Abstract
Actinobacteria are known for their metabolic potential of producing diverse secondary metabolites such as antibiotics. Actinobacteria also playimportant roles in biogeochemical cycling and how soils develop. However, little is known about the effect of the vegetation type on the actinobacterial community structures in soils from arid regions. For these reasons, we have analyzed the actinobacterial communities of five types of ecosystem (tree grove, shrub, meadow, desert, and farm) in the Heihe river basin. Using 16S rRNA high-throughput sequencing, we found 11 classes of Actinobacteria, with dominant classes of Actinobacteria (36.2%), Thermoleophilia (28.3%), Acidimicrobiia (19.4%). Five classes, 15 orders, 20 families and 36 genera were present in all samples. The dominant generalist genera were Gaiella, Solirubrobacter, Nocardioides, Mycobacterium, and Pseudonocardia. The actinobacterial community structures were significantly affected by the environment and vegetation type. The diversity of the actinobacterial community in the desert ecosystem was high, and this ecosystem harbored the highest proportion of unclassified sequences, representing rare Actinobacteria. Functional metagenomic prediction, using PICRUSt, indicated that Actinobacteria play an important role in nitrogen cycling in both desert and cultivated farm ecosystems.
Keywords: actinobacterial community, diversity, vegetation gradient, arid region, Heihe river
Introduction
The phylum Actinobacteria is a primary eubacterial phylogenetic clade containing diverse gram-positive bacteria (Sun et al., 2010) that belong to several classes such as the Acidimicrobiia, Actinobacteria, and Thermoleophila. Most Actinobacteria are aerobic saprophytes, and many can produce bioactive compounds (Eisenlord and Zak, 2010; Miao and Davies, 2010). Many of these secondary metabolites are used in medicine and agriculture, including antibacterial, antifungal and antitumor drugs (Hill et al., 2011). Unexplored and underexplored niche habitats are important resources of rare actinomycetes, and consequently potential new bioactive compounds. The distribution of different Actinobacteria is associated with environmental factors, such as soil type and pH (Hayakawa et al., 1988; Matsukawa et al., 2007; Hayakawa, 2008).
Actinobacteria are typically dominant soil microbes (Hill et al., 2011). They are important for the cycling of carbon, nitrogen, phosphorus, potassium, and several other elements in the soil (Goodfellow and Williams, 1983; Holmalahti et al., 1994; Hill et al., 2011). As saprophytes, they produce a range of extracellular hydrolytic enzymes which can degrade animal and plant polymers, including lignin, cellulose, chitin, and other organic compounds (Eisenlord and Zak, 2010). The actinomycete genus Frankia are root symbionts incorporated into nodules of a wide range of perennial woody dicotyledonous plants (Normand et al., 1996). However, in many cases the ecological roles of Actinobacteria in soils are poorly understood (Miao and Davies, 2010). A better understanding of the diversity and distribution of Actinobacteria can provide insight into microbial ecology and guide the discovery of novel bioactivities such as new antibiotics (Beattie et al., 2011; Hill et al., 2011).
The diversity of Actinobacteria has been investigated in several special or extreme environments such as marine sediments (Zhang et al., 2014; Duran et al., 2015), volcanic caves (Riquelme et al., 2015), sediments of cold springs (Yang et al., 2015), microbial mats of hot springs (Jiang et al., 2012), glacial forelands (Zhang et al., 2016b), lakes (Parveen et al., 2011), associations with organisms (Inthasotti and Pathom-aree, 2015), and deserts (Ding et al., 2013). In this study, we have investigated the Heihe River basin located in the arid northwest region of China. It offers both a special environment and an untapped resource of potential new actinobacterial diversity. Previous research into microbial diversity in the Heihe River has been limited to studies that associate watershed quality with bacterial community structures in the middle reach of the river basin (Borruso et al., 2015). In addition, Su et al. (2004) investigated bacterial diversity in the lower reach of the river using culture-dependent methods. Moreover, Tai et al. (2013) studied the diversity of nitrogen-fixing bacteria in the upper reaches of the Heihe River.
Habitat types can significantly affect soil bacterial diversity (Han et al., 2007; Xu et al., 2014). The Heihe River basin is an ideal place to research microbial diversity in relation to the environment. The vegetation types of the region vary from low to high altitudes (Wang et al., 2009; Tai et al., 2013). The abiotic (e.g., soil) and biotic (e.g., vegetation) varied with slope aspect (Qin et al., 2016). Soil organic carbon density ranged from 9.73 to 35.21 kg m–2 at 0–60 cm at the hill scale (Zhu et al., 2017). Measures of actinobacterial diversity in relation to vegetation can be expected to provide insights into the ecological role of the bacteria. Insight into soil ecology can be gained from understanding how microbial communities influence functional diversity in soil (Xu et al., 2014). However, studies into community composition based on16S rRNA gene sequences have generally neglected the functional and metabolic properties of a microbial community (Langille et al., 2013). The aims of this study were to: (1) investigate actinobacterial communities in soils of the Heihe River basin with samples taken from tree groves, shrubland, meadows, desert and farmland, (2) assess any relation of the vegetation gradient (tree groves, shrubland, meadow, desert) to the actinobacterial communities, and (3) assess to what extent metabolic pathways differ among the Actinobacteria found in the soils of these different habitats.
Materials and Methods
Site Description and Soil Sampling
The Heihe River basin in northwestern China represents the second-largest inland river basin in this arid region (37°41′-42°42′N and 96°42′-102°00′E) (Wang et al., 2009). The Heihe River basin extends from its upper reaches located on the northern slopes of the Qilian Mountains (Li et al., 2001). Precipitation mainly takes place in the summer months and decreases from east to west while increasing from approximately 200 mm at low altitudes, to 600 mm at high altitudes. The types of environments found consist of the following zones: desert steppe, arid shrubbery grassland, forest grassland, sub-alpine shrubbery meadow, alpine cold-and-desert meadow, and alpine perma-frost-snow-ice from low to high altitudes, respectively (Wang et al., 2009; Tai et al., 2013). The lower reaches of the Heihe River basin are located on the northern Alxa Highland. It is one of the most arid regions in China. The precipitation is concentrated during the summer. Annual mean precipitation is 42 mm, with an annual mean evaporation of 3755 mm (Li et al., 2001; Yin et al., 2012).
Soil samples were collected from five types of natural soil environments tree groves, shrubland, meadow, desert, and farmland throughout July 27th to Aug 14th, 2012 (Table 1 and Supplementary Figure S1). For tree grove, shrub and meadow ecosystems, four different representative plant species typical to each habitat were chosen for soil sampling. In the desert, we chose soils representing four different degrees of desertification. For farmland, soil samples treated with four different fertilizers were chosen. More detailed information about sample sites is provided in Table 1. The desert soil samples were obtained exclusively from the lower reaches of the Heihe River basin. In each sample site, we collected three independent replicate samples and each replicate was pooled from five sample soils (four corner and one centre sample) that were 5 cm deep within an area of 2 m2. For each sample, approximately 10 g of soil was obtained with a trowel and sieved to remove non-particulate content. Samples from shrub and tree groves were obtained from soil adjacent to the relevant non-mixed plant species. The samples were kept below 4°C during transport to the laboratory and were then stored at −20°C until DNA extraction.
TABLE 1.
Overview of the soil sample locations.
| Biotopes | Vegetation/ | Abbr. | Latitude | Longitude | Elevation |
| Soil Type | |||||
| Tree grove soil | Sabina przewalskii | T1 | 38.0706 | 100.2402 | 3479 |
| Picea crassifolia | T2 | 38.1271 | 100.1485 | 3094 | |
| Salix babylonica | T3 | 38.1742 | 100.2164 | 2820 | |
| Populus tremula | T4 | 38.1736 | 100.2138 | 2814 | |
| Shrubland soil | Caragana jubata | S1 | 38.0300 | 100.2333 | 3894 |
| Potentilla fruticosa | S2 | 38.0652 | 100.2146 | 3624 | |
| Hippophae rhamnoides | S3 | 38.0642 | 100.2656 | 3250 | |
| Salix wilhelmsiana | S4 | 38.0681 | 100.2964 | 3075 | |
| Meadowland soil | Carex tristachya | M1 | 38.1216 | 100.1914 | 3128 |
| Stipa capillata | M2 | 38.2508 | 100.2181 | 2911 | |
| Kobresia pygmaea | M3 | 38.1769 | 100.2150 | 2905 | |
| Achnatherum splendens | M4 | 38.2593 | 100.1809 | 2631 | |
| Desert soil | Mild desertification | D1 | 42.0203 | 101.2385 | 922 |
| Moderate desertification | D2 | 42.0179 | 101.2587 | 915 | |
| Severe desertification | D3 | 42.0211 | 101.2618 | 933 | |
| Extreme desertification | D4 | 42.0328 | 101.2952 | 916 | |
| Farmland soil | M fertilizer | F1 | 38.3518 | 100.1319 | 1380 |
| N/P/M fertilizer | F2 | 38.3532 | 100.1344 | 1380 | |
| N/P/K fertilizer | F3 | 38.3515 | 100.1323 | 1380 | |
| N/P/K/M fertilizer | F4 | 38.3529 | 100.1338 | 1380 |
DNA Extraction and Sequencing
DNA was isolated from representative 0.5 g samples of the triplicate mixed soils or from 2 g samples of desert soil, using a PowerSoil DNA Isolation Kit (MoBio Mo Bio Laboratories, Inc., Carlsbad, CA). We amplified the V4 region of the bacterial 16S rRNA genes with the bacterial universal primers 515F and 806R. The PCR amplification conditions were set exactly as described previously (Zhang et al., 2016a). The amplicons were sequenced with an Illumina platform.
Sequence Analyses
Raw sequence reads were sorted with barcodes and quality trimmed using QIIME 1.91 (Caporaso et al., 2010) in Bio-Linux 8.0.5 (Field et al., 2006). The following parameters were used: minimum 200 bp in length, with perfect matches to the sequence tag (barcode) and the 16S rRNA gene primer, and exclusion of sequences with undetermined bases, including chimeric sequences. Remaining sequences were clustered into operational taxonomic units (OTUs) with a minimum of 97% identity, using usearch v11.0 (Edgar, 2010) on QIIME followed by taxonomically assigning each sequence with RDP (Wang et al., 2007) according to the best matches in the SILVA ribosomal database (version 132) (Quast et al., 2013; Yilmaz et al., 2014). Sequences belonging to the phylum Actinobacteria were identified with QIIME from each library (filter_taxa_from_otu_table.py, –positive_tax p__Actinobacteria). Specialists were defined as either families or genera that were present in a maximum of two out of 20 samples, whereas generalists were defined as families of genera present in more than 18 samples. Occupancy indicates from how many of the sites a family or genus was recovered. Sequences obtained from this research were deposited at DDBJ/EMBL/GenBank with accession number KCVG00000000.
Metagenome Analyses
We used PICRUSt to predict actinobacterial community functional content based on 16S rRNA sequences (Langille et al., 2013). To identify operational taxonomic units (OTU) within each ecosystem, the 16S rRNA sequences were compared with sequences in the Greengenes ribosomal database (version 13-5). A minimum of 97% identity was used to classify each OTU using pick_closed_reference_otus.py in QIIME (DeSantis et al., 2006). The data for each ecosystem was normalized in terms of the numbers of sequences representing each OTU, using normalize_by_copy_number.py in PICRUSt. Subsequently, the script predict_metagenomes.py in PICRUSt was used to predict metagenome function for a given OTU, with reference to the Kyoto Encyclopedia of Genes and Genomes database (Kanehisa and Goto, 2000). A full description of the methods is detailed in Cleary et al. (2015). The contributions of genes and selected orders for each sample were analyzed with the metagenome contributions.py script in PICRUSt.
Statistical Analyses
The alpha diversity of samples was calculated with alpha_diversity.py in QIIME with the lowest number of sequences of all samples. The dissimilarity of the actinobacterial communities and metagenome gene functional content were determined using beta_diversity.py in QIIME following the Bray-Curtis method (beta_diversity.py -i otu_table.biom -o otu_beta_diversity -m bray_curtis). Non-metric multidimensional scaling (NMDS), based on the Bray-Curtis distance, was used to visualize the composition of actinobacterial communities using the vegan package in R3.1.3 (R Core Team, 2012). We used an ANOSIM test to assess whether a significant variation of the environment was related to the actinobacterial composition or environment, to predict the metagenome function using script compare_categories.py in QIIME. The number of permutations was set at 999; all other arguments used the default values set. One-way PERMANOVA tests were used to test significant variation of actinobacterial community composition for each pair of environments with script compare_categories.py in QIIME. The number of permutations was set at 999. An assessment of the high-dimensional biomarker discovery and an explanation of the actinobacterial communities and metagenome gene functional content were performed using the LEfSe analysis (Segata et al., 2011). We used network analyses to explore co-occurrence patterns of actinobacterial communities for each environment. We first calculated the Pearson correlation coefficient of actinobacterial communities at the taxonomic level from species to class. Gephi software (version 0.9.2) was then used to visualize strongly correlated data with a p value < 0.01 and r > 0.6 or r < −0.6. The network layout algorithm used was the Fruchterman-Reingold Algorithm.
Results
Community Composition of Actinobacteria in Soils of the Heihe River Basin
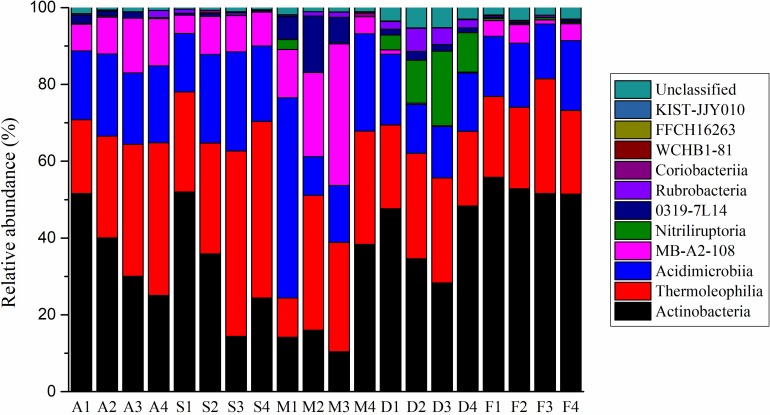
A total of 110,773 (average 5 539 sample–1) actinobacterial sequences from the 20 soil sites were obtained from a total of 362 554 sequences. When grouped at the level of 97% similarity, 17,714 actinobacterial OTUs were formed. 12,010 OTUs contained only one sequence. The most abundant 20 OTUs were represented by 22,440 sequences or 20.3% of the total. The top 100 and 1,000 OTUs were 32.2% and 59.1%, respectively, of the entire data set. Ninety-seven percent of the actinobacterial sequences could be grouped into 11 classes (Figure 1 and Supplementary Figure S2). The mean relative abundances for each class were Actinobacteria (36.1%), Thermoleophilia (28.1%), Acidimicrobiia (19.4%), MB-A2-108 (8.4%), Nitriliruptoria (2.5%), 0319-7L14 (2.1%), Rubrobacteria (1.2%), Coriobacteriia (0.14%), WCHB1-81 (0.02%), FFCH16263 (0.006%) and KIST-JJY010 (0.005%). Only one class on the SILVA database, RBG-16-55-12, was not represented. At the lower level of classification, there were 33 orders, 83 families, 214 genera, and 247 species found in the Heihe river basin. Streptomyces genus sequences accounted for less than 1% of the total.
FIGURE 1.
The actinobacterial community composition at the class level for the 20 sample sites.
Actinobacterial Communities of Habitat Generalists and Specialists
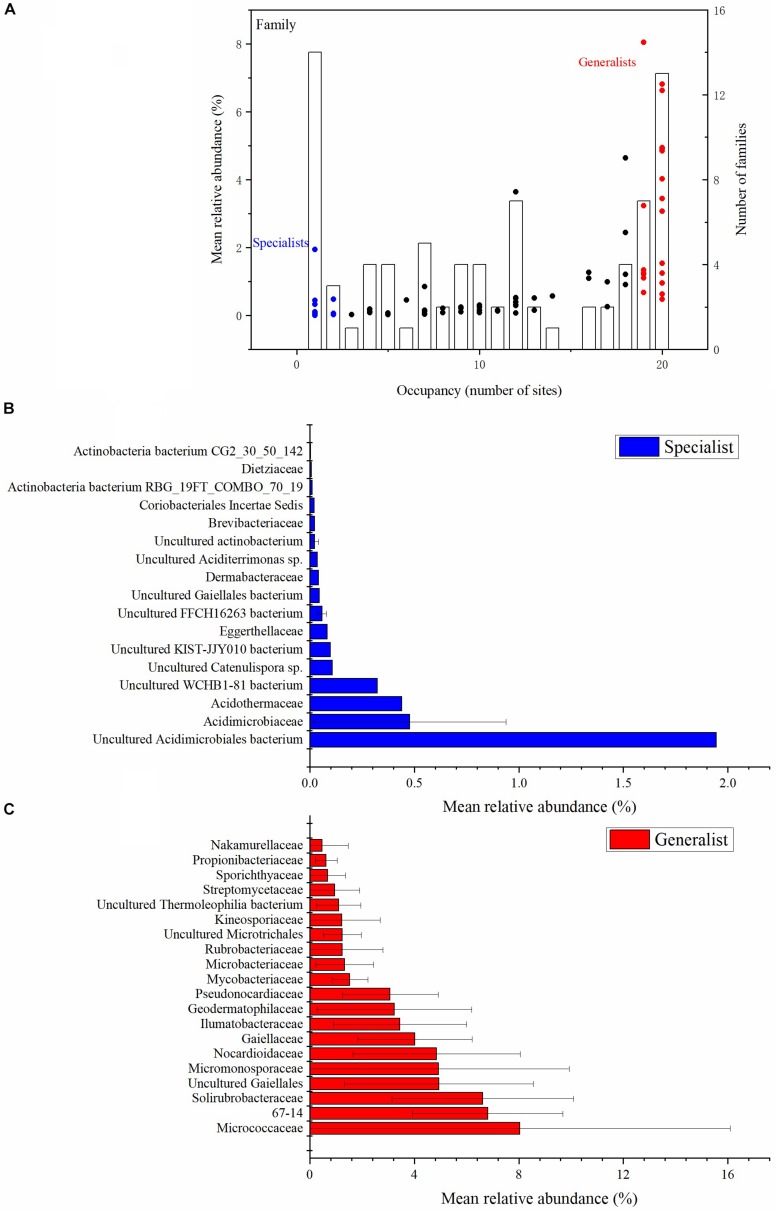
Each of the actinobacterial taxonomic units were assigned with the relative abundance vs. occupancy plot to split the set of taxa into two general categories: soil generalists and specialists (Figure 2 and Supplementary Figure S3). There were five class, 15 order, 20 family and 36 genus habitat generalists, and three class, seven order, 17 family and 69 genus habitat specialists. At the class taxonomic level, Actinobacteria, Thermoleophilia, Acidimicrobiia, MB-A2-108, and Rubrobacteria were the generalists, whereas WCHB1-81, FFCH16263, and KIST-JJY010 were the specialists. At the family taxonomic level, Micrococcaceae, 67-14, and Solirubrobacteraceae were the main generalists, whereas uncultured Acidimicrobiales bacteria, Acidimicrobiaceae, and Acidothermaceae were the main specialists (Figure 2). At the genus taxonomic level, Gaiella, Solirubrobacter, Nocardioides, and Blastococcus were the main generalists, with uncultured Acidimicrobiales bacterium, Collinsella, and uncultured prokaryote Gaiellales being the main specialists (Supplementary Figure S3). The number of generalist genera was much higher than non-generalist genera (Supplementary Figure S3). More than half of the specialist genera belonged to the Actinobacteria class.
FIGURE 2.
Abundance (y axis) and occupancy (x axis) plot for the actinobacterial communities at the family taxonomic level (A). Occupancy indicates the number of sites that a family recovered. The mean relative abundance of families is indicated by dots (left-hand y axis). The specialist families are shown with blue dots. The generalist families are shown with red dots. The number of families at each occupancy is indicated by the bars (right-hand y axis) (A). The mean relative abundance of the top 17 habitat specialist families (B) and top 20 habitat generalist families (C) are plotted with histograms (Unclassified sequences are not included). All the relative abundances in the figures are the proportion of the Actinobacteria phylum.
Dissimilarity of Actinobacterial Communities Between Different Vegetation Ecosystems
In accordance with the taxonomic information from the SILVA database (Quast et al., 2013), the sequences could be grouped into unclassified, uncultured, and accurate classification. With a decrease of classification level, the ratio of unclassified and uncultured sequences increased (Supplementary Figure S2). The ratio of uncultured and accurate classified sequences from meadowlands were the highest of the five types of ecosystems. More differences appeared among unclassified, uncultured, and accurate classified sequences with a decrease in classification level across the five ecosystems.
We used Chao1 and the ACE index to estimate the richness of actinobacterial communities, and the Shannon-Wiener and Simpson index to estimate the diversity of actinobacterial communities in the five different ecosystems (Figure 3 and Supplementary Figure S4). The results indicated that both richness and diversity of the actinobacterial communities followed the same trend. The highest was for the farmland ecosystem, followed by desert, tree grove, shrub, and meadow ecosystems. The diversity of samples belonging to the meadow ecosystem exhibited a greater variation range than for the other ecosystems, likely reflecting an association between community structure and plant species, as each of the four samples were obtained from soils adjacent to different plant species. The diversity in samples obtained from farmland and tree grove ecosystems was narrower than for the other ecosystems.
FIGURE 3.
Boxplots showing Shannon-Wiener index (A) and Chao1 index (B) of actinobacterial communities in five biome types.
Dissimilarities in the actinobacterial communities from the 20 sites were identified with a NMDS biplot (Figure 4). All sites, except M4, were aggregated with their environment. Notably, the composition of the actinobacterial communities was significantly affected by the environment (ANOSIM test, R = 0.6804, p = 0.001). The effect of five kinds of ecosystems on the actinobacterial communities was different (Supplementary Table S1). The four samples within the farmland environment shared the greatest similarity with a value of 0.82 ± 0.03. In contrast, the four samples from the meadowland had the greatest dissimilarity, with a value of 0.45 ± 0.18. Comparing the similarity of actinobacterial communities between each pair of environments, indicated that shrubland and tree grove ecosystems were the most similar environments (0.67 ± 0.09), whereas shrubland compared with either farmland or desert ecosystems exhibited the largest dissimilarity (0.36 ± 0.03, 0.36 ± 0.05). In addition, we used one-way PERMANOVA tests (Bray-Curtis distance and Euclidean distance) to analyze differences in actinobacterial communities for each environment pair, indicating that tree grove and shrubland communities were not significantly different. Pairwise comparisons of tree grove and meadowland communities, and shrubland and meadowland communities indicated marginal significant differences (Supplementary Tables S2, S3).
FIGURE 4.
Non-metric multidimensional scaling (NMDS) biplot of a Bray-Curtis dissimilarity matrix that shows the actinobacterial community structure (A) and the functional metagenomic analysis (B) of the 20 sample sites.
At the taxonomic class level, the Actinobacteria class were dominant in tree grove, desert and farm environments; Thermoleophilia was the largest class in shrub and meadow environments (Figure 1). At the level of family, there was more dissimilarity between the ecosystems (Supplementary Figure S5). Using LefSe analysis, we obtained actinobacterial biomarkers from class to species for each environment (Supplementary Figure S5). There were 29 biomarkers identified in tree grove samples, 22 in shrubland samples, two in meadowland samples, 45 in desert samples and 41 in farmland samples.
Dissimilarity of Potential Function of Actinobacterial Communities
We categorized the KEGG results from metagenome analysis into hierarchies of functional pathways. Although there were different actinobacterial communities in the different habitats, the functional predicted pathways in the Actinobacteria from these different habitats were similar. The top five common metabolic predicted pathways were purine metabolism, butanoate metabolism, valine, leucine, and isoleucine degradation, propanoate metabolism and fatty acid metabolism. However, the top two most abundant specific functions were the Transporters and ABC transporters. They belong to the category Environmental Information Processing and took up to about 11% of all counts of the gene.
The dissimilarities in the potential functions (KO composition) of the actinobacterial communities among the 20 sites were identified with a NMDS biplot (Figure 4). Just as for the dissimilarity of actinobacterial communities, all sites, except M4, were aggregated with their environment. The metabolic potential function of the actinobacterial communities was significantly affected by the environment (Anosim test, R = 0.7317, p = 0.001). The analysis underlined that the different actinobacterial communities have different metabolic functions related to the ecosystem that they occupy.
In addition, LefSe analysis also indicated significant differences in potential metabolic functions in each environment (Supplementary Figure S6). This analysis identified 20 biomarkers for shrubland, 15 in meadowland, nine in farmland, 16 in desert and three in tree-grove soil.
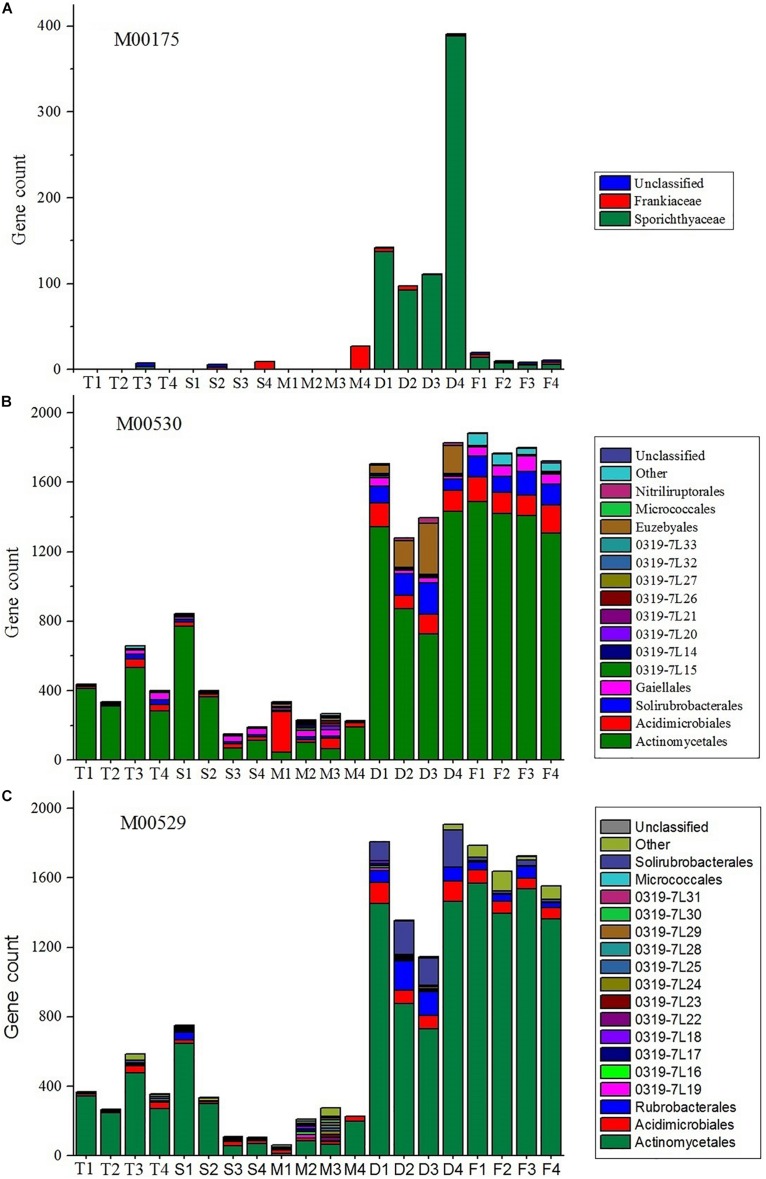
It is evident that nitrogen metabolism is an important feature of the actinobacterial communities, especially in farmland and non-cultivated desert ecosystems (Figure 5). Sporichthyaceae, abundant in the desert environment, were the main source of nitrogen fixation pathways (module M00175). The Frankiaceae, present in S4 and M4 samples, can also fix nitrogen. The dissimilatory nitrate reduction and denitrification pathways (modules M00530 and M00529, respectively), were also abundant in desert and farm ecosystems.
FIGURE 5.
Stacked bar plots showing the estimated gene count contributions (y-axis) of the orders (≥2%) for M00175 (A, nitrogen fixation), M00530 (B, dissimilatory nitrate reduction), and M00529 (C, denitrification).
Discussion
Ecosystems That Vary in Vegetation Significantly Affect Actinobacterial Community Composition
Our analyses indicate that soil vegetation systems significantly influence the composition of actinobacterial communities in the Heihe river basin. The type of vegetation ecosystem plays a more important role in actinobacterial communities than the component plant species. This phenomenon is consistent with previous observations for how bacterial communities are affected by the type of ecosystem (Fierer et al., 2012b; Zhang et al., 2013; Xu et al., 2014). However, the influence of each of the different vegetation ecosystems on soil actinobacterial communities was different. For example, in this study, meadowland soil was the least rich and least diverse ecosystem (Figure 3) but contained non-clustering community structures (Figure 4), likely reflecting that actinobacterial communities vary according to the vegetation type. These results differ to the bacterial community analysis of Xu et al. (2014). They found that sample sites belonging to grasslands were intensively clustered together based on the bacterial community Bray-Curtis similarity scores. This suggests differences for how actinobacterial and overall bacteria communities can be influenced in the same kind of environment.
A previous analysis has indicated that the soil bacterial community structure is dependent on the specific plant species growing in the sample sites (Rich et al., 2003; Chu et al., 2011; Zhang et al., 2013; Xu et al., 2014). Our analyses of the Heihe river basin soils indicate that the actinobacterial communities in tree grove and shrubland soils are closely related, while they are distinct for three meadowland soil samples (Figure 4A). The exception was for site M4 that clustered with tree grove and shrubland soils. The soil from this site is associated with the plant Achnatherum splendens that may provide a soil micro-environment similar to the tree grove and shrubland soils. A functional metagenomic analysis also indicated a relation between the community structure of this site and that of tree grove and shrubland soils (Figure 4B). In these ecosystems, amino acid and carbohydrate metabolism pathways were better represented than in other sites. Different biomes typically harbor distinct plant species (Fierer et al., 2012b). Due to the diverse types of litter and rooting systems, the soil organic materials associated with different vegetation can vary (Chan et al., 2008; Zeng et al., 2016). This can consequently influence the soil microbial community structure.
The Desert Ecosystem of the Heihe River Basin Is a Rich Resource for Actinobacteria
The desert ecosystem of the Heihe river basin is characterized as an abundant resource for Actinobacteria. It has a high alpha diversity index for uncultivated land (Shannon, Chao1, ACE) (Figure 3 and Supplementary Figure S4), has the lowest relative abundance of accurate classification sequences (Supplementary Figure S2), has the largest number of biomarker species and the lowest modularity index. The actinobacterial community in this desert ecosystem was different to those characterized in other desert ecosystems. Whereas the genera Micromonospora, Streptosporangium and Cellulomonas were found in desert soils from the northeast Qinghai-Tibet Plateau (Ding et al., 2013), they were not detected in the Heihe desert ecosystem. The genera Microbispora and Microtetraspora were found in soils from the Mojave Desert, California (Takahashi et al., 1996), but not in the desert soil of this study. In addition, the genus Lechevalieria was identified in soils from the Atacama Desert, Chile (Okoro et al., 2009), but was absent in this study. Geodermatophilaceae was the dominant group in both the Badain Jaran (27.4%) and Tengger (18.2%) deserts, but only 6.8% in this study (Sun et al., 2018). This suggests that the Actinobacteria communities in the Heihe desert are different from other deserts. Indeed, a variety of rare actinomycetes have been isolated from desert soils in the past few years (Tiwari and Gupta, 2013). More than two thirds of the rare actinomycete genera identified between 2006 and 2018, that are known to produce new natural products (Zhang et al., 2019), were present in the Heihe river basin desert soil. In contrast, the relative abundance of species belonging to the genus Streptomyces was no more than 1%.
Different Actinobacterial Communities Inhabit Copiotrophic and Oligotrophic Ecosystems
The distribution of Actinobacteria has been associated with different environmental factors such as soil type and pH (Hayakawa et al., 1988; Matsukawa et al., 2007; Hayakawa, 2008). In many studies the abundance of actinobacterial communities is positively correlated with soil carbon and nitrogen content and are consequently considered copiotrophic bacteria. For example, the relative abundance of many dominant Actinobacteria was observed to increase with N gradients in agricultural fields (Fierer et al., 2012a). In addition, the abundance of Actinobacteria was positively correlated with soil carbon and nitrogen in tropical rain forest soils (Nemergut et al., 2010). Moreover, in nine different ecosystems examined in North America, nitrogen addition consistently led to an increase in soil Actinobacteria (Ramirez et al., 2012). Eilers et al. (2010) found that low molecular weight carbon compounds had significant effects on relative increases in Actinobacteria. However, we found that the relative abundance of Actinobacteria was higher in the oligotrophic environments (desert soil) and its community structure was significantly different compared to copiotrophic environments. For example, the class of Rubrobacteria and Nitriliruptoria were enriched in the desert environment. This suggests that some members of the phylum Actinobacteria may be able to live in an oligotrophic environment. Arocha-Garza et al. (2017) isolated 350 different strains from an oligotrophic desert oasis. The Actinobacteria can produce a variety of extracellular hydrolytic enzymes to degrade animal and plant residues, litter and other organic compounds in soil, enabling them to thrive in oligotrophic environments (Eisenlord and Zak, 2010). Shange et al. (2012) found that the orders of Actinomycetales and Solirubrobacterales showed their highest abundance in the lowest soil organic carbon values in a diverse agroecosystem. The genus Saccharomonospora was identified as the most abundant in relatively humus-poor alkaline soils (Hayakawa, 2008).
Actinobacterial Communities May Play an Important Role in the Nitrogen Cycle in the Oligotrophic Desert Ecosystem
It is believed that actinobacterial communities play an important role in the ecosystems they colonize (Goodfellow and Williams, 1983; Holmalahti et al., 1994; Rich et al., 2003; Hill et al., 2011), although a functional description of their contribution is often lacking. A bioinformatic metagenome analysis provided us with insight into the potential function of Actinobacteria in the Heihe river basin ecosystems, suggesting an important role in nitrogen cycling in the oligotrophic desert ecosystem. In this environment, Sporichthyaceae and Frankiaceae would appear to be important for nitrogen fixation and may consequently enable other organisms to become established. In addition, members of the actinomycetales contribute significantly to nitrogen cycling in this environment. Previously, Navarrete et al. (2015) identified a close relationship between actinobacterial communities and nitrogen metabolism in copiotrophic Amazon forest soils. Our analysis indicates a potential role in nitrogen cycling for Actinobacteria as well as in the fertilized copiotrophic farmland ecosystem.
Conclusion
This detailed description of actinobacterial communities in five different ecosystems of the Heihe river basin indicates that community structures vary according to the dominant vegetation and trophic status of the soils. This provides the data point to a surprisingly diverse community structure in oligotrophic desert soil, where actinobacteria could play an important role in nitrogen fixation. This habitat is also remarkable for its abundance of rare actinobacteria that are likely sources of new bioactive compounds.
Data Availability Statement
The datasets generated for this study can be found in NCBI, KCVG00000000.
Author Contributions
BZ, TC, and GL designed the study. XW, XT, and MW performed the field observation and sampling activities. BZ, LS, and XC analyzed the Illumina sequencing data. BZ, GZ, and WZ performed the statistical analysis. BZ, TC, GL, and PD interpreted the results and wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was funded by the National Natural Science Foundation of China (Nos. 41801045 and 31570498), the Bureau of International Cooperation, Chinese Academy of Sciences (131B62KYSB20160014), the CAS “Light of West China” Program and the State Key Laboratory of Cryospheric Science, and the Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences (SKLCS-OP-2018-10).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02209/full#supplementary-material
References
- Arocha-Garza H. E., Castillo R. C., Eguiarte L. E., Souza V., Torre-Zavala S. D. (2017). High diversity and suggested endemicity of culturable Actinobacteria in an extremely oligotrophic desert oasis. PeerJ 5:e3247. 10.7717/peerj.3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie A. J., Hay M., Magnusson B., de Nys R., Smeathers J., Vincent J. F. (2011). Ecology and bioprospecting. Austral Ecol. 36 341–356. 10.1111/j.1442-9993.2010.02170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruso L., Zerbe S., Brusetti L. (2015). Bacterial community structures as a diagnostic tool for watershed quality assessment. Res. Microbiol. 166 38–44. 10.1016/j.resmic.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O. C., Casper P., Sha L. Q., Feng Z. L., Fu Y., Yang X., et al. (2008). Vegetation cover of forest, shrub and pasture strongly influences soil bacterial community structure as revealed by 16S rRNA gene T-RFLP analysis. FEMS Microbiol. Ecol. 64 449–458. 10.1111/j.1574-6941.2008.00488.x [DOI] [PubMed] [Google Scholar]
- Chu H. Y., Neufeld J. D., Walker V. K., Grogan P. (2011). The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low arctic tundra landscape. Soil Sci. Soc. Am. J. 75 1756–1765. 10.2136/sssaj2011.0057 [DOI] [Google Scholar]
- Cleary D. F. R., de Voogd N. J., Polonia A. R. M., Freitas R., Gomes N. C. M. (2015). Composition and predictive functional analysis of bacterial communities in seawater, sediment and sponges in the spermonde archipelago, indonesia. Microb. Ecol. 70 889–903. 10.1007/s00248-015-0632-5 [DOI] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Chen G. C., Wang B. C., Wang Q. L., Liu D. M., Peng M., et al. (2013). Culturable actinomycetes from desert ecosystem in northeast of qinghai-tibet plateau. Ann. Microbiol. 63 259–266. 10.1007/s13213-012-0469-9 [DOI] [Google Scholar]
- Duran R., Bielen A., Paradzik T., Gassie C., Pustijanac E., Cagnon C., et al. (2015). Exploring Actinobacteria assemblages in coastal marine sediments under contrasted human influences in the west istria sea, Croatia. Environ. Sci. Pollut. Res. 22 15215–15229. 10.1007/s11356-015-4240-1 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Eilers K. G., Lauber C. L., Knight R., Fierer N. (2010). Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 42 896–903. 10.1016/j.soilbio.2010.02.003 [DOI] [Google Scholar]
- Eisenlord S. D., Zak D. R. (2010). Simulated atmospheric nitrogen deposition alters actinobacterial community composition in forest soils. Soil Sci. Soc. Am. J. 74 1157–1166. 10.2136/sssaj2009.0240 [DOI] [Google Scholar]
- Field D., Tiwari B., Booth T., Houten S., Swan D., Bertrand N., et al. (2006). Open software for biologists: from famine to feast. Nat. Biotechnol. 24 801–803. 10.1038/nbt0706-801 [DOI] [PubMed] [Google Scholar]
- Fierer N., Lauber C. L., Ramirez K. S., Zaneveld J., Bradford M. A., Knight R. (2012a). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. Isme J. 6 1007–1017. 10.1038/ismej.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N., Leff J. W., Adams B. J., Nielsen U. N., Bates S. T., Lauber C. L., et al. (2012b). Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. U.S.A. 109 21390–21395. 10.1073/pnas.1215210110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow M., Williams S. T. (1983). Ecology of actinomycetes. Annu. Rev. Microbiol. 37 189–216. 10.1146/annurev.mi.37.100183.001201 [DOI] [PubMed] [Google Scholar]
- Han X. M., Wang R. Q., Liu R., Wang M. C., Zhou J., Guo W. H. (2007). Effects of vegetation type on soil microbial community structure and catabolic diversity assessed by polyphasic methods in North China. J. Environ. Sci. China 19 1228–1234. 10.1016/S1001-0742(07)60200-9 [DOI] [PubMed] [Google Scholar]
- Hayakawa M. (2008). Studies on the isolation and distribution of rare actinomycetes in soil. Actinomycetologica 22 12–19. 10.3209/saj.SAJ220103 [DOI] [Google Scholar]
- Hayakawa M., Ishizawa K., Nonomura H. (1988). Distribution of rare actinomycetes in Japanese soils. J. Fermentation Technol. 66 367–373. 10.1016/0385-6380(88)90001-5 [DOI] [Google Scholar]
- Hill P., Krištùfek V., Dijkhuizen L., Boddy C., Kroetsch D., van Elsas J. D. (2011). Land use intensity controls actinobacterial community structure. Microb. Ecol. 61 286–302. 10.1007/s00248-010-9752-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmalahti J., Wright A., Raatikainen O. (1994). Variations in the spectra of biological activities of actinomycetes isolated from different soils. Lett. Appl. Microbiol. 18 144–146. 10.1111/j.1472-765X.1994.tb00829.x [DOI] [Google Scholar]
- Inthasotti T., Pathom-aree W. (2015). Diversity of actinobacteria associated with nostoc commune vaucher ex Bornet & Flahault macrocolonies. Ann. Microbiol. 65 2229–2240. 10.1007/s13213-015-1063-8 [DOI] [Google Scholar]
- Jiang H., Dong C. Z., Huang Q., Wang G., Fang B., Zhang C., et al. (2012). Actinobacterial diversity in microbial mats of five hot springs in central and central-eastern tibet, China. Geomicrobiol. J. 29 520–527. 10.1080/01490451.2011.590872 [DOI] [Google Scholar]
- Kanehisa M., Goto S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 27–30. 10.1093/Nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille M. G. I., Zaneveld J., Caporaso J. G., McDonald D., Knights D., Reyes J. A., et al. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31 814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lu L., Cheng G. D., Xiao H. L. (2001). Quantifying landscape structure of the heihe river basin, north-west China using FRAGSTATS. J. Arid Environ. 48 521–535. 10.1006/jare.2000.0715 [DOI] [Google Scholar]
- Matsukawa E., Nakagawa Y., Iimura Y., Hayakawa M. (2007). Stimulatory effect of indole-3-acetic acid on aerial mycelium formation and antibiotic production in Streptomyces spp. Actinomycetologica 21 32–39. 10.3209/saj.SAJ210105 [DOI] [Google Scholar]
- Miao V., Davies J. (2010). Actinobacteria: the good, the bad, and the ugly. Antonie Van Leeuwenhoek 98 143–150. 10.1007/s10482-010-9440-6 [DOI] [PubMed] [Google Scholar]
- Navarrete A. A., Tsai S. M., Mendes L. W., Faust K., de Hollander M., Cassman N. A., et al. (2015). Soil microbiome responses to the short-term effects of Amazonian deforestation. Mol. Ecol. 24 2433–2448. 10.1111/mec.13172 [DOI] [PubMed] [Google Scholar]
- Nemergut D. R., Cleveland C. C., Wieder W. R., Washenberger C. L., Townsend A. R. (2010). Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol. Biochem. 42 2153–2160. 10.1016/j.soilbio.2010.08.011 [DOI] [Google Scholar]
- Normand P., Orso S., Cournoyer B., Jeannin P., Chapelon C., Dawson J., et al. (1996). Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int. J. Syst. Bacteriol. 46 1–9. 10.1099/00207713-46-1-1 [DOI] [PubMed] [Google Scholar]
- Okoro C. K., Brown R., Jones A. L., Andrews B. A., Asenjo J. A., Goodfellow M., et al. (2009). Diversity of culturable actinomycetes in hyper-arid soils of the atacama desert, Chile. Antonie Van Leeuwenhoek 95 121–133. 10.1007/s10482-008-9295-2 [DOI] [PubMed] [Google Scholar]
- Parveen B., Reveilliez J. P., Mary I., Ravet V., Bronner G., Mangot J. F., et al. (2011). Diversity and dynamics of free-living and particle-associated betaproteobacteria and actinobacteria in relation to phytoplankton and zooplankton communities. FEMS Microbiol. Ecol. 77 461–476. 10.1111/j.1574-6941.2011.01130.x [DOI] [PubMed] [Google Scholar]
- Qin Y. Y., Feng Q., Holden N. M., Cao J. J. (2016). Variation in soil organic carbon by slope aspect in the middle of the qilian mountains in the upper heihe river basin, China. CATENA 147 308–314. 10.1016/j.catena.2016.07.025 [DOI] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K. S., Craine J. M., Fierer N. (2012). Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 18 1918–1927. 10.1111/j.1365-2486.2012.02639.x [DOI] [Google Scholar]
- R Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available at: https://www.r-project.org/ [Google Scholar]
- Rich J. J., Heichen R. S., Bottomley P. J., Cromack K., Myrold D. D. (2003). Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 69 5974–5982. 10.1128/Aem.69.10.5974-5982.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme C., Hathaway J. J. M., Dapkevicius M. D. N. E., Miller A. Z., Kooser A., Northup D. E., et al. (2015). Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 6:1342. 10.3389/fmicb.2015.01342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. 10.1186/Gb-2011-12-6-R60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shange R. S., Ankumah R. O., Ibekwe A. M., Zabawa R., Dowd S. E. (2012). Distinct soil bacterial communities revealed under a diversely managed agroecosystem. PLoS One 7:e40338. 10.1371/journal.pone.0040338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. P., Wu Y. Q., Ma X. J., Zhang G. S., Feng H. Y., Zhang Y. H. (2004). Soil microbial counts and identification of culturable bacteria in an extreme by arid zone. Folia Microbiol. 49 423–429. 10.1007/Bf02931604 [DOI] [PubMed] [Google Scholar]
- Sun W., Dai S., Jiang S., Wang G., Liu G., Wu H., et al. (2010). Culture-dependent and culture-independent diversity of actinobacteria associated with the marine sponge Hymeniacidon perleve from the South China Sea. Antonie Van Leeuwenhoek 98 65–75. 10.1007/s10482-010-9430-8 [DOI] [PubMed] [Google Scholar]
- Sun Y., Shi Y. L., Wang H., Zhang T., Yu L. Y., Sun H., et al. (2018). Diversity of bacteria and the characteristics of actinobacteria community structure in badain jaran desert and tengger desert of China. Front. Microbiol. 9:1068. 10.3389/fmicb.2018.01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X. S., Mao W. L., Liu G. X., Chen T., Zhang W., Wu X. K., et al. (2013). High diversity of nitrogen-fixing bacteria in the upper reaches of the heihe river, northwestern China. Biogeosciences 10 5589–5600. 10.5194/bg-10-5589-2013 [DOI] [Google Scholar]
- Takahashi Y., Matsumoto A., Seino A., Iwai Y., Omura S. (1996). Rare actinomycetes isolated from desert soils. Actinomycetologica 10 91–97. 10.3209/saj.10_91 [DOI] [Google Scholar]
- Tiwari K., Gupta R. K. (2013). Diversity and isolation of rare actinomycetes: an overview. Crit. Rev. Microbiol. 39 256–294. 10.3109/1040841X.2012.709819 [DOI] [PubMed] [Google Scholar]
- Wang N. L., Zhang S. B., He J. Q., Pu J. C., Wu X. B., Jiang X. (2009). Tracing the major source area of the mountainous runoff generation of the heihe river in northwest China using stable isotope technique. Chin. Sci. Bull. 54 2751–2757. 10.1007/s11434-009-0505-8 [DOI] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. F., Hansen M. A., Hansen L. H., Jacquiod S., Sorensen S. J. (2014). Bioinformatic approaches reveal metagenomic characterization of soil microbial community. PLoS One 9:e93445. 10.1371/journal.pone.0093445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li X. Y., Huang L. Q., Jiang H. C. (2015). Actinobacterial diversity in the sediments of five cold springs on the qinghai-tibet plateau. Front. Microbiol. 6:1345. 10.3389/Fmicb.2015.01345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz P., Parfrey L. W., Yarza P., Gerken J., Pruesse E., Quast C., et al. (2014). The SILVA and “All-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 42 D643–D648. 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Zhao L., Ruan Y., Xiao H., Cheng G.-D., Zhou M.-X., et al. (2012). Study of the replenishment sources of typical ecosystems water and dominant plant water in the lower reaches of Heihe, China. J. Glaciol. Geocryol. 34 1478–1486. [Google Scholar]
- Zeng Q., Dong Y., An S. (2016). Bacterial community responses to soils along a latitudinal and vegetation gradient on the loess plateau, China. PLoS One 11:e0152894. 10.1371/journal.pone.0152894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wu X., Zhang G., Li S., Zhang W., Chen X., et al. (2016a). The diversity and biogeography of the communities of actinobacteria in the forelands of glaciers at a continental scale. Environ. Res. Lett. 11:054012 10.1088/1748-9326/11/5/054012 [DOI] [Google Scholar]
- Zhang B., Wu X., Zhang W., Chen X., Zhang G., Ai X., et al. (2016b). Diversity and succession of actinobacteria in the forelands of the tianshan glacier, China. Geomicrobiol. J. 33 716–723. 10.1080/01490451.2015.1085468 [DOI] [Google Scholar]
- Zhang G. Y., Cao T. F., Ying J. X., Yang Y. L., Ma L. Q. (2014). Diversity and novelty of actinobacteria in arctic marine sediments. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 105 743–754. 10.1007/s10482-014-0130-7 [DOI] [PubMed] [Google Scholar]
- Zhang J., Yang X., Zhou H., Yang Y., Ding Z. (2019). New natural products of rare actinomycetes from 2006 to 2018. Chin. J. Org. Chem. 39 1–40. 10.6023/cjoc201810006 [DOI] [Google Scholar]
- Zhang X. F., Zhao L., Xu S. J., Liu Y. Z., Liu H. Y., Cheng G. D. (2013). Soil moisture effect on bacterial and fungal community in Beilu River (Tibetan Plateau) permafrost soils with different vegetation types. J. Appl. Microbiol. 114 1054–1065. 10.1111/jam.12106 [DOI] [PubMed] [Google Scholar]
- Zhu M., Feng Q., Qin Y. Y., Cao J. J., Li H. Y., Zhao Y. (2017). Soil organic carbon as functions of slope aspects and soil depths in a semiarid alpine region of Northwest China. CATENA 152 94–102. 10.1016/j.catena.2017.01.011 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in NCBI, KCVG00000000.