Abstract
Background
Abdominal pain‐related functional gastrointestinal disorders (FGIDs) are among the most common medical problems in paediatric medicine. Frequently, physicians prescribe antidepressants as a second‐line treatment for children and adolescents with FGIDs. To date, the evidence on the benefits and harms of antidepressants for the treatment of abdominal pain‐related FGIDs has not been assessed systematically.
Objectives
The primary objectives were to conduct a systematic review to evaluate the efficacy and safety of antidepressants for the treatment of abdominal pain‐related FGIDs in children and adolescents.
Search methods
We searched The Cochrane Library, PubMed, EMBASE, IPA, CINAHL, PsycINFO, ISI Web of Science, Biosis Previews and the International Clinical Trials Registry Platform of the World Health Organization with appropriate filters (from inception to January 31, 2011).
Selection criteria
For efficacy we included double‐blind, randomised controlled trials (RCTs) of antidepressants for treatment of abdominal pain‐related FGIDs in children and adolescents 18 years or younger. Open‐label and uncontrolled experimental studies, as well as observational studies were eligible for the assessment of harms. The minimum study duration was 4 weeks. The minimum study size was 30 participants.
Data collection and analysis
Two authors independently assessed all abstracts and full text articles, and rated the risk of bias for included studies. Data were extracted independently by one author and checked for accuracy by another author. Data were analysed using RevMan 5.
Main results
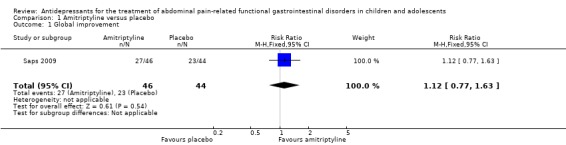
Two RCTs (123 participants), both using amitriptyline, met the pre‐specified inclusion criteria. These studies provided mixed findings on the efficacy of amitriptyline for the treatment of abdominal pain‐related FGIDs. The larger, publicly‐funded study reported no statistically significant difference in efficacy between amitriptyline and placebo in 90 children and adolescents with FGIDs after 4 weeks of treatment. On intention‐to‐treat (ITT)‐ analysis, 59% of the children reported feeling better in the amitriptyline group compared with 53% in the placebo group (RR 1.12; 95% CI: 0.77 to 1.63; P = 0.54). The risk of bias for this study was rated as low.
The second RCT enrolled 33 adolescents with irritable bowel syndrome. Patients receiving amitriptyline experienced greater improvements in the primary outcome, overall quality of life, at weeks 6, 10, and 13 compared with those on placebo (P= 0.019, 0.004, and 0.013, respectively). No effect estimates were calculated for the quality of life outcome because mean quality of life scores and standard deviations were not reported. For most secondary outcomes no statistically significant differences between amitriptyline and placebo could be detected. The risk of bias for this study was rated as unclear for most items. However, it was rated as high for other bias due to multiple testing. The results of this study should be interpreted with caution due to the small number of patients and multiple testing.
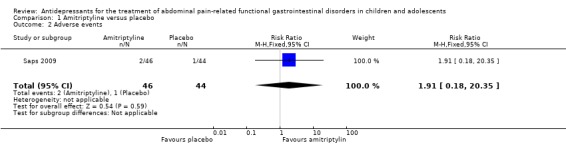
The larger study reported mild adverse events including fatigue, rash and headache and dizziness. On ITT analysis, 4% of the amitriptyline group experienced at least one adverse event compared to 2% of the placebo group. There was no statistically significant difference in the proportion of patients who experienced at least one adverse event (RR 1.91; 95% CI 0.18 to 20.35; P = 0.59). The smaller study reported no adverse events. The methods of adverse effects assessment was poorly reported in both studies and no clear conclusions on the risks of harms of amitriptyline can be drawn.
Authors' conclusions
Clinicians must be aware that for the majority of antidepressant medications no evidence exists that supports their use for the treatment of abdominal pain‐related FGIDs in children and adolescents. The existing randomised controlled evidence is limited to studies on amitriptyline and revealed no statistically significant differences between amitriptyline and placebo for most efficacy outcomes. Amitriptyline does not appear to provide any benefit for the treatment of FGIDs in children and adolescents. Studies in children with depressive disorders have shown that antidepressants can lead to substantial, sometimes life‐threatening adverse effects. Until better evidence evolves, clinicians should weigh the potential benefits of antidepressant treatment against known risks of antidepressants in paediatric patients.
Keywords: Adolescent; Child; Humans; Abdominal Pain; Abdominal Pain/drug therapy; Abdominal Pain/psychology; Amitriptyline; Amitriptyline/adverse effects; Amitriptyline/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/adverse effects; Antidepressive Agents, Tricyclic/therapeutic use; Gastrointestinal Diseases; Gastrointestinal Diseases/drug therapy; Gastrointestinal Diseases/psychology; Irritable Bowel Syndrome; Irritable Bowel Syndrome/drug therapy; Randomized Controlled Trials as Topic
Antidepressants for the treatment of children and adolescents with functional abdominal pain
Abdominal pain‐related functional gastrointestinal disorders (FGIDs) are common in childhood and adolescence. In most cases no medical reason for the pain can be found. Various drug treatment approaches for the different types of abdominal pain‐related FGIDs exist. These drug treatments include: prokinetics and antisecretory agents for functional dyspepsia; pizotifen, propranolol, cyproheptadine or sumatriptane for abdominal migraine; and antispasmodic and antidiarrhoeal regimen for irritable bowel syndrome. Antidepressants have been shown to be effective in some studies of adults with functional gastrointestinal disorders. As a result young patients with similar complaints are sometimes treated with antidepressants. The purpose of this review was to examine the evidence assessing the advantages and disadvantages of such an approach. Only two studies met the inclusion criteria. Both of these studies were randomised controlled trials and assessed the effectiveness and safety of amitriptyline in children with FGIDs. Amitriptyline is a first generation antidepressant (tricyclic antidepressant). Amitriptyline is no longer an agent of first choice for the treatment of depressive disorders because of potentially serious side effects including overdose. Amitriptyline has not been approved for the treatment of functional abdominal pain in children or adolescents.
The larger study (n = 90) showed no difference in the proportion of patients feeling better between the treatment ‐ and the placebo (sugar pill) groups. Side effects were mild and included fatigue, rash and headache and dizziness.The authors of the other, much smaller study (n = 33) reported a significant improvement in overall quality of life and a reduction in pain for specific areas of the abdomen and certain follow up times. However, the results of this study should be interpreted with caution due to poor methodological quality and the small number of patients enrolled. Amitriptyline does not appear to provide any benefit for the treatment of FGIDs in children and adolescents. At present, the evidence for the treatment of children and adolescents with abdominal pain‐related diseases with antidepressants does not support the use of antidepressant agents in this group of patients. We suggest considering alternative treatments that are supported by stronger evidence. There is need for larger, well‐conducted trials with adequate patient relevant outcomes such as quality of life and pain relief to provide more information regarding this common condition.
Summary of findings
Summary of findings for the main comparison.
|
Antidepressants for the treatment of abdominal pain‐related functional gastrointestinal disorders in children and adolescents. | ||||||
|
Patient or population: adolescents with newly diagnosed irritable bowel syndrome according to Rome II criteria Settings: Outpatients at private practice clinic Intervention: amitriptyline Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| [placebo] | [amitriptyline] | |||||
|
Patients' overall assessment of satisfactory relief [Overall, how do you feel your problem is? Answers: better, same, or worse] [4 weeks] |
53% (95% CI not reported) of children reported feeling better | 59% (95% CI not reported) of children reported feeling better (P not reported) | RR 1.12 (0.77, 1.63) | 90 [1] |
low1,2 | |
|
Quality of life [IBS ‐ QOL Questionnaire] 13 weeks |
The mean change of IBS‐QOL scores was 1.9 points (95% CI not reported) in the control group | The mean change of IBS‐QOL scores in the intervention groups was 16.8 points (95% CI not reported); 14.9 points higher than control group; P = 0.013 |
33 [1] | low1,2 | ||
|
Adverse events [methods of adverse effects assessment not reported] |
2% (95% CI not reported) of children reported at least one adverse event | 4% (95 CI not reported) of children reported at least one adverse event | RR 1.91 (0.18, 20.35) | 90 [1] |
very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; IBS‐QOL: Irritable Bowel Syndrome‐Quality of Life scale; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Unclear risk of bias
2 Imprecision of results
3 High probability of outcomes reporting bias
Background
Description of the condition
Apley 1958introduced the term “recurrent abdominal pain” (RAP) for clinically apparent, non‐organic, chronic or recurrent abdominal pain in children, with 3 or more episodes within 3 months that are severe enough to interfere with daily activities. The term “abdominal pain‐related functional gastrointestinal disorders” (FGIDs) replaced "recurrent abdominal pain" in the Rome III system.
The Rome III criteria (Drossman 2006) divide abdominal pain‐related FGIDs into the following subcategories and conceptualise these functional disorders within a biopsychosocial context:
‐Functional dyspepsia (H2a)
‐Irritable bowel syndrome (H2b)
‐Abdominal migraine (H2c)
‐Childhood functional abdominal pain (H2d)
‐Childhood functional abdominal pain syndrome (H2d1)
In Western countries the prevalence of recurrent abdominal pain in children ranges from 0.3% to 19% (Chitkara 2005). Observational studies indicate that this condition substantially interferes with activities of daily living and the functional capacity of children. Furthermore, recurrent abdominal pain is responsible for a considerable proportion of office and emergency room visits (up to 25%) and up to 33% of emergency appendectomies (Scharff 1995). Children suffering from recurrent abdominal pain miss substantially more school days than healthy peers (Stordal 2005; Walker 1998). An investigation published in 2008 by Gieteling et al (Gieteling 2008) concluded that in 29.1% of patients with recurrent abdominal pain, symptoms persisted for a median of 5 years (range 1 to 29 years). Physical examinations and diagnostic tests in the majority of patients do not reveal any organic causes of the symptoms.
Symptoms of abdominal pain‐related FGIDs have been attributed to several known pathophysiological determinants, including altered gastrointestinal motility, enhanced visceral hypersensitivity, altered mucosal immune and inflammatory function (with changes in bacterial flora), as well as altered central nervous or enteric nervous regulation ‐ influenced by psychosocial and sociocultural factors and exposures (Drossman 2006). In child and adolescent psychiatry FGIDs are classified as “somatoform disorders” (Code F 45), emphasizing the psychosocial triggers and psychiatric co‐morbidities of FGIDs (WHO 1996). Although the debate regarding the relation of biological, psychiatric, and behavioural factors in children and adolescents with FGIDs continues, little has changed in treatment approaches over the past 15 years (Schurman 2010).
Various therapies for patients with abdominal pain‐related FGIDs exist. Most commonly, therapy begins with a health professional first acknowledging the patient's pain and then explaining to the patient that no physical cause for the pain could be identified (Schurman 2010). Frequently, a drug treatment is prescribed to relieve specific, predominant symptoms. Agents used for the treatment of abdominal pain‐related FGIDs include prokinetics, antisecretory agents (H2 blockers or proton pump inhibitors), peppermint oil for functional dyspepsia and pizotifen, propranolol and cyproheptadine for abdominal migraine (Weydert 2003; Huertas‐Ceballos 2008a). Antidepressants are often used as second line therapies if other treatment approaches are unsuccessful. In North America, 62% of paediatric gastroenterologists reported prescribing tricyclic antidepressants (TCAs), and up to 20% chose selective serotonin reuptake inhibitors (SSRIs) for the treatment of abdominal pain‐related FGIDs (Schurman 2010).
Description of the intervention
Antidepressants include first‐generation drugs such as tricyclic antidepressants as well as second‐generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and other drugs with related mechanisms of action that selectively target neurotransmitters.The exact mechanism of action of antidepressants, particularly for the treatment of abdominal pain‐related FGIDs in children and adolescents is poorly understood. In general, these drugs work through their effect on prominent neurotransmitters in the central nervous system. Although the drugs can be grouped based on their primary mechanism of action as SSRIs, SNRIs, and other antidepressants, drugs within these groups are not homogenous and the specific activity may vary.
In adult patients with major depressive disorders, first‐ and second‐generation antidepressants have equivalent efficacy (Williams 2000). However, first‐generation drugs often are accompanied by multiple adverse events that many patients find intolerable. For example, tricyclic antidepressants tend to cause anticholinergic effects including dry mouth and eyes, urinary hesitancy, and sometimes urinary retention and constipation. In addition, they have a high rate of lethality when overdose occurs. Thus, first‐generation antidepressants are no longer considered first‐line agents of choice for the treatment of psychiatric disorders in most circumstances.
Second‐generation antidepressants can also lead to substantial adverse effects (e.g. nausea, vomiting, diarrhoea, sexual dysfunction, and others) in up to 90% of adult patients. In patients with depressive disorders, some of these adverse effects can be life threatening (e.g. suicidality, seizures). The potential for lethality when overdose occurs, however, is low compared with tricyclic antidepressants (Gartlehner 2007).
Currently, the evidence on the efficacy and safety for antidepressant agents in children and adolescents is limited. No antidepressants are currently approved by regulatory agencies for treating abdominal pain‐related FGIDs in children and adolescents.
Objectives
The primary objectives were to evaluate the efficacy and safety of antidepressant agents for the treatment of abdominal pain‐related FGIDs in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
For efficacy we included double‐blind randomised controlled trials comparing antidepressants with placebo or active comparators with a study duration of at least 4 weeks.
For the assessment of adverse effects open‐label and uncontrolled experimental studies as well as controlled and uncontrolled observational studies with a study duration of at least 4 weeks and a minimum sample size of 30 participants were considered for inclusion.
Types of participants
Participants included children and adolescents (in‐ and outpatients) ≤ 18 years with abdominal pain‐related FGIDs as defined by the Rome criteria.
Types of interventions
Interventions of interest included all commonly prescribed antidepressant agents. We included all second‐generation antidepressants such as SSRIs or SNRIs as well as some first‐generation antidepressants. The following medications were of interest for our report:
tricyclic antidepressants: amitriptyline, amoxapine, clomipramine, desipramine, dibenzepine, dothiepin, doxepin, imipramine, lofepramine, nortriptyline, protriptyline, trimipramine;
selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline;
selective serotonin‐norepinephrine reuptake inhibitors: desvenlafaxine, duloxetine, milnacipran, venlafaxine; and
antidepressants with other mechanisms of action: bupropion, maprotiline, mirtazapine, reboxetine, trazodone.
Types of outcome measures
Primary outcomes
The primary outcome of interest was the proportion of patients achieving global or clinical improvement as defined by the included studies and expressed as a percentage of the total number of patients randomised (intention‐to‐treat analysis).
Secondary outcomes
Secondary outcome measures included:
1. quality of life; and
2. occurrence of adverse effects. We were interested in overall rates of adverse effects as well as the frequencies of specific adverse effects that have been associated with antidepressant medications.
Search methods for identification of studies
Electronic searches
We searched the following online databases from inception to January 31, 2011: PubMed, The Cochrane Library, EMBASE, International Pharmaceutical Abstracts, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, ISI Web of Science, and Biosis Previews. The detailed search strategy for PubMed, The Cochrane Library, and EMBASE can be found in Appendices 1, 2, and 3, respectively.
We used either Medical Subject Headings or subject searching (Emtree) as search terms when available or key words when appropriate. We combined terms for selected indications (functional dyspepsia, irritable bowel syndrome, migraine disorders, abdominal migraine, childhood functional abdominal pain syndrome, recurrent abdominal pain) and the relevant drugs (amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine, desvenlafaxine, dibenzepin, dothiepin, doxepin, duloxetine, escitalopram, fluoxetine, fluvoxamine, imipramine, lofepramine, maprotiline, milnacipran, mirtazapine, nortriptyline, protriptyline, paroxetine, reboxetine, sertraline, trazodone, trimipramine, and venlafaxine). We limited the electronic searches to "human" and "children".
Searching other resources
Additionally, we searched the grey literature database OpenSIGLE ( System for Information on Grey Literature in Europe ), Dissertations & Theses, and the British Library Online Catalogue, as well as conference proceedings at the Canadian Association of Gastroenterology, The American Gastroenterological Association, and the United European Gastroenterology Federation.
We have also searched the International Clinical Trials Registry Platform of the World Health Organization (http://apps.who.int/trialsearch/; accessed on January 31, 2011) with the terms "Functional Gastrointestinal Disorder" OR "Recurrent Abdominal Pain" combined with the names of each of the antidepressant drugs.
Finally, we handsearched reference lists of relevant narrative reviews and editorials and conducted citation analysis using Scopus to identify publications that had cited RCTs that met our eligibility criteria.
Data collection and analysis
Selection of studies
We dually reviewed abstracts and selected full text articles to determine their eligibility for inclusion based on the eligibility criteria described above. Studies published in abstract form were to be included only if authors could be contacted for further information. Any disagreements between reviewers were discussed and resolved by consensus or the involvement of a third party.
Data extraction and management
We developed a standardized data form to extract data from included studies. Data were extracted by one author and checked for accuracy by a second author. Any disagreements were discussed and resolved by consensus. Data were analysed using Review Manager 5.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias for each included study and assigned a rating for the risk of bias. Any disagreements were resolved by discussion and consensus or by consulting a third, independent party. The evaluation of the risk of bias for RCTs included methods of randomisation and allocation concealment, blinding, incomplete outcome data, selective outcome reporting and any other potential sources of bias (Higgins 2008).
Measures of treatment effect
For the primary outcome, the proportion of patients who achieved global or clinical improvement was recorded and a relative risk with 95% confidence intervals was calculated. No effect estimates were calculated for the quality of life outcome because the included study did not report mean quality of life scores along with standard deviations.
Assessment of heterogeneity
Heterogeneity was not assessed because no studies were pooled for analyses.
Data synthesis
No studies were pooled for meta‐analysis. Data were analysed using Review Manager 5 (RevMan 5.0.25). We rated the quality of the evidence based on the system developed by the GRADE (Grading of Recommendations Assessment, Development and Evaluation) Working Group (Atkins 2004).
Subgroup analysis and investigation of heterogeneity
We did not conduct any subgroup analyses.
Sensitivity analysis
We did not conduct sensitivity analyses.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies .
Results of the search
The literature search identified 729 citations. We retrieved 57 publications as full text articles for further assessment. Three potentially eligible studies were identified (Bahar 2008; Saps 2009; Campo 2004). An ongoing randomized trial was also identified (Campo NCT00962039).
We identified two eligible studies assessing the efficacy and safety of antidepressant treatments for children and adolescents with abdominal pain‐related FGIDs (Bahar 2008; Saps 2009). A third, open‐label, uncontrolled study did not meet eligibility criteria (Campo 2004).
Both included studies assessed the efficacy and safety of amitriptyline for the treatment of abdominal pain‐related FGIDs. The larger study was funded by the American College of Gastroenterology and by the National Institutes of Health (Saps 2009). The second study was supported by the James L. Brooks and the Diana Brooks Medical Research Foundation of the California Community Foundation, as well as by Astra Zeneca, LP (Bahar 2008). Overall, these studies provided data on 123 paediatric patients with abdominal, pain‐related FGID.
The publicly funded, larger trial included 90 children aged 8 to 17 years (mean age,12.7 years) with a diagnosis of functional abdominal pain, functional dyspepsia, or irritable bowel syndrome according to the Rome II criteria (Saps 2009). This double‐blind RCT recruited patients from paediatric gastroenterology clinics of 6 tertiary care centres in the United States. Eligible patients were enrolled into a 1‐week run‐in phase during which investigators assessed symptoms on a daily basis employing age‐appropriate, validated questionnaires and a pain scale. Children who suffered from weekly average pain of more than 25 mm on a 100‐mm visual analogue/ Likert pain scale during the run‐in phase were randomised to receive either placebo (n = 44) or amitriptyline (n = 46). Patients weighing less than 35 kg received 10 mg amitriptyline per day and those weighing more than 35 kg received 20 mg daily for 4 weeks. Almost 90% of the participating children had previously failed a pharmacologic treatment. Seventy‐three percent of the enrolled study population were female.
The primary outcomes of the study were the patients' overall assessment of satisfactory relief of symptoms and the satisfaction with treatment over the 4 week treatment period. The primary outcomes were assessed with two questions at week 4:
Question 1: "Overall how do you feel your problem is ? " Answers: better/same/worse; and
Question 2: "How did the medication relieve your pain ?" Answers: excellent/good/fair/poor/failed.
Secondary outcomes of the study included the effects of the intervention on psychosocial traits and daily functioning. The Pain Response Inventory, the Children's Depression Inventory (CDI), the State‐Trait Anxiety Inventory for Children (STAIC), The Children Somatization Inventory Questionnaire and the Pediatric Functional Disability Inventory, all validated self‐report and age‐appropriate psychological questionnaires, were completed at the beginning and the end of the study.
Bahar 2008 compared the efficacy and safety of amitriptyline and placebo in adolescents aged 12 to 18 years with newly diagnosed IBS based on the Rome II criteria. This double‐blind RCT enrolled 33 adolescent (24 females) outpatients from a suburban, private‐practice paediatric gastroenterology clinic in California. Patients on concurrent medication for depression, anxiety, or chronic pain syndromes were excluded. The study period consisted of 2 weeks of enrollment and symptom scoring, 8 weeks of therapy with amitriptyline or placebo and three weeks of "wash out" and symptom scoring. From weeks 3 to 10, patients were randomised to receive either amitriptyline (n = 16) or placebo (n = 17) in a double‐blinded fashion. Amitriptyline was administered at different dosages according to bodyweight (30 to 50 kg ‐ one 10 mg capsule orally by bedtime; 50 to 80 kg: two 10 mg capsules orally at bedtime; 80 kg or higher: three 10 mg capsules orally at bedtime). At weeks 0, 2, 6 and 10 each of the participants was asked to complete the data collection packet consisting of an IBS‐QOL (Quality of life) questionnaire, a symptoms checklist to assess 13 IBS‐associated symptoms, a pain‐rating scale/Likert‐like Scale and a visual analogue scale.
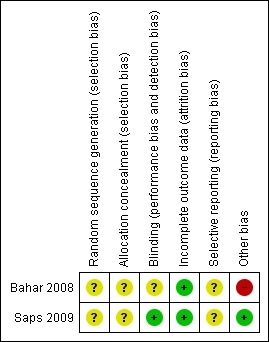
Risk of bias in included studies
The risk of bias in the two included studies (Bahar 2008; Saps 2009) varied (Figure 1). Saps et al. was a well designed, presumably concealed, and double blinded RCT. The drop out rate was low (8%) and investigators employed an intention‐to‐treat analysis. We rated the risk of bias for this study as low.
Figure 1.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The reporting of the methods of the second study (Bahar 2008) was sub optimal and did not adhere to the CONSORT (Consolidated Standards of Reporting Trials) statement (Schulz 2010). Our assessment of relevant domains of study design such as randomisation, allocation concealment, or methods of blinding was compromised by a lack of information in the publication. We rated the risk of bias for this study as unclear.
Allocation
Saps 2009 did not report the exact method of randomisation and allocation concealment. They state, however, that all investigators were blinded to the randomisation codes. Baseline characteristics of treatment groups appeared to be similar after randomisation.
Bahar 2008 did not report methods of randomisation, nor indicate any attempts of allocation concealment. The risk of selection bias, therefore, remains unclear. Furthermore, the publication does not provide a table presenting patient characteristics at baseline. Because of the small sample size (n = 33) and the play of chance, an imbalance of prognostic factors at baseline is conceivable, even with adequate methods of randomisation and allocation concealment. For example, authors indicate a substantial difference in baseline quality of life scores between the amitriptyline and the placebo groups.
Blinding
In the Saps 2009 study both patients and outcome assessors were masked. Treatment (amitriptyline or placebo) was provided in identical capsules and data were analysed independently at a central coordinating site which did not have access to the randomisation code until analysis was completed.
Bahar 2008 did not describe how adequate blinding of investigators and patients was achieved.
Incomplete outcome data
Seven female patients (8%) did not complete the Saps 2009 study. No drop outs were reported in the Bahar 2008 study.
Selective reporting
Saps 2009 and Bahar 2008 reported on all pre‐specified outcome measures assessing efficacy. However, in both studies it remains unclear how investigators assessed adverse effects. Thus selective reporting was assessed as unclear for both studies.
Other potential sources of bias
No other potential sources of bias could be detected for the Saps 2009 study.
For the Bahar 2008 study it remains unclear whether the statistical analyses were based on an ITT principle. The multiple subgroup analyses that the authors conducted are likely to be subject to type I and type II errors and should be interpreted with caution. Bahar 2008 did not employ a test of interaction for subgroup analyses or correct for multiple testing. Statistically significant results based on chance findings (type I error), therefore, are likely. Conversely, because of the small sample (n = 33) size, the absence of statistical significance for other results might be attributable to a lack of statistical power (type II error). As mentioned above, the publication does not provide a table presenting patient characteristics at baseline. Thus, the Bahar 2008 study was assessed as a high risk of bias for other potential sources of bias.
Effects of interventions
See: Table 1
The results on the efficacy of amitriptyline for the treatment of abdominal pain‐related FGIDs in children were mixed. Bahar 2008 reported statistically significantly greater improvements on the IBS‐QOL scale for patients treated with amitriptyline compared to placebo. The larger, and methodologically sounder trial by Saps 2009 however, did not detect any statistically significant differences between amitriptyline and placebo for any of the primary efficacy outcomes.
Saps 2009 The primary outcome in the Saps 2009 study was the overall response to treatment (child's assessment of pain relief and sense of improvement), while secondary outcomes were the effect of treatments on psychosocial traits and daily functioning. On ITT analysis, 59% of children treated with amitriptyline reported feeling better, compared to approximately 53% of patients treated with placebo (RR 1.12; 95% CI 0.77 to 1.63; P = 0.54). Four percent of children in the amitriptyline group and 2% in the placebo group reported feeling worse after the course of treatment (P = 0.81). Children in both treatment arms achieved a statistically significant decrease in pain from baseline (P < 0.0001), without a statistically significant difference between the groups (P=0.18). No statistically significant differences between amitriptyline and placebo could be detected for any of the secondary outcomes such as depression, coping, disability, or somatization except for the outcome anxiety. The anxiety score improved significantly in the amitriptyline group compared with the placebo group.
Overall, 83 children (92 %) completed the study (amitriptyline, 43 children [35 girls]; placebo, 40 children [30 girls]). Seven girls discontinued the study, three of them because of mild adverse events. In the amitriptyline group one girl dropped out because of fatigue and one because of rash and headaches. In the placebo group one girl stopped the medication because of dizziness. On ITT analysis, 4% of the amitriptyline group experienced at least one adverse event compared to 2% of the placebo group. There was no statistically significant difference in the proportion of patients who experienced at least one adverse event (RR 1.91; 95% CI 0.18 to 20.35; P = 0.59) Three patients had to be excluded because of intercurrent viral illnesses, non adherence to the protocol, or improper consent. One girl discontinued because of lack of interest.
Bahar 2008 The primary outcome in the Bahar 2008 study was the overall score on a modified IBS‐QOL scale. At weeks 6, 10, and 13 (after 4 and 8 weeks of treatment and 3 weeks post‐treatment), patients on amitriptyline experienced significantly greater improvements in overall IBS‐QOL scores than patients treated with placebo (P = 0.019, P = 0.004, and P = 0.013, respectively). Furthermore, Bahar 2008 reported that at weeks 10 and 13 patients treated with amitriptyline were significantly more likely than patients on placebo to have achieved an at least 15% improvement on the IBS‐QOL scale (data reported as figure only.; P = 0.007 and P = 0.002). The authors did not provide any justification for choosing a 15% improvement as a cut‐off level. The clinical significance of a 15% improvement seems questionable. Subgroup analyses of individual subscales of the IBS‐QOL tool yielded mixed findings including some statistically significant differences between amitriptyline and placebo. For most of the symptoms associated with abdominal pain‐related FGIDs (e.g. constipation, tenesmus, abdominal distension, diffuse abdominal pain, and others), however, no statistically significant differences in patients treated with amitriptyline or placebo could be detected at any recorded time. Moreover, no statistically significant differences were apparent for intensity of pain or interference with schoolwork, sports, or interaction with friends (data not reported; P >0.05). These findings should be interpreted with caution due to multiple testing and lack of statistical power. No adverse effects were reported.
Discussion
Summary of main results
This systematic review reveals that the evidence on the efficacy and safety of antidepressant medications in children and adolescents with abdominal pain‐related FGIDs is sparse. We found only two double‐blind RCTs of mixed methodological quality comparing amitriptyline to placebo for treatment of abdominal pain‐related FGIDs (Bahar 2008; Saps 2009). An open‐label, uncontrolled trial (n = 25) that assessed the efficacy and safety of citalopram in children with recurrent abdominal pain did not meet eligibility criteria (Campo 2004). This study reported an improvement in symptoms after 12 weeks of treatment without the occurrence of serious adverse effects. No studies of other antidepressant medications of interest were found. A search in the International Clinical Trials Registry Platform of the WHO detected one ongoing additional RCT on the efficacy and safety of citalopram (Campo NCT00962039) in paediatric patients with abdominal pain‐related FGIDs (n = 100). To date, however, no results of this study are available. The WHO trial registry did not contain any additional ongoing or planned RCTs on antidepressant medications in patients younger than 18 years.
The findings of the two included studies were mixed. The larger and methodologically sounder trial (Saps 2009) did not detect any statistically significant differences in most efficacy outcomes between patients on amitriptyline or placebo. In this study, patients in both treatment arms experienced high rates of therapeutic response (amitriptyline: 59%, placebo: 53% on ITT analysis). Similar results were apparent for other outcomes including pain, psychosocial traits and daily functioning. The smaller study did not detect a statistically significant difference for most outcomes either (Bahar 2008). An exception, however, were the assessments of differences in quality of life and pain. Patients who were treated with amitriptyline experienced statistically significantly greater improvements in quality of life and abdominal pain than the adolescents randomised to a placebo. Due to methodological limitations, however, the results of this study should be interpreted with caution.
Overall completeness and applicability of evidence
Patients in the included studies were recruited from six tertiary care centres in the United States (Saps 2009) and from a suburban, private‐practice paediatric gastroenterology clinic in California (Bahar 2008). In the Saps 2009 study only patients with reported weekly pain of more than 25 mm on a 100‐mm visual analogue/Likert pain scale were randomised. Consequently, the applicability of the study results for children with lower pain levels is uncertain. The results of this review should not be extrapolated to antidepressant medications other than amitriptyline.
Quality of the evidence
The GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria were used to assess the quality of evidence. The quality of evidence for patient's overall assessment of satisfactory relief and quality of life were rated as low. The quality of evidence for safety outcomes was rated as very low.
Potential biases in the review process
Despite extensive literature searches, studies for most antidepressant medications could not be found. It remains unclear whether this reflects a lack of research or whether such studies have not been published.
Agreements and disagreements with other studies or reviews
A recent meta‐analysis on pharmacological interventions for recurrent abdominal pain and irritable bowel syndrome in children found only weak evidence supporting the use of pizotifen, peppermint oil and famotidine (Huertas‐Ceballos 2008a). Neither this review, which was updated in 2008, nor a 2003 systematic review of treatments for recurrent abdominal pain in children (Weydert 2003) included any antidepressant drugs. In contrast, various studies on the efficacy and safety of antidepressant drugs in adults with FGIDs exist. Data obtained from studies conducted in adults are often utilized to tailor treatment to children with functional gastrointestinal disorders (Chitkara 2006). A Cochrane Review on bulking agents, antispasmodic, and antidepressant medication (amitriptyline, doxepine, desipramine and trimipramine) for the treatment of patients with irritable bowel syndrome (Quartero 2005) did not find clear evidence for the benefit of antidepressants, which correlates with the findings of the present review. Results from a recent systematic review, however, showed the efficacy of antidepressants for the treatment of irritable bowel syndrome in adult patients (Ford 2009). An evidence‐based position statement on irritable bowel syndrome by the American College of Gastroenterologists from 2009 suggests that, based on good quality trials with a limited number of patients, tricyclic antidepressants and selective serotonin reuptake inhibitors are effective for patients with all subtypes of irritable bowel syndrome (Brandt 2009).
Authors' conclusions
Clinicians must be aware that, for the majority of antidepressant medications, no evidence exists that support their use for the treatment of abdominal pain‐related FGIDs in children and adolescents. The included studies revealed no statistically significant differences between amitriptyline and placebo for most efficacy outcomes. Amitriptyline does not appear to provide any benefit for the treatment of FGIDs in children. Studies in children with depressive disorders have shown that antidepressants can lead to substantial, sometimes life‐threatening adverse effects (Anonymous 2004). Taking into consideration that there is only weak evidence for the efficacy of any medical intervention in children and adolescents with recurrent abdominal pain (Huertas‐Ceballos 2008a) and some evidence that cognitive behavioural therapy may be useful in this group of patients (Huertas‐Ceballos 2008b) the use of antidepressant treatment in paediatric patients has to be weighed against potential adverse effects.
Currently, only placebo‐controlled trials on antidepressant medications in children and adolescents are available. Future research needs to focus on comparisons that are relevant for clinicians and patients; head‐to‐head comparisons of non‐pharmacological treatments with antidepressants could provide valuable information about the direct advantages and disadvantages of either treatment. Methodological limitations with respect to small sample sizes and lack of power could be overcome by large multicenter studies. Studies with potentially less harmful pharmacological and non‐pharmacological interventions are needed. If antidepressants are considered to be indispensable for severe cases, there is a need for pragmatic studies with patients in non‐clinical surroundings and adequate study durations that reflect a clinical course of treatment.
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. PubMed search strategy
| #1 | Search functional dyspepsia OR "Irritable Bowel Syndrome"[Mesh] OR IBS OR "Migraine Disorders"[Mesh] OR abdominal migraine OR childhood functional abdominal pain OR childhood functional abdominal pain syndrome OR recurrent abdominal pain OR RAP | 33456 |
| #2 | Search amitriptyline OR amoxapine OR clomipramine OR desipramine OR dibenzepin OR dothiepin OR doxepin OR imipramine OR lofepramine OR nortriptyline OR protriptyline OR trimipramine OR citalopram OR escitalopram OR fluoxetine OR fluvoxamine OR paroxetine OR sertraline OR desvenlafaxine OR duloxetine OR milnacipran OR venlafaxine OR bupropion OR maprotiline OR mirtazapine OR reboxetine OR trazodone | 47765 |
| #3 | Search "Amitriptyline"[Mesh] OR "Amoxapine"[Mesh] OR "Clomipramine"[Mesh] OR "Desipramine"[Mesh] OR "dibenzepin "[Substance Name] OR "Dothiepin"[Mesh] OR "Doxepin"[Mesh] OR "Imipramine"[Mesh] OR "Lofepramine"[Mesh] OR "Nortriptyline"[Mesh] OR "Protriptyline"[Mesh]OR "Trimipramine"[Mesh] OR "Citalopram"[Mesh] OR escitalopram OR "Fluoxetine"[Mesh] OR "Fluvoxamine"[Mesh] OR "Paroxetine"[Mesh] OR "Sertraline"[Mesh] OR "Bupropion"[Mesh] OR "Maprotiline"[Mesh] OR "mirtazapine "[Substance Name] OR "reboxetine "[Substance Name] OR "Trazodone"[Mesh] | 37730 |
| #4 | Search #1 AND (#2 OR #3) Limits: Humans, All Child: 0‐18 years | 83 |
Appendix 2. Cochrane Library Search Strategy
| #1 | MeSH descriptor Irritable Bowel Syndrome explode all trees | 252 |
| #2 | MeSH descriptor Migraine Disorders explode all trees | 1485 |
| #3 | functional dyspepsia OR Irritable Bowel Syndrome OR IBS OR Migraine Disorders OR abdominal migraine OR childhood functional abdominal pain OR recurrent abdominal pain OR RAP | 3401 |
| #4 | (#1 OR #2 OR #3) | 3450 |
| #5 | MeSH descriptor Amitriptyline explode all trees | 975 |
| #6 | MeSH descriptor Amoxapine explode all trees | 27 |
| #7 | MeSH descriptor Clomipramine explode all trees | 384 |
| #8 | MeSH descriptor Desipramine explode all trees | 374 |
| #9 | MeSH descriptor Dothiepin explode all trees | 61 |
| #10 | MeSH descriptor Doxepin explode all trees | 141 |
| #11 | MeSH descriptor Imipramine explode all trees | 1004 |
| #12 | MeSH descriptor Lofepramine explode all trees | 29 |
| #13 | MeSH descriptor Nortriptyline explode all trees | 337 |
| #14 | MeSH descriptor Protriptyline explode all trees | 14 |
| #15 | MeSH descriptor Trimipramine explode all trees | 63 |
| #16 | MeSH descriptor Citalopram explode all trees | 505 |
| #17 | MeSH descriptor Fluoxetine explode all trees | 1031 |
| #18 | MeSH descriptor Fluvoxamine explode all trees | 339 |
| #19 | MeSH descriptor Paroxetine explode all trees | 673 |
| #20 | MeSH descriptor Sertraline explode all trees | 515 |
| #21 | MeSH descriptor Bupropion explode all trees | 354 |
| #22 | MeSH descriptor Maprotiline explode all trees | 158 |
| #23 | MeSH descriptor Trazodone explode all trees | 155 |
| #24 | amitriptyline OR amoxapine OR clomipramine OR desipramine OR dibenzepin OR dothiepin OR doxepin OR imipramine OR lofepramine OR nortriptyline OR protriptyline OR trimipramine OR citalopram OR escitalopram OR fluoxetine OR fluvoxamine OR paroxetine OR sertraline OR desvenlafaxine OR duloxetine OR milnacipran OR venlafaxine OR bupropion OR maprotiline OR mirtazapine OR reboxetine OR trazodone | 12254 |
| #25 | (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) | 12254 |
| #26 | child* OR kid* OR teen* OR adolescen* OR Kind OR Kinder OR Jugend* OR Heranwachsende* | 134527 |
| #27 | (#4 AND #25 AND #26) | 40 |
Appendix 3. Embase search strategy
| #1 | 'functional dyspepsia' | 1,822 |
| #2 | 'irritable bowel syndrome'/exp OR 'irritable bowel syndrome' | 10,743 |
| #3 | ibs | 4,708 |
| #4 | 'migraine disorders'/exp OR 'migraine disorders' | 30,494 |
| #5 | 'abdominal migraine' | 117 |
| #6 | 'childhood'/exp OR childhood AND functional AND abdominal AND ('pain'/exp OR pain) | 825 |
| #7 | 'childhood'/exp OR childhood AND functional AND abdominal AND ('pain'/exp OR pain) AND ('syndrome'/exp OR syndrome) | 258 |
| #8 | recurrent AND abdominal AND ('pain'/exp OR pain) | 7,903 |
| #9 | rap | 3,690 |
| #10 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 | 55,736 |
| #11 | 'amitriptyline'/exp OR 'amitriptyline' OR 'amoxapine'/exp OR 'amoxapine' OR 'clomipramine'/exp OR 'clomipramine' OR 'desipramine'/exp OR 'desipramine' OR 'dibenzepin'/exp OR 'dibenzepin' OR 'dothiepin'/exp OR 'dothiepin' OR 'doxepin'/exp OR 'doxepin' OR 'imipramine'/exp OR 'imipramine' OR 'lofepramine'/exp OR 'lofepramine' OR 'nortriptyline'/exp OR 'nortriptyline' OR 'protriptyline'/exp OR 'protriptyline' OR 'trimipramine'/exp OR 'trimipramine' OR 'citalopram'/exp OR 'citalopram' OR 'escitalopram'/exp OR 'escitalopram' OR 'fluoxetine'/exp OR 'fluoxetine' OR 'fluvoxamine'/exp OR 'fluvoxamine' OR 'paroxetine'/exp OR 'paroxetine' OR 'sertraline'/exp OR 'sertraline' OR 'desvenlafaxine'/exp OR 'desvenlafaxine' OR 'duloxetine'/exp OR 'duloxetine' OR 'milnacipran'/exp OR 'milnacipran' OR 'venlafaxine'/exp OR 'venlafaxine' OR 'bupropion'/exp OR 'bupropion' OR 'maprotiline'/exp OR 'maprotiline' OR 'mirtazapine'/exp OR 'mirtazapine' OR 'reboxetine'/exp OR 'reboxetine' OR 'trazodone'/exp OR 'trazodone' | 115,259 |
| #12 | #10 AND #11 AND ([infant]/lim OR [child]/lim OR [adolescent]/lim) AND [humans]/lim | 291 |
Data and analyses
Comparison 1.
Amitriptyline versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.63] |
| 2 Adverse events | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.18, 20.35] |
Analysis 1.1.
Comparison 1 Amitriptyline versus placebo, Outcome 1 Global improvement.
Analysis 1.2.
Comparison 1 Amitriptyline versus placebo, Outcome 2 Adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2011 | Amended | Minor edit to Acknowledgements section |
Differences between protocol and review
The study duration and the age of the included participants differ from the protocol. The study duration was changed from 12 to 4 weeks and the age of the included participants was changed from ≤ 16 to ≤18 years.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial | |
| Participants | Adolescents with newly diagnosed irritable bowel syndrome according to Rome II criteria | |
| Interventions | Amitriptyline versus placebo | |
| Outcomes | Irritable Bowel Syndrome ‐ Quality of Life scale (34 items, 7 subscales) Symptoms checklist Pain rating scale (Likert Like Scale) Visual analogue scale for pain intensity |
|
| Notes | Methods of adverse effects assessment not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method not reported |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | No drop outs |
| Selective reporting (reporting bias) | Unclear risk | Methods of adverse effects assessment not reported |
| Other bias | High risk | Multiple testing and lack of power for subgroup analyses could cause type I and type II errors. |
| Methods | Randomized controlled trial | |
| Participants | Children and adolescents from 8‐17 years with a diagnosis of functional abdominal pain, functional dyspepsia and Irritable Bowel Syndrome | |
| Interventions | Amitriptyline vs. placebo | |
| Outcomes | 1: Patient's satisfactory relief and satisfaction with treatment assessed by the questions: "Overall how do you feel your problem is ?" (Better/Same/Worse) "How did the medication relieve your pain ?" (Excellent/Good/Fair/Poor/Failed) 2: Effect on psychosocial traits and ability to perform daily activities, measured by the Pain Response Inventory Children's Depression Inventory (CDI) State‐Trait Anxiety Inventory for Children (STAIC) Children Somatization Inventory Questionnaire Pediatric Functional Disability Inventory Visual analogue scale for pain intensity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules as medical intervention, data were analysed independently of the investigators at a central coordinating site, which did not have access to the code until analysis was completed |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | 7 dropouts |
| Selective reporting (reporting bias) | Unclear risk | Methods of adverse effects assessment not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Campo 2004 | 12 week, flexible dose, uncontrolled, open‐label trial of citalopram in a population with functional recurrent abdominal pain and comorbid internalising disorders aged 7 to 18 years Excluded because the sample size was too small (n = 25). |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Anxiety and Recurrent Abdominal Pain in Children |
| Methods | Randomized, double‐blind, placebo‐controlled trial |
| Participants | Children and adolescents aged 7 to 18 years with functional recurrent abdominal pain (FAP) |
| Interventions | Citalopram (10 to 40 mg/day) or placebo for 8 weeks. |
| Outcomes | Global clinical improvement is the primary outcome. Secondary outcomes include: Abdominal Pain Index (API) and adverse events |
| Starting date | July 2004 to April 2010 |
| Contact information | Principal Investigator: John V Campo, The Research Institute at Nationwide Children's Hospital |
| Notes | ClinicalTrials.gov Identifier: NCT00962039 |
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double‐blind placebo‐controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr 2008;152(2):685‐9. [PUBMED: 18410774] [DOI] [PubMed] [Google Scholar]
- Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomized, placebo‐controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137(4):1261‐9. [PUBMED: 19596010] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Campo JV, Perel J, Lucas A, Bridge J, Ehmann M, Kalas C, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: an exploratory study. J Am Acad Child Adolesc Psychiatry 2004;43(10):1234‐42. [PUBMED: 15381890] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Anxiety and Recurrent Abdominal Pain in Children. Ongoing study July 2004 to April 2010.
Additional references
- Anonymous. Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants. Medicines and Healthcare Products Regulatory Agency2004, issue http://www.mhra.gov.uk/home/groups/pl‐p/documents/drugsafetymessage/con019472.pdf.
- Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child 1958;33(168):165‐70. [PUBMED: 13534750] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt LJ, Chey WD, Foxx‐Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, et al. An evidence‐based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104 Suppl 1:S1‐35. [PUBMED: 19521341] [DOI] [PubMed] [Google Scholar]
- Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol 2005;100(8):1868‐75. [PUBMED: 16086724] [DOI] [PubMed] [Google Scholar]
- Chitkara DK, Lorenzo C. Pharmacotherapy for functional gastrointestinal disorders in children. Curr Opin Pharmacol 2006;6(6):536‐40. [PUBMED: 16949871] [DOI] [PubMed] [Google Scholar]
- Drossman DA. Rome III: the new criteria. Chin J Dig Dis 2006;7(4):181‐5. [PUBMED: 17054578] [DOI] [PubMed] [Google Scholar]
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta‐analysis. Gut 2009;58(3):367‐78. [PUBMED: 19001059] [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Thieda P, DeVeaug‐Geiss AM, Gaynes BN, Krebs EE, et al. Comparative Effectiveness of Second‐generation Antidepressants in the Pharmacologic Treatment of Adult Depression.. Comparative Effectiveness Review No. 7‐EHC007‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [PubMed]
- Gieteling MJ, Bierma‐Zeinstra SM, Passchier J, Berger MY. Prognosis of chronic or recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr 2008;47(3):316‐26. [PUBMED: 18728528] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. [Google Scholar]
- Huertas‐Ceballos A, Logan S, Bennett C, Macarthur C. Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003017.pub2] [DOI] [PubMed] [Google Scholar]
- Huertas‐Ceballos A, Logan S, Bennett C, Macarthur C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003014.pub2] [DOI] [PubMed] [Google Scholar]
- Quartero AO, Meineche‐Schmidt V, Muris J, Rubin G, Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003460.pub2] [DOI] [PubMed] [Google Scholar]
- Scharff L. Psychogical treatment of children with abdominal pain. Dissertation Abstracts International Database UMI 95354731995.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [PUBMED: 20334633] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. J Pediatr Gastroenterol Nutr 2010;50(1):32‐7. [PUBMED: 19915496] [DOI] [PubMed] [Google Scholar]
- Stordal K, Nygaard EA, Bentsen BS. Recurrent abdominal pain: a five‐year follow‐up study. Acta Paediatr 2005;94(2):234‐6. [PUBMED: 15981760] [DOI] [PubMed] [Google Scholar]
- Walker LS, Guite JW, Duke M, Barnard JA, Greene JW. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. J Pediatr 1998;132(6):1010‐5. [PUBMED: 9627595] [DOI] [PubMed] [Google Scholar]
- Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics 2003;111(1):e1‐11. [PUBMED: 12509588] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Multiaxial classification of child and adolescent psychiatric disorders. The ICD‐10 classification of mental and behavioural disorders in children and adolescents. Cambridge University Press, 1996.
- Williams JW Jr, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med 2000;132(9):743‐56. [PUBMED: 10787370] [DOI] [PubMed] [Google Scholar]


