Abstract
Background
The 'off‐label' effect of alprazolam on depression has not been systematically evaluated.
Objectives
To determine the antidepressant effect, including tolerability and acceptability, of alprazolam as monotherapy for major depression, when compared to placebo and conventional antidepressants in outpatients and patients in primary care.
Search methods
We searched the Cochrane Central Register of Controlled Trials and the Cochrane Depression, Anxiety and Neurosis Group Register, which includes relevant randomised controlled trials from the following bibliographic databases: The Cochrane Library (all years to February 2012); EMBASE (1970 to February 2012); MEDLINE (1950 to February 2012) and PsycINFO (1960 to February 2012). Two review authors identified relevant trials by assessing the abstracts of all possible studies. We applied no language restrictions.
Selection criteria
We selected randomised controlled trials (RCTs) of alprazolam versus placebo or conventional antidepressants for depression in adults, excluding studies with inpatients only.
Data collection and analysis
Two review authors performed the data extraction and 'Risk of bias' assessment independently with disagreements resolved through discussion with a third review author. Primary outcomes included the mean difference (MD) in reduction of depression on a continuous measure of depression symptoms, and the risk ratio (RR) of the clinical response based on a dichotomous measure, with 95% confidence intervals (CI).
Main results
We identified 21 alprazolam studies (22 reports) with a total of 2693 participants. Seven studies used a placebo (n = 771) and 20 used cyclic antidepressants (n = 1765). The typical duration of the studies was four to six weeks. We considered six studies to have a high risk of bias.
When alprazolam was compared with placebo for reduction in symptoms all estimates indicated a positive effect for alprazolam. Pooled estimates of efficacy data showed a moderately large continuous mean difference (MD) at the end of trial (‐5.34, 95% CI ‐7.48 to ‐3.20; I2 = 68%). The risk difference (RD) for the dichotomous measure of clinical response (50% improvement) was 0.32 in favour of alprazolam (95% CI 0.22 to 0.42; I2 = 0%), with a number needed to treat to benefit (NNTB) of 3 (95% CI 2 to 5). The RD of all‐cause withdrawals did not differ between alprazolam and placebo.
When depression severity was measured as a continuum the effect of alprazolam did not differ statistically or clinically from the effects of any of the conventional antidepressants combined (MD 0.25, 95% CI ‐0.93 to 1.43; I2 = 55%). However, for dichotomised depression severity, alprazolam had less effect than antidepressants (RR 0.86, 95% CI 0.75 to 0.99; I2 = 37%; RD ‐0.11, 95% CI ‐0.24 to 0.01; I2 = 58%; NNTB 9, 95% CI 4 to 100). The RD of all‐cause withdrawals was ‐0.04 (95% CI ‐0.07 to 0.00; I2 = 35%), in favour of alprazolam.
Authors' conclusions
Alprazolam appears to reduce depressive symptoms more effectively than placebo and as effectively as tricyclic antidepressants. However, the studies included in the review were heterogeneous, of poor quality and only addressed short‐term effects, thus limiting our confidence in the findings. Whilst the rate of all‐cause withdrawals did not appear to differ between alprazolam and placebo, and withdrawals were less frequent in the alprazolam group than in any of the conventional antidepressants combined group, these findings should be interpreted with caution, given the dependency properties of benzodiazepines.
Keywords: Adult, Aged, Humans, Middle Aged, Alprazolam, Alprazolam/therapeutic use, Antidepressive Agents, Antidepressive Agents/therapeutic use, Depression, Depression/drug therapy, Randomized Controlled Trials as Topic
Plain language summary
Alprazolam for depression
Additional options to help those with depression control their mood, besides psychotherapy and antidepressants, can be important, especially when there is also anxiety involved. One of the drug options is alprazolam, a benzodiazepine. We evaluated the effect of alprazolam for depression. The best evidence currently available suggests that alprazolam may be moderately more effective than a placebo, and as effective as conventional antidepressants, in the treatment of major depression. We cannot conclude whether this is due to its specific antidepressant effect or to a non‐specific effect on sleep and anxiety. There were relatively few short‐term side effects. However, the multiple shortcomings of the currently available evidence, including probable sponsorship bias, publication bias, the age of the studies and the heterogeneity of the results, limit confidence in these findings.
Background
Description of the condition
Depression is a broad and heterogeneous diagnostic grouping, central to which is depressed mood or loss of interest or pleasure in most activities. Depressive symptoms are frequently accompanied by symptoms of anxiety, but may also occur on their own. Sleeping problems, lack of energy, eating problems, abnormal feelings of guilt, concentration problems, psychomotor agitation or retardation, and suicidal ideation are other depressive symptoms. Symptoms should be present for at least two weeks or more and every symptom should be present for most of every day (APA 2000). It is doubtful whether the severity of the depressive illness can realistically be captured in a single symptom count. Clinicians will consider family and previous history, as well as the degree of associated disability, in making this assessment.
Description of the intervention
In most countries, the vast majority of patients with major depression are treated in primary care or as outpatients. Specific antidepressant drugs, such as the tricyclics (TCAs) and the selective serotonin reuptake inhibitors (SSRIs), are generally recommended as the primary classes of drugs for these patients, if and when drug treatment is indicated. However, treatment with antidepressants may be difficult in primary care for several reasons: a) antidepressants have a low acceptance and compliance rate and more than 50% of patients who start antidepressant treatment may cease taking their medication (Hansen 2004; Lawrenson 2000); b) depressive symptoms frequently co‐occur with symptoms of stress and anxiety; c) antidepressants have a long latency time of several weeks; and d) depression in primary care or outpatient settings frequently starts with mild symptoms, which are not severe enough to warrant long‐term conventional antidepressant treatment.
Primary care physicians sometimes prescribe brief courses of benzodiazepines to patients with mild to moderate major depression, who represent the majority of their depression caseload (Rijswijk 2007). However, most depression treatment guidelines do not support this indication (Furukawa 2001; NICE 2009; Van Marwijk 2003). Evidence of a specific antidepressant effect of benzodiazepines as a single treatment is inconclusive, although benzodiazepines can have additional effects when combined with antidepressants (Furukawa 2001; NICE 2009). Caution with long‐term psychotropic drugs, as well as with high‐potency tranquillisers, such as alprazolam, may however be a good clinical policy (Committee 1980). Benzodiazepines may lose their efficacy with long‐term administration (Committee 1980).
How the intervention might work
Alprazolam, a triazolo 1,4‐benzodiazepine, is one of the high‐potency benzodiazepines. Early claims were that it combined an anxiolytic effect with a specific and fast‐onset antidepressant effect (Sethy 1982). Alprazolam differs from the classic benzodiazepines by the incorporation of a triazolo ring in the basic molecular structure. The addition of this ring is believed to have provided alprazolam with antidepressant properties. Benzodiazepines bind to a specific area of the GABA‐A benzodiazepine receptor and may modulate transmission of the inhibitory neurotransmitter GABA as agonists, by their allosteric actions facilitating the opening of the receptor's chloride channel.
No independent systematic evaluation of its antidepressant effect has ever been undertaken. In daily practice, a number of patients still use alprazolam. Although as a class benzodiazepines act rapidly and are well tolerated for anxiety, their use presents clinical issues such as dependence, rebound anxiety, memory impairment and discontinuation syndrome (Schweizer 1998). Accident‐proneness, including traffic accidents and falls, are other particularly important considerations (Barbone 1998). These side effects occur early in the course of treatment (Neutel 1996).
Why it is important to do this review
As doubts about the magnitude of the specific antidepressant effect of antidepressants remain, it may be worthwhile to evaluate alternatives (Moncrieff 2004). One non‐systematic review in 1995 showed that benzodiazepines were less effective than conventional antidepressants in treating major depression (Birkenhager 1995). Alprazolam is internationally registered for the treatment of anxiety, panic disorder and anxiety associated with depression (Jonas 1993). However, there is still debate about its efficacy for the treatment of depression alone (Petty 1995). Therefore, a systematic review to evaluate whether alprazolam is a suitable alternative for outpatients with major depression, requiring drug treatment but not wishing to take conventional antidepressants, may generate clinically useful information.
Objectives
To determine the effectiveness, including tolerability and acceptability, of alprazolam as monotherapy for major depression in comparison with placebo and conventional antidepressants in outpatients and patients in primary care.
Methods
Criteria for considering studies for this review
Types of studies
We selected double‐blind randomised controlled trials (RCTs). Double‐blind indicates that both provider and participant are unaware of the exact nature of the intervention or control. We did not apply any language restriction and we included both published and unpublished trials.
Types of participants
Participant characteristics and setting
Trial participants were adults (18 years of age and over), both male and female.
Diagnosis
The primary diagnosis for trial participants was major depression according to the Research Diagnostic Criteria (RDC) (Feighner 1972); Diagnostic and Statistical Manual (DSM III (APA 1980) or DSM IV (APA 1994)); a depressive episode according to the International Classification of Diseases (ICD) (WHO 2003); or if the clinician considered the patient to be depressed and eligible for antidepressant treatment.
Setting
Studies were included if they were conducted in an outpatient or primary care setting. However, studies conducted in mixed inpatient and outpatient settings were included in the review.
Exclusion criteria
We excluded patients with a primary diagnosis of another major psychiatric condition, such as anxiety disorder, or important medical problems.
We excluded studies limited to inpatient populations, as the severity of their depressive symptoms is likely to be considerably higher (Hubain 1990; Lenox 1984). Hubain 1990
Types of interventions
Intervention
At least one of the treatment arms had to include alprazolam as a monotherapy (variable dosages and exposure times). There were no restrictions on dose or duration of treatment. We excluded studies that combined alprazolam with other interventions, such as alprazolam plus forms of psychotherapy.
Control conditions
Alprazolam had to be compared with placebo, conventional antidepressants or both.
Types of outcome measures
Primary outcomes
1) Our primary continuous outcome was the last mean assessment score on a depression severity measure (end of trial): Hamilton Depression Rating Scale (HDRS), or the equivalent Montgomery‐Asberg Depression Rating Scale (MADRS) in an intention‐to‐treat analysis (Hamilton 1960; Montgomery 1979). The primary dichotomous outcome was 'improvement of depression' which was dichotomised as 50% reduction on the initial mean depression severity score (end of trial HDRS or MADRS). We used the HDRS‐based response as the primary outcome measure when multiple measures were reported.
Secondary outcomes
2) Our primary measure of harm was the number of reported drug adverse events and data on tolerability, which were abstracted by collecting 'all‐cause' withdrawals from each treatment group, including the reason attributed for withdrawal from therapy (lack of efficacy and adverse effects).
3) We assessed withdrawals, rebound symptoms and tolerance, which may not have been manifested as a loss of efficacy due to concomitant dose increase.
Search methods for identification of studies
Electronic searches
We identified relevant trials from systematic searches in the following electronic databases: CCDANCTR‐Studies and CCDANCTR‐References (Specialised Registers of the Cochrane Depression, Anxiety and Neurosis Group. For a description see Appendix 1).
We also carried out complementary searches in: PubMed, EMBASE (Elsevier, EMBASE and MEDLINE combined) and PsycINFO.
We conducted searches using a controlled vocabulary of terms related to ALPRAZOLAM, DEPRESSIVE DISORDERS and DEPRESSION (using the APA Thesaurus of Psychological Index Terms in PsycINFO, Medical Subject Headings (MeSH) in PubMed, and EMTREE in EMBASE.com). Search strategies are listed below:
CCDANCTR‐Studies
Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" AND Intervention = Alprazolam
CCDANCTR‐References
Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" AND Free‐text = Alprazolam
PubMed (MEDLINE)
("Depressive Disorder"[mh] OR Depression[mh]) AND "Alprazolam"[mh] AND humans[mh] NOT case reports[pt]
EMBASE.com
'major depression'/exp AND 'alprazolam'/de AND [humans]/lim NOT 'case report'/exp OR 'major depression'/exp AND 'alprazolam'/dd_ae,dd_ct
PsycINFO
DE=("depression emotion" OR "major depression") AND DE="alprazolam"
Searching other resources
Reference lists
We checked the reference lists of selected reviews and published studies. We also searched for additional trials in the reference lists of studies initially identified and by scrutinising other relevant review articles.
Personal communication
We consulted authors of studies included and experts in the field to find out if they know of any relevant published or unpublished RCTs, which had not been identified through the electronic searches. We mailed and emailed four traceable authors, however they were unable to provide any information.
Pharmaceutical companies
We also contacted the company that had developed alprazolam.
Unpublished studies
We searched the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/), Clinical Studies Results (http://www.clinicalstudyresults.org/) and Current Controlled Trials (http://www.controlled‐trials.com/) for ongoing trials on depression.
We searched the four open databases suggested in the Cochrane Handbook for Systematic Reviews of Interventions as suitable for locating grey literature for alprazolam and Xanax. We searched Open Sigle (opensigle.inist.fr), the National Technical Information Service (NTIS), which provides access to the results of both US and non‐US government‐sponsored research (www.ntis.gov), PsycExtra (www.apa.ort/psycextra) and Healthcare Management Information Consortium (HMIC). The large literature databases we used cover conference reports and abstracts published in journals.
Data collection and analysis
Selection of studies
Two review authors independently assessed the abstracts from all the studies potentially eligible for inclusion against relevant study inclusion criteria (FW, HvM). We made decisions about selection of studies through discussion and consensus. Any disagreement was resolved through consultation with an independent third party (GA).
Data extraction and management
Both review authors extracted data independently on the inclusion and exclusion criteria for each study, the dose and regimen of alprazolam and the medication or placebo compared, the number of patients randomised, dropouts, length of follow‐up, age, in or outpatient status, relevant clinical outcomes reported (such as HDRS score) and also noted side effects. Any disagreement about the data extraction process was resolved through discussion and consensus, or through consultation with GA. We used the results of the data extraction mainly to consider the generalisability of study findings (external validity) and to evaluate clinical heterogeneity across trials. We set no minimum quality score for inclusion.
Comparisons
Alprazolam versus placebo
Alprazolam versus tricyclic antidepressants
Alprazolam versus heterocyclic antidepressants
Alprazolam versus SSRIs
Assessment of risk of bias in included studies
We assessed risk of bias for each included study using the Cochrane Collaboration 'Risk of bias' assessment tool (Higgins 2009). We considered the following six domains:
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated treatment adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
Selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias? Additional items included here are therapist qualifications, treatment fidelity and researcher allegiance/conflict of interest.
We provided a description of what was reported to have happened in each study, and made a judgement on the risk of bias for each domain within and across studies, based on the following three categories: low risk of bias, unclear risk of bias and high risk of bias.
Two independent review authors assessed the risk of bias in the selected studies. Any disagreement was discussed with a third review author. Where necessary, we contacted the authors of the studies for further information. All 'Risk of bias' data are presented graphically and described in the text.
Two review authors (FW, (AB) and HvM) independently assessed the methodological quality or internal validity of each trial using the Cochrane Handbook for Systematic Reviews of Interventions criteria (Higgins 2009).
Measures of treatment effect
Standardised mean differences (SMD) are reported for continuous outcomes, together with 95% confidence intervals (CI). The SMD is the difference between the group means divided by the combined standard deviation. We used these to calculate a standard measure of effect for each trial. We calculated the mean difference (MD) where the same outcome scale was used. We defined change in mood at the end of treatment as the outcome of interest. We selected observer‐rated measures in preference to patient‐rated ones as we expected these to be employed most consistently at the time that most alprazolam studies were undertaken. Many different outcome measures are used in depression studies. It is assumed that these all measure an underlying construct which we called mood. We reported both risk ratios (RR) and risk differences (RD) for dichotomous data, as RRs are more precise and RDs allow calculation of numbers needed to treat to benefit (NNTb) and numbers needed to treat to harm (NNTh), with 95% CI for those studies which were statistically significant.
Unit of analysis issues
We expected few problems in this area, as most studies used participants as the unit of analysis. Some studies had multiple treatment groups. We used the relevant data separately in each comparison (alprazolam versus placebo, alprazolam versus other antidepressant). Where more than one active treatment group with the same drug was eligible for inclusion in a comparison, we pooled the groups for comparison against the control group, to avoid including the same group of participants twice in the same meta‐analysis.
Dealing with missing data
We either analysed missing continuous data on an endpoint basis, including only participants with a final assessment, or analysed them using last observation carried forward to the final assessment (LOCF) if LOCF data had been reported by the trial authors.
For dichotomous outcomes, we assigned the worst possible outcome to dropouts (intention‐to‐treat). As many of the studies on the antidepressant effects of alprazolam were published some years ago, it was difficult to recover missing data. To estimate standard deviations (SDs), we used the method described by Furukawa et al (Furukawa 2006). Where data were available in graphic format only, we made an approximation of the mean to assess the outcomes.
Assessment of heterogeneity
We explored inconsistency across studies visually. We also used a Chi2 test, the Q‐statistic, with a P value set at 0.1. Furthermore, we used the I2 statistic, a measure of effect size estimates that is due to heterogeneity rather than sampling error (Higgins 2009), with 30% to 50% representing moderate heterogeneity, 50% to 80% substantial, and 80% to 100% considerable heterogeneity.
Assessment of reporting biases
We addressed publication bias and other reporting biases by means of visual inspection for signs of asymmetry, and generated the funnel plots using Review Manager 5.1 software (RevMan 2011).
Data synthesis
We pooled discrete outcomes (recovered/not recovered) and, where possible, continuous outcomes, using both fixed and random‐effects approaches. Fixed‐effect models assume that the underlying true treatment effect in each trial is the same and that the observed differences are due to chance. Random‐effects models assume the true treatment effects in different trials are randomly placed around some central value and incorporates the within and between‐study variation into the calculation, generating a wider confidence interval if heterogeneity is present, and allowing for an appropriate degree of statistical caution (DerSimonian 1986). The fixed‐effect approach used was the Mantel‐Haenszel‐Peto method which allows the calculation of an estimate known as the 'typical' or pooled odds ratio with a 95% confidence interval (DerSimonian 1986). We chose random‐effects models when there was more than 50% heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned to perform two subgroup analyses for:
speed of recovery; and
alprazolam dosage.
Given that these trial characteristics may have influenced the observed treatment effect, they were planned to identify potential sources of heterogeneity. The use of multiple statistical analyses leads to an increase in the probability of type I errors.
Sensitivity analysis
We planned to perform two sensitivity analyses to determine whether certain methodological decisions made during the review process were robust. We planned to test these decisions by removing studies from the main analysis to investigate the effect of their inclusion. The following two analyses were planned:
to test the inclusion of studies with divergent diagnostic criteria for depression; and
to test the reliance on self reported measures of depression only.
We also conducted a post hoc sensitivity analysis:
to investigate the effects of bias on the results of the meta‐analysis by excluding studies classified as having a high risk of bias.
Results
Description of studies
See: Characteristics of included studies.
Results of the search
Electronic searches
The search of the CCDAN registers yielded 391 references of potentially eligible studies. We excluded papers that were not relevant (mainly because they did not fulfil the inclusion criteria or were non‐randomised studies). We included 21 randomised controlled alprazolam trials. Seven studies used a placebo comparison (n = 771) and 18 used tricyclic or heterocyclic antidepressants (n = 1697). The studies typically lasted four to six weeks.
Reference lists
We found three reviews of alprazolam by checking the reference lists of selected reviews and published studies (Birkenhager 1995; Jonas 1993; Srisurapanont 1997). The findings of these other reviews are summarised below in the Agreements and disagreements with other studies or reviews section. No additional studies were found through checking reference lists.
Personal communication
Few authors of included studies were available for advice on relevant published or unpublished RCTs not identified through electronic searches. We contacted one author (Carl Rickels). No new studies were identified.
Pharmaceutical companies
We contacted the company that developed alprazolam: the Upjohn Company. In 1995, Upjohn merged with Pharmacia AB to form Pharmacia & Upjohn. Today, through a series of mergers, the remainder of Upjohn is owned by Pfizer. The Dutch branch of Pharmacia/Pfizer was unable to provide information as alprazolam is no longer a priority or a marketable drug for them. Alprazolam is generically available.
Unpublished studies
We found no unpublished studies in the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/), in Clinical Studies Results (http://www.clinicalstudyresults.org/) or in Current Controlled Trials (http://www.controlled‐trials.com/). The latter database is where we expected pharmaceutical companies to post their results (although alprazolam is mainly prescribed for anxiety and panic disorders).
We searched the three open databases advised in the Cochrane Handbook for Systematic Reviews of Interventions as suitable for locating grey literature for alprazolam and Xanax, but we found no relevant information for our research question. The fourth database mentioned, the Healthcare Management Information Consortium (HMIC), is only accessible through a license at OVID. Our two universities do not have such a license. The search results were: Open Sigle (opensigle.inist.fr) (two hits, none relevant); the National Technical Information Service (NTIS) which provides access to the results of both US and non‐US government‐sponsored research (www.ntis.gov) (five hits, none relevant); and PsycExtra (www.apa.org/psycextra) (one hit, but again not relevant).
Included studies
Design
Length of the studies
Five studies were four‐week trials (Banerji 1989; Bassi 1990; Cropper 1987; di Perri 1990; Imlah 1985). One study was a five‐week trial (Mendels 1986). Fifteen studies were six‐week trials (Ansseau 1984; Borison 1989; Draper 1983; Fabre 1980; Feighner 1983a; Goldberg 1986; Laakman 1995; Lapierre 1994; Murthy 1991; Overall 1987; Remick 1988; Rickels 1985; Rickels 1987; Rush 1985; Singh 1988).
Sample size
The mean number of participants who entered the studies was 129.2 (SD 111.5), with a minimum sample size of 43 (Lapierre 1994) and a maximum of 504 (Rickels 1985).
Setting
In 19 studies, the participants were outpatients (Ansseau 1984; Banerji 1989; Bassi 1990; Borison 1989; Cropper 1987; Draper 1983; Fabre 1980; Feighner 1983a; Goldberg 1986; Laakman 1995; Lapierre 1994; Mendels 1986; Murthy 1991; Overall 1987; Remick 1985; Remick 1988; Rickels 1985; Rickels 1987; Singh 1988). Two studies included both in‐ and outpatients, and one study failed to provide information about the setting (di Perri 1990; Imlah 1985; Rush 1985).
Participants
Age
Studies were limited to adults and excluded elderly patients (one used 55 years of age as the upper age limit, four used 60, five used 65, eight used 69/70 and one used 75). Two studies did not provide any details on age (Murthy 1991; Remick 1988).
Diagnosis
Most patients were diagnosed with depressive disorder according to explicit diagnostic criteria (16 studies), with added severity criteria. In 14 studies, a Raskin Depression Scale (RDS) score of at least six (Banerji 1989), eight (Ansseau 1984; Draper 1983; di Perri 1990; Fabre 1980; Feighner 1983a; Laakman 1995; Lapierre 1994; Murthy 1991; Rickels 1985; Rickels 1987; Singh 1988) or nine was added (Bassi 1990; Raskin 1970). In 16 studies, a Hamilton Depression Rating Scale (HDRS) score of at least 17 (Draper 1983), 18 (Bassi 1990; Borison 1989; di Perri 1990; Fabre 1980; Feighner 1983a; Goldberg 1986; Laakman 1995; Lapierre 1994; Murthy 1991; Rickels 1985; Rickels 1987; Rush 1985; Singh 1988), 20 (Mendels 1986) or 21 (Remick 1985; Remick 1988) was required. The Covi Anxiety Score (CAS) had to be less than or equal to the RDS score in many studies (Ansseau 1984; Banerji 1989; di Perri 1990; Draper 1983; Feighner 1983a; Goldberg 1986; Lapierre 1994; Mendels 1986; Murthy 1991; Rickels 1985; Rickels 1987; Singh 1988), while anxiety was not addressed in seven studies (Bassi 1990; Borison 1989; Covi 1976; Laakman 1995; Overall 1987; Remick 1988; Rush 1985).
Diagnostic criteria were:
DSM‐III or its predecessors (Bassi 1990; di Perri 1990; Goldberg 1986; Murthy 1991; Rickels 1987); the Feighner Diagnostic Criteria (di Perri 1990; Draper 1983; Feighner 1983a; Goldberg 1986; Mendels 1986; Murthy 1991; Overall 1987; Rickels 1985; Rickels 1987); or the Research Diagnostic Criteria (RDC) (Remick 1985; Remick 1988; Rush 1985);
ICD‐9 (di Perri 1990; Laakman 1995; Singh 1988); or
unspecified criteria (Ansseau 1984; Banerji 1989; Cropper 1987; Imlah 1985; Lapierre 1994).
Two studies included patients with anxiety: mixed symptoms of anxiety and depression, and neurotic depression with or without anxiety (Cropper 1987; Imlah 1985).
Interventions
Eight studies included a placebo arm (Borison 1989; Fabre 1980;Feighner 1983a; Imlah 1985; Laakman 1995; Mendels 1986; Rickels 1985; Rickels 1987). Three studies presented a comparison between four arms (alprazolam‐amitriptyline‐lorazepam‐placebo, alprazolam‐amitriptyline‐doxepin‐placebo and alprazolam‐imipramine‐diazepam‐placebo, respectively (Laakman 1995; Rickels 1985; Rickels 1987). One study presented a comparison between three arms (alprazolam‐imipramine‐placebo) (Mendels 1986). In seven studies, alprazolam was compared with amitriptyline (Banerji 1989; Imlah 1985; Laakman 1995; Lapierre 1994; di Perri 1990; Rickels 1985; Rush 1985; Singh 1988). In six studies it was compared with imipramine (Feighner 1983a; Goldberg 1986; Mendels 1986; Murthy 1991; Overall 1987; Rickels 1987). In five separate studies it was compared with other heterocyclic antidepressants ('other TCAs'): mianserin (Bassi 1990), dothiepin (Cropper 1987), desipramine (Remick 1985; Remick 1988) or doxepin (Rickels 1985).
Dosage of study drugs
The maximum alprazolam dose allowed was within the recommended therapeutic range for anxiety (1.5 to 8 mg; Bandelow 2008) in all studies. The mean alprazolam dose (2.9 mg; SD 0.7) was also within the recommended therapeutic range in all studies, although it was not reported in one study (Imlah 1985). Doses therefore did not seem to be extraordinarily high. Drugs in the control groups were within the recommended therapeutic range, although there was considerable variation in the mean dose between control groups. One further option is to classify mean dosages for the purposes of subgroup analyses in the first revision.
Outcomes
For the continuous outcomes, the HDRS was used in all but two studies (Cropper 1987; Imlah 1985). Dichotomous outcomes, a 50% reduction of the initial depression score, were reported in six studies (di Perri 1990; Laakman 1995; Lapierre 1994; Mendels 1986; Rickels 1987; Rush 1985). All studies reported all‐cause withdrawals, but withdrawals due to adverse effects and ineffectiveness were not specified in five studies (Feighner 1983a; Lapierre 1994; Murthy 1991; Rickels 1987; Rush 1985) and 10 studies respectively (Cropper 1987; di Perri 1990; Feighner 1983a; Goldberg 1986; Imlah 1985; Lapierre 1994; Mendels 1986; Murthy 1991; Rickels 1987; Rush 1985).
Sponsorship
Seven studies were clearly supported by the manufacturer of alprazolam (Cropper 1987; Goldberg 1986; Imlah 1985; Remick 1988; Rickels 1985; Rickels 1987; Rush 1985) and although the other studies did not offer any disclosures in the text, they were remarkably similar in methodology, suggesting sponsorship by the manufacturer of alprazolam in all studies.
Risk of bias in included studies
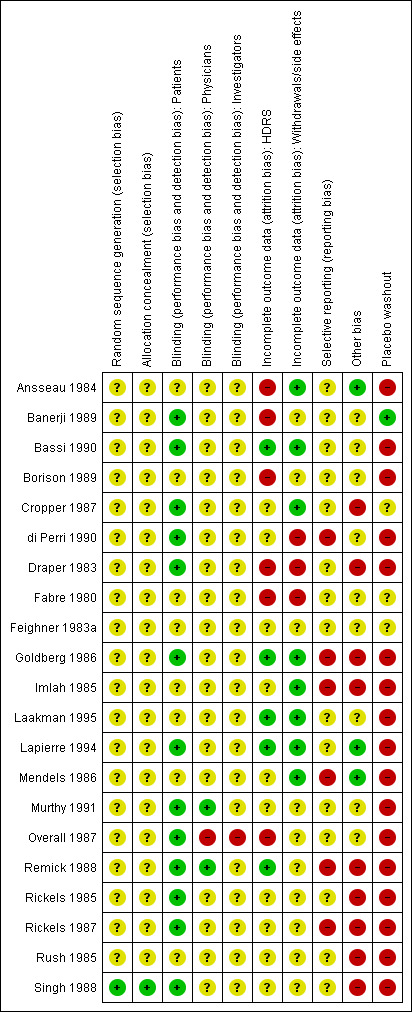
See Figure 1 and Figure 2 for a graphical summary of the methodological quality for the 22 included studies. Most of the studies were older, and many of the recent developments to enhance the quality of reporting of clinical trials such as the requirement of a CONSORT statement did not apply at that time. On the basis of the assessments, we considered six studies to have a high risk of bias (Ansseau 1984; Banerji 1989; Borison 1989; Draper 1983; Fabre 1980; Overall 1987). One study we presumed to be a duplicate of another study by the same authors and so we considered it a secondary reference to that study (Remick 1985).
1.
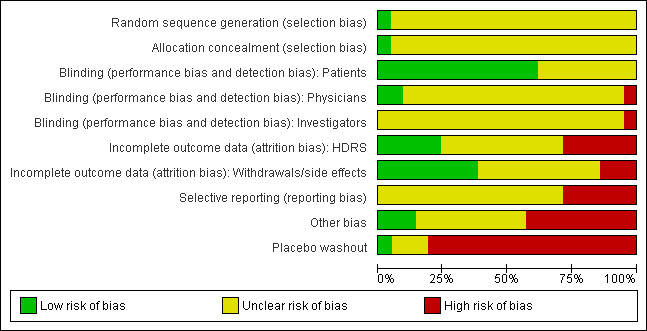
2.
Allocation
All studies were reported to be randomised trials, however only one study reported sufficient details on allocation, and used a computer‐generated randomisation list (Singh 1988).
Blinding
All studies were reported to be double‐blind trials, however none of the studies reported sufficient details on blinding. The best study described that weekly assessments were completed by the research psychiatrist (Remick 1988), but did not further describe the blinding procedures. Independent outcome assessment was a rarity and was scored unclearly at best in most studies.
Incomplete outcome data
To have adequately addressed incomplete outcome data, studies had to demonstrate that an intention‐to‐treat (ITT) analysis was performed based on all persons randomised, or that attrition was balanced in numbers with similar reasons for dropout across treatment groups, or that outcome data were complete. Incomplete outcome data were judged to have been adequately addressed in eight studies.
Selective reporting
We only included trials in which the primary outcome was severity of depressive symptoms. However, trial investigators may have used still other depression rating scales, and only reported data from the scale that showed a positive effect. Investigators may have also selectively reported outcomes at the time point(s) at which the largest effect was found. Selective reporting was difficult to assess as few trials had pre‐published study protocols.
Other potential sources of bias
Over half of the included studies were explicitly supported by the manufacturer of alprazolam. Another potential source of bias is the placebo washout phase that all studies bar two used before entry (Banerji 1989; Cropper 1987).
Effects of interventions
1. Alprazolam versus placebo
Primary outcome
1.1 Hamilton Depression Rating Scale (HDRS) end of trial
Alprazolam produced a moderately better effect than placebo, based on data from seven studies and 771 persons. For continuous depression severity, the mean difference (MD) was ‐5.34 (95% confidence interval (CI) ‐7.48 to ‐3.20; I2 = 68%), which was higher than the UK National Institute for Clinical Excellence (NICE) cut‐off of three as being clinically meaningful. When applying a sensitivity analysis to exclude the two studies of low quality, with 131 participants, the MD changed slightly to ‐6.22 (95% CI ‐7.42 to ‐5.02; I2 = 23%; fixed‐effect model). For depression severity, dichotomised as a 50% reduction in the initial mean depression severity score, the risk ratio (RR) was 2.47 (95% CI 1.78 to 3.43; I2 = 0%; fixed‐effect model), but only three studies with 312 participants, none of them high‐risk, were available for this comparison. The risk difference (RD) was 0.32 (95% CI 0.22 to 0.42; I2 = 0%; fixed‐effect model) and the number needed to treat to benefit (NNTB) was 3 (95% CI 2 to 5).
Secondary outcomes
1.2 Tolerability
Tolerability was expressed as all‐cause withdrawals, based on data from four studies and 640 participants. The RR of all‐cause withdrawals was 0.78 (95% CI 0.48 to 1.27; I2 = 76 %; random‐effects model) for alprazolam versus placebo, indicating that alprazolam did not result in significantly more all‐cause withdrawals than placebo. Without two high‐risk studies , this was 0.68 (95% CI 0.37 to 1.26; I2 = 72%; random‐effects model). The RD was ‐0.09 (95% CI ‐0.26 to 0.08 ; I2 = 87 %; random‐effects model); without the high‐risk studies it was ‐0.11 (95% CI ‐0.30 to 0.09; I2 = 87%; random‐effects model). The number needed to treat to harm (NNTH) was 11 (95% CI 4 to 13 ). Drowsiness, dry mouth and dizziness were more common among alprazolam users than placebo users.
1.2 Adverse effects
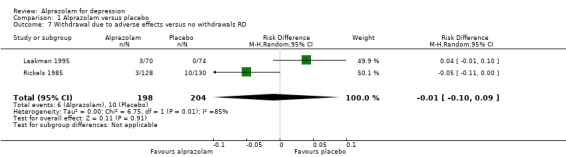
For alprazolam versus placebo, the RR of withdrawals due to adverse effects was 1.14 (95% CI 0.05 to 26.35; I2 = 75%; random‐effects model), based on data from two studies with 402 participants. The RD was ‐0.01 (95% CI ‐0.10 to 0.09; I2 = 85%; random‐effects model). The NNTH was 25 (95% CI ‐8 to 58).
1.3 Lack of efficacy
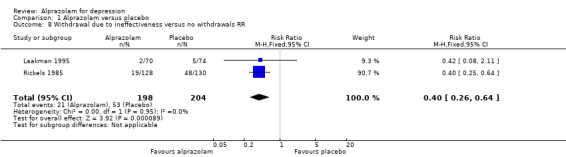
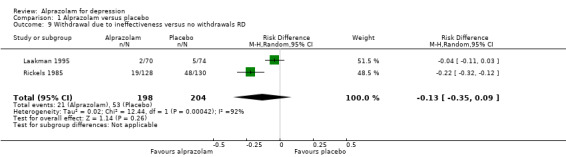
For alprazolam versus placebo, the RR of withdrawals due to ineffectiveness was 0.40 (95% CI 0.26 to 0.64; I2 = 0%; fixed‐effect model) favouring alprazolam over placebo, with a RD of ‐0.13 (95% CI ‐0.35 to 0.09; I2 = 92%; random‐effects model) and a NNTH of 8 (95% CI 3 to 11), in two studies with 402 participants.
Subgroup analysis
Speed of recovery
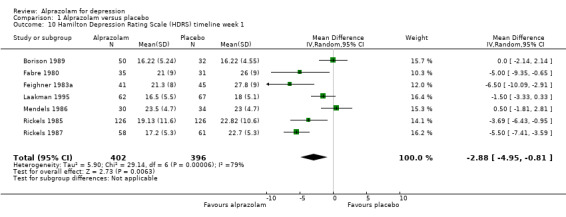
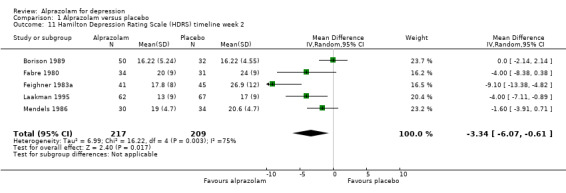
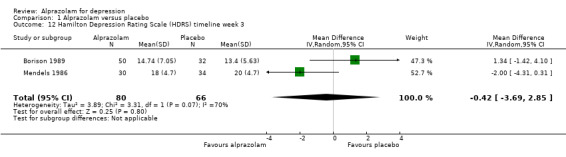
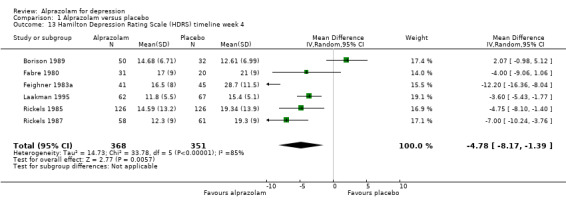
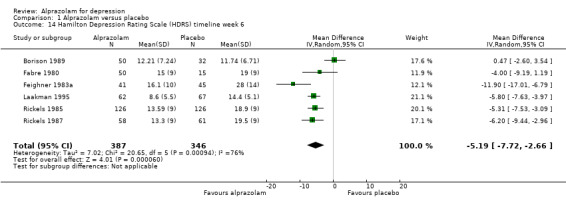
For alprazolam versus placebo, the following MD pattern for depression severity emerged, favouring alprazolam over placebo at all time points but three weeks: ‐2.88 (95% CI ‐4.95 to ‐0.81; I2 = 79%) at one week; ‐3.34 (95% CI ‐6.07 to ‐0.61; I2 = 75%) at two weeks; ‐0.42 (95% CI ‐3.69 to 2.85; I2 = 70%) at three weeks; ‐4.78 (95% CI ‐8.17 to ‐1.39; I2 = 85%) at four weeks; and ‐5.19 (95% CI ‐7.72 to ‐2.66; I2 = 76%) at six weeks. Without the high‐risk studies, these results were MD ‐3.20 (95% CI ‐5.66 to ‐0.74; I2 = 82%); ‐4.55 (95% CI ‐8.48 to ‐0.63; I2 = 78%); ‐2.00 (95% CI ‐4.31 to 0.31; single study: no I2 estimate possible); ‐6.58 (95% CI ‐9.96 to ‐3.20; I2 = 80%); and ‐6.07 (95% CI ‐7.33 to ‐4.82; I2 = 46%). All models but the last one were random‐effects.
2. Alprazolam versus tricyclic antidepressants
Primary outcome
2.1 Hamilton Depression Rating Scale (HDRS) end of trial
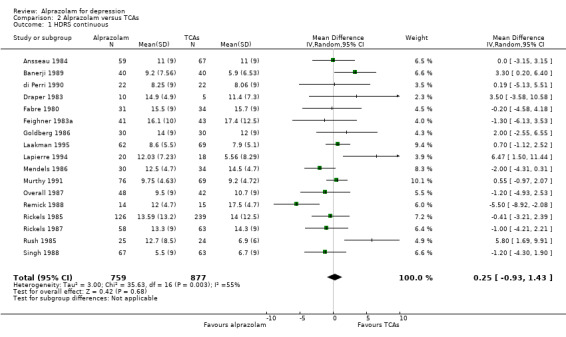
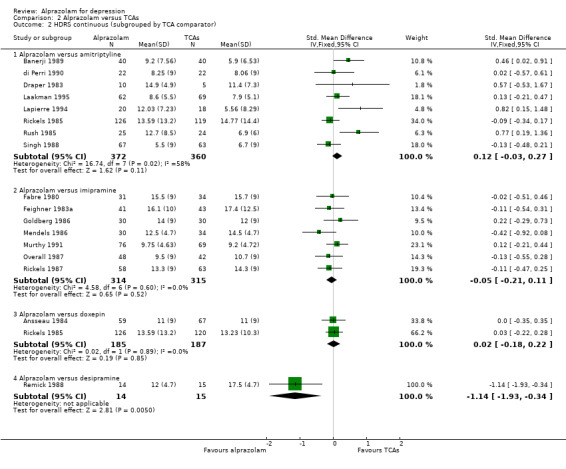
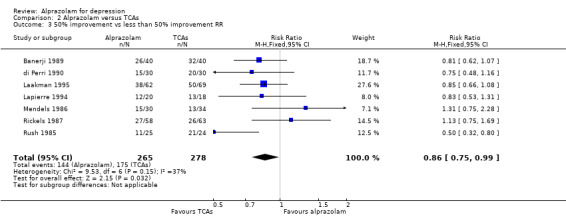
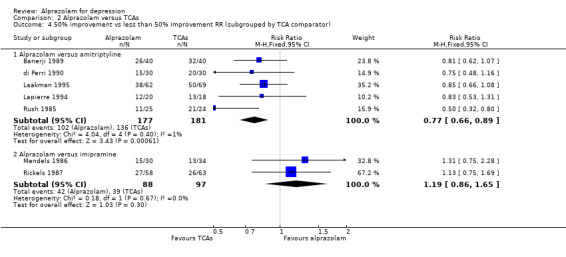
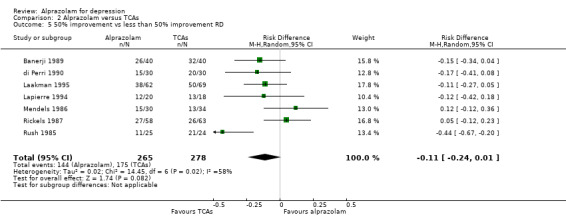
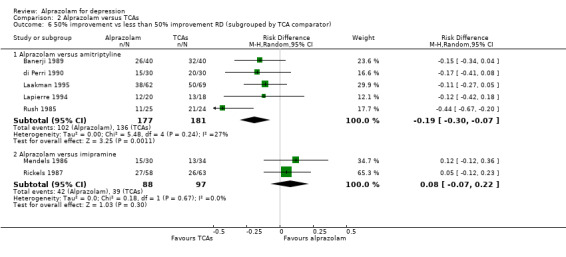
The effect of alprazolam did not differ from the effects of all tricyclic antidepressants (TCAs) combined, based on 17 studies and 1636 participants. For continuous depression severity, the pooled mean difference (MD) was 0.25 (95% CI ‐0.93 to 1.43; I2 = 55%; random‐effects model), which was similar to the estimate without the five studies with a high risk of bias: MD 0.06 (95% CI ‐1.40 to 1.52 ; I2 = 63%; random‐effects model). For 50% reduction in the initial mean depression severity score, the RR was 0.86 (95% CI 0.75 to 0.99; I2 = 37%; fixed‐effect model), which was available for 543 participants in seven studies. Without the one high‐risk study, this was 0.87 (95% CI 0.74 to 1.02; I2 = 47%; fixed‐effect model). The RD was ‐0.11 (95% CI ‐0.24 to 0.01; I2 = 58%; random‐effects model); without the one high‐risk study it remained ‐0.11 (95% CI ‐0.26 to 0.04; I2 = 65%, random‐effects model). The NNTB on the basis of the RD was 9 (95% CI 4 to 100). All TCA subgroups gave similar HDRS estimates (amitriptyline, imipramine, doxepin) to alprazolam, except for one study with desipramine, which did worse (‐1.14, 95% CI ‐1.93 to ‐0.34). Amitriptyline produced a better dichotomous HDRS outcome than alprazolam with a RR of 0.77 (95% CI 0.66 to 0.89; I2 = 1%; fixed‐effect model) and a RD of ‐0.19 (95% CI ‐0.30 to ‐0.07; I2 = 27%; fixed‐effect model).
Secondary outcomes
2.2 Tolerability
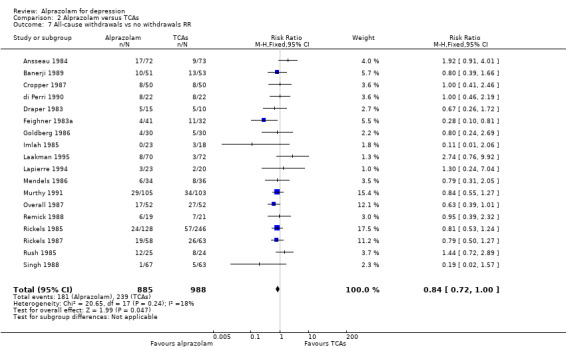
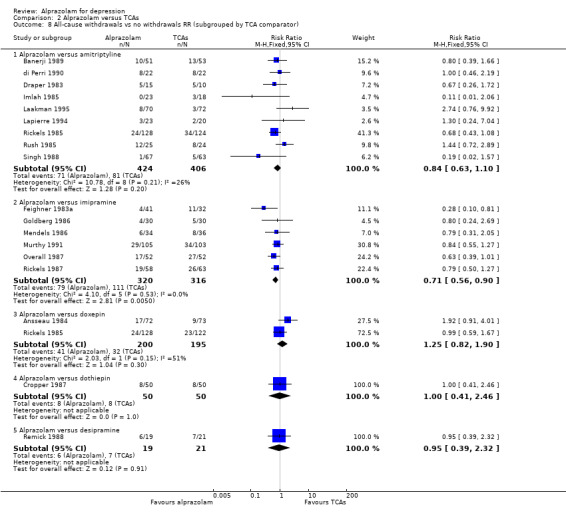
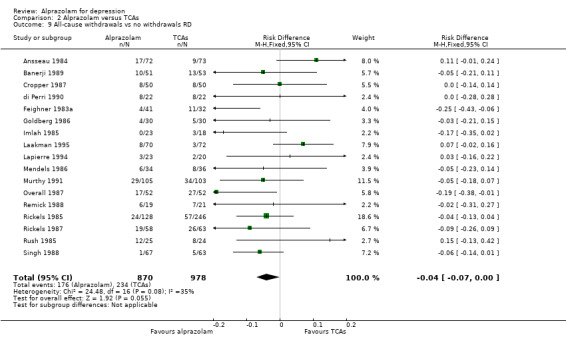
For alprazolam versus all TCAs, based on data from 18 studies and 1873 participants, the RR for all‐cause withdrawals was 0.84 (95% CI 0.72 to 1.00; I2 = 18%; fixed‐effect model), indicating that alprazolam was better tolerated than the group of TCAs as a whole. Without the four high‐risk studies with 378 participants, the RR was 0.83 (95% CI 0.69 to 1.01; I2 = 9%; fixed‐effect model). The RD was ‐0.04 (95% ‐0.07 to 0.00; I2 = 35%; fixed‐effect model), while without the three high‐risk studies it was still ‐0.04 (95% CI ‐0.08 to 0.00; I2 = 20%; fixed‐effect model). The NNTH was 25 (95% CI 14 to 100). The tolerability of alprazolam did not differ from that of most TCAs, but imipramine had more all‐cause withdrawals (RR 0.71, 95% CI 0.56 to 0.90, I2 = 0%; RD ‐0.10, 95% CI ‐0.17 to ‐0.03; I2 = 0%).
2.3 Adverse effects
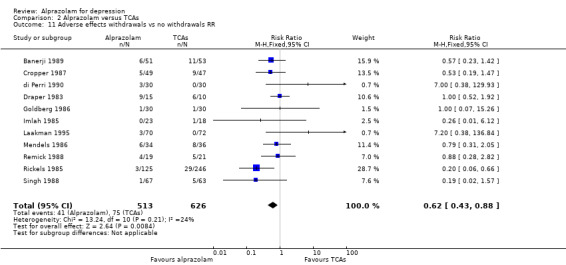
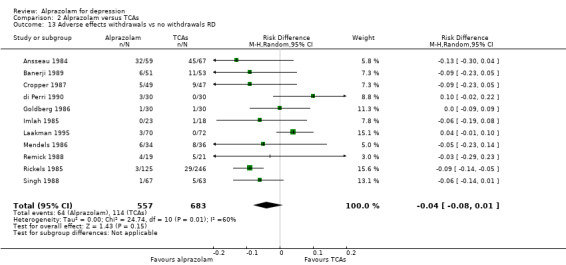
For alprazolam versus all TCAs, the RR of withdrawals due to adverse effects was 0.62 (95% CI 0.43 to 0.88; I2 = 24%; fixed‐effect model), in favour of alprazolam, based on 11 studies with 1139 participants. Without the high‐risk studies, the RR was 0.57 (95% CI 0.37 to 0.90; I2 = 28%; fixed‐effect model). However, the RD was only ‐0.04 (95% CI ‐0.08 to 0.01; I2 =60%; random‐effects model) and without the high‐risk studies ‐0.03 (95% CI ‐0.08 to 0.03; I2 = 62%; random‐effects model). The NNTH was 25 (95% CI 11 to 100). Alprazolam had fewer withdrawals due to adverse effects than amitriptyline with a RR of 0.58 (95% CI 0.37 to 0.90, I2 = 55%) but with a RD of ‐0.03 (95% CI ‐0.10 to 0.04, I2 = 77%) and doxepin (RR 0.24, 95% CI 0.07 to 0.82; RD ‐0.08, 95% CI ‐0.14 to ‐0.03).
2.4 Lack of efficacy
For alprazolam versus all TCAs, the RR of withdrawals due to lack of efficacy was 1.66 (95% CI 0.99 to 2.79; I2 = 0%; fixed‐effect model), with a RD of 0.02 (95% CI ‐0.04 to 0.08; I2 = 67%; random‐effects model) and a NNTH of 50 (95% CI 25 to 13) in four studies with 686 participants. It had more ineffectiveness withdrawals than doxepin (RR 2.26, 95% CI 1.03 to 4.98; RD 0.08, 95% CI 0.01 to 0.16).
Subgroup analysis
Speed of recovery
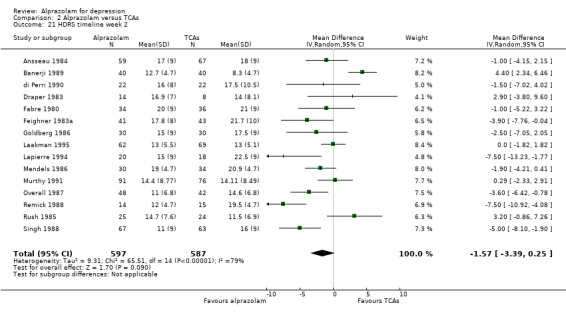
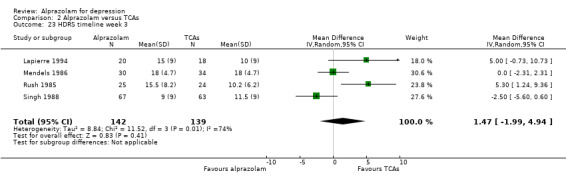
The MD for depression severity for alprazolam versus all TCAs was ‐1.48 (95% CI ‐2.77 to ‐0.18; I2 = 73%) at one week; ‐1.57 (95% CI ‐3.39 to 0.25; I2 = 79%) at two weeks; 1.47 (95% CI ‐1.99 to 4.94; I2 = 74%) at three weeks; 0.25 (95% CI ‐1.20 to 1.70; I2 = 64%) at four weeks; 0.83 (95% CI ‐5.13 to 6.80; I2 = 85%) at five weeks and 0.24 (95% CI ‐1.06 to 1.54; I2 = 54%) at six weeks. At two weeks, the difference lost significance. Without the high‐risk studies, the difference lost significance at three weeks: MD ‐2.04 (95% CI ‐3.30 to ‐0.77; I2 = 64%); ‐2.42 (95% CI ‐4.39 to ‐0.46; I2 = 72%); 1.47 (95% CI ‐1.99 to 4.94 ; I2 = 74%); ‐0.13 (95% CI ‐1.81 to 2.08; I2 = 70%); 0.83 (95% CI ‐5.13 to 6.08; I2 = 85%) and 0.33 (95% CI ‐1.32 to 1.99; I2 = 66%). All models were random‐effects.
3. Alprazolam versus heterocyclic antidepressants
Primary outcome
3.1 Hamilton Depression Rating Scale (HDRS) end of trial
The effect of alprazolam did not differ from the effects of mianserin, based on one study and 61 participants: the MD was ‐2.50 (95% CI ‐7.02 to 2.02).
Secondary outcomes
3.2 Tolerability
The RR of all‐cause withdrawals for alprazolam versus mianserin, based on data from one study and 61 participants, was 0.24 (95% CI 0.03 to 2.04), indicating that alprazolam did not result in significantly more all‐cause withdrawals than mianserin. The RD was ‐0.10 (95% CI ‐0.24 to 0.04).
3.3 Adverse effects
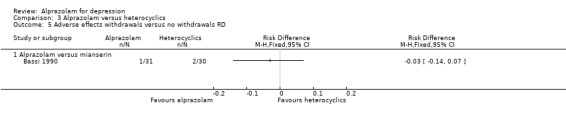
For alprazolam versus mianserin, the RR of withdrawals due to adverse effects was 0.48 (95% CI 0.05 to 5.06), with a RD of ‐0.03 (95% CI ‐0.14 to 0.07).
3.4 Lack of efficacy
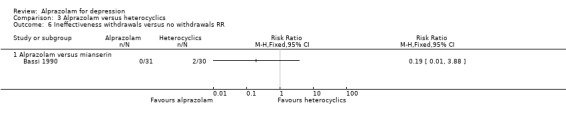
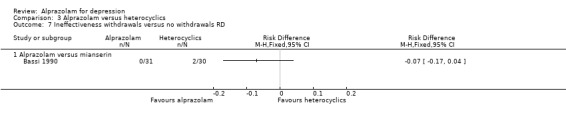
For alprazolam versus mianserin, the RR of withdrawals due to ineffectiveness was 0.19 (95% CI 0.01 to 3.88), with a RD of ‐0.07 (95% CI ‐0.17 to 0.04).
4. Alprazolam versus SSRIs
We found no trials that assessed the effects of alprazolam versus SSRIs.
Sensitivity analysis
Two of the three planned sensitivity analyses were not considered useful post hoc as all but one study used formal diagnostic criteria (see 'Types of participants' 'Diagnosis'). We were also able to use a physician‐rated outcome for all studies and, therefore, no self reports were required. We added two other sensitivity analyses:
To test the effect of including trials with imputed SD values in three of the seven studies of alprazolam versus placebo, we excluded these, but this did not change the MD much (MD ‐5.35, 95% CI ‐9.29 to ‐1.40; I2 = 83%). Alprazolam did worse than all TCAs combined after we excluded eight studies with imputed SDs, as the MD point estimate became 1.44 (95% CI ‐0.05 to 2.93; I2 = 50%; random‐effects model).
To test for the effect of including sub‐samples of inpatients, we excluded a study on alprazolam versus all TCAs with 17 inpatients of 49 patients available at three weeks who perhaps had a more severe depression (Rush 1985). This did not alter the MD point estimate (‐0.06, 95% CI ‐1.16 to 1.05; I2 = 47%; fixed‐effect model). The other study with some inpatients did not report a usable primary outcome, therefore this study did not affect any of the effect estimates. We could not include it in a sensitivity analysis (Imlah 1985).
Discussion
Summary of main results
Alprazolam versus placebo
At the end of trial, alprazolam was more effective than placebo, based on the mean difference (MD) of ‐5.34 (95% confidence interval (CI) ‐7.48 to ‐3.20; I2 = 68%) of reduction in symptoms on the Hamilton Depression Rating Scale (HDRS). The high heterogeneity found for this important comparison indicates that the positive effects of alprazolam in treating depression are to some extent provisional and should be weighed against the potential adverse effects of the medication. However, several studies were of low quality and when applying a sensitivity analysis the results changed to a slightly higher MD of ‐6.22 (95% CI ‐7.42 to ‐5.02), but with less heterogeneity (I2 = 23%).
Based on the dichotomous 50% reduction in the initial mean depression severity scores, alprazolam was also more effective than placebo, with a risk ratio (RR) of 2.47 (95% CI 1.78 to 3.43; I2 = 0%) and risk difference (RD) of 0.32 (95% CI 0.22 to 0.42; I2 = 0%). There was less heterogeneity in this comparison.
Tolerability was expressed as all‐cause withdrawals, with a RR of 0.78 (95% CI 0.48 to 1.27; I2 = 76 %), indicating that alprazolam did not have significantly fewer all‐cause withdrawals than placebo. Leaving the high‐risk studies out did not reduce heterogeneity substantially (I2 = 72%).
Alprazolam versus tricyclic antidepressants
Alprazolam was as effective as its tricyclic comparators. Based on 17 studies and 1636 participants, the pooled mean difference (MD) for depression severity was 0.25 (95% CI ‐0.93 to 1.43; I2 = 55%), which was similar to the estimate without studies with a high risk of bias: MD 0.06 (95% CI ‐1.40 to 1.52; I2 = 63%). However, based on the dichotomous 50% reduction on the initial mean depression severity scores, and considerably fewer studies, alprazolam was less effective than the tricyclics with a RR of 0.86 (95% CI 0.75 to 0.99; I2 = 37%). There was some evidence that alprazolam produces a response faster than placebo and than tricyclic antidepressants (TCAs). The RR of all‐cause withdrawals was 0.84 (95% CI 0.72 to 1.00; I2 = 18%) for alprazolam versus the tricyclics, indicating that alprazolam had significantly fewer all‐cause withdrawals. Leaving the high‐risk studies out further reduced heterogeneity (I2 = 9%) in this comparison.
Alprazolam versus heterocyclic antidepressants
The effects of alprazolam did not differ from those of mianserin in any comparison.
Overall completeness and applicability of evidence
Most of the included studies are older trials; comparisons with newer antidepressants would have been informative, but these were not found. We doubt whether there will be many new studies on the subject but in a future update, we will assess anxiety as a secondary outcome as alprazolam’s effect on depression may be due to underlying anxiety. Undiagnosed anxiety disorders or substantial sub‐threshold anxiety symptoms may have placed the benzodiazepine at an advantage. We will also add inpatient studies and a severity subgroup analysis as it may exert its effect on depression through non‐specific sedative effects on improved sleep and reduced agitation, particularly for mild to moderate depression. One of the key limitations of the review is that the crucial issue of dependence on benzodiazepines (and the allied problems of tolerance, dose escalation and difficult withdrawal) cannot be captured by the present methodology. Indeed, it is even possible when using the conventional methodology, including all‐cause withdrawals as the main measure of tolerability, that a drug which causes dependence and is difficult to withdraw from may inevitably be associated with fewer withdrawals than one that does not. After long‐term treatment with benzodiazepines (e.g. over two to eight months), dependency may occur in a substantial number of patients (Bandelow 2008; Rickels 1990; Schweizer 1990), especially in predisposed persons with, for instance, an alcohol problem.
Quality of the evidence
There is ample room for methodological improvement as Figure 1 and Figure 2 show: important quality criteria such as allocation concealment and adequate randomisation procedures were largely absent. Very few studies also used independent outcome assessments: doctors typically assessed subjects themselves. Another potentially worrying issue is publication bias as was demonstrated in other antidepressant studies (Kirsch 2008). The role of the sponsor in the presentation of results could have been large. Many studies, for instance, used exactly the same set of instruments, and it is not clear what results or studies have not been published. Psychotropic drug studies frequently have methodological problems that tend to weaken the contrast between the drug and placebo, and inflate claimed effects (Van Marwijk 2006). To give two examples: many studies used a placebo washout period. This design feature severely limits the ability to generate accurate estimates of the placebo response rate (Fournier 2010). Because early placebo responders are removed from the trial before they can contribute data, the true rate of placebo response may be underestimated in trials that use this feature. Another example is the difficulty of blinding: subjects quickly experience alprazolam's sedative effects. Most of the studies were performed before the publication and implementation of current quality criteria for conducting and reporting randomised controlled clinical trials (CONSORT).
Potential biases in the review process
Although this review has several strengths, such as a pre‐published protocol, an experienced librarian who performed thorough searches, two authors to select studies/data extract/assess risk of bias and a third to resolve disputes, there were also post hoc decisions. The management of antidepressant classes was one. We would have also liked to analyse the effects of alprazolam dosage and the loss of efficacy due to concomitant dose increase but this was not possible due to insufficient data. The review authors have the impression that all studies were in some way sponsored by the pharmaceutical company that manufactured alprazolam. Publication bias cannot be excluded.
Agreements and disagreements with other studies or reviews
There are no related non‐Cochrane systematic reviews, however we did identify three literature reviews (Birkenhager 1995; Jonas 1993; Srisurapanont 1997). In Birkenhager 1995 alprazolam was found to be effective in mild to moderate depression, but inferior to TCAs in patients with endogenous or melancholic depression. It may not cause amelioration of core symptoms. In Jonas 1993 alprazolam demonstrated efficacy for depression, equal in efficacy to comparison agents. Medical events were reported infrequently or not at all for alprazolam and the comparator drugs; there were no marked differences between drug classes. In Srisurapanont 1997, the antidepressant effect of alprazolam was comparable to that of low‐dose TCAs, but the lack of long‐term treatment studies makes the issue of alprazolam's benefits and disadvantages still undetermined. The results of these three reviews are therefore consistent with our findings here.
Authors' conclusions
Implications for practice.
The best evidence currently available suggests that alprazolam may be moderately more effective than a placebo and as effective as conventional antidepressants in the treatment of major depression. We cannot conclude whether this is due to its specifically antidepressant effect or rather to its non‐specific effect on sleep and anxiety. There were relatively few short‐term side effects. However, the multiple shortcomings of the evidence, including probable sponsorship bias, publication bias, age of the studies and heterogeneity of the results, lessen our confidence in the estimates of its effectiveness based on the currently available evidence. The negative effects of benzodiazepine treatment, such as dependence and withdrawal reactions, cast further doubt on the risk‐benefit ratio of the use of alprazolam as an antidepressant. It is also likely that some participants had undiagnosed anxiety disorders or substantial sub‐threshold anxiety symptoms, which may have placed the benzodiazepine at an advantage.
Implications for research.
We found no studies that compared alprazolam to newer antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), which would have been informative. SSRIs are as effective as other antidepressants but may have a more favourable risk–benefit ratio (NICE 2009; Van Marwijk 2003), however, the included studies compared alprazolam to antidepressants which may no longer be used as first‐line treatment for depression. In view of the remarkable similarity of the design of nearly all studies, we have the distinct impression that the manufacturer of alprazolam funded all of them. An independently funded study comparing the effects of alprazolam to placebo or to SSRIs would, therefore, be desirable. Claims of the clinical utility of benzodiazepines would best be tested using a cost‐benefit analysis. All studies looked at short‐term effects, but many of the potential side effects of benzodiazepines, except accident‐proneness, are to be expected in the longer term. Research into the effects of alprazolam in different patient subgroups (the elderly and severely depressed) should be conducted. Investigation of the contribution of possible methodological sources of heterogeneity observed in this study, such as different medication doses, is also warranted. An interesting possibility for future studies would be to evaluate core items on the Hamilton Depression Rating Scale (HDRS) (for example, excluding sleep) to consider non‐specific effects.
Acknowledgements
We thank the reviewers of the protocol for their detailed feedback.
Appendices
Appendix 1. CCDANCTR
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 25,300 reports of trials in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Co‐ordinator for further details.
Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE, EMBASE and PsycINFO; quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization’s trials portal (ICTRP) (http://apps.who.int/trialsearch/), drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN’s generic search strategies can be found in the ‘Specialised Register’ section of the Cochrane Depression, Anxiety and Neurosis Group’s module text.
Data and analyses
Comparison 1. Alprazolam versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hamilton Depression Rating Scale (HDRS) continuous | 7 | 771 | Mean Difference (IV, Random, 95% CI) | ‐5.34 [‐7.48, ‐3.20] |
| 2 50% improvement versus less than 50% improvement RR | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.78, 3.43] |
| 3 50% improvement versus less than 50% improvement RD | 3 | 312 | Risk Difference (M‐H, Fixed, 95% CI) | 0.32 [0.22, 0.42] |
| 4 All‐cause withdrawals versus no withdrawals RR | 5 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.48, 1.27] |
| 5 All‐cause withdrawals versus no withdrawals RD | 5 | 742 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.26, 0.08] |
| 6 Withdrawal due to adverse effects versus no withdrawals RR | 2 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.05, 26.35] |
| 7 Withdrawal due to adverse effects versus no withdrawals RD | 2 | 402 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 8 Withdrawal due to ineffectiveness versus no withdrawals RR | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.26, 0.64] |
| 9 Withdrawal due to ineffectiveness versus no withdrawals RD | 2 | 402 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.35, 0.09] |
| 10 Hamilton Depression Rating Scale (HDRS) timeline week 1 | 7 | 798 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐4.95, ‐0.81] |
| 11 Hamilton Depression Rating Scale (HDRS) timeline week 2 | 5 | 426 | Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐6.07, ‐0.61] |
| 12 Hamilton Depression Rating Scale (HDRS) timeline week 3 | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐3.69, 2.85] |
| 13 Hamilton Depression Rating Scale (HDRS) timeline week 4 | 6 | 719 | Mean Difference (IV, Random, 95% CI) | ‐4.78 [‐8.17, ‐1.39] |
| 14 Hamilton Depression Rating Scale (HDRS) timeline week 6 | 6 | 733 | Mean Difference (IV, Random, 95% CI) | ‐5.19 [‐7.72, ‐2.66] |
1.1. Analysis.
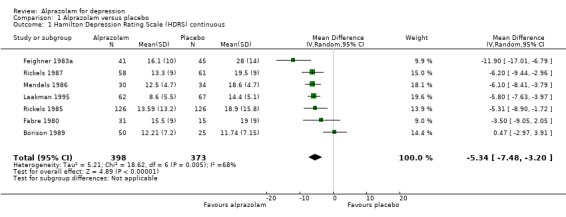
Comparison 1 Alprazolam versus placebo, Outcome 1 Hamilton Depression Rating Scale (HDRS) continuous.
1.2. Analysis.
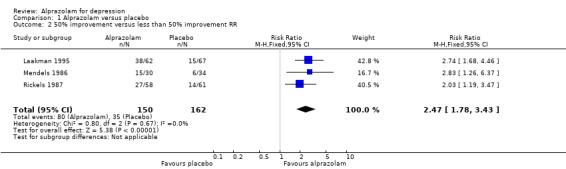
Comparison 1 Alprazolam versus placebo, Outcome 2 50% improvement versus less than 50% improvement RR.
1.3. Analysis.
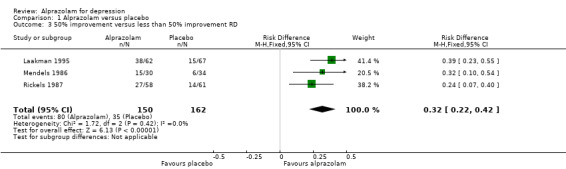
Comparison 1 Alprazolam versus placebo, Outcome 3 50% improvement versus less than 50% improvement RD.
1.4. Analysis.
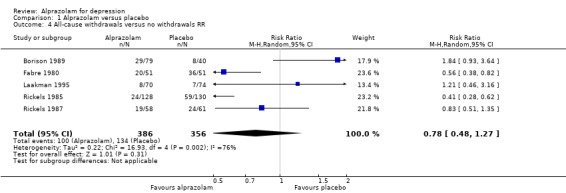
Comparison 1 Alprazolam versus placebo, Outcome 4 All‐cause withdrawals versus no withdrawals RR.
1.5. Analysis.
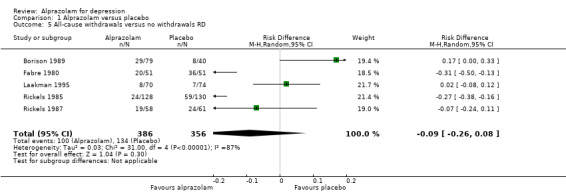
Comparison 1 Alprazolam versus placebo, Outcome 5 All‐cause withdrawals versus no withdrawals RD.
1.6. Analysis.
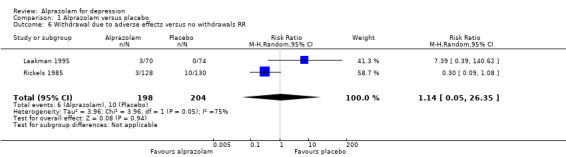
Comparison 1 Alprazolam versus placebo, Outcome 6 Withdrawal due to adverse effects versus no withdrawals RR.
1.7. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 7 Withdrawal due to adverse effects versus no withdrawals RD.
1.8. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 8 Withdrawal due to ineffectiveness versus no withdrawals RR.
1.9. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 9 Withdrawal due to ineffectiveness versus no withdrawals RD.
1.10. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 10 Hamilton Depression Rating Scale (HDRS) timeline week 1.
1.11. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 11 Hamilton Depression Rating Scale (HDRS) timeline week 2.
1.12. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 12 Hamilton Depression Rating Scale (HDRS) timeline week 3.
1.13. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 13 Hamilton Depression Rating Scale (HDRS) timeline week 4.
1.14. Analysis.
Comparison 1 Alprazolam versus placebo, Outcome 14 Hamilton Depression Rating Scale (HDRS) timeline week 6.
Comparison 2. Alprazolam versus TCAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HDRS continuous | 17 | 1636 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.93, 1.43] |
| 2 HDRS continuous (subgrouped by TCA comparator) | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alprazolam versus amitriptyline | 8 | 732 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.03, 0.27] |
| 2.2 Alprazolam versus imipramine | 7 | 629 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 2.3 Alprazolam versus doxepin | 2 | 372 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.18, 0.22] |
| 2.4 Alprazolam versus desipramine | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.93, ‐0.34] |
| 3 50% improvement vs less than 50% improvement RR | 7 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.75, 0.99] |
| 4 50% improvement vs less than 50% improvement RR (subgrouped by TCA comparator) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Alprazolam versus amitriptyline | 5 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.89] |
| 4.2 Alprazolam versus imipramine | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.86, 1.65] |
| 5 50% improvement vs less than 50% improvement RD | 7 | 543 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.24, 0.01] |
| 6 50% improvement vs less than 50% improvement RD (subgrouped by TCA comparator) | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Alprazolam versus amitriptyline | 5 | 358 | Risk Difference (M‐H, Random, 95% CI) | ‐0.19 [‐0.30, ‐0.07] |
| 6.2 Alprazolam versus imipramine | 2 | 185 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.07, 0.22] |
| 7 All‐cause withdrawals vs no withdrawals RR | 18 | 1873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 1.00] |
| 8 All‐cause withdrawals vs no withdrawals RR (subgrouped by TCA comparator) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Alprazolam versus amitriptyline | 9 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.10] |
| 8.2 Alprazolam versus imipramine | 6 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.56, 0.90] |
| 8.3 Alprazolam versus doxepin | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.82, 1.90] |
| 8.4 Alprazolam versus dothiepin | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.46] |
| 8.5 Alprazolam versus desipramine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.39, 2.32] |
| 9 All‐cause withdrawals vs no withdrawals RD | 17 | 1848 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.07, 0.00] |
| 10 All‐cause withdrawals vs no withdrawals RD (subgrouped by TCA comparator) | 17 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
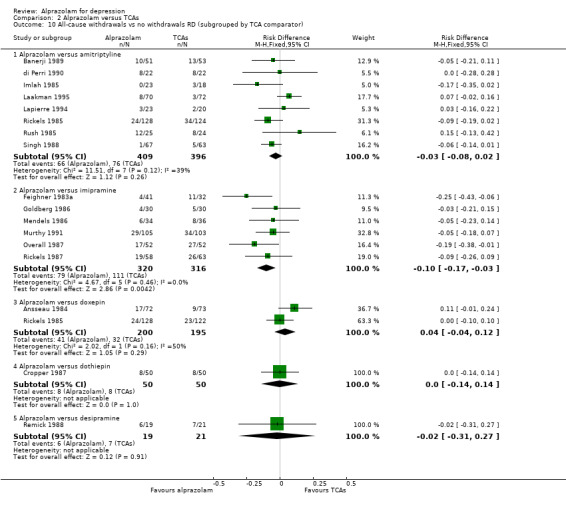
| 10.1 Alprazolam versus amitriptyline | 8 | 805 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 10.2 Alprazolam versus imipramine | 6 | 636 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.17, ‐0.03] |
| 10.3 Alprazolam versus doxepin | 2 | 395 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.04, 0.12] |
| 10.4 Alprazolam versus dothiepin | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.14, 0.14] |
| 10.5 Alprazolam versus desipramine | 1 | 40 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.31, 0.27] |
| 11 Adverse effects withdrawals vs no withdrawals RR | 11 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.43, 0.88] |
| 12 Adverse effects withdrawals vs no withdrawals RR (subgrouped by TCA comparator) | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
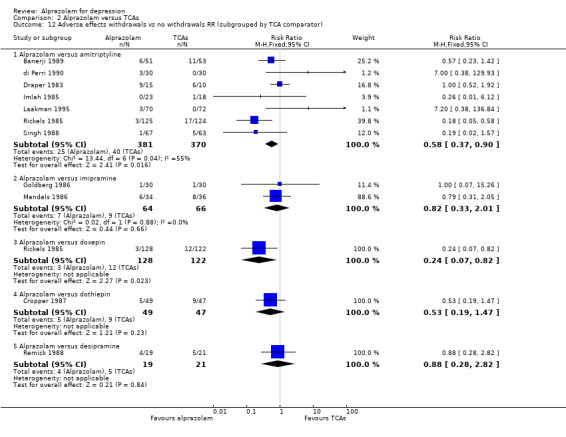
| 12.1 Alprazolam versus amitriptyline | 7 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.90] |
| 12.2 Alprazolam versus imipramine | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.33, 2.01] |
| 12.3 Alprazolam versus doxepin | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.82] |
| 12.4 Alprazolam versus dothiepin | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.47] |
| 12.5 Alprazolam versus desipramine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.28, 2.82] |
| 13 Adverse effects withdrawals vs no withdrawals RD | 11 | 1240 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.01] |
| 14 Adverse effects withdrawals vs no withdrawals RD (subgrouped by TCA comparator) | 11 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Alprazolam versus amitriptyline | 6 | 726 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 14.2 Alprazolam versus imipramine | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 14.3 Alprazolam versus doxepin | 2 | 376 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.14, ‐0.03] |
| 14.4 Alprazolam versus dothiepin | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 14.5 Alprazolam versus desipramine | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.29, 0.23] |
| 15 Ineffectiveness withdrawals vs no withdrawals RR | 4 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.99, 2.79] |
| 16 Ineffectiveness withdrawals vs no withdrawals RR (subgrouped by TCA comparator) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Alprazolam versus amitriptyline | 3 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.82, 3.04] |
| 16.2 Alprazolam versus doxepin | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.03, 4.98] |
| 16.3 Alprazolam versus desipramine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.32, 3.82] |
| 17 Ineffectiveness withdrawals vs no withdrawals RD | 4 | 686 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 18 Ineffectiveness withdrawals vs no withdrawals RD (subgrouped by TCA comparator) | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Alprazolam versus amitriptyline | 3 | 524 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 18.2 Alprazolam versus desipramine | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.23, 0.27] |
| 18.3 Alprazolam versus doxepin | 1 | 250 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.01, 0.16] |
| 19 HDRS timeline week 1 | 17 | 1692 | Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.77, ‐0.18] |
| 20 HDRS timeline week 1 (subgrouped by TCA comparator) | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Alprazolam versus amitriptyline | 8 | 742 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.41, 0.31] |
| 20.2 Alprazolam versus imipramine | 7 | 675 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.50, ‐0.13] |
| 20.3 Alprazolam versus doxepin | 2 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.32, 0.09] |
| 20.4 Alprazolam versus desipramine | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.05, ‐0.44] |
| 21 HDRS timeline week 2 | 15 | 1184 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐3.39, 0.25] |
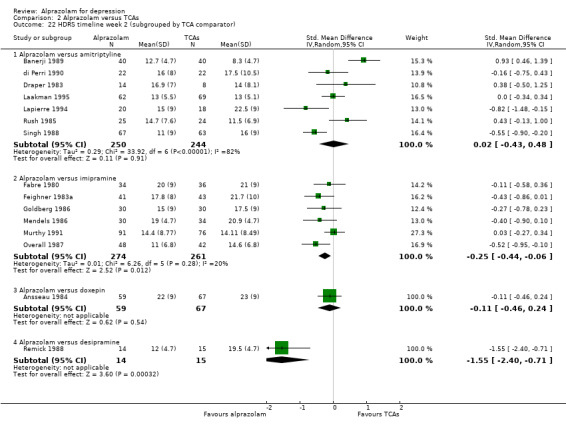
| 22 HDRS timeline week 2 (subgrouped by TCA comparator) | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Alprazolam versus amitriptyline | 7 | 494 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.43, 0.48] |
| 22.2 Alprazolam versus imipramine | 6 | 535 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.44, ‐0.06] |
| 22.3 Alprazolam versus doxepin | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.46, 0.24] |
| 22.4 Alprazolam versus desipramine | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐2.40, ‐0.71] |
| 23 HDRS timeline week 3 | 4 | 281 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐1.99, 4.94] |
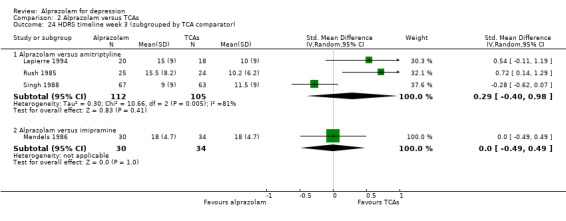
| 24 HDRS timeline week 3 (subgrouped by TCA comparator) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Alprazolam versus amitriptyline | 3 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.40, 0.98] |
| 24.2 Alprazolam versus imipramine | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.49, 0.49] |
| 25 HDRS timeline week 4 | 14 | 1406 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.20, 1.70] |
| 26 HDRS timeline week 4 (subgrouped by TCA comparator) | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 26.1 Alprazolam versus amitriptyline | 6 | 561 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.00, 0.65] |
| 26.2 Alprazolam versus imipramine | 6 | 570 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.14] |
| 26.3 Alprazolam versus doxepin | 2 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.26, 0.14] |
| 26.4 Alprazolam versus desipramine | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.93, ‐0.34] |
| 27 HDRS timeline week 5 | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐5.13, 6.80] |
| 28 HDRS timeline week 5 (subgrouped by TCA comparator) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 Alprazolam versus amitriptyline | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.00, 1.14] |
| 28.2 Alprazolam versus imipramine | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.92, 0.08] |
| 29 HDRS timeline week 6 | 14 | 1448 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.06, 1.54] |
| 30 HDRS timeline week 6 (subgrouped by TCA comparator) | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 30.1 Alprazolam versus amitriptyline | 6 | 608 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.08, 0.24] |
| 30.2 Alprazolam versus imipramine | 6 | 565 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.17, 0.16] |
| 30.3 Alprazolam versus doxepin | 2 | 372 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.18, 0.22] |
| 30.4 Alprazolam versus desipramine | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.93, ‐0.34] |
2.1. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 1 HDRS continuous.
2.2. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 2 HDRS continuous (subgrouped by TCA comparator).
2.3. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 3 50% improvement vs less than 50% improvement RR.
2.4. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 4 50% improvement vs less than 50% improvement RR (subgrouped by TCA comparator).
2.5. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 5 50% improvement vs less than 50% improvement RD.
2.6. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 6 50% improvement vs less than 50% improvement RD (subgrouped by TCA comparator).
2.7. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 7 All‐cause withdrawals vs no withdrawals RR.
2.8. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 8 All‐cause withdrawals vs no withdrawals RR (subgrouped by TCA comparator).
2.9. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 9 All‐cause withdrawals vs no withdrawals RD.
2.10. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 10 All‐cause withdrawals vs no withdrawals RD (subgrouped by TCA comparator).
2.11. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 11 Adverse effects withdrawals vs no withdrawals RR.
2.12. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 12 Adverse effects withdrawals vs no withdrawals RR (subgrouped by TCA comparator).
2.13. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 13 Adverse effects withdrawals vs no withdrawals RD.
2.14. Analysis.
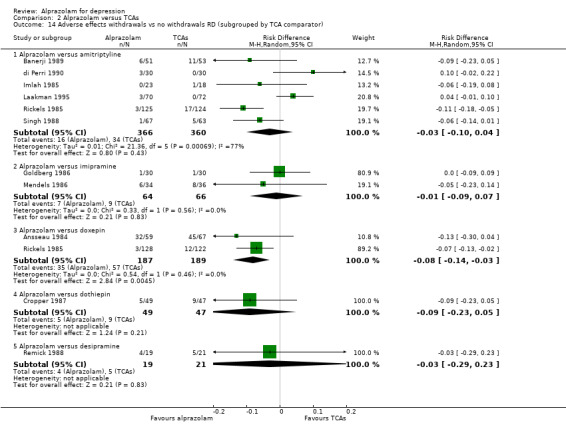
Comparison 2 Alprazolam versus TCAs, Outcome 14 Adverse effects withdrawals vs no withdrawals RD (subgrouped by TCA comparator).
2.15. Analysis.
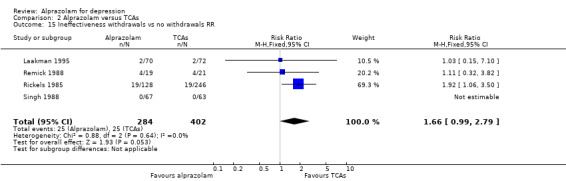
Comparison 2 Alprazolam versus TCAs, Outcome 15 Ineffectiveness withdrawals vs no withdrawals RR.
2.16. Analysis.
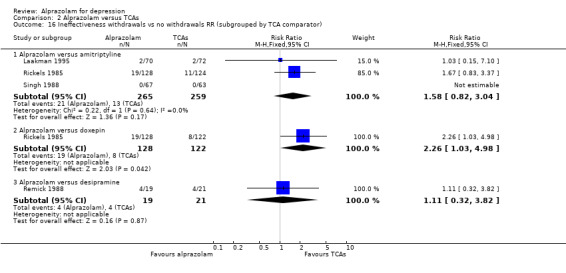
Comparison 2 Alprazolam versus TCAs, Outcome 16 Ineffectiveness withdrawals vs no withdrawals RR (subgrouped by TCA comparator).
2.17. Analysis.
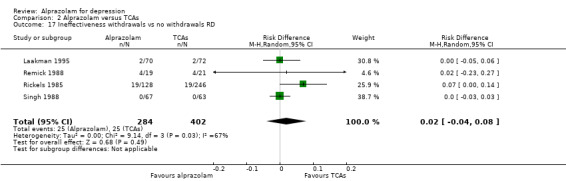
Comparison 2 Alprazolam versus TCAs, Outcome 17 Ineffectiveness withdrawals vs no withdrawals RD.
2.18. Analysis.
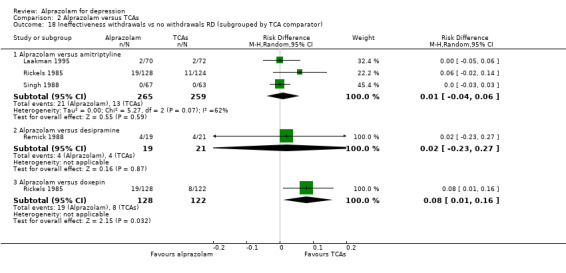
Comparison 2 Alprazolam versus TCAs, Outcome 18 Ineffectiveness withdrawals vs no withdrawals RD (subgrouped by TCA comparator).
2.19. Analysis.
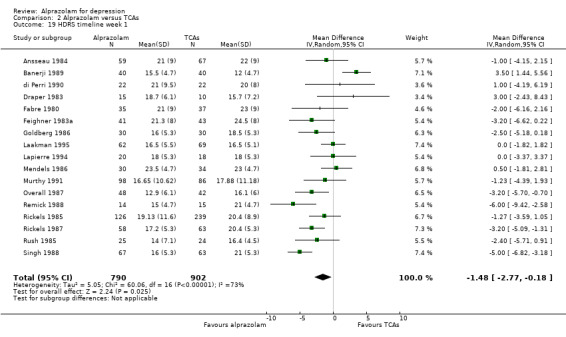
Comparison 2 Alprazolam versus TCAs, Outcome 19 HDRS timeline week 1.
2.20. Analysis.
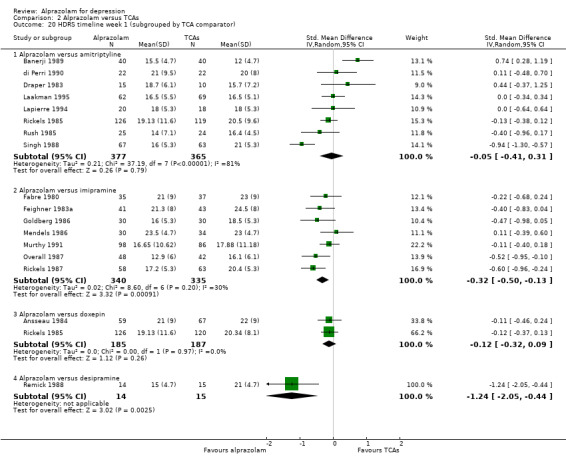
Comparison 2 Alprazolam versus TCAs, Outcome 20 HDRS timeline week 1 (subgrouped by TCA comparator).
2.21. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 21 HDRS timeline week 2.
2.22. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 22 HDRS timeline week 2 (subgrouped by TCA comparator).
2.23. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 23 HDRS timeline week 3.
2.24. Analysis.
Comparison 2 Alprazolam versus TCAs, Outcome 24 HDRS timeline week 3 (subgrouped by TCA comparator).
2.25. Analysis.
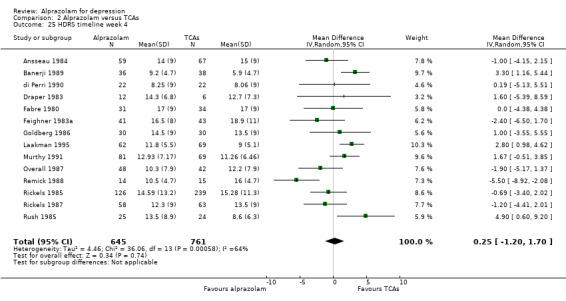
Comparison 2 Alprazolam versus TCAs, Outcome 25 HDRS timeline week 4.
2.26. Analysis.
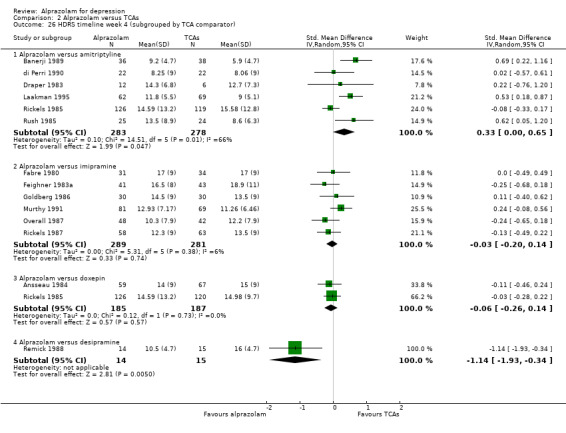
Comparison 2 Alprazolam versus TCAs, Outcome 26 HDRS timeline week 4 (subgrouped by TCA comparator).
2.27. Analysis.
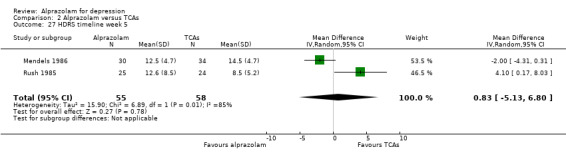
Comparison 2 Alprazolam versus TCAs, Outcome 27 HDRS timeline week 5.
2.28. Analysis.
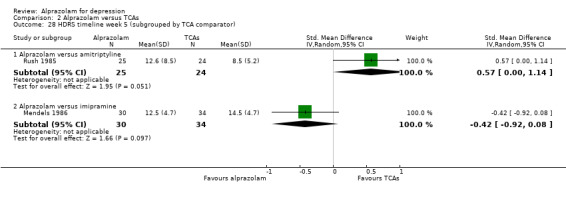
Comparison 2 Alprazolam versus TCAs, Outcome 28 HDRS timeline week 5 (subgrouped by TCA comparator).
2.29. Analysis.
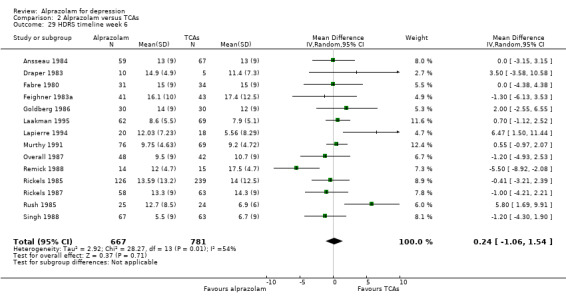
Comparison 2 Alprazolam versus TCAs, Outcome 29 HDRS timeline week 6.
2.30. Analysis.

Comparison 2 Alprazolam versus TCAs, Outcome 30 HDRS timeline week 6 (subgrouped by TCA comparator).
Comparison 3. Alprazolam versus heterocyclics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hamilton Depression Rating Scale (HDRS) continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Alprazolam versus mianserin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 All‐cause withdrawals versus no withdrawals RR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Alprazolam versus mianserin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 All‐cause withdrawals versus no withdrawals RD | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Alprazolam versus mianserin | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects withdrawals versus no withdrawals RR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Alprazolam versus mianserin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects withdrawals versus no withdrawals RD | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Alprazolam versus mianserin | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Ineffectiveness withdrawals versus no withdrawals RR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Alprazolam versus mianserin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Ineffectiveness withdrawals versus no withdrawals RD | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Alprazolam versus mianserin | 1 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Hamilton Depression Rating Scale (HDRS) timeline week 2 | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Alprazolam versus mianserin | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Hamilton Depression Rating Scale (HDRS) timeline week 4 | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Alprazolam versus mianserin | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
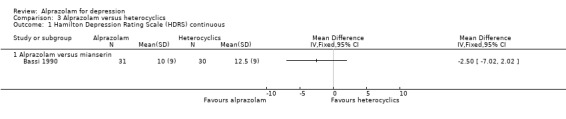
Comparison 3 Alprazolam versus heterocyclics, Outcome 1 Hamilton Depression Rating Scale (HDRS) continuous.
3.2. Analysis.
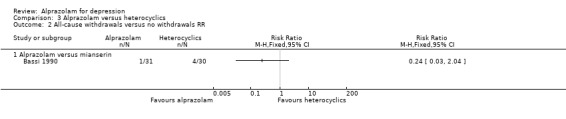
Comparison 3 Alprazolam versus heterocyclics, Outcome 2 All‐cause withdrawals versus no withdrawals RR.
3.3. Analysis.
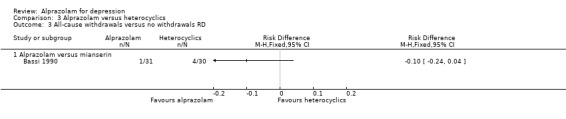
Comparison 3 Alprazolam versus heterocyclics, Outcome 3 All‐cause withdrawals versus no withdrawals RD.
3.4. Analysis.
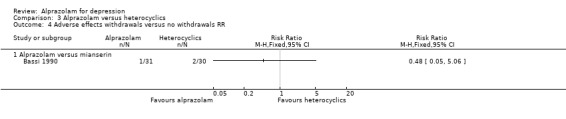
Comparison 3 Alprazolam versus heterocyclics, Outcome 4 Adverse effects withdrawals versus no withdrawals RR.
3.5. Analysis.
Comparison 3 Alprazolam versus heterocyclics, Outcome 5 Adverse effects withdrawals versus no withdrawals RD.
3.6. Analysis.
Comparison 3 Alprazolam versus heterocyclics, Outcome 6 Ineffectiveness withdrawals versus no withdrawals RR.
3.7. Analysis.
Comparison 3 Alprazolam versus heterocyclics, Outcome 7 Ineffectiveness withdrawals versus no withdrawals RD.
3.8. Analysis.
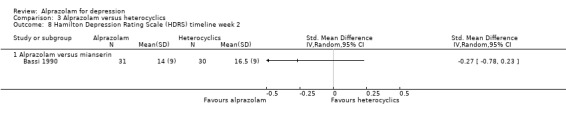
Comparison 3 Alprazolam versus heterocyclics, Outcome 8 Hamilton Depression Rating Scale (HDRS) timeline week 2.
3.9. Analysis.
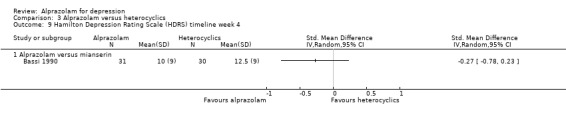
Comparison 3 Alprazolam versus heterocyclics, Outcome 9 Hamilton Depression Rating Scale (HDRS) timeline week 4.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansseau 1984.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 126 outpatients with primary affective non‐psychotic depression of at least moderate severity, required to have a Raskin Depression Scale (RDS) score of at least 8, at least 5 items on Feighner Depression Checklist (FDC), a Covi Anxiety Score (CAS) equal to or less than RDS, a HDRS score of at least 18 on the 21‐item HDRS, and an anxiolytic antidepressant was warranted 145 participants were enrolled of whom 19 were not available; 126 outpatients were therefore available Aged 18 to 70 years |
|
| Interventions | Alprazolam versus doxepin Placebo washout for 4 to 7 days Maximum dose for alprazolam 4.5 mg and for doxepin 225 mg Mean final doses 2.7 mg for alprazolam and 137.5 mg for doxepin |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 11 (initial 24.9), doxepin 11 (initial 25.1) Secondary outcomes: All‐cause withdrawals: 23% for alprazolam, 12% for doxepin Side effects: drowsiness (alprazolam 22%, doxepin 28%), dry mouth (alprazolam 3%, doxepin 36%), constipation (alprazolam 3%, doxepin 28%), lightheadedness (alprazolam 15%, doxepin 18%) 32 alprazolam patients (of 59) reported 142 side effects (mean of 4.4 per reporting patient), versus 45 (of 67) doxepin patients who reported 357 side effects (mean of 8.5 per patient) |
|
| Notes | Results reported in table 1 (change scores) and figure 1 do not match | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly assigned to treatment.” “... no statistical differences between the two treatment groups except for sex.” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | “Double‐blind study” “Initial dose of 2 tablets...” They probably used identical tablets, but this is not described in the study |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | They used a standard scheme for dose increase |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not described how they managed to keep the medication separated for each patient so that the investigators were blinded for the therapy as well. No information is provided about whether they used an investigator to do the assessments |
| Incomplete outcome data (attrition bias) HDRS | High risk | Initially, “13 patients in the alprazolam group and 6 patients in the doxepin group were not available”. Thus, 19/145 (13%) of the enrolled patients enrolled were not available. They further describe that 7 patients in the alprazolam group and 4 patients in the doxepin group were not available at final assessment; but it is not clear why these are left out of the evaluation ”Concomitant medical events, which may or may not have been adverse reactions to treatment, prompted the investigators to discontinue treatment for 11 patients.” |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | Unclear why numbers do not seem to match |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Dosing: Both drugs were given in the therapeutic range |
| Placebo washout | High risk | Yes |
Banerji 1989.
| Methods | Randomised controlled trial, 4 weeks | |
| Participants | 104 patients in general practice suffering from neurotic or reactive depression (for at least 2 weeks) entered the study, 3 were lost to follow‐up, 104 patients were included. An RDS score of at least 6 with a CAS score of no more than the RDS was required to be eligible for entry Patients who were withdrawn from the study before week 2 were not included in the analysis of data for therapeutic efficacy, but were evaluated for side effects. 21 patients failed to complete 2 weeks treatment ‐> 80 participants (40 each group) were evaluated for treatment Aged 18 to 70 years |
|
| Interventions | Alprazolam versus amitriptyline Maximum dose: alprazolam 3 mg and amitriptyline 150 mg Average daily dose alprazolam 1.8 mg and for amitriptyline 63.8 mg | |
| Outcomes | Primary outcome: Mean HDRS after 4 weeks: alprazolam 9.2 (initial 21.9) and amitriptyline 5.9 (initial 21.1) 50% reduction of baseline HDRS score: alprazolam 26/40 (72%), 32/40 amitriptyline (84%) Secondary outcome: All‐cause withdrawals: alprazolam 10/51 (20%), amitriptyline 13/53 (25%) Withdrawals due to side effects: alprazolam 6/51 (12%), amitriptyline 11/53 (21)% Physicians' evaluation of no side effects: alprazolam 36%, amitriptyline 54% Side effects: insomnia alprazolam 22%, amitriptyline 6%, headache alprazolam 20%, amitriptyline 4%, dry mouth 30%, amitriptyline 65% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised design". No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "The drugs were presented in identical capsules." The drugs looked the same, but there appears to have been a difference in side effect profile. |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | "The drugs were presented in identical capsules." No further information provided; unclear how physicians administered the drugs, while the tablets looked the same. |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | "The drugs were presented in identical capsules" |
| Incomplete outcome data (attrition bias) HDRS | High risk | 20% of the included patients were withdrawn from the study and were not evaluated for treatment |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | “...21 patients failed to complete two weeks treatment with the investigational drug (seven protocol violations and 14 withdrawals)” From table: 7 protocol violations: 5 alprazolam and 2 amitriptyline, 13 withdrawals due to side effects: 4 alprazolam and 9 amitriptyline and 1 withdrawal due to feeling improved: alprazolam |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Low therapeutic dosage of amitriptyline in comparison to that of alprazolam |
| Placebo washout | Low risk | No information provided |
Bassi 1990.
| Methods | Randomised controlled trial, 4 weeks | |
| Participants | 61 outpatients diagnosed with depressive neurosis according to DSM‐III. Score of at least 9 on RDS and at least 18 on HDRS Aged 19 to 70 years |
|
| Interventions | Alprazolam versus mianserin (30/31 patients) Placebo washout 7 days Maximum dose: alprazolam 5 mg and mianserin 90 mg Mean maximum daily dose: alprazolam 2.4 mg and mianserin 47 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 4 weeks: alprazolam 10, mianserin 12.5 (estimate from figure) Secondary outcome: All‐cause withdrawals: alprazolam 1 (3%) and mianserin 4 (13%) Withdrawals due to side effects: alprazolam 1 (3%), mianserin 2 (7) |
|
| Notes | In the abstract, "depression neurosis (or dysthymic disorder)" is described as the diagnosis for inclusion, but the results are not specified for major depressive episode and/or dysthymia. As all participants had at least a HDRS score of 18, we assume that some patients had so called double depression HDRS version not specified, HDRS‐21 assumed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized allocation". No other information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "Double blind". No other information is provided |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Low risk | No information is provided |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | No information is provided |
| Selective reporting (reporting bias) | Unclear risk | SD not reported |
| Other bias | Unclear risk | No information provided Dosages are in adequate range |
| Placebo washout | High risk | 7 days |
Borison 1989.
| Methods | Randomised, double‐blind study, 6 weeks | |
| Participants | 119 outpatients, who met DSM III criteria for major depressive disorder and scored at least 18 on the HDRS 82 patients, data analysis on those who completed a minimum of 28 days of treatment (25 patients on 3 mg and 25 patients on 6 mg of alprazolam and 32 patients on placebo) Aged 18 to 65 years |
|
| Interventions | Alprazolam versus placebo (25/25/32) Placebo washout 7 days |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 7.2 (initial 23.86), placebo 6.71 (initial 22.16) Secondary outcome: All withdrawals: alprazolam 3 mg 38%, alprazolam 6 mg 36% and placebo 20% Side effects: Drowsiness alprazolam 3 mg 73%, alprazolam 6 mg 59%, placebo 35%; lightheadedness alprazolam 3 mg 35%, alprazolam 6 mg 21%, placebo 15%; dry mouth: alprazolam 3 mg 28%, alprazolam 6 mg 23%, placebo 13%; confusion: alprazolam 3 mg 23%, alprazolam 6 mg 18%, placebo 5%; nervousness alprazolam 3 mg 18%, alprazolam 6 mg 8%, placebo 5% |
|
| Notes | We collapsed both alprazolam arms (3 mg and 6 mg) into 1 arm and pooled means and SDs for the HDRS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomized double‐blind phase of the study.” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | "randomised double‐blind phase of the study." Identical tablets Medication: alprazolam was compared to placebo; the experience of (side) effects could influence the patient; thinking they are using the ‘real’ medication |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Three investigators are used, but no other information is provided |
| Incomplete outcome data (attrition bias) HDRS | High risk | “The early dropout rate in the active treatment groups was primarily due to adverse effects of medication, whereas placebo dropouts were due to administrative reasons.” 69% of the 119 patients who entered the study could be analysed |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | There were different reasons for early dropout between the patients using medication and those using placebo |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | 6 mg alprazolam is a very high, not recommended dose |
| Placebo washout | High risk | 7 days |
Cropper 1987.
| Methods | Randomised controlled trial, 4 weeks | |
| Participants | 100 patients in general practice with mixed symptoms of anxiety and depression. Minimum score of at least 9 in both components of the Hospital Anxiety and Depression Scale (HADS) Aged 18 to 69 years |
|
| Interventions | Alprazolam versus dothiepin (50/50 patients) Maximum dose: alprazolam 3 mg, dothiepin 150 mg Mean maximum daily dose: alprazolam 2.33 mg, dothiepin 115 mg |
|
| Outcomes | Primary outcomes: not described Secondary outcomes: All‐cause withdrawals after 4 weeks: alprazolam 8 (4.0%), dothiepin 8 (4.0%) Withdrawals due to side effects: alprazolam 2 (4.1%), dothiepin 5 (10.6%) Number drug‐related adverse effects: alprazolam 5 (10.2%), dothiepin 9 (19.2%) Physician's global assessment of severity of illness after 4 weeks: alprazolam 0.95 (initial score of 2.47, SD 0.58), dothiepin 0.76 (initial score of 2.38, SD 0.66) HADS, self report, depression scale score after 4 weeks: alprazolam: 6.6 (SD 3.76) and dothiepin 5.9 (SD 3.11) |
|
| Notes | Patients must have complied with the protocol for 2 weeks to be included in the analysis of the treatment. Side effect data are available for those who attended the first week follow‐up assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly and blindly allocated to treatment groups..." No other information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "The study medications were presented in identical capsules, each containing..." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | "...and a new bottle was dispensed for the next study period." No other information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | 16 patients were not included for analysis due to protocol violations (alprazolam 6, dothiepin 3) and adverse effects (alprazolam 2, dothiepin 5). Different reasons for dropout were not equally distributed between the treatment groups; there was no intention‐to‐treat analysis. |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | All data for patients who attended the week 1 follow‐up assessment were analysed |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Patients included with mixed anxiety and depression Maximum dose of alprazolam is adequate therapeutic maximum dose in contrast to the maximum dose of dothiepin, which is lower than the recommended maximum therapeutic range (100 to 200 mg) Support from alprazolam manufacturer |
| Placebo washout | Unclear risk | No information provided |
di Perri 1990.
| Methods | Randomised controlled trial, 4 weeks | |
| Participants | 60 patients with moderate neurotic depression according to DSM‐III and ICD‐9 criteria (endogenous depression excluded) with a RDS score of at least 8 and a HDRS score of at least 18 Aged 18 to 70 years |
|
| Interventions | Alprazolam versus amitriptyline (30/30 patients) Placebo washout period for 4 to 7 days Maximum dose: alprazolam 4.5 mg and amitriptyline 225 mg Mean daily dose: alprazolam 2.3mg and amitriptyline 97.7 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 4 weeks: alprazolam 8.25 (initial score 26), amitriptyline 8.06 (initial score 26) Secondary outcomes: All‐cause withdrawals: alprazolam 8 (27%), amitriptyline 8 (27%) Withdrawals due to side effects: alprazolam 3 (10%), amitriptyline 0 (0%) |
|
| Notes | HDRS‐21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomized allocation”. No further information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Patients | Low risk | “...double blind study... active drug supplied in capsules of identical aspect.” No further information provided |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | "...double blind study". No further information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | Lack of clarity concerning analysis, probably endpoint analysis of remaining patients |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | High risk | No information provided |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | Unclear risk | No information provided |
| Placebo washout | High risk | 4 to 7 days |
Draper 1983.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 36 o utpatients with neurotic/reactive depression and a minimum RDS of 8 which had to equal or exceed the CAS score indicating that depression predominated. They had to satisfy FDC criteria for primary affective disorder and achieve a minimum score of 17 on the HDRS. 201 had a diagnosis of depression (151 of whom had neurotic depression). 60 did not fulfil severity criteria, 30 refused, or had other treatment etc., 25 left because of physical illness, 16 had wrong age, 11 responded to existing therapy, and 7 had the wrong clinical presentation. Of 52 participants remaining, 51 had neurotic depression and of these 36 met the inclusion criteria, 11 failed to reattend and 15 were ultimately available for the final assessments. Aged 18 to 60 years |
|
| Interventions | Alprazolam versus amitriptyline Placebo wash‐out period 4 to 7 days Mean dosage: 2.15 mg alprazolam per day and 85 mg amitriptyline per day |
|
| Outcomes | Primary outcome: Mean HDRS after 4 weeks: alprazolam 8.25 (initial 26), amitriptyline 8.06 (initial 26) Secondary outcome: All‐cause withdrawals: alprazolam 8 (27%), amitriptyline 8 (27%) Withdrawals due to side effects: Alprazolam 3 (10%), amitriptyline 0 (0%) |
|
| Notes | At 6 weeks n = 10, alprazolam and n = 10 to n = 5 for amitriptyline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The design of the study was double blind with random allocation.” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Patients | Low risk | “...in matched capsules.” “The design of the study was double blind” We assume patients were blinded |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) HDRS | High risk | 31% of the recruited patients were non‐available |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | High risk | “Two‐thirds of the alprazolam treated group remained in trial at week six compared with one half of the amitriptyline treated group.” Of the 15 patients in the alprazolam group, 10 were available at the end, while for the amitriptyline group, of the 10 patients, 5 were available |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Dosing of medication, at least for amitriptyline |
| Placebo washout | High risk | 4 to 7 days |
Fabre 1980.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 154 outpatients with primary depression of moderate to severe degree, a RDS score of at least 8, at least 5 associated items on Feighner depression checklist, CAS equal or less than the Raskin and a HDRS score of at least 18. Patients were selected from the outpatient population at the Fabre Clinic in Houston, Texas 104 participants were included in the statistical analysis Aged 17 to 65 years |
|
| Interventions | Alprazolam versus imipramine versus placebo Placebo washout 4 to 7 days Mean dosage of alprazolam 2.7 mg/day and for imipramine 126.5 mg/day |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 15.5 (initial 28), imipramine 15.7 (initial 28), placebo 19 (initial 28) Secondary outcomes: All‐cause dropouts: alprazolam 20 /51 (3 9 % ) , imipramine 1 8 /52 (3 5 % ) , placebo 36/51 (71 % ) |
|
| Notes | No numbers in the text; results were read from figure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The design of the study was double blind with random allocation to test drug, standard drug and placebo. The randomisation was forced so that in each consecutive group of six patients there were two on each drug treatment.” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No further information on the 'blocked randomisation' provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | “...double blind...” use of capsules We assume that identical capsules were used, but this is not stated in the paper |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | High risk | Unclear whether patients with ineffective medication were included in the analysis "31 of 35 alprazolam patients completed the study and 34 of 38 receiving imipramine completed the study as compared to only 15 of the 31 receiving placebo." |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | High risk | 154 entered the study, but 104 were included in the statistical analysis “Sixteen of the unavailable patients were in the alprazolam group, fourteen in the imipramine group and twenty in the placebo group.” The majority were lost to follow‐up: 13, 13 and 19. Others: one inter‐current life event, 2 moved away and 1 refused to co‐operate with the investigator Unclear whether the analysis at week 6 is with all patients or only with those who completed the study |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No information provided |
| Placebo washout | Unclear risk | 4 to 7 days |
Feighner 1983a.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 129 outpatients suffering from moderate to severe symptoms of unipolar major depressive disorder, RDS score at least 8, CAS less or equal RDS, HDRS score at least 18 Age: 18 to 70 years |
|
| Interventions | Alprazolam versus imipramine versus placebo (41/43/45) Placebo 'washout' period 4 to 7 days Maximum dose alprazolam: 4.5 mg, imipramine 225 mg, placebo 12 capsules Mean daily dose alprazolam: 2.7 mg, imipramine 117.3 mg, placebo 7.2 capsules |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 16.1 (initial score of 30.5, SD 14.4), imipramine 17.4 (initial score of 30.4, SD 12.5), placebo 28.0 (initial score of 30.0, SD 14) Secondary outcome: All‐cause withdrawals: alprazolam 4 (9.8%), imipramine 11 (25.6%), placebo 21 (46.7%) |
|
| Notes | Endpoint analysis HDRS‐21 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | "Patients...took identical appearing capsules daily for 42 days." Drugs compared to placebo; patients taking placebo will not experience (as many) side effects. |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | "Patients could be terminated if they showed clinical deterioration or minimal response." Minimal response is not specified and it is not reported when patients dropped out. |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | Dropouts due to ineffective medication and due to side effects are grouped together |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | Unclear risk | No information is provided |
| Placebo washout | Unclear risk | 4 to 7 days |
Goldberg 1986.
| Methods | Randomised controlled trial | |
| Participants | 60 symptomatic volunteers with major depressive disorder according to DSM‐III. At least 18 on HDRS, at least 3 (out of 5) Bielski and Friedel criteria | |
| Interventions | Alprazolam versus imipramine (30/30 patients) Placebo 'washout' period 1 week Maximum dose alprazolam 4.5 mg, imipramine 225 mg Mean daily dose: alprazolam 3.9 mg and imipramine 193.3 mg Aged 18 to 55 years |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 14 (initial score of 26), imipramine 12 (initial score of 26) Secondary outcome: All‐cause withdrawals: alprazolam 4 (13.3%), imipramine 5 (16.7%) Withdrawals due to side effects after 6 weeks: alprazolam 1 (3.3%), imipramine 1 (3.3%) |
|
| Notes | HDRS‐21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "....patients were randomly assigned..." No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "The drugs were in identical appearing capsules..." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Low risk | All dropouts are reported and endpoint analysis is used |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | All dropouts are reported |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | High risk | Special group of patients: volunteers, recruited through a newspaper advertisement Support from alprazolam manufacturer |
| Placebo washout | High risk | 1 week |
Imlah 1985.
| Methods | Randomised controlled trial, 4 weeks | |
| Participants | 65 out‐ and inpatients with reactive or neurotic depression with or without anxiety, and a score of at least 18 on Hamilton Anxiety Rating Scale (HARS) and 2 on the depressed mood component of the HARS Age 18 to 60 years |
|
| Interventions | Alprazolam versus amitriptyline versus placebo (23/18/20 patients) Placebo 'washout' period 1 week Maximum dose: alprazolam 3 mg, amitriptyline 150 mg, placebo 6 capsules |
|
| Outcomes | Primary outcome: Not described Secondary outcome: All‐cause withdrawals: alprazolam 0 (60%), amitriptyline 0 (16.7%), placebo 5 (25%) Physician's assessment of severity after 4 weeks; differences: alprazolam ‐2.4, amitriptyline ‐1.6, placebo ‐1.2 |
|
| Notes | Not clear how many inpatients Endpoint analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...on a double‐blind randomised group comparative basis..." No information is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | Unclear if identical capsules were used |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | Not measured. Patients who did not benefit after 2 weeks could be withdrawn! |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | All dropouts are described |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | High risk | Mean or average dose for the treatment groups is not given Support from alprazolam manufacturer |
| Placebo washout | High risk | 1 week |
Laakman 1995.
| Methods | Randomised controlled trial | |
| Participants | 288 of 342 outpatients suffering from mild to moderate depression were available for analysis Aged 19 to 75 years |
|
| Interventions | Alprazolam versus lorazepam versus amitriptyline versus placebo (70/66/72/74 patients) Placebo 'washout' period 3 to 7 days The 6 weeks of drug treatment were followed by a drug taper period Maximum dose: alprazolam 4 mg, lorazepam 10 mg, amitriptyline 200 mg, placebo 4 tablets Mean daily dose: alprazolam 2.08 mg, lorazepam 4.93 mg, amitriptyline 102 mg, placebo 2.79 tablets |
|
| Outcomes | Primary outcomes: Mean HDRS after 6 weeks: alprazolam 8.6 (initial score of 20.2, SD 5.5), lorazepam 8.5 (initial score of 19.6, SD 5.7), amitriptyline 7.9 (initial score of 19.7, SD 5.1), placebo 14.4 (initial score of 19.2, SD 5.1) 50% or greater reduction on HDRS after 6 weeks: alprazolam 38 (61%), lorazepam 39 (66%), amitriptyline 50 (73%), placebo 15 (22%) Secondary outcome: All‐cause withdrawals: alprazolam 8 (11.4%), lorazepam 7 (10.6%), amitriptyline 3 (4.2%), placebo 7 (9.5%) Withdrawals due to side effects: alprazolam 3 (4.3%), lorazepam 1 (1.5%), amitriptyline and placebo 0 Adverse effects after dose reduction, after 8 weeks: alprazolam 5 (8.1%), amitriptyline 2 (2.9%), lorazepam and placebo 0 |
|
| Notes | Analysis of treatment for all patients who participated for at least 1 week; sample size varies at each point (no endpoint analysis) HDRS‐17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the four treatment groups..." No further information is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | "...under double‐blind conditions..." Unclear if identical capsules were used. |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Low risk | “Of the 342 depressive patients who were admitted to the study, 282 were available, 257 finished 6 weeks” All withdrawals are listed and there are no significant differences between the treatment groups |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | All withdrawals are well documented |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | Unclear risk | No information is provided |
| Placebo washout | High risk | 3 to 7 days |
Lapierre 1994.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 43 outpatients with depression of at least a moderate degree. Score at least 8 on RDS, at least 18 on HDRS, and equal or less on CAS Age 18 to 70 years |
|
| Interventions | Alprazolam versus amitriptyline (23/20 patients) Placebo 'washout' period 4 to 7 days Maximum dose: alprazolam 4.5 mg, amitriptyline 225 mg Mean final dosage: alprazolam 3.2 mg, amitriptyline 115 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 12.03 (initial score of 26.86, SD 7.23), amitriptyline 5.56 (initial score of 26.50, SD 8.29). 50% decrease of total HDRS after 6 weeks: alprazolam 12 (60%), amitriptyline 13 (73%) Secondary outcome: All‐cause withdrawals: alprazolam 3 (13%), amitriptyline 2 (10%) |
|
| Notes | HDRS version not specified, HDRS‐21 assumed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...assigned to two treatment groups by forced randomization such that in each consecutive group of six enrolled patients, three were assigned to each drug." No further information is provided. |
| Allocation concealment (selection bias) | Unclear risk | No further information is provided. Since there were fixed groups of 6 patients (3 per drug), one could predict the treatment group for the last patient in the group, but no information is provided on how they assigned patients to the groups. |
| Blinding (performance bias and detection bias) Patients | Low risk | "Both active study medications were supplied in capsules of identical appearance." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Low risk | Patients who dropped out due to inefficacy were equally distributed between the 2 treatment groups |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | Dropped out patient well documented |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | Low risk | No information is provided |
| Placebo washout | High risk | 4 to 7 days |
Mendels 1986.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 107 outpatients with major depressive disorder (12 agitated, 47 anxious, 39 retarded depression). At least 8 on RDS and equal or less on CAS, at least 5 associated items on FDC and at least 20 on HDRS. Age 18 to 60 years |
|
| Interventions | Alprazolam versus imipramine versus placebo (34/36/37 patients) Placebo washout period 7 days Maximum dose: alprazolam 5 mg, imipramine 250 mg, placebo 5 tablets Mean daily doses: alprazolam 3.67 mg, imipramine 167 mg, placebo 3.7 tablets |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 12.5 (initial score of 23.8), imipramine 14.5 (initial score of 23.7), placebo 18.6 (initial score of 23.9) 50% decrease of total HDRS after 6 weeks: alprazolam 15 (50%), imipramine 13 (38.2%), placebo 6 (17.7%) Secondary outcome: All‐cause withdrawals: alprazolam 13 (38.2%), imipramine 20 (55.6%), placebo 21 (56.8%) Withdrawals due to side effects: alprazolam 6 (17.6%), imipramine 8 (22.2%), placebo 1 (2.7%) |
|
| Notes | Endpoint analysis HDRS‐17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | "...double‐blind study." Unclear if they used identical tablets. |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | The additional reasons for dropping out are not specified for each treatment group. Other dropouts are equally distributed and endpoint analysis is used. |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Low risk | Side effects are listed for all included patients |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | Low risk | No information is provided |
| Placebo washout | High risk | 7 days |
Murthy 1991.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 208 outpatients with moderate to severe depression meeting Feighner’s criteria for primary depression and DSM‐III major depression criteria | |
| Interventions | Alprazolam versus imipramine (105/103 patients) Placebo 'washout' period: 3 to 7 days Maximum dosages: alprazolam 4.5 mg per day, imipramine 225 mg per day Mean dosages: alprazolam 2.5 mg per day, imipramine 125 mg per day, and 80% required less than 6 capsules of 0.5 mg per day |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 9.75 (initial score 23.81), SD 4.63; imipramine 9.20 (initial score of 23.44); SD 4.72 Secondary outcome: All‐cause withdrawals: alprazolam 29 (28%), imipramine 34 (33%) "The frequency of side effects was higher for imipramine compared to alprazolam. Significantly higher number of patients reported insomnia (P < 0.01) and tremor as side effects (P < 0.01). None of the side effects reported was significantly more in the alprazolam group." |
|
| Notes | HDRS 21 items | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned in double blind random fashion." In each consecutive group, there were 3 persons who received alprazolam and 3 imipramine; but there is no further information |
| Allocation concealment (selection bias) | Unclear risk | There is no information, except that "The drug code of each patient was kept in a sealed envelope which could be opened in case of emergency." |
| Blinding (performance bias and detection bias) Patients | Low risk | Yes, identical capsules (there could have been a specific and rapid tranquillising effect of alprazolam) |
| Blinding (performance bias and detection bias) Physicians | Low risk | Yes, identical capsules and the code was kept in a sealed envelope: "The drugs were dispensed in identical capsules, each capsule containing alprazolam 0.5 mg or imipramine 25 mg. Patients were started with one capsule twice daily. The drug code for each patient was kept in a sealed envelope which could be opened in case of emergency." |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No further information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | Unclear, although dropouts are documented |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | Unclear risk | It seems that Upjohn sponsored the study |
| Placebo washout | High risk | 3 to 7 days |
Overall 1987.
| Methods | Randomised controlled study, 6 weeks | |
| Participants | 104 outpatients with depressive disorder according to DSM‐III and Zung Self Rating Depression Scale score of 55 or higher. 96% fulfilled DSM‐III criteria for major depressive disorder and 4% for dysthymic disorder 104 entered the study, but only 90 returned for at least one follow‐up, 60 (35/25) completed the full 6 weeks Aged 18 to 60 years |
|
| Interventions | Alprazolam versus imipramine Placebo washout period 4 to 7 days Maximum dosages: alprazolam 6 mg, imipramine 300 mg Median dosage at the end: alprazolam 3 mg and imipramine 200 mg (mean dosage at week 5: alprazolam 3.6 mg and imipramine 201.0 mg) |
|
| Outcomes | Primary outcome: Mean HDRS, with the last observation carried forward strategy (LOCF). Imipramine dropouts had a significantly poorer response at the last available point than imipramine patients who completed the 6 weeks. In the imipramine there were more early dropouts who were included as poor responders HDRS (LOCF) after 6 weeks: alprazolam 9.5 (initial score of 23.4), imipramine 10.7 (initial score of 23.3) HDRS (completers) after 6 weeks: alprazolam 7.3 (initial score of 23.4), imipramine 6.2 (initial score of 23.3) Secondary outcome: All dropouts after inclusion. 14 participants (4 in alprazolam arm and 10 in imipramine arm) did not return after baseline evaluation and were not included in efficacy analysis |
|
| Notes | Two analytic strategies are presented: 1) the last available observation on each patient to represent the best estimate of response to treatment (LOCF), and 2) completers: only those who remained in treatment at each point were included. The LOCF data are used. The paper reports SDs that are much lower than in other studies (for instance, 1.4). We assume they have calculated SEs instead of SDs. We recalculated SDs (table 4 SD alprazolam at 6 weeks 1.800 and SD imipramine 1.605). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomised, parallel‐group design.” "Patients were then randomised to treatment”. No further information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | “Drugs were supplied in identical appearing capsules...” |
| Blinding (performance bias and detection bias) Physicians | High risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | High risk | It is not clear whether the investigator was the same person who administered the medication. No information is provided on how they managed to keep the capsules apart. |
| Incomplete outcome data (attrition bias) HDRS | High risk | For both analysis there seems a great risk of bias, since the dropout rate was much greater for the imipramine group. LOCF may underestimate the effect of imipramine and analysing only the patients who stayed in treatment overestimates the imipramine effect. |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | 60/90 (67%) completed the 6‐week study. Two analyses have been done; LOCF and for those who remained in treatment |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | Unclear risk | No information is provided |
| Placebo washout | High risk | 4 to 7 days |
Remick 1988.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 52 outpatients suffering from major depressive disorder (12 dropped out before analysis), with a score of at least 21 on HDRS | |
| Interventions | Alprazolam versus desipramine (19/21 patients) Placebo 'washout' period 3 to 14 days Maximum dose: alprazolam 4.5 mg, desipramine 225 mg Mean daily dose at the end: alprazolam 3.34 mg, desipramine 192 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 12.0 (initial 26.3), desipramine 17.5 (initial 26.0) Secondary outcome: All‐cause withdrawals: alprazolam 6 (31.6%), desipramine 7 (33.3%) Withdrawals due to side effects: alprazolam 4 (21.1%), desipramine 5 (23.8%) |
|
| Notes | There seems to be an overlap with the Remick 1985 paper. The 2 car accidents and the side effects profile are the same, but this study has larger samples and allows for a higher maximum dosage. 21 patients completed an all‐night polysomnographic recording and 37 completed a modified dexamethasone suppression test HDRS‐17 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were blindly assigned to either alprazolam or desipramine." No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "Medicine was dispensed in opaque gelatine capsules containing...in a dose‐dispensing system administered by a pharmacist." |
| Blinding (performance bias and detection bias) Physicians | Low risk | "Weekly assessments were completed by the research psychiatrist..." Drugs were distributed by the pharmacist. |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Low risk | 40 patients enrolled of whom 29 were analysed for 6 weeks treatment. There was also an analysis for all 40 patients. Number and reasons for dropping out are equally distributed between the treatment groups. |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | It is not specified when the patients dropped out during the study |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | High risk | "There was a trend for desipramine patients to have more previous episodes than the alprazolam group. In addition, more desipramine patients had their current episode characterized as an exacerbation of a chronic condition while more alprazolam patients were having their first occurrences with no previous psychiatric illness." Group differences Support from alprazolam manufacturer |
| Placebo washout | High risk | 3 to 14 days |
Rickels 1985.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 504 outpatients with major depressive disorder, conducted in 3 treatment centres. Patients with a RDS score of at least 8 and an equal or less score on the CAS and at least 18 on HDRS Aged 18 to 70 years |
|
| Interventions | Alprazolam versus amitriptyline versus doxepin versus placebo (126/119/120/126) Placebo 'washout' period 4 to 7 days Maximum dose: alprazolam 4.5 mg, amitriptyline 225 mg, doxepin 225 mg, placebo 9 capsules Mean daily dose during last 2 weeks: alprazolam 3 mg, amitriptyline 148 mg, doxepin 143 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 13.59 (initial 25.19), amitriptyline 14.77 (initial 25.48), doxepin 13.23 (initial 25.85), placebo 18.90 (initial 26.38) Secondary outcome: All‐cause withdrawals: alprazolam 24 (18.8%), amitriptyline 34 (27.4%), doxepin 23 (18.9%), placebo 59 (45.4%) Withdrawals due to side effects: alprazolam 3 (2.3%), amitriptyline 17 (13.7%), doxepin 12 (9.8%), placebo 10 (7.7%) |
|
| Notes | Endpoint analysis HDRS‐21 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." No further information is provided. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "Medication,..., was administered in identical capsules containing..." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Unclear if they used independent investigators for the assessments |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | "... persistent side effects and worsening of symptoms or lack of improvement permitted removal of the patient from the study." “... statistically significant differences among treatment groups with respect to dropout rate, with significantly more patients given placebo dropping out, end‐point analyses, including patients with at least one week of efficacy data, were also performed for all efficacy variables.” “... but the set of analyses based on actual patients numbers reached at each evaluation period provided rather similar results.” |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | Not all causes for withdrawal are described. Dropout rates are different between the groups due to ineffectiveness (alprazolam 19 patients, amitriptyline 11, doxepin 8 and placebo 48), but these last scores are taken in the analysis. There were also differences for dropouts due to side effects: alprazolam 3 patients, amitriptyline 17, doxepin 12 and placebo 10. It is not clear for which side effects patients were withdrawn from the study, so there can be a bias regarding patients who dropped out too soon before there was any effect of treatment. |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | High risk | The mean doses are given, but it is unclear how fast they increased the dosages Support from alprazolam manufacturer |
| Placebo washout | High risk | 4 to 7 days |
Rickels 1987.
| Methods | Randomised controlled trial | |
| Participants | 241 outpatients (48% family practice, 52% psychiatric practice) suffering from major depressive disorder (DSM‐III), and a RDS score of at least 8, equal or less on CAS score, and at least 18 on the HDRS Aged 18 to 65 years |
|
| Interventions | Alprazolam versus imipramine versus diazepam versus placebo (58/63/59/61 patients) Placebo washout period 7 days Maximum dose: alprazolam 4.5 mg, diazepam 45 mg, imipramine 225 mg, placebo 9 capsules. Mean daily dose: alprazolam 3.1 mg, diazepam 24 mg, imipramine 143 mg, placebo 6.8 capsules |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 13.3 (initial 23.2), imipramine 14.3 (initial 24.4), diazepam 16.3 (initial 23.7), placebo 19.5 (initial 24.5) 50% decrease of total HDRS after 6 weeks: alprazolam 70%, imipramine 70%, diazepam 45%, placebo 39% Secondary outcome: All‐cause withdrawals: alprazolam 33%, imipramine 41%, diazepam 42.4%, placebo 39% |
|
| Notes | Endpoint analysis and analysis for all patients available at a given moment HDRS‐21 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "Medication was randomized and prepared in identical looking capsules..." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Unclear if they used independent investigators for the assessments |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | Side effects were the major reason for dropout for the active treatment groups, whereas this was ineffectiveness for placebo. 61% completed 6 weeks of therapy |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | Actual reasons for dropping out are not listed |
| Selective reporting (reporting bias) | High risk | SD not reported |
| Other bias | High risk | Maximum doses are in the same range for the different drugs Support from alprazolam manufacturer |
| Placebo washout | High risk | 7 days |
Rush 1985.
| Methods | Randomised controlled trial, 6 weeks | |
| Participants | 52 out‐ and inpatients suffering from major depression, non‐psychotic type. At least 18 on HDRS and at least a mean REM latency less or equal to 65. Aged 18 to 65 years |
|
| Interventions | Alprazolam versus amitriptyline (26/26 patients) Placebo washout 10 to 14 days Maximum dose: alprazolam 6 mg, amitriptyline 300 mg Mean final dose: alprazolam 4.4 mg, amitriptyline 190 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 12.7 (initial score of 24.1, SD 8.5), amitriptyline 6.9 (initial score of 26.1, SD 6.0) 50% decrease of total HDRS after 6 weeks: alprazolam 11 (44%), amitriptyline 21 (87.5%) Secondary outcome: All‐cause withdrawals: alprazolam 12 (48%), amitriptyline 8 (33%) |
|
| Notes | Endpoint and raw score data analyses were employed HDRS‐17 (After 3 weeks, 32 outpatients and 17 inpatients had assessments) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatments were assigned on a randomized, double‐blind basis and were independent of all pretreatment clinical and laboratory evaluations." No information is provided |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Unclear risk | Unclear if they used identical tablets |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | Only endpoint analysis is given while dropout rate is not equally distributed between the treatment groups |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | The reasons for dropping out are not listed |
| Selective reporting (reporting bias) | Unclear risk | No information is provided |
| Other bias | High risk | A selective group of patients, due to the requirement of a shortened REM latency Support from alprazolam manufacturer |
| Placebo washout | High risk | 10 to 14 days |
Singh 1988.
| Methods | Randomised controlled trial | |
| Participants | 130 outpatients suffering from moderate to severe nonpsychotic depression. Score of at least 8 on RDS and equal or less on CAS, presence of at least 5 items on FDC and at least a score of 18 on the HDRS. Aged 18 to 65 years |
|
| Interventions | Alprazolam versus amitriptyline (67/63 patients) Placebo 'washout' period of 4 to 7 days, single‐blind Max dose: alprazolam 4.5 mg, amitriptyline 225 mg Mean daily dose: alprazolam 2.4 mg, amitriptyline 135 mg |
|
| Outcomes | Primary outcome: Mean HDRS after 6 weeks: alprazolam 5.5 (initial 23.8), amitriptyline 6.7 (initial 23.9) Secondary outcome: All‐cause withdrawals: alprazolam 1 (1.5%), amitriptyline 5 (7.9%) |
|
| Notes | HDRS version not specified, HDRS‐21 assumed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were assigned sequentially by a computer‐generated randomisation list to receive treatment with alprazolam or amitriptyline..." |
| Allocation concealment (selection bias) | Low risk | No information is provided |
| Blinding (performance bias and detection bias) Patients | Low risk | "...were provided in identical appearing capsules that were packaged and labelled for each patient." |
| Blinding (performance bias and detection bias) Physicians | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) HDRS | Unclear risk | Unclear which method they used for analysing patients' outcome, endpoint or the patients at any given moment |
| Incomplete outcome data (attrition bias) Withdrawals/side effects | Unclear risk | Dropouts are listed |
| Selective reporting (reporting bias) | Unclear risk | SD not reported |
| Other bias | High risk | Dosing of medication: both the daily doses for alprazolam and amitriptyline are lower than the recommended doses of 3.0 mg and 150 mg |
| Placebo washout | High risk | 4 to 7 days |
CAS: Covi Anxiety Score FDC: Feighner Depression Checklist HADS: Hospital Anxiety and Depression Scale HARS: Hamilton Anxiety Rating Scale HDRS: Hamilton Depression Rating Scale LOCF: last observation carried forward RDS: Raskin Depression Scale SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aden 1983 | Clinically anxious patients with depressed mood |
| Beutler 1987 | Psychotherapy arm and complex factorial design |
| Ceskova 1989 | Insufficient information |
| Eriksson 1987 | Patients with no clinical response to adequate dosages of tricyclic antidepressants during the present episode were excluded |
| Ettigi 1988 | Outpatients; cortisol primary outcome |
| Fawcett 1987 | Treatment was not double‐blind: desipramine blood levels were monitored and dose adjustments were made |
| Feighner 1983b | Contains combined data from (probably) previously published studies |
| Hubain 1990 | Inpatients suffering from severe endogenous depression |
| Keller 1993 | Primary diagnosis anxiety syndrome |
| Kravitz 1990 | Secondary analysis of trial reported by Fawcett 1987, which we consider not to be double‐blind |
| Lenox 1984 | Inpatients only |
| Pitts 1983 | Inpatients, open label study |
| Pollack 1994 | Results reported by Keller et al, primary diagnosis was an anxiety disorder |
| Rickels 1982 | Lack of usable outcomes, the authors report only change scores |
| Rimon 1991 | Oxazepam control condition |
| Rothblum 1982 | Treatment was combined with interpersonal psychotherapy |
| Weissman 1985 | Treatment was combined with interpersonal psychotherapy |
| Weissman 1992 | Treatment was combined with interpersonal psychotherapy |
| Zung 1983 | Alprazolam versus natural history; different comparison |
Differences between protocol and review
Clinical Global Impression (CGI) scale analysis was not performed: in several studies, the CGI was not studied or reported (Borison 1989; Goldberg 1986; Overall 1987; Rush 1985). In the remaining studies the CGI version was not specified (Ansseau 1984; Banerji 1989; Bassi 1990; Cropper 1987; di Perri 1990; Fabre 1980; Feighner 1983a; Imlah 1985; Lapierre 1994; Mendels 1986; Rickels 1985; Rickels 1987; Singh 1988). Insufficient data were reported in the other studies (di Perri 1990; Lapierre 1994; Mendels 1986; Remick 1988; Singh 1988). The effects of alprazolam dosage and the loss of efficacy due to concomitant dose increase could not be analysed due to insufficient data on alprazolam dosages in all the studies.
Contributions of authors
Harm van Marwijk drafted the original idea in a national Dutch guideline committee meeting for the drug treatment of depression. Arjan Bax was vocational trainee in General Practice and developed the protocol in close co‐operation with van Marwijk. Gideon Allick and Froukje Wegman reassessed all the papers, assessed the risk of bias, helped with the searches and analysed and drafted the text of the review. All authors contributed to the final draft of the manuscript.
Sources of support
Internal sources
VU University Medical Centre, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Harm van Marwijk has not received any payments from relevant stakeholders, such as pharmaceutical industries, for this project. He is involved with research in mental health in primary care. The majority of his projects are sponsored by the Dutch government. He is a member of a Dutch working party on revision of national guidelines for anxiety and depression. GA, FW, AB and IR: none declared.
New
References
References to studies included in this review
Ansseau 1984 {published data only}
- Ansseau M, Ansoms C, Beckers G, Bogaerts M, Botte L, Buck R, et al. Double‐blind clinical study comparing alprazolam and doxepin in primary unipolar depression. Journal of Affective Disorders 1984;7(3‐4):287‐96. [DOI] [PubMed] [Google Scholar]
Banerji 1989 {published data only}
- Banerji JR, Brantingham P, McEwan GD, Mason J, Munt DF, Renton RL, et al. A comparison of alprazolam with amitriptyline in the treatment of patients with neurotic or reactive depression. A report of a randomised, double blind study by a general practitioner working party. Irish Journal of Medical Science 1989;158(5):110‐3. [DOI] [PubMed] [Google Scholar]
Bassi 1990 {published data only}
- Bassi S, Albizzati MG, Sbacchi M, Corsini GU, Pancheri P, Romiti R, et al. Clinical evaluation of alprazolam versus mianserin in the treatment of patients with depressive neurosis [Valutazione clinica dell'alprazolam nei confronti della mianserina nel trattamento dei pazienti affetti da nevrosi depressiva]. Giornale di Neuropsicofarmacologia 1990;12(1):35‐8. [Google Scholar]
Borison 1989 {published data only}
- Borison RL, Sinha D, Albrecht W, Geber S, Diamond BI. Double‐blind comparison of 3‐ and 6‐mg fixed doses of alprazolam vs. placebo in outpatients with major depressive disorder. Psychopharmacology Bulletin 1989;25(2):186‐9. [PubMed] [Google Scholar]
Cropper 1987 {published data only}
- Cropper M, Garner A, McEwan GD, Munt FDF, Rushbrook LAW, Stevens V, et al. A double‐blind comparative study of alprazolam and dothiepin hydrochloride in the treatment of anxiety associated with depression. Pharmatherapeutica 1987;5(2):76‐82. [PubMed] [Google Scholar]
di Perri 1990 {published data only}
- Perri R, Rosa AE, Morgante L, Rosa A, Sannino V. Alprazolam versus amitriptyline in the treatment of patients with nervous depression [Alprazolam versus amitriptilina nel trattamento di pazienti con nevrosi depressiva]. Rivista N.P.S. Neurologia Psichiatria Scienze Umane 1990;10:43‐53. [Google Scholar]
Draper 1983 {published data only}
- Draper RJ, Daly I. Alprazolam and amitriptyline: a double blind comparison of anxiolytic and anti‐depressant activity. Irish Medical Journal 1983;76(11):453‐6. [PubMed] [Google Scholar]
Fabre 1980 {published data only}
- Fabre LF, McLendon DM. A double‐blind comparison of alprazolam, imipramine, and placebo in primary depression [conference abstract]. Clinical Research 1979;27(2):542A. [Google Scholar]
- Fabre LF, McLendon DM. A double‐blind study comparing the efficacy and safety of alprazolam with imipramine and placebo in primary depression. Current Therapeutic Research 1980;27(5):474‐82. [Google Scholar]
Feighner 1983a {published data only}
- Feighner JP, Meredith CH, Frost NR, Chammas S, Hendrickson G. A double‐blind comparison of alprazolam vs. imipramine and placebo in the treatment of major depressive disorder. Acta Psychiatrica Scandinavica 1983;68(4):223‐33. [DOI] [PubMed] [Google Scholar]
Goldberg 1986 {published data only}
- Goldberg SC, Ettigi P, Schulz PM, Hamer RM, Hayes PE, Friedel RO. Alprazolam versus imipramine in depressed out‐patients with neurovegetative signs. Journal of Affective Disorders 1986;11(2):139‐45. [DOI] [PubMed] [Google Scholar]
Imlah 1985 {published data only}
- Imlah NW. An evaluation of alprazolam in the treatment of reactive or neurotic (secondary) depression. British Journal of Psychiatry 1985;146:515‐9. [DOI] [PubMed] [Google Scholar]
Laakman 1995 {published data only}
- Laakman G, Faltermaier‐Temizel M, Bossert‐Zaudig S, Baghai T, Lorkowski G. Treatment of depressive outpatients with lorazepam, alprazolam, amitriptyline and placebo. Psychopharmacology 1995;120:109‐15. [DOI] [PubMed] [Google Scholar]
- Laakmann G, Faltermaier‐Temizel M, Bossert‐Zaudig S, Baghai T, Lorkowski G. Treatment of mild to moderate depressive syndrome. A placebo‐controlled double‐blind trial on therapy with benzodiazepines or antidepressants [German]. Munchener Medizinische Wochenschrift 1996;138(5):39‐44. [Google Scholar]
Lapierre 1994 {published data only}
- Lapierre YD, Browne M, Oyewumi K, Sarantidis D. Alprazolam and amitriptyline in the treatment of moderate depression. International Clinical Psychopharmacology 1994;9:41‐5. [DOI] [PubMed] [Google Scholar]
Mendels 1986 {published data only}
- Mendels J, Schless AP. Comparative efficacy of alprazolam, imipramine, and placebo administered once a day in treating depressed patients. Journal of Clinical Psychiatry 1986;47(7):357‐61. [PubMed] [Google Scholar]
Murthy 1991 {published data only}
- Murthy RS, Chatterjee S, Sriram TG, Shah LP, Parikh R, Elavia S, et al. A double‐blind evaluation of alprazolam and imipramine in the treatment of major depression. Indian Journal of Psychiatry 1991;33(2):131‐5. [PMC free article] [PubMed] [Google Scholar]
Overall 1987 {published data only}
- Overall JE, Biggs J, Jacobs M, Holden K. Comparison of alprazolam and imipramine for treatment of outpatient depression. Journal of Clinical Psychiatry 1987;48(1):15‐9. [PubMed] [Google Scholar]
- Overall JE, Donachie ND, Faillace LA. Implications of restrictive diagnosis for compliance to antidepressant drug therapy: alprazolam versus imipramine. Journal of Clinical Psychiatry 1987;48(2):51‐4. [PubMed] [Google Scholar]
Remick 1988 {published data only}
- Remick RA, Fleming JAE, Buchanan RA, Keller FD, Hamilton P, Loomer F, et al. A comparison of the safety and efficacy of alprazolam and desipramine in moderately severe depression. Canadian Journal of Psychiatry 1985;30(8):597‐601. [DOI] [PubMed] [Google Scholar]
- Remick RA, Keller D, Buchanan RA, Gibson RE, Fleming JAE. A comparison of the efficacy and safety of alprazolam and desipramine in depressed outpatients. Canadian Journal of Psychiatry 1988;33:590‐4. [DOI] [PubMed] [Google Scholar]
Rickels 1985 {published data only}
- Rickels K, Feighner JP, Smith WT. Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Archives of General Psychiatry 1985;42(2):134‐41. [DOI] [PubMed] [Google Scholar]
Rickels 1987 {published data only}
- Rickels K, Chung HR, Csanalosi IB, Hurowitz AM, London J, Wiseman K, et al. Alprazolam, diazepam, imipramine, and placebo in outpatients with major depression. Archives of General Psychiatry 1987;44(10):862‐6. [DOI] [PubMed] [Google Scholar]
Rush 1985 {published data only}
- Rush AJ, Erman MK, Schlesser MA, Roffwarg HP, Vasavada N, Khatami M, et al. Alprazolam vs amitriptyline in depression with reduced REM latencies. Archives of General Psychiatry 1985;42:1154‐9. [DOI] [PubMed] [Google Scholar]
Singh 1988 {published data only}
- Singh AN, Nair NPV, Suranyi‐Cadotte B, Schwartz G, Lizondo E. A double blind comparison of alprazolam and amitriptyline hydrochloride in the treatment of nonpsychotic depression. Canadian Journal of Psychiatry 1988;33(3):218‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aden 1983 {published data only}
- Aden GC. Alprazolam in clinically anxious patients with depressed mood. Journal of Clinical Psychiatry 1983;44(1):22‐4. [PubMed] [Google Scholar]
Beutler 1987 {published data only}
- Beutler LE, Scogin F, Kirkish P, Schretlen D, Corbishley A, Hamblin D, et al. Group cognitive therapy and alprazolam in the treatment of depression in older patients. Journal of Consulting and Clinical Psychology 1987;55(4):550‐6. [DOI] [PubMed] [Google Scholar]
Ceskova 1989 {published data only}
- Ceskova E, Svestka J, Nahunek K, Obrovska V. Double blind comparison of alprazolam with dosulepine in endogenous depression. Activitas Nervosa Superior 1989;31(4):290‐1. [PubMed] [Google Scholar]
Eriksson 1987 {published data only}
- Eriksson B, Nagy A, Starmark J‐E, Thelander U. Alprazolam compared to amitriptyline in the treatment of major depression. Acta Psychiatrica Scandinavica 1987;75:656‐63. [DOI] [PubMed] [Google Scholar]
Ettigi 1988 {published data only}
- Ettigi PE, Goldberg SC, Schulz PM, Hamer RM, Blackard WG. Cortisol differentially predicts response to imipramine and alprazolam. Psychopharmacology Bulletin 1988;24(1):206‐9. [PubMed] [Google Scholar]
Fawcett 1987 {published data only}
- Fawcett J, Edwards JH, Kravitz HM, Jeffriess H. Alprazolam: an antidepressant? Alprazolam, desipramine, and an alprazolam‐desipramine combination in the treatment of adult depressed outpatients. Journal of Clinical Psychopharmacology 1987;7(5):295‐310. [PubMed] [Google Scholar]
Feighner 1983b {published data only}
- Feighner JP, Aden GC, Fabre LF, Rickels K, Smith WT. Comparison of alprazolam, imipramine, and placebo in the treatment of depression. JAMA 1983;249(22):3057‐64. [PubMed] [Google Scholar]
Hubain 1990 {published data only}
- Hubain PP, Castro P, Mesters P, Maertelaer V, Mendlewicz J. Alprazolam and amitriptyline in the treatment of major depressive disorder: a double‐blind clinical and sleep EEG study. Journal of Affective Disorders 1990;18:67‐73. [DOI] [PubMed] [Google Scholar]
Keller 1993 {published data only}
- Keller MB, Lavori PW, Goldenberg IM, Baker LA, Pollack MH, Sachs GS, et al. Influence of depression on the treatment of panic disorder with imipramine, alprazolam and placebo. Journal of Affective Disorders 1993;28:27‐38. [DOI] [PubMed] [Google Scholar]
Kravitz 1990 {published data only}
- Kravitz HM, Fogg L, Fawcett J, Edwards J. Antidepressant or antianxiety? A study of the efficacy of antidepressant medication. Psychiatry Research 1990;32:141‐9. [DOI] [PubMed] [Google Scholar]
Lenox 1984 {published data only}
- Lenox RH, Shipley JE, Peyser JM, Williams JM, Weaver LA. Double‐blind comparison of alprazolam versus imipramine in the inpatient treatment of major depressive illness. Psychopharmacology Bulletin 1984;20(1):79‐82. [PubMed] [Google Scholar]
Pitts 1983 {published data only}
- Pitts WM, Fann WE, Sajadi C, Snyder S. Alprazolam in older depressed patients. Journal of Clinical Psychiatry 1983;44:213‐5. [PubMed] [Google Scholar]
Pollack 1994 {published data only}
- Pollack MH, Otto MW, Sachs GS, Leon A, Shear MK, Deltito JA, et al. Anxiety psychopathology predictive of outcome in patients with panic disorder and depression treated with imipramine, alprazolam and placebo. Journal of Affective Disorders 1994;30(4):273‐81. [DOI] [PubMed] [Google Scholar]
Rickels 1982 {published data only}
- Rickels K, Cohen D, Csanalosi I, Harris H, Koepke H, Werblowsky J. Alprazolam and imipramine in depressed outpatients: a controlled study. Current Therapeutic Research 1982;32(6):157‐64. [Google Scholar]
Rimon 1991 {published data only}
- Rimon R, Kultalahti ER, Kalli A, Koskinen T, Lepola U, Naarala M, et al. Alprazolam and oxazepam in the treatment of anxious out‐patients with depressive symptoms: a double‐blind multicenter study. Pharmacopsychiatry 1991;24(3):81‐4. [DOI] [PubMed] [Google Scholar]
Rothblum 1982 {published data only}
- Rothblum ED, Sholomskas AJ, Berry C, Prusoff BA. Issues in clinical trials with depressed elderly. Journal of the American Geriatrics Society 1982;30(11):694‐9. [DOI] [PubMed] [Google Scholar]
Weissman 1985 {published data only}
- Weissman MM, Prusoff BA, Kleber HD, Sholomskas AJ, Rounsaville BJ. Alprazolam (Xanax) in the treatment of major depression. Clinical and Pharmacological Studies in Psychiatric Disorders. John Libbey, 1985:52‐8. [Google Scholar]
Weissman 1992 {published data only}
- Weissman MM, Prushoff B, Sholomskas AJ, Greenwald S. A double‐blind clinical trial of alprazolam, imipramine, or placebo in the depressed elderly. Journal of Clinical Psychopharmacology 1992;12(3):175‐82. [PubMed] [Google Scholar]
Zung 1983 {published data only}
- Zung WWK, King RE. Identification and treatment of masked depression in a general medical practice. Journal of Clinical Psychiatry 1983;44(10):365‐8. [PubMed] [Google Scholar]
Additional references
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Bandelow 2008
- Bandelow B, Zohar J, Hollander E, Kasper S, Moller HJ, WFSB task force on treatment guidelines for anxiety obsessive‐compulsive post‐traumatic stress disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive‐compulsive and post‐traumatic stress disorders ‐ first revision. World Journal of Biological Psychiatry 2008;9(4):248‐312. [DOI] [PubMed] [Google Scholar]
Barbone 1998
- Barbone F, McMahon AD, Davey PG, Morris AD, Reid IC, McDevitt DG, et al. Association of road‐traffic accidents with benzodiazepine use. Lancet 1998;352(9137):1331‐6. [DOI] [PubMed] [Google Scholar]
Birkenhager 1995
- Birkenhager TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. International Clinical Psychopharmacology 1995;10:181‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Committee 1980
- Committee on the Review of Medicines. Systematic review of the benzodiazepines: guidelines for the data sheets for diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, flurazepam. British Medical Journal 1980;1:910‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covi 1976
- Lipman RS, Covi L. Outpatient treatment of neurotic depression: medication and group psychotherapy. In: Spitzer RL, Klein DF editor(s). Evaluation of Psychological Therapies. Baltimore, MD: Baltimore University Press, 1976:178‐218. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26(1):57‐63. [DOI] [PubMed] [Google Scholar]
Fournier 2010
- Fournier JC, DeRubeis RR, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient‐level meta‐analysis. JAMA 2010;303(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2001
- Furukawa TA, Streiner D, Young LT, Kinoshita Y. Antidepressants plus benzodiazepines for major depression. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001026] [DOI] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2004
- Hansen DG, Vach W, Rosholm JU, Sondergaard J, Gram LF, Kragstrup J. Early discontinuation of antidepressants in general practice: association with patient and prescriber characteristics. Family Practice 2004;21:623‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Greene S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [Updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Jonas 1993
- Jonas JM, Cohon MS. A comparison of the safety and efficacy of alprazolam versus other agents in the treatment of anxiety, panic and depression: a review of the literature. Journal of Clinical Psychiatry 1993;54:S25‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Kirsch 2008
- Kirsch I, Deacon BJ, Huedo‐Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta‐analysis of data submitted to the Food and Drug Administration. Plos Medicine 2008;5(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrenson 2000
- Lawrenson RA, Tyrer F, Newson RB, Farmer RD. The treatment of depression in UK general practice: selective serotonin inhibitors and tricyclic antidepressants compared. Journal of Affective Disorders 2000;59:149‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely F, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Neutel 1996
- Neutel CI, Hirdes JP, Maxwell CJ, Patten SB. New evidence on benzodiazepine use and falls: the time factor. Age and Ageing 1996;25(4):273‐8. [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Clinical Excellence (NICE). Depression. The Treatment and Management of Depression in Adults [CG90]. London: National Institute for Clinical Excellence, 2009. [Google Scholar]
Petty 1995
- Petty F, Trivedi MH, Fulton M, Rush AJ. Benzodiazepines as antidepressants: does GABA play a role in depression?. Biological Psychiatry 1995;38(9):578‐91. [DOI] [PubMed] [Google Scholar]
Raskin 1970
- Raskin A, Schulterbrandt JG, Reatig N, Chase C, McKeen JJ. Differential response to chlorpromazine, imipramine and placebo. Archives of General Psychiatry 1970;23:164‐73. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rickels 1990
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long‐term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Archives of General Psychiatry 1990;47(10):899‐907. [DOI] [PubMed] [Google Scholar]
Rijswijk 2007
- Rijswijk E, Borghuis M, Lisdonk E, Zitman F, Weel C. Treatment of mental health problems in general practice: a survey of psychotropics prescribed and other treatments provided. International Journal of Clinical Pharmacology and Therapeutics 2007;45(1):23‐9. [DOI] [PubMed] [Google Scholar]
Schweizer 1990
- Schweizer E, Rickels K, Case WG, Greenblatt DJ. Long‐term therapeutic use of benzodiazepines. II. Effects of gradual taper. Archives of General Psychiatry 1990;47(10):908‐15. [DOI] [PubMed] [Google Scholar]
Schweizer 1998
- Schweizer E, Rickels K. Benzodiazepine dependence and withdrawal: a review of the syndrome and its clinical management. Acta Psychiatrica Scandinavica, Supplementum 1998;393:95‐101. [DOI] [PubMed] [Google Scholar]
Sethy 1982
- Sethy VH, Hodges DH Jr. Alprazolam in a biochemical model of depression. Biochemical Pharmacology 1982;31(19):3155‐7. [DOI] [PubMed] [Google Scholar]
Srisurapanont 1997
- Srisurapanont M, Boonyanaruthee V. Alprazolam and standard antidepressants in the treatment of depression: a meta‐analysis of antidepressant effect. Journal of the Medical Association of Thailand 1997;80(3):183‐8. [PubMed] [Google Scholar]
Van Marwijk 2003
- Marwijk HW, Grundmeijer HGLM, Bijl D, Gelderen MG, Haan M, Weel‐Baumgarten EM, et al. Dutch College of General Practitioners Guideline Depression, first revision. Huisarts en Wetenschap 2003;46:614‐23. [Google Scholar]
Van Marwijk 2006
- Marwijk H. "A catalogue of biases": the need for a European perspective on antidepressants' evidence in general practice. European Journal of General Practice 2006;12(3):98‐9. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. International Classification of Diseases (ICD‐10). Geneva: World Health Organization, 2003. [Google Scholar]


