Abstract
Background
Nasal polyps frequently occur in people with cystic fibrosis. Sinus infections have been shown to be a factor in the development of serious chest complications in these people. Nasal polyps have been linked to a higher risk of lower respiratory tract infections with Pseudomonas aeruginosa . Topical nasal steroids are of proven efficacy for treating nasal polyposis in the non‐cystic fibrosis population. There is no clear current evidence for the efficacy of topical steroids for nasal polyps in people with cystic fibrosis. This is an updated version of a previously published review.
Objectives
To assess the effectiveness of topical nasal steroids for treating symptomatic nasal polyps in people with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.
Latest search: 10 June 2015.
Selection criteria
Randomised and quasi‐randomised controlled comparing the effects of topical nasal steroids to placebo in people with nasal polyps with cystic fibrosis.
Data collection and analysis
Two authors independently assessed risk of bias in the included trial and extracted data.
Main results
One single‐centred trial (46 participants) was identified comparing a topical steroid (betamethasone) given as nasal drops to placebo. Treatment was given twice daily for six weeks; 22 participants received the active drug.
Subjective symptom scores, change in polyp size, and side effects were assessed. There was no difference in nasal symptom scores between the treatment and placebo groups. Betamethasone was effective in reducing the size of polyps, but was associated with increased reports of mild side effects, nasal bleeding and discomfort.
Risk of bias was high since over 50% of people enrolled did not complete the study. Follow‐up of participants was short (six weeks) also reducing the significance of the results for clinical practice.
Authors' conclusions
This review suggests topical steroids for nasal polyposis in people with cystic fibrosis have no demonstrable effect on subjective nasal symptom scores. They have some effect in reducing the size of the polyps, but due to the small sample size, poor completion rates and lack of follow‐up, the trial is at high risk of bias and evidence for efficacy is limited. Overall there is no clear evidence for using topical steroids in people with cystic fibrosis and nasal polyposis.
A well‐designed randomised controlled trial of adequate power and long‐term follow‐up is needed. Validated measures of symptoms and physical findings should be performed and quality of life issues addressed.
Keywords: Adult; Humans; Administration, Intranasal; Administration, Intranasal/methods; Betamethasone; Betamethasone/administration & dosage; Betamethasone/adverse effects; Cystic Fibrosis; Cystic Fibrosis/complications; Glucocorticoids; Glucocorticoids/administration & dosage; Glucocorticoids/adverse effects; Nasal Polyps; Nasal Polyps/complications; Nasal Polyps/drug therapy
Plain language summary
Steroids applied directly to polyps in the nose in people with cystic fibrosis
Review question
We reviewed the evidence on the effect of applying steroids directly to polyps in the noses of people with cystic fibrosis.
Background
People with cystic fibrosis often have polyps in their nose which can cause discharge and also a blocked nose. We know that people with cystic fibrosis and polyps in their nose also have more of some types of bacteria in their lungs. This can lead to serious chest complications later on. If we treat the polyps effectively at an early stage, we may prevent these chest complications.
Steroid sprays or drops are often applied directly to polyps in the nose.These drugs are usually a safe treatment but can have some important side effects, like affecting the immune system and how the body can regulate blood sugar. They also add to the existing burden of treatment, especially if they need to be taken for the rest of a person's life. However, these drugs have been shown to be successful in treating polyps in people who do not have cystic fibrosis.
Search date
The evidence is current to: 10 June 2015.
Study characteristics
Our search found one trial with 46 adult volunteers with cystic fibrosis which compared nasal drops containing a steroid (betamethasone) to identical drops containing no active treatment. Two drops were applied directly to polyps twice a day for six weeks. Volunteers were put into the different treatment groups at random and a total of 22 volunteers received the steroid drops and 24 received the dummy treatment.
Key results
The trial measured each person's perception of their symptom scores, but found no difference to the scores whether volunteers were using the steroid drops or the dummy treatment. The trial also measured the change in polyp size and found that they shrank. There were no major side effects linked to using betamethasone, and only three volunteers reported increased nose bleeds and discomfort.
The small number of volunteers in this trial means the calculations and results should be regarded with some caution. More trials are needed to confirm the findings and these trials should report on measures of symptoms and quality of life issues.
Quality of the evidence
We think that volunteers had a truly equal chance of being put in either the steroid group or the control group and wouldn't have known which treatment they were receiving, so these issues would not have influenced the results in any way. However, over 50% of people enrolled did not complete the study and the reasons for dropping out were not very clearly explained. We think it is important to take this fact into account when considering the trial results. Also, the trial only followed the volunteers for six weeks, which is a short time when evaluating a treatment that could be needed for the rest of a person's life.
Background
Description of the condition
Cystic fibrosis (CF) is an autosomal recessive condition that is common in people of a Northern European background but less common in people of Hispanic, African or Asian background (Bobadilla 2002). The genetic defect causes an abnormality of salt transport that has a detrimental effect on different parts of the body, most notably the airways and pancreas. In the airways the salt transport defect causes an abnormal airway surface liquid, which results in chronic airway infection and inflammation leading eventually to airway damage and respiratory failure (Southern 2007).
The upper airways are frequently affected in people with CF with rhinosinusitis (sinusitis) and nasal polyps regularly described (Robertson 2008). Nasal polyps are benign growths of water‐logged tissue that arise from the lining tissue in the roof of the nasal cavity. They have a grape‐like appearance and can sometimes be large enough to cause complete nasal obstruction and occasionally disfigurement with broadening of the bridge of the nose. Nasal polyposis affects about four per cent of the non‐CF population (Hedman 1999), but polyposis and rhinosinusitis is much commoner in the CF population (reports of 25% to 40% of individuals with CF (Fokkens 2007b)). There are reports of children and adults being diagnosed with CF following presentation with nasal polyps, although this is uncommon.
Description of the intervention
The accessibility of the nasal cavity makes the delivery of topical treatments very straightforward, either by spray or drops. For many years corticosteroids have been delivered topically to treat nasal conditions including polyposis. A variety of corticosteroids are available in once or twice‐daily dosing regimes. Other topical treatments include nasal washings with medicated salt water (saline douches) (Fokkens 2007a). For more severe polyposis endoscopic surgery is the most common treatment, although systemic steroids are also prescribed (Robertson 2008).
How the intervention might work
It is not clear how topical steroids might work for CF nasal polyposis (or rhinosinusitis); however, corticosteroids are potent anti‐inflammatory agents that reduce the activation and function of inflammatory cells, in this case within the nasal lining (Burgel 2004). They have been investigated extensively in individuals without CF and have proved their clinical effectiveness at reducing nasal symptoms of blockage, discharge, polyp size and improving quality of life (Lund 1998).
Why it is important to do this review
Nasal polyposis (or rhinosinusitis) is a common and distressing complication for children and adults with CF. It is associated with significant morbidity and reduced quality of life and therefore it is imperative to have a clear evidence base on which to establish treatment strategies. Whilst topical steroids are generally a safe treatment, they may be associated with significant side effects, most notably systemic absorption with potential adrenal suppression. They also represent a significant treatment burden for individuals with daily dosing which is potentially a life‐long intervention. The evidence for clinical efficacy of topical and system steroids in nasal polyps and rhinosinusitis has already been assessed for non‐CF patients (Kim 2007; Patiar 2007). It is particularly important to establish the efficacy in people with CF as infections originating from the sinuses have been shown to be a causative factor in the development of serious chest complications later on (Roby 2008). Furthermore, people with CF who also have nasal polyps have a higher frequency of chronic colonisation of Pseudomonas aeruginosa in the lower respiratory tract (Henriksson 2002). Early effective management of the nasal disease may prevent this. In addition, if the disease process in the nose is halted, surgical intervention may be avoided with its own inherent complications.
This is an updated version of a previously published review (Beer 2011).
Objectives
To test the hypothesis that topical nasal steroids are an effective form of treatment for symptomatic nasal polyps in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised trials.
Types of participants
Adults and children with diagnosed CF (diagnosed by sweat or genetic testing or both) who present with symptomatic nasal polyps are to be included in both the treatment and control groups. Participants will not be excluded on the basis of previous surgery for nasal polyps.
Types of interventions
We will compare different pharmaceutical topical steroid agents versus saline douches or placebo at the standard optimum therapeutic dose and regimen as recommended by the British National Formulary (British National Formulary 2009).
Types of outcome measures
Primary outcomes
Quality of life (QOL) (as measured by a validated QOL score i.e. CFQoL (Gee 2000), CFQ‐R (Quittner 2009))
Nasal symptom score (as measured by a validated score i.e. SNOT (Hopkins 2009))
Secondary outcomes
Treatment burden (as measured by validated score)
Need for surgery
Polyp size (determined by a validated score (MacKay 1997))
-
Adverse events
Mild (not requiring significant treatment) e.g. limited epistaxis, discomfort
Moderate (requiring significant treatment or admission) e.g. epistaxis, adrenal suppression
Severe (life threatening) e.g. epistaxis, adrenal failure
-
Respiratory function
forced expiratory volume at one second (FEV1) % of predicted value
FEV1/forced vital capacity (FVC) ratio
New CF pathogens recognised from culture of upper or lower airway secretions
Search methods for identification of studies
Electronic searches
We identified relevant trials identified from the Group's Cystic Fibrosis Trials Register using the term 'nasal polyps'.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the last search of the Cystic Fibrosis Trials Register: 10 June 2015.
Searching other resources
Reference lists of all relevant papers were screened to identify additional relevant citations and other potential studies.
Data collection and analysis
Selection of studies
Two authors (HB, ACS) independently selected trials for inclusion into the review and resolved any disagreements by discussion with a third author. The authors documented the reason for excluding trials. We planned to clearly categorise studies where important information is lacking (including foreign language studies awaiting translation) and report these as 'Studies awaiting classification'.
Data extraction and management
Two review authors (HB, ACS) independently undertook data extraction using a data extraction form, adapted from a form developed by the Cochrane Cystic Fibrosis and Genetic Disorders Review Group. The review authors resolved any disagreements by consensus or through discussion with a third author, which was then recorded on the final data extraction form. One review author (HB) transcribed these into RevMan 5 (Review Manager 2008). Another review author verified all data entry for discrepancies.
We planned to report outcomes at three months, six months, nine months, one year and at further six‐monthly intervals thereafter, but data were only available for the three‐month time‐point.
Assessment of risk of bias in included studies
Two review authors assessed each trial using a simple form, following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
We assessed the following domains, stating low risk, unclear risk and high risk of bias:
randomisation of participants;
concealment of allocation;
blinding (of participants, personnel and outcome assessors);
incomplete outcome data addressed;
selective outcome reporting;
other sources of bias.
We compared the assessments and discussed any inconsistencies between the review authors in the interpretation of inclusion criteria and their significance to the selected studies. We resolved any disagreements through discussion with a third author. We did not automatically exclude any study as a result of being judged to have an unclear or high risk of bias. We presented the evaluation of the risk of bias in included studies in the 'Results' section of the review, both as text and within the risk of bias tables.
Measures of treatment effect
For dichotomous data, we calculated an estimate of treatment effect for each outcome across studies using risk ratio (RR) where appropriate. For continuous outcomes, we planned to record either mean relative change from baseline for each group or mean post‐treatment or intervention values and standard deviation. If standard errors (SEs) were reported, where possible, we planned to convert these to standard deviations (SDs). We planned to calculate a pooled estimate of treatment effect by calculating the standardized mean difference (SMD) and 95% confidence intervals (CIs).
Unit of analysis issues
We will include data from any eligible cross‐over trials in the review; we plan to analyse these using a method recommended by Elbourne (Elbourne 2002).
Dealing with missing data
We identified that raw data from the symptom scores (individual and combined) were missing. However, narrative information was available for the outcomes in question and we are of the opinion that the missing data would not alter our conclusions.
We are planning to contact the original investigators to seek these missing data in order to set up a meta‐analysis. We will contact them in time for the first update of this review.
If we were unable to obtain the missing data by this method, we will assume the missing data are random and impute mean data adjusted to SEs. We will discuss the potential impact of the missing data in the review findings.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by examining the characteristics of the trials; the similarity between the types of participants, the interventions and the outcomes as specified in the criteria for included trials. We also planned to assess statistical homogeneity using the I2 statistic, where I2 values over 50% indicate moderate to high heterogeneity (Higgins 2003).
Assessment of reporting biases
We carried out a comprehensive search to obtain all available studies, and systematically reviewed these to compare the study protocols or the 'Methods' sections of published papers against the 'Results' section to ascertain that data had been addressed for all stated measured outcomes. If we had identified sufficient studies (more than 10), we would have used funnel plots to make a visual assessment of small study effects and possible publication bias, or other causes (Higgins 2003).
Data synthesis
We planned to use fixed‐effect analysis if heterogeneity was low. If we found that heterogeneity is moderate to high (I2 more than 50%), we will use the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to assess for clinical heterogeneity by examining the subgroups for:
age of participants;
severity of disease;
intervention type used (spray versus drops);
previous surgery for nasal polyps or sinusitis or both.
Sensitivity analysis
We planned to test the robustness of our results by performing sensitivity analysis by allocation method (including and excluding the quasi‐randomised trials) and risk of bias (comparing all trials against those of only low risk of bias).
Results
Description of studies
Results of the search
One trial was identified from our search and included in our review (Hadfield 2000).
Included studies
Trial Characteristics
The included trial is a randomised, double‐blind trial of the treatment of nasal polyps in adults with CF. The trial was single‐centred and of parallel design lasting six weeks (Hadfield 2000).
Participants
There were 46 participants; all of whom were adults (over 16 years old) with CF and nasal polyps. Individuals were excluded if they were: pregnant or breast feeding; taking oral steroids; taking more than 1500 micrograms of inhaled steroid per day; or if they had a severely deviated nasal septum or had undergone a surgical nasal polypectomy within the preceding six months.
Interventions
Participants were either given active treatment or placebo; 22 participants were prescribed the active drug and 24 the placebo. Treatment was in the form of either betamethasone sodium diphosphate nasal drops or passive placebo drops containing an identical vehicle, prescribed as two drops to be used twice a day for six weeks, in a head down and forwards position.
Outcomes measured
Polyp grading and subjective nasal symptom scores were assessed prior to the start of the trial and after six weeks.
Side‐effects were also reported (see Risk of bias in included studies).
Excluded studies
No other trials were identified.
Risk of bias in included studies
See risk of bias table (Characteristics of included studies)
Allocation
Generation of the allocation sequence
The Hadfield trial was described as being randomised using random number tables. The randomisation method was deemed adequate and so we judged this trial to have a low risk of bias from the generation of the randomisation sequence (Hadfield 2000).
Concealment of allocation
For the Hadfield study allocation was concealed by a pharmacist at the institution and was thus judged as adequate and thus having a low risk of bias (Hadfield 2000).
Blinding
Blinding was provided by the pharmacy producing nasal drops in the form of either betamethasone sodium diphosphate nasal drops or passive placebo contained in an identical vehicle. The participants and clinicians were completely blind to this process, therefore the risk of bias was judged to be low.
Incomplete outcome data
The percentage of participants enrolled that were not available for analysis was 52%. Of the 46 patients enrolled into the trial, 22 completed and the results were analysed on an available case basis. The trial authors reported no bias as they felt both groups were comparable due to similar drop‐out rates and pre‐treatment polyp sizes. They report no statistical difference between the two groups using the Mann‐Whitney Rank Sum test.
The trial authors contacted non‐completing participants by telephone or letter to ascertain the reasons for dropping out. Those participants who replied, stated their reasons for dropping out as difficulty in returning for the follow‐up or indeed not continuing treatment due to lack of symptoms at the commencement of the trial.
Given that 52% of participants did not complete the trial, the risk of bias was judged to be high.
Selective reporting
One of the primary outcomes of the study was to measure subjective nasal symptoms by the visual analogue staging system at commencement and at six weeks. The individual scores from the raw data are not included, although analysis of these showed there to be no statistical difference between the two groups. Absence of these individual results lead to a high risk of bias.
Other potential sources of bias
There were no other identified potential sources of bias.
Effects of interventions
Only one study has so far been identified which compares betamethasone drops against placebo (Hadfield 2000).
The results are presented by outcome in the order in which they appear in the aims of the review. Within each outcome, data are described as a narrative account and data have been entered into graphs for results of treatment on polyp size and side effects.
Primary outcome measures
1. Quality of life
This outcome was not reported in the included trial.
2. Nasal symptom score
The included trial pre‐stated an outcome measure of subjective nasal symptoms using a visual analogue score. The individual scores from the raw data were not included in the publication. The overall symptom scores for the combined cohort of participants receiving either betamethasone or placebo drops showed improvement, but there was no significant change for the betamethasone or placebo groups nor any change for individual symptoms.
Secondary outcome measures
1. Treatment burden
This outcome was not reported in the included trial.
2. Need for surgery
This outcome was not reported in the included trial.
3. Polyp size
Polyp size was assessed by endoscopic grading using the Mackay and Lund system (MacKay 1997). These were graded by the same observer at commencement of treatment and at six weeks for both sides of the nose. Change in polyp size was compared using the Wilcoxon signed rank test. Data for the left side of the nose and right side of the nose were presented and graded separately. There was a significant reduction in size of polyps for those on active treatment (right P = 0.016, left P = 0.004), whereas placebo had no effect (right P = 0.06, left P = 0.5) The results show that the RR for a reduction in size of polyp overall to be statistically significant in the treatment group as compared to the placebo group, RR 2.74 (95% CI 1.42 to 5.31) (Analysis 1.1).
1.1. Analysis.
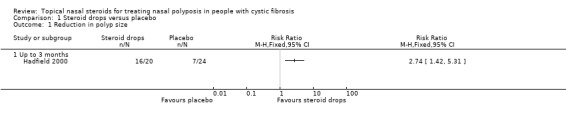
Comparison 1 Steroid drops versus placebo, Outcome 1 Reduction in polyp size.
4. Adverse events
a. Mild (not requiring significant treatment) e.g. limited epistaxis, discomfort
Three participants suffered mild side effects and all completed the trial. They were all within the active treatment group, and suffered with bleeding and discomfort. No breakdown of the side effects was provided. The results presented for mild adverse events were not statistically significant, RR 8.27 (95% CI 0.48 to 143.35) (Analysis 1.2).
1.2. Analysis.

Comparison 1 Steroid drops versus placebo, Outcome 2 Mild adverse events.
b. Moderate (requiring significant treatment or admission) e.g. epistaxis, adrenal suppression
This outcome was not reported in the included trial.
c. Severe (life threatening) e.g. epistaxis, adrenal failure
This outcome was not reported in the included trial.
5. Respiratory function
a. FEV1 % of predicted value
This outcome was not reported in the included trial.
b. FEV1/FVC ratio
This outcome was not reported in the included trial.
6. New CF pathogens recognised from culture of upper or lower airway secretions
This outcome was not reported in the included trial.
Discussion
The primary aim of this review was to evaluate the effectiveness of topical nasal steroids in individuals with CF and nasal polyposis.
We identified one trial with a total of 46 participants which satisfied our criteria for eligibility. Only 22 participants completed the trial and were included in analysis.
The included trial did not report on the review's first primary outcome (quality of life).The reported results did not show any measurable difference between the groups in nasal symptoms when examining either individual symptoms or overall symptom scores. The betamethasone nasal drops were effective, however, in reducing the size of the polyps in this group of patients, as measured by a clinician blinded to the intervention. The trial authors reported that there were only a few minor side effects and no moderate or major side effects from the drops.
The study design was adequately blinded and randomised with validated outcome measures examining symptomatic improvement, polyp size and adverse events secondary to the treatment.
The major concern with this trial is that over 50% of the initial cohort did not complete the study. Attempts to establish reason for losses to the trial were attempted but were poor. Reasons given ranged from lack of symptoms prior to entering the trial and distance to follow‐up. The small number of participants and significant drop out rate reduced the power of the calculations and validity of the results considerably.
Reporting of results was also selective leading to a high risk of bias with regards the subjective symptom scores. Raw data from the symptom scores (individual and combined) were not included. We are planning to contact the original investigators to seek missing data to set up a meta‐analysis.
Another perceived problem was the length of the trial, which was six weeks. It is useful to have examined the impact of topical steroids on short‐term symptoms and polyp size; however, studies of longer duration are required to more rigorously assess the relevance to patients.
Further work is required to assess the impact of topical steroids on the quality of life of patients and the longer term consequences of treating nasal polyposis.
Authors' conclusions
Implications for practice.
Nasal polyposis is a significant problem affecting many people with CF. As the outlook for this condition improves, this problem will continue to affect increasing numbers of adults with CF. The results of this review do not provide a clear evidence base to support the use of topical steroids, but do not provide a strong argument against their use, aside from a possible increase in the incidence of mild adverse events.
Implications for research.
This is a major clinical problem that adversely affects the quality of life of many people with CF. There is an urgent need for a large and well‐designed clinical trial examining medical therapies for CF nasal polyposis. Such a trial must be adequately powered to show a difference in a meaningful primary outcome that has direct impact on patients, such as quality of life. Other outcomes should use validated measures to assess symptoms and polyp size. Given the lack of evidence and uncertainty over the natural history, such a trial should be placebo‐controlled and over a duration that is relevant to patients (at least six months). A pragmatic multi‐centre design is desirable to achieve adequate recruitment and ensure sufficient trial completion and intention‐to‐treat analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 10 June 2015 | New search has been performed | A search of the Cystic Fibrosis & Genetic Disorders Group's Cystic Fibrosis Trials Register did not identify any references potentially eligible for inclusion in this review. The Plain Language Summary has been updated in line with new guidance from the Cochrane Cystic Fibrosis & Genetic Disorders Group. |
| 10 June 2015 | New citation required but conclusions have not changed | As no new information has been added to this review, our conclusions remain the same. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 5, 2011
| Date | Event | Description |
|---|---|---|
| 26 February 2013 | New citation required but conclusions have not changed | No new references were included in this review, therefore the conclusions of the review remain the same. |
| 26 February 2013 | New search has been performed | A search of the Cystic Fibrosis Trials Register did not identify any new references potentially eligible for inclusion in this review. |
Data and analyses
Comparison 1. Steroid drops versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in polyp size | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Up to 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mild adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Bleeding and discomfort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hadfield 2000.
| Methods | Randomised, double blind, placebo‐controlled. Parallel design. | |
| Participants | 46 adults (over 16 years old) with CF and nasal polyps, excluding those who were pregnant or breast feeding, taking oral steroids, taking more than 1500 micrograms of inhaled steroid per day, had a severely deviated nasal septum or had undergone a surgical nasal polypectomy within the preceding 6 months. | |
| Interventions | Nasal drops in the form of either betamethasone sodium diphosphate nasal drops or passive placebo drops containing an identical vehicle, prescribed as two drops to be used twice a day for 6 weeks, in a head down and forwards position. 22 participants received the active form of the drug with only 10 completing the course. 24 participants were prescribed the placebo and 11 of this group completed. |
|
| Outcomes | Primary outcome was polyp size. Secondary outcomes were subjective symptom scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using random number tables by the study centre. |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Nasal drops in the form of either betamethasone sodium diphosphate nasal drops or passive placebo drops containing an identical vehicle produced by the pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial was analysed on an available case basis. No patients were excluded. Dropouts were reported but not included in analysis. Only 22 participants completed the treatment of the 46 that commenced. This was rationalised by comparing the completing and non‐completing groups which were comparable for both treatment and placebo groups. Non‐completers were contacted by telephone or letter but reasoning for study dropout was notably weak or absent. |
| Selective reporting (reporting bias) | High risk | One of the secondary outcomes of subjective nasal symptoms by the visual analogue staging system is not fully reported which was pre‐specified measurements. Analysis is reported but none of the raw results are stated. |
| Other bias | Low risk | None identified. |
CF: cystic fibrosis
Contributions of authors
Beer H. Design of the review, coordinating the review, writing the protocol and review, taking principal responsibility for updating the review.
Southern K. Critically evaluating protocol and review, providing general advice on the review.
Swift A. Conception of the review, critically evaluating protocol and review, appraising quality of papers, data analysis and interpretation, providing general advice on conduct of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hadfield 2000 {published data only}
- Hadfield PJ, Rowe‐Jones JM, Mackay IS. A prospective treatment trial of nasal polyps in adults with cystic fibrosis. Rhinology 2000;38(2):63‐5. [PubMed] [Google Scholar]
Additional references
Bobadilla 2002
- Bobadilla JL, Macek M Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations‐‐correlation with incidence data and application to screening. Human mutation 2002;19(6):575‐606. [PUBMED: 12007216] [DOI] [PubMed] [Google Scholar]
British National Formulary 2009
- Joint Formulary Committee. British National Formulary. 8th Edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, September 2009. [Google Scholar]
Burgel 2004
- Burgel PR, Cardell LO, Ueki IF, Nadel JA. Intranasal steroids decrease eosinophils but not mucous expression in nasal polyps. European Respiratory Journal 2004;24(4):594‐600. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fokkens 2007a
- Fokkens W, Lund V, Mullol J. EPOS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology 2007;45(2):97‐101. [PubMed] [Google Scholar]
Fokkens 2007b
- Fokkens W, Lund V, Mullol J, European Position Paper on Rhinosinusitis and Nasal Polyps group. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology. Supplement 2007;20:1‐136. [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway S, Etherington C, Webb A. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946‐54. [DOI: 10.1136/thorax.55.11.946] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedman 1999
- Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population‐based study. International Journal of Epidemiology 1999;28(4):717‐22. [DOI] [PubMed] [Google Scholar]
Henriksson 2002
- Henriksson G, Westrin KM, Karpati F, Wikström AC, Stierna P, Hjelte L. Nasal polyps in cystic fibrosis: clinical endoscopic study with nasal lavage fluid analysis. Chest 2002;121(1):40‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hopkins 2009
- Hopkins C, Gillet S, Slack R, Lund V, Browne J. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clinical Otolaryngology 2009;34(5):447‐54(8). [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim SSY, Wong ECK, Kalish L, Craig JC. Topical steroids for nasal polyps. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006549] [DOI] [Google Scholar]
Lund 1998
- Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Archives of Otolaryngology ‐ Head and Neck Surgery 1998;124(5):513‐8. [DOI] [PubMed] [Google Scholar]
MacKay 1997
- Mackay IS, Lund VJ. Imaging and staging. In: Mygind N, Lildholdt T editor(s). In Nasal Polyposis: An Inflammatory Disease and Its Treatment. Copenhagen: Munksgaard, 1997:137‐44. [Google Scholar]
Patiar 2007
- Patiar S, Reece P. Oral steroids for nasal polyps. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005232.pub2] [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Robertson 2008
- Robertson JM, Friedman EM, Rubin BK. Nasal and sinus disease in cystic fibrosis. Paediatric Respiratory Review 2008;9(3):213‐9. [PUBMED: 18694713] [DOI] [PubMed] [Google Scholar]
Roby 2008
- Roby BB, McNamara J, Finkelstein M, Sidman J. Sinus surgery in cystic fibrosis patients: comparison of sinus and lower airway cultures. International Journal of Pediatric Otorhinolaryngology 2008;72(9):1365‐9. [DOI] [PubMed] [Google Scholar]
Southern 2007
- Southern KW. Cystic fibrosis and formes frustes of CFTR‐related disease. Respiration 2007;74(3):241‐51. [PUBMED: 17534127] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Beer 2011
- Beer H, Southern KW, Swift AC. Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008253.pub2] [DOI] [PubMed] [Google Scholar]


