Abstract
Parasites of animals and plants can encounter trade-offs between their specificity to any single host and their fitness on alternative hosts. For parasites that manipulate their host's behaviour, the added complexity of that manipulation may further limit the parasite's host range. However, this is rarely tested. The recently described crypt-keeper wasp, Euderus set, changes the behaviour of the gall wasp Bassettia pallida such that B. pallida chews a significantly smaller exit hole in the side of its larval chamber and ‘plugs’ that hole with its head before dying. Euderus set benefits from this head plug, as it facilitates the escape of the parasitoid from the crypt after it completes development. Here, we find direct and indirect evidence that E. set attacks and manipulates the behaviour of at least six additional gall wasp species, and that these hosts are taxonomically diverse. Interestingly, each of E. set's hosts has converged upon similarities in their extended phenotypes: the galls they induce on oaks share characters that may make them vulnerable to attack by E. set. The specialization required to behaviourally manipulate hosts may be less important in determining the range of hosts in this parasitoid system than other dimensions of the host–parasitoid interaction, like the host's physical defences.
Keywords: host specificity, behavioural manipulation, extended phenotype, Euderus set, gall wasp
1. Introduction
Trade-offs present limits to adaptation [1], as resources are finite and maximal optimization of all traits at once is impossible. For example, when energy is dedicated towards growth, it may not be available for reproduction [2]. One common trade-off observed in nature is that when parasites adapt to attack one group of hosts, their adaptations may reduce their ability to attack other hosts. This trade-off has been used to explain, in part, the tendency for parasites to specialize on a subset of available hosts (e.g. herbivorous insects on plant hosts or blood feeding insects on animal hosts) [3]. However, few studies quantify the host ranges of behaviour-manipulating parasites, and this is particularly true for parasitoids (insects that parasitize and eventually kill their hosts), which constitute perhaps the most speciose group of all animals [4].
Two recent papers [5,6] describe the discovery and life history of the parasitoid ‘crypt keeper wasp’ (Euderus set), so named because of its parasitism and behavioural manipulation of the ‘crypt-gall wasp’ (Bassettia pallida) in live oaks (Quercus virginiana and Q. geminata). Oviposition by a B. pallida female into a young stem induces the formation of a swollen internal gall (known as a ‘crypt’) inside the stem, where the developing larval wasp will then feed and grow. Unparasitized adult B. pallida later chew a small exit hole in their gall—made of woody plant material—and then fly away. However, when parasitized by E. set, B. pallida chew significantly smaller exit holes, do not (or cannot) leave the gall and stop moving with their heads blocking (or ‘plugging’) the exit hole. The parasitoid then feeds on the disabled body of the host wasp, and, upon maturing into an adult wasp, chews through the ‘head plug’ and exits the gall (figure 1). In the absence of information on mechanisms, we are unable to determine if E. set is a ‘simple’ manipulator that debilitates/reduces host activity in a specific favourable context or a more ‘complex’ manipulator (e.g. one that interacts with central nervous system functions) [7,8]. Because B. pallida itself manipulates oaks to develop galls in their young branches, E. set is also a ‘hypermanipulator’—a rarely quantified phenomenon in which a parasite manipulates a parasite that itself manipulates its host. Though many species of Euderus have previously been described [9], E. set represents the first definitive example of behavioural manipulation in this genus.
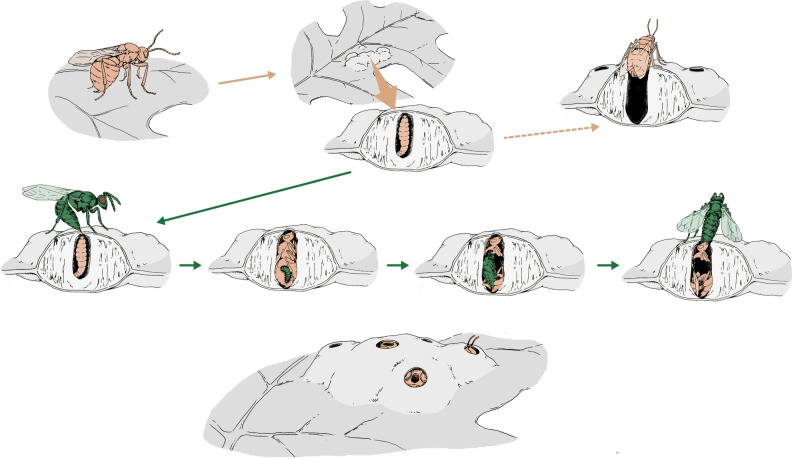
Figure 1.
Life cycles of an oak gall wasp (in this case, Melikaiella ostensackeni; pink), and the crypt keeper wasp, E. set (green). It is not known whether the larvae of E. set are ecto- or endoparasitic, so this illustration is intentionally ambiguous. Also, although the stage of adult attack is shown to be at the host's late larval stage, it is not yet known exactly when attack occurs. Artwork by Mona Luo.
Given the potential trade-off between specialization and host range, does this strategy of behavioural manipulation limit the host range of E. set? The parasitoid communities of most oak gall wasps are understudied or unknown, such that it is premature to assert that the manipulation of B. pallida by E. set is a unique relationship. Indeed, Weinersmith et al. [6] report additional examples of ‘head plugging’ in the gall wasp Bassettia ligni on the host plants Q. lobata and Q. douglasii. They also report evidence of head-plugging in an unidentified cynipid on Q. nigra in southeast Texas [6], which has since been keyed to genus Bassettia (probably B. aquaticae; A.A.F. 2018, unpublished data). Further, our own parasitoid-rearing studies have yielded several unidentified Euderus from non-Bassettia galls (A.K.G.W. & A.A.F. 2018, unpublished data). These findings suggest the possibility that E. set may have a wide host range, but requires assessment of whether these Euderus are all the same species and whether they are inducing the same behavioural changes in their gall hosts.
Here, we ask whether the host head-plugging manipulation by E. set is (i) limited to the B. pallida host and its close relatives or (ii) found across multiple gall wasp species. If (ii), then what properties of hosts make them vulnerable to E. set? We identify hosts and non-hosts of E. set using wide-ranging collections (approx. 100 species of oak gall wasp) and DNA barcoding. We assess associations between Euderus infection and ‘head-plugging’ behaviour in Euderus' hosts. This study addresses a critical question and a gap in our current knowledge regarding how manipulation of insect behaviour by parasitoids is (or is not) translatable across disparate hosts.
2. Material and methods
(a). Collections and discovery of new Euderus/galler associations
From August 2015 to August 2018, we collected more than 23 000 galls from more than 10 oak species (electronic supplementary material, table S1), with a focus on maximizing the diversity of gall wasp species and on collecting mature galls that were most likely to have been parasitized. All galls were North American, and most collections (approx. 60%) were made in Midwestern states, but at least some collections extended farther afield, including to, for example, New England, North Carolina and Texas. Details on rearing conditions are provided in the electronic supplementary material. We identified Euderus to species using Yoshimoto [9] and Egan et al. [5]. We extracted DNA from two to six individual Euderus reared from each of the six different hosts using a CTAB/PCI method based on Chen et al. [10]. For each sample, we sequenced a segment of the mitochondrial cytochrome oxidase I (mtCOI) gene (see electronic supplementary material).
(b). Observations of Euderus behaviour and emergence phenology
We chose one galler species for a focused study of the natural history, behaviour and phenology of Euderus host manipulation in a non-Bassettia gall. From 15 June to 20 July 2018, we collected Melikaiella ostensackeni galls weekly from a single, heavily infested pin oak (Quercus palustris) tree in Iowa City, IA, USA. Galls of M. ostensackeni manifest as parenchymal thickenings that project on both sides of the leaf. Each gall contains anywhere from a dozen to upwards of a hundred larval chambers (figure 2a). Each week we collected galls representing from 319 to 1040 larval chambers (electronic supplementary material, table S2). Upon collection, we cut each multi-chambered gall from its leaf, assigned it a number, photographed it from above using a Canon EOS Rebel T1i camera with an MP-E 65 mm f/2.8 1-5x Macro Lens (Canon USA, New York, NY, USA) mounted on a StackShot automated macro rail (Cognisys Inc., Traverse City, MI, USA) and placed it into an individual closed container. Individual chambers in each gall were visible as light green subcircles, and, especially in later collections, dark circles with an exit hole indicated that a galler, parasitoid or inquiline had emerged (figure 2a). Twice daily, we checked galls for (i) emergent animals in cups, (ii) appearance of new emergence holes, (iii) signs of active chewing/movement at the gall surface, (iv) apparent head plugs, and (v) previously identified head plugs that had been chewed through or otherwise destroyed. Head plugs were usually obvious, and defined as when gallers had chewed incomplete holes and had then stopped moving (figure 2c,d). We softly poked putative head plugs with the blunt end of a 0.20 mm pin (BioQuip, Rancho Dominquez, CA, USA) and only recorded an observation as a head plug when the insect made no movement in response. All observations were recorded daily on printed black and white photographs of each gall (figure 2b), such that newly emergent animals (gallers or parasites) could be traced back to a specific gall chamber or head plug. After Euderus had ceased emerging, we dissected six of the remaining head plugs to capture details of Euderus interactions with its host while inside the gall. In two chambers, we found gall wasps along with an apparently dead larva (electronic supplementary material, figure S3). We extracted DNA from these larvae and sequenced mtCOI as above.
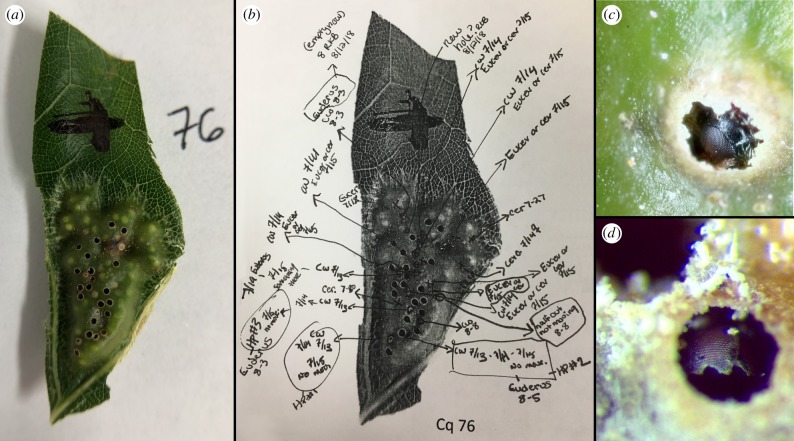
Figure 2.
Details of methods for the M. ostensackeni/Euderus emergence study. (a) Photograph of an M. ostensackeni gall 1 h after collection, showing both intact chambers (light green circles) and galls from which an insect had emerged prior to the gall's collection (dark circles). Very light tan-coloured circles with a small pinprick of black in the centre are animals that were either actively chewing out of their gall or had stopped chewing and were already ‘head plugs’. (b) Example of notations made across the course of the study. Any changes to the gall were noted daily, such that all emergent animals found in the cup on any given day could be associated with the individual chamber from which they emerged. This particular gall had four Euderus emerge from gall chambers for which chewing (CW) and/or a ‘head plug’ (HP) had previously been observed. Other notation refers to dates of observations, initials of the observer (e.g. ‘RKB’) or the genus of the emergent animal (e.g. ‘Eucer’ for the inquiline Euceroptres); (c) close-up of a ‘head plug’ where a M. ostensackeni galler has chewed a partial hole and then stopped moving; (d) close-up of M. ostensackeni head after emergence of a Euderus parasitoid. (Online version in colour.)
3. Results
(a). Collections and discovery of new Euderus/galler associations
Between 2015 and 2018, we collected more than 23 000 galls representing approximately 100 oak gall wasp species (electronic supplementary material, table S1) and subsequently reared more than 15 000 individual parasitoids, inquilines and hyperparasitoids (A.K.G.W. & A.A.F. 2018, unpublished data). Among these collections, we reared Euderus wasps from six different gall wasp host species (table 1): Andricus quercuspetiolicola (n = 68), Callirhytis flavipes (14), Callirhytis quercusscitula (34), M. ostensackeni (157, including the 44 below), Melikaiella tumifica (10) and Neuroterus quercusbatatus (22). All Euderus keyed morphologically to E. set.
Table 1.
Description of oak gall wasps, tree habitats and geographical locations from which E. set has been reared and identified.
| cynipid oak gall-wasp | oak host(s) species and section | locations | description of gall | timing of galls collections and Euderus emergences | record |
|---|---|---|---|---|---|
| Andricus quercuspetiolicola |
Q. alba; Q. bicolor (section: Quercus) |
IA | multichambered integral leaf gall, swelling of leaf petiole and sometimes basal midrib projecting on both sides of leaf | galls collected May–August, most E. set emergences June–July | this study |
| Bassettia pallida |
Q. geminata;
Q. virginiana (section: Quercus) |
FL, GA, LA, MS, TX | integral stem gall (a ‘crypt’) | galls collected July–October, most E. set emergences February–March | [11,12] |
| Callirhytis flavipes |
Q. macrocarpae (section: Quercus) |
IA | integral leaf gall, elongated swelling of the midrib | galls collected May–July, most Euderus emergences June–July | this study |
| Melikaiella ostensackeni |
Q. palustris Q. rubra (section: Lobatae) |
IA, MO | integral leaf gall, parenchyma of leaf swelling, projecting on both sides, multiple chambers | galls collected May–August, most E. set emergences July–August | this study |
| Callirhytis quercusscitula |
Q. imbricaria (section: Lobatae) |
IA, MO | swelling of stem at the base of where new leaves attach | galls collected June, most E. set emergences June–July | this study |
| Melikaiella tumifica |
Q. coccinea
(section: Lobatae) |
PA | integral leaf gall, swelling of midrib on the bottom third of leaf | galls collected May–June, most E. set emergences July | this study |
| Neuroterus quercusbatatus |
Q. bicolor
(section: Quercus) |
IA | integral leaf gall | galls collected June, most E. set emergences June–July | this study |
COI sequences also suggest that all Euderus wasps in this study were E. set. The 16 adult and two larval Euderus sequenced in this study had mtCOI sequences that were 95–100% identical to one another (electronic supplementary material, figure S1). Though some sequences were relatively different (approx. 5%) from one another, percentage sequence similarity should not alone be used to define species [13], and wasps with some of the most divergent mtCOI sequences in the dataset were reared from the same collections, suggesting these are all the same species with variable mtCOI haplotypes. The alternative—that two very closely related Euderus species attack the same gall wasps on the same trees and attack no other gall wasps in our widespread collections—remains possible, but seems unlikely.
(b). Observations of Euderus behaviour and emergence
Across 128 M. ostensackeni galls, we reared 291 adult M. ostensackeni gallers, 44 Euderus wasps and 649 other parasitoids and inquilines (electronic supplementary material, table S3 and figure S2). During the course of the study, we observed 63 M. ostensackeni ‘head plugs’. Thirty-nine emergent Euderus were conclusively linked to a specific head plug that had been noted during a previous observation period and that, after Euderus emergence, had a visible hole in the gall wasp's head. The 24 head plugs from which no Euderus emerged did not produce any other adult parasitoids, suggesting that Euderus may have died in an early stage of development as in the two we found during dissections (electronic supplementary material, figure S3). In five cases, a Euderus emerged from a gall where we had not previously observed a head plug, indicating either a failure of detection or a genuine lack of a plug. No non-Euderus parasitoid emerged from any gall previously characterized as a head plug.
For the five additional non-Bassettia gall wasp species from which Euderus emerged in the laboratory (table 1), we inspected a subset of post-emergence galls for evidence of head plugs, chewed heads or other signs of hypermanipulation previously described in the B. pallida/E. set interaction. We observed more than 10 head plugs, both intact and eviscerated, on A. quercuspetilicola galls and found one or more incidences of head plugs in each of the other four additional hosts (electronic supplementary material, figure S4). Head plugs were not observed in gall collections from which Euderus did not emerge, as was the case in the previous study [6].
4. Discussion
Given that trade-offs present limits to adaptation, we hypothesized that for parasites that manipulate their host's behaviour, the complexity of that manipulation would limit the parasite's host range. Thus, we predicted that E. set-like wasps from different hosts should be genetically and morphologically distinct lineages tightly coevolving with their hosts. In contrast with our prediction, the combination of natural history observations, with morphological and genetic data imply that all Euderus wasps reared in this study are E. set, the species described previously from B. pallida. We now, therefore, have direct (2 gall wasp species) and indirect (5 species) evidence that E. set attacks and manipulates at least seven gall wasp hosts spanning five genera (table 1). Notably, many potential gall wasp hosts were collected from the same tree host species at the same time as the E. set-associated gallers, and yet were not apparently manipulated or infected by E. set. Our collections (electronic supplementary material, table S1) represent approximately 1/7 of the approximately 700 described species of Nearctic oak gall wasps [14]. So, while E. set is oligophagous, widespread (Iowa to Pennsylvania; south to Texas and Florida) and attacks representatives of five different genera, it is also not a broad generalist on all oak gall wasps. This pattern of host use is unexpected. Many insect parasites of plants and animals are taxonomically specialized [4,15,16], and for parasites that manipulate the behaviour of their hosts, the symbiotic intimacy implied by behavioural control might be expected to further restrict host range—though the literature to date is equivocal on this point [7,11,17].
Remarkably, each of E. set's hosts show similar extended gall phenotypes suggesting that behavioural manipulation may be less important to its host range than other dimensions of the host–parasitoid interaction, such as host's physical defences. The Enemy Hypothesis states that galls provide defence against natural enemies and, in turn, pressures from those natural enemies have led to a wide diversity of defensive gall structures [12,18]. Even though gall wasps are often heavily attacked by parasites, this hypothesis is supported by findings showing correlations between gall characteristics and their corresponding parasitoid communities, suggesting that gall phenotypes may limit host ranges for parasitoids [19].
Consistent with the Enemy Hypothesis, all of E. set's hosts induce integral galls (i.e. enclosed within the epidermis; gall is not detachable without causing significant damage to the plant tissue). All known hosts also lack the baroque structural defences found on many other galls, which can include spines, fuzz or larval cells suspended deep inside otherwise empty chambers [20]. These defences grow more substantial as the gall grows, which may render many galls inaccessible to Euderus and other parasitoids of gall wasp pupae. Euderus are late-instar larval or pupal parasitoids [9,21], and lack the long, exerted ovipositors typical of some other genera of late-attacking galler parasitoids [22], such that they may be limited to attacking hosts that are not buried deep within a gall nor protected by complex defence structures. Closer study of the phenology of attack in the M. ostensackeni system supports the hypothesis that E. set successfully attacks only at this late stage of development (i.e. at a stage of development where the host's body nearly fills the chamber, and is easier to find by the parasitoid's ovipositor) (electronic supplementary material, figure S5).
We have discovered that the parasitoid wasp E. set is specialized on a taxonomically diverse subset of available gall wasp hosts, a finding that contrasts with our expectation that this behaviour-manipulating insect would have a taxonomically limited host range. Further, all of the known hosts of E. set induce galls with similar characteristics, suggesting that the extended phenotypes of gall wasps better explain E. set's host range than does its ability to manipulate host behaviour.
Supplementary Material
Acknowledgements
Robin Bagley, Will Carr, Sara Devine, Rachel Erikson, Leo Gastel, Alaine Hippee, Emily Manders, Danny McGarry, Moe Shakally, Joseph Verry and Caleb Wilson assisted with gall collections and rearing and preservation of insects. Thanks to Robbins Park Environmental Education Center in Ambler, PA, USA, for permission to collect M. tumifica galls from a scarlet oak on their property. Figure 1 was illustrated by Mona Luo (http://www.monaluo.com/).
Data accessibility
All data are available as electronic supplementary material.
Authors' contributions
A.K.G.W. and A.A.F. conceived of the study. A.K.G.W. and O.S.K. conducted the M. ostensackeni study. All authors contributed to gall collections, insect rearing and writing of this paper. All authors agree to be held accountable for the content and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This study was funded by University of Iowa's Center for Global and Regional Environmental Research (CGRER).
References
- 1.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 2.Reznick D. 1985. Costs of reproduction: an evaluation of the empirical evidence. Oikos 44, 257–267. ( 10.2307/3544698) [DOI] [Google Scholar]
- 3.Forister ML, et al. 2015. The global distribution of diet breadth in insect herbivores. Proc. Natl Acad. Sci. USA 112, 442–447. ( 10.1073/pnas.1423042112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes AA, Bagley RK, Beer MA, Hippee AC, Widmayer HA. 2018. Quantifying the unquantifiable: why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 18, 21 ( 10.1186/s12898-018-0176-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan SP, Weinersmith KL, Liu S, Ridenbaugh RD, Zhang YM, Forbes AA. 2017. Description of a new species of Euderus Haliday from the southeastern United States (Hymenoptera, Chalcidoidea, Eulophidae): the crypt-keeper wasp. ZooKeys 645, 37–49. ( 10.3897/zookeys.645.11117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinersmith KL, Liu SM, Forbes AA, Egan SP. 2017. Tales from the crypt: a parasitoid manipulates the behaviour of its parasite host. Proc. R. Soc. B 284, 20162365 ( 10.1098/rspb.2016.2365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredensborg B. 2014. Predictors of host specificity among behavior-manipulating parasites. Am. Zool. 54, 149–158. ( 10.1093/icb/icu051) [DOI] [PubMed] [Google Scholar]
- 8.Lafferty KD, Shaw JC. 2013. Comparing mechanisms of host manipulation across host and parasite taxa. J. Exp. Biol. 216, 56–66. ( 10.1242/jeb.073668) [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto CM. 1971. Revision of the genus Euderus of America north of Mexico (Hymenoptera: Eulophidae). Can. Entomol. 103, 541–578. ( 10.4039/Ent103541-4) [DOI] [Google Scholar]
- 10.Chen H, Rangasamy M, Tan SY, Wang H, Siegfried BD. 2010. Evaluation of five methods for total DNA extraction from western corn rootworm beetles. PLoS ONE 5, e11963 ( 10.1371/journal.pone.0011963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez R, Fredensborg BL. 2015. Experimental test of host specificity in a behaviour-modifying trematode. Parasitology 142, 1631–1639. ( 10.1017/S0031182015001171) [DOI] [PubMed] [Google Scholar]
- 12.Price PW, Fernandes GW, Waring GL. 1987. Adaptive nature of insect galls. Environ. Entomol. 16, 15–24. ( 10.1093/ee/16.1.15) [DOI] [Google Scholar]
- 13.Cognato AI. 2006. Standard percent DNA sequence difference for insects does not predict species boundaries. J. Econ. Entomol. 99, 1037–1045. ( 10.1093/jee/99.4.1037) [DOI] [PubMed] [Google Scholar]
- 14.Stone GN, Schönrogge K, Atkinson RJ, Bellido D, Pujade-Villar J. 2002. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 47, 633–668. ( 10.1146/annurev.ento.47.091201.145247) [DOI] [PubMed] [Google Scholar]
- 15.Price PW. 1980. Evolutionary biology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Hawkins B. 1994. Pattern and process in host–parasitoid interactions. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Moore J, Gotelli NJ. 1996. Evolutionary patterns of altered behavior and susceptibility in parasitized hosts. Evolution 50, 807–819. ( 10.1111/j.1558-5646.1996.tb03890.x) [DOI] [PubMed] [Google Scholar]
- 18.Stone GN, Schönrogge K. 2003. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 18, 512–522. ( 10.1016/S0169-5347(03)00247-7) [DOI] [Google Scholar]
- 19.Bailey R, Schönrogge K, Cook JM, Melika G, Csóka G, Thuróczy C, Stone GN. 2009. Host niches and defensive extended phenotypes structure parasitoid wasp communities. PLoS Biol. 7, e1000179 ( 10.1371/journal.pbio.1000179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weld LH. 1959. Cynipid galls of eastern United States Ann Arbor, MI: Privately printed. [Google Scholar]
- 21.Bennett FD. 1995. Parasites of the pepper flower-bud moth (Lepidoptera: Gelechiidae) in Florida. Florida Entomol. 78, 546 ( 10.2307/3495543) [DOI] [Google Scholar]
- 22.Grissell E. 1976. A revision of western nearctic species of Torymus Dalman (Hymenoptera, Torymidae). Berkeley, CA: University of California Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available as electronic supplementary material.