Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library 2008, Issue 3.
Upper abdominal surgical procedures are associated with a high risk of postoperative pulmonary complications. The risk and severity of postoperative pulmonary complications can be reduced by the judicious use of therapeutic manoeuvres that increase lung volume. Our objective was to assess the effect of incentive spirometry compared to no therapy or physiotherapy, including coughing and deep breathing, on all‐cause postoperative pulmonary complications and mortality in adult patients admitted to hospital for upper abdominal surgery.
Objectives
Our primary objective was to assess the effect of incentive spirometry (IS), compared to no such therapy or other therapy, on postoperative pulmonary complications and mortality in adults undergoing upper abdominal surgery.
Our secondary objectives were to evaluate the effects of IS, compared to no therapy or other therapy, on other postoperative complications, adverse events, and spirometric parameters.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 8), MEDLINE, EMBASE, and LILACS (from inception to August 2013). There were no language restrictions. The date of the most recent search was 12 August 2013. The original search was performed in June 2006.
Selection criteria
We included randomized controlled trials (RCTs) of IS in adult patients admitted for any type of upper abdominal surgery, including patients undergoing laparoscopic procedures.
Data collection and analysis
Two authors independently assessed trial quality and extracted data.
Main results
We included 12 studies with a total of 1834 participants in this updated review. The methodological quality of the included studies was difficult to assess as it was poorly reported, so the predominant classification of bias was 'unclear'; the studies did not report on compliance with the prescribed therapy. We were able to include data from only 1160 patients in the meta‐analysis. Four trials (152 patients) compared the effects of IS with no respiratory treatment. We found no statistically significant difference between the participants receiving IS and those who had no respiratory treatment for clinical complications (relative risk (RR) 0.59, 95% confidence interval (CI) 0.30 to 1.18). Two trials (194 patients) IS compared incentive spirometry with deep breathing exercises (DBE). We found no statistically significant differences between the participants receiving IS and those receiving DBE in the meta‐analysis for respiratory failure (RR 0.67, 95% CI 0.04 to 10.50). Two trials (946 patients) compared IS with other chest physiotherapy. We found no statistically significant differences between the participants receiving IS compared to those receiving physiotherapy in the risk of developing a pulmonary condition or the type of complication. There was no evidence that IS is effective in the prevention of pulmonary complications.
Authors' conclusions
There is low quality evidence regarding the lack of effectiveness of incentive spirometry for prevention of postoperative pulmonary complications in patients after upper abdominal surgery. This review underlines the urgent need to conduct well‐designed trials in this field. There is a case for large RCTs with high methodological rigour in order to define any benefit from the use of incentive spirometry regarding mortality.
Plain language summary
Incentive spirometry for prevention of postoperative pulmonary complications after upper abdominal surgery
Background
Previous studies have suggested that between 17% and 88% of people having surgery on the upper abdomen will suffer complications that affect their lungs after the operation (postoperative pulmonary complications). The lung volume tends to fall after such surgeries. These complications can be made less likely and less severe with the careful use of treatments designed to encourage breathing in (inspiration) and thus increasing the volume of the lungs, as these volumes tend to fall after such surgeries. Incentive spirometers are mechanical devices developed to help people take long, deep, and slow breaths to increase lung inflation.
Objective
We reviewed the evidence about the effect of incentive spirometry (IS), compared to no intervention or other therapy, to prevent postoperative pulmonary complications (for example, pneumonia, fever, death) in people following upper abdominal surgery.
Study characteristics
We included adults (aged 18 years and above) admitted for any type of upper abdominal surgery. The evidence is current to August 2013. We found 12 studies with a total of 1834 participants. The maximum period of time that a patient was followed by the doctor was seven days postoperatively. The quality of the included studies was uncertain because of poor reporting in the published articles.
Key results
The following results were examined in this review: clinical complications, respiratory failure (that is, inadequate gas exchange by the respiratory system), and pulmonary complications. The results from participants receiving IS were the same as for those receiving either no treatment, deep breathing exercises (DBE) or physiotherapy in the meta‐analyses for clinical complications, respiratory failure, and pulmonary complications.
Quality of evidence
Because of poorly conducted studies (results not similar across studies; some issues with study design and; not enough data collected and organized) we ranked the overall quality of the evidence reported in this review as low.
Conclusion and future research
There is low quality evidence showing a lack of effectiveness of incentive spirometry for prevention of postoperative pulmonary complications in patients after upper abdominal surgery. This review underlines the urgent need to conduct well‐designed trials in this field.
Summary of findings
for the main comparison.
| Incentive spirometry (IS) compared with no treatment, deep‐breathing exercise (DBE), or physiotherapy for prevention of postoperative pulmonary complications in upper abdominal surgery | |||
|
Patient or population: postoperative pulmonary complications in upper abdominal surgery Intervention: incentive spirometry Comparison: no treatment, DBE, or physiotherapy | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
|
Pulmonary complications Follow up: until the fifth postoperative day (Craven 1974); and until the seventh postoperative day (Hall 1991) Comparison: physiotherapy |
RR 0.83 (0.51 to 1.34) | 946 (2 studies)a | ⊕⊕⊝⊝ low |
|
Respiratory failure Follow up: until the fourth postoperative day (Celli 1984); and not reported (Hall 1996) Comparison: DBE |
RR 0.67 (0.04 to 10.50) | 194 (2 studies)b | ⊕⊕⊝⊝ low |
|
Clinical complications Follow up: until the fourth postoperative day (Celli 1984; Schwieger 1986); 1‐7 days postoperatively (Kulkarni 2010); and until the second postoperative day (O'Connor 1988) Comparison: no treatment |
RR 0.59 (0.30 to 1.18) | 152 (4 studies)c | ⊕⊕⊝⊝ low |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
aSubstantial heterogeneity in the overall effect due to different IS evaluated.
bLower risk patients (no chronic respiratory disease and non‐smokers).
cNo blinding of investigators in Kulkarni 2010 study.
Background
Description of the condition
This is an update of a Cochrane Review first published in The Cochrane Library in 2008 (Issue 3) (Guimarães 2009). The original review included 11 studies (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985) and did not provide any evidence from randomized controlled trials of differences in patient outcomes with incentive spirometry compared to no such therapy (or other therapy) in adult patients admitted for upper abdominal surgery.
Upper abdominal surgical procedures are associated with a high risk of postoperative pulmonary complications (PPCs). These are defined as pulmonary abnormalities occurring in the postoperative period which produce clinically significant identifiable disease or dysfunction that adversely affects the patient's clinical course (Kips 1997; O'Donohue 1992). The reported risk rates of PPCs in upper abdominal surgery range from 17% to 88% (Overend 2001). Pulmonary complications include atelectasis, pneumonia, respiratory failure, and tracheobronchial infection. The commonest of these complications is pulmonary atelectasis, though pneumonia is considered to be the main cause of mortality (Kips 1997; Martin 1984).
Shallow, monotonous breathing may decrease ventilation to dependent lung regions and may contribute to the development of atelectasis. Incisional pain, residual anaesthetic effects, and lying in bed for prolonged periods are also contributive factors. Although postoperative atelectasis usually improves spontaneously, if the collapsed regions of the lungs do not become re‐inflated then infection may arise as a secondary event (Chumillas 1998; Kips 1997; Stock 1985). Other serious postoperative complications such as intraperitoneal infection, wound infection, cardiac and haemodynamic complications (Hall 1996) can also develop and worsen the patients' outcomes. It is particularly important to identify patients at risk of postoperative pulmonary complications as these are the most frequently reported cause of morbidity and mortality in the postoperative period (Doyle 1999). The occurrence of pulmonary complications is linked to the presence of risk factors, many of which are identifiable at the patient's preoperative evaluation. They include age, smoking status, obesity, pre‐existing chronic lung disease, and co‐morbidities (Chumillas 1998; Kips 1997). The anaesthetic factors include the type and duration of anaesthesia as well as the different drugs used for anaesthesia and postoperative pain relief. The surgical factors include the type and duration of surgery and the extent of the surgical incision (Celli 1993; Chumillas 1998; Strandberg 1986; Sykes 1993).
Description of the intervention
Physiotherapy is designed to enhance inspiration and is aimed at increasing the abnormally low postoperative functional residual capacity (FRC) (Celli 1993; Hall 1991; Hall 1996). Deep‐breathing exercises, such as inspiring to total lung capacity with particular emphasis on the use of the diaphragm, have been shown to inflate alveoli and reverse postoperative hypoxaemia (Celli 1993). Incentive spirometers are mechanical devices developed to reach this aim. The spirometer is designed to imitate maximum deep inspirations and encourages the patient to take long, deep, slow breaths that increase lung inflation (AARC 1991; Bartlett 1970; Chuter 1990). The first documented report of incentive spirometry (IS) as a treatment technique appears to be that of Van de Water et al (Van de Water 1972). Bartlett et al developed an incentive spirometer that both provided visual feedback to the patient and recorded the number of successful breathing manoeuvres (Bartlett 1973; Craven 1974). The Bartlett‐Edward incentive spirometer was used as the standard model for many years but now cheaper units are often substituted (Overend 2001). There are two kinds of incentive spirometer, based on the flow and volume of air. In the flow incentive model the flow may be turbulent, which increases the work of breathing. The Respiron and Triflo devices are examples. The volume incentive model is thought to be more 'physiological' because the training volume is constant until it reaches the maximum inspiratory capacity or the level preset by the physiotherapist, but it is more expensive. The Voldyne spirometer is one such device.
IS can produce undesirable side effects, such as pulmonary hyperventilation. This may be because an inaccurate dose of IS was prescribed or because the physiotherapist did not adequately supervise the spirometry. The use of IS may also be limited by the high cost of some devices (AARC 1991). IS is not indicated for patients with chronic obstructive pulmonary disease.
How the intervention might work
After surgery, it may be hard to take deep breaths and if patients do not breathe deeply enough this can lead to pulmonary complications. IS can be helpful to aid recovery and keep the lungs healthy. Patient should place the IS mouthpiece into the mouth and inhale slowly. A piece in the device rises as the patient takes a breath in. The healthcare provider or physician determine how big a breath the patient should take.
Why it is important to do this review
There is still controversy about the clinical benefits of IS (Celli 1984; Chumillas 1998; Fagevik Olsen 1997). We set out to gather the best available evidence on the effectiveness of this form of therapy.
Objectives
Our primary objective was to assess the effect of incentive spirometry (IS), compared to no such therapy or other therapy, on postoperative pulmonary complications and mortality in adults undergoing upper abdominal surgery. Our secondary objectives were to evaluate the effects of IS, compared to no therapy or other therapy, on other postoperative complications, adverse events, and spirometric parameters in adults undergoing upper abdominal surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs).
Types of participants
We included adults (aged 18 years and above) admitted for any type of upper abdominal surgery, including patients undergoing laparoscopic procedures.
We included adult patients with co‐morbidities (for example, pre‐existing pulmonary disease, smokers, and people who were obese). We planned to analyse these patients separately.
We included patients who developed any other type of complications (for example, haemodynamic complications) in the postoperative period.
Types of interventions
We planned to study the following interventions:
IS versus breathing exercises;
IS versus intermittent positive pressure breathing (IPPB);
IS versus other chest physiotherapy techniques;
IS versus no intervention.
We included all of these groups in the review. This is because there were a number of different types of therapeutic manoeuvres that increase lung volume and we felt it was important to be able to distinguish between the effects of different techniques, if possible.
Types of outcome measures
Primary outcomes
We planned to include the following outcomes.
-
Pulmonary complications, defined as:
atelectasis (radiographic, tomographic, or bronchoscopic diagnosis: in patients whose clinical signs were acute respiratory symptoms such as dyspnoea, cough, wheeze);
respiratory failure (radiographical diagnosis: in patients with signs of acute respiratory symptoms such as tracheobronchial purulent secretions, fever (greater than 38 ºC), or increased white blood cell count (greater than 10,000/mm3);
tracheobronchial infection or pneumonia.
Other types of complications in the postoperative period.
Total mortality from respiratory causes.
Evidence of harms from IS.
All‐cause mortality.
The radiological alterations were defined as segmental or subsegmental atelectasis with infiltration or consolidation, according to the criteria set by the radiologist. The postoperative clinical pulmonary complications were defined according to clinical (symptoms and physical examination) and also radiological criteria as atelectasis and pneumonia. When there were only radiological alterations, without clinical symptoms or alterations in auscultation, the complications were considered to be subclinical (Chumillas 1998).
Secondary outcomes
We planned to use the following as secondary outcomes:
average tidal volume (TV), or volume of air inspired and expired in each normally ventilated cycle;
forced expiratory volume in one second (FEV1), the expiratory volume obtained during the first second of execution of the forced vital capacity;
vital capacity (VC), the largest amount of air that a person can expel from the lungs shortly after having filled them to the maximum capacity;
functional residual capacity (FRC), the amount of air that stays in the lungs at the end of normal expiration;
forced vital capacity (FVC), the total amount of air expired during a forced expiration after a maximum inspiration;
length of stay in hospital;
evidence of harms from IS;
all‐cause mortality;
cost analysis.
We planned to perform subgroup analyses to estimate the efficacy of the different types of devices:
Triflo;
Voldyne;
Spirocare;
Coach;
Bartlett‐Edwards incentive spirometer.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 8), see Appendix 1 for the detailed search strategy; MEDLINE (OvidSP) (1966 to August 2013), see Appendix 2; EMBASE (OvidSP) (1980 to August 2013), see Appendix 3; Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS), via BIREME (1982 to August 2013), see Appendix 4; and CINAHL (EBSCOhost) (1980 to 2013), see Appendix 5.
We used a comprehensive search strategy and searched the databases using both subject headings and free text words. We used search strategies that were optimal for identifying RCTs (Castro 1999; Dickersin 1994) together with specific subject terms. We adapted our MEDLINE search strategy to use with other electronic databases such as LILACS and CENTRAL.
Searching other resources
We handsearched the reference lists of identified studies for additional citations and contacted specialists in the field and the authors of studies for information regarding unpublished data. There were no language restrictions.
Data collection and analysis
Selection of studies
Two authors (PNJ and RPED) independently assessed the titles and abstracts of all trials identified by the electronic searching. We obtained full‐text hard copies of any study which appeared to fulfil our selection criteria. We independently assessed and analysed the selected papers and resolved any disagreements in consensus meetings.
Data extraction and management
Two authors (PNJ and RPED) independently extracted data and resolved any discrepancies by discussion. We initially used a standard data extraction form to extract the following information:
characteristics of the study (design, methods of randomization);
participants;
interventions;
outcomes (types of outcome measures, timing of outcomes, adverse events).
Assessment of risk of bias in included studies
We used the risk of bias approach for Cochrane Reviews to assess study quality (Higgins 2011). Two review authors (PNJ and RPED) independently assessed the following six criteria. We resolved any discrepancies by discussion.
Adequate sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
In the first step, we copied the information that was relevant for making a judgement on a criterion from the original publication into an assessment table. If additional information was available from the study authors we entered this in the table along with an indication that this was unpublished information. Two review authors (PNJ and RPED) independently made a judgment as to whether the risk of bias for each criterion was considered to be 'low', 'uncertain', or 'high'. We resolved disagreements by discussion. We considered that trials categorized as 'low risk' for all six criteria were low bias‐risk trials.
We considered trials which were classified as low risk of bias in sequence generation, allocation concealment, blinding, incomplete data, and selective outcome reporting as low bias‐risk trials. We recorded this information for each included trial in 'Risk of bias' tables in RevMan 5 (RevMan 5.2) and summarized the risk of bias for each study in a summary ’Risk of bias’ figure and graph.
Measures of treatment effect
Binary outcomes
For dichotomous data, we used the relative risk (RR) as the effect measure with 95% confidence interval (CI).
Continuous outcomes
For continuous data, we presented the results as mean differences (MD) with 95% CIs. When pooling data across studies we estimated the MD if the outcomes were measured in the same way between trials. We used the standardized mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
The unit of analysis was one outcome for each participant.
Dealing with missing data
An intention‐to‐treat analysis (ITT) is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received the intervention or not. For each trial we planned to report whether or not the investigators stated if the analysis was performed according to the ITT principle. If participants were excluded after allocation, we reported any details that were provided in full.
Assessment of heterogeneity
We intended to quantify inconsistency among the pooled estimates using the I2 statistic. This illustrates the percentage of variability in effect estimates that results from heterogeneity rather than sampling error (Higgins 2003; Higgins 2011). We also intended to examine forest plots for CI overlap and to calculate the Chi2 test for homogeneity with a 10% level of significance. We used values for the I2 statistic to categorize heterogeneity: less than 25%; 26% to 50%; 51% to 75%, and greater than 75%. To allow meta‐analysis we considered an I2 less than 75%.
Assessment of reporting biases
Apart from assessing the risk of selective outcome reporting, considered under assessment of risk of bias in included studies, we intended to assess the effects of small studies by using funnel plots when at least 10 trials were identified.
Data synthesis
We planned to use the fixed‐effect model to analyse data. If the I2 was greater than 50%, we intended to use a random‐effects model. We planned to undertake quantitative analyses of outcomes on an ITT basis.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses by types of interventions and personal characteristics such as age, gender, and type of surgery (for example, laparoscopic versus standard abdominal surgery) if sufficient data were available. However, the number of studies was insufficient for performance of any of these subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the causes of heterogeneity and the robustness of the results by means of the following factors:
trials with low risk of bias versus those with high risk of bias; and
rates of withdrawal for each outcome (< 20% versus ≥ 20%).
However, the planned analyses could not be carried out because of lack of relevant data in the included studies.
Results
Description of studies
See the Characteristics of included studies table.
Results of the search
We identified a total of 775 citations from the database searches for the original review (Guimarães 2009) (see Figure 1 for search results). After screening by title and then abstract, we obtained full‐text copies for 21 citations that were potentially eligible for inclusion in the review. Of these, 10 did not fulfil our inclusion criteria and were excluded for the reasons described in the table Characteristics of excluded studies (Carmini 2000; Gale 1977; Genç 2004; Indihar 1982; Lederer 1980; Minschaert 1982; Pereira 2000; Pfenninger 1977; Sleszynski 1993; Vilaplana 1990). Eleven studies with a total of 1754 participants met the minimal methodological requirements and they were included in this review (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985).
1.
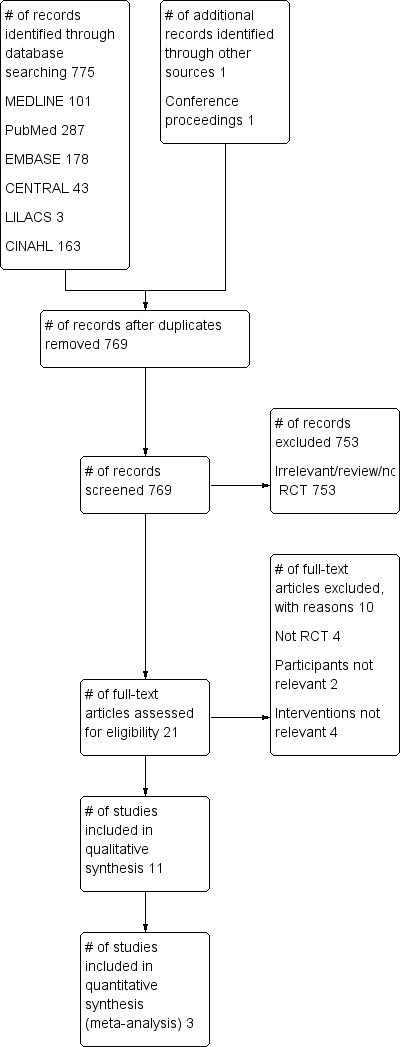
Study flow diagram for the original review (Guimarães 2009).
For the first update of the review in 2013, 309 references (post‐deduplication) were identified by the searches. We selected five references for careful reading and obtained them in full text when available. After assessing the full articles, we included only one study (Kulkarni 2010) and added four studies (Cattano 2010; Dronkers 2008; Kundra 2010; Westwood 2007) to the exclusion table (see Figure 2). Therefore, there are 12 studies with a total of 1834 participants that met the minimal methodological requirements in this review (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Kulkarni 2010; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985).
2.
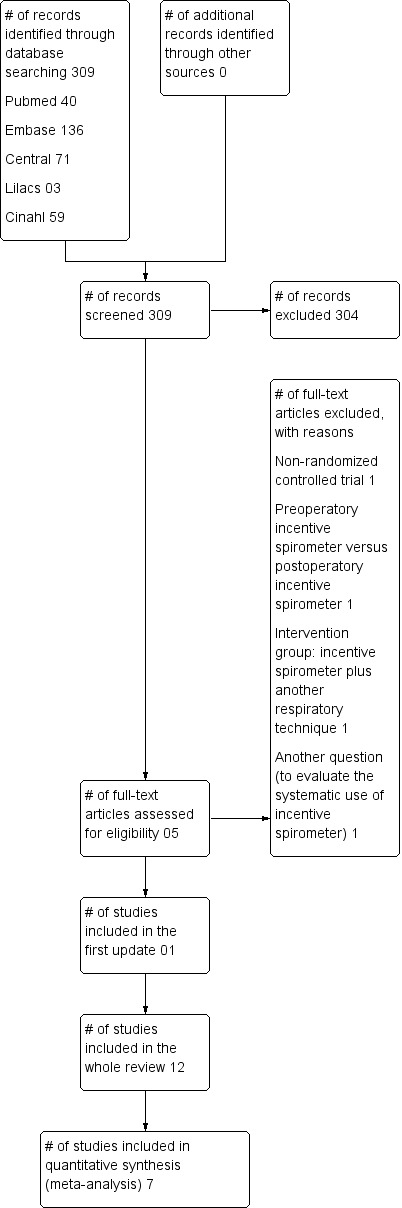
Study flow diagram for this review update.
Included studies
In the first update of this review, we have included 12 studies with a total of 1834 participants (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Kulkarni 2010; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985). These studies enrolled a total of 1834 participants.
Design of the studies
All included studies claimed to be RCTs.
Types of study participants
Celli 1984 assessed 172 participants (59 male and 113 female) with a mean age of 47 years.
Craven 1974 evaluated 70 participants (39 male and 31 female) with a mean age of 52.4 years. The authors of this study evaluated high‐risk patients, defined as smokers and those with chronic respiratory disease.
Dohi 1978 studied 64 participants with a mean age of 55.5 years; the gender of the participants was not reported.
Hall 1991 evaluated 876 participants (436 male and 440 female) with a mean age of 55.0 years.
Hall 1996 assessed 456 participants with a mean age of 51.8 years, of whom 155 (68 male and 87 female) were eligible.
Jung 1980 enrolled 126 individuals (29 male and 97 female) with a mean age of 40.7 years.
Kulkarni 2010 studied 80 randomized participants and 66 were analysed, their age and gender were not reported.
Lyager 1979 studied 103 participants (9 patients were excluded from the study and from the analysis); their gender and mean age were not reported.
O'Connor 1988 assessed 40 participants with a mean age of 23.0 years; the participants' gender was not reported.
Ricksten 1986 evaluated 43 participants (21 male and 22 female) with a mean age of 53.7 years.
Schwieger 1986 enrolled 40 participants (9 male and 31 female) with a mean age of 53.5 years.
Stock 1985 assessed 65 participants; their gender and mean age were not reported.
Types of intervention
In Celli 1984, the control group received no respiratory treatment (n = 44); the first intervention group received intermittent positive pressure breathing therapy (IPPB) at a pressure of 15 cm H2O for 15 minutes, four times daily (n = 45); the second intervention group received incentive spirometry (IS) with a visual signal to indicate that the volume goal was met (a 3 sec breathhold signal was used to sustain maximal inspiration) four times daily (n = 42); and the third intervention group undertook deep breathing exercises (DBE) under supervision for 15 minutes, four times daily (n = 41). All treatments were applied for a minimum of 10 breaths at volumes ranging from 100 to 1800 ml, starting at one half of the preoperative VC until at least 70% of the VC was achieved.
In the Craven 1974 study the interventions were IS (n = 35) versus preoperative physiotherapy (twice a day, or more frequently if clinically indicated) (n = 35). Patients in the IS group received no chest physiotherapy but they received preoperative instructions about how to use the spirometer and practised using it. Postoperatively the IS group was encouraged to use the machine at least 10 times each hour for the first five postoperative days.
In the Dohi 1978 study the intervention groups were: (1) deep breathing using an incentive spirometric three‐ball, flow‐measuring device (Triflo), for five consecutive postoperative days five times every hour for about eight waking hours daily (n = 34, of whom only 23 underwent upper abdominal surgery); and (2) standard episodic IPPB (0.5 ml of a solution (Bronkosol) containing isoetharine hydrochloride and phenylephrine hydrochloride and 4.5 ml of saline) as the control group (n = 30, of whom only 13 underwent upper abdominal surgery). Each treatment lasted 15 minutes, averaging four treatments per day. In the Hall 1991 study, patients received an Airx incentive spirometer fitted with a one‐way valve (Airlife Inc, California, USA) which the patients were initially instructed on the use of (n = 431) (patients were encouraged to take slow maximal inspirations and to hold each breath for as long as possible) or chest physiotherapy (CP) (n = 445): 115 (IS) and 104 (CP); upper transverse or oblique incision: 129 (IS) and 116 (CP); upper vertical incision: 37 (IS) and 55 (CP). In Hall 1996, patients were randomized to receive either deep breathing therapy, where they were seen once and encouraged to take 10 deep breaths each hour, or IS, where they were provided with a laminated information sheet and an Airx incentive spirometer fitted with a one‐way valve (Airlife Inc, California, USA) that they used at least 10 times each hour by taking slow maximal inspirations and holding each breath for as long as possible.
In Jung 1980, one group was prescribed IPPB (n = 36) set at 15 cm H2O; the second group was prescribed resistance breathing used as often as possible (using a blow glove) (n = 45); and the third group was prescribed sustained maximal inhalations using an incentive spirometer (Spirocare) (n = 45) with a preset volume goal between 1400 and 1750 ml, which was to be held for three seconds using the breath‐hold signal incorporated into the spirometer. All patients received instructions on the use of the assigned device. Each treatment was undertaken four times daily, spread out during the waking hours, for 15 to 20 minutes through to the third postoperative day. No attempt was made to assure an absolute minimum number of breaths with any of the devices.
In Kulkarni 2010, the patients were divided in four groups: group A (n = 20), no training; group B (n = 20), deep breathing exercises; group C (n = 20), IS (Spiroball); and group D (n = 20), inspiratory muscle training (IMT) (patients were trained with Powerbreathe). In the postoperative period there were 14 lost to follow‐up: group A (n = 3), group B (n = 3), group C (n = 5), and group D (n = 3). Patients were expected to train twice daily, each session lasting 15 min, for two weeks minimum up to the day before surgery. They were also instructed in the technique by the researcher. The initial device resistance loading was set to 20% to 30% of baseline maximal inspiratory pressure (MIP) and according to ease of use in the first session. The load varied from one to nine and was increased incrementally by half a level daily for the first week.
In Lyager 1979, the patients were divided into two groups: the Bartlett group (exercise repeated at least four times per hour, starting the morning of the first postoperative day and continuing up to the end of the fourth day), and a control group. Patients in both groups received respiratory physiotherapy.
In O'Connor 1988, there were two groups: patients in one group used an IS as part of their postoperative chest physiotherapy (n = 20), those in the other group received routine postoperative physiotherapy (n = 20). Patients were encouraged to inhale maximally and the leak on the IS was adjusted to maintain the ball at the top of the tube for three seconds, three times every hour, postoperatively.
In Ricksten 1986, the three groups received: (1) deep‐breathing exercises by taking 30 sustained maximal inspirations every waking hour with the aid of a deep‐breathing exerciser (Triflo), (2) periodic continuous positive airway pressure (CPAP) via a face mask for 30 breaths every waking hour with a positive end‐expiratory pressure of 10 to 15 cm H2O, or (3) a positive expiratory pressure (PEEP) for 30 breaths every waking hour. The patients were trained preoperatively with individually chosen expiratory resistances. Treatment was started one hour after surgery and was continued for three postoperative days.
Schwieger 1986 randomized the patients to an IS group (n = 20) or a control group (n = 20) where patients did not receive any respiratory treatment before or after surgery. In the IS group, patients were trained before surgery with a volumetric incentive spirometer (Inspiron). The treatment started on the day of surgery and it consisted of increasingly deep and prolonged inspirations with the IS for five minutes hourly, at least 12 times per day, during the first three days after surgery.
In Stock 1985, there were three groups: (1) coughing and deep‐breathing group (CDB) (n = 20), (2) IS group (n = 22), and (3) the CPAP group (n = 23). All treatments lasted 15 minutes and were delivered every two hours during waking hours, starting from four to 72 hours after the operation. The IS device was adjusted to contain from 200 to 2000 ml to keep the bulb lit for three seconds. If inspiratory effort improved during the 15 minutes treatment, the volume was increased.
Types of outcome measures
Celli 1984 measured the patients' weight, height, temperature, heart rate, forced vital capacity (FVC), forced expiratory volume in one second (FEV1), forced expiratory flow from 25% to 75% of vital capacity (VC), postoperative pulmonary complications, and length of stay in hospital.
Craven 1974 assessed pulmonary complications, temperature, pulse, respiratory rate, production of sputum, and recorded the use of analgesics.
Dohi 1978 measured FEV1, FVC, peak expiratory flow rate, and pulmonary complications.
Hall 1991 evaluated pulmonary complications, arterial blood gas analysis, and length of hospital stay.
Hall 1996 assessed respiratory complications and the time that staff devoted to prophylactic respiratory therapy.
Jung 1980 measured the presence of fever, increased respiratory rate, cough and sputum, abnormalities on auscultation of the chest, and pulmonary atelectasis.
Kulkarni 2010 measured patients' physical ability, pain score, discharge date, and the respiratory variables. Primary outcomes were absolute and relative change in all respiratory variables while secondary outcomes included length of stay, time in intensive care unit (ICU) postoperatively, time on a ventilator, respiratory rates, oxygen saturations, proven respiratory infections, and other pulmonary complications.
Lyager 1979 studied the severity of coughing, expectoration, and dyspnoea; the degree of mobility; arterial blood oxygenation and respiratory rate; pulse rate; body temperature; and carried out auscultation of the lungs. They recorded the degree of atelectasis, infiltration, stasis, and pleural effusion.
O'Connor 1988 evaluated pulmonary complications (cough, wheeze, basal crepitations, bronchial breathing), FEV1, FVC, arterial blood gas analysis, and length of hospital stay.
Ricksten 1986 assessed arterial blood gases, alveolar‐arteriolar oxygen difference, and peak expiratory flow.
Schwieger 1986 studied arterial blood gas analyses, body temperature, white blood cell count (WBC) and differential cell count, FVC, and FEV1, atelectasis and pulmonary complications.
Stock 1985 measured atelectasis, FVC, and FEV1.
Excluded studies
We excluded 15 studies (Carmini 2000; Cattano 2010; Dronkers 2008; Gale 1977; Genç 2004; Indihar 1982; Kundra 2010; Lederer 1980; Minschaert 1982; Pereira 2000; Pfenninger 1977; Sleszynski 1993; Vilaplana 1990; Westwood 2007). See the table Characteristics of excluded studies for the reasons for exclusion.
Studies awaiting assessment
No study is awaiting assessment.
Risk of bias in included studies
3.
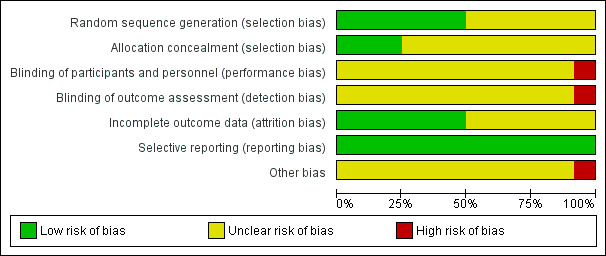
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
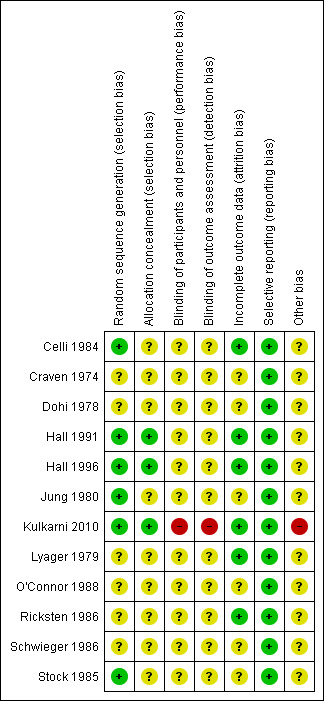
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only the Hall 1991; Hall 1996; and Kulkarni 2010 studies described both the methods for generation of allocation sequence (computer‐generated numbers) and allocation concealment (sealed opaque envelopes) in an adequate manner. Therefore, these studies were graded as at low risk of bias using the criteria of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The Celli 1984; Jung 1980; and Stock 1985 studies described an adequate method for allocation, as drawing of a number to either the control or treatment group for the first study and computer‐generated random numbers for the latter studies, therefore they were ranked as low risk of bias for this domain; however, related to the allocation concealment the studies were ranked as unclear risk of bias (not reported). The other studies (Craven 1974; Dohi 1978; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986) were graded as unclear risk of bias because the allocation procedures were not described.
Blinding
The following included studies did not report whether there was blinding for personnel, participants, or outcome assessors: Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985. They were ranked as at unclear risk of bias for this domain.
In Kulkarni 2010, the authors mentioned that there was no blinding assessment and we ranked the study as high risk of bias for this domain.
Incomplete outcome data
Six studies reported withdrawals (Celli 1984; Hall 1991; Hall 1996; Kulkarni 2010; Lyager 1979; Ricksten 1986); all were less than 20% of the total number of participants and they were ranked as at low risk of bias for this domain. The other studies (Craven 1974; Dohi 1978; Jung 1980; O'Connor 1988; Schwieger 1986; Stock 1985) did not report either the withdrawals or the dropouts, therefore they were ranked as at unclear risk of bias. For the purposes of the ITT analysis we assumed that dropouts had a worse outcome.
Selective reporting
No evidence of selective reporting was noted in any of the included studies (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Kulkarni 2010; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985), therefore they were all ranked as low risk of bias.
Other potential sources of bias
No evidence of other biases was found in any of the included studies (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Kulkarni 2010; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985) except the Kulkarni 2010 study, which reported a conflict of interest and was ranked as at high risk of bias for this domain.
Effects of interventions
See: Table 1
See Table 1.
It was not possible to address most of the outcomes specified in the protocol as the included studies either did not report them or did not provide sufficient data for analysis.
Incentive spirometry (IS) versus no respiratory treatment
(See Analysis 1.1)
1.1. Analysis.
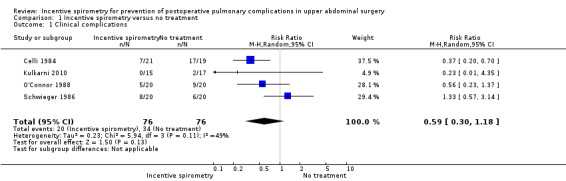
Comparison 1 Incentive spirometry versus no treatment, Outcome 1 Clinical complications.
Outcome: clinical complications
We found no statistically significant difference between the participants receiving IS and those who had no respiratory treatment in the meta‐analysis of four studies (Celli 1984; Kulkarni 2010; O'Connor 1988; Schwieger 1986). The relative risk (RR) was 0.59 (95% CI 0.30 to 1.18) for the incidence of clinical complications.
Incentive spirometry versus deep‐breathing exercises (DBE)
(See Analysis 2.1)
2.1. Analysis.
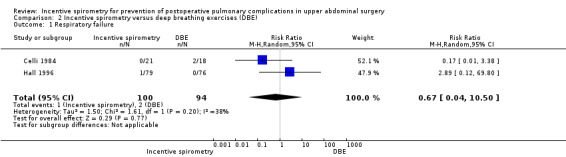
Comparison 2 Incentive spirometry versus deep breathing exercises (DBE), Outcome 1 Respiratory failure.
Outcome: respiratory failure
We found no statistically significant differences between the participants receiving IS compared to those receiving DBE in the meta‐analysis of two studies (Celli 1984; Hall 1996) (RR 0.67, 95% CI 0.04 to 10.50).
Incentive spirometry versus physiotherapy
(See Analysis 3.1)
Outcome: pulmonary complications as determined by chest X‐ray
We found no statistically significant differences between the participants receiving IS compared to those receiving physiotherapy in the risk of developing a pulmonary condition or complication in the overall meta‐analysis of two studies (Craven 1974; Hall 1991). Some possible benefits (favouring IS in high‐risk patients and the use of the Bartlett‐Edwards spirometer, in the Craven 1974 study) were not borne out by the meta‐analysis as this small trial was not weighted highly in comparison to the larger Hall 1991 study (RR 0.83, 95% CI 0.51 to 1.34).
Discussion
Summary of main results
We have found no evidence to support the use of incentive spirometry (IS) in the prevention of pulmonary complications after upper abdominal surgery. We set out to identify the best clinical evidence available to answer our question, and performed an extensive search with careful quality assessment, but only limited conclusions can be drawn from the trials that were included. This review has been limited mainly by the low quality of the trials available for inclusion. The methodological descriptions reported inadequate methods of randomization and allocation concealment, and there were limitations to the blinding. Only two studies (Hall 1991; Hall 1996) showed adequate allocation concealment; the other studies did not report adequate concealment. Further, the majority of the included trials did not address the same outcomes and for this reason the pooling of data was seldom possible. Some included studies did not provide separate data for patients undergoing upper abdominal surgery. This meant that their data could not be used in the meta‐analysis. The small number of trials and the sometimes low methodological quality meant that our intended sensitivity analyses were not possible. In particular, we would have welcomed the opportunity to establish a possible difference between trials where compliance with the prescribed therapy was recorded and those trials where it was not. Although compliance with treatment is not a standard methodological quality marker, it is important in this context. There is a great deal of heterogeneity amongst the studies in the different physiotherapy techniques described, both in the IS groups and in the comparison groups. The comparison groups may not strictly be control groups, and some papers did not report what constituted standard therapy or standard care. This variation is inherent in physiotherapy practice and may reflect the lack of a 'gold standard' method. In some studies, additional therapeutic procedures were applied to either the IS or control group, or both, thus making it difficult to assess the pure effect of the experimental intervention. The poor coverage of the different devices also made our planned subgroup analyses impossible.
The age of some of the devices, and the trials evaluating them, is also relevant. A number of older studies are of poor methodological quality and might also describe practice which is less applicable to modern physiotherapy. For instance, we have included trials of the Bartlett‐Edwards spirometer from the 1970s (Craven 1974; Dohi 1978; Lyager 1979) and 1980s (Celli 1984; Jung 1980; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985). Clinicians should consider this point in applying the findings to their clinical practice as the Bartlett‐Edwards spirometer is an obsolete technique. The need to compare disposable or cleanable ISs is strongly advised.
We had hoped to be able to comment on the effective use of resources. However, both the cost of any intervention and the benefit it offers must be taken into account. As we have been unable to make a clear statement on effectiveness there can be no further consideration of overall cost‐effectiveness.
Further well‐designed research studies are necessary, with long‐term follow up.
Overall completeness and applicability of evidence
Because of our comprehensive search strategy, including handsearches of the reference lists of identified studies for additional citations and contact with experts in the field, we are confident that we have mapped all the clinical trials comparing IS to no therapy or other therapy on postoperative pulmonary complications and mortality in adults undergoing upper abdominal surgery.
Quality of the evidence
The methodological quality of the included studies was generally unclear (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985). Methodological aspects of one study (Kulkarni 2010) had a high risk of introducing bias, with inadequate blinding of outcome assessment and conflict of interest.
Potential biases in the review process
Even though we included 12 studies in this review, the overall methodological quality of the studies was unclear. Most of the studies that we assessed were classified as showing unclear methodological quality due to the lack of information. This would reflect on any conclusions drawn from this review. Many of the studies included in this review are old and were conducted before any awareness related to the issues involved in the internal validity of RCTs. Another area of concern was that the included studies have no standardized outcomes as yet, and this makes the performance of a meta‐analysis more difficult.
Agreements and disagreements with other studies or reviews
The results of this review reflect those of previous versions of the review (Guimarães 2009). A recent systematic review, undertaken to look at the effects of breathing exercises on the recovery of pulmonary function and prevention of postoperative pulmonary complications after upper abdominal surgery, showed that breathing exercises seem to be effective on respiratory muscle strength, however the lack of good quality primary studies makes it difficult to draw a consistent conclusion on this topic (Grams 2012). Another review evaluated the efficacy of IS, IPPB, and DBE in the prevention of postoperative pulmonary complications in patients undergoing upper abdominal surgery. The authors found that IS and DBE appear to be more effective than no physical therapy intervention, however there is no evidence comparing the three modalities themselves (Thomas 1994).
Overend 2001 published a systematic review of the literature with the same aim as our review. Our search is more recent, more extensive, had no language restrictions, and made an explicit assessment of study quality, but nevertheless we reached the same overall conclusions.
Authors' conclusions
Implications for practice.
There is low quality evidence regarding the lack of effectiveness of incentive spirometry for prevention of postoperative pulmonary complications in patients after upper abdominal surgery.
Implications for research.
Future randomized controlled clinical trials should have standardized outcome measures such as pulmonary complications, total mortality from respiratory causes, and all‐cause mortality. Dropouts and losses to follow up need to be clearly reported.
Future studies should also address the issue of compliance with treatment, for example, the study must be designed to address efficacy or efficiency. Besides that, future studies must be adequately powered and assess patients at a common time point, or points, following surgery. Treatment modalities described as standard care, or similar, must be carefully defined and must be identically applied in both the control and experimental groups.
What's new
| Date | Event | Description |
|---|---|---|
| 4 February 2014 | New search has been performed | This review is an update of the previous Cochrane systematic review (Guimarães 2009) that included 11 RCTs (Celli 1984; Craven 1974; Dohi 1978; Hall 1991; Hall 1996; Jung 1980; Lyager 1979; O'Connor 1988; Ricksten 1986; Schwieger 1986; Stock 1985) and enrolled 1754 participants. We updated the methods section, plain language summary, risk of bias tables and included a summary of findings table. |
| 4 February 2014 | New citation required but conclusions have not changed | The previous authors Andrew Smith and Delcio Matos decided not to update the review (new authors: Paulo do Nascimento Junior, Norma SP Módolo, Michele Guimarães, Silvia Aline dos Santos Andrade, Leandro Gobbo Braz and Regina P El Dib have updated this version). We found only one new trial (Kulkarni 2010) that met our inclusion criteria. In general, our review reaches the same conclusions as the original review (Guimarães 2009). (Previously we stated there was 'no evidence'. In this update we state the evidence is 'low quality'.) |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 2 May 2011 | Amended | Contact details updated. |
| 31 March 2011 | Amended | Contact details updated. |
| 4 February 2011 | Amended | Contact details updated. |
| 10 July 2009 | Amended | Author's name corrected, previously: Regina El Dib; now: Regina P El Dib. |
| 19 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Prof Nathan Pace, Dr Tom Overend, Prof Shigemasa Ikeda, and Anne Peticolas for their help and editorial advice during the preparation of this review. We thank Karen Hovhannisyan (Trial Search Co‐ordinator, Cochrane Anaesthesia Review Group) for the initial search strategy developed for the protocol for the review. We would also like to thank Jane Cracknell (Review Group Co‐ordinator, Cochrane Anaesthesia Review Group), Janet Wale (copy editor), Dr Delcio Matos, and Dr Andrew F Smith for their help with the original review.
We would like to thank Andrew Smith (content editor), Nathan Pace (Statistical editor), Jane Cracknell (Review Group Co‐ordinator), Shigemasa Ikeda and Tom Overend (peer reviewers) and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Spirometry] explode all trees #2 (incentiv* near spiromet?r*) or spiromet?r*:ti,ab or (breath* near exercis*) #3 MeSH descriptor: [Breathing Exercises] explode all trees #4 #1 or #2 or #3 #5 MeSH descriptor: [Bronchial Spasm] explode all trees #6 MeSH descriptor: [Respiratory Distress Syndrome, Adult] explode all trees #7 MeSH descriptor: [Pulmonary Atelectasis] explode all trees #8 MeSH descriptor: [Pneumonia] explode all trees #9 ((lung or pulmonary) near complication*) or tracheo?bronchial:ti,ab or bronchospasm:ti,ab or (breath* near (inadequacy or insufficiency or failure)) #10 #5 or #6 or #7 or #8 or #9 #11 #4 and #10
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. exp Spirometry/ or (incentiv* adj3 spiromet?r*).mp. or spiromet?r*.ti,ab. or exp breathing exercises/ or (breath* adj3 exercis*).mp. 2. exp Bronchial Spasm/ or exp Respiratory Distress Syndrome, Adult/ or exp Atelectasis/ or exp Pneumonia/ or ((lung or pulmonary) adj3 complication*).mp. or tracheo?bronchial.ti,ab. or bronchospasm.ti,ab. or (breath* adj3 (inadequacy or insufficiency or failure)).mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (OvidSP)
1. exp spirometry/ or (incentiv* adj3 spiromet?r*).mp. or spiromet?r*.ti,ab. or exp breathingexercise/ or (breath* adj3 exercis*).mp. 2. bronchospasm/ or exp adult respiratory distress syndrome/ or atelectasis/ or pneumonia/ or ((lung or pulmonary) adj3 complication*).mp. or tracheo?bronchial.ti,ab. or Bronchial Spasm/ or (breath* adj3 (inadequacy or insufficiency or failure)).mp. 3. exp abdominal surgery/ or exp thorax surgery/ or ((abdom?n* or thora*) adj3 surg*).mp. or (surg* or operat*).ti,ab. 4. 1 and 2 and 3 5. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 6. 4 and 5
Appendix 4. LILACS (BIREME) search strategy
spiromet?r$ or (breath$ and exercise$) [Palavras] and ( Bronchial Spasm or Respiratory Distress Syndrome or Atelectasis or Pneumonia or ((lung or pulmonary) AND complication$) or tracheo?bronchial or bronchospasm or (breath$ AND (inadequacy or insufficiency or failure))) [Palavras]
Appendix 5. Search strategy for CINAHL (EBSCOhost)
S1 ( (MM "Breathing Exercises") OR (MM "Spirometry") ) OR ( (incentiv* N3 spiromet?r*) or (breath* N3 exercis*) ) OR AB spiromet?r* S2 ((MM "Bronchial Spasm") OR (MH "Respiratory Distress Syndrome+") OR (MM "Atelectasis") OR (MH "Pneumonia") ) OR ( ((lung or pulmonary) N3 complication*) or AB tracheo?bronchial or AB bronchospasm or (breath* N3 (inadequacy or insufficiency or failure))) S3 S1 and S2
Data and analyses
Comparison 1. Incentive spirometry versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical complications | 4 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.18] |
Comparison 2. Incentive spirometry versus deep breathing exercises (DBE).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.04, 10.50] |
Comparison 3. Incentive spirometry versus physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary complications | 2 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.34] |
| 1.1 IS (Bartlett‐Edwards) versus physiotherapy | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.97] |
| 1.2 IS (Airlife) versus chest physiotherapy | 1 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.41] |
3.1. Analysis.
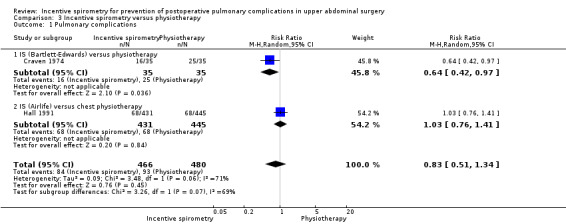
Comparison 3 Incentive spirometry versus physiotherapy, Outcome 1 Pulmonary complications.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Celli 1984.
| Methods | Design: randomized controlled trial Multicentre or single‐centre: not reported Period: not reported Sample size: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: reported (less than 20%). Intention‐to‐treat analysis: not used. Follow up: until the fourth postoperative day |
|
| Participants | 172 participants. Sex (male/female): 59/113. Age (mean): 46.95 years. Setting: private hospital in Maracaibo, Venezuela. Inclusion criteria: patients undergoing abdominal surgery. Exclusion criteria: not reported | |
| Interventions | Control group (no respiratory treatment): n = 44 patients. IPPB group (intermittent positive pressure breathing therapy for 15 minutes, four times daily): n = 45 patients). IS group (incentive spirometry four times daily): n = 42 patients). DBE group (deep breathing exercises under supervision for 15 min, four times daily): n = 41 patients) | |
| Outcomes | Patients' body temperature, heart rate, forced vital capacity, forced expiratory volume in one second, forced expiratory flow from 25% to 75% of vital capacity, postoperative pulmonary complications, and length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of a number to either the control or treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total of the participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Craven 1974.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: not reported. Intention‐to‐treat analysis: not used. Follow up: until the fifth postoperative day | |
| Participants | 70 participants. Sex (male/female): 39/31. Age (mean): 52.4 years. Setting: not reported. Inclusion criteria: patients undergoing elective surgery through upper abdominal incisions. Exclusion criteria: not reported | |
| Interventions | Spirometer group (n = 35) or physiotherapy group (n = 35) | |
| Outcomes | Pulmonary complications, temperature, pulse, respiratory rate, production of sputum, and use of analgesics were recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Not reported |
| Other bias | Unclear risk | Conflict of interest: not reported |
Dohi 1978.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: not reported. Intention‐to‐treat analysis: not used. Follow up: for five consecutive postoperative days | |
| Participants | 64 participants. Sex (male/female): not reported. Age (mean): 55.5 years. Setting: not reported. Inclusion criteria: patients scheduled for elective intra‐abdominal surgery. Exclusion criteria: patients with a history of ischaemic heart disease or paraplegia | |
| Interventions | Deep breathing using an incentive spirometric three‐ball, flow‐measuring device (Triflo) or standard episodic intermittent positive‐pressure breathing (IPPB) | |
| Outcomes | Forced expiratory volume in one second, forced vital capacity, peak expiratory flow rate, and pulmonary complications | |
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Hall 1991.
| Methods | Design: randomized controlled trial. Single‐centre. Period: July 1988‐August 1989. Sample size calculations: reported (the prevalence of pulmonary complications was predicted to be 20%, a sample size of 874 patients was necessary to detect an absolute 10% difference in the prevalence of pulmonary complications by use a two‐tailed comparison with a probability of a type I error of 1% and a power of 80%). Generation of allocation sequence: adequate (computer‐generated pseudo‐random numbers). Allocation concealment: adequate (sealed opaque envelopes). Blinding of assessment of treatment effect: not reported. Withdrawals: reported (less than 20%). Intention‐to‐treat analysis: reported, but did not include patients who were randomized and did not subsequently undergo abdominal surgery. Follow up: until the seventh postoperative day | |
| Participants | 876 participants. Sex (male/female): 436/440. Age (mean): 55.0 years. Setting: Royal Perth Hospital, Australia. Inclusion criteria: patients who underwent a laparotomy which included manipulation of viscera. Exclusion criteria: patients who had elective operations for groin hernia, patients who did not give consent, were under 14 years of age, or had a pre‐existing pulmonary complication | |
| Interventions | Incentive spirometry (at least five minutes in every waking hour) or chest physiotherapy | |
| Outcomes | Pulmonary complication, blood gas analysis, and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total of the participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Hall 1996.
| Methods | Design: stratified randomized trial. Single‐centre. Period: not reported. Sample size calculations: reported (the prevalence of respiratory complications was predicted to be between 10% and 15%. an overall sample size of 430 patients was estimated to be necessary to detect an absolute 10% difference in the prevalence of postoperative respiratory complications with type I error of 5% and a power of 70%). Generation of allocation sequence: adequate (computer generated numbers). Allocation concealment: adequate (sealed opaque envelopes). Blinding of assessment of treatment effect: adequate (outcome checked by a clinician who was unaware of the nature of the respiratory therapy). Withdrawals: reported (less than 20%). Intention‐to‐treat analysis: reported, but did not include patients who were randomized and did not subsequently undergo abdominal surgery. Follow up: not reported | |
| Participants | 155 eligible participants. Sex (male/female): 68/87. Age (median): 38 years, incentive spirometry group and 34 years, deep breathing group. Setting: general surgical service of an Australian urban teaching hospital. Inclusion criteria: patients undergoing abdominal surgery, less than 60 years of age with an American Society of Anesthesiologists' physical status classification of I (low risk). Exclusion criteria: pulmonary embolism and pulmonary oedema (both cardiogenic and non‐cardiogenic) were not regarded as respiratory complications | |
| Interventions | Patients randomized to receive deep breathing therapy were seen once and encouraged to take 10 deep breaths each hour. Patients randomized to receive incentive spirometry were provided with a laminated information sheet, an Airx Incentive Spirometer fitted with a one way valve and were encouraged to use the incentive spirometer at least 10 times each hour by taking slow maximal inspirations and holding each breath for as long as possible | |
| Outcomes | Respiratory complications and the time that staff devoted to prophylactic respiratory therapy | |
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total of the participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Jung 1980.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation sequence: adequate (system of computer‐generated random numbers). Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: not reported. Intention‐to‐treat analysis: not used. Follow up: until the third postoperative day | |
| Participants | 126 participants. Sex (male/female): 29/97. Age (mean): 40.7 years. Inclusion criteria: patients who were undergoing elective upper‐abdominal surgery. Exclusion criteria: not reported | |
| Interventions | Group 1: intermittent positive‐pressure breathing ‐ IPPB (n = 36). Group 2: resistance breathing (blow glove) (n = 45). Group 3: sustained maximal inhalations using an incentive spirometer (Spirocare) (n = 45) | |
| Outcomes | Presence of fever, increased respiratory rate, cough and sputum, or abnormalities on auscultation of the chest, pulmonary atelectasis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Kulkarni 2010.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size: not reported. Follow up: 1‐7 days postoperatively | |
| Participants | N = 80 randomized and 76 analysed Sex: not reported Mean age: not reported Inclusion criteria: (i) over 18 years of age; and (ii) undergoing major abdominal general surgery (defined as deliberate breach of peritoneum), or major urological surgery, with ASA (American Society of Anesthesiologists) grades I–IV requiring any length of hospital stay Exclusion criteria: if they were ASA grade V, had suspected or established respiratory infection, were likely to undergo surgery to be performed within 2 weeks of initial assessment, had previous spontaneous pneumothorax Setting: not reported |
|
| Interventions | Group A: no training (n=20) Group B: deep breathing exercises (n=20) Group C: incentive spirometry Spiroball® (n=20) Group D: Powerbreathe® |
|
| Outcomes | Change in all respiratory variables following training before surgery and after surgery; length of stay of patients, time in ICU postoperatively, time on a ventilator, respiratory rates and oxygen saturations from charts at a fixed time postoperatively off oxygen, proven respiratory infection (positive sputum culture) and other pulmonary complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were not blinded; however blinding for participants was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total of the participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | High risk | Conflict of interest: Professor Alison McConnell acts as a consultant to HaB Ltd |
Lyager 1979.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: reported (lesser than 20%). Intention‐to‐treat analysis: not used. Follow up: four days postoperative | |
| Participants | 103 participants (nine patients were excluded from the study and from the analysis). Sex (male/female): not reported. Age (mean): not reported. Setting: not reported. Inclusion criteria: patients undergoing elective surgery for gallstones or peptic ulcers. Exclusion criteria: all patients over 75 years old | |
| Interventions | The patients were divided in two groups: the Bartlett group (the exercise should be repeated at least four times per hour during waking hours starting in the morning of the first postoperative day and continuing up to the end of the fourth day) and the control group (respiratory physiotherapy) | |
| Outcomes | The severity of coughing, expectoration and dyspnoea, the degree of mobility, arterial blood gas analysis, respiratory rate, pulse rate, body temperature, and auscultation of the lungs. Besides that, the degree of atelectases, infiltration, stasis or pleural effusion were recorded | |
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total of the participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
O'Connor 1988.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation sequence: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: not reported. Intention‐to‐treat analysis: not used. Follow up: until the second postoperative day | |
| Participants | 40 participants. Sex (male/female): not reported. Age (mean): 23 years. Setting: not reported. Inclusion criteria: patients ASA class 1 or 2, scheduled for elective cholecystectomy. Exclusion criteria: not reported | |
| Interventions | There were two groups: patients in one group used an IS as part of their postoperative chest physiotherapy (n = 20); those in the other group received routine postoperative physiotherapy (n = 20) | |
| Outcomes | Pulmonary complications (cough, wheeze, basal crepitations or bronchial breathing), forced expiratory volume in one second, forced vital capacity, arterial blood gas analysis, and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Ricksten 1986.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: reported (less than 20%). Intention‐to‐treat analysis: not used. Follow up: for three postoperative days | |
| Participants | 43 participants. Sex (male/female): 21/22. Age (mean): 53.7 years. Setting: not reported. Inclusion criteria: patients undergoing elective upper abdominal surgery. Exclusion criteria: not reported | |
| Interventions | Intervention group (deep breathing exercises taking 30 sustained maximal inspirations every waking hour with the aid of a deep breathing exerciser ‐ Triflo), CPAP group (periodic continuous positive airway pressure by face mask for 30 breaths every waking hour with a positive end‐expiratory pressure between 10 to 15 cm H2O) or PEP group (positive expiratory pressure for 30 breaths every waking hour) The control group carried out deep breathing exercises taking 30 sustained maximal inspirations every waking hour with the aid of a deep breathing exerciser (Triflo) |
|
| Outcomes | Arterial blood gas analysis, alveolar‐arteriolar oxygen difference and peak expiratory flow | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% of the total participants |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Schwieger 1986.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation: not reported. Allocation concealment: not reported. Blinding of assessment of treatment effect: not reported. Withdrawals: not reported. Intention‐to‐treat analysis: not reported. Follow up: for four postoperative days | |
| Participants | 40 participants. Sex (male/female): 9/31. Age (mean): 53.5 years. Setting: not reported. Inclusion criteria: patients undergoing elective cholecystectomy ASA class 1. Exclusion criteria: patients with a ratio of weight to height greater than 0.45, patients over 65 years of age and those with acute infection | |
| Interventions | Incentive spirometry (n = 20) or control group (n = 20) (these patients did not receive any respiratory treatment before or after surgery) | |
| Outcomes | Arterial blood gas analysis, body temperature, white blood cell count (WBC), and differential cell count, forced vital capacity and forced expiratory volume in one second, atelectasis and pulmonary complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
Stock 1985.
| Methods | Design: randomized controlled trial. Multicentre or single‐centre: not reported. Period: not reported. Sample size calculations: not reported. Generation of allocation: adequate (computer random number generator). Allocation concealment: not reported. Blinding of assessment of treatment effect. Withdrawals: not reported. Intention‐to‐treat analysis: not reported. Follow up: for 72 hours | |
| Participants | 65 participants. Sex (male/female): not reported. Age (mean): not reported. Setting: not reported. Inclusion criteria: adults scheduled for elective upper abdominal operations. Exclusion criteria: not reported | |
| Interventions | Coughing and deep breathing (CDB) (n = 20), incentive spirometry (IS) (n=22) or continuous positive airway pressure (CPAP) (n=23). All treatments lasted 15 min, and were delivered every two hours during waking hours from the fourth to the 72 hours after operation | |
| Outcomes | Atelectasis, forced vital capacity and forced expiratory volume in one second | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Conflict of interest: not reported |
RCT = Randomized controlled trial IPPB = Intermittent positive pressure breathing IS = Incentive spirometry DBE = Deep breathing exercises CPAP = Continuous positive airway pressure CDB = Coughing and deep breathing
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carmini 2000 | Intervention group: incentive spirometer plus another respiratory technique |
| Cattano 2010 | Another question (to evaluate the systematic use of incentive spirometer) |
| Dronkers 2008 | Intervention group: incentive spirometer plus another respiratory technique |
| Gale 1977 | Clinical situation: cardiac surgery |
| Genç 2004 | Intervention group: incentive spirometer plus another respiratory technique such as forced expiration and coughing techniques |
| Indihar 1982 | Quasi‐randomized controlled trial |
| Kundra 2010 | Preoperative incentive spirometer versus postoperative incentive spirometer |
| Lederer 1980 | The study compared the use of three types of deep‐breathing devices |
| Minschaert 1982 | Intervention group: incentive spirometer plus chest physical therapy |
| Pereira 2000 | Cohort study |
| Pfenninger 1977 | Case series |
| Sleszynski 1993 | Quasi‐randomized controlled trial |
| Vilaplana 1990 | Patients with chest and oesophageal surgery |
| Westwood 2007 | Non‐randomized controlled trial |
Differences between protocol and review
This review is an update of the previous Cochrane Review (Guimarães 2009). We updated the methods section, plain language summary, risk of bias tables, and included a summary of findings table.
Contributions of authors
Conceiving the review: Paulo do Nascimento Junior (PNJ) and Regina El Dib (RED) Co‐ordinating the review: RED Undertaking manual searches: Michele MF Guimarães (MMFG) and Silvia Aline Andrade (SAA) Screening search results: MMFG and SA Organizing retrieval of papers: MMFG and SAA Screening retrieved papers against inclusion criteria: PNJ and RED Appraising quality of papers: PNJ and RED Abstracting data from papers: Norma Sueli Pinheiro Modolo (NSPM) Writing to authors of papers for additional information: MMFG and SAA Providing additional data about papers: NSPM Obtaining and screening data on unpublished studies: Leandro Gobbo Bras (LGB) Data management for the review: PNJ, LGB and RED Entering data into Review Manager (RevMan 5.2): PNJ and RED RevMan statistical data: RED Other statistical analysis not using RevMan: RED Double entry of data: (data entered by person one: PNJ; data entered by person two: RED) Interpretation of data: PNJ, NSPM, LGB and RED Statistical analysis: PNJ and RED Writing the review: PNJ, NSPM, LGB and RED Securing funding for the review: PNJ, NSPM, LGB and RED Guarantor for the review (one author): RED Responsible for reading and checking the review before submission: PNJ, MMFG, NSPM, LGB and RED
Sources of support
Internal sources
New Source of support, Brazil.
External sources
No sources of support supplied
Declarations of interest
Paulo do Nascimento Junior: none known.
Norma SP Módolo: none known.
Michele MF Guimarães: none known.
Sílvia Andrade: none known.
Leandro G Braz: none known.
Regina P El Dib: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Celli 1984 {published data only}
- Celli BR, Rodriguez K, Snider GL. A controlled trial of intermittent positive pressure breathing incentive spirometry and deep breathing exercises in preventing pulmonary complications after abdominal surgery. The American Review of Respiratory Disease 1984;130:12‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Craven 1974 {published data only}
- Craven JL, Evans GA, Davenport PJ, Williams RH. The evaluation of the incentive spirometer in the management of postoperative pulmonary complications. The British Journal of Surgery 1974;61:793‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dohi 1978 {published data only}
- Dohi S, Gold MI. Comparison of two methods of postoperative respiratory care. Chest 1978;73(5):592‐5. [DOI] [PubMed] [Google Scholar]
Hall 1991 {published data only}
- Hall JC, Tarala R, Harris J, Tapper J, Christiansen K. Incentive spirometer versus routine chest physiotherapy for prevention of pulmonary complications after abdominal surgery. Lancet 1991;337:953‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall JC, Tarala RA, Tapper J, Hall, JL. Prevention of respiratory complications after abdominal surgery: a randomised clinical trial. BMJ 1996;312:148‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 1980 {published data only}
- Jung R, Wight J, Nusser R, Rosoff L. Comparison of three methods of respiratory care following upper abdominal surgery. Chest 1980;78(1):31‐5. [DOI] [PubMed] [Google Scholar]
Kulkarni 2010 {published data only}
- Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre‐operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery ‐ a randomised pilot study. Annals of the Royal College of Surgeons of England 2010;92(8):700‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyager 1979 {published data only}
- Lyager S, Wernberg M, Rajani N, Boggild‐Madsen B, Nielsen L, Nielsen HC, et al. Can postoperative pulmonary conditions be improved by treatment with the Bartlett‐Edwards incentive spirometer after upper abdominal surgery?. Acta Anaesthesiologica Scandinavica 1979;23(4):312‐9. [DOI] [PubMed] [Google Scholar]
O'Connor 1988 {published data only}
- O'Connor M, Tattersall MP, Carter JA. An evaluation of the incentive spirometer to improve lung function after cholecystectomy. Anaesthesia 1988;43(9):785‐7. [DOI] [PubMed] [Google Scholar]
Ricksten 1986 {published data only}
- Ricksten SE, Bengtsson A, Soderberg C, Thorden M, Kvist H. Effects of periodic positive airway pressure by mask on postoperative pulmonary function. Chest 1986;89(6):774‐81. [DOI] [PubMed] [Google Scholar]
Schwieger 1986 {published data only}
- Schwieger I, Gamulin Z, Forster A, Meyer P, Gemperle M, Suter PM. Absence of benefit of incentive spirometry in low‐risk patients undergoing elective cholecystectomy. A controlled randomized study. Chest 1986;89(5):652‐6. [DOI] [PubMed] [Google Scholar]
Stock 1985 {published data only}
- Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 1985;87(2):151‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carmini 2000 {published data only}
- Carmini V, Damignani R, Brooks D, Graveline C. Preoperative physiotherapy teaching in paediatric patients. Physiotherapy Canada 2000;52(4):312‐4. [Google Scholar]
Cattano 2010 {published data only}
- Cattano D, Altamirano A, Vannucci A, Melnikov V, Cone C, Hagberg CA. Preoperative use of incentive spirometry does not affect postoperative lung function in bariatric surgery. Translational Research: the journal of laboratory and clinical medicine 2010;156(5):265‐72. [DOI] [PubMed] [Google Scholar]
Dronkers 2008 {published data only}
- Dronkers J, Veldman A, Hoberg E, Waal C, Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clinical Rehabilitation 2008;22(2):134‐42. [DOI] [PubMed] [Google Scholar]
Gale 1977 {published data only}
- Gale GD, Sanders DE. The Bartlett‐Edwards incentive spirometer: a preliminary assessment of its use in the prevention of atelectasis after cardio‐pulmonary bypass. Canadian Anaesthetists Society 1977;24(3):408‐16. [DOI] [PubMed] [Google Scholar]
Genç 2004 {published data only}
- Genc A, Yildirim Y, Gunerli A. Researching of the effectiveness of deep breathing and incentive spirometry in postoperative early stage. Fizyoterapi‐Rehabilitasyon 2004;15(1):28‐33. [Google Scholar]
Indihar 1982 {published data only}
- Indihar FJ, Forsberg DP, Adams AB. A prospective comparison of three procedures used in attempts to prevent postoperative pulmonary complications. Respiratory Care 1982;27(5):564‐8. [Google Scholar]
Kundra 2010 {published data only}
- Kundra P, Vitheeswaran M, Nagappa M, Sistla S. Effect of preoperative and postoperative incentive spirometry on lung functions after laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2010;20(3):170‐2. [DOI] [PubMed] [Google Scholar]
Lederer 1980 {published data only}
- Lederer DH, Water JM, Indech RB. Which deep breathing device should the postoperative patient use?. Chest 1980;77(5):610‐3. [DOI] [PubMed] [Google Scholar]
Minschaert 1982 {published data only}
- Minschaert M, Vincent JL, Ros AM, Kahn RJ. Influence of incentive spirometry on pulmonary volumes after laparotomy. Acta Anaesthesiologica Belgica 1982;33(3):203‐9. [PubMed] [Google Scholar]
Pereira 2000 {published data only}
- Pereira ED, Farensin SM, Fernandes AL. Respiratory morbidity in patients with and without pulmonary obstructive syndrome after upper abdominal surgery [Morbidade respiratoria nos pacientes com e sem sindrome pulmonar obstrutiva submetidos a cirurgia abdominal alta]. Revista da Associação Médica Brasileira 2000;46(1):15‐22. [DOI] [PubMed] [Google Scholar]
Pfenninger 1977 {published data only}
- Pfenninger J, Roth F. Intermittent positive pressure breathing (IPPB) versus incentive spirometer (IS) therapy in the postoperative period. Intensive Care Medicine 1977;3(4):279‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sleszynski 1993 {published data only}
- Sleszynski SL, Kelso AF. Comparison of thoracic manipulation with incentive spirometry in preventing postoperative atelectasis. The Journal of the American Osteopathic Association 1993;93(8):834‐8. [PubMed] [Google Scholar]
Vilaplana 1990 {published data only}
- Vilaplana J, Sabaté A, Ramon R, Gasolibe V, Villalonga R. Ineffectiveness of incentive spirometry as coadjuvant of conventional physiotherapy for the prevention of postoperative respiratory complications after thoracic and esophageal surgery [Ineficacia de la espirometría incentiva como coadyuvante de la fisioterapia convencional en la prevención de las complicaciones respiratorias postoperatorias de la cirugía torácica y esofágica]. Revista Espanola de Anestesiologia y Reanimacion 1990;37(6):321‐5. [PubMed] [Google Scholar]
Westwood 2007 {published data only}
- Westwood K, Griffin M, Roberts K, Williams M, Yoong K, Digger T. Incentive spirometry decreases respiratory complications following major abdominal surgery. Surgeon 2007;5(6):339‐42. [DOI] [PubMed] [Google Scholar]
Additional references
AARC 1991
- No authors listed. AARC (American Association for Respiratory Care) clinical practice guideline. Incentive spirometry. Respiratory Care 1991;36:1402‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Bartlett 1970
- Bartlett RH, Krop P, Hanson EL, Moore FD. Physiology of yawning and its application to postoperative care. Surgical Forum 1970;21:223‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Bartlett 1973
- Bartlett RH, Gazzaniga AB, Geraghty TR. Respiratory maneuvers to prevent postoperative pulmonary complications. A critical review. JAMA 1973;224:1017‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Castro 1999
- Castro AA, Clark OA, Atallah AN. Optimal search strategy for clinical trials in the Latin American and Caribbean Health Science Literature database (LILACS database): update. São Paulo Medical Journal/ Revista Paulista de Medicina 1999;117(3):138‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Celli 1993
- Celli BR. Perioperative respiratory care of the patient undergoing upper abdominal surgery. Clinics in Chest Medicine 1993;14(2):253‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Chumillas 1998
- Chumillas S, Ponce JL, Delgado F, Viciano V, Mateu M. Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Archives of Physical Medicine and Rehabilitation 1998;79:5‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chuter 1990
- Chuter TA, Weissman C, Mathews DM, Starker PM. Diaphragmatic breathing maneuvers and movement of the diaphragm after cholecystectomy. Chest 1990;97:1110‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 1999
- Doyle LR. Assessing and modifying the risk of postoperative pulmonary complications. Chest 1999;115(5):77‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fagevik Olsen 1997
- Fagevik Olsen M, Hahn I, Nordgren S, Lonroth H, Lundholm K. Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. The British Journal of Surgery 1997;84:1535‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grams 2012
- Grams ST, Ono LM, Noronha MA, Schivinski CI, Paulin E. Breathing exercises in upper abdominal surgery: a systematic review and meta‐analysis. Revista Brasileira de Fisioterapia 2012;16(5):345‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Kips 1997
- Kips JC. Preoperative pulmonary evaluation. Acta Clinica Belgica 1997;52(5):301‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martin 1984
- Martin LF, Asher EF, Casey JM, Fry DE. Postoperative pneumonia. Determinants of mortality. Archives of Surgery 1984;119(4):379‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Donohue 1992
- O'Donohue WJ Jr. Postoperative pulmonary complications: When are preventive and therapeutic measures necessary?. Postgraduate Medicine 1992;91:167‐70. [DOI] [PubMed] [Google Scholar]
Overend 2001
- Overend TJ, Anderson CM, Lucy SD, Bhatia C, Jonsson BI, Timmermans C. The effect of incentive spirometry on postoperative pulmonary complications. Chest 2001;120(3):971‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.2. Copenhagen: The NordicCochrane Centre, The Cochrane Collaboration, 2011.
Strandberg 1986
- Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Atelectasis during anaesthesia and in the postoperative period. Acta Anaesthesiologica Scandinavica 1986;30(2):154‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sykes 1993
- Sykes LA, Bowe EA. Cardiorespiratory effects of anaesthesia. Clinics in Chest Medicine 1993;14(2):211‐26. [MEDLINE: ] [PubMed] [Google Scholar]
Thomas 1994
- Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta‐analysis. Physical Therapy 1994;74(1):3‐10. [DOI] [PubMed] [Google Scholar]
Van de Water 1972
- Water JM, Watring WG, Linton LA, Murphy M, Byron RL. Prevention of postoperative pulmonary complications. Surgery, Gynecology & Obstetrics 1972;135:229‐33. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Guimarães 2009
- Guimarães MMF, Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006058] [DOI] [PubMed] [Google Scholar]


