Abstract
Phenotypic integration and modularity influence morphological disparity and evolvability. However, studies addressing how morphological integration and modularity change for long periods of genetic isolation are scarce. Here, we investigate patterns of phenotypic integration and modularity in the skull of phenotypically and genetically distinct populations of the Artic fox (Vulpes lagopus) from the Commander Islands of the Aleutian belt (i.e. Bering and Mednyi) that were isolated ca 10 000 years by ice-free waters of the Bering sea. We use three-dimensional geometric morphometrics to quantify the strength of modularity and integration from inter-individual variation (static) and from fluctuating asymmetry (random developmental variation) in both island populations compared to the mainland population (i.e. Chukotka) and we investigated how changes in morphological integration and modularity affect disparity and the directionality of trait divergence. Our results indicate a decrease in morphological integration concomitant to an increase in disparity at a developmental level, from mainland to the smallest and farthest population of Mednyi. However, phenotypic integration is higher in both island populations accompanied by a reduction in disparity compared to the population of mainland at a static level. This higher integration may have favoured morphological adaptive changes towards specific feeding behaviours related to the extreme environmental settings of islands. Our study demonstrates how shifts in phenotypic integration and modularity can facilitate phenotypic evolvability at the intraspecific level that may lead to lineage divergence at macroevolutioanry scales.
Keywords: phenotypic integration, modularity, skull, Commander Islands, Vulpes lagopus, evolvability
1. Introduction
Phenotypic integration and modularity determine how different traits within a given structure evolve independently or in a coordinate fashion (e.g. [1]). Accordingly, both phenotypic integration and modularity influence phenotypic disparity and evolvability (e.g. [2]). Patterns of phenotypic integration and modularity can be investigated through quantitative analysis of morphology at different levels such as evolutionary (i.e. among species means of an evolutionary lineage), static (i.e. among adult individuals of the same species) or developmental (i.e. random changes in development or fluctuating asymmetry) [3].
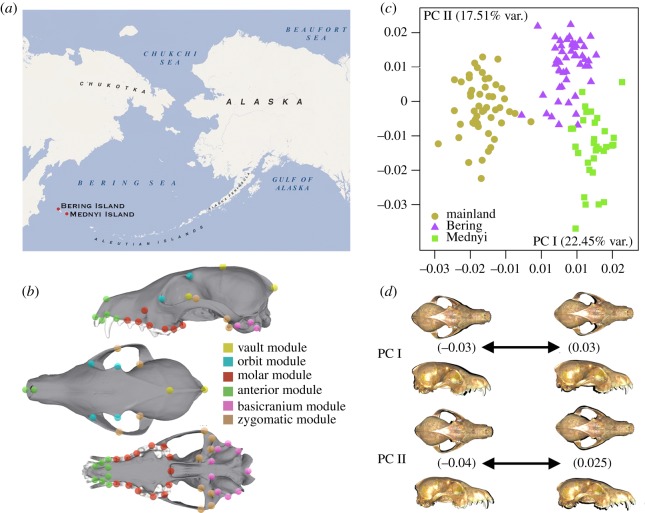
As genetic isolation influences development due to genomic coadaptation [4], isolated populations with different environmental settings represent a good opportunity to investigate emerging modularity and integration shifts. Moreover, population bottlenecks and genetic drift can influence changes that are not necessarily coadapted or in response to different environmental settings [4]. These changes may later facilitate or constrain functional adaptations to these new ecological venues at a macroevolutionary scale (e.g. [1,5]). Moreover, genetic isolation might indeed facilitate ecological specialization when environmental conditions are relatively stable, and eventually, lead to population decline after abrupt environmental changes [6]. However, studies on phenotypic integration and modularity among isolated populations of the same species adapted to different environmental conditions are scarce. Here, we investigate the effect of geographical isolation on phenotypic integration, evolvability and ecological specialization by comparing the patterns of integration and modularity in the skull of three populations of the Artic fox (Vulpes lagopus), one from the mainland (Chukotka) and two isolated from the Commander Islands of the Aleutian belt (i.e. Mednyi and Bering Islands; figure 1a).
Figure 1.
Phenotypic analysis in Artic fox populations. (a) Area of study (Chukotka/Bering and Mednyi Islands); (b) landmarks digitized in an Artic fox's skull; (c) bivariate graph depicted from the first two PCs of SC; (d) shape changes accounted for by each PC. In black are the deviations from the consensus to each extreme shape.
The Artic foxes from Commander Islands represent genetically unique populations as a consequence of their extended isolation (ca 10 000 years) by ice-free waters from mainland Arctic foxes of the Chukchi Peninsula in Far East continental Russia [7–10]. Indeed, genetic data indicate that these two populations are separated from all the other populations of Arctic foxes, and they also exhibit the lowest genetic diversity (i.e. low levels of mtDNA and microsatellite variation) among Arctic foxes [9,11,12].
Food resources available to Arctic foxes living on the Commander Islands differ from those available to the mainland population [13–16]. While voles and other rodents are generally the main prey of Arctic foxes on the mainland throughout the year [13,14,17], they play a minor role in the diet of foxes from Bering Island and they are absent from Mednyi [15]. In contrast, the main food resource of Artic foxes from Commander Islands range from sea birds (e.g. Fulmarus glacialis) and newborn seal pups (Callorhinus ursinus) during the summer season to carcasses of sea otters (Enhydra lutris) and fur seals [15,16,18,19]. Therefore, clear differences at the genetic, morphological and ecological levels have been found among mainland and island foxes.
In this study, we specifically test: (i) whether there is an increase of developmental instability in the skull of Artic foxes associated with the degree of geographical isolation; (ii) whether the genetic isolation of island populations correlates with changes in the developmental integration and modularity, using fluctuating asymmetry (FA, e.g. [20]) or random developmental variation; (iii) whether the static (between adult individuals) integration patterns of each population are similar to the developmental patterns and (iv) how changes in morphological integration and modularity affect disparity and the directionality of trait divergence. These questions assess the extent to which patterns of developmental integration and modularity can be modified intraspecifically in genetically isolated populations and how this can facilitate or constrain phenotypic evolvability and ecological differentiation at macroevolutionary scales.
2. Material and methods
A sample of 134 skulls belonging to adult individuals of three different populations of Artic foxes was scanned in three-dimensions using a Next Engine surface scanner. These included 51 from Bering Island, 32 from Mednyi Island and 51 from the mainland. All skulls analysed in this paper from Mednyi Island were collected before the late 1970s to avoid the effects of the severe bottleneck population and the high levels of mercury experienced by this population [6,8]. All the skulls are housed at the Zoological Museum of the Lomonosov Moscow State University (electronic supplementary material, table S1).
A total of 52 homologous landmarks (LMs) were digitized (figure 1b, electronic supplementary material, table S2) using the Landmark software package (IDAV 2002–2006). All specimens were digitized twice to assess for data collection error. We used generalized Procrustes superimposition alignment.
To test for skull allometric effects, we performed a Procrustes ANOVA assessing the covariation of shape and size [21–24]. We included the three populations as a grouping factor to test whether there were differences in the allometric pattern among populations.
FA was studied for all three populations (mainland and two island populations). We performed an independent Procrustes ANOVA for each population of foxes with two main factors: individual (each skull) and side (left and right sides), plus the two digitizations per skull as the measurement error [25,26] (for the effect of sexual dimorphism, see electronic supplementary material, table S5).
To assess the level of integration and modularity for the three populations independently, we divided the 52 LMs into six regions (figure 1b) following Goswami [5]. To study static modularity, we used the symmetric component of shape variation (SC) and to study developmental modularity we used FA.
We calculated the average covariance ratio (CR) to test for the modular hypothesis described above [27]. We tested the significance of the CR coefficient with a permutation test with 1000 random modular partitions. In addition, to compare the CR distributions from different populations, we performed a bootstrap procedure to compute the confidence intervals for each CR. We also corrected the CR values to the CR distribution obtained by bootstrapping in the following way: CRobs + (1/n * Σ (CRboot –CRrobs); where CRobs is the observed CR value of the actual sample; CRboot is the values of CR obtained by bootstrapping, and n the number of iterations.
We performed a series of partial least squares analysis between pairs of modules for each of the three populations to quantify patterns of morphological integration. We also compared the strength of integration among populations using the mean of the z-scores obtained in each pairwise comparison among modules for each population [28]. This analysis calculates effect sizes as standard deviates of the rPLS statistic (z) and performs two-sample z-tests, using the pooled standard error from the sampling distributions of the PLS analyses.
To assess how integration/modularity affects phenotypic evolution (e.g. [1,29–31]), we performed a set of analyses to investigate how the craniofacial variation is structured in the three populations. First, we calculated shape disparity to explore how integration and modularity affected overall disparity. Second, we conducted separate PCAs with each of the populations to gain insight into how this variance is structured along specific axes of trait variation. Third, we compared the distribution of shape variance among principal axes within each PCA, comparing the angles of the first three eigenvectors.
All the analyses were performed with the Geomorph [32] package of R with the exception of PC axes comparisons that were conducted in MorphoJ [33].
3. Results
The Procrustes ANOVA yielded a significant relationship between skull size and shape (table 1), which indicates that there is an allometric shape change in the Artic fox skull.
Table 1.
Results of the Procrustes ANOVA computed to test the effect of allometry for each population of Artic foxes. Log(Cs) refers to the effect of skull size among individuals (total allometry); populations is the differences in skull shape among the three populations of Artic foxes analysed; and log(Cs): populations refers to allometric differences among the three populations; residuals is the morphological variance in the skull of Artic foxes explained by other factors.
| symmetric component | d.f. | SS | MS | Rsq | F | Z | p-value |
|---|---|---|---|---|---|---|---|
| log(CS) | 1 | 0.012 | 0.012 | 0.099 | 19.899 | 7.444 | 0.001 |
| Pop | 2 | 0.029 | 0.015 | 0.250 | 25.020 | 11.744 | 0.001 |
| log(CS): Pop | 2 | 0.001 | 0.001 | 0.012 | 1.234 | 3.253 | 0.001 |
| residuals | 128 | 0.074 | 0.001 | 0.639 | |||
| total | 133 | 0.116 |
| fluctuating asymmetry | d.f. | SS | MS | Rsq | F | Z | Pr(>F) |
|---|---|---|---|---|---|---|---|
| log(CS) | 1 | 0.001 | 0.001 | 0.010 | 1.367 | 1.332 | 0.099 |
| Pop | 2 | 0.002 | 0.001 | 0.031 | 2.142 | 3.956 | 0.001 |
| log(CS): Pop | 2 | 0.001 | 0.001 | 0.023 | 1.584 | 2.565 | 0.009 |
| residuals | 128 | 0.047 | 0.000 | 0.936 | |||
| total | 133 | 0.051 |
Figure 1c shows the bivariate graph resulting from the first two principal components obtained from the PCA of SC. The three populations of Arctic foxes occupy different regions of the morphospace. The first PC separates foxes from the mainland population from island foxes (figure 1c). The mainland foxes have skulls with deeper rostra and more anteriorly positioned zygomatic arches than the skulls of island foxes (figure 1d). The second PC slightly separates both populations of island foxes with the foxes of the mainland scoring in between (figure 1c). The skulls of Mednyi foxes are shorter, wider and deeper than the skulls of Bering Island foxes (figure 1d).
The effects of FA are significant for the three populations of Artic foxes (electronic supplementary material, table S3) but the degree of FA is similar among them. As indicated by the CR values, island populations show lower modularity than the population of mainland for SC and for FA (table 2). This is particularly evident when comparing the Mednyi and mainland populations (electronic supplementary material, figures S1 and S2). The z-score (Zs) obtained from PLS analysis of SC indicated an increase of integration among modules from the population of mainland (Zsma = 6.630) to the population of Bering (Zsb = 6.960) and Mednyi (Zsme = 6.674) (electronic supplementary material, table S4). In contrast, the Zs obtained from the PLS of FA reveal a decrease in integration from the mainland (Zsma = 7.181) and Bering populations (Zsb = 8.025) to the population of Mednyi (Zsme = 3.646) (electronic supplementary material, table S4).
Table 2.
Modularity results. Covariance ratio (CR) obtained for the hypothesis of modularity tested for each population using SC and FA. The observed (CR) and corrected (CRc) values, as well as the confidence intervals (CI) are shown. Higher CR values indicate lower skull shape modularity.
| CR | CRc | p-value | CI | ||
|---|---|---|---|---|---|
| symmetric component | |||||
| mainland | 0.622 | 0.696 | 0.001 | 0.651 | 0.746 |
| Bering | 0.653 | 0.713 | 0.001 | 0.656 | 0.776 |
| Mednyi | 0.766 | 0.819 | 0.001 | 0.742 | 0.898 |
| fluctuating asymmetry | |||||
| mainland | 0.575 | 0.651 | 0.001 | 0.595 | 0.717 |
| Bering | 0.596 | 0.670 | 0.001 | 0.610 | 0.745 |
| Mednyi | 0.648 | 0.737 | 0.001 | 0.674 | 0.812 |
The analysis of disparity shown that the population of Mednyi (Dme = 0.00045) has lower disparity values than the populations of Bering Island (Db = 0.00061) and the mainland (Dma = 0.00056) for SC (electronic supplementary material, table S6). In contrast, the analysis of disparity from FA indicated that the population of Mednyi has significantly higher disparity values (Dme = 0.00047) than the populations of mainland (Dma = 0.00030) and Bering Island (Db = 0.00033) (electronic supplementary material, table S6).
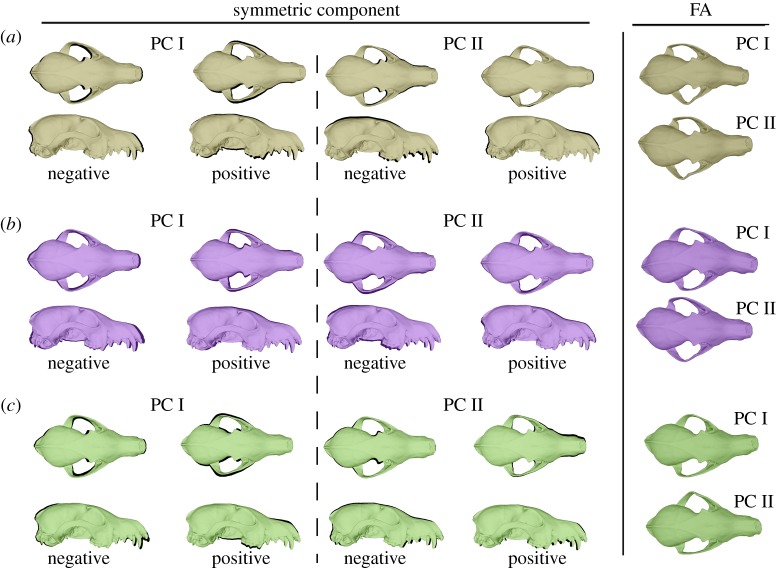
The shape variance accounted for by the first two eigenvectors obtained from the PCA analyses of the SC and FA are shown in figure 2, and the percentages of variance explained for by the first 10 eigenvectors are shown in electronic supplementary material, figure S3. The results of the angular comparisons between these axes (the first three eigenvectors) are shown in electronic supplementary material, table S7.
Figure 2.
Maximum shape variance obtained from the PCAs computed for the population of (a) the mainland, and (b) Bering and (c) Mednyi Islands for both the symmetric component and fluctuating asymmetry (FA).
4. Discussion
Genetic variation is crucial for the evolution of functionally and developmentally related characters [34], and therefore, genetic drift and inbreeding depression may substantially impact patterns of modularity [35]. We hypothesized that both genetic drift and inbreeding depression have modified developmental modularity patterns in both island populations of Artic foxes compared to the population of the mainland.
Theoretical models have predicted an increase of integration in the presence of drift [36,37] and, although empirical evidence for changes in covariation among morphological modules in island mammals is scarce, domesticated mammals show changing patterns of developmental integration [38]. Our results indicate that genetic isolation and drift have reduced developmental integration, particularly in Mednyi foxes, compared to those from the mainland (electronic supplementary material, table S4).
The strength of integration/modularity affects the direction of phenotypic evolution (e.g. [30]), with integration expected to produce phenotypic disparity more constrained along specific axes of variation (e.g. [1]) and morphological modularity ‘allows’ a more even exploration of trait space (i.e. unconstrained to axes of maximum covariation) [1,39,40]. However, a stronger integration could also be associated with higher values of morphological disparity (e.g. [41]). Our results indicate a decrease in developmental integration accompanied by a significant increase in disparity or developmental instability (electronic supplementary material, table S6). This disparity is not structured along the same axes of variation across populations, and therefore, is not constrained to specific axes of maximum variation (figure 2; electronic supplementary material, table S7). Moreover, morphological disparity in those populations with more integrated skulls at developmental level is more concentrated in the first axes of variation (electronic supplementary material, figure S3 and table S8). More than a cause–effect relationship between developmental integration and disparity, our results suggest that genetic drift could be a cause to increase developmental variation and ‘breaking’ canalization [4].
These patterns of integration and disparity at the developmental level are different than at a static level. Morphological integration among individuals significantly increases in both island populations compared to the population of mainland (electronic supplementary material, table S4), and this is accompanied by a significant decrease in disparity in the population of Mednyi (electronic supplementary material, table S6). Moreover, among individual disparity seems to be constrained to specific axes of shape variation (figure 2; electronic supplementary material, figure S3 and tables S7 and S8).
Our analyses suggest that there is a decoupling between the developmental and static levels, which might indicate that phenotypic variation among populations is not only a direct outcome of the developmental variation. Developmental variation is altered by a decrease in morphological integration concomitant with an increase in phenotypic disparity, specially in the smallest population of Mednyi Island, and most probably as a consequence of genetic drift. However, developmental shape variation was ‘restructured’ leading to an increase in morphological integration and a reduction in morphological disparity at inter-individual variation in both island populations. Whereas genetic drift might alter phenotypic variation, directional selection might ‘push’ the population distribution towards the optimum [42,43]. This may be the case of the population of Mednyi, as it is the one with most extreme feeding behaviours and the one with more derived morphological traits. Accordingly, changes in skull shape developmental integration seem to facilitate morphological adaptive changes towards the extreme environmental conditions of islands, and hence, decreasing disparity.
In summary, our results demonstrate that in ca 10 000 years of genetic isolation and 20 000 generations [40], the strength of developmental integration could change in natural conditions facilitating phenotypic divergence (evolvability) that would further constitute independent lineages adapted to particular environmental conditions.
Supplementary Material
Supplementary Material
Acknowledgements
Dr Dean Adams and other two anonymous reviewers provided constructive comments.
Data accessibility
The dataset has been included as text file under the category of electronic supplementary material with the name ‘Dataset.txt’.
Authors' contributions
A.-M.S. analysed data, designed the study, interpreted results and drafted the article. O.N. and G.O. collected data and drafted the article. C.V.-G. interpreted data and reviewed the article. B.F. conceived and designed the study, assisted in data collection and drafted the paper. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Secretaría de Estado de Investigación, Desarrollo e Innovación (grant nos. CGL2015-68300P and CGL2017-92166-EXP (B.F.)) and Russian Foundation for Basic Research (grant no. RFBR 19-04-00111) and grant no. RSCF 14-50-00029 (O.N.).
References
- 1.Goswami A, Smaers JB, Soligo C, Polly PD. 2014. The macroevolutionary consequences of phenotypic integration: from development to deep time. Phil. Trans. R. Soc. B 369, 20130254 ( 10.1098/rstb.2013.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner GP, Altenberg L. 1996. Complex adaptations and the evolution of evolvability. Evolution 50, 967–976. ( 10.1111/j.1558-5646.1996.tb02339.x) [DOI] [PubMed] [Google Scholar]
- 3.Klingenberg CP. 2013. Cranial integration and modularity: insights into evolution and development from morphometric data. Hystrix 24, 43–58. [Google Scholar]
- 4.Clarke GM. 1993. The genetic basis of developmental stability. I. Relationships between stability, heterozygosity and genomic coadaptation. Genetica 89, 15–23. ( 10.1007/BF02424502) [DOI] [Google Scholar]
- 5.Goswami A. 2006. Cranial modularity shifts during mammalian evolution. Am. Nat. 168, 270–280. ( 10.1086/505758) [DOI] [PubMed] [Google Scholar]
- 6.Bocharova N, Treu G, Czirják GÁ, Krone O, Stefanski V, Wibbelt G, Goltsman M. 2013. Correlates between feeding ecology and mercury levels in historical and modern arctic foxes (Vulpes lagopus). PLoS ONE 8, e60879 ( 10.1371/journal.pone.0060879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goltsman M, Kruchenkova EP, Macdonald DW. 1996. The Mednyi Arctic foxes: treating a population imperilled by disease. Oryx 30, 251–258. ( 10.1017/S0030605300021748) [DOI] [Google Scholar]
- 8.Goltsman M, Kruchenkova EP, Sergeev S, Volodin I, Macdonald DW. 2005. ‘Island syndrome' in a population of Arctic foxes (Alopex lagopus) from Mednyi Island. J. Zool. (London) 267, 405–418. ( 10.1017/S0952836905007557) [DOI] [Google Scholar]
- 9.Geffen ELI, Waidyaratne S, Dalén L, Angerbjörn A, Vila C, Hersteinsson P, Wayne RK. 2007. Sea ice occurrence predicts genetic isolation in the Arctic fox. Mol. Ecol. 16, 4241–4255. ( 10.1111/j.1365-294X.2007.03507.x) [DOI] [PubMed] [Google Scholar]
- 10.Dzhykiya EL. 2008. Genetic polymorphism of Commander Islands' Arctic foxes (Alopex lagopus semenovi, Ognev 1931, Alopex lagopus beringensis, Merriam 1902). D. Phil. thesis, Moscow State University of MV Lomonosov. [Google Scholar]
- 11.Ploshnitsa AI, Goltsman ME, Macdonald DW, Kennedy LJ, Sommer S. 2012. Impact of historical founder effects and a recent bottleneck on MHC variability in Commander Arctic foxes (Vulpes lagopus). Ecol. Evol. 2, 165–180. ( 10.1002/ece3.42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploshnitsa AI, Goltsman ME, Happ G, Macdonald DW, Kennedy LJ. 2013. Historical and modern neutral genetic variability in Mednyi Arctic foxes passed through a severe bottleneck. J. Zool. (London) 289, 68–76. ( 10.1111/j.1469-7998.2012.00964.x) [DOI] [Google Scholar]
- 13.Angerbjorn A, Tannerfeldt M, Erlinge S. 1999. Predator–prey relationships: arctic foxes and lemmings. J. Anim. Ecol. 68, 34–49. ( 10.1046/j.1365-2656.1999.00258.x) [DOI] [Google Scholar]
- 14.Anthony RM, Barten NL, Seiser PE. 2000. Foods of arctic foxes (Alopex lagopus) during winter and spring in western Alaska. J. Mammal. 81, 820–828. () [DOI] [Google Scholar]
- 15.Zagrebelnyi SV. 2000. Feeding ecology of Commander Islands arctic fox subspecies: from Bering Island (Alopex lagopus beringensis Merriam 1902) and Mednyi Island (Al semenovi Ognev 1931; Carnivora, Canidae). Zool. Z. 79, 595–697. ( 10.1515/mammalia-2018-0165) [DOI] [Google Scholar]
- 16.Goltsman ME, Nanova OG, Sergeev SN, Shienok AN. 2010. Ispolzovanie kormovyh resursov reproduktivnymi semjami pescov (Alopex lagopus semenovi Ognev, Mammalia: Canidae) na ostrove Mednyi (Komandorskie ostrova). Zool. Ž. 89, 1246–1263. ( 10.1134/s1062359010151014) [DOI] [Google Scholar]
- 17.Eide NE, Jepsen JU, Prestrud PÅL. 2004. Spatial organization of reproductive Arctic foxes Alopex lagopus: responses to changes in spatial and temporal availability of prey. J. Anim. Ecol. 73, 1056–1068. ( 10.1111/j.0021-8790.2004.00885.x) [DOI] [Google Scholar]
- 18.Chelnokov FG. 1970. On relationship between Arctic foxes and fur seal pups. Iss. Kamchatka Geograph. 6, 151–158. [Google Scholar]
- 19.Naumov NP, Goltsman ME, Kruchenkova EP, Ovsijnikov NG, Smirin VM. 1981. Social behaviour of Arctic fox on the Mednyi Island. In Ecology, population organization and communicative processes in the mammals (ed. Naumov NP.), pp. 31–75. Moscow, Russia: Nauka; (In Russian.) [Google Scholar]
- 20.Klingenberg CP. 2014. Studying morphological integration and modularity at multiple levels: concepts and analysis. Phil. Trans. R. Soc. B 369, 20130249 ( 10.1098/rstb.2013.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardia KV, Bookstein FL, Moreton IJ. 2000. Statistical assessment of bilateral symmetry of shapes. Biometrika 87, 285– 300 ( 10.1093/biomet/87.2.285) [DOI] [Google Scholar]
- 22.Dryden IL, Mardia K. 1998. Statistical analysis of shape. Chichester, UK: Wiley. [Google Scholar]
- 23.Bookstein FL. 1991. Morphometric tools for landmark data: geometry and biology. London, UK: Cambridge University Press. [Google Scholar]
- 24.Collyer ML, Sekora DJ, Adams DC. 2015. A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Heredity 115, 357–365. ( 10.1038/hdy.2014.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klingenberg CP, McIntyre GS. 1998. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 52, 1363–1375. ( 10.1111/j.1558-5646.1998.tb02018.x) [DOI] [PubMed] [Google Scholar]
- 26.Klingenberg CP. 2015. Analyzing fluctuating asymmetry with geometric morphometrics: concepts, methods, and applications. Symmetry 7, 843–934. ( 10.3390/sym7020843) [DOI] [Google Scholar]
- 27.Adams DC. 2016. Evaluating modularity in morphometric data: challenges with the RV coefficient and a new test measure. Methods Ecol. Evol. 7, 565–572. ( 10.1111/2041-210X.12511) [DOI] [Google Scholar]
- 28.Adams DC, Collyer ML. 2016. On the comparison of the strength of morphological integration across morphometric datasets. Evolution 70, 2623–2631. ( 10.1111/evo.13045) [DOI] [PubMed] [Google Scholar]
- 29.Frafjord K. 1993. Circumpolar size variation in the skull of the arctic fox Alopex lagopus. Polar Biol. 13, 235–238. ( 10.1007/BF00238758) [DOI] [Google Scholar]
- 30.Felice RN, Goswami A. 2018. Developmental origins of mosaic evolution in the avian cranium. Proc. Natl Acad. Sci. USA 115, 555–560. ( 10.1073/pnas.1716437115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villmoare B. 2013. Morphological integration, evolutionary constraints, and extinction: a computer simulation-based study. Evol. Biol. 40, 76–83. ( 10.1007/s11692-012-9186-3) [DOI] [Google Scholar]
- 32.Adams DC, Collyer M, Sherratt E. 2015. Geomorph: software for geometric morphometric analyses. R package version 2.1.7-1.
- 33.Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Res. 11, 353–357. ( 10.1111/j.1755-0998.2010.02924.x) [DOI] [PubMed] [Google Scholar]
- 34.Cheverud JM, Ehrich TH, Vaughn TT, Koreishi SF, Linsey RB, Pletscher LS. 2004. Pleiotropic effects on mandibular morphology II: differential epistasis and genetic variation in morphological integration. J. Exp. Zool. 302, 424–435. ( 10.1002/jez.b.21008) [DOI] [PubMed] [Google Scholar]
- 35.Cheverud JM. 1996. Developmental integration and the evolution of pleiotropy. Am. Zool. 36, 44–50. ( 10.1093/icb/36.1.44) [DOI] [Google Scholar]
- 36.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. New York, NY: Longman. [Google Scholar]
- 37.Melo D, Marroig G. 2015. Directional selection can drive the evolution of modularity in complex traits. Proc. Natl Acad. Sci. USA 112, 470–475. ( 10.1073/pnas.1322632112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Villagra MR, Geiger M, Schneider RA. 2016. The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals. R. Soc. open sci. 3, 160107 ( 10.1098/rsos.160107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klingenberg CP. 2005. Developmental constraints, modules, and evolvability. In Variation (eds Hallgrímsson B, Hall BK), pp. 219–247. New York, NY: Academic Press. [Google Scholar]
- 40.Wagner GP, Zhang J. 2011. The pleiotropic structure of the genotype–phenotype map: the evolvability of complex organisms. Nat. Rev. Gen. 12, 204–213. ( 10.1038/nrg2949) [DOI] [PubMed] [Google Scholar]
- 41.Randau M, Goswami A. 2017. Unravelling intravertebral integration, modularity and disparity in Felidae (Mammalia). Evol. Dev. 19, 85–95. ( 10.1111/ede.12218) [DOI] [PubMed] [Google Scholar]
- 42.Wagner GP. 1996. Homologues, natural kinds and the evolution of modularity. Am. Zool. 36, 36–43. ( 10.1093/icb/36.1.36) [DOI] [Google Scholar]
- 43.Schluter D, Clifford EA, Nemethy M, McKinnon JS. 2004. Parallel evolution and inheritance of quantitative traits. Am. Nat. 163, 809–822. ( 10.1086/383621) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset has been included as text file under the category of electronic supplementary material with the name ‘Dataset.txt’.