Abstract
Background
Spina bifida is a fetal neural tube defect (NTD), which may be diagnosed in utero and is compatible with life postnatally, albeit often with significant disability and morbidity. Although postnatal repair is possible, with increasing in utero diagnosis with ultrasound, the condition has been treated during pregnancy (prenatal repair) with the aim of decreased morbidity for the child. The procedure that is performed during pregnancy does have potential morbidities for the mother, as it involves maternal surgery to access the fetus.
Objectives
To compare the effects of prenatal versus postnatal repair and different types of repair of spina bifida on perinatal mortality and morbidity, longer term infant outcomes and maternal morbidity.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2014).
Selection criteria
All published, unpublished, and ongoing randomised controlled trials comparing prenatal and postnatal repair of meningomyelocele for fetuses with spina bifida and different types of prenatal repair.
Data collection and analysis
Two review authors independently evaluated trials for inclusion and methodological quality without consideration of their results according to the stated eligibility criteria and extracted data.
Main results
Our search strategy identified six reports for potential inclusion. Of those, we included one trial (four reports) involving 158 women, which was at low risk of bias.
The one included trial examined the effect of prenatal repair versus postnatal repair. For the primary infant outcome of neonatal mortality, there was no clear evidence of a difference identified for prenatal versus postnatal repair (one study, 158 infants, risk ratio (RR) 0.51, 95% confidence interval (CI) 0.05 to 5.54), however event rates were uncommon and so the analysis is likely to be underpowered to detect differences.
Prenatal repair was associated with an earlier gestational age at birth (one study, 158 infants, mean difference (MD) ‐3.20 weeks, 95% CI ‐3.93 to ‐2.47) and a corresponding increase in both the risk of preterm birth before 37 weeks (one study, 158 infants, RR 5.30, 95% CI 3.11 to 9.04) and preterm birth before 34 weeks (one study, 158 infants, RR 9.23, 95% CI 3.45 to 24.71). Prenatal repair was associated with a reduction in shunt dependent hydrocephalus and moderate to severe hindbrain herniation. For women, prenatal repair was associated with increased preterm ruptured membranes (one study, 158 women, RR 6.15, 95% CI 2.75 to 13.78), although there was no clear evidence of difference in the risk of chorioamnionitis or blood transfusion, although again, event rates were uncommon.
A number of this review's secondary infant and maternal outcomes were not reported. For the infant: days of hospital admission; survival to discharge; stillbirth; need for further surgery (e.g. skin grafting); neurogenic bladder dysfunction; childhood/infant quality of life. For the mother: admission to intensive care; women's emotional wellbeing and satisfaction with care.
Authors' conclusions
This review is based one small well‐conducted study. There is insufficient evidence to recommend drawing firm conclusions on the benefits or harms of prenatal repair as an intervention for fetuses with spina bifida. Current evidence is limited by the small number of pregnancies that have been included in the single conducted randomised trial to date.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Gestational Age; Pregnancy Outcome; Premature Birth; Prenatal Care; Randomized Controlled Trials as Topic; Spinal Dysraphism; Spinal Dysraphism/mortality; Spinal Dysraphism/surgery
Plain language summary
Spina bifida repair and infant and maternal health
Spina bifida is the term used to describe a group of neural tube conditions where the fetal spinal cord does not close properly during the first month of pregnancy. With open spina bifida some of the vertebrae are not completely formed but are split or divided and the spinal cord and its coverings (the meninges) protrude through the opening. The most severe is where the spinal cord and meninges come out of the child's back (myelomeningocele). Open spina bifida is often associated with hindbrain herniation, where the cerebellum and brainstem tissue extend into the large opening in the base of the skull, and hydrocephalus (enlargement of the fluid filled cavities in the brain). Resulting disabilities include bladder and bowel incontinence, difficulties in moving about due to limb weakness, paralysis, deformity and loss of sensation. Conventional treatment of spina bifida is surgical repair within two days of birth, which may include the placement of a shunt between the ventricles of the baby’s brain and the belly (peritoneum) to relieve hydrocephalus. Spina bifida can be diagnosed with prenatal ultrasound or maternal serum alpha‐feto protein and in utero treatment could improve outcomes; although it involves surgical incision into the mother’s abdomen and uterus to access the unborn baby.
This review aimed to compare the effects of in utero repair versus repair as a newborn. We included one randomised controlled trial involving 158 women who were from 19 to 27 weeks pregnant with a baby with severe spina bifida and evidence of hindbrain herniation. For neonatal mortality, there was no clear difference identified for prenatal versus postnatal repair. However, the numbers of neonates who died were low and so the review was likely underpowered to detect any difference. Prenatal repair was associated with reduced need for shunt placement and a reduction in the risk of moderate to severe hindbrain herniation after birth. No direct complications of the repair procedure were evident, including orthopaedic deformities. Prenatal repair was associated with an increased risk of the women experiencing preterm ruptured membranes and subsequent preterm birth (both before 34 and 37 weeks). Severe maternal illness (infection and need for blood transfusion) were not clearly different; although the review was underpowered to detect any difference in these important, less common outcomes. The included trial was of high quality (low risk of bias) but included a small number of pregnancies. There is currently insufficient evidence to recommend in utero repair for unborn babies with spina bifida.
Background
Description of the condition
The term spina bifida is from the Latin words 'spina' meaning spine and 'bifida' meaning split or divided. The human brain, spinal cord and spine develop from a plate of cells along the back of the embryo (developing human). During development this plate of cells forms a closed tube which is called the neural tube. The human spine is made up of separate bony components called vertebrae, which normally cover and protect the spinal cord (the nerve tissue that carries messages between the brain and the body).
If problems occur during development, neural tube defects (NTDs) such as spina bifida result (Mitchell 2004; Sutton 2008; Walsh 2003). NTDs result in the brain or spinal cord structures not being protected and these structures developing outside the body. In spina bifida, some of the vertebrae are not completely formed but are split or divided and the defective spinal cord and its coverings protrude through the opening.
There are different categories of spina bifida including open and closed defects. An open spina bifida is more severe and is classified either as a meningocele or meningomyelocele. A meningocele is a fluid filled sac that protrudes through the defect in the spine. The outer part of the spinal bony components (vertebrae) is split but the spinal cord is normal. Only the meninges (the membrane covering the spinal cord) is damaged and pushed out through the opening. Myelomeningoceles are the more severe form and involve the outer part of the vertebrae being split with the spinal cord and meninges damaged and protruding from the opening in the bone, muscle and skin. The majority of spina bifida abnormalities involve the lower back (lumbar and sacral area), whilst a smaller proportion involve the upper back (thoracic and cervical) (Mitchell 2004; Sutton 2008; Walsh 2003).
Epidemiology
The incidence of NTDs and spina bifida varies with time, geography and ethnicity. The incidence in some countries has decreased over the last 20 years, but variations still remain between regions and countries.
In Australia, the incidence of NTDs is approximately 0.5/1000 births (livebirths and stillbirths).The majority of these abnormalities are spina bifida (0.3/1000), with anencephaly and encephalocele making up the remainder. The incidence of spina bifida varies with inclusion of terminations of pregnancy, being up to 0.6 per 1000 pregnancies (live births, stillbirths and terminations) (AIHW 2004). Incidence is similar in the United States, although some countries experience much higher rates, for example China has reported rates up to 2.92/1000 pregnancies (Mitchell 2004). It is estimated that one‐quarter of pregnancies where a NTD is diagnosed, are terminated (Sutton 2008).
NTDs have a multifactorial causation; risk factors include teratogens (particularly the anti‐epileptic medications valproic acid and carbamazepine), diet (particularly folate deficiency), family history, geographical location, genetics and obesity (Mitchell 2004). The following factors are be well established risk factors (with relative risks) and variations in these may contribute to different incidence rates across time and geography; history of previous affected pregnancy with same partner (RR = 30), inadequate maternal intake of folic acid (RR = 2–8), pre‐gestational maternal diabetes (RR = 2–10), exposure to valproic acid and carbamazepine (RR = 10‐20) (Mitchell 2004).
Diagnosis of the condition
Spina bifida and associated structural abnormalities are able to be diagnosed with prenatal ultrasound or maternal serum alpha‐feto protein. Amniocentesis (when a sample of amniotic fluid is taken with a needle, for testing) is also utilised, predominantly to detect fetal chromosomal abnormalities. Prenatal magnetic resonance imaging (a sophisticated medical imaging test) may be used to further characterise the associated brain structural abnormalities (Chiari II malformation). Prenatal ultrasound can also be used to monitor and assess the presence of leg and spine abnormalities and spontaneous leg movements.
Outcomes of spina bifida
Associated anomalies
Open spina bifida is often associated with abnormalities of brain structure and hydrocephalus (enlargement of the fluid filled cavities in the brain). There may be other structural anomalies present, in addition to the NTD, and in a small percentage a recognisable "malformation syndrome" may be diagnosed (Mitchell 2004).
Later health for the infant and child
The amount and type of health issues caused by a spina bifida varies on its type and the level of the defect in the spinal cord. Abnormalities occurring at a higher level in the spinal cord generally cause more severe disability. The main disabilities resulting when a child has spina bifida are bladder and bowel incontinence and difficulties in ambulation due to weakness, paralysis deformity and loss of sensation. When hydrocephalus occurs, there is frequently need for surgical intervention after birth in the form of a shunt (Mitchell 2004).
Description of the intervention
Conventional treatment of spina bifida has been to undertake a postnatal repair within two days of birth. Immediate postnatal treatment may also include the placement of a ventriculo‐peritoneal shunt to relieve ventriculomegaly or hydrocephalus (Mitchell 2004).
It has been observed by repeated ultrasound examination that affected babies may show neurological deterioration as pregnancy progresses, i.e. spontaneous leg movement may be seen early in pregnancy which evolves to paralysis and deformity later in pregnancy. It has been hypothesised that as pregnancy progresses the sac that contains neural tissue is exposed to amniotic fluid or trauma with resultant damage to the tissue. This observation has led to the proposal that in utero treatment of spina bifida might lead to improved postnatal outcomes. In utero repair by hysterotomy was first reported in 1998 (Adzick 1998; Tulipan 1998), and initial observations suggested that those infants who received in utero therapy had less requirement for shunting after birth. Endoscopic repair has also been described (Bruner 1999). Outcomes in a subsequent larger group of infants receiving in utero treatment, compared with historical controls seemed to show improvement in terms of shunt requirements and brain abnormalities (Tulipan 1999; Tulipan 2003).
How the intervention might work
It has been hypothesised that prenatal repair of a spina bifida lesion (by closure of an open lesion) might prevent damage of neural tissue as pregnancy progresses and thereby prevent deterioration of function and morbidity after birth. Advantages may therefore include improved neurologic function, and less severe or absent hindbrain herniation (Walsh 2003). Disadvantages of prenatal repair include the potential side effects of maternal procedures that involve opening the maternal abdomen and uterus at a preterm stage of pregnancy, in addition to anaesthetic complications (Sutton 20080. There is also the potential for subsequent rupture of membranes, preterm labour and maternal infection (Sutton 2008).
Why it is important to do this review
Spina bifida is a type of NTD which may be diagnosed in utero and is compatible with life postnatally, albeit with associated disability and morbidity. Although postnatal repair is possible, with increasing in utero diagnosis with ultrasound, the condition has been treated during pregnancy (prenatal repair) with the aim of decreased morbidity for the child. However, the prenatal repair procedure does have potential morbidities for the woman, in particular preterm labour. It is important to review this topic in a systematic way and assess the benefits and risks of this approach to treatment.
Objectives
To compare the effects of prenatal versus postnatal repair and different types of repair of spina bifida on perinatal mortality and morbidity, longer term infant outcomes and maternal morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials that compare prenatal and postnatal repair of antenatally diagnosed spina bifida and also those trials that compare different approaches to in utero repair.
Cluster‐randomised trials and trials using a cross‐over design were not eligible for inclusion. Studies presented in abstract form were eligible for inclusion, depending on the amount of information present in the abstract.
Types of participants
Women with a singleton pregnancy in which the fetus has been diagnosed with open spina bifida/meningomyelocele.
Types of interventions
Prenatal (in utero) repair of myelomeningocele compared with postnatal repair, and also different types of prenatal repair.
Types of outcome measures
Primary outcomes
Infant outcomes
Neonatal mortality
Secondary outcomes
Infant outcomes
Shunt dependent hydrocephalus
Moderate/severe hindbrain herniation after birth
Gestational age at birth
Preterm birth before 37 weeks
Preterm birth before 34 weeks
Perinatal mortality
Stillbirth
Days of hospital admission
Survival to discharge
Direct complications of the repair procedure
Perinatal mortality
Need for further surgery after initial repair procedure, e.g. skin grafting
Orthopaedic deformities after birth
Neurogenic bladder dysfunction
Childhood/infant quality of life
Maternal outcomes
Preterm ruptured membranes
Maternal infectious morbidity (any of: endometritis, wound infection, chorioamnionitis)
Maternal blood transfusion
Admission to intensive care
Emotional wellbeing and satisfaction with care
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 July 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We planned to resolve any disagreement through discussion or, if required, by consulting the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We planned to resolve discrepancies through discussion or, if required, by consulting the third person. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
If information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for the one included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for the included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies would be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. In general we planned to use a cut‐off point of 20% missing data to assess a study as adequate. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias .
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included study any important concerns we had about other possible sources of bias.
We assessed whether the study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether the one included study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
We did not plan to include cluster‐randomised trials or trials with a cross‐over design. It is unlikely that any trials will be identified that use more than one treatment group. Multiple pregnancies are not the focus of our review and it is unlikely that they would be included in any study of this type of intervention.
Dealing with missing data
For the included study, we noted levels of attrition. If we had included more than one study, we planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. In future updates of this review, if more trials are included, we will regard heterogeneity as substantial if an I² is greater than 30% and either the Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had we suspected reporting bias (see 'Selective reporting bias' above), we planned to attempt to contact study authors to ask them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, if asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). Had we included more than one trial, we planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We planned to treat the random‐effects summary as the average range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not combine trials.
In future updates of the review, if we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, we would have used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
repair at less than 24 weeks versus repair at greater than 24 weeks (for those studies looking at in utero repair);
quasi‐randomised controlled trials versus randomised controlled trials.
We planned to use the following outcomes in subgroup analysis: neonatal mortality and preterm labour before 34 weeks.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We planned to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value
Sensitivity analysis
Sensitivity analyses were not required.
Results
Description of studies
Results of the search
There were six trial reports for consideration in the Pregnancy and Childbirth Group's Trials Register. The primary trial report for the "MOMS trial" is included in the meta‐analysis and is the only included study (Adzick 2011) (see:Figure 1).
1.
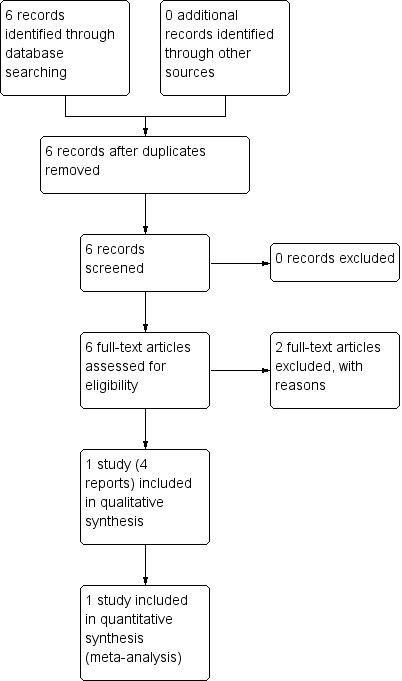
Study flow diagram.
Included studies
One study was included (Adzick 2011) (with four associated reports). This randomised trial was conducted in three maternal‐fetal surgery centres in the United States and compared prenatal surgery for fetal myelomeningocele (MMC) with postnatal repair (standard care) for fetuses/infants with a MMC with an upper boundary spinal level between T1 and S1, evidence of hindbrain herniation, gestational age between 19 and 27 weeks and normal karyotype. For women assigned to the prenatal surgery group, maternal laparotomy and uterine incision (hysterotomy) was performed and then the lesion repaired using standardised techniques. Birth was by subsequent caesarean section at 37 weeks. Women and their infants in the standard care group, underwent caesarean delivery at 37 weeks and then postnatal repair of the MMC. The first primary outcome of this trial was a composite outcome of perinatal death and shunt placement by 12 months, and the second primary outcome was infant neurodevelopment at 30 months of age.
Excluded studies
Two studies (one report each) excluded from the review were studies examining methods for uterine entry and not surgical repair (Almodin 2006; Bruner 1999a).
Risk of bias in included studies
Overall, the methodological quality of the one included trial was good. See Figure 2 and Figure 3 for a summary of Risk of bias' assessments.
2.
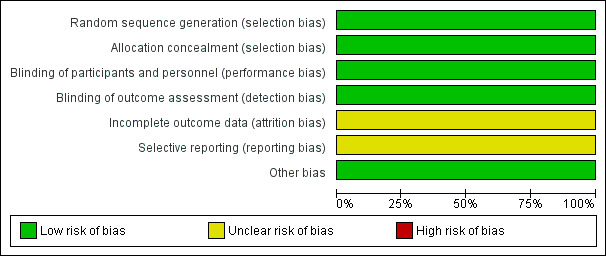
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
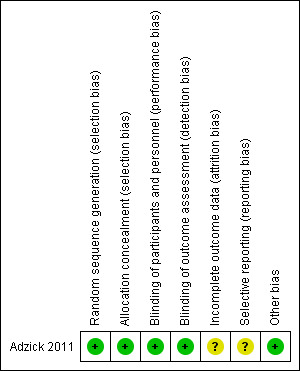
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study was stated to be randomised, using a randomisation method based on a computer‐generated allocation sequence and a website for allocation concealment.
Blinding
Blinding of outcome assessor was stated to be blinded; an independent assessor undertook assessment for the neurodevelopmental outcomes. Participants and clinicians were not able to be blinded.
Incomplete outcome data
The study reported a very low rate of loss to follow‐up at 30 months however, the 30‐month data included in the paper (complete on 132/134 infants) was based only on those infants recruited until December 1st, 2007. After that time another 24 women and their infants were recruited to the trial and these participants are not reported on for the 30‐month outcome.
Selective reporting
The study was assessed as at unclear risk of bias due to incomplete reporting, due to data not being reported for all participants.
Other potential sources of bias
There were no other identified potential sources of bias.
Effects of interventions
The one included trial involved 158 women (Adzick 2011) and compared the effect of prenatal surgical repair of myelomeningocele (via maternal laparotomy and hysterotomy) with standard (postnatal) repair.
Primary outcomes
For the primary infant outcome of neonatal mortality there was no difference detected between the groups that underwent prenatal repair and postnatal repair (one study, 158 infants, risk ratio (RR) 0.51, 95% confidence interval (CI) 0.05 to 5.54) (Analysis 1.1).
1.1. Analysis.
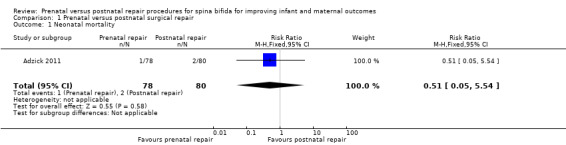
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 1 Neonatal mortality.
Secondary outcomes
For infants, prenatal repair was associated with a reduction in shunt dependent hydrocephalus (reported by the trial authors as shunt placement) (one study, 158 women, RR 0.48, 95% CI 0.36 to 0.64) (Analysis 1.2), and was also associated with a significant decrease in the risk of moderate to severe hindbrain herniation after birth (one study, 158 women, RR 0.56, 95% CI 0.38 to 0.81) (Analysis 1.3). For the infant, prenatal repair was not associated with any difference in the risk of direct complications of the repair procedure (Analysis 1.7), perinatal mortality (Analysis 1.8,) or orthopaedic deformities (Analysis 1.9).
1.2. Analysis.
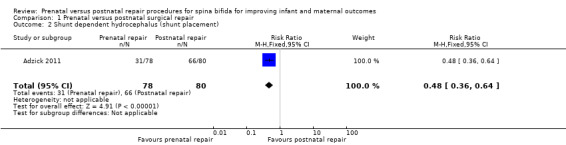
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 2 Shunt dependent hydrocephalus (shunt placement).
1.3. Analysis.
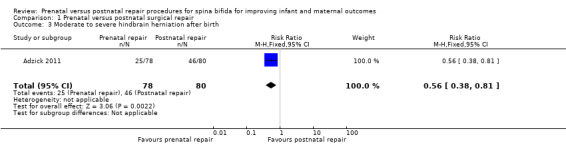
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 3 Moderate to severe hindbrain herniation after birth.
1.7. Analysis.
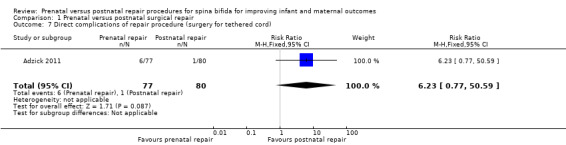
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 7 Direct complications of repair procedure (surgery for tethered cord).
1.8. Analysis.
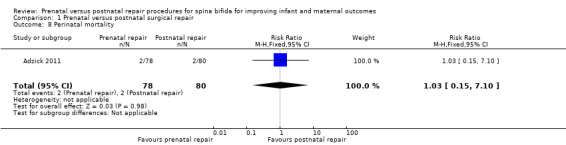
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 8 Perinatal mortality.
1.9. Analysis.
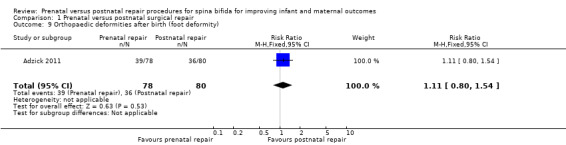
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 9 Orthopaedic deformities after birth (foot deformity).
For the outcome of preterm birth, the intervention was associated with a reduction in gestational age at birth (one study, 158 infants, mean difference (MD) ‐3.20 weeks, 95% CI ‐3.93 to ‐2.47) (Analysis 1.4) and a corresponding increase in both the risk of preterm birth before 37 weeks (one study, 158 infants, RR 5.30, 95% CI 3.11 to 9.04) (Analysis 1.5) and preterm birth before 34 weeks (one study, 158 infants, RR 9.23, 95% CI 3.45 to 24.71) (Analysis 1.6). Prenatal intervention was also associated with an increase in the chance of preterm ruptured membranes (one study, 158 women, RR 6.15, 95% CI 2.75 to 13.78) (Analysis 1.10).
1.4. Analysis.
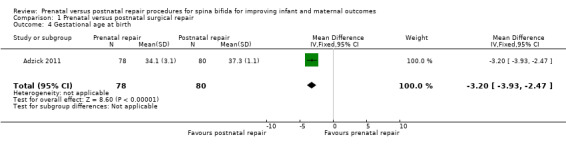
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 4 Gestational age at birth.
1.5. Analysis.
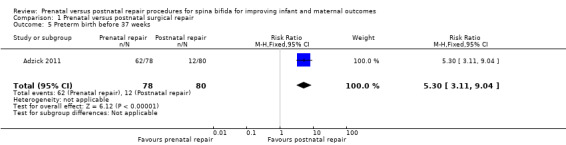
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 5 Preterm birth before 37 weeks.
1.6. Analysis.
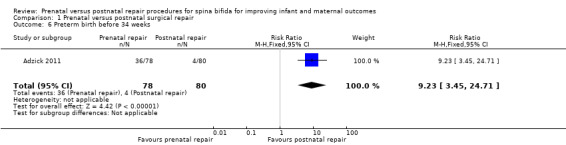
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 6 Preterm birth before 34 weeks.
1.10. Analysis.
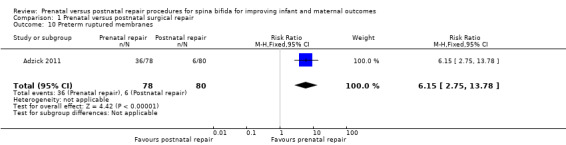
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 10 Preterm ruptured membranes.
For the woman, prenatal repair was associated with a non significant increase in blood transfusion at birth (one study, 158 women, RR 7.18, 95% CI 0.90 to 57.01) (Analysis 1.12) and no significant increase in the risk of chorioamnionitis (maternal infectious morbidity) (Analysis 1.11).
1.12. Analysis.
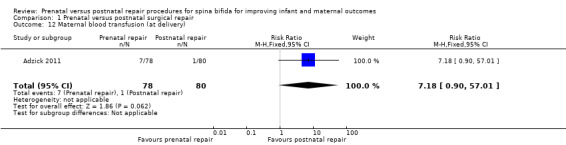
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 12 Maternal blood transfusion (at delivery).
1.11. Analysis.
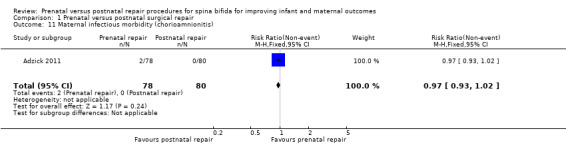
Comparison 1 Prenatal versus postnatal surgical repair, Outcome 11 Maternal infectious morbidity (chorioamnionitis).
For some of this systematic review's outcomes, it was not possible to include data from the study by Adzick 2011 because the information was not available. These outcomes included:
Infant outcomes: days of hospital admission; survival to discharge; stillbirth; need for further surgery (e.g. skin grafting); neurogenic bladder dysfunction; childhood/infant quality of life.
Maternal outcomes: admission to intensive care; women's emotional wellbeing and satisfaction with care
Discussion
This systematic review included one study with a total of 158 women and infants. In this single study, which recruited participants over the course of seven years, the effect of prenatal repair versus postnatal repair was examined. While some of this review's outcomes were reported by the single included study, other important secondary outcomes were not reported.
Summary of main results
The included study, which compared prenatal and postnatal repair, reported a range of neonatal and maternal outcomes able to be included in this systematic review. For the infant, prenatal repair is associated with a reduced need for shunt placement after birth and a reduction in the risk of hindbrain herniation. Prenatal repair is associated with an increased risk of preterm ruptured membranes and subsequent preterm birth for women and their infants. Other outcomes were either not reported, or not statistically significant.
Overall completeness and applicability of evidence
The aim of this systematic review was to evaluate the effect of prenatal and postnatal repair for spina bifida on maternal and neonatal outcomes, and pre‐specified outcomes were chosen as the most important and representative clinical measures of effectiveness and complications. Unfortunately, some of these outcomes, were not assessed by the included study. Some outcomes of interest are particularly uncommon, (for example, neonatal mortality (primary outcome) and severe maternal morbidity measures) and this review is therefore underpowered to detect any significant difference in these important outcomes.
Quality of the evidence
This review has included only one randomised trial, which although recruiting patients for seven years, only recruited 158 women, a reflection of the uncommon nature of the fetal condition in question and perhaps the small number of centres participating in the trial. The review therefore reflects the findings of this single randomised controlled trial, which was conducted with a low risk of bias.
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and assessed risk of bias independently; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
This Cochrane review has summarised the only randomised controlled trial examining the effect of prenatal versus postnatal repair of spina bifida, and as such there is very little other high‐quality evidence with which it can be compared.
Authors' conclusions
Implications for practice.
There is insufficient evidence to draw firm conclusions on the benefits or harms of prenatal repair as an intervention for fetuses with spina bifida. Current evidence is limited by the small numbers of pregnancies that have been included in the single conducted randomised trial to date.
Implications for research.
Prenatal spina bifida repair should only be conducted as part of a well designed and conducted randomised trial. More studies are needed to further examine the effect of a prenatal intervention on long‐term maternal health in particular, given the invasive nature of the intervention for women.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Prenatal versus postnatal surgical repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.54] |
| 2 Shunt dependent hydrocephalus (shunt placement) | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.64] |
| 3 Moderate to severe hindbrain herniation after birth | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.81] |
| 4 Gestational age at birth | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐3.93, ‐2.47] |
| 5 Preterm birth before 37 weeks | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.30 [3.11, 9.04] |
| 6 Preterm birth before 34 weeks | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.23 [3.45, 24.71] |
| 7 Direct complications of repair procedure (surgery for tethered cord) | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.23 [0.77, 50.59] |
| 8 Perinatal mortality | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 7.10] |
| 9 Orthopaedic deformities after birth (foot deformity) | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.80, 1.54] |
| 10 Preterm ruptured membranes | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.15 [2.75, 13.78] |
| 11 Maternal infectious morbidity (chorioamnionitis) | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.02] |
| 12 Maternal blood transfusion (at delivery) | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.18 [0.90, 57.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adzick 2011.
| Methods | Randomised controlled trial. | |
| Participants | Singleton pregnancy, MMC with upper boundary T1‐S1, evidence of hindbrain herniation, gestational age 19.0 to 25.9 weeks, normal karyotype, US residency and maternal age > 18 years. | |
| Interventions | Prenatal surgery via maternal laparotomy and hysterotomy versus postnatal repair. | |
| Outcomes | Primary = perinatal death or need for shunt at 12 months and developmental scores at 30 months of age. Secondary maternal and infant outcomes = membrane separation, pulmonary oedema, biophysical profile, oligohydramnios, placental abruption, gestational diabetes, chorioamnionitis, pre‐eclampsia or gestational hypertension, spontaneous membrane rupture, blood transfusion at delivery, status of hysterotomy site at delivery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Website for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Those assessing outcomes, checking eligibility criteria, etc. were blinded, however it is unclear at what point the investigators were unblinded as to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of childhood neurodevelopment were blinded as were those checking shunt placement criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the original 158 participants, only 134 had presumably reached the 30‐month age when the data set was closed and reported on. Another 24 participants from the original 158 were not included in the report of the 30‐month outcome. |
| Selective reporting (reporting bias) | Unclear risk | Risk unclear as data not available or reported for all participants. |
| Other bias | Low risk | Interim analysis was conducted as pre‐specified. |
MMC: myelomeningocele
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almodin 2006 | Not an RCT of repair methods. |
| Bruner 1999a | Not an RCT of repair methods. |
RCT: randomised controlled trial
Differences between protocol and review
In response to editorial feedback at the review stage, the following changes were made.
Background ‐ the background has been modified.
Outcomes ‐ the review's outcomes have also been altered.
The maternal primary outcome 'Preterm labour before 34 weeks' has been deleted.
The maternal secondary outcome 'Preterm labour before 37 weeks' has been deleted.
We have clarified 'Maternal infectious morbidity' by adding (any of: endometritis, wound infection, chorioamnionitis).
Contributions of authors
Rosalie Grivell (RG) is guarantor for the review. RG registered the title and wrote the protocol with advice on content and outcomes from Chad Andersen and Jodie Dodd. All authors commented on drafts and approved the published protocol.
Declarations of interest
None known.
New
References
References to studies included in this review
Adzick 2011 {published data only}
- Adzick NS, Thom EA, Spong CY, Brock JW III, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. New England Journal of Medicine 2011;364(11):993‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. MOMS management of myelomeningocele study. http://www.spinabifidamoms.com/english/overview.html (accessed 22 July 2003).
- Spong C. Myelomeningocele repair randomized trial. http://clinicaltrials.gov/ct2/show/NCT00060606 (accessed 18 March 2011) 2011.
- Thom E, Burrows PK, Adzick S, Yang E, Spong C. Management of myelomeningocele study: status after five years. Prenatal Diagnosis 2008;28:S23. [Google Scholar]
References to studies excluded from this review
Almodin 2006 {published data only}
- Almodin CG, Moron AF, Cavaliero S, Yamashita A, Hisaba W, Piassi J. The Almodin‐Moron trocar for uterine entry during fetal surgery. Fetal Diagnosis and Therapy 2006;21(5):414‐7. [DOI] [PubMed] [Google Scholar]
Bruner 1999a {published data only}
- Bruner JP, Boehm FH, Tulipan N. The tulipan‐bruner trocar for uterine entry during fetal surgery. American Journal of Obstetrics and Gynecology 1999;181(5 Pt 1):1188‐91. [DOI] [PubMed] [Google Scholar]
Additional references
Adzick 1998
- Adzick NS, Sutton LN, Crombleholme TM, Flake AW. Successful fetal surgery for spina bifida. Lancet 1998;352:1675‐6. [DOI] [PubMed] [Google Scholar]
AIHW 2004
- AIHW National Perinatal Statistics Unit. Australia's babies: their health and wellbeing. Bulletin no. 21. AIHW cat. no. AUS 54. Canberra: AIHW NPSU, 2004. [Google Scholar]
Bruner 1999
- Bruner JP, Richards WO, Tulipan NB, Arney TL. Endoscopic coverage of fetal myelomeningocele in utero. Americal Journal of Obstetrics and Gynecology 1999;180(1 Pt 1):153‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mitchell 2004
- Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet 2004;364(9448):1885‐95. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sutton 2008
- Sutton LN. Fetal surgery for neural tube defects. Best Practice and Research Clinical Obstetrics and Gynaecology 2008;22(1):175‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulipan 1998
- Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatric Neurosurgery 1998;28(4):177‐80. [DOI] [PubMed] [Google Scholar]
Tulipan 1999
- Tulipan N, Bruner JP, Hernanz‐Schulman M, Lowe LH, Walsh WF, Nickolaus D, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatric Neurosurgery 1999;31:183‐8. [DOI] [PubMed] [Google Scholar]
Tulipan 2003
- Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt dependent hydrocephalus. Pediatric Neurosurgery 2003;38(1):27‐33. [DOI] [PubMed] [Google Scholar]
Walsh 2003
- Walsh DS, Adzick NS. Foetal surgery for spina bifida. Seminars in Neonatology 2003;8:197‐205. [DOI] [PubMed] [Google Scholar]


