efficacy of hydergine, the goals of this overview were to assess its overall effect in patients with possible dementia, and to investigate potential moderators of an effect.
Search methods
The trials were identified from a searches of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group, The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS, clinical trials registries and grey literature sources on 2 March 2009 using the terms hydergin*, ergoloid* and dihydroergo*. Two proprietary databases were also searched. Published reviews were inspected for further sources.
Selection criteria
Trials to be included must be randomized, double‐blind, parallel‐group, and unconfounded comparisons of hydergine with placebo for a treatment duration of greater than one week in subjects with dementia or symptoms consistent with dementia.
Data collection and analysis
Data were extracted independently by the reviewers, pooled where appropriate and possible, and the pooled odds ratios (95% CI) or the average differences (95% CI) were estimated. Where possible, intention‐to‐treat data were used.
Outcomes of interest included clinical global impressions of change and comprehensive rating scales. Potential moderating variables of a treatment effect included: inpatient/outpatient status, trial duration, age, sex, medication dose, publication year, and diagnostic grouping.
Main results
There were a total of 19 trials that met inclusion criteria and that had data sufficient for analysis.
Thirteen trials reported sufficient information to use a global rating of improvement and nine trials provided information on a comprehensive rating scale. Three trials provided both outcome measures.
It was not possible to use many of the published results in a combined analysis owing to the lack of sufficient data to perform statistical analyses.
For the 12 trials that used global ratings, there was a significant effect favouring hydergine (OR 3.78, 95% CI, 2.72 to 5.27). For the nine trials that used comprehensive ratings, there was a significant mean difference favouring hydergine (WMD 0.96, 95%CI, 0.54 to 1.37).
Hydergine was well tolerated in these trials, with 78% of randomized subjects available for data analyses.
Greater effect sizes on global ratings were associated with younger age, and possibly higher dose, although most of the subgroup analyses were statistically insignificant.
Authors' conclusions
As in an earlier systematic review, we found hydergine to show significant treatment effects when assessed by either global ratings or comprehensive rating scales (based here on a smaller set of trials than in the earlier published systematic review because trials were required to have data that could conform with MetaView, the Cochrane Collaboration statistics software). The small number of trials available for analysis, however, limited the ability of subgroup analyses to identify statistically significant moderating effects.
Unfortunately, most of the randomized, double‐blind, and placebo‐controlled trials of hydergine were conducted and published before the advent of consensus‐based diagnostic standards of dementia in 1984; therefore diagnostic criteria were less specific. As a result, uncertainty remains regarding hydergine's efficacy in dementia.
Plain language summary
Uncertainty about the efficacy of hydergine in dementia
Hydergine has been used to treat patients with either dementia, or 'age‐related' cognitive symptoms. Hydergine may offer benefit to some patients, possibly at doses of 4.5 to 9.0 mg per day, with possibly greater benefit to younger subjects and inpatients. The statistical evidence for efficacy in 'possible or probable Alzheimer's disease' patients was so modest however that one additional statistically non‐significant trial would have reduced the results to non significance; evidence for efficacy in vascular dementia rested on relatively stronger effects for hydergine on clinical ratings; and effective doses may be higher than 3 mg/d (i.e., than that currently approved in the USA). Despite its availability for 40 years, the circumstances of hydergine's efficacy in dementia syndromes have not been adequately researched, and have yet to be precisely defined.
Background
Hydergine, the brand name for a specific combination of four dihydro derivatives of ergotoxine, also referred to as ergoloid mesylates, was introduced to clinical medicine in 1949. It has been used in the past as a treatment for peripheral vascular disease, hypertension, angina pectoris, and tinnitus. Currently, it is used almost exclusively for treating patients with either dementia, or 'age‐related' cognitive symptoms. In the United States, the Food and Drug Administration has approved hydergine for 'idiopathic decline in mental capacity'. In some countries hydergine is marketed as a non‐disease‐specific cognitive enhancer.
Of note, the drug has also been advocated as a so‐called 'smart drug', for use by cognitively intact young and older adults to 'increase mental abilities'.
As early as 1981, Jarvik 1981 identified limitations in the hydergine medical literature, including the lack of diagnostic rigor, and the use of crude outcome instruments, finding that there was "no convincing consistent improvement in memory, learning, or other cognitive functions." Yet, she also observed that statistically significant improvement in confusion was reported in approximately 50% of studies. Jarvik 1981 concluded that hydergine "may help some patients with some of their activities of daily living, some of their symptoms, and their self‐care...But, overall, the improvements...are very small..."
Since that time there have been over a dozen more clinical trials, yet hydergine's efficacy remains uncertain. Although previous reviews offer generally favorable support for hydergine's efficacy, they were, however, limited by a bias with respect to the particular clinical studies chosen (eg, the inclusion of case reports, and uncontrolled trials), and by authors' impressionistic assessments of results. Not surprisingly, there has been a lack of consensus among reviewers with regard to the efficacy of hydergine.
In 1994, a meta‐analysis was published by the present reviewers (Schneider 1994) who reported that overall, hydergine was more effective than placebo as assessed by comprehensive ratings, clinical global ratings, and combined cognitive measures. Inpatient status, high daily doses, and vascular dementia were generally associated with larger effect sizes. The effect in patients who likely would have fulfilled criteria for 'possible or probable Alzheimer's disease' (ie, DSM III or NINCDS‐ADRA criteria) was significant only for combined neuropsychological measures in the five trials identified.
The present reviewers observed that the statistical evidence for efficacy in 'possible or probable Alzheimer's disease' patients was so modest that one additional statistically non‐significant trial would have reduced the results to non significance; evidence for efficacy in vascular dementia rested on relatively stronger effects for hydergine on clinical ratings; and effective doses may be higher than 3 mg/d (ie, than that currently approved in the USA).
Objectives
The aim of this review is to assess the overall effect of hydergine in patients with dementia and to investigate variables that might moderate the effect.
Additional reasons to undertake this review are to test for significant consensus among trials that may have contradictory findings; to gather potential information about efficacy which can be revealed only by assessing systematic variations in study design, data characteristics, and methodology; and to assess the development of a particular research domain.
Methods
Criteria for considering studies for this review
Types of studies
Studies were selected for this review if they fulfilled the following criteria:
(1) they comprised a clinical trial (2) the trial was double‐blind, parallel‐group, placebo‐controlled, with randomized and unconfounded treatment assignment to placebo or hydergine (3) sample selection criteria were specified (4) subjects included elderly patients with clinical descriptions or diagnostic syndromes consistent with dementia (this criterion is intended to accommodate varying definitions of dementia over the last 30 years and to ensure adequate description of study samples) (5) absence of other psychiatric disorders (6) outcome instruments were specified (7) sufficient statistical information was provided to calculate an effect size between drug and placebo treatment groups using Cochrane Review Manager software (version 3.0)
Types of participants
Elderly patients with possible dementia, as evidenced by clinical descriptions or diagnostic syndromes consistent with dementia (e.g., cerebral insufficiency, vascular dementia, cerebral deterioration, senile dementia, 'possible or probable AD' (NINCDS‐ADRA or DSM III criteria), presence of confusion or memory impairment, absence of other psychiatric disorders).
Two reviewers independently assigned clinical trials into five categories based on the selection of subjects as follows: (1) those including patients judged to have dementia consistent with possible Alzheimer's disease (AD) (2) those including patients judged to have dementia consistent with possible vascular dementia (3) trials which included patients whose syndromes were consistent with a primary dementia but of undetermined type (4) trials with patients described as having "cerebral insufficiency" (5) and trials with patients described as having "cerebral or senile deterioration."
Categories (4) and (5) were operational definitions in the literature from the late 1960s to the early 1980s.
Types of interventions
1) Any oral dose of hydergine (including sublingual, oral tablet, or liquid capsule) 2) Placebo with or without multivitamin supplement
Types of outcome measures
1. Comprehensive rating scores. These typically consisted of multi‐item rating scales (eg, Sandoz Clinical Assessment Geriatric; Shader 1974). 2. Clinical global impressions. These typically were seven‐point clinician's ratings of change (eg, Clinical Global Impression of Change; Guy 1976). 3. Neuropsychological tests.
Search methods for identification of studies
See Cochrane Dementia and Cognitive Improvement Group methods used in reviews.
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) was searched on 2 March 2009. This register contains records from the following major healthcare databases: The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing trial databases and other grey literature sources. The following search terms were used: hydergin*, ergoloid* and dihydroergo*.
The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, many ongoing trial databases and other grey literature sources were searched separately on 2 March 2009 for the latest records. The search terms used to identify relevant controlled trials on dementia, Alzheimer's disease and mild cognitive impairment for the Group's Specialized Register can be found in the Group's module on The Cochrane Library. These search terms were combined with the following search terms and adapted for each database, where appropriate: hydergin*, ergoloid* and dihydroergo*. See Appendix 1 for the sources searched and number of hits retrieved.
Further trials were identified by Sandoz Pharmaceutical Corporation in 1991, who provided lists of published and unpublished reports under their sponsorship. These included reports supporting an original submission to a National Academy of Sciences‐‐Medical Research Panel in 1974, and a subsequent submission to the Drug Safety and Efficacy Study Implementation.
Published reviews were inspected for additional sources.
Data collection and analysis
Selection of studies
A single reviewer (JO) discarded irrelevant citations, based on the title of the publication and its abstract. Any suggestion that an article could possibly be relevant, caused it to be retrieved for further assessment.
Two reviewers (AL and SL) independently selected the trials for inclusion in the review from the culled citation list. Disagreements were resolved by discussion by a third reviewer (JO).
Quality Assessment
The same two reviewers (AL and SL) assessed the methodological quality of each trial using the Cochrane Collaboration guidelines (Mulrow 1996).
Category A (adequate) is where the report describes allocation of treatment by: (i) some form of centralized randomized scheme, such as having to provide details of an enrolled participant to an office by phone to receive the treatment group allocation; (ii) some form of randomization scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administrated sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (vi) other combinations of described elements of the process that provides assurance of adequate concealment.
Category B (intermediate) is where the report describes allocation of treatment by: (i) use of a "list" of "table" to allocate assignments; (ii) use of "envelopes" or "sealed envelopes"; (iii) stating the study as "randomised" without further detail.
Category C (inadequate) is where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of week, or any other such approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments.
Empirical research has shown that lack of adequate allocation concealment is associated with bias. Trials with unclear concealment measures have been shown to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but less pronounced than inadequately concealed trials (Chalmers 1983; Schulz 1995). Thus trials are included if they conform to categories A or B, and those falling into category C were excluded.
Other aspects of trial quality are not assessed by a scoring system although details were noted of blinding, whether intention‐to‐treat analyses were extractable from the published data, and the number of patients lost to follow‐up.
Data extraction
Data were independently extracted by the same two reviewers and cross‐checked by a third reviewer (AN). Any discrepancies were discussed.
For comprehensive rating scales, data were required to provide either: (a) means and standard deviations for pre‐ and post‐tests, (b) means and standard deviations for change scores, (c) individual data for each patient in the trial. For global ratings, data were required to provide either: (a) the percentage of responders for both drug and placebo groups, (b) frequencies of responders for both drug and placebo groups, (c) individual data for each patient in the trial.
In studies where a crossover design was used, only data from the first treatment period were eligible for inclusion.
Data analysis
For continuous or ordinal variables, such as comprehensive rating scales, the main outcome of interest was the change in score from baseline (i.e. pre‐randomisation or at randomisation) to the final assessment. If ordinal scale data appear to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then the outcome measures were treated as continuous data.
For binary outcomes such as global ratings, the endpoint itself is of interest and the Peto method of the 'typical odds ratio' used.
A test for heterogeneity of the treatment effect between the trials was made using a standard chi‐squared statistic. If a test of heterogeneity was negative then a weighted estimate of the typical treatment effect across trials, the 'typical odds ratio' (i.e. the odds of an unfavourable outcome amongst treatment‐allocated patients to the corresponding odds amongst controls) was calculated using Peto's log‐rank test adapted for ordinal data (EBCTCG, 1990). If, however, there was evidence of heterogeneity of the treatment effect between trials then either only homogeneous results were pooled, or a random effects model used (in which case the confidence intervals would be broader than a fixed effects model).
Additional hypotheses tested were that hydergine had no differential effect, when compared with placebo, for certain subgroups of patients:
1. Age (stratified into 65‐75 and 75‐85 years). 2. Sex (stratified into < 51% male, >50% male). 3. Diagnosis (Alzheimer's Disease, Vascular Dementia, Primary Dementia, Cerebral Deterioration, Cerebral Insufficiency). 4. Dose (1.5‐3.0mg/d and 4.0‐9.0mg/d). 5. Duration of treatment (<10 weeks, 12‐16 weeks, >23 weeks). 6. Inpatient or outpatient status. 7. Publication year (stratified into 1955‐1973 and 1974‐1990).
Results
Description of studies
Nineteen trials were identified which met the inclusion criteria for the review, and provided sufficient outcome data for analysis using the Cochrane Review Manager software.
Methods
All 19 trials were parallel group designs. Treatment lasted from 9 weeks to 15 months.
Participants
The number of participants randomised in the trials ranged from 19 to 458, with completers ranging from 19 to 274.
Interventions
Eighteen trials were designed to compare hydergine with placebo. One trial compared hydergine to placebo with a multivitamin and 600mg of potassium (t.i.d.). The dose of hydergine and method of dose determination varied widely. In some trials, different doses of hydergine were used within the same study. Eighteen trials tested a single dose of hydergine. One trial tested 1.5mg per day, 7 trials tested 3mg per day, 6 trials tested 4.5mg per day, 2 trials tested 6mg per day, 1 trial tested 7.5mg per day, and 2 trials had different fixed doses within the same study. One trial (Hollister 1955) started at 4mg per day for 30 days, reducing the dose to 3mg per day for 15 days and 2mg per day for the final 15 days. Another trial (van Loveren 1984) started at 4.5mg per day for 12 weeks and increased the dose to 7.5mg per day for the remaining 12 weeks.
Outcome measures
The outcome measures fall within the general groupings of (1) comprehensive rating scales, (2) clinical global impressions and (3) neuropsychological tests scores. Neuropsychological tests were not used as a separate outcome because there were insufficient trials reporting data for analysis (N=7). Within these groups there is a wide variety of scales. A few of the better known ones were used in the majority of trials, but there are many scales which are less well known and which display varying degrees of comparability whilst purporting to measure the same outcome. The number of subjects who failed to compete the trial, and of subjects who were omitted from analyses were reported.
The following outcomes were recorded:
1) Comprehensive Rating Scales SCAG ‐ Sandoz Clinical Assessment Geriatric (n=8) MACC Behavioral Adjustment Scale (n=1)
2) Scores of Global Function Unnamed Global Rating Scale (n=9) SCAG global rating (n=1) Proportion based on composite of all measures administered (n=1)
3) Neuropsychological Tests Benton Visual Retention Test (n=1) Buschke Selective Reminding Test (n=3) Digit Symbol Substitution Test (n=1) Divided Attention Task (n=1) Labyrinth Test (n=1) Mini‐Mental State Examination (n=2) Orientation (n=1) Nowlis Test (n=1) Raven's Progressive Matrices (n=1) Toulouse‐Pieron Test (n=1) Trail Making Test (n=3) Wechsler Adult Intelligence Scale (n=1) Wechsler Block Design (n=1) Wechsler Digit Span (n=2) Wechsler Digit Symbol (n=4) Wechsler Memory Scale Logical Memory (n=1) Wechsler Memory Scale Visual Reproduction (n=1) Word Recall (n=1)
Risk of bias in included studies
Most of the included trials have significant methodological limitations by current standards, primarily due to sample selection. Eleven of the included trials were published prior to 1980, with one paper published as early as 1955. Only one trial used NINCDS‐ADRA criteria for AD. All trials were parallel group designs, and most subjects (77.9%) were able to complete the protocol.
Effects of interventions
There were a total of 19 trials that met inclusion criteria and that had data sufficient for analysis. Trials were published between 1955 and 1990 (modal year =1973).
There were 12 trials that provided data on global improvement. The analyses gave statistically significant results in favour of treatment (OR 3.78; 95%CI 2.52 ‐ 5.27), though only 7 of the trials showed statistically significant effects. In addition, the trials were significantly heterogeneous.
Nine trials that provided data regarding change on comprehensive rating scales were significantly heterogeneous. The analyses gave statistically significant results in favour of treatment (WMD 0.96; 95%CI 0.54 ‐ 1.37), though only five trials showed statistically significant effects.
SUBGROUP ANALYSES
Age
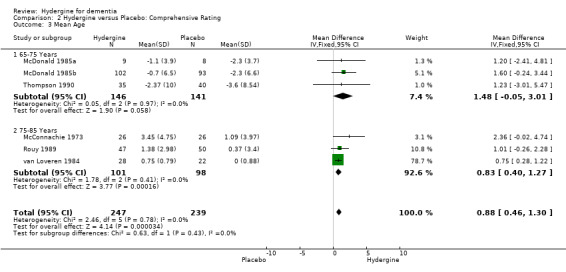
Trials were divided into two groups based on younger (65‐75 years) and older (76‐85 years) subjects. Using global ratings, the four trials with younger subjects showed a greater effect (OR 7.72; 95%CI 3.41 ‐ 17.52) than the seven trials with older subjects (OR 3.09; 95%CI 2.11 ‐ 4.52), though the difference was not statistically significant. Using comprehensive rating scales, a similar finding occurred with the three trials with younger subjects showing a greater effect (WMD 1.48; 95%CI ‐0.05 ‐ 3.01), than the three trials with older subjects (WMD 0.83; 95%CI 0.40 ‐ 1.27), though the difference was non‐significant, and the trials with younger subjects, the treatment effect was statistically significant.
Sex
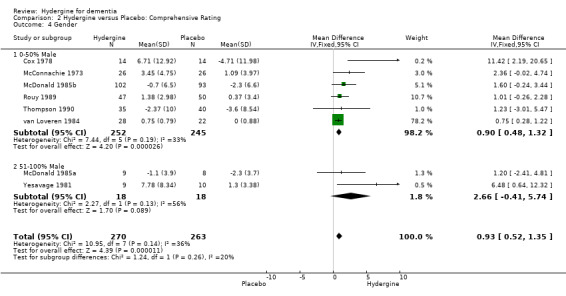
Trials were divided into two groups based on the presence of male subjects (0‐50%, 51‐100%). For global ratings, the seven trials with fewer male subjects showed a similar effect (OR 3.65; 95%CI 2.37 ‐ 5.63) to the four trials with more male subjects (OR 3.61 95%CI 2.02 ‐ 6.46). For comprehensive rating scales, the six trials with fewer male subjects showed a smaller effect (WMD 0.90; 95%CI 0.48 ‐ 1.32) than the two trials with more male subjects (WMD 2.66; 95%CI ‐0.41 ‐ 5.74) though the difference was not statistically significant, and the latter mean effect was not statistically significant.
Diagnosis
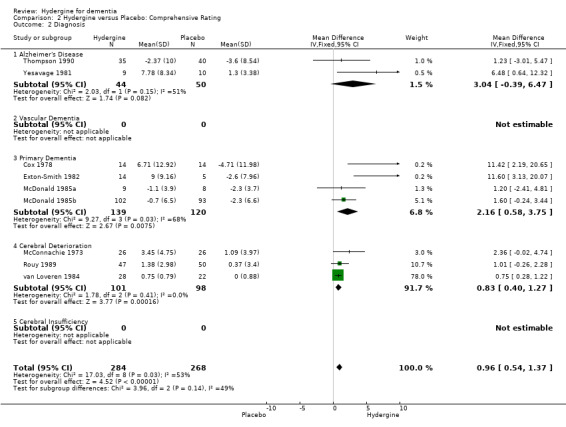
The trials were divided into five diagnostic groups (AD, Vascular Dementia, Primary Dementia, Cerebral Deterioration, Cerebral Insufficiency). Using global ratings, the three trials with primary dementia diagnoses (OR 7.46; 95%CI 3.35 ‐ 16.54) showed the greatest effect size, followed by the four trials with cerebral insufficiency diagnoses (OR 4.65; 95%CI 2.74 ‐ 7.89), the three trials with cerebral deterioration diagnoses (OR 3.07; 95%CI 1.67 ‐ 5.63), and the two trials with vascular dementia (OR 1.43; 95%CI 0.58 ‐ 3.49). None of the differences among diagnostic groupings was statistically significant, and the effect for the vascular dementia trial was nonsignificant. Using comprehensive ratings, the two trials with AD diagnoses did not show a statistically significant treatment effect (WMD 3.04; 95%CI ‐0.39 ‐ 6.47), though the effect was greater than in the four trials with primary dementia diagnoses (WMD 2.16; 95%CI ‐0.58 ‐ 3.75) and the three trials with cerebral deterioration diagnoses (WMD 0.83; 95%CI 0.40 ‐ 1.27).
Dose Response
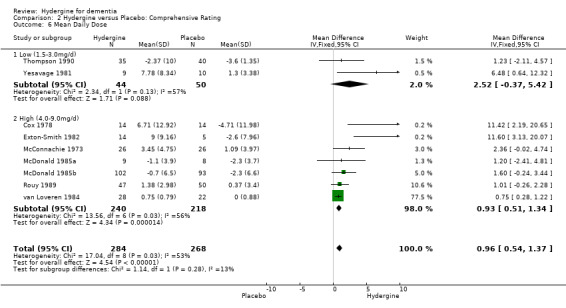
Studies were divided into two groups based on low (1.5‐3.0mg/d) and high (4.0‐9.0mg/d) daily dose. Using global ratings, the 8 trials with higher doses had a greater effect (OR 4.39; 95%CI 2.99 ‐ 6.45) than the 4 trials with lower doses (OR 2.48; 95%CI 1.29 ‐ 4.75), though the difference was not statistically significant. Using comprehensive rating scales, the 2 trials with lower doses (WMD 2.52; 95%CI ‐0.37 ‐ 5.42) showed a greater effect than the 7 trials with higher doses (WMD 0.93; 95%CI 0.51 ‐ 1.34), though the difference was not statistically significant. In addition, the trials with lower doses did not show an overall statistically significant effect.
Duration
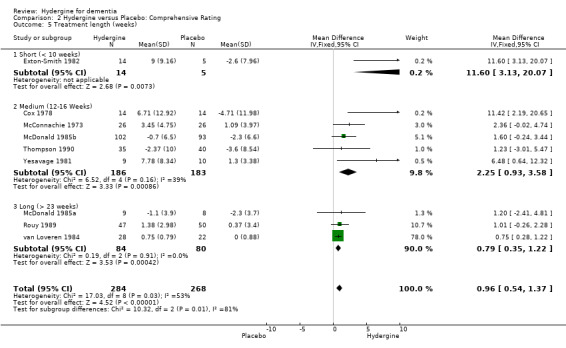
Trials were divided into three groups: short (< 10 weeks), medium (12‐16 weeks), and long (> 23 weeks). There were no significant differences between groupings, with the 6 trials of medium duration showing similar efficacy (OR 4.07; 95%CI 2.40 ‐ 6.88) to the four trials of long duration (OR 3.86; 95%CI 2.44 ‐ 6.09). The two trials of short duration did not show a statistically significant effect (OR 2.28; 95%CI 0.69 ‐ 7.55). Using comprehensive ratings, there was only one short duration trial compared with five medium duration (WMD 2.26; 95%CI 0.93 ‐ 3.58) and three long duration trials (WMD 0.79; 95%CI 0.35 ‐ 1.22). The trials of medium duration showed a statistically significant difference from trials of long duration.
Inpatient Status
Trials were divided into an inpatient and outpatient group. Using global ratings, all ranges showed significant effects. There was only one outpatient trial compared to nine inpatient trials (OR 3.46; 95%CI 2.30 ‐ 5.19). Using comprehensive ratings, the three inpatient trials showed a greater effect (WMD 1.46; 95%CI 0.34 ‐ 2.57) compared with the four outpatient trials (WMD 0.85; 95%CI 0.40 ‐ 1.29); though the difference was not statistically significant.
Publication Year
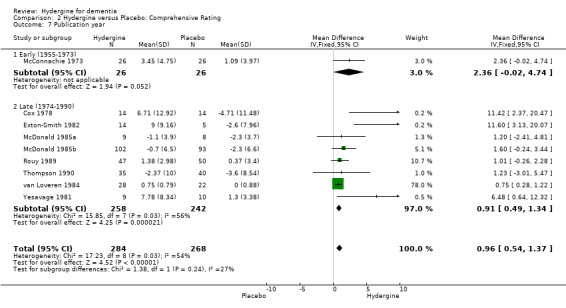
Trials were divided into two groups based on year of publication: early (1955‐1973) and late (1974‐1990). Using global ratings, all ranges showed significant effects. The early trials showed a similar effect (OR 3.76; 95%CI 2.33 ‐ 6.06) to the late trials (OR 3.81; 95%CI 2.41 ‐ 6.03). Using comprehensive ratings, there was only one early trial compared with eight late trials (WMD 0.91; 95%CI 0.49 ‐ 1.33); thus, a subgroup analysis was made.
Adverse events were not reported in a systematic manner in most of the trials so making formal comparison difficult. Seventy‐seven percent of the subjects completed the trials and had data available for analysis.
Discussion
In the two rating areas assessed, the results of this overview seem to demonstrate a heterogeneous, statistical treatment effect for hydergine, consistent with our earlier report, though about half of the trials individually showed nonsignificant effects. In particular, our results suggest that hydergine may be more likely to benefit younger subjects rather than older and inpatients rather than outpatients, though these findings were not statistically significant. In addition, higher doses may offer a greater benefit.
To maintain an adequate level of methodological rigor, only trials that were double‐blind, placebo‐controlled, and parallel‐group in design were included in this overview. In addition trials were required to report sufficient data to use the Cochrane Collaboration software. Despite these selection criteria, individual trials still may have contained methodological problems.
For example, historical factors may have biased findings in favour of hydergine: Most of the trials were published prior to 1980 and earlier trials tended to report their outcomes more selectively. The use of analyses ‐ wherein only subjects who complete a trial are analysed for efficacy ‐ in the vast majority of published trials, may have biased the outcomes toward greater effects for hydergine because dropouts may have been less likely to benefit from treatment. Historical factors in the rendering of diagnoses could have biased effect sizes because the majority of trials were reported before stringent diagnostic standards were in widespread use. Apart from methodological biases that could positively skew effect sizes, these analyses, by design, provide no direct information about onset or duration of action within each trial; nor do they provide information on direction of change over time for hydergine or placebo. Thus, it remains unclear whether the hydergine group actually improved, or merely deteriorated more slowly than the placebo group.
The small sample sizes of most trials (Mean, 67 subjects; range, 19‐274) contributed to the frequency of statistically nonsignificant trials. The statistical power of the average‐sized hydergine trial in our earlier published review suggested that 144 subjects would be needed to ensure a type II error rate of 20 percent. Even a trial of this size may have been insensitive to cognitive change in dementia, as evidenced by current trials of drugs considered more potent than hydergine.
A general 'publication bias', whereby trials with significant results tend to be reported and those with nonsignificant results do not, may have increased the reported effect sizes. This becomes a critical issue in any meta analysis where the effect sizes are modest in magnitude. An effort was made, however, to identify all trials, including unpublished ones. Three unpublished trials were included in these analyses. These trials largely resulted in nonsignificant outcomes, tending to give a more conservative estimate of hydergine's effect.
Hydergine in possible Alzheimer's and vascular dementia
None of the Alzheimer's trials included a global rating. The two trials with comprehensive rating scales showed a nonsignificant effect. On the other hand, the two vascular dementia trials did not have a comprehensive rating that could be analysed and when global ratings were used, the effect was again equivocal. These results are difficult to compare with our earlier review because of the smaller number of trials.
A total of only 94 subjects were included in these two trials, much less than the number of subjects in typical, current multicenter trials for an Alzheimer's drug, and about a third the number from our earlier review. Clearly, the effect of hydergine in patients with possible Alzheimer's dementia has not been adequately researched.
An adequately designed clinical trial would need to include over 200 moderately impaired subjects, require a dose ranging between 4.5 and 9.0 mg/day (ie, greater than the approved dosage in the United States), track outcome over a duration of at least 24 weeks, and a relevant neuropsychological test battery.
Conclusions
Little evidence for the efficacy of hydergine in placebo‐controlled, parallel‐group trials rests on observations that hydergine shows statistical effects on global and comprehensive measures, and the suggestion that effects are stronger with higher doses. The apparent dose‐response relationship (ie, doses greater than 4.5 mg/day were more effective than lower doses) suggests that an adequate hydergine dose may be higher than the dose approved by the Food and Drug Administration.
There was little evidence that hydergine's effect varied by diagnosis, owing, probably, to the small number of trials.
Because hydergine's effects were significant at higher doses, it may be a useful comparator medication in clinical trials with new, investigational drugs with putative cognitive activity (as has been done in one study previously). Overall, patients with a primary dementia show effects with hydergine. Yet, despite its availability for 40 years, the circumstances of hydergine's efficacy in dementia syndromes have not been adequately researched, and have yet to be precisely defined.
Authors' conclusions
Implications for practice.
Hydergine showed a significant treatment effect compared with placebo. The results of this review suggest that hydergine may offer benefit to some patients, possibly at doses of 4.5 to 9.0 mg per day, with possibly greater benefit to younger subjects and inpatients.
Implications for research.
Despite the large number of trials performed using hydergine, future trials are needed in clinically diagnosed subjects with Alzheimer's disease or vascular dementia. In particular, trials using higher doses of the medication (e.g., 9 mg/d) are warranted. Finally, studies that assess long‐term use of hydergine and its ability to affect the course of disease would be appropriate.
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2009 | New search has been performed | Update searches were run on 2 March 2009, and no new studies were found for inclusion in the review; a number of studies have been excluded |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 20 June 2008 | Amended | Converted to new review format. |
| 3 November 2003 | Amended | November 2003: Jason Olin has withdrawn as author of this review due to a new conflict of interest; we are presently recruiting a reviewer to take on the update of the review. |
| 28 March 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Particular acknowledgements are due to Marion Finkel, PhD, of Sandoz Pharmaceuticals Inc (now Novartis) for facilitating access to unpublished data.
Appendices
Appendix 1. Sources searches and hits retrieved
| Source | Date Range Searched | Hits Retrieved |
| Medline (Pubmed) | Jan 2000‐ 2 March 09 | 10 |
| Embase (Ovid SP) | Jan 2000‐ 3 March 09 | 13 |
| PsycInfo (Ovid SP) | Jan 2000‐ 3 March 09 | 0 |
| Cinahl (Ovid SP) | Jan 2000‐ 3 March 09 | 1 |
| Lilacs (bireme) | Jan 2000‐ 2 March 09 | 0 |
| CDCIG SR* | Searched 2 March 09 from 2000 onward | 9 |
| CENTRAL (The Cochrane Library) | Issue 1 2009 | 13 |
| ISTP Conference Proceedings http://portal.isiknowledge.com/portal.cgi | Jan 2000‐ 2 March 09 | 5 |
| Australian Digital Theses Program http://adt.caul.edu.au/ |
Searched 3 March 2009 | 0 |
| Canadian Theses and Dissertations http://www.collectionscanada.ca/thesescanada/index‐e.html |
Searched 3 March 2009 | 0 |
| WHO trials register | Searched 3 March 2009 | 0 |
| Current Controlled trials: Meta Register of Controlled trials (mRCT) http://www.controlled‐trials.com/ |
Searched 3 March 2009 | 0 |
| ISRCTN Register |
Searched 3 March 2009 | 0 |
| Nederlands Trial Register http://www.trialregister.nl/trialreg/index.asp | Searched 3 March 2009 | 0 |
| ClinicalTrials.gov http://www.ClinicalTrials.gov |
Included in WHO portal | // |
| IPFMA Clinical Trials Register www.ifpma.org/clinicaltrials.html |
Searched 3 March 2009 | 0 |
| UMIN Japan Trial Register http://www.umin.ac.jp/ctr/ |
Searched 3 March 2009 | 0 |
| OPENsigle | Searched 3 March 2009 | 0 |
Data and analyses
Comparison 1. Hydergine versus Placebo: Global Rating (% improved).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient status | 9 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.46 [2.30, 5.19] |
| 1.1 Inpatient | 9 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.46 [2.30, 5.19] |
| 1.2 Outpatient | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Diagnosis | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 2.1 Alzheimer's Disease | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Vascular Dementia | 2 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.58, 3.49] |
| 2.3 Primary Dementia | 2 | 61 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.66 [3.11, 30.02] |
| 2.4 Cerebral Deterioration | 3 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.07 [1.67, 5.63] |
| 2.5 Cerebral Insufficiency | 4 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.65 [2.74, 7.89] |
| 3 Mean Age | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 3.1 65‐75 Years | 4 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.72 [3.41, 17.52] |
| 3.2 75‐85 Years | 7 | 515 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.09 [2.11, 4.52] |
| 4 Gender | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 4.1 0‐50% Male | 7 | 339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.65 [2.37, 5.63] |
| 4.2 51‐100% Male | 4 | 278 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.61 [2.02, 6.46] |
| 5 Treatment length (weeks) | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 5.1 Short (< 10 weeks) | 2 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [0.69, 7.55] |
| 5.2 Medium (12‐16 Weeks) | 6 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.07 [2.40, 6.88] |
| 5.3 Long (> 23 weeks) | 3 | 331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.57 [2.17, 5.88] |
| 6 Mean Daily Dose | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 6.1 Low (1.5‐3.0mg/d) | 3 | 111 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.74, 3.62] |
| 6.2 High (4.0‐9.0mg/d) | 8 | 506 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.39 [2.99, 6.45] |
| 7 Publication year | 11 | 617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.57, 5.14] |
| 7.1 Early (1955‐1973) | 8 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.76 [2.33, 6.06] |
| 7.2 Middle (1974‐1990) | 3 | 329 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.51 [2.13, 5.80] |
1.1. Analysis.
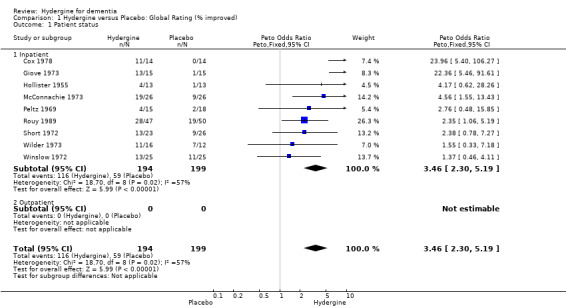
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 1 Patient status.
1.2. Analysis.
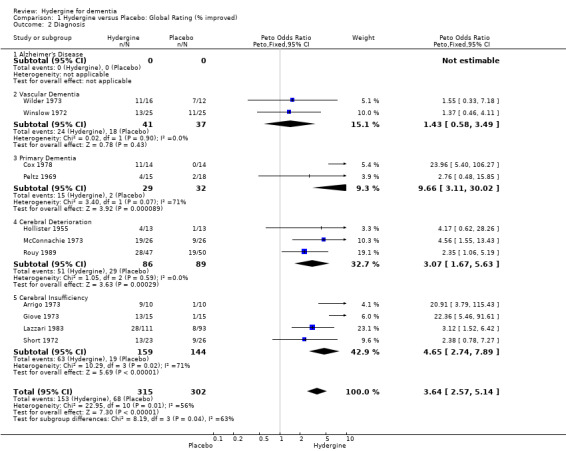
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 2 Diagnosis.
1.3. Analysis.
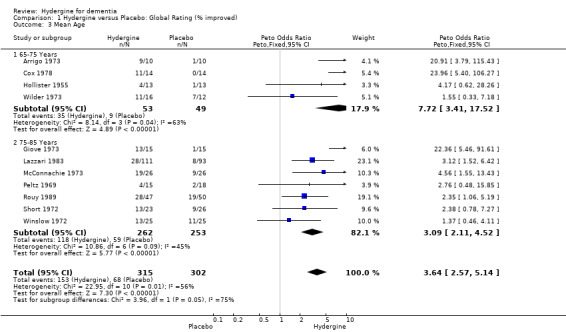
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 3 Mean Age.
1.4. Analysis.
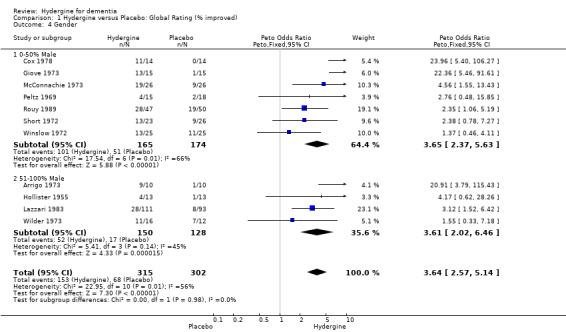
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 4 Gender.
1.5. Analysis.
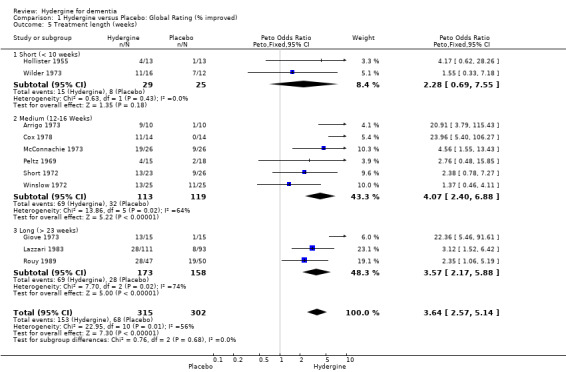
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 5 Treatment length (weeks).
1.6. Analysis.
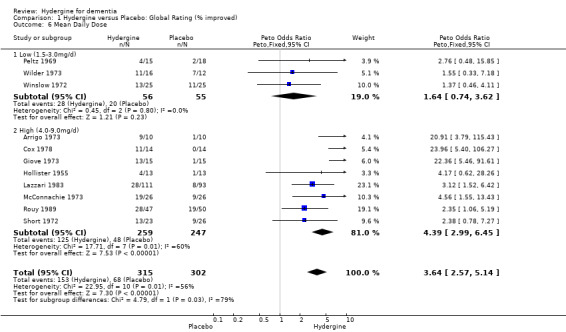
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 6 Mean Daily Dose.
1.7. Analysis.
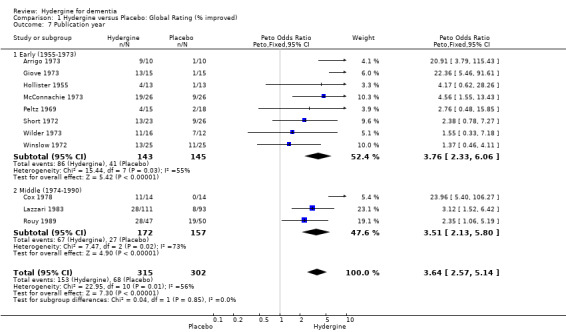
Comparison 1 Hydergine versus Placebo: Global Rating (% improved), Outcome 7 Publication year.
Comparison 2. Hydergine versus Placebo: Comprehensive Rating.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient status | 8 | 533 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [0.52, 1.35] |
| 1.1 Inpatient | 3 | 177 | Mean Difference (IV, Fixed, 95% CI) | 1.46 [0.34, 2.57] |
| 1.2 Outpatient | 5 | 356 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.40, 1.29] |
| 2 Diagnosis | 9 | 552 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [0.54, 1.37] |
| 2.1 Alzheimer's Disease | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 3.04 [‐0.39, 6.47] |
| 2.2 Vascular Dementia | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Primary Dementia | 4 | 259 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [0.58, 3.75] |
| 2.4 Cerebral Deterioration | 3 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.40, 1.27] |
| 2.5 Cerebral Insufficiency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mean Age | 6 | 486 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [0.46, 1.30] |
| 3.1 65‐75 Years | 3 | 287 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [‐0.05, 3.01] |
| 3.2 75‐85 Years | 3 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.40, 1.27] |
| 4 Gender | 8 | 533 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [0.52, 1.35] |
| 4.1 0‐50% Male | 6 | 497 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.48, 1.32] |
| 4.2 51‐100% Male | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.66 [‐0.41, 5.74] |
| 5 Treatment length (weeks) | 9 | 552 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [0.54, 1.37] |
| 5.1 Short (< 10 weeks) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 11.60 [3.13, 20.07] |
| 5.2 Medium (12‐16 Weeks) | 5 | 369 | Mean Difference (IV, Fixed, 95% CI) | 2.25 [0.93, 3.58] |
| 5.3 Long (> 23 weeks) | 3 | 164 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.35, 1.22] |
| 6 Mean Daily Dose | 9 | 552 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [0.54, 1.37] |
| 6.1 Low (1.5‐3.0mg/d) | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [‐0.37, 5.42] |
| 6.2 High (4.0‐9.0mg/d) | 7 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [0.51, 1.34] |
| 7 Publication year | 9 | 552 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [0.54, 1.37] |
| 7.1 Early (1955‐1973) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.36 [‐0.02, 4.74] |
| 7.2 Late (1974‐1990) | 8 | 500 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [0.49, 1.34] |
2.1. Analysis.
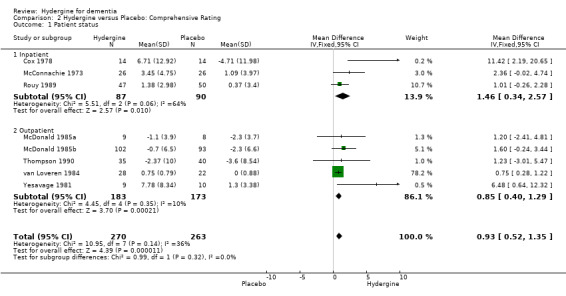
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 1 Patient status.
2.2. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 2 Diagnosis.
2.3. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 3 Mean Age.
2.4. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 4 Gender.
2.5. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 5 Treatment length (weeks).
2.6. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 6 Mean Daily Dose.
2.7. Analysis.
Comparison 2 Hydergine versus Placebo: Comprehensive Rating, Outcome 7 Publication year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arrigo 1973.
| Methods | Randomized Double‐blind Placebo‐controlled Parallel‐group Duration: 12 weeks | |
| Participants | Country: Italy No. of centers: 1 Diagnosis: cerebrovascular insufficiency Diagnosis defined by: 7‐point rating scale Total No. of patients: 20 Setting: not described Age: Hydergine mean age = 66.1 (range = 59‐75), Placebo mean age = 70.5 (range = 63‐79) Gender: 19 males, 1 female Exclusions: not described | |
| Interventions | Route: oral Treatment: Hydergine 1.5 mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Rating scale (1‐7 points) assessing physical parameters, subjective complaints, Activities of Daily Living, cognitive and emotional functioning, EEG, global rating | |
| Notes | No. excluded after randomization: Total=0, Treatment=0, Control=0. No. not included in analysis: Total=0, Treatment=0, Control=0. | |
Chierichetti 1985.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Cox 1978.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: England No. of centers: 1 Diagnosis: varying degrees of dementia Diagnosis defined by: SCAG (Sandoz Clinical Assessment Geriatric Scale) Total No. of patients: 37 Setting: inpatient Age: Hydergine (range= 62‐93 years), Placebo (range= 68‐89 years) Gender: 5 males, 32 females Inclusion: Sandoz Clinical Assessment Geriatric > 2 points in 6 symptoms areas, and > 3 points in more than 2 designated symptoms. Exclusion: psychosis, post‐traumatic brain disease, post‐infective brain disease, cerebral neoplasm, marked mental deterioration, psychotropic drugs, hypotensive drugs containing reserpine or with severe intercurrent illness. | |
| Interventions | Route: oral Treatment: Hydergine 1.5 mg t.i.d. Control: Placebo with multivitamin tablets and 600 mg of potassium t.i.d. | |
| Outcomes | SCAG (Sandoz Clinical Assessment Geriatric), other mental functioning tests, global rating | |
| Notes | No. excluded after randomization: Total=9, Treatment=5, Control=4. No. not included in analysis at 6 weeks: Total=7, Treatment=3, Control=4. No. not included in analysis at 12 weeks: Total=9, Treatment=5, Control=4. | |
Exton‐Smith 1982.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 9 weeks | |
| Participants | Country: England No. of centers: 1 Diagnosis: multi‐infarct dementia with vascular component, or senile dementia Alzheimer's type Diagnosis defined by: Crichton Royal Rating Scale for SDAT and HIS (Hachinski Scale) >6 for MID Total No. of patients: 20 Setting: not described Age: range= 68‐94 Gender: not described Exclusion: medications such as vasodilators, CNS stimulants, major tranquilizers, antidepressants, daytime sedatives, psychiatric illness other than dementia, physical disorders which might change and affect assessment of mental status or ability to complete the study. | |
| Interventions | Route: oral Treatment: Hydergine 3mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Automated Picture Matching Task Raven's Progressive Matrices Word recall (immediate and delayed) Orientation (general) activities of daily living The MACC Behavioural Adjustment Scale | |
| Notes | No. excluded after randomization: Total=1. Treatment=1 Control=0. No. not included in analysis: Total=1. Treatment=1, Control=0. | |
Gaitz 1977.
| Methods | RandomizedDouble‐blindPlacebo‐controlledParallel‐groupDuration: 6 months | |
| Participants | Country: United StatesNo. of centers: 1Diagnosis: Nursing home residents with organic brain syndrome associated with senile dementia, cerebral arteriosclerosis, or both.Diagnosis defined by: SCAG (Sandoz Clinical Assessment Geriatric Scale)Total No. of patients: 54 (Hydergine = 28, Placebo = 26)Setting: nursing homeAge: > 59 years of ageGender: not describedInclusion: Sandoz Clinical Assessment Geriatric (SCAG) score > 4 on at least 6 items and a score > 4 on 2 of 7 symptoms.Exclusion: significant preexisting or concomitant "functional" psychosis (schizophrenia, major affective disorders), marked mental retardation, posttraumatic or postinfective brain damage, cerebral neoplasm, and any other organic disorder not related to senile cortical atrophy or cerebrovascular disease. | |
| Interventions | Route: sublingualTreatment: Hydergine 1 mg t.i.d. (2 tablets [.5mg each] 30 minutes before meals) Control: 2 Placebo tablets t.i.d. (30 minutes before meals) | |
| Outcomes | SCAG, 7‐point rating scale, vital signs, labs, physical examinations, MSCL (Mental Status Check List), global rating | |
| Notes | No. excluded after randomization: Total=7, Treatment=5, Control=2.No. not included in analysis: Total:7, Treatment=5, Control=2.Note: Total of 19 subjects (Treatment=12, Control=7) were not observed for full 24 weeks (only 12 weeks) but used in analysis because of data substitution rule if in trial >49% of time. | |
Giove 1973.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 6 months | |
| Participants | Country: Italy No. of centers: 1 Diagnosis: cerebrovascular insufficiency Diagnosis defined by: 7‐point rating scale Total No. of patients: 30 Setting: Inpatient Age: Mean= 79 Gender: not described Exclusions: not described | |
| Interventions | Route: oral Treatment: Hydergine 1.5 mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Rating scale (1‐7 points) assessing physical parameters, subjective complaints, activities of daily living, cognitive and emotional functioning, EEG, global rating | |
| Notes | No. excluded after randomization: not described No. not included in analysis: not described | |
Hollister 1955.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 60 days | |
| Participants | Country: United States No. of centers: 1 Diagnosis: Hospitalized patients with various diagnosis (chronic brain syndrome, cerebral arteriosclerosis, pre‐senile brain disease, arterial hypertension, involutional psychotic reaction, schizophrenic reaction, syphillis, Korsakoff). Diagnosis defined by: "careful clinical assessment" Total No. of patients: 28 Setting: Inpatient Age: Median age= 66 (range= 56‐83) Gender: 27 males, 1 female Inclusion: hospitalized patients with various diagnosis (chronic brain syndrome, cerebral arteriosclerosis, pre‐senile brain disease, arterial hypertension, involutional psychotic reaction, schizophrenic reaction, syphillis, Korsakoff). Exclusion: unclear | |
| Interventions | Route: sublingual Treatment: Hydergine 30d= 1mg q.i.d., 15d= 1mg t.i.d., 15d= 1mg b.i.d. Control: Placebo 30d= q.i.d., 15d= t.i.d., 15d= b.i.d. | |
| Outcomes | Hospital Adjustment Scale, physical examination, blood pressure, global rating | |
| Notes | No. excluded after randomization: Total = 2, Treatment = unclear, Control = unclear. No. not included in analysis: Total = 2, Treatment = unclear, Control = unclear. | |
Lazzari 1983.
| Methods | RandomizedDouble‐blindPlacebo‐controlledParallel‐groupDuration: 12 months | |
| Participants | Country: Italy No. of centers: 40 Diagnosis: chronic senile cerebral insufficiency Diagnosis defined by: clinical examination Total no. of patients: 458 Age: Hydergine mean= 75.8 (SD=6.9), Placebo= 76.0 (SD=7.9) Gender: Hydergine: 73 males, 122 females, Placebo: 82 males, 111 females Inclusion: Scores > 2 on Sandoz Clinical Assessment Geriatric (SCAG) items (confusion, attention, short term memory, disorientation, anxiety, depression, emotional lability), Hachinski score between 5‐14. Exclusion: Severe hepatic, renal or cardiac disease, psychosis, alcoholism, pregancy, vascular accident, recent trauma that would affect stability, physical problems affecting testing (e.g., tremor, involuntary movements); psychotropic or CNS medications within 4 months of the study (e.g., antihypertensives, beta‐blockers). | |
| Interventions | Route: not describedTreatment: Hydergine 1.5mg t.i.d.Control: Placebo t.i.d. | |
| Outcomes | SCAG, Geriatric Rating Scales, Northwestern University Disability Scales, Nowlis test, Digit Symbol, Digit Span, Toulouse‐Pieron test | |
| Notes | No. excluded after randomization and excluded from analysis: Total=184. Treatment=84, Control=100. | |
McConnachie 1973.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: England No. of centers: 2 Diagnosis: mild‐moderate symptoms of cerebrovascular insufficiency or senile dementia Diagnosis defined by: Crichton Royal Behavioral Rating Scale (RBRS) Total No. of patients: 58 Age: Hydergine mean= 81, Placebo mean= 82 Gender: 10males, 42 females Inclusion: hospital inpatients; nursing home residents; score 2 or 3 on 7/10 factors on Crichton RBRS with total score of 22‐28. Exclusion: psychoses; post‐traumatic brain damage; regular use of psychotropic or hypotensive medications. | |
| Interventions | Route: oral Treatment: Hydergine 1.5mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Physical examination, Physician assessment ‐ 3 point scale on four main symptom complexes: 1) Physical manifestations (anorexia, headache, vertigo, tremor, muscle cramps, paraesthesia, fatigue), 2) Daily living activities (walking, eating, washing, dressing, general self care), 3) Attitudes and mood (hostility, emotional lability, unsociability, depression, uncooperativeness, indifference to surroundings, lack of motivation, anxiety, fear), 4) Motor activity (disinclination for activity or restlessness), global rating | |
| Notes | No. excluded after randomization: Total = 6, Treatment = 4, Control = 2. No. not included in analysis: Total = 6, Treatment = 4, Control = 2. | |
McDonald 1985a.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 24 weeks (12 weeks, option for additional 12 weeks) | |
| Participants | Country: United States No. of centers: 1 Diagnosis: primary degenerative dementia Diagnosis defined by: DSM‐III Duration of disease: Treatment=3.9 years (S.D.=1.66, range2‐7), Placebo=2.7 years (SD=1.42, range 1‐5). No. of patients: 20 Age: Hydergine mean: 64.6 (SD=6.1), Control: 65.1 (SD=8.9) Sex: 19 men, 1 woman Inclusion: normal ECG, vital signs, clinical labs, and GDS range (2‐4) Exclusions: physical abnormalities that would interfere with study, neurological or psychiatric diagnosis other than primary degenerative dementia. | |
| Interventions | Route: oral Treatment: Hydergine 2 mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | GDS (Global Deterioration Scale), MMSE (Mini‐Mental Status Exam) HDRS (Hamilton Depression Rating Scale), SCAG (Sandoz Clinical Assessment Geriatric Scale), IPSC‐E (The Inventory of Psychic and Somatic Complaints of the Elderly), Self‐Rating Scale, GDR (The Geriatric Depression Rating Scale) DAT (Divided Attention Task), SRT (Buschke Selective Reminding Test) Digit Symbol (WAIS), Trail‐Making Test | |
| Notes | Initial 4 week single‐blind, placebo‐controlled design (prior to eligibility). No. excluded after randomization: Total=0. Treatment=0, Control=0. No. not included in analysis: (12 weeks) Total=0. Treatment=0, Control=0. No. not included in analysis : (24 weeks) Total=3. Treatment=1, Control=2. | |
McDonald 1985b.
| Methods | Randomized Double‐blind Placebo‐controlled Parallel‐group Duration: 12 weeks | |
| Participants | Country: United States No. of centers: 5 Diagnosis: primary degenerative dementia Diagnosis defined by: DSM‐III Total No. of patients: 236 Age: Hydergine mean age was 69.8, range (55‐79), Placebo mean age was 68.5, range (55‐79) Sex: Hydergine: 59 females, 43 males, Placebo: 46 females, 47 males. Inclusion: Global deterioration score 2‐4; Hamilton Depression Rating Scale (HDRS) <17; normal pre‐treatment ECG, vitals, labs; no additional neurological or psychiatric diagnosis. Exclusion: Significant abnormal findings that would interfere with study objectives. | |
| Interventions | Route: oral Treatment: Hydergine 2mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | GDS (Global Deterioration Scale), MMS (Mini Mental Status), HRDS (Hamilton), (Depression Rating Scale), SCAG (Sandoz Clinical Assessment Geriatric), IPSC‐E (Inventory of Psychic and Somatic Complaints), GDR (Geriatric Depression Rating Scale), DSST (Digit Symbol Substitution Test: subtest of WAIS: Wechsler) Trail‐Making Test, BSRT (Buschke Selective Reminding Test), physical examination labs, vitals, ECG, adverse reactions | |
| Notes | Initial 2 week single‐blind, placebo controlled design (prior to elegibility). No. excluded after randomization: Total = 41. Treatment = 17, Control = 24. No. not included in analysis: Total: 41. Treatment = 17, Control = 24. | |
Peltz 1969.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 16 weeks | |
| Participants | Country: United States No. of centers: 1 Diagnosis: Symptoms attributable to cerebral arteriosclerosis or simple senile degeneration Diagnosis defined by: Munroe III Visual Test Total No. of patients: 48 Age: Mean age was 82.5, range (64‐96), Hydergine mean age was 81.7, range (72‐95), Placebo mean age was 84, range (64‐96). Sex: 20 females, 13 males Inclusions: Hospitalized geriatric patients manifesting symptoms of cerebral arteriosclerosis or simple cerebral degeneration and exhibiting impairment in general daily living skills and social adaptability. Exclusions: schizophrenia, post‐traumatic brain damage, post‐infective brain disease, cerebral neoplasm; severe degeneration causing inability to participate in testing; psychotropic or sedative medications within 3 weeks prior to study. | |
| Interventions | Route: sublingual Treatment: Hydergine .5mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Munroe III Visual Test, Physical examinations, labs, vital signs, Daily Living and Behavior Rating Scale, Geriatric Daily Living Rating Scale, Overall Rating of Severity (of degeneration), Global Rating of Improvement or Regression | |
| Notes | No. excluded after randomization: Total = 15. Treatment = unclear, Control = unclear. No. not included in analysis: Total = 15. Treatment = unclear, Control = unclear.n | |
Rodriguez 1971.
| Methods | RandomizedDouble‐blindPlacebo‐controlledParallel‐groupDuration: 12 weeks | |
| Participants | Country: United StatesNo. of centers: 1Diagnosis: chronic cerebrovascular insufficiency associated with arteriosclerosisDiagnosis defined by: > 1 of the following (confusion, impairment of recent memory, impairment of mental alertness, deviations from normal mood)Total No. of patients: 60Age: Hydergine mean age was 71.9, range (62‐92), Placebo mean age was 73.0, range (62‐91)Sex: Hydergine: 15 female, 15 male, Placebo: 15 female, 15 maleInclusion: > 1 of the following (confusion, impairment of recent memory, impairment of mental alertness, deviations from normal mood)Exclusion: (1) psychosis, post‐traumatic brain damage, post infective brain disease, cerebral neoplasm, marked mental deterioration; (2) regular use of psychotropic drugs, sedatives, vasodilators; (3) physical conditions of such severity as to preclude participation; (4) unstable chronic physical illness; (4) instability in hospital setting; (5) expectation of discharge in < 12 weeks | |
| Interventions | Route: sublingualTreatment: Hydergine 2, .5 mg tablets t.i.d.Control: Placebo 2, .5mg tablets t.i.d. | |
| Outcomes | Sandoz Clinical Assessment Geriatric, Clinical Global Impression | |
| Notes | No. excluded after randomization and not included in analyses: Total=1. Treatment=1, Control=0. | |
Rouy 1989.
| Methods | Randomized Double‐blind Placebo‐controlled Parallel‐group Duration: 6 months | |
| Participants | Country: France No. of centers: 1 Diagnosis: mild‐moderate mental deterioration due to cerebral aging Diagnosis defined by: SCAG (Sandoz Clinical Assessment Geriatric Scale) Total No. of patients: 97 Age: Hydergine mean age was 82.9, range (63‐98), Placebo mean age was 82.6, range (71‐95) Sex: Hydergine: 34 female, 13 male, Placebo: 32 female, 18 male Inclusion: geriatric in‐patients diagnosed with mild‐moderate mental deterioration due to cerebral aging and SCAG score of 3‐5 on 11 items. Exclusion: vascular accident < 3mth prior to study; cardiac arrhythmias; carotid souffle; severe psychosis; organic brain lesion; metabolic, toxic, and infectious encephalopathy; severe mental shock or physical disease inducing psychic effects; no medications affecting metabolism, cerebral or peripheral vascular system. | |
| Interventions | Route: oral Treatment: Hydergine 4.5mg once‐a‐day Control: Placebo 1 tablet per day | |
| Outcomes | EACG ( a French version of SCAG), NOSIE (Nurse's Observation Scale for In‐Patients) Record of adverse effects, Global Rating | |
| Notes | No. excluded after randomization: Total = 10. Treatment = 5, Control = 5. No. not included in analysis: Total = 10. Treatment = 5, Control = 5. | |
Short 1972.
| Methods | Randomized Double‐blind Placebo‐controlled Parallel‐group Duration: 12 weeks | |
| Participants | Country: United States No. of centers: 1 Diagnosis: general symptoms of aging attributed to chronic degenerative processes such as cerebrovascular insufficiency associated with arteriosclerosis. Diagnosis defined by: unclear Total No. of patients: 60 Age: Hydergine mean age was 85, range (68‐96), Placebo mean age was 81, range (71‐93). Sex: Hydergine: 21 females, 2 males, Placebo: 22 females, 4 males. Inclusion: hospitalized geriatric patients manifesting general symptoms of aging such as deficits in mental alertness, recent memory, mood (depression), and thinking (confusion). Exclusion: severe mental disorders affecting treatment; upcoming traumatic events; psychoactive or vasodilator medications three weeks prior to study. | |
| Interventions | Route: sublingual Treatment: Hydergine 2.5mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Clinical status (17 symptoms, 7‐point scale), Overall clinical status, Global therapeutic response, Mental status, labs, vitals, physical examinations, untoward effects (5 symptoms, 7‐point scale) | |
| Notes | No. excluded after randomization: Total = 11. Treatment = 7, Control = 4. No. not included in analysis: Total = 11. Treatment = 7, Control = 4. | |
Thompson 1990.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 24 weeks | |
| Participants | Country: United States No. of Centers: 1 Diagnosis: Probable Alzheimer's disease Diagnosis defined by: DSM‐III (Diagnostic and Statistical Manual of Mental Disorders‐ Third Edition), NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Strokes, and the Alzheimer's Disease and Related Disorders Association). Inclusion: Modified Hachinski Ischemic Scoring Scale < 4. Mini Mental State Examination: range (10‐23) Hamilton Psychiatric Rating Scale for Depression <19 Inventory of Psychic and Somatic complaints in the Elderly Exclusions: evidence of multi‐infact on CT, MRI, labs, or physical exam Total No. of patients: 80 Sex: 46 women, 34 men Age: Hydergine mean was 72, Placebo mean was 70, range (55‐79) | |
| Interventions | Route: oral Treatment: Hydergine‐LC 1mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | WAIS (Wechsler Adult Intelligence Scale), Digit Symbol Substitution Test, Russell revision of the Wechsler Memory Scale (Logical Memory and Visual Reproduction), IPSC‐E (Inventory of Psychic and Somatic Complaints in the Elderly), SCAG (Sandoz Clinical Assessment Geriatric Scale), GERRI (Geriatric Evaluation by Relatives Rating Instrument), HDRS (Hamilton Psychiatric Rating Scale for Depression) | |
| Notes | No. excluded after randomization: not stated No. not included in analysis: Total=12, Treatment=6, Control=6. | |
van Loveren 1984.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 24 weeks | |
| Participants | Country: Netherlands No. of centers: 3 Diagnosis: mild‐moderate senile mental deterioration Diagnosis defined by: SCAG (Sandoz Clinical Assessment Geriatric Scale) Total No. of patients: 58 Setting: Old‐peoples homes Age: Hydergine mean age = 83.7, Placebo mean age = 82.7 Sex: unclear Inclusion: geriatric residents diagnosed with mild‐moderate senile mental deterioration, including SCAG scores of 3‐5 on >5 symptoms and 4‐5 on >2 key symptoms and HIS (Hachinski Ischemic Score) of <7. Exclusion: HIS >7; psychiatric, neurologic, or other severe systemic disorders; vasodilator or CNS medications; SCAG score of 7 on 1 or more items. | |
| Interventions | Route: oral Treatment: Hydergine 1.5mg t.i.d. (1st 12 wks) and Hydergine 1.5mg (AM) and 3mg b.i.d. (Noon and PM) for 2nd 12wks. Control: Placebo t.i.d. | |
| Outcomes | SCAG Behavior, assessed by 45 min. conversation Hamburg Wechsler Adult Intelligence Scale subtests: Digit Span Digit Symbol Block Design Labyrinth Test Benton Visual Retention Trails A & B Questionnaires on biographical data, activities of daily living, personality, and activities Physical examination, labs, vitals, ECG | |
| Notes | No. excluded after randomization: Total = 8. Treatment = 2, Control = 6. No. not included in analysis: Total = 8. Treatment = 2, Control = 8. | |
Wilder 1973.
| Methods | Randomized Double‐blind Placebo‐controlled Parallel‐group Duration: 6 weeks | |
| Participants | Country: United States No. of centers: 1 Diagnosis: cerebrovascular insufficiency associated with thrombo‐embolic stroke Diagnosis defined by: physician assessment and hospital record Total No. of patients: 28 Age: Hydergine mean age was 68, range (55‐90), Placebo mean age was 68, range (56‐79). Sex: 28 male Inclusion: Hospitalized geriatric patients who had thrombo‐embolic stroke resulting in fixed neurological deficits 4‐15 weeks prior to study; mild‐moderate in >1 of 5 CVI (cerebrovascular insufficiency) symptoms. Exclusion: unclear | |
| Interventions | Route: sublingual Treatment: Hydergine 2 tablets .5mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | SCAG (Sandoz Clinical Assessment Geriatric Scale), Mental Status Exam, Neurologic Status (Cortical Function, Cranial Nerves, Reflexes, Sensation, Miscellaneous, Global Change), EEG, EKG, labs, vitals, physical examination, Global Rating | |
| Notes | No. excluded after randomization: Total = 0. Treatment = 0, Control = 0. No. not included in analysis: Total = 0. Treatment = 0, Control = 0. | |
Winslow 1972.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: United States No. of centers: 1 Diagnosis: chronic cerebrovascular insufficiency due to cerebral arteriosclerosis Diagnosis defined by: Assessment of Clinical Status Total No. of patients: 59 Age: range (60‐94), Hydergine mean was 77.9, range (60‐94); Placebo mean age was 76.3, range (62‐89). Sex: Hydergine: 15 females, 10 males; Placebo: 12 females, 13 males Inclusion: hospitalized geriatric patients suffering from symptoms attributable to diagnosis of chronic cerebrovascular insufficiency due to cerebral arteriosclerosis Exclusion: psychosis, post‐traumatic brain damage, post‐infective brain disease, cerebral neoplasm, mental deterioration; regular use of psychotropic, sedatives or vasodilator medications 3 weeks prior to study; insufficient symptoms for diagnosis; physical conditions precluding participation; acute physical disorders affecting assessment; expectation of transfer, traumatic events, or discharge before end of study. | |
| Interventions | Route: sublingual Treatment: Hydergine 1mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | Assessment of Clinical Status (19 items, 7‐point rating scale), vitals, labs, physical examination, Drug Reaction Record, Global Rating of Change, Major Symptom Inventory | |
| Notes | No. excluded after randomization: Total = 9. Treatment = 4, Control = 5. No. not included in analysis: Total = 9. Treatment = 4, Control = 5. | |
Yesavage 1981.
| Methods | Randomized Double‐blind Parallel group Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: United States No. of centers 1 Diagnosis: mild to moderate senile dementia Total number of patients: 19 Sex: 19 males Age: greater than 54 years Inclusion: SCAG (Sandoz Clinical Assessment Geriatric Scale) >4 points for 6 target symptoms of affect state and cognitve function; at least >5 symptoms had to be related to cognitive function. SCAG overall impression rating of 4‐5 indicating mild to moderate degree of senile dementia Exclusions: Parkinson's disease, Huntington's chorea, affective, toxic, organic, or presile psychosis, schizophrenia, posttraumatic encephalopathy, delirium, drug induced brain changes, syphillis, cerebral neoplasm, normal pressure hydrocephalus, mental retardation, alcoholic brain syndrome, cerebral vascular accident with residual neurological sequelae, acute brain disease associated with systematic disease, or endocrine, metabolic, or hematologic disorders; recent history of long‐term psychoactive chemotherapy, known hypersensitivity to DEM, blindness, deafness, language difficulties, current alcohol or hallucinogen abuse, administration of an investigational drug 4 weeks immediately preceding admission to study, administration of any drug known to cause major organ system toxicity during three months preceding participation in current study. | |
| Interventions | Route: oral Hydergine 1 mg t.i.d. Control: Placebo t.i.d. | |
| Outcomes | SCAG (Sandoz Clinical Assessment Geriatric Scale), Raskin Depression Scale, Buschke Word Recall Test | |
| Notes | No. excluded after randomization: Total=0, Treatment=0, Control=0. No. not included in analysis: Total=0; Treatment=0, Control=0. | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ammon 1995 | Review article |
| Anon 1970 | letter to the editor with insufficient information |
| Anon 1990 | not a clinical trial; a critique |
| Arrigo 1969 | outcome was not reported adequately |
| Arrigo 1972 | outcome measure was irrelevant to clinical efficacy |
| Arrigo 1975 | outcome was presented in other reports |
| Arrigo 1989 | intravenous administration of Hydergine |
| Atarashi 1988 | trial was not placebo‐controlled |
| Baldoni 1971 | trial was not placebo‐controlled |
| Balestreri 1984 | trial was not placebo‐controlled |
| Banen 1972 | outcome was not reported adequately |
| Bargheon 1973 | outcome not adequately reported |
| Bastos 1980 | trial was not placebo‐controlled |
| Battaglia 1990 | trial was not placebo controlled |
| Bazo 1972 | trial was not placebo‐controlled |
| Bazo 1973 | trial was not placebo‐controlled |
| Bente 1979 | outcome measure was irrelevant to clinical efficacy |
| Benton 1951 | patient sample was not appropriate for medication (sample not homogeneous) |
| Biel 1976 | cross‐over trial without first‐period data |
| Bochner 1973 | patient sample was not appropriate for medication (stroke patients ‐motor functioning assessment) |
| Bohard 1967 | patient sample was not appropriate for medication (psychotic patients) |
| Brage 1984 | data reported do not permit an empirical analysis of outcome |
| Breeze 1985 | outcome measure was irrelevant to clinical efficacy |
| Breeze 1988 | outcome measure was irrelevant to clinical efficacy |
| Cai 2003 | No placebo group |
| Casale 1989 | intravenous administration of Hydergine |
| Chen 1997 | No placebo control |
| Cheng 1998 | no placebo control group |
| Chien 1980 | patient sample was not appropriate for medication (data not explicit; sample population = tardive dyskinesia) |
| Chudnovsky 1979 | data reported do not permit an empirical analysis of outcome |
| Cid 1975 | outcome measure was irrelevant to clinical efficacy |
| Cook 1982 | intravenous administration of Hydergine |
| Dartigues 1983 | data reported do not permit an empirical analysis of outcome |
| de Brito‐Paiva 1982 | data reported do not permit an empirical analysis of outcome |
| DeLaRevilla 1967 | patient sample was not appropriate for medication (hypertension) |
| Dennler 1979 | trial was not placebo‐controlled |
| Desimirovic 1988 | trial was not placebo‐controlled |
| Ditch 1971 | outcome was not reported adequately |
| Einspruch 1976 | trial was not placebo‐controlled |
| Forster 1955 | treatment duration was not described |
| Gentili 1976 | data reported do not permit an empirical analysis of outcome |
| Gerin 1969 | outcome was not adequately reported |
| Gerin 1970 | |
| Glover 1980 | letter to the editor with insufficient information |
| Good 1982 | data reported do not permit an empirical analysis of outcome |
| Grill 1969 | outcome was not adequately reported |
| Grobe Einsler 1993 | Review article |
| Gross 1979 | outcome measure was irrelevant to clinical efficacy |
| Heiss 1971 | data reported do not permit an empirical analysis of outcome |
| Herzfeld 1972a | data reported do not permit an empirical analysis of outcome |
| Herzfeld 1972b | outcome measure was irrelevant to clinical efficacy |
| Hofstatter 1956 | data reported do not permit an empirical analysis of outcome |
| Hollingsworth 1974 | |
| Hollingsworth 1980 | outcome was not adequately reported |
| Hoyer 1969 | letter to the editor with insufficient information |
| Huang 2003 | no placebo control |
| Huber 1986 | patient sample was not appropriate for medication (sample has no disorder) |
| Hughes 1976 | Review article |
| Irfan 1978a | outcome was not adequately reported |
| Jansen 1985 | trial was not placebo controlled |
| Jarvik 1981 | data reported do not permit an empirical analysis of outcome |
| Jenike 1986 | cross‐over trial without first‐period data |
| Jenike 1989 | Review article |
| Jenike 1990 | No placebo control |
| Jennings 1972 | outcome was not adequately reported |
| Junod 1978 | data reported do not permit an empirical analysis of outcome |
| Kaiser 1956 | open trial |
| Kanowski 1988 | outcome was not adequately reported |
| Klein 1985 | open trial |
| Koberle 1984 | |
| Kugler 1978 | data reported do not permit an empirical analysis of outcome |
| Kugler 1985 | trial was not placebo‐controlled |
| Kuskowski 1990 | |
| Labecki 1954 | intravenous administration of Hydergine |
| Ladurner 1991 | trial was not placebo controlled |
| Li 2004 | no placebo control |
| Liao 2004 | no placebo control |
| Linden 1975 | data reported do not permit an empirical analysis of outcome |
| Linden 1976 | |
| Linder 1977 | |
| Liu 2005 | no placebo control |
| Lozeron 1974 | open trial |
| Luo 2001 | No placebo control |
| Mars 1970 | trial was not placebo‐controlled |
| Martucci 1986 | outcome was not adequately reported |
| Matejcek 1979 | data reported do not permit an empirical analysis of outcome |
| Matejcek 1980 | |
| Matejcek 1986 | outcome measure was irrelevant to clinical efficacy |
| McConnachie 1978 | data reported do not permit an empirical analysis of outcome |
| McDonald 1979 | review article |
| McDonald 1989 | outcome was not adequately reported |
| Mellini 1973 | trial was not placebo‐controlled |
| Memin 1973 | trial was not placebo‐controlled |
| Misra 1982 | data reported do not permit an empirical analysis of outcome |
| Misurec 1978 | data reported do not permit an empirical analysis of outcome |
| Moglia 1983 | outcome was not adequately reported |
| Nelson 1973 | trial was not plalcebo‐controlled |
| Nelson 1975 | trial was not placebo‐controlled |
| Nicrosini 1976 | trial was not placebo‐controlled |
| Novo 1978 | outcome was not adequately reported |
| Odanische 1981 | trial was not placebo‐controlled |
| Olivella 1981 | trial was not placebo‐controlled |
| Orma 1956 | treatment was not double‐blind |
| Oswald 1979 | outcome was not adequately reported |
| Oswald 1980 | trial was not placebo‐controlled |
| Oswald 1982 | trial was not placebo‐controlled |
| Ouaniche 1981 | trial was not placebo‐controlled |
| Parade 1978 | trial was not placebo‐controlled |
| Patin 1981 | outcome was not adequately reported |
| Paux 1975 | outcome was not adequately reported |
| Pfeiff 1980 | trial was not placebo‐controlled |
| Piguet 1981 | trial was not placebo‐controlled |
| PiresDeOliveira 1973 | outcome measure was irrelevant to clinical efficacy |
| Pomara 1983 | trial was not placebo‐controlled |
| Popkin 1956 | intravenous administration of Hydergine |
| Puxty 1989 | data reported do not permit an empirical analysis of outcome |
| Puxty 1991 | |
| Pöpperl 2004 | No placebo control |
| Rao 1972 | outcome was not adequately reported |
| Rehman 1973a | data reported do not permit an empirical analysis of outcome |
| Rehman 1973b | data reported do not permit an empirical analysis of outcome |
| Riccardi 1978 | trial was not placebo‐controlled |
| Ronge 1982 | trial was not placebo‐controlled |
| Rosen 1975 | trial was not placebo‐controlled |
| Roubicek 1971 | |
| Roubicek 1972 | Data reported do not permit an empirical analysis of the outcome. |
| Roubicek 1973 | |
| Roubicek 1975 | |
| Saletu 1990 | Sample were not demented |
| Saletu 1994 | trial was not placebo‐controlled |
| Samorajski 1982 | outcome measure was irrelevant to clinical efficacy |
| Schardt 1982 | patient sample was not appropriate for medication (hypertension) |
| Schartl 1978 | trial was not placebo‐controlled |
| Schneider 1994 | review article |
| Schnell 1967 | outcome measure was irrelevant to clinical efficacy |
| Setnikar 2001 | Healthy participants |
| Seus 1969 | data reported do not permit an empirical analysis of outcome |
| Shoptaw 2005 | Cocaine‐dependent participants |
| Soni 1975 | outcome was not adequately reported |
| Spiegel 1983 | |
| Spilich 1996 | trial was not placebo‐controlled |
| Stewart 1996 | trial was not placebo‐controlled |
| Strauss 1951 | open trial |
| Strauss 1954 | patient sample was not appropriate for medication (organic peripheral vascular disease) |
| Tartara 1974 | |
| Tecce 1981 | outcome was presented in other reports |
| Tecce 1983a | outcome measure was irrelevant to clinical efficacy |
| Tecce 1983b | outcome was presented in other reports |
| Teixeira 1970 | cross‐over trial without first‐period data |
| Thibault 1974 | data reported do not permit an empirical analysis of outcome |
| Thienhaus 1987 | data reported do not permit an empirical analysis of outcome |
| Tinklenberg 1986 | outcomes not adequately reported |
| Triboletti 1969 | outcome was not adequately reported |
| Tucker 1982 | open trial |
| Winslow 1974 | trial was not placebo‐controlled |
| Yan 2001 | No placebo control |
| Yesavage 1979 | trial was not placebo‐controlled |
| Yesavage 1981b | trial was not placebo‐controlled |
| Yoshikawa 1983 | trial was not placebo‐controlled |
| Zhao 1999 | No placebo control |
Contributions of authors
‐Jason Olin assisted in article collection, data analysis, and wrote the primary draft of the manuscript. ‐Lon Schneider co‐wrote and assisted with editing the manuscript, and assessed search results for the 2009 update. ‐Adrian Novit and Susan Luczak selected articles, coded article content, input article related data into Revman, and edited the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Mental Health (MH01368, MH19074, MH48759), USA.
Sandoz Pharmaceuticals, Inc., Switzerland.
National Institute on Aging (AG05142), USA.
Declarations of interest
The authors (JO, LS) were involved in a previous meta‐analysis of this literature that was commissioned by Sandoz Pharmaceuticals Corporation (now Novartis).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arrigo 1973 {published data only}
- Arrigo A, Braun P, Kauchtschischwili GM, Moglia A, Tartara A. Influence of treatment on symptomatology and correlated electroencephalopathy (EEG) changes in the aged. Cr. Ther. Res. 1973;15:417‐26. [PubMed] [Google Scholar]
Chierichetti 1985 {published data only}
- Chierichetti S, Cucinotta D, Santini V. Implications of long‐term study of Hydergine in elderly patients with chronic senile cerebral insufficiency. In: CM Gaitz, T Samorajski editor(s). Aging 2000: Our health care destiny. New York: Springer‐Verlag, 1985:377‐93. [Google Scholar]
Cox 1978 {published data only}
- Cox JR. Selected patients treated with Hydergine ‐ a double‐blind trial. Brit. J. Clin. Pract 1982;16:9‐11. [Google Scholar]
- Cox JR, Pandurangi VR, Wallace, MG. Drugs will help if dementing patients are caught early. Modern Geriatrics 1978;8(12):14‐15. [Google Scholar]
Exton‐Smith 1982 {published data only}
- Exton‐Smith AN, Piper M, Phillips M, Simpson J. Clinical experience with ergot alkaloids. Clinical experience with ergot alkaloids 1983;23:323‐328. [Google Scholar]
- Exton‐Smith AN, Piper ME, Phillips MJ, Simpson, JM. Management of elderly patients with dementia: A clinical trial using high doses of Hydergine. British Journal of Clinical Practice 1982;16:55‐58. [Google Scholar]
Gaitz 1977 {published data only}
- Gaitz CM, Varner RV. Community‐based comprehensive geriatric services [Community‐based comprehensive geriatric services]. Scientific exhibit presented at the 128th Annual Meeting of the American Psychiatric Association, Anaheim, CA 1975.
- Gaitz CM, Varner RV. Pharmacoptherapy of late life organic brain syndromes: evaluation of hydergine (an ergot derivative) versus placebo using double blind technique. Gerontologist 1974;14(5(II)):44. [Google Scholar]
- Gaitz CM, Varner RV, Overall JE. Pharmacotherapy for organic brain syndrome in late life. Arch. Gen. Psychiatry 1977;34(7):839‐845. [Google Scholar]
Giove 1973 {unpublished data only}
- Giove C, Necchi A. Effect of Hydergine on EEG and clinical symptomatology in the aged. Report for the Xth International Neurology Congress, Barcelona. 1973.
Hollister 1955 {published data only}
- Hollister LE. Combined hydrogenated alkaloids of ergot in mental and nervous disorders associated with old age. Diseases of the Nervous System 1955;16:259‐62. [PubMed] [Google Scholar]
Lazzari 1983 {published data only}
- Lazzari R, Passeri M, Chierichetti SM. Dihydroergotoxine mesylate in the treatment of chronic senile cerebral insufficiency. Result of one long‐term multicenter controlled double‐blind clinical trial against placebo. Press Med 1983;12:3179‐85. [PubMed] [Google Scholar]
McConnachie 1973 {published data only}
- McConnachie RW. A clinical trial comparing Hydergine with placebo in the treatment of cerebrovascular insufficiency in elderly patients. Current Medical Research and Opinion 1973;1:463‐8. [DOI] [PubMed] [Google Scholar]
McDonald 1985a {unpublished data only}
- McDonald RJ. A double‐blind, placebo‐controlled study of the effect of 6 mg/day Hydergine on attention and other behavioral parameters in outpatients with symptoms of primary degenerative dementia. Unpublished manuscript No. 66, submitted by Sandoz Pharmaceuticals Inc, East Hanover, NJ 1985.
McDonald 1985b {unpublished data only}
- McDonald RJ. A double‐blind, placebo‐controlled study of the effect of 6 mg/day Hydergine on attention and other behavioral parameters in outpatients with symptoms of primary degenerative dementia. Unpublished manuscript No. 65, submitted by Sandoz Pharmaceuticals Inc, East Hanover, NJ 1985.
Peltz 1969 {unpublished data only}
- Peltz KS. Clinical evaluation of Hydergine tablets. Unpublished manuscript. Sandoz Pharmaceuticals, East Hanover, NJ 1969.
Rodriguez 1971 {unpublished data only}
- Rodriguez JM. Clinical evaluation of Hydergine. Unpublished manuscript No. 15, Sandoz Pharmaceuticals Inc., East Hanover, NJ 1971.
Rouy 1989 {published data only}
- Rouy JM, Douillon AM, Compan B, Wolmark Y. Ergoloid mesylates ('Hydergine') in the treatment of mental deterioration in the elderly: a 6‐month double‐blind, placebo‐controlled trial. Current Medical Research and Opinion 1989;11:380‐9. [DOI] [PubMed] [Google Scholar]
Short 1972 {published data only}
- Short MJ, Benway M. Delivery of mental health services to the elderly...the state hospital and the community. Presented at the American Psychiatric Association Annual Meeting, Dallas, TX; May 1‐5 1972.
Thompson 1990 {published data only}
- Thompson TL, Filley CM, Mitchell WD, Culig KM, LoVerde M, Bvyny R. Lack of efficacy of Hydergine in patients with Alzheimer's disease. The New England Journal of Medicine 1990;323:445‐8. [DOI] [PubMed] [Google Scholar]
- Walker C. Ergoloid Mesylates Vs. Alzheimer's: The Latest Round. Geriatrics 1990;45(12):22‐24. [MEDLINE: ] [PubMed] [Google Scholar]
van Loveren 1984 {published data only}
- Loveren‐Huyben CMS, Engeiaar HFWJ, Hermans MBM, Bom JA, Leering C, Munnichs JMA. Double‐blind clinical and psychologic study of Ergoloid Mesylates (Hydergine) in subjects with senile mental deterioration. Journal of the American Geriatrics Society 1984;32:584‐8. [DOI] [PubMed] [Google Scholar]
Wilder 1973 {published data only}
- Wilder BJ, Gonyea EF. The effects of the dihydrogenated ergot alkaloids on symptoms of aging. A controlled pilot study of clinical, neurologic and EEG changes. Presented at the Scientific Exhibit, American Medical Association Annual Convention, New York, NY, June 23‐27 1973.
Winslow 1972 {published data only}
- Rosen HJ, Nelson JJ, Winslow IE, Einspruch BC, Hamot HB. Mental decline in the aged: Aspects of etiology and therapy. Scientific exhibit presented at the 123rd Annual Meeting of the American Medical Association, Chicago, Illinois 1974.
- Winslow IE. Clinical evaluation of Hydergine. Unpublished manuscript, Book No. 20, submitted by Sandoz Pharmaceuticals, Inc. East Hanover, NJ 1972.
Yesavage 1981 {published data only}
- Yesavage J, Leirer VO, Becker L, Holman C. Effect of an ergot preparation on cognitive ability and depression. Journal of Psychiatric Treatment and Evaluation 1981;3:153‐6. [Google Scholar]
References to studies excluded from this review
Ammon 1995 {published data only}
- Ammon R, Sharma R, Gambert SR, Gupta KL. A statistical analysis of studies showing efficacy in the treatment of cognitively impaired elderly. Age 1995;18(1):5‐9. [Google Scholar]
Anon 1970 {published data only}
- Anon. Dihydrogenated ergot alkaloids (Hydergine) and cerebrovascular insufficiency. Medical Letter on Drugs and Therapeutics 1970;12:27‐28. [PubMed] [Google Scholar]
Anon 1990 {published data only}
- Anon. Hydergine in Alzheimer's disease. Nurses' Drug Alert 1990c;14(11):84. [Google Scholar]
Arrigo 1969 {published data only}
- Arrigo A, Mille T. The use of Visergil in the prophylaxis and treatment of cerebral sclerosis ("Degenerative Cerebropathy"). Neuropsychiatria 1969;35:1‐15. [Google Scholar]
Arrigo 1972 {published data only}
- Arrigo A, Kauchtschischwili GM, Tartara A. EEG and clinical effects of Hydergine in cerebral vascular insufficiency. 9th International Congress of Gerontology. 1972; Vol. 3:68.
Arrigo 1975 {published data only}
- Arrigo, A. Influence of Hydergine on senile cerebrovascular disorders and its correlation with changes in the electroencephalogram. Investigacion Medica Internacional 1975;45:45‐60. [Google Scholar]
Arrigo 1989 {published data only}
- Arrigo A, Casale R, Giorgi I, Guarnaschelli C, Zelaschi F. Effects of intravenous high dose co‐dergocrine mesylate ('Hydergine') in elderly patients with severe multi‐infarct dementia: A double‐blind, placebo‐controlled trial. Current Medical Research and Opinion 1989;11:491‐500. [DOI] [PubMed] [Google Scholar]
Atarashi 1988 {published data only}
- Atarashi J, Ohtomo E, Kogure K, Hirai S, Tazaki Y, Araki G, Itoh E, Sawada T, Fujishima M. Clinical utility of HYG‐FAS in treatment of cerebrovascular disorders: Multi‐center double‐blind study in comparison with Hydergine tablet 2mg. Rinsho Hyoka (Clin. Eval) 1988;16:425‐486. [Google Scholar]
Baldoni 1971 {published data only}
- Baldoni E, Serentha P, Cuttin S, Galetti G. Therapeutic action of nimergoline in geriatric arteriosclerosis with cerebrovascular insufficiency. Gazzetta Internazionale di Medicina e Chirurgia 1971;76:965‐974. [Google Scholar]
Balestreri 1984 {published data only}
- Balestreri R, Bompani R, Cerrato G, Cuzzupoli M, Feliciantonio RD, Grasso A, Orefice G, Turrisi E. Comparative study of Suloctidil and Dihydroergotoxine in chronic cerebrovascular insufficiency. Results of a double‐blind dummy multicentric trial. Acta Therapeutics 1984;10:163‐175. [Google Scholar]
Banen 1972 {published data only}
- Banen DM. An ergot preparation (Hydergine) for relief of symptoms of cerebrovascular insufficiency. Journal of the American Geriatrics Society 1972;20:22‐24. [DOI] [PubMed] [Google Scholar]
Bargheon 1973 {published data only}
- Bargheon J. Double‐blind study of Hydergine in elderly patients. La Nouvelle Presse Medicale 1973;2:2053‐5. [PubMed] [Google Scholar]
Bastos 1980 {published data only}
- Bastos AC, Celestino CA, Almeida SML. Avaliacao da eficacia clinica do buflomedil no tratamento de pacientes portadores de arteriosclerose senil [Buflomedil clinical efficacy evaluation in the treatment of senile dementia]. Folha Med 1980;80:73‐82. [Google Scholar]
Battaglia 1990 {published data only}
- Battaglia Angelo, Bruni G, Sacchetti G, Pamparana F, et al. A double‐blind randomized study of two ergot derivatives in mild to moderate dementia.. Current Therapeutic Research 1990;48(4):597‐612. [Google Scholar]
Bazo 1972 {unpublished data only}
- Bazo AJ. Symptoms of senility: Recognition and management. Presented at the Scientific Exhibit, American College of Angiology, Phoenix, Arizona, January 27‐30 1972.
Bazo 1973 {published data only}
- Bazo AJ. An ergot alkaloid preparation (Hydergine) versus papaverine in treating common complaints of the aged: double‐blind study. Journal of the American Geriatrics Society 1973;21:63‐71. [DOI] [PubMed] [Google Scholar]
Bente 1979 {published data only}
- Bente VD, Glatthaar G, Ulrich G, Lewinsky M. Quantitative EEG‐Untersuchungen zur vigilanzfordernden Wirkung von Nicergolin [Quantitative EEG‐Untersuchungen zur vigilanzfordernden Wirkung von Nicergolin]. Arzneimittel‐Forsch 1979;29:1804‐8. [PubMed] [Google Scholar]
Benton 1951 {published data only}
- Benton JG, Brown H, Rinzler SH. Objective evaluation of physical and drug therapy in the rehabilitation of the hemiplegic patient. American Heart Journal 1951;42:719‐32. [DOI] [PubMed] [Google Scholar]
Biel 1976 {published data only}
- Biel ML, Seus R, Struppler A. Drug therapy of the organic brain psychosyndrome in geriatric patients. Med. Klin. 1976;71:2177‐84. [PubMed] [Google Scholar]
Bochner 1973 {published data only}
- Bochner F, Eadie MJ, Tyrer JH. Use of an ergot preparation (Hydergine) in the convalescent phase of stroke. Journal of the American Geriatric Society 1973;21:10‐17. [DOI] [PubMed] [Google Scholar]
Bohard 1967 {published data only}
- Bohard F, Guennoc A, Klein H, Borgne Y, Masanes P. Hydergine in the treatment of chronic psychosis [L'hydergine dans le traitement des psychoses chroniques]. Ann‐Med‐Psychol‐Paris 1967;125(3):438‐446. [PubMed] [Google Scholar]
Brage 1984 {published data only}
- Brage D, Brage D. Insuficiencia vasculo‐encefalcia cronica: Ensayo terapeutico [Insuficiencia vasculo‐encefalcia cronica: Ensayo terapeutico]. Prensa Medica Argentina 1984;71:761‐4. [Google Scholar]
Breeze 1985 {published data only}
- Breeze RW. Event related evoked potentials in dementia. British Journal of Clinical Practice 1985;39:43‐4. [PubMed] [Google Scholar]
Breeze 1988 {published data only}
- Breeze RW, Cox S, Rodgers‐Cox J. Changes in P‐300 latency as a result of co‐dergocrine mesylate therapy in patients with senile dementia. International Journal of Geriatric Psychiatry 1988;3:263‐6. [Google Scholar]
Cai 2003 {published data only}
- Cai J, Du J, Huang J‐S, Lin Q‐C. Clinical effect of the principle of tonifying‐kidney, invigorating‐spleen, nourishing and activating‐blood on patients with vascular dementia. Chinese Journal of Clinical Rehabilitation 2003;7(31):4251‐3. [Google Scholar]
- Cai J, Du J, Huang JS. Effect of kangxin capsule on homocysteine and beta‐amyloid protein in vascular dementia patients. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(9):664‐7. [PubMed] [Google Scholar]
Casale 1989 {published data only}
- Casale R, Buonocore M, Arrigo A. Role of i.v. administration of Hydergine in the initial stage of treatment of multi‐infarct dementia: Influence on the psychomotor performances and the shortening of the effects' onset period. In: Carlsson A, Kanowski S, Allain H, Spiegel R editor(s). Cerebral Insufficiency: Trends in Research and Treatment. Vol. 4, Carnforth, Lancs, UK/Park Ridge, NJ: The Parthenon Publishing Group, 1989:193‐209. [Google Scholar]
Chen 1997 {published data only}
- Chen K, Chen KJ, Zhou WQ. Clinical study of effect of yizhi capsule on senile vascular dementia [Chung Kuo Chung Hsi I Chieh Ho Tsa Chih]. Zhongguo‐Zhong‐Xi‐Yi‐Jie‐He‐Za‐Zhi 1997;17(7):393‐397. [ISSN: 1003‐5370] [PubMed] [Google Scholar]
Cheng 1998 {published data only}
- Cheng W, Zhou W, Chen K. Clinical study on effect of huancongdan capsule in treating senile vascular dementia. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18(2):81‐4. [PubMed] [Google Scholar]
Chien 1980 {published data only}
- Chien C, Marder SR, Putten T, Escobar J. A double‐blind study of dihydrogenated ergot alkaloids (Hydergine) in the treatment of tardive dyskinesia: preliminary report. 12th Congress of the Collegium Internationale Neuro‐Pharmacologicum, Goteborg (S), 22‐26 June. Oxford, New York: Pergamon Press, 1980; Vol. 120:105‐106.
Chudnovsky 1979 {published data only}
- Chudnovsky N. Community‐based management of mild to moderate mental deterioration in older age outpatients. Scientific Exhibit presented at the American Academy of Family Physicians, 31st Annual Scientific Assembly, Atlanta, GA. 1979:8‐11.
Cid 1975 {published data only}
- Cid MA. Modificaciones farmacoligias del EEG en la insuficiencia vascular cerebral. In: Sandoz SAE editor(s). VIIth Symposium Internacional "Insuficiencia Circulatoria Cerebra". Madrid, Spain: Sandoz SAE, 1975. [Google Scholar]
Cook 1982 {published data only}
- Cook PJ, Burgoyne W, James IM. Effect of intravenous Hydergine on cerebral blood flow in patients following subarachnoid haemorrhage. British Journal of Clinical Practice 1982;16:32‐4. [Google Scholar]
Dartigues 1983 {published data only}
- Dartigues JF, Pere JJ. Methodologie d'un essai controle de la dihydroergotoxine dans les deteriorations intellectuelles acquises de l'adulte. Presse Med. 1982;12:3173‐5. [PubMed] [Google Scholar]
de Brito‐Paiva 1982 {published data only}
- Brito‐Paiva E, Junod JP. Prevencion a largo plazo del deterioro mental del paciente anciano [Long‐term prevention of mental decline in the elderly patient]. In: Sandoz S.A.E editor(s). Symposium Internacional sobre Involucion Cerebral, Fisiopatologia, Clinica y Terapeutica, Estoril, 6‐7 II 1981. Barcelona (E.): Impr. Hermanos Vila Sala S.A., 1982:81‐4. [Google Scholar]
DeLaRevilla 1967 {published data only}
- Revilla L, Alvarado JJ. La Hydergina en la terapeutica geriatrica. Med. Esp. 1967;58:105‐13. [Google Scholar]
Dennler 1979 {published data only}
- Dennler HJ, Bachmann H. Hydergin therapy of cerebrovascular insufficiency in patients requiring digitalis. Results of a double‐blind study Hydergin therapy of cerebrovascular insufficiency in patients requiring digitalis. Results of a double‐blind study (author's transl)] Hydergin therapy of cerebrovascular insufficiency in patients requiring digitalis. Results of a double‐blind study (author's transl)] HydHydergin therapy of cerebrovascular insufficiency in patients requiring digitalis. Results of a double‐blind study (author's transl)] Hydergin therapy of cerebrovascular insufficiency in patients requiring digitalis. Results of a double‐blind study (author's transl)] [Behandlung der zerebrovaskularen insuffizienz. Einsatz von Hydergin bei digitalisbedurftigen patienten: Ergebnisse einer doppelblindstudie]. Munch, Med, Wschr 1979;121:1615‐8. [PubMed] [Google Scholar]
Desimirovic 1988 {published data only}
- Desimirovic V, Vujic D, Vlajkovic J. Comparative longterm study of dihydroergotoxine and cinarizine effects on incipient dementia syndrome. Therapie 1988;43:143‐63. [Google Scholar]
Ditch 1971 {published data only}
- Ditch M, Kelly FJ, Resnick, O. An ergot preparation (Hydergine) in the treatment of cerebrovascular disorders in the geriatric patient: Double‐blind Study. Journal of the American Geriatrics Society 1971;19:208‐17. [DOI] [PubMed] [Google Scholar]
Einspruch 1976 {published data only}
- Einspruch BC. Helping to make the final years meaningful for the elderly residents of nursing homes. Diseases of the Nervous System 1976;37:439‐42. [PubMed] [Google Scholar]
Forster 1955 {published data only}
- Forster W, Schultz S, Henderson AL. Combined hydrogenated alkaloids of ergot in senile and arteriosclerotic psychoses. Geriatrics 1955;10:26‐30. [PubMed] [Google Scholar]
Gentili 1976 {published data only}
- Gentili E, Guerra A. Studio clinico sull'azione dell'Hydergina nelle insufficienze cerebro‐vasculari acute e croniche. Archives of Internal Medicine 1976;28:101‐12. [Google Scholar]
Gerin 1969 {published data only}
- Gerin J. Symptomatic treatment of cerebrovascular insufficiency with Hydergine. Current Therapeutic Research 1969;1:539‐46. [PubMed] [Google Scholar]
Gerin 1970 {published data only}
- Gerin J. Physiopathologie de la sclerose cerebrale et experiences cliniques avec les derives dihydrogenes de l'ergot de seigle [Physiopathologie de la sclerose cerebrale et experiences cliniques avec les derives dihydrogenes de l'ergot de seigle]. Ars Medici 1970;25:663‐9. [Google Scholar]
Glover 1980 {published data only}
- Glover BH. Drug therapy of confusional states in the elderly. JAMA 1980;244:1497. [Google Scholar]
Good 1982 {published data only}
- Good WR, Griffiths RA. Hydergine in depression and dementia.. British Journal of Clinical Practice 1982;16:38‐42. [Google Scholar]
Grill 1969 {published data only}
- Grill P, Broicher B. Therapy for cerebral insufficiency. Dtsch. Med. Wschr. 1969;94:2429‐35. [DOI] [PubMed] [Google Scholar]
Grobe Einsler 1993 {published data only}
- Grobe Einsler R. Clinical aspects of nimodipine. Clinical Neuropharmacology 1993;16(Suppl 1):S39‐S45. [DOI] [PubMed] [Google Scholar]
Gross 1979 {published data only}
- Gross RJ, Eisdorfer CE, Schiller HS, Cox G. Effect of ergot alkaloids on serum prolactin in non‐psychotic organic brain syndrome of the elderly. Experimental Aging Research 1979;5:293‐302. [DOI] [PubMed] [Google Scholar]
Heiss 1971 {published data only}
- Heiss R, Seus R, Fahrenberg J. Study on the psychodynamic effect of dihydroergotoxin [Eine studie prufung der psychodynamischen wirkung von dihydroergotoxin]. Arzneimittel‐Forsch 1971;21:797‐800. [PubMed] [Google Scholar]
Herzfeld 1972a {published data only}
- Herzfeld U, Christian W, Oswald WD, Ronge J, Wittgen M. Analysis of effectiveness of Hydergine in a long‐term trial: An interdisciplinary study. Med. Klin. 1972;67:1118‐25. [PubMed] [Google Scholar]
Herzfeld 1972b {published data only}
- Herzfeld U, Christian W, Ronge J, Wittgen M. Determinants estimating cerebral functions after long term therapy with Hydergine. Aerztl. Forsch 1972;26:215‐28. [PubMed] [Google Scholar]
Hofstatter 1956 {published data only}
- Hofstatter L, Ossorio A, Mandl B, Kohler LH, Busch AK, Hyman A. Pharmaceutical treatment of patients with senile brain changes. Archives of Neurology 1956;75:316‐22. [DOI] [PubMed] [Google Scholar]
Hollingsworth 1974 {published data only}
- Hollingsworth SW. Effective nursing home care for elderly patients: Evaluation and treatment. Scientific exhibit, American Medical Association, Clinical Convention, Portland, OR 1974.
Hollingsworth 1980 {published data only}
- Hollingsworth SW. Response of geriatric patients from the satellite nursing homes of Maricopa County to Hydergine therapy: A double‐blind study. Current Therapeutic Research 1980;27:401‐10. [Google Scholar]
Hoyer 1969 {published data only}
- Hoyer S. Concerning therapy of cerebrovascular insufficiency [Zur therapie der zerebralen insuffizienz]. Dtsch. Med. Wschr. 1969;94:2654‐5. [Google Scholar]
Huang 2003 {published data only}
- Huang JS, Lin QC, Huang RZ. Clinical study on effect of yuantong capsule in treating vascular dementia. Chinese Journal of Integrated Traditional and Western Medicine 2003;23(11):815‐8. [PubMed] [Google Scholar]
Huber 1986 {published data only}
- Huber F, Koberle S, Prestele H, Spiegel R. Effects of long‐term ergoloid mesylates ('Hydergine') administration in healthy pensioners: 5‐year results.. Current Medical Research Opinion 1986;10:256‐79. [DOI] [PubMed] [Google Scholar]
Hughes 1976 {published data only}
- Hughes JR, Williams JG, Currier RD. An ergot alkaloid preparation (Hydergine) in the treatment of dementia: critical review of the clinical literature. Journal of the American Geriatics Society 1976;24(11):490‐497. [DOI] [PubMed] [Google Scholar]
Irfan 1978a {published data only}
- Irfan S, Linder L. The effect of dihydrogenated ergot alkaloids in the treatment of geriatric patients suffering from senile mental deterioration. XI International Congress of Gerontolgy. Tokyo, Japan: SCIMED Publishers, Inc., 1978:142. [Google Scholar]
Jansen 1985 {published data only}
- Jansen W, Bruckner GW, Jansen P. The treatment of senile dementia associated with cerebrovascular insufficiency: a comparative study of buflomedil and dihydrogenated ergot alkaloids. Journal of International Medical Research 1985;13(1):48‐53. [DOI] [PubMed] [Google Scholar]
Jarvik 1981 {published data only}
- Jarvik LF. Hydergine as a treatment for organic brain syndrome in late life. Psychopharmocology 1981;36:3680‐2. [Google Scholar]
Jenike 1986 {published data only}
- Jenike MA, Albert MS, Heller H, LoCastro S, Gunther J. Combination therapy with Lecithin and Ergoloid Mesylates for Alzheimer's disease. Journal of Clinical Psychiatry 1986;47:249‐51. [PubMed] [Google Scholar]
Jenike 1989 {published data only}
- Jenike MA. Alzheimer's Disease and Other Dementias. In: Jenike MA editor(s). Geriatric Psychiatry and Psychopharmacology: A Clinical Approach. Saint Louis, MO, USA: Mosby‐Year Book, Inc., 1989:127‐202. [Google Scholar]
Jenike 1990 {published data only}
- Jenike MA, Albert M, Baer L, Gunther J, et al. Ergot mesylates for Alzheimer's disease: A year‐long double‐blind trial of 3mg vs 12 mg daily. International Journal of Geriatric Psychiatry 1990;5(6):375‐380. [Google Scholar]
Jennings 1972 {published data only}
- Jennings WG. An ergot alkaloid preparation (Hydergine) versus placebo for treatment of symptoms of cerebrovascular insufficiency: Double‐blind study. Journal of the American Geriatrics Society 1972;20:407‐12. [DOI] [PubMed] [Google Scholar]
Junod 1978 {published data only}
- Junod JP. Long‐term study of chronic cerebrovascular insufficiency [Etude longitudinale de l'insuffisance cerebro‐vasculaire chronique]. Med. et Hyg. 1978;36:3680‐2. [Google Scholar]
Kaiser 1956 {published data only}
- Kaiser G, Tschabitscher H. Erfahrungen mit Hydergin in der Behandlung zerebraler Durchblutungsstorungen im hoheren [Erfahrungen mit Hydergin in der Behandlung zerebraler Durchblutungsstorungen im hoheren]. Alter. Wien. Klin. Wschr. 1956;68:150‐4. [PubMed] [Google Scholar]
Kanowski 1988 {published data only}
- Kanowski S, Fischhof P, Hiersemenzel R, Rohmel J, Kern U. Therapeutic efficacy of nootropic drugs ‐ a discussion of clinical phase III studies with Nimodipine as a model. Zeitschrift fur Gerontopsychologie und ‐psychiatrie 1988;1:35‐44. [Google Scholar]
Klein 1985 {published data only}
- Klein L, Cohen VJ. Insuficiencia cerebrovascular su tratamiento con dihidroergotoxina, 4.5mg [Insuficiencia cerebrovascular su tratamiento con dihidroergotoxina, 4.5mg]. Sem. Med 1985;167:189‐95. [Google Scholar]
Koberle 1984 {published data only}
- Koberle S, Spiegel R. A long‐term study with co‐dergocrine mesylate (Hydergine) in healthy pensioners ‐ Results after 3 years. Gerontology (Basel) 1984;30:3‐52. [DOI] [PubMed] [Google Scholar]
Kugler 1978 {published data only}
- Kugler J, Oswald WD, Herzfeld U, Seus R, Pingel J, Welzel D. Long‐term treatment of age‐related cerebral insufficiency. Dtsch. Med. Wschr. 1978;103:456‐62. [DOI] [PubMed] [Google Scholar]
Kugler 1985 {published data only}
- Kugler VJE, Meurer‐Krull BCh. mesylate] [Electroencephalography and psychometric measurements during the treatment of cerebral insufficiency with nicergoline and dihydroergotamine mesylate] Electroencephalography and psychometric measurements during the treatment of cerebral insufficiency with nicergoline and dihydroergotamine mesylate] mesylate] [Elektroenzephalographische und psychometrische messungen bei der behandlung der zerebralen insuffizienz mit Nicergolin und Dihydroergotoxin‐Mesylat]. Arzneimittel‐Forsch 1985;35:1865‐70. [PubMed] [Google Scholar]
Kuskowski 1990 {published data only}
- Kuskowski MA, Morley G, Malone SM, Dysken MW, Okaya A. Hydergine treatment and psychophysiological measures in primary degenerative dementia. Journal of Psychiatry and Neurology 1990;3:41‐7. [DOI] [PubMed] [Google Scholar]
Labecki 1954 {published data only}
- Labecki TD, Busby CL. Dihydrogenated ergot derivatives in cerebral arteriosclerosis. Journal of Gerontology 1954;9:485. [Google Scholar]
Ladurner 1991 {published data only}
- Ladurner G, Erhart P, Erhart C, Scheiber V. Therapy of organic brain syndrome with nicergoline given once a day. ORIGINAL TITLE: Therapie des hirnorganischen Psychosyndroms mit Nicergolin in taglicher Einmalgabe.. Wien‐Klin‐Wochenschr 1991;103(1):8‐14. [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li H, Liu F, Xu LR. Effect of huannao yicong capsule on senile mild cognitive impairment. Chinese Journal of Integrated Traditional and Western Medicine 2004;24(8):689‐93. [PubMed] [Google Scholar]
Liao 2004 {published data only}
- Liao XL, Chen KY, Zheng ZR, Liu H, Zheng KY. Clinical study of shoulingjiannao capsule in improvement of brain function for patients with vascular dementia. Chinese Journal of Clinical Rehabilitation 2004;8(1):112‐14. [Google Scholar]
Linden 1975 {published data only}
- Linden ME. Mental decline in elderly patients. Scientific exhibit, National Association of Private Psychiatric Hospitals, Palm Springs, CA 1976.
- Linden ME. Retirement and the elderly patients. Problems and practical therapy. Scientific Exhibit, American Geriatrics Society, 32nd Annual Meeting, Miami Beach, FL 1975; Vol. 4:16‐17.
Linden 1976 {published data only}
- Linden ME. Mental decline in elderly patients. Scientific exhibit, National Association of Private Psychiatric Hospitals, Palm Springs, CA 1976.
Linder 1977 {published data only}
- Linder L, Irfan S. Hydergine in the treatment of geriatric patients suffering from senile mental deterioration. VIth World Congress of Psychiatry, Honolulu, HI. Washington, DC: American Psychiatric Association., 1977; Vol. 1025:188.
Liu 2005 {published data only}
- Liu XD, Wan Q, Wei D, Wang XM. Cognitive and non‐cognitive functions in patients with vascular dementia and the interventional efficacy of dihydroergotamine mesilate plus nimodipine. Chinese Journal of Clinical Rehabilitation 2005;9(20):48‐9. [Google Scholar]
Lozeron 1974 {published data only}
- Lozeron H. Cerebral circulatory disorders and arteriosclerosis. Their treatment with Hydergine [Troubles circulatoires cerebraux et arteriosclerose. Leur traitement par l'Hydergine]. Praxis 1974;63:660‐5. [PubMed] [Google Scholar]
Luo 2001 {published data only}
- Luo ZG, Zhou WQ, Gao P. Clinical observation on effect of Xianlong capsule in treating vascular dementia. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(8):565‐8. [PubMed] [Google Scholar]
Mars 1970 {published data only}
- Mars G, Nebuloni G. Treatment of cerebral vascular insufficiency syndromes in old age. Controlled research by double blind technique [Sul trattamento delle sindromi da insufficienza cerebrale vascolare nell'eta avanzata]. G. Geront 1970;18:153‐76. [PubMed] [Google Scholar]
Martucci 1986 {published data only}
- Martucci N, Manna V, Mailand F. EEG‐Pharmacological and neuropsychological study of Dihydroergocristine Mesylate in patients with chronic cerebrovascular disease. Advances in Therapy 1986;3:210‐23. [Google Scholar]
Matejcek 1979 {published data only}
- Matejcek M, Knor K, Piguet PV, Weil C. Electroencephalographic and clinical changes as correlated in geriatric patients treated three months with an ergot alkaloid preparation. Journal of the American Geriatrics Society 1979;27:198‐202. [DOI] [PubMed] [Google Scholar]
Matejcek 1980 {published data only}
- Matejcek M. Cortical correlates of vigilance regulation and their use in evaluating the effects of treatment. In: Goldstein M, Calne DB, Lieberman A, Thorner MO editor(s). Series advances in biochemical psychopharmacology. Vol. 23, New York: Raven Press, 1980:339‐348. [PubMed] [Google Scholar]
Matejcek 1986 {published data only}
- Matejcek M, Blasowitsch R, Schweingruber M, Abt K. Some correlations in geriatric patients between EEG parameters and clinical status as evaluated using the observer‐rated SCAG Rating Scale. Neuropsychology 1986;15:49‐50. [DOI] [PubMed] [Google Scholar]
McConnachie 1978 {published data only}
- McConnachie RW. The clinical assessment of brain failure in the elderly. Pharmacology 1978;16:27‐35. [DOI] [PubMed] [Google Scholar]
McDonald 1979 {published data only}
- McDonald RJ. Hydergine: a review of 26 clinical studies. Pharmakopsychiatr Neuropsychopharmakol. 1979;12(6):407‐422. [DOI] [PubMed] [Google Scholar]
McDonald 1989 {unpublished data only}
- McDonald S, Patin J. A double‐blind, placebo‐controlled study of the safety and efficacy of 4.5mg and 9.0 mg/day of Hydergine FAS capsules in patients with Alzheimer's disease. Unpublished manuscript , submitted by Sandoz Research Institute, East Hanover, NJ 1989.
Mellini 1973 {published data only}
- Mellini M. Clinical use of a new vasoactive drug: Nicergoline [Considerazioni sull'impiego clinico di un nuovo farmaco vasoattivo: la nicergolina]. Minerva Med. 1973;64:1844‐53. [PubMed] [Google Scholar]
Memin 1973 {published data only}
- Memin Y, Hueber EN. Double‐blind study according to a quantitative scale of evaluation of a treatment for chronic cerebrovascular disorders [Etude a double insu selon une echelle d'appreciation quantitative d'un traitement des troubles vasculaires cerebraux chroniques]. Entretiens de Bichat 1973;Vol. Expansion Scientif. Francais, Paris:239‐42. [Google Scholar]
Misra 1982 {published data only}
- Misra SC, Patnaik BC, Patnaik SR, Panda RK, Maharana SN. Dehydroergotoxine mesylate (Hydergine) in cerebrovascular insufficiency (Summary). J. Assoc. Physicians India 1982;30:696. [Google Scholar]
Misurec 1978 {published data only}
- Misurec J, Moravek Z, Nahunek K, Ceskova E, Slama B, Svestka J. DH ergotoxin (Spofa) sublingual tablets in the treatment of cerebrovascular insufficiency in involution: clinical and experimental psychologic study. Act. Nerv. Sup. (Praha) 1978;20:266‐7. [PubMed] [Google Scholar]
Moglia 1983 {published data only}
- Moglia A, Bono G, Sinforiani E, Alfonsi E, Zandrini C, Pistarini C, Arrigo A, Franch F. Dihydroergotoxine Mesylate in chronic cerebrovascular insufficiency: Spectral analysis of EEG and neuropsychological correlates. Il Farmaco ‐ Pr. 1983;38:97‐102. [PubMed] [Google Scholar]
Nelson 1973 {unpublished data only}
- Nelson JJ. Comprehensive care for the aging patient. Presented at the Scientific Exhibit, Interstate Postgraduate Medical Association, Chicago, IL. 1973:1‐16.
Nelson 1975 {published data only}
- Nelson JJ. Relieving select symptoms of the elderly. Geriatrics 1975;3:133‐42. [PubMed] [Google Scholar]
Nicrosini 1976 {published data only}
- Nicrosini F, Pasotti C. Controlled clinical study on the activity and tolerability of Trivastan [Studio clinico controllato sull'attivita e sulla tollerabilita del Trivastan]. Minerva Med. 1976;67:1745‐50. [PubMed] [Google Scholar]
Novo 1978 {published data only}
- Novo FP, Ryan RP, Frazier, EL. Dihydroergotoxine Mesylate in the treatment of symptoms of idiopathic cerebral dysfunction in geriatric patients. Clin. Ther. 1978;1:359‐69. [Google Scholar]
Odanische 1981 {published data only}
- Odanische D, Eisinger JM. [Estudio comparative doble ciego/cruzado de la asociacion vincamina‐pracetam frente a la dihidroergotoxina en la insuficiencia vascular cerbral]. ed. Klin. (Ed. Esp.) 1981;21:24‐31. [Google Scholar]
Olivella 1981 {published data only}
- Olivella J, Espadaler JM. Estudio comparativo doble ciego/cruzado de la asociacion vincamina‐piracetam frente a la dihidroergotoxina en la insuficiencia vascular cerebral [Estudio comparativo doble ciego/cruzado de la asociacion vincamina‐piracetam frente a la dihidroergotoxina en la insuficiencia vascular cerebral]. Med. Klin. (ed. Esp) 1981;21:24‐31. [Google Scholar]
Orma 1956 {published data only}
- Orma EJ. Hydrogenated ergot alkaloids (Hydergine) in the treatment of geriatric postural dizziness. Ann. Med. Int. Fenniae 1956;45:39. [PubMed] [Google Scholar]
Oswald 1979 {published data only}
- Oswald WD. Psychometric procedures and questionnaires in geronto‐psychological research] [Psychometrische Verfahren und Fragebogen fur gerontopsychologische Untersuchungen]. Z. Gerontologie 1979;12:341‐50. [PubMed] [Google Scholar]
Oswald 1980 {published data only}
- Oswald WD, Lang E. The effect of treatment on performance and self‐appraisal in geriatric patients [Therapeutische beeinflussung von leistung und selbstbild bei geriatrischen patienten]. Munch. Med. Wschr. 1980;122:59‐62. [PubMed] [Google Scholar]
Oswald 1982 {published data only}
- Oswald VWD, Matejcek M, Lukaschek K, Dennler HJ, Oswald B. [On the meaning of psychometrically operationalized therapeutic effects in the treatment of brain insufficiency phenomena caused by old age demonstrated by the "Nurnberger‐Alters‐Inventar" (author's transl)] On the meaning of psychometrically operationalized therapeutic effects in the treatment of brain insufficiency phenomena caused by old age demonstrated by the "Nurnberger‐Alters‐Inventar" (author's transl)] [Uber die relevanz psychometrisch operationalisierter theapie‐effekte bei der behandlung altersbedingter insuffizienzerscheinungen des gehirns am beispiel des nurnberger‐alters‐inventars]. Arzneimittel‐Forsch 1982;32:584‐90. [PubMed] [Google Scholar]
Ouaniche 1981 {published data only}
- Ouaniche D, Eisinger J. Etude comparative en double aveugle du Cervilane contre la Dihydroergotoxine [Etude comparative en double aveugle du Cervilane contre la Dihydroergotoxine]. Lyon Mediter. Med. 1981;17:4223‐5. [Google Scholar]
Parade 1978 {published data only}
- Parade D. Therapie bei kardialer und zerebraler insuffizienz: Ergebnisse einer vergleichenden doppelblindprufung [Therapie bei kardialer und zerebraler insuffizienz: Ergebnisse einer vergleichenden doppelblindprufung]. Aerztl. Prax 1978;30:2783‐5. [Google Scholar]
Patin 1981 {unpublished data only}
- Patin J, Hamot HB. A double‐blind placebo‐controlled, multi‐clinic study to evaluate the safety and efficacy of a total daily dose of 6.0 mg of Hydergine for the treatment of cognitive, affective and behavioral symptoms associated with aging. Unpublished manuscript: Statistical report for Sandoz Pharmaceutical Research and Development 1981.
Paux 1975 {published data only}
- Paux G, Boismare F, Delaunay P. Double‐blind study with Hydergine in the elderly subject [Etude en double aveugle de l'Hydergine chez le sujet age]. Nouv. Presse. Med. 1975;4:2529. [PubMed] [Google Scholar]
Pfeiff 1980 {published data only}
- Pfeiff E, Schlegel HE, Seus R, Dennler HJ, Kugler J. Therapy of organic psychosyndrome with hydergine and digitalis. A component study using double blind technics [Therapie des hirnorganischen psychosyndroms mit Hydergin und digitalis. Eine komponentenstudie in doppelblindtechnik]. Med. Welt. (Stuttg) 1980;31:1403‐7. [PubMed] [Google Scholar]
Piguet 1981 {published data only}
- Piguet PV, Zoufal Z. Controlled double‐blind study on the clinical equivalence of 2 Hydergine dosage regimens: 1 x 4.5mg compared to 3 x 1.5mg per day [Etude controlee en double insu de l'equivalence clinique de deux regimes posologiques d'Hydergine: 1 x 4.5mg compare a 3 x 1.5mg par jour]. Rev. Med. Suisse Rom. 1981;101:157‐63. [PubMed] [Google Scholar]
PiresDeOliveira 1973 {published data only}
- Pires De Oliveira RS. Electroencephalographic alterations after administration of vaso‐active substance (Hydergine) [Alteracoes Eletrencefalograficas apos administracao de substancias Vaso‐Ativas (Hydergine)]. Arch. Neuro‐psiquiat. 1973;31:107‐22. [PubMed] [Google Scholar]
Pomara 1983 {published data only}
- Pomara N, Block R, Abraham J, Domino EF, Gershon S. Combined cholinergic precursor treatment and dihydroergotoxine mesylate in Alzheimer's disease. IRCS Med. Sci: Psychology and Psychiatry 1983;11:1048‐9. [Google Scholar]
Popkin 1956 {published data only}
- Popkin RJ. The hydrogenated alkaloids of ergot (Hydergine) in geriatrics ‐ follow up study. American Pract. Digest Treatment 1956;7:1594‐7. [PubMed] [Google Scholar]
Puxty 1989 {published data only}
- Puxty J. Community screening for dementia and evaluation of treatment. In: Carlsson A, Kanowski S, Allain H, Spiegel R editor(s). Proceedings of a Symposium Basle (CH). Basil, Switzerland: Parthenon Publishing Group, 1989:211‐230. [Google Scholar]
Puxty 1991 {published data only}
- Puxty J. High‐dose Ergoloid Mesylates (Hydergine) in community residing patients with age‐related mental decline. 7th submission to the Journal of the American Geriatric Association 1991.
Pöpperl 2004 {published data only}
- Pöpperl G, Tatsch K, Ruzicka E, Storch A, Gasser T, Schwarz J. Comparison of alpha‐dihydroergocryptine and levodopa monotherapy in Parkinson's disease: assessment of changes in DAT binding with [123I]IPT SPECT. Journal of Neural Transmission 2004;111(8):1041‐52. [DOI] [PubMed] [Google Scholar]
Rao 1972 {published data only}
- Rao DB, Norris JR. A double‐blind investigation of Hydergine in the treatment of cerebrovascular insufficiency in the eldery. Hopkins Med. J. 1972;130:317‐24. [PubMed] [Google Scholar]
Rehman 1973a {published data only}
- Rehman SA. Two trials comparing Hydergine with placebo in the treatment of patients suffering from cerebrovascular insufficiency. Current Medical Research and Opinion 1973;1:456‐62. [DOI] [PubMed] [Google Scholar]
Rehman 1973b {published data only}
- Rehman SA. Two trials comparing Hydergine with placebo in the treatment of patients suffering from cerebrovascular insufficiency. Current Medical Research and Opinion 1973;1:456‐62. [DOI] [PubMed] [Google Scholar]
Riccardi 1978 {published data only}
- Riccardi T, Passeri F, Locatelli F. Controlled clinical study of the effects of Anasclerol in hospitalized patients with cerebrovascular disorders [Studio clinico controllato sugli effetti dell'Anasclerol in pazienti cerebro‐vasculopatici ospedalizzati]. Minerva Med. 1978;69:2873‐8. [PubMed] [Google Scholar]
Ronge 1982 {published data only}
- Ronge J, Dennler HJ. Comparison of subjective tolerance of hydergine specific and hydergine in gerontologic therapy [Vergleich der subjektiven vertraglichkeit von Hydergin spezial und Hydergin in der gerontotherapie]. Med. Welt. (Stuttg) 1982;33:1485‐9. [PubMed] [Google Scholar]
Rosen 1975 {published data only}
- Rosen HJ. Mental decline in the elderly: Pharmacotherapy (Ergot Alkaloids versus Papaverine). J. Amer. Geriat. Soc. 1975;23:169‐174. [DOI] [PubMed] [Google Scholar]
Roubicek 1971 {published data only}
- Roubicek J, Geiger C, Abt K. Hydergine therapy in geriatric patients. VIth European Congress of Clinical Gerontology. Berne, Switzerland, abstract no.89 1971.
- Roubicek J, Geiger C, Abt K. Hydergine therapy in geriatric patients. 5th World Congress of Psychiatry, Mexico. American Psychiatric Association, 1971:501. [Google Scholar]
Roubicek 1972 {published data only}
- Roubicek J, Geiger CH, Abt K. An ergot alkaloid preparation (Hydergine) in geriatric therapy. Journal of the American Geriatrics Society 1972;5:222‐9. [DOI] [PubMed] [Google Scholar]
Roubicek 1973 {published data only}
- Roubicek J, Geiger CH, Abt K, Huber F. Hydergine therapy in geriatric patients. In: Steinmann B, Huber H editor(s). Proceedings of the VI European Congress of Clinical Gerontology. Berne, Switzerland: Verlag, 1973:468‐81. [Google Scholar]
- Roubicek J, Huber F. Influence des alcaloides hydrogenes de l'ergot sur la fonction cerebrale dans la vieillesse [Influence des alcaloides hydrogenes de l'ergot sur la fonction cerebrale dans la vieillesse]. In: Geraud J, Lazorthes G, Bes A editor(s). L'ischemie cerebrale dans la territoire carotidien. Journees internationales de circulation cerebrales. Toulouse, France: Revue de Medecine de Toulouse, 1973:509‐13. [Google Scholar]
Roubicek 1975 {published data only}
- Roubicek J. Hydergina en la terapia geriatrica [Hydergina en la terapia geriatrica]. Invest. Medica Internacional 1975;2:61‐71. [Google Scholar]
Saletu 1990 {published data only}
- Saletu B, Grunberger J, Anderer R. On brain protection of co‐dergocrine mesylate (Hydergine) against hypoxic hypoxidosis of different severity: double‐blind placebo‐controlled quantitative EEG and psychometric studies. Int J Clin Pharmacol Ther Toxicol. 1990;28(12):510‐524. [PubMed] [Google Scholar]
Saletu 1994 {published data only}
- Saletu B, Grunberger J, Anderer P, Linzmayer L, Pakesch G, Zyhlarz G. Effect‐kinetics on brain protection of two codergocrine‐mesylate preparations (Aramexe retard registered and Hydergine registered) by EEG mapping and psychometry under hypoxia. Archives of Gerontology and Geriatrics 1994;18(2):81‐99. [DOI] [PubMed] [Google Scholar]
Samorajski 1982 {published data only}
- Samorajski T, Ho BT, Kralik PM, Hartford JT. Serum prolactin changes with age, senile dementia, and dihydroergotoxine mesylate treatment. In: Giacobini E, Filogamo G, Giacobini G, Vernadakis A editor(s). Aging: Aging Brain. Cellular and Molecular Mechanisms of Aging in the Nervous System. Vol. 20, New York: Raven Press, 1982:259‐69. [Google Scholar]
Schardt 1982 {published data only}
- Schardt F, Dennler HJ. Behandlung des hochdrucks im alter mit Hydergin [Behandlung des hochdrucks im alter mit Hydergin]. Therapiewoche 1982;32:4039‐40, 4042‐3, 4046‐7. [Google Scholar]
Schartl 1978 {published data only}
- Schartl W, Bratke G, Kemper R. Uber die wirkung von Card‐Hydergin in doppelblindversuch [Uber die wirkung von Card‐Hydergin in doppelblindversuch]. Therapiewoche 1978;28:9117‐8, 9120, 9123‐6. [Google Scholar]
Schneider 1994 {published data only}
- Schneider LS, Olin JT. Overview of clinical trials of hydergine in dementia. Archives of Neurology 1994;51(8):787‐798. [DOI] [PubMed] [Google Scholar]
Schnell 1967 {published data only}
- Schnell R, Oswald WD. Geriatric pharmacotherapy of the neurasthenic syndrome (an experimental psychological and clinical long term study [Geriatrische pharmakotherapie des neurasthenischen syndroms]. Aerztl. Forsch 1967;21:464‐70. [PubMed] [Google Scholar]
Setnikar 2001 {published data only}
- Setnikar I, Schmid K, Rovati LC, Vens‐Cappell B, Mazur D, Kozak I. Bioavailability and pharmacokinetic profile of dihydroergotoxine from a tablet and from an oral solution formulation. Arzneimittelforschung 2001;51(1):2‐6. [DOI] [PubMed] [Google Scholar]
Seus 1969 {published data only}
- Seus R. A model study on geriatric drug testing under realistic practical conditions (Summary). Proceedings of the 8th International Congress of Gerontology. 1969; Vol. 2:52.
Shoptaw 2005 {published data only}
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, et al. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST). Addiction 2005;100(Suppl):178‐90. [DOI] [PubMed] [Google Scholar]
Soni 1975 {published data only}
- Soni SD, Soni SS. Dihydrogenated alkaloids of ergotoxine in non‐hospitalised elderly patients. Curr. Med. Res. Opinion. 1975;3:464‐8. [Google Scholar]
Spiegel 1983 {published data only}
- Spiegel R, Huber F, Koberle S. A controlled long‐term study with ergoloid mesylates (Hydergine) in healthy, elderly volunteers: results after three years. Journal of the American Geriatrics Society 1983;31:549‐55. [DOI] [PubMed] [Google Scholar]
Spilich 1996 {published data only}
- Spilich GJ, Wannenmacher W, Duarte A, Buendia R, Gomez JT, Ramirez S, Anaya A, Otero E. Effiacy of pyritinol versus hydergine upon cognitive performance in patients with senile dementia of the Alzheimer's type: A double‐blind multi‐center trial. Alzheimer's Research 1996;2(3):79‐84. [Google Scholar]
Stewart 1996 {published data only}
- Stewart RB, Marks RG, Caranasos GC, Hale WE. Prevalence of ergoloid mesylates use in an ambuolatory elderly popualtion over a 14 year period. Journal of Geriatric Drug Therapy 1996;10:63‐72. [Google Scholar]
Strauss 1951 {published data only}
- Strauss HL. Clinical experiences with Hydergine [Klinische erfahrungen mit Hydergin]. Med. Welt. 1951;20:113‐6. [PubMed] [Google Scholar]
Strauss 1954 {published data only}
- Strauss LH. Clinical experience with Hydergine [Klinische erfahrunger mit Hydergin]. Cardiologia 1954;25:1. [PubMed] [Google Scholar]
Tartara 1974 {published data only}
- Tartara A, Kauchtschischvili G, Arrigo A, Moglia A. EEG and clinical effects of diidroergotoxinemethansulphonate in cerebrovascular insufficiency. G. Geront. 1974;22:298‐309. [Google Scholar]
Tecce 1981 {published data only}
- Tecce JJ, Boehner MB, Cattanach L, Branconnier R, Cole JO. Hydergine treatment and brain‐functioning (CNV rebound) in Alzheimer's patients: preliminary findings. Psychoparmacology Bulletin 1981;17:202‐6. [Google Scholar]
Tecce 1983a {published data only}
- Tecce JJ, Cattanach L, Boehner‐Davis MB, Branconnier RJ, Cole JO. Neuropsychological assessment of impaired attention and drug treatment in Alzheimer's patients. Presse Med. 1983;12:3155‐2. [PubMed] [Google Scholar]
Tecce 1983b {published data only}
- Tecce JJ, Cattanach L, Boehner‐Davis MB, Branconnier RJ, Cole JO. CNV rebound, attention performance, and Hydergine treatment in Alzheimer patients. British Journal of Clinical Practice. Symposium Supplement 1983;30:19‐22. [Google Scholar]
Teixeira 1970 {published data only}
- Teixeira JE. Hydergine in symptomatic treatment of cerebrovascular insufficiency. Comparative study with placebo [Hydergine no tratamento sintomatico da insuficiencia vascular cerebral. Ensaio clinico comparativo con placebo]. Hospital 1970;77:1907‐31. [PubMed] [Google Scholar]
Thibault 1974 {published data only}
- Thibault A. A double‐blind evaluation of Hydergine and placebo in the treatment of patients with organic brain syndrome and cerebral arteriosclerosis in a nursing home. Current Medical Research and Opinion 1974;2:482‐8. [DOI] [PubMed] [Google Scholar]
Thienhaus 1987 {published data only}
- Thienhaus OJ, Wheeler, BG, Simon, BS, Zemlan, FP, Hartford, JT. A controlled double‐blind study of high‐dose Dihydroergotoxine Mesylate (Hydergine) in mild dementia. Journal of the American Geriatrics Society 1987;35:219‐23. [DOI] [PubMed] [Google Scholar]
Tinklenberg 1986 {unpublished data only}
- Tinklenberg JR, Davies HD, Miller TP, Moylan AE. High dose hydergine treatment of alzheimer's patients: Final report. Unpublished manuscript 1993.
Triboletti 1969 {published data only}
- Triboletti F, Ferri H. Hydergine for treatment of symptoms of cerebrovascular insufficiency. Current Therapeutic Research 1969;11:609‐20. [PubMed] [Google Scholar]
Tucker 1982 {published data only}
- Tucker JS. An assessment of the effect of intramuscular Hydergine in elderly patients with early dementia using critical flicker fusion thresholds. British Journal of Clinical Practice 1982;16:16‐17. [Google Scholar]
Winslow 1974 {unpublished data only}
- Winslow IE. The hospitalized geriatric patient: Guidelines for effective therapy. Presented at the Scientific Exhibit, American Medical Association, 28th Clinical Convention, Portland, Oregon. 1974:1‐24.
Yan 2001 {published data only}
- Yan XP, Wang H, Zhang TZ. Clinical observation of effect on tongmai yizhi capsule in treating vascular dementia. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(8):573‐5. [PubMed] [Google Scholar]
Yesavage 1979 {published data only}
- Yesavage JA, Hollister LE, Burian E. Dihydroergotoxine: 6mg versus 3mg dosage in the treatment of senile dementia. Preliminary report. Journal of the American Geriatrics Society 1979;27:80‐2. [DOI] [PubMed] [Google Scholar]
Yesavage 1981b {published data only}
- Yesavage JA, Westphal J, Rush L. Senile dementia: Combined pharmacologic and psychologic treatment. Journal of the American Geriatrics Society 1981;29:164‐71. [DOI] [PubMed] [Google Scholar]
Yoshikawa 1983 {published data only}
- Yoshikawa M, Hirai S, Aizawa T, Kuroiwa Y, Goto F, Sofue I, Toyokura Y, Yamamura H, Iwasaki Y. A dose‐response study with Dihydroergotoxine Mesylate in cerebrovascular disturbances. Journal of the American Geriatrics Society 1983;31:1‐7. [DOI] [PubMed] [Google Scholar]
Zhao 1999 {published data only}
- Zhao Y, Zhou W, Gao P. Clinical study on effect of xianlong capsule on senile vascular dementia. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19(10):585‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Klebel 1992 {published data only}
- Klebel E, Hutter M, Maritsch F. The influence of co‐derogocrine mesilate (hydergine FAS) on psychometric performance of elderly patients with organic brain syndrome. Zeitschrift Fur Gerontolgie and Psychiatrie 1992;5(1):43‐55. [Google Scholar]
Additional references
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H, Junior. Bias in Treatment Assignment in Controlled Clinical Trials. N Eng J Med, 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
DSM III
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association. 3rd Edition. Washington DC: APA, 1987. [Google Scholar]
Guy 1976
- Guy W (ed). ECDEU Assessment manual for psychopharmacology.. Publication number 76‐388.. Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Mulrow 1996
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. In: Mulrow CD, Oxman AD editor(s). Available in The Cochrane Library [database on disk and CDROM], Issue 1 [updated 9 December 1996]. Updated quarterly. Oxford: Update Software, 1997. [Google Scholar]
NINCDS‐ADRA
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease, Neurolog. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease, Neurology 1984;4:939‐44. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shader 1974
- Shader RL, Harmatz JS, Salzman C. A new scale for clinical assessment in geriatric populations: Sandoz‐Clinical‐Assessment‐Geriatric(SGAG). J Am Geriatr Soc 1974;22:107‐113. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Olin 2000
- Olin J, Schneider L, Novit A, Luczak S. Hydergine for dementia. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000359] [DOI] [PubMed] [Google Scholar]


