Abstract
Background
Respiratory distress syndrome (RDS) is considered one of the major contributors to severe pulmonary dysfunction and consequent death in preterm infants. Despite widespread improvements in care, including increased utilization of antenatal steroids, use of surfactant replacement therapy, and advances in conventional mechanical ventilation (CMV), chronic lung disease (CLD) occurs in 42% of surviving preterm infants born at less than 28 weeks gestational age (GA). High frequency ventilation (HFV) aims to optimize lung expansion while minimizing tidal volume (Vt) to decrease lung injury. Two methods of HFV ‐ high frequency oscillatory ventilation (HFOV) and high frequency jet ventilation (HFJV) ‐ are widely used, but neither has demonstrated clear superiority in elective or rescue mode.
Objectives
To compare the benefits and side effects of HFJV versus HFOV for mortality and morbidity in preterm infants born at less than 37 weeks GA with pulmonary dysfunction in both elective and rescue modes.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 11), MEDLINE via PubMed (1966 to November 30, 2015), EMBASE (1980 to November 30, 2015), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to November 30, 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials. We imposed no date, language, or publication restrictions.
Selection criteria
We planned to include randomized, cluster‐randomized, and quasi‐randomized controlled trials if study authors stated explicitly that groups compared in the trial were established by a random or systematic method of allocation. We planned to exclude cross‐over studies, as they would not allow assessment of the outcomes of interest.
Data collection and analysis
We used the standard methods of the Neonatal Cochrane Review Group, including independent trial assessment and data extraction. We intended to analyze the data by using risk ratios (RRs) and risk differences (RDs) and 1/RD. We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH).
Main results
We found no studies that met our inclusion criteria.
Authors' conclusions
We found no evidence to support the superiority of HFJV or HFOV as elective or rescue therapy. Until such evidence is available, comparison of potential side effects or presumed benefits of either mode is not feasible.
Plain language summary
High frequency jet ventilation versus high frequency oscillatory ventilation for pulmonary dysfunction in preterm infants
Background: Breathing difficulty due to respiratory distress syndrome (RDS) is one of the major causes of death in babies born prematurely. Breathing machines providing what is known as conventional mechanical ventilation (CMV), which is currently used to support these babies, potentially contribute to longer‐term lung injury known as chronic lung disease (CLD). CLD occurs frequently in preterm babies who require breathing machines, and the type of breathing machine used may affect whether CLD occurs. Two new types of breathing machines (known as high frequency jet ventilation (HFJV) and high frequency oscillatory ventilation (HFOV)) have been tested in the hope that these methods of breathing support might reduce lung injury (CLD).
Our review question: In preterm infants (born before term) at risk for or having RDS, we planned to compare the risks and benefits of two modes of breathing machines: HFJV and HFOV.
What the studies showed: We identified no studies that compared these two modes of breathing support.
Overall: This review found no evidence for comparison of the superiority or harmful side effects of HFJV over HFOV, or of HFOV over HFJV, in infants at risk for or having breathing difficulty due to RDS.
Background
Description of the condition
Pulmonary dysfunction is a major cause of mortality and morbidity in preterm infants. In a population‐based study in the United Kingdom, 49% of deaths in preterm infants born between 2002 and 2008 at less than 32 weeks gestational age (GA) were due to respiratory illness (Berrington 2012). In addition to lung immaturity and infection, respiratory distress syndrome (RDS) is considered the major contributor to severe pulmonary dysfunction and consequent death in preterm infants (Stichtenoth 2012). Provision of conventional mechanical ventilation (CMV) has improved the survival of preterm infants, although ventilator‐induced lung injury and oxygen toxicity contribute to ongoing chronic lung disease (CLD) in this vulnerable group (Jobe 2000; Attar 2002). Despite widespread administration of antenatal steroids, use of surfactant replacement therapy, and advances in CMV with gentler strategies and improved technology, CLD occurred in 42% of surviving preterm infants born at less than 28 weeks GA between 2003 and 2007 in the USA (Stoll 2010). CLD is associated with poor neurodevelopmental outcomes (Singer 1997; Hintz 2005). In a recent study, infants with CLD had lower weight and smaller head circumference, greater likelihood of cerebral palsy, and impairment in language and cognition at 18 to 22 months of age compared with infants without CLD (Natarajan 2012). In addition, infants with CLD are at greater risk for rehospitalization during the first year of life (Smith 2004). Introduction of early continuous positive airway pressure (CPAP) in the delivery room, as well as emerging use of noninvasive ventilation, is thought to decrease the need for mechanical ventilation and consequently the risk of CLD. Nevertheless, in a large clinical trial comparing early CPAP versus surfactant and CMV, 83.1% of infants in the CPAP group needed intubation (SUPPORT 2010).
Description of the intervention
High frequency ventilation (HFV) aims to optimize lung expansion while minimizing tidal volumes to decrease lung injury. Animal studies have demonstrated that HFV is associated with improved pulmonary mechanics and reduced pulmonary inflammation (Yoder 2000). Human studies have shown that HFV maintains gas exchange with lower peak and mean airway pressures (Frantz 1983; Spitzer 1989). As many different manufacturers produce equipment to provide HFV, we planned to classify ventilators according to the mechanism by which gas exchange is achieved. We planned to include in this review two types of HFV.
High frequency oscillatory ventilation (HFOV) delivers tidal volume (Vt) smaller than the dead space through an electronically controlled piston‐diaphragm unit at rates of 300 to 900 breaths/min (5 to 15 Hz). The forward and backward action of the piston produces alternating positive and negative pressure changes, superimposed on the mean airway pressure (MAP), which results in a biphasic pressure waveform. MAP is adjusted to achieve the target lung volume and is controlled by adjusting the flow in the patient circuit. The operator sets the amplitude, frequency, MAP, and fraction of inspired oxygen (FiO2), and sets inspiratory time (Ti) as a proportion of the respiratory cycle. The default is 33% and changes with frequency. In HFOV, both expiration and inspiration are active processes, and a single ventilator provides both ventilation and oxygenation to patients (Cotten 2001; Courtney 2002;Courtney 2006).
High frequency jet ventilation (HFJV) requires use of the jet ventilator in conjunction with a conventional ventilator. It uses a standard endotracheal tube (ETT), but a special three‐way adaptor allows the tandem connection of the two ventilators. A patient box that contains a pinch valve, placed proximal to the ETT adaptor, is used to deliver high frequency gas pulses through the side nozzle at frequencies of 240 to 660 breaths/min (4 to 11 Hz). Lung volume is maintained by positive end‐expiratory pressure (PEEP) generated by the conventional ventilator with or without sigh breaths. Inspiratory time is set at 0.02 seconds and is occasionally modified. In HFJV, inspiration is active and expiration is passive through lung recoil (Cotten 2001; Courtney 2006).
Two strategies are used for initiation of HFV: elective and rescue (Bhuta 1998; Joshi 2006; Cools 2009; Henderson‐Smart 2009).
Elective: initiation of HFJV or HFOV as the primary mode of treatment or early in the course of RDS soon after CMV is commenced (Bhuta 1998; Cools 2009).
Rescue: initiation of HFJV or HFOV after failure to adequately ventilate or oxygenate a preterm infant on CMV, or when complications of CMV develop or are likely to develop (Bhuta 1998; Joshi 2006).
How the intervention might work
Neither HFOV nor HFJV has demonstrated clear superiority over CMV in Cochrane reviews, as outlined below. This fact may be due to lack of sufficient power in certain subpopulations such as extremely preterm infants or those with air leak syndromes. Basic physiology (Pfenninger 1988) and animal studies (Boros 1989) suggest that HFV offers some advantages over CMV. Both modes of HFV are widely used. The distinctive air flow pattern produced during HFJV may offer better ventilation of lungs affected by nonhomogenous disease processes (Loughnan 2010). Benefits for both modes of HFV could be due to the lower Vt. HFJV offers theoretical benefits through its passive expiration, along with lower MAP, when compared with HFOV. In animal studies, HFJV produced better ventilation at lower pressures than HFOV (Boros 1989; Zobel 1994). Both modalities of HFV are used for various indications; however, HFJV is thought to be more beneficial in nonhomogenous lung disease and air leak, as it allows for passive expiration and adjustment of inspiratory time, and it enables adequate gas exchange with lower mean airway pressures (Zobel 1994; Cotten 2001).
Elective use of HFOV
Cools 2009 found no clear evidence that elective HFOV offers important advantages over CMV when used as the initial ventilation strategy to treat preterm infants with acute lung disease. Investigators noted a small reduction in the rate of CLD with HFOV, but effects across trials were inconsistent, as were effects on short‐term and long‐term neurological outcomes. One large trial found a detrimental effect of HFOV on long‐term neurodevelopment, but the other five trials that reported this outcome did not describe such an effect (Cools 2010).
Rescue use of HFOV
One Cochrane review (Bhuta 2009) cited only one multicenter trial that randomized preterm infants with pulmonary interstitial emphysema (PIE), or at risk of developing PIE, to HFOV or CMV. Investigators noted no differences in mortality rate, in the need for intermittent positive‐pressure ventilation at 28 days of age, or in the incidence of PIE or gross pulmonary leak (e.g. pneumothorax, pneumomediastinum) between the two groups. In subgroup analysis of participants without PIE at study entry, infants assigned to HFOV had less risk of developing pulmonary air leak (typical risk ratio (RR) 0.73, 95% confidence interval (CI) 0.55 to 0.96). This fact was balanced against an increased rate of intraventricular hemorrhage (IVH) of any grade in the HFOV group (typical RR 1.77, 95% CI 1.06 to 2.96) (HIFO 1993).
Elective use of HFJV
Bhuta 1998 conducted a pooled analysis of three trials comparing elective HFJV with CMV. Trial authors reported that although infants who were treated with HFJV had a reduced rate of bronchopulmonary dysplasia, a worrisome trend showed increased risk of brain injury in infants ventilated by a low volume HFJV strategy.
Rescue use of HFJV
We found limited data about rescue use of HFJV in comparison with CMV among studies performed in the late 1980s, before surfactant became generally available. The Cochrane review conducted by Joshi and Bhuta (Joshi 2006) stated that because of the small numbers randomized and methodological problems of the single included trial, information is insufficient to permit assessment of the effectiveness of rescue HFJV in preterm infants.
HFJV and HFOV provide respiratory support via different mechanisms, and their safety and efficacy profiles may differ, particularly among different subgroups of patients.
Why it is important to do this review
Many clinicians continue to use HFV as elective and rescue therapy, and evidence for its benefits and harms versus CMV in certain subpopulations is incomplete. As the two modes use different mechanics, direct comparison of both elective HFJV versus elective HFOV, and rescue HFJV versus rescue HFOV, is needed to examine the safety and efficacy of both modes of ventilation to ascertain assumed benefits and potential harms.
Objectives
To compare the benefits and side effects of HFJV versus HFOV for mortality and morbidity in preterm infants born at less than 37 weeks GA with pulmonary dysfunction in both elective and rescue modes.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include randomized controlled trials (RCTs) and quasi‐RCTs. We considered a trial to be randomized when study author(s) stated explicitly that groups compared in the trial were established by random allocation. We intended to include quasi‐RCTs when a systematic method of allocation was used. We intended to include cluster‐randomized trials for which the unit of analysis would be the whole group, not individual patients assigned to the group.
We intended to exclude cross‐over studies, as they would not allow assessment of the outcomes of interest (CLD and death) as they would occur after both modes are used.
Types of participants
Preterm infants born at less than 37 weeks gestational age (GA) with pulmonary dysfunction requiring intubation and CMV.
Types of interventions
HFOV defined as ventilation R from 10 to 20 Hz, a fractional inspiratory time of 0.3, and pressure amplitude sufficient to produce visible chest wall motion (Henderson‐Smart 2009).
HFJV defined as high‐flow, short‐duration pulses of pressurized gas inserted directly into the upper airway through a specifically designed endotracheal lumen, at rates of 150 to 600 breaths per minute (Joshi 2006).
Elective when randomization occurred before initiation of ventilation or early in the course of pulmonary dysfunction soon after mechanical ventilation was commenced.
Rescue when randomization occurred after CMV failed to adequately ventilate, or when complications of CMV developed or were likely to develop.
We intended to include HFOV or HFJV provided with any type of device. We intended to explore in a subgroup analysis differences in outcomes attributable to the type of device used.
Types of outcome measures
Primary outcomes
Composite outcome of death from any cause before initial hospital discharge, or CLD (need for supplemental oxygen at 36 weeks GA).
Secondary outcomes
Death from any cause before initial hospital discharge.
-
CLD.
Need for supplemental oxygen at 28 days of age.
Need for supplemental oxygen at 36 weeks GA or at discharge for infants born at less than 32 weeks GA.
Duration of hospital admission (days).
Duration of CMV (days of all types of CMV including HFV and CMV).
Duration of respiratory support (days of all types of respiratory support including CMV and CPAP).
Duration of oxygen supplementation (days).
Weight gain (from birth to hospital discharge).
Air leak syndrome: pneumothorax or PIE (study author defined).
IVH grade 3 or 4 (Papile 1978).
Periventricular leukomalacia (PVL).
Retinopathy of prematurity (ROP): defined as stage 2 or higher (ICROP 1984; ICROP 1987; ICROP 2005).
Neurodevelopmental outcomes including motor, mental, and sensory outcomes at two years of age (study author defined).
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 11); MEDLINE via PubMed (1996 to November 30, 2015); EMBASE (1980 to November 30, 2015); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to November 30, 2015) with use of the following search terms: {high frequency ventilation (explode) [MeSH heading] or high frequency oscillatory ventilation (explode) [MeSH heading] or high frequency jet ventilation (explode) [MeSH heading] or oscillatory ventilation (explode) [MeSH heading] or jet ventilation (explode) [MeSH heading]}, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategy for each database).
We imposed no date, language, or publication restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the International Standard Randomized Controlled Trials Number (ISRCTN) Registry (controlled‐trials.com)).
We searched Proceedings of Society for Pediatric Research/American Pediatric Society meetings for the years 2002 to 2012 at www.abstracts2view.com/pasall/.
Searching other resources
We consulted national and international trials registers and experts in this field for ongoing studies. We checked the reference lists of all relevant articles to identify other relevant studies. We intended to contact authors of any identified abstracts or unpublished studies to ascertain the nature of the study design and outcome measures if not reported. We intended to include abstracts and unpublished studies in the meta‐analysis if they provided sufficient information on study design and outcome measures in the publication, or if we could obtain this information by contacting study authors.
Data collection and analysis
Selection of studies
Two review authors (YE, AA) independently identified studies and assessed whether they met the inclusion criteria (see Figure 1). We used an eligibility checklist developed by these review authors that was based on targeted participants (preterm infants) and interventions of interest (HFJV, HFOV) to determine eligibility for inclusion. We piloted this approach to determine interobserver agreement between the two review authors. We planned to resolve disagreements by discussion between all five review authors. We intended to identify studies that were potentially eligible for the review on the basis of abstract review, and to retrieve full‐text articles when possible. We intended to state independently reasons for exclusion.
1.
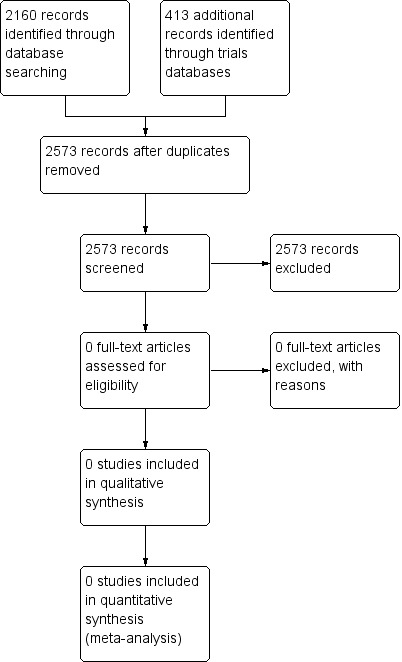
Study flow diagram.
Data extraction and management
Two review authors (YE, AA) intended to independently extract data using a standardized data extraction form that included:
study details: title, names of authors, publication status, and year of publication;
study eligibility and characteristics: study type, participant characteristics, diagnostic criteria for respiratory failure, elective HFV, rescue HFV, ventilation strategy (low vs high volume), definition of CLD, death, coincidental complications, and other secondary outcomes;
methodological quality: method of sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, selective reporting, and intention‐to‐treat (ITT) analysis; and
outcomes: number of infants in each study group and number of infants with each outcome measure reported. We intended to abstract outcomes using the measures reported in the study for all outcomes. If results were not reported in a way that they could be included in the review, we planned to contact study authors to request clarification and additional data. We planned to analyze infants in the group to which they were randomized, regardless of other modes of ventilation used.
We identified no trials for inclusion and found no discrepancies between review authors.
Assessment of risk of bias in included studies
Two review authors (YE, AA) intended to independently assess the methodological quality of all included studies by using the following template.
Adequate sequence generation
We intended to categorize risk of selection bias as:
low risk: adequate (any truly random process; e.g. random numbers table, computer random numbers generator);
high risk: inadequate (any non‐random process; e.g. odd or even date of birth, hospital or clinic record number); or
unclear risk: no or unclear information provided.
Allocation concealment
We intended to categorize risk of bias regarding allocation concealment as:
low risk: adequate (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk: inadequate (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or
unclear risk: no or unclear information provided.
Performance bias
We intended to categorize methods used to blind study personnel from knowledge of which intervention a participant received as:
low risk: adequate for personnel aware of group assignment (e.g. no difference in response attributable to behavioral responses of participants or researchers to knowledge that a participant is in or is not included in either group);
high risk: inadequate for personnel aware of group assignment; or
unclear risk: no or unclear information provided.
Note: As our study population consists of neonates, all would be blinded to the study intervention.
Detection bias
We intended to categorize methods used to blind outcome assessors from knowledge of which intervention a participant received. We intended to assess blinding separately for different outcomes or classes of outcomes. We planned to categorize methods used with regard to detection bias as:
low risk: adequate; follow‐up was performed with assessors blinded to groups;
high risk: inadequate; assessors at follow‐up were aware of group assignments; or
unclear risk: no or unclear information provided.
Note: As our study population consists of neonates, all would be blinded to the study intervention.
Attrition bias
We intended to describe completeness of data including attrition and exclusions from analysis for each outcome in each study. We intended to report numbers included in the analysis at each stage (compared with total numbers of randomized participants), reasons for attrition or exclusion when provided, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we intended to re‐include missing data in the analyses. We intended to categorize methods with respect to risk of attrition bias as:
low risk: adequate (< 10% missing data);
high risk: inadequate (> 10% missing data); or
unclear risk: no or unclear information provided.
Reporting bias
We intended to describe how we investigated the risk of selective outcome reporting bias and to present our findings. We intended to assess these methods as:
low risk: adequate (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest for the review have been reported);
high risk: inadequate (when not all of the study's prespecified outcomes have been reported):
One or more reported primary outcomes were not prespecified;
Outcomes of interest were reported incompletely and so cannot be used; or
Study failed to provide results of a key outcome for which we would have expected to receive data.
unclear risk: no or unclear information provided (study protocol was not available).
Other bias
We intended to describe any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early as the result of some data‐dependent process). We intended to assess whether each study was free of other problems that could put it at risk of bias as:
low risk: no concerns of other bias raised;
high risk: concerns raised about multiple looks at the data with results made known to investigators, differences between the abstract and the final published paper in the numbers of participants enrolled; or
unclear: concerns raised about potential sources of bias that could not be verified by contacting study authors.
Overall risk of bias
We intended to make explicit judgments about whether studies were at high risk of bias, according to criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to assess the likely magnitude and direction of bias, and whether we considered it likely to impact study findings. We intended to explore the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
We intended to use Review Manager (RevMan 2011) software for all statistical analyses. We intended to express dichotomous data as risk ratios (RRs) with their 95% confidence intervals (CIs). We intended to calculate the risk difference (RD) and its 95% CI if the RD was statistically significant, and we intended to convert the RD to the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). We intended to report continuous data as mean differences (MDs) and to report 95% CIs on all estimates.
Unit of analysis issues
When cluster trials were identified, we intended to analyze the whole group rather than the individual patient.
Dealing with missing data
We intended to contact study authors if we found that data were missing. We intended to exclude studies from the analysis if missing data could not be adequately explained.
Assessment of heterogeneity
We intended to assess heterogeneity for outcomes by using the Chi2 test in RevMan 5.1, with the null hypothesis being no heterogeneity for treatment effect (Higgins 2011; RevMan 2011). The I2 statistic assesses the impact of heterogeneity on the meta‐analysis. The magnitude is roughly interpreted as follows: less than 25% unimportant, 25% to 49% low, 50% to 74% moderate, 75% or greater high. We planned to calculate summary treatment effects by using RevMan 5.1 (RevMan 2011).
Assessment of reporting biases
We intended to assess funnel plot symmetry to detect publication bias when possible.
Data synthesis
We intended to summarize data statistically if we identified similar trials of adequate quality (meeting inclusion criteria). We intended to consider conducting a meta‐analysis if we identified more than one eligible trial, and if homogeneity among the studies was sufficient with respect to participants and reported outcomes. We intended to perform statistical analysis according to statistical guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to use standard methods of the Cochrane Neonatal Review Group and a fixed‐effect model. We intended to use the typical RR and the typical RD with 95% CIs to assess treatment effects for dichotomous outcomes. We intended to use MDs with 95% CIs for outcomes measured on a continuous scale.
Subgroup analysis and investigation of heterogeneity
We intended to undertake the following subgroup analyses.
GA at birth: < 30 weeks, 30 weeks to 36 weeks and 6 days.
Birth weight strata: ≤ 1000 grams and > 1000 grams.
Surfactant replacement therapy: treatment or nontreatment with surfactant regardless of timing or type.
Management of HFV: trials with and without high volume strategy, defined as initial use of higher mean airway pressure than on CMV; initial weaning of FiO2 before mean airway pressure; and use of alveolar recruitment maneuvers including sigh breaths (Bhuta 1998; Cools 2009).
Type of HFV ventilator: true oscillator versus flow interrupter for HFOV; different brand for HFJV.
Gas humidification: trials with and without adequate humidification (30 mg H2O/L (Restrepo 2012) of inspired gas (Joshi 2006)).
Inhaled nitric oxide (iNO) for prophylaxis of bronchopulmonary dysplasia (BPD) ‐ study author‐defined prophylaxis (Schreiber 2003).
Cause of pulmonary dysfunction: RDS, PIE/air leak, pneumonia, as defined by study author.
Sensitivity analysis
We intended to perform a sensitivity analysis for methodological quality using the risk of bias domains. We intended to conduct sensitivity analysis with and without inclusion of cluster‐randomized trials.
Results
Description of studies
We found no study that met the inclusion criteria.
Results of the search
We found no study that met the inclusion criteria.
Included studies
We found no study that met the inclusion criteria.
Excluded studies
None.
Risk of bias in included studies
N/A.
Allocation
N/A.
Blinding
N/A.
Incomplete outcome data
N/A.
Selective reporting
N/A.
Other potential sources of bias
N/A.
Effects of interventions
N/A.
Discussion
High frequency ventilation (HFV) seems to hold great promise, but effects in clinical studies are equivocal. Neither high frequency oscillatory ventilation (HFOV) (Cools 2009; Cools 2010) nor high frequency jet ventilation (HFJV) (Bhuta 1998; Joshi 2006) has demonstrated clear superiority over conventional mechanical ventilation (CMV) in human studies. However, basic physiology (Pfenninger 1988) and animal studies (Boros 1989) suggest that HFV offers some advantages over CMV. Both modes of HFV are widely used. Given that HFJV and HFOV provide respiratory support via different mechanisms, their safety and efficacy profiles may differ, particularly in different subgroups of patients. As noted previously, animal studies have demonstrated that HFV is associated with improved pulmonary mechanics and reduced pulmonary inflammation (Yoder 2000). Human studies have demonstrated that HFV maintains gas exchange with lower peak inspiratory pressure (PIP) and positive end expiratory pressure (PEEP) (Frantz 1983; Spitzer 1989). The two modes (HFOV and HFJV) are notably mechanically different, and their clinical effects may differ. HFOV deliver tidal volumes smaller than the dead space by using a piston or a diaphragm with active inspiration and expiration (Cotten 2001; Courtney 2002;Courtney 2006), whereas HFJV is used in conjunction with CMV and delivers pulses of gas into the trachea with active inspiration and passive expiration (Cotten 2001; Courtney 2006). Both can be used as elective or rescue treatment (Bhuta 1998; Joshi 2006; Cools 2009; Henderson‐Smart 2009).
We found no studies that directly compared the two modes of HFV so could not evaluate their presumed physiological benefits and side effects.
Summary of main results
We found no studies that met the inclusion criteria.
Overall completeness and applicability of evidence
We found no studies that met the inclusion criteria.
Quality of the evidence
We found no studies that met the inclusion criteria.
Potential biases in the review process
N/A.
Agreements and disagreements with other studies or reviews
N/A.
Authors' conclusions
Implications for practice.
Despite theoretical differences between the two modes of HFV, we found no clinical evidence by which to evaluate the superiority of HFJV or HFOV, whether given as elective or rescue therapy.
Implications for research.
We identified the need for continued research to examine the effects of HFV as a whole, and of HFJV and HFOV as subtypes, in current preterm infants. These studies should reflect high risk subpopulations and should be powered to follow up on previous concerns about increased risk of intraventricular hemorrhage (IVH) versus reduction in chronic lung disease (CLD).
Notes
N/A.
Acknowledgements
None.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) The Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Differences between protocol and review
All review authors contributed to editing the protocol and review. Two review authors conducted the independent search.
Contributions of authors
Yahya Ethawi: writing the protocol, performing literature search, identifying studies, abstracting data, analyzing data, writing and editing the review. Ayman Abou Mehrem: writing the protocol, identifying studies (literature search), abstracting data, analyzing data. John Minski: writing the protocol, writing the review. Chelsea Ruth: writing the protocol, writing and editing the review. Peter Davis: writing the protocol, writing and editing the review.
Sources of support
Internal sources
-
University of Manitoba Library, Canada.
As source of search for articles for registering the title, writing the protocol, and conducting the review.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None known.
New
References
Additional references
Attar 2002
- Attar MA, Donn SM. Mechanisms of ventilator‐induced lung injury in premature infants. Seminar Neonatology 2002;7(5):353‐60. [PUBMED: 12464497 ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. The Journal of Pediatrics 2012;160(1):49‐53.e1. [PUBMED: 21868028 ] [DOI] [PubMed] [Google Scholar]
Bhuta 1998
- Bhuta T, Henderson‐Smart DJ. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000328; PUBMED: 10796194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhuta 2009
- Bhuta T, Henderson‐Smart DJ. Rescue high frequency oscillatory ventilation versus conventional ventilation for pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2009, Issue CD000438. DOI: 10.1002/14651858.CD000438. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boros 1989
- Boros SJ, Mammel MC, Coleman JM, Horcher P, Gordon MJ, Bing DR. Comparison of high‐frequency oscillatory ventilation and high‐frequency jet ventilation in cats with normal lungs. Pediatric Pulmonology. New York: Theime medical publication inc, 1989; Vol. 7, issue 1:35‐41. [PUBMED: 2771469] [DOI] [PubMed]
Cools 2009
- Cools F, Henderson‐Smart DJ, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD000104.pub3; PUBMED: 19588317] [DOI] [PubMed] [Google Scholar]
Cools 2010
- Cools F, Askie LM, Offringa M, Asselin JM, Calvert SA, Courtney SE, et al. Elective high‐frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta‐analysis of individual patients' data. The Lancet 2010;375(9731):2082‐91. [DOI: 10.1016/S0140-6736(10)60278-4; PUBMED: 20569978] [DOI] [PubMed] [Google Scholar]
Cotten 2001
- Cotten M, Clark RH. The science of neonatal high‐frequency ventilation. Respiratory Care Clinics of North America 2001;7(4):611‐31. [PUBMED: 11926759] [DOI] [PubMed] [Google Scholar]
Courtney 2002
- Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT, Neonatal Ventilation Study Group. High‐frequency oscillatory ventilation versus conventional mechanical ventilation for very‐low‐birth‐weight infants. New England Journal of Medicine 2002;347(9):643‐52. [PUBMED: 12200551] [DOI] [PubMed] [Google Scholar]
Courtney 2006
- Courtney SE, Asselin JM. High‐frequency jet and oscillatory ventilation for neonates: which strategy and when. Respiratory Care Clinics of North America 2006;12(3):453‐67. [PUBMED: 16952804] [DOI] [PubMed] [Google Scholar]
Frantz 1983
- Frantz ID 3rd, Werthammer J, Stark AR. High‐frequency ventilation in premature infants with lung disease: adequate gas exchange at low tracheal pressure. Pediatrics 1983;71(4):483‐8. [PUBMED: 6835731] [PubMed] [Google Scholar]
Henderson‐Smart 2009
- Henderson‐Smart DJ, Paoli AG, Clark RH, Bhuta T. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002974.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
HIFO 1993
- HiFO Study Group. Randomized study of high‐frequency oscillatory ventilation in infants with severe respiratory distress syndrome. The Journal of Pediatrics 1993;122(4):609‐19. [PUBMED: 8463913] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hintz 2005
- Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD, NICHD Neonatal Research Network. Changes in neurodevelopmental outcomes at 18 to 22 months' corrected age among infants of less than 25 weeks' gestational age born in 1993‐1999. Pediatrics 2005;115(6):1645‐51. [PUBMED: 15930228] [DOI] [PubMed] [Google Scholar]
ICROP 1984
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] [DOI] [PubMed] [Google Scholar]
ICROP 1987
- The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. Archives of Ophthalmology 1987;105(7):906‐12. [PUBMED: 3606449] [PubMed] [Google Scholar]
ICROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Jobe 2000
- Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant treatment era. Annual Review of Physiology 2000;62:825‐46. [PUBMED: 10845113] [DOI] [PubMed] [Google Scholar]
Joshi 2006
- Joshi VH, Bhuta T. Rescue high frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000437.pub2] [DOI] [PubMed] [Google Scholar]
Loughnan 2010
- Loughnan P. High frequency jet ventilation (HFJV) in mature infants with non‐homogeneous lung disease and severe gas rapping. http://www.bunl.com/Clinical%20PDFs/Loughnan.pdf 2010 (accessed 30 November 2015).
Natarajan 2012
- Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Human Development 2012;88(7):509‐15. [PUBMED: 22236557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Pfenninger 1988
- Pfenninger J, Minder C. Pressure‐volume curves, static compliances and gas exchange in hyaline membrane disease during conventional mechanical and high‐frequency ventilation. Intensive Care Medicine 1988;14(4):364‐72. [DOI] [PubMed] [Google Scholar]
Restrepo 2012
- Restrepo RD, Walsh BK, American Association for Respiratory Care. Humidification during invasive and noninvasive mechanical ventilation: 2012. Respiratory Care 2012;57(5):782‐8. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schreiber 2003
- Schreiber MD, Gin‐Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. The New England Journal of Medicine 2003;349(22):2099‐107. [DOI: 10.1056/NEJMoa031154; PUBMED: 14645637] [DOI] [PubMed] [Google Scholar]
Singer 1997
- Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics 1997;100(6):987‐93. [PUBMED: 9374570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2004
- Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. The Journal of Pediatrics 2004;144(6):799‐803. [PUBMED: 15192629] [DOI] [PubMed] [Google Scholar]
Spitzer 1989
Stichtenoth 2012
- Stichtenoth G, Demmert M, Bohnhorst B, Stein A, Ehlers S, Heitmann F, et al. Major contributors to hospital mortality in very‐low‐birth‐weight infants: data of the birth year 2010 cohort of the German Neonatal Network. Klinische Padiatrie [Clinical Pediatrics] 2012;224(4):276‐81. [PUBMED: 22441803] [DOI] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [PUBMED: 20732945 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SUPPORT 2010
- SUPPORT Study Group, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. The New England Journal of Medicine 2010;362(21):1970‐9. [PUBMED: 20472939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoder 2000
- Yoder BA, Siler‐Khodr T, Winter VT, Coalson JJ. High‐frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine 2000;162(5):1867‐76. [PUBMED: 11069828] [DOI] [PubMed] [Google Scholar]
Zobel 1994
- Zobel G, Dacar D, Rodl S. Proximal and tracheal airway pressures during different modes of mechanical ventilation: an animal model study . Pediatric Pulmonology 1994 ; 18 ( 4 ):239‐43. [PUBMED: 7838623] [DOI] [PubMed] [Google Scholar]


