Abstract
Micronutrient supplements are often recommended during pregnancy, yet their role and necessity remain poorly understood in the Australian population. This study aimed to determine the essential mineral intake of a population of pregnant women in South East Queensland and investigate the effects of supplements on their micronutrient status and birth outcomes. Women completing the Oral Glucose Tolerance Test at two South East Queensland hospitals between 180 and 210 days gestation provided fasting blood samples and dietary data using the Maternal Outcomes and Nutrition Tool (n = 127). Birth outcomes were sourced from medical records. Serum elemental profiles were determined by inductively coupled plasma mass spectrometry (ICP-MS) analysis. Intake of 8 essential minerals was compared with Australian dietary recommendations; matched serum mineral levels were compared with the current Queensland pregnancy reference ranges. Data were examined using cross-sectional cohort design and independent sample t-tests. Supplement use had no significant influence on serum values of trace elements or the incidence of hypertensive disorders, gestational diabetes, preterm birth or infant birthweight. Dietary selenium, zinc and iodine were significantly higher in women birthing beyond 41 completed weeks; selenium (P = .026) and zinc (P = .034) both made unique contributions to the regression models when controlling for confounders. Women exhibited adequate to excessive serum micronutrient levels compared with pregnancy reference ranges, a finding consistent with dietary intake calculations. Data suggest that excessive essential mineral intake contributed to prolonged pregnancy in this cohort, supporting previous studies in this population. Further research is required to determine individual needs and eliminate the potential for harm before recommending pregnancy supplements.
Keywords: Micronutrients, Pregnancy, Birth weight, Pregnancy, prolonged, Australia
Introduction
Micronutrient supplements are often recommended during pregnancy, yet their efficacy remains poorly understood in Australia’s geographically and culturally diverse population.1 While research regarding birth outcomes in women with a known risk for nutrition deficits such as those in low- and middle-income regions and with socio-economic disadvantage has found improved outcomes with both multiple and individual vitamin and mineral supplementation, little research has been undertaken in high-income countries.2-4 Furthermore, research conducted in wealthy countries has thus far focused on pathological pregnancy.5,6 The therapeutic application of micronutrient supplements and the potential benefit of their anti-inflammatory and antioxidant properties in the amelioration of preterm birth and pre-eclampsia are promising.7,8 However, the effects of these properties on uncomplicated pregnancies are yet to be determined.
Current antenatal care guidelines support the recommendation of folic acid and iodine in the first trimester of pregnancy.9 The role of folate in neural tube formation is well understood, with folic acid supplements proven to be effective in the prevention of neural tube deformities.10 However, while the importance of available minerals for foetal cognitive development is known,11 supplementation in Australia is less well supported by research, with current recommendations released in the absence of quality evidence.9,12 Australia’s micronutrient intake during pregnancy has previously been examined, finding data to be of insufficient quality and quantity to enable reliable meta-analyses.13 As such, a low level of evidence informs practice, resulting in conflicting and anecdotal advice regarding supplementation during pregnancy. This situation is exacerbated by social norms,14 advertising,14 and an overarching belief that supplements are harmless.15 However, a lack of evidence exists surrounding the potential effects of subclinical excess, and as such, remains an essential but unexplored area of investigation.
In order to examine dietary intake of essential minerals, their corresponding serum levels and the potentially beneficial or detrimental effects of these on birth outcomes in a South East Queensland cohort, this research has taken advantage of recently developed inductively coupled plasma mass spectrometry (ICP-MS) serum element extraction processes16,17 and the Maternal Outcomes and Nutrition Tool (MONT). Based on previously validated food frequency questionnaires, the MONT collects self-reported data regarding 259 individual foods, micronutrient supplements, 190 constituent nutrients, their related components, and 8 birth outcomes.18 These combined methods offer a novel approach to assessing the intake of trace minerals during pregnancy, the effect of diet and supplements on serum mineral levels and their potential influence on birth outcomes.
Participants and Methods
Pregnant women between 180 and 210 days of gestation residing in South East Queensland were the focus of this research. Women presenting to either the Gold Coast University Hospital or the Royal Brisbane and Women’s Hospitals for an Oral Glucose Tolerance Test between September 5, 2017 and June 26, 2018 were approached and offered study participation by the researcher. Written informed consent was obtained prior to both biological and data sampling.
Fasting blood samples were collected at recruitment into serum separator tubes (SSTs) and conveyed to the School of Medical Science, Griffith University Gold Coast Campus for processing. Samples were rested for 30 minutes and then centrifuged under refrigeration at 1500g for 10 minutes. Serum was subsequently aliquoted into Eppendorf tubes and stored at −80°C until analysis. Serum elemental values were determined using an inductively coupled plasma mass spectrometer (ICP-MS). Samples were prepared in a 1:10 dilution with a solution of 2.8% ammonia, 10% isopropanol, 0.2% triton X solution, 0.1% ethylenediaminetetraacetic acid (EDTA), and high purity H2O.19,20 Samples were measured on an Agilent 7900 ICP-MS. Quality control standards between 10 and 100 µg/L were analysed every 12 samples. Sc, Y, In, and Tb were added to all samples (final concentration of 10 µg/L) as an internal standard to account for instrument drift. ClinCheck trace element controls for serum (Levels I and II, ref 8880-8882) were used for an external quality-assurance test. The limit of detection was set at 3 times the standard deviation of 20 blank replicates, and limit of quantification set at 10 times the standard deviation of the 20 blank replicates. This methodology has recently been validated in a number of studies and been found to be reliable with regard to 29 trace elements;16 in particular, magnesium (Mg), calcium (Ca), iron (Fe), copper (Cu), zinc (Zn), molybdenum (Mo), selenium (Se), and iodine (I) levels have been validated in ICP-MS analysis of serum samples processed using this technique.17 As such, this research will focus on these essential elements.
Dietary data were self-reported by participants using the MONT, an instrument designed for exclusive administration in a digital format.18 Food frequency questionnaires (FFQs) in the tool were adapted from the previously validated Norwegian Mother and Child (MoBa) Study21 with reference to the Harvard,22 the Block,23 and diet history questionnaires.24 Surveys were designed with a minimal language component, facilitating engagement with women with English literacy limitations. Respondents were asked to report food and micronutrient supplement frequency in the month prior to participation; resulting values therefore represented second trimester intake. Each response was allocated a numerical value, facilitating calculation of the average equivalent serves consumed by the respondent per day. Nutrient equivalents for each food were then calculated using the Australian Food Composition Database values.25 Data relating to Mg, Ca, Fe, Cu, Zn, Mo, Se, and I were extracted from the database along with information regarding the demographic characteristics and obstetric outcomes of eligible participants. Dietary mineral intake was compared with the Australian Dietary Guidelines;26 serum values were compared with the current reference ranges for each of the elements during the second and third trimesters of pregnancy.27-29
A total of 196 women were recruited to the study and provided blood samples. Women were excluded from this analysis if they did not complete the full MONT survey set (n = 38), were not between 180 and 210 days gestation at recruitment (n = 18), birthed at a nonparticipating hospital (n = 7), or were outside the target age range (18-44 years, n = 1). Biological samples were missing at time of analysis in 5 cases. The final data set comprised 127 women.
Descriptive statistics were used to detail the cohort (Table 1). Demographics examined include maternal age (continuous and categorical – under 24, 25-29, 30-34, and over 35 years), parity (nulliparous/multiparous), cultural or linguistically diversity (defined as identifying with an ethnicity other than white and/or birth in a traditionally non-English speaking country – dichotomous), education (categorical – did not attend/finish high school, finished high school, TAFE trade or apprenticeship, university degree), income (categorical – <50k, 50-70k, 70-120k, and >120k), smoking (dichotomous), supplement use (multiple micronutrient formulations and/or individual supplements, dichotomous), and body mass index (BMI; dichotomous – lean/overweight and categorical – underweight <18.5 kg/m2, healthy weight 18.5-24.99 kg/m2, overweight 25.0-29.99 kg/m2, obese class I 30.0-34.99 kg/m2, obese class II 35.0-39.99 kg/m2, and morbid obesity ⩾40.0 kg/m2); induction of labour (where labour occurred, dichotomous), preterm (<37 completed weeks, dichotomous), and postdates birth (>41 completed weeks, dichotomous), method of due date calculation (dates/scan), hypertensive disorders (HDPs) (dichotomous), gestational diabetes (GDM) (dichotomous), and low birthweight babies (<2500 g, dichotomous) were examined in terms of birth outcomes (No., %).
Table 1.
Demographic and outcome characteristics of the MONT pilot cohort.
| No. (%) | Supplement usea |
χ2 | df | P value | ||
|---|---|---|---|---|---|---|
| No (n = 38) | Yes (n = 89) | |||||
| Age group | ||||||
| ⩽24 | 27 (21.2) | 12 (44.4) | 15 (55.6) | 3.537 | 3 | .316 |
| 25–29 | 40 (31.5) | 10 (25.0) | 30 (75.0) | |||
| 30–34 | 39 (30.7) | 10 (25.6) | 29 (74.4) | |||
| ⩾35 | 21 (16.5) | 6 (28.6) | 15 (71.4) | |||
| BMI | ||||||
| Lean (<25 kg/m2) | 73 (57.5) | 18 (24.7) | 55 (75.3) | 1.337 | 1 | .248 |
| Overweight (⩾25 kg/m2) | 50 (39.4) | 18 (36.0) | 32 (64.0) | |||
| Cultural and linguistic diversity (CALD) | ||||||
| No | 91 (71.7) | 23 (25.3) | 68 (74.7) | 2.570 | 1 | .109 |
| Yes | 36 (28.3) | 15 (41.7) | 21 (58.3) | |||
| Income | ||||||
| <50k | 40 (31.5) | 15 (37.5) | 25 (62.5) | 2.638 | 3 | .451 |
| 50–70k | 23 (18.1) | 8 (34.8) | 15 (65.2) | |||
| 70–120k | 34 (26.8) | 8 (23.5) | 26 (76.5) | |||
| >120k | 30 (23.6) | 7 (23.3) | 23 (76.7) | |||
| Education | ||||||
| Did not attend/finish high school | 23 (18.5) | 11 (47.8) | 12 (52.2) | 7.974 | 3 | .047b |
| Finished high school | 34 (26.8) | 13 (38.2) | 21 (61.8) | |||
| TAFE trade or apprenticeship | 22 (17.3) | 4 (18.2) | 18 (81.8) | |||
| University degree | 48 (37.8) | 10 (20.8) | 38 (79.2) | |||
| Parity | ||||||
| Nulliparous | 73 (57.5) | 15 (20.5) | 58 (79.5) | 6.181 | 1 | .013b |
| Multiparous | 54 (42.5) | 23 (42.6) | 31 (57.4) | |||
| Smoker | ||||||
| No | 118 (92.9) | 32 (27.1) | 86 (72.9) | 6.040 | 1 | .014b |
| Yes | 9 (7.1) | 6 (75.0) | 2 (25.0) | |||
| Hypertensive disorders | ||||||
| No | 115 (86.6) | 34 (29.6) | 81 (70.4) | 0.000 | 1 | .000 |
| Yes | 12 (9.4) | 2 (33.3) | 8 (66.7) | |||
| Gestational diabetes | ||||||
| No | 110 (86.6) | 31 (28.2) | 79 (71.8) | 0.647 | 1 | .276 |
| Yes | 17 (13.4) | 7 (41.2) | 10 (58.8) | |||
| Preterm birth (idiopathic) | ||||||
| No | 105 (82.7) | 31 (29.5) | 74 (70.5) | 0.728 | 1 | .394 |
| Yes | 16 (12.6) | 7 (43.8) | 9 (56.2) | |||
| Birth >41 weeks | ||||||
| No | 114 (89.8) | 36 (31.6) | 78 (68.4) | 0.789 | 1 | .374 |
| Yes | 13 (10.2) | 2 (15.4) | 11 (84.6) | |||
| Induction of labour (excluding no labour) | ||||||
| No | 54 (42.5) | 17 (31.5) | 37 (68.5) | 0.115 | 1 | .734 |
| Yes | 42 (33.1) | 11 (26.2) | 31 (73.8) | |||
| Low birthweight baby (excluding multiple births) | ||||||
| No | 101 (79.5) | 32 (31.7) | 69 (68.3) | 0.000 | 1 | 1.000 |
| Yes | 19 (15.0) | 6 (31.6) | 13 (68.4) | |||
Abbreviations: BMI, body mass index; CALD, cultural and linguistic diversity; MONT, Maternal Outcomes and Nutrition Tool.
Includes multiple and individual micronutrient supplements.
Statistically significant.
Relationships between variables were assessed with two-tailed Pearson’s product-moment correlation analysis; variables P ⩽ .05 were retained as covariates (Supplemental Appendices 1 and 2). Variables with correlation coefficients ⩾0.7 were considered collinear and treated as a single variable for analysis purposes. Correlations between supplement use and descriptive variables were analysed with chi-square analyses (χ2, df, P value – Table 1) and subsequently analysed with simple logistic regression (Table 2). Independent samples t-tests were used to examine the differences in mean values for dietary (Table 3) and serum values (Table 4) between supplement users and nonusers. Linear regression analysis was performed using serum and dietary elemental values (continuous and dependent) and birth outcomes (categorical and independent); multiple regression was subsequently performed adding identified covariates into the regression models (Tables 5 and 6). Data analysis was performed using SPSS, version 25.0, IBM Corp. 2017.
Table 2.
Simple logistic regression of demographic variables with significant supplement associations.
| Variable | No. (%) | β | WALD | OR | 95% CI AOR |
P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Parity | Multiparous | 54 (42.5) | Referent | |||||
| Nulliparous | 73 (57.5) | 1.118 | 8.968 | 3.059 | 1.429 | 6.549 | .004a | |
| Education | <high school | 23 (18.5) | Referent | |||||
| High school | 34 (26.8) | 0.742 | 1.826 | 0.716 | 2.100 | 6.173 | .177 | |
| TAFE | 22 (17.3) | 1.243 | 3.806 | 3.472 | 0.994 | 12.048 | .051 | |
| University | 48 (37.8) | 1.475 | 7.382 | 4.367 | 1.508 | 12.658 | .007a | |
| Smokers | No | 118 (92.9) | Referent | |||||
| Yes | 9 (7.1) | −1.882 | 5.017 | 0.152 | 0.029 | 0.790 | .025a | |
Abbreviation: AOR, adjusted odds ratio.
Statistically significant.
Table 3.
MONT dietary data – multiple micronutrient supplement (MuMS) versus nonsupplement groups.
|
MONT dietary data No MuMS
|
N |
MONT dietary data MuMS
|
N | T value | P value | RDI |
MONT data total
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | 95% CI | ||||||
| Ia, μg/day | 193 | 162-206 | 43 | 348 | 329-370 | 83 | −9.171 | <.001b | 220 | 295 | 274-316 |
| Mo, μg/day | 72 | 64-82 | 40 | 78 | 72-84 | 80 | −1.270 | .208 | 50 | 76 | 71-79 |
| Zna, mg/day | 16 | 14-18 | 43 | 24 | 23-25 | 82 | −6.779 | <.001b | 11 | 22 | 20-23 |
| Sea, μg/day | 79 | 69-88 | 42 | 122 | 116-130 | 80 | −7.046 | <.001b | 65 | 107 | 98-111 |
| Fea, mg/day | 31.5 | 18-48 | 43 | 69.1 | 58-82 | 84 | −4.447 | <.001b | 27 | 32.3 | 47-65 |
| Caa, mg/day | 1441 | 1225-1623 | 43 | 1470 | 1335-1550 | 84 | −0.258 | .798 | 1000 | 1474 | 1362-1559 |
| Mga, mg/day | 803 | 562-1042 | 43 | 731 | 615-745 | 84 | 0.594 | .555 | 350–360 | 597 | 668-849 |
| Cua, mg/day | 2.03 | 1.7-2.2 | 39 | 2.46 | 2.3-2.6 | 76 | −2.696 | .009b | 1.3c | 2.3 | 2.2-2.4 |
Abbreviation: MONT, Maternal Outcomes and Nutrition Tool.
Included in MuMS
Statistically significant
Adequate intake.
Table 4.
ICP-MS serum analysis – multiple micronutrient supplement (MuMS) versus non-supplement groups.
|
MONT ICP-MS No MuMS
|
N |
MONT ICP-MS MuMS
|
N | T value | P value | Trimester 2/3 ref range |
MONT ICP-MS
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||||
| Ia, μg/L | 90.1 | 84.7-93.7 | 42 | 91.2 | 86.1-92.2 | 81 | −0.403 | .688 | 40-92 | 90.8 | 88.2-93.5 |
| Mo, μg/L | 0.66 | 0.55-0.72 | 39 | 0.67 | 0.61-0.71 | 78 | −0.316 | .753 | 0.3-2.0 | 0.67 | 0.63-0.70 |
| Zna, mg/L | 0.78 | 0.71-0.81 | 43 | 0.77 | 0.71-0.81 | 84 | 0.233 | .816 | 0.50-0.80 | 0.78 | 0.75-0.80 |
| Sea, μg/L | 74.1 | 70.2-79.0 | 43 | 75.5 | 71.2-78.0 | 84 | −0.484 | .630 | 71-145 | 75.0 | 72.1-77.8 |
| Fea, mg/L | 1062 | 814-1341 | 38 | 1076 | 990-1167 | 84 | −0.112 | .911 | 279.2-1954.6 | 1071 | 979-1164 |
| Caa, mg/L | 54.3 | 53.5-54.7 | 42 | 54.2 | 53.6-54.7 | 79 | 0.229 | .820 | 44-53 | 54.2 | 53.8-54.7 |
| Mga, mg/L | 17.8 | 17.0-17.9 | 43 | 17.5 | 16.8-17.5 | 84 | 1.045 | .299 | 11.2-22.4 | 17.6 | 17.3-17.9 |
| Cua, mg/L | 2.49 | 2.30-2.67 | 40 | 2.39 | 2.24-2.44 | 84 | 1.138 | .259 | 1.3-2.4 | 2.42 | 2.34-2.50 |
Abbreviations: ICP-MS, inductively coupled plasma mass spectrometer; MONT, Maternal Outcomes and Nutrition Tool.
Included in MuMS
Table 5.
Multiple linear regression – outcomes, covariates, and micronutrient intake – MONT dietary data.
| Birth outcome (covariates) | Micronutrient | N | β | Standardised β | 95% CI β |
P value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Hypertensive disordersa (4, 5, 7, 8) | Seleniumb | 89 | −7.624 | −0.053 | −41.324 | 26.077 | .654 |
| Molybdenum | 87 | 7.099 | 0.084 | −14.015 | 28.213 | .505 | |
| Magnesiumc | 93 | −0.047 | −0.065 | −0.214 | 0.120 | .577 | |
| Iron | 84 | 2.481 | 0.024 | −22.849 | 27.810 | .846 | |
| Copper | 84 | −0.247 | −0.084 | −0.954 | 0.459 | .488 | |
| Gestational diabetesd (7) | Seleniumb | 123 | −7.451 | −0.065 | −29.191 | 14.288 | .499 |
| Molybdenum | 120 | −0.879 | −0.012 | −14.251 | 12.493 | .897 | |
| Magnesiumc | 127 | 0.042 | 0.066 | −0.076 | 0.161 | .481 | |
| Iron | 115 | −4.543 | −0.052 | −21.615 | 12.528 | .599 | |
| Copper | 115 | −0.385 | −0.160 | −0.858 | 0.088 | .110 | |
| Induction of laboure (3, 6) | Seleniumb | 92 | −10.976 | −0.142 | −27.289 | 5.337 | .185 |
| Molybdenum | 90 | −2.678 | −0.054 | −14.055 | 8.698 | .641 | |
| Magnesiumc | 96 | −0.044 | −0.109 | −0.132 | 0.044 | .324 | |
| Iron | 87 | 2.508 | 0.044 | −10.897 | 15.914 | .711 | |
| Copper | 87 | 0.206 | 0.125 | −0.172 | 0.583 | .282 | |
| Gestation < 37 weeksf (1, 3, 8, 10) | Seleniumb | 115 | 0.412 | 0.004 | −28.874 | 29.697 | .978 |
| Molybdenum | 112 | 4.183 | 0.058 | −13.845 | 22.210 | .646 | |
| Magnesiumc | 118 | −0.025 | −0.039 | −0.192 | 0.141 | .763 | |
| Iron | 108 | −7.380 | −0.090 | −28.582 | 13.822 | .492 | |
| Copper | 107 | 0.059 | 0.022 | −0.685 | 0.803 | .876 | |
| Gestation > 41 weeksf (2, 4, 8, 9, 11) | Seleniumb | 90 | 32.092 | 0.289 | 10.860 | 53.324 | .003* |
| Molybdenum | 88 | 3.277 | 0.045 | −13.345 | 19.899 | .696 | |
| Magnesiumc | 91 | 0.027 | 0.047 | −0.099 | 0.154 | .670 | |
| Iron | 85 | 7.281 | 0.089 | −8.986 | 23.548 | .376 | |
| Copper | 85 | 0.193 | 0.083 | −0.334 | 0.721 | .468 | |
| Birthweight < 2500 gg (5, 7) | Seleniumb | 115 | 6.169 | 0.058 | −20.386 | 32.723 | .646 |
| Molybdenum | 112 | −13.031 | −0.191 | −29.640 | 3.578 | .123 | |
| Magnesiumc | 119 | 0.002 | 0.004 | −0.150 | 0.155 | .975 | |
| Iron | 108 | 12.805 | 0.170 | −6.274 | 31.885 | .186 | |
| Copper | 107 | 0.285 | 0.116 | −0.376 | 0.946 | .395 | |
| Gestation at birthh (3, 6, 8) | Seleniumb | 123 | −0.011 | −0006 | −0.663 | 0.641 | .973 |
| Molybdenum | 120 | −0.043 | −0.031 | −0.493 | 0.407 | .852 | |
| Magnesiumc | 120 | −0.002 | −0.123 | −0.005 | 0.002 | .380 | |
| Iron | 116 | −0.029 | −0.018 | −0.602 | 0.543 | .919 | |
| Copper | 115 | −0.004 | −0.099 | −0.020 | 0.011 | .579 | |
| Birthweighth (1, 3, 5, 6) | Seleniumb | 115 | −0.003 | −0.051 | −0.016 | 0.010 | .674 |
| Molybdenum | 112 | 0.004 | 0.116 | −0.004 | 0.013 | .340 | |
| Magnesiumc | 119 | 0.000 | −0.118 | 0.000 | 0.000 | .333 | |
| Iron | 108 | 0.001 | 0.022 | −0.010 | 0.011 | .866 | |
| Copper | 107 | 0.000 | 0.083 | 0.000 | 0.000 | .527 | |
Statistically significant (P ⩽ .05).
Note: 1 = cultural and/or linguistic diversity; 2 = parity; 3 = hypertensive disorders of pregnancy; 4 = induction of labour; 5 = birth <37 weeks; 6 = birth >41 weeks; 7 = gestation at birth; 8 = birthweight <2500 g; 9 = lean/overweight; 10 = birthweight; 11 = supplement use.
Reference categories:
No hypertensive disorder.
Collinearity with iodine and zinc.
Collinearity with calcium.
No gestational diabetes.
Spontaneous labour.
Gestation at birth 37–41 weeks.
Birthweight ⩾2500 g.
Continuous variable.
Table 6.
Multiple linear regression – outcomes and covariates – ICP-MS micronutrient values.
| Birth outcome (covariates) | Micronutrient | N | β | Standardised β | 95% CI β |
P value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Hypertensive disordersa (4, 5, 7, 8) | Selenium | 127 | 0.001 | 0.067 | −0.004 | 0.006 | .615 |
| Molybdenum | 117 | −0.217 | −0.160 | −0.501 | 0.067 | .132 | |
| Zinc | 127 | −0.345 | −0.164 | −0.856 | 0.166 | .182 | |
| Magnesium | 127 | 0.039 | 0.251 | −0.005 | 0.084 | .079 | |
| Iron | 122 | 0.000 | −0.024 | 0.000 | 0.000 | .820 | |
| Calcium | 121 | −0.032 | −0.253 | −0.063 | −0.001 | .045* | |
| Copper | 124 | −0.031 | −0.048 | −0.170 | 0.108 | .657 | |
| Gestational diabetesb (7) | Selenium | 127 | 0.001 | 0.035 | −0.005 | 0.006 | .793 |
| Molybdenum | 117 | 0.112 | 0.071 | −0.216 | 0.440 | .499 | |
| Zinc | 127 | 0.126 | 0.052 | −0.460 | 0.712 | .670 | |
| Magnesium | 127 | −0.016 | −0.088 | −0.067 | 0.034 | .529 | |
| Iron | 122 | 0.000 | −0.068 | 0.000 | 0.000 | .514 | |
| Calcium | 121 | 0.029 | 0.196 | −0.007 | 0.065 | .114 | |
| Copper | 124 | −0.039 | −0.052 | −0.198 | 0.120 | .628 | |
| Induction of labourc (3, 6) | Selenium | 127 | 0.000 | 0.010 | −0.008 | 0.009 | .944 |
| Molybdenum | 117 | 0.125 | 0.054 | −0.368 | 0.618 | .615 | |
| Zinc | 127 | 0.600 | 0.168 | −0.282 | 1.483 | .180 | |
| Magnesium | 127 | −0.038 | −0.142 | −0.114 | 0.038 | .324 | |
| Iron | 122 | 0.000 | −0.117 | 0.000 | 0.000 | .273 | |
| Calcium | 121 | 0.027 | 0.128 | −0.028 | 0.083 | .327 | |
| Copper | 124 | 0.044 | 0.040 | −0.194 | 0.282 | .715 | |
| Gestation < 37 weeksd (1, 3, 6, 8, 10) | Selenium | 127 | −0.001 | −0.030 | −0.005 | 0.004 | .762 |
| Molybdenum | 117 | 0.150 | 0.095 | −0.109 | 0.409 | .252 | |
| Zinc | 127 | −0.015 | −0.006 | −0.460 | 0.430 | .948 | |
| Magnesium | 127 | –0.041 | –0.223 | –0.080 | –0.002 | .042* | |
| Iron | 122 | 0.000 | −0.018 | 0.000 | 0.000 | .816 | |
| Calcium | 121 | 0.000 | 0.003 | −0.027 | 0.028 | .978 | |
| Copper | 124 | 0.021 | 0.028 | −0.101 | 0.144 | .729 | |
| Gestation > 41 weeksd (2, 4, 8, 9) | Selenium | 127 | 0.002 | 0.088 | −0.004 | 0.007 | .548 |
| Molybdenum | 117 | 0.149 | 0.106 | −0.171 | 0.469 | .356 | |
| Zinc | 127 | 0.192 | 0.088 | −0.388 | 0.773 | .511 | |
| Magnesium | 127 | −0.008 | −0.048 | −0.058 | 0.042 | .757 | |
| Iron | 122 | 0.000 | 0.039 | 0.000 | 0.000 | .733 | |
| Calcium | 121 | 0.025 | 0.188 | −0.012 | 0.061 | .180 | |
| Copper | 124 | −0.008 | −0.012 | −0.175 | 0.160 | .928 | |
| Birthweight < 2500 ge (5, 7) | Selenium | 127 | −0.001 | −0.037 | −0.005 | 0.004 | .703 |
| Molybdenum | 117 | −0.189 | −0.111 | −0.446 | 0.068 | .148 | |
| Zinc | 127 | 0.020 | 0.008 | −0.440 | 0.480 | .932 | |
| Magnesium | 127 | 0.028 | 0.144 | −0.012 | 0.068 | .163 | |
| Iron | 122 | 0.000 | 0.059 | 0.000 | 0.000 | .436 | |
| Calcium | 121 | 0.017 | 0.105 | −0.012 | 0.045 | .249 | |
| Copper | 124 | −0.027 | −0.034 | −0.152 | 0.098 | .670 | |
| Gestation at birthf (3, 6, 8) | Selenium | 127 | 0.050 | 0.043 | −0.116 | 0.215 | .553 |
| Molybdenum | 117 | −40.066 | −0.047 | −130.952 | 50.819 | .416 | |
| Zinc | 127 | 30.145 | 0.023 | −140.388 | 200.679 | .722 | |
| Magnesium | 127 | 0.205 | 0.020 | −10.310 | 10.721 | .788 | |
| Iron | 122 | 0.001 | 0.014 | −0.004 | 0.005 | .797 | |
| Calcium | 121 | −0.307 | −0.038 | −10.411 | 0.797 | .582 | |
| Copper | 124 | −0.569 | −0.014 | −50.347 | 40.209 | .814 | |
| Birthweightf (1, 3, 5, 6) | Selenium | 127 | 10.368 | 0.030 | −80.505 | 110.241 | .784 |
| Molybdenum | 117 | −1820.107 | −0.052 | −7760.157 | 4110.944 | .544 | |
| Zinc | 127 | 1240.280 | 0.023 | −9200.659 | 11 690.219 | .814 | |
| Magnesium | 127 | −30.125 | −0.008 | −950.112 | 880.862 | .946 | |
| Iron | 122 | −0.082 | −0.056 | −0.325 | 0.161 | .504 | |
| Calcium | 121 | −50.074 | −0.016 | −700.743 | 600.595 | .878 | |
| Copper | 124 | 2310.404 | 0.141 | −520.331 | 5150.138 | .109 | |
Statistically significant (P ⩽ .05).
Note: 1 = cultural and/or linguistic diversity; 2 = parity; 3 = hypertensive disorders of pregnancy; 4 = induction of labour; 5 = birth <37k; 6 = birth >41k; 7 = gestation at birth; 8 = birthweight <2500 g; 9 = lean/overweight; 10 = birthweight.
Reference categories:
No hypertensive disorder.
No gestational diabetes.
Spontaneous labour.
Gestation at birth 37–41 weeks.
Birthweight ⩾2500 g.
Continuous variable.
This research was approved by the Gold Coast Hospital and Health Service Human Ethics (HREC 16/QGC/70) and Griffith University Human Ethics Committees (HREC 2016/423). All women included in this cohort gave written consent for the release of their perinatal data from their medical record.
Results
Women of this cohort were most commonly white, nonsmoking, university educated, nulliparous, aged 25 to 29 years or with a BMI in the healthy range (Table 1). Pregnancy complication rates reflected state-wide statistics.30 The majority of estimated due dates were calculated by early ultrasound scan rather than last menstrual period (72.4%, n = 92 vs 27.6%, n = 35). No significant association was found between the method of calculation and gestation at birth (P = .069). Mean gestational length between the 2 groups did not significantly differ (P = .109).
Significant correlations were demonstrated between demographic, outcome, supplement use, and dietary mineral intake (Supplemental Appendix 1). Collinearity was found between numerous variables; these have been considered on an analysis by analysis basis. Maternal age, education, and income exhibited significant associations (P < .001); smoking status was associated with education (P = .009), while cultural and linguistic diversity demonstrated association with income (P = .042) and education (P = .017). Household income was associated with lean or overweight status, rather than BMI as a continuous variable. Birth beyond 41 completed weeks was inversely associated with parity but positively correlated with induction of labour (P = .047); hypertensive disorders of pregnancy demonstrated positive correlation with induction of labour (P < .001) but a negative correlation with gestational length at birth (P = .011).
Supplement use was significantly associated with education (P = .047), smoking (P = .014), and parity (P = .013, Supplemental Appendix 1), which were retained in the chi-square (Table 1) and logistic regression analysis (Table 2). The use of supplements was more likely in nulliparous women (P = .004) and most significantly associated with university-level education (P = .007); smokers were less likely to have used supplements than nonsmokers (P = .025, Table 2).
Dietary iodine, zinc and selenium were found to be collinear; selenium was retained as the representative variable for these in subsequent regression analysis. Similarly, electrolytes calcium and magnesium demonstrated collinearity (Supplemental Appendix 1), with magnesium considered representative of the two. Serum elemental values demonstrated correlations with several descriptive variables (Supplemental Appendix 2), including body mass index (Fe; P = .021; Cu; P < .001), maternal age (Ca; P = .017), level of education (Ca; P = .017), and cultural and/or linguistic diversity (Mo; P = .022). Serum iodine was correlated with all other reported elements (Supplemental Appendix 2). None of the correlation coefficients attained the r = 0.7 level, and as such each was retained in the regression models individually. Supplement use was not associated with any examined outcome in either the dietary intake or serum sample data; relationships with descriptive groups were considered in the final regression models.
Calculated mean daily dietary values exceeded national recommended daily intakes during trimester 2 and 3 for all elements; these results were exacerbated by the inclusion of supplements in dietary calculations (Table 3). Dietary totals of iodine, zinc, iron, and selenium demonstrated a significant mean increase with the use of supplements (P < .001). Mean copper levels (P = .007) were also significantly higher in women using these supplements compared to women declaring no multivitamin use (Table 3). Women using supplements were found to exhibit mean dietary values up to double the recommended daily intake (iodine: 350 vs 220 μg/day [59%], zinc: 24 vs 11 mg/day [118%], iron: 70 vs 27 mg/day [159%], selenium: 123 vs 65 μg/day [89%], copper 2.44 vs 1.3 [87%]). However, these excesses did not translate to serum values, as no significant differences were found between elemental means in supplement and nonsupplement users (Table 4).
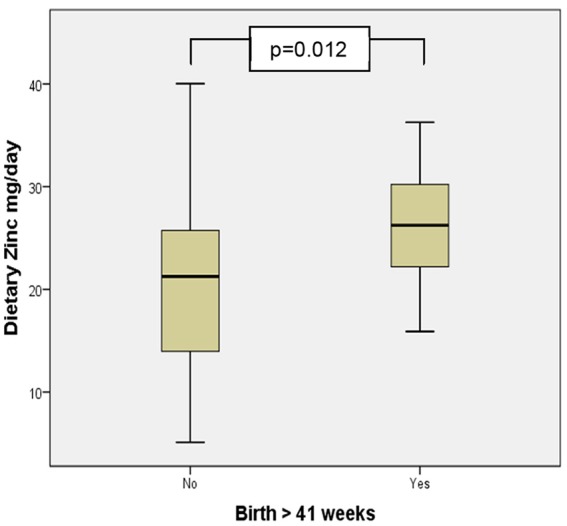
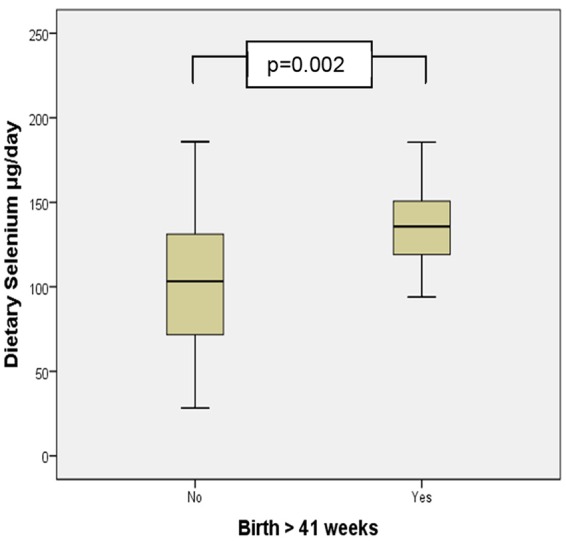
Mean dietary values of zinc and selenium were significantly higher in women birthing beyond 41 completed weeks (Figures 1 and 2). Multiple regression analysis examining this relationship demonstrated a significant increase in the odds of birth beyond 41 weeks gestation with increasing dietary intake of selenium when controlling for confounders (β = 32.092, 95% CI = 10.860-53.324, P = .003; Table 4). Serum values reported by ICP-MS analysis all fell within the current pregnancy reference ranges for the second and third trimester except for mean calcium and copper; these values exceeded pregnancy reference ranges (Table 4). Multiple regression analysis demonstrated that the risk of HDP increased with decreasing serum calcium levels (β = −0.032, 95% CI = −0.063 to −0.001, P = .045; Table 6); decreasing serum magensium levels were associated with an increase in the risk of pre-term birth (β = −0.041, 95% CI = −0.080 to −0.002, P = .042; Table 6). No significant relationship was found between serum or dietary mineral levels, the incidence of low birthweight infants, GDM, or preterm birth.
Figure 1.
Mean dietary selenium intake in births before and after 41 completed weeks gestation.
Figure 2.
Mean dietary zinc intake in births before and after 41 completed weeks gestation.
Discussion
This research aimed to assess selected dietary and serum trace element levels in a sample of pregnant women of South East Queensland, and determine any evidence of an effect of supplements on their health and birth outcomes. Women of this cohort exhibited dietary mineral consumption in excess to recommended daily intake,26 while mean serum values fell largely within the reference ranges for trimesters 2 and 3 of pregnancy,31 irrespective of supplement use. This finding suggests that the use of supplements had no effect on serum elemental profiles, a result further supported by the lack of significant difference in the mean values of individual elements in those who did and did not consume supplements. High levels of dietary selenium, zinc, iodine, and copper were evident in this group of women. Seafood, including crustaceans/shellfish and white-fleshed fish, were high-quality, easily-accessed sources of these minerals for the cohort. Their consumption was reported by 26.8% and 73.2% of participants respectively, potentially accounting for the high dietary intake calculations. Despite homeostatic mechanisms working to store, utilise, and excrete these potentially toxic elements, supplemental intake of these minerals in this cohort of women may result in subclinical excesses and subsequent hypo and hyperthyroid states.32 However, data regarding these elements and their excess on parturition is sparse and mixed, with most of the research examining these nutrients and outcomes in states of deficiency.
While the exact mechanisms linking endocrine function and the timing of spontaneous labour are yet to be elucidated, research strongly supports a relationship between thyroid function and natural timing of birth.33 Recent literature has reported the potential role of thyroid hormones in placental development, function, and protection, adding to the existing body of evidence regarding placental integrity and oxidative stress in premature labour.34,35 In addition, maternal thyroid function largely dictates that of the foetus,36 knowledge underpinning seminal work surrounding the necessity of thyroid hormones for brown fat and lung development in-utero.37 Given the role of lung maturation in foetal-maternal signalling and initiation of parturition,38 it could be argued that the influence of thyroid hormones and their mediators – including selenium, zinc, and copper – extends beyond the maternal physiology into that of the foetus and its contribution to the initiation of labour.
Zinc and selenium are vital to endocrine function. In addition to their roles in protection against oxidative stress in the thyroid, these micronutrients interact with iodine in the form of thyroid hormones, facilitating the conversion of prohormone thyroxine (T4) to its biologically active form 3,5,3′-triiodothyronine (T3).39 Selenocysteine and zinc perform pivotal roles in this conversion; as such, suboptimal iodine, copper, selenium, or zinc levels have the potential to affect the amount of active thyroid hormone available for normal endocrine function.39,40 In turn, these levels have the capacity to affect the regulatory relationship between the thyroid, pituitary, and hypothalamic (HPT axis) hormones, in particular, the downregulation of this axis with advancing pregnancy in response to changing levels of human chorionic gonadotropin (hCG).41 In addition, the increase in thyroid-binding globulin (TBG) in the second and third trimesters result in a functional reduction in active T3 in healthy women with uncomplicated pregnancies.41 It is possible that dietary excess of these minerals disrupts these mechanisms, affecting the processes involved in spontaneous labour.
Zinc is a pivotal mineral in the maintenance of normal function, demonstrating antioxidant and anti-inflammatory properties and a key role in protein synthesis, cellular division, and immunity.42,43 In the context of labour, these properties may have the capacity to inhibit the systemic inflammatory response required for spontaneous parturition.44,45 Previous research reports an association between low maternal zinc levels and preterm labour,46 and reduced odds of preterm labour in at-risk groups with zinc supplementation.3,47 This study supports both of these findings and those of prior research regarding the positive association between zinc supplementation and extended gestational length in healthy women with uncomplicated pregnancies.48
Selenium is crucial to many biological functions and is central to the human selenoproteome. This family of 25 known selenoproteins dictate function across numerous mechanisms, including inflammatory and immune response.8 Current literature has highlighted the role of low selenium intake in the aetiology of pre-eclampsia,6,49,50 a finding supported by the association between HDPs, induction of labour, and reduced selenium levels found in this analysis. Furthermore, emerging evidence suggests that low selenium levels influence the incidence of idiopathic preterm birth,51,52 with placental observations between term and preterm gestations demonstrating lower concentrations of anti-inflammatory elements including zinc and selenium than the term specimens. This finding is thought to be due to the actions of enzymes such as selenium-dependent glutathione peroxidase (GPx-3) and copper/zinc superoxide dismutase, antioxidant proteins requiring these trace elements.52 Deficiencies in these minerals may result in a failure to regulate inflammatory processes in the quiescent uterus leading to spontaneous labour. This perspective is supported by evidence of a relationship between maternal selenium status, cord serum GPx-3 levels and gestational age at birth.53 As such, high dietary selenium may have the capacity to inhibit inflammatory processes necessary for spontaneous labour, potentially contributing to the extended length of gestation in this group of women.
Examination of the MONT FFQ data found the median energy intake of the cohort to be 12 743 kJ/day (5925-33 970 kJ/day), a figure within the calculated requirements of pregnant women in this group (7200-14 600 kJ/day).26 While over and under-reporting are potential limitations of this analysis, the trimmed mean did not significantly differ from the standard; both fell within the recommended energy intake for the eligible gestation and as such outliers were retained, contributing to final intake calculations. As such, the data collected by the MONT reflects a realistic level of mineral intake and dietary behaviour across the cohort. Despite the ongoing validation of the MONT representing a potential limitation in this study, to date, results in this research support its use and reliability in terms of dietary data collection.
No evidence of benefit regarding supplement use was evident in the outcomes examined in this cohort. In contrast to literature published in low and middle-income countries and high-risk groups, the lack of association between supplementation, serum values, and preterm birth in this cohort suggest that the protective effect of minerals on the incidence of idiopathic preterm labour are moot in this nutritionally replete population. Conversely, excessive dietary selenium, iodine, and zinc were associated with an increase in births beyond 41 weeks gestation. Further research is required to examine methods that identify individual needs and assess the potential for harm before recommending multiple micronutrient or mineral supplements during pregnancy.
Conclusion
Women of this cohort exhibited serum mineral levels within current pregnancy reference ranges, a finding consistent with the dietary data declarations. Prolonged pregnancy was associated with excessive dietary intake of zinc, iodine, and selenium in this cohort, irrespective of maternal supplement use. This nutritionally replete population demonstrated no evidence of benefit concerning supplements and their effect on birth outcomes. Further research is required to determine individual need and minimise the potential for harm prior to recommending pregnancy supplements.
Supplemental Material
Supplemental material, Appendix_1_-_Correlations_table_-_MONT_dietary_data_V2_xyz247585339b45f for Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia by Janelle M McAlpine, Daniel R McKeating, Lisa Vincze, Jessica J Vanderlelie and Anthony V Perkins in Nutrition and Metabolic Insights
Supplemental Material
Supplemental material, Appendix_2_-_Correlations_table_-_ICPMS_micronutrient_values_v2_xyz2475897b2fdfa_1 for Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia by Janelle M McAlpine, Daniel R McKeating, Lisa Vincze, Jessica J Vanderlelie and Anthony V Perkins in Nutrition and Metabolic Insights
Acknowledgments
The authors gratefully acknowledge the support and assistance of the midwives and management of the Gold Coast University and Royal Brisbane and Women’s Hospitals and the Griffith University School of Medical Science pregnancy research team on this project.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a Griffith University Post-Graduate Research Scholarship and the Griffith University Postgraduate support funding scheme.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JMMc conceptualisation, design, research and writing the initial draft (65%). DRMc: content and authorship regarding the ICP-MS methodology and analysis (20%). LV, JJV and AVP contributed 5% each in guidance, supervision and reviewing the manuscript.
ORCID iDs: Janelle M McAlpine  https://orcid.org/0000-0002-1157-9527
https://orcid.org/0000-0002-1157-9527
Lisa Vincze  https://orcid.org/0000-0003-0669-7147
https://orcid.org/0000-0003-0669-7147
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Shand AW, Walls M, Chatterjee R, Nassar N, Khambalia AZ. Dietary vitamin, mineral and herbal supplement use: a cross-sectional survey of before and during pregnancy use in Sydney, Australia. Aust N Z J Obstet Gynaecol. 2016;56:154-161. [DOI] [PubMed] [Google Scholar]
- 2. De-Regil LM, Palacios C, Lombardo L, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;2:CD008873. [DOI] [PubMed] [Google Scholar]
- 3. Ota E, Mori R, Middleton P, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015;2:CD000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;4: CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gernand AD, Simhan HN, Baca KM, Caritis S, Bodnar LM. Vitamin D, pre-eclampsia, and preterm birth among pregnancies at high risk for pre-eclampsia: an analysis of data from a low-dose aspirin trial. BJOG. 2017;124:1874-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu M, Guo D, Gu H, Zhang L, Lv S. Selenium and preeclampsia: a systematic review and meta-analysis. Biol Trace Elem Res. 2016;171:283-292. [DOI] [PubMed] [Google Scholar]
- 7. Baltaci AK, Mogulkoc R, Baltaci SB. Review: the role of zinc in the endocrine system. Pak J Pharm Sci. 2019;32:231. [PubMed] [Google Scholar]
- 8. Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Department of Health. Clinical Practice Guidelines: Pregnancy Care. Canberra, ACT, Australia: Australian Government; 2018. [Google Scholar]
- 10. Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJL, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US preventive services task force. JAMA. 2017;317:190-203. [DOI] [PubMed] [Google Scholar]
- 11. Harding KB, Pena-Rosas JP, Webster AC, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev. 2017:CD011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf HT, Hegaard HK, Huusom LD, Pinborg AB. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404e30. [DOI] [PubMed] [Google Scholar]
- 13. Blumfield ML, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. 2013;71:118-132. [DOI] [PubMed] [Google Scholar]
- 14. Malek L, Umberger WJ, Makrides M, Collins CT, Zhou SJ. Understanding motivations for dietary supplementation during pregnancy: a focus group study. Midwifery. 2018;57:59-68. [DOI] [PubMed] [Google Scholar]
- 15. Marinello V, Buckton C, Combet E. Harmless? Mixed perception and awareness of vitamin and mineral supplements. Proc Nutr Soc. 2016;75:E66. [Google Scholar]
- 16. Konz T, Migliavacca E, Dayon L, et al. ICP-MS/MS-based ionomics: a validated methodology to investigate the biological variability of the human ionome. J Proteome Res. 2017;16:2080-2090. [DOI] [PubMed] [Google Scholar]
- 17. Meyer S, Markova M, Pohl G, et al. Development, validation and application of an ICP-MS/MS method to quantify minerals and (ultra-)trace elements in human serum. J Trace Elem Med Biol. 2018;49:157-163. [DOI] [PubMed] [Google Scholar]
- 18. McAlpine JM, Perkins AV, Vanderlelie JJ. Design, development, and evaluation of the Maternal Outcomes and Nutrition Tool (MONT). Matern Child Nutr. 2018:e12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnett MP, Chiang VS, Milan AM, et al. Plasma elemental responses to red meat ingestion in healthy young males and the effect of cooking method. Eur J Nutr. 2019;58:1047-1054. [DOI] [PubMed] [Google Scholar]
- 20. De Blas Bravo I, Sanz Castro R, Lopez Riquelme N, Tormo Diaz C, Apraiz Goyenaga D. Optimization of the trace element determination by ICP-MS in human blood serum. J Trace Elem Med Biol. 2007;21:14-17. [DOI] [PubMed] [Google Scholar]
- 21. Brantsaeter AL, Haugen M, Alexander J, Meltzer HM. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr. 2008;4:28-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122: 51-65. [DOI] [PubMed] [Google Scholar]
- 23. Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84-93. [DOI] [PubMed] [Google Scholar]
- 24. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the eating at America’s Table Study. Am J Epidemiol. 2001;154:1089-1099. [DOI] [PubMed] [Google Scholar]
- 25. Food Standards Australia and New Zealand. Australian food composition database. http://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/default.aspx. Updated 2017. Accessed February 4, 2018.
- 26. Australian National Health and Medical Research Council & New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand. https://www.nrv.gov.au/home. Updated 2017. Accessed 23 March, 2019.
- 27. Pathology Queensland. Reference Intervals for General Chemistry, General Immunoassay and Blood Gas Analyser Tests Performed in Chemical Pathology. Gold Coast, QLD, Australia: Queensland Health; 2018. [Google Scholar]
- 28. Focus Information Technology. Normal Reference Ranges and Laboratory Values in Pregnancy. http://perinatology.com/Reference/Reference%20Ranges/Reference%20for%20Serum.htm. Updated 2018. Accessed 5 March, 2019.
- 29. James D, Steer P, Weiner C, et al. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2010;115:868. [DOI] [PubMed] [Google Scholar]
- 30. Australian Institute of Health and Welfare (AIHW). Australia’s Mothers and Babies 2016—In Brief. Canberra, ACT, Australia: AIHW; 2018. [Google Scholar]
- 31. Zhang Z, Yuan E, Liu J, et al. Gestational age-specific reference intervals for blood copper, zinc, calcium, magnesium, iron, lead, and cadmium during normal pregnancy. Clin Biochem. 2013;46:777-780. [DOI] [PubMed] [Google Scholar]
- 32. Shi X, Han C, Li C, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in china. J Clin Endocrinol Metab. 2015;100:1630-1638. [DOI] [PubMed] [Google Scholar]
- 33. Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239-245. [DOI] [PubMed] [Google Scholar]
- 34. Khera A, Vanderlelie JJ, Holland O, Perkins AV. Overexpression of endogenous anti-oxidants with selenium supplementation protects trophoblast cells from reactive oxygen species-induced apoptosis in a Bcl-2-dependent manner. Biol Trace Elem Res. 2017;177:394-403. [DOI] [PubMed] [Google Scholar]
- 35. Chen C-Y, Chen C-P, Lin K-H. Biological functions of thyroid hormone in placenta. Int J Mol Sci. 2015;16:4161-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Symonds ME. Pregnancy, parturition and neonatal development: interactions between nutrition and thyroid hormones. Proc Nutr Soc. 1995;54:329-343. [DOI] [PubMed] [Google Scholar]
- 38. Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2017;170:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ventura M, Melo M, Carrilho F. Selenium and thyroid disease: from pathophysiology to treatment. Int J Endocrinol. 2017:1297658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Kane SM, Mulhern MS, Pourshahidi LK, Strain JJ, Yeates AJ. Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutr Rev. 2018;76:418-431. [DOI] [PubMed] [Google Scholar]
- 41. King N, Bernardi LA. Thyroid function and pregnancy. In: Eaton JL, ed. Thyroid Disease and Reproduction. Cham, Switzerland: Springer; 2019: 69-78. [Google Scholar]
- 42. Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res. 2016:6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chaffee BW, King JC. Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26:118-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stephen G, Lui S, Hamilton S, Stevens A, Jones R. PLD.24 Gene expression profiling of human decidua during term labour: inflammation as a key driver of labour. Arch Dis Child Fetal Neonatal Ed. 2014;99:A113. [Google Scholar]
- 45. Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204:223.e1-223.e5. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Cao Z, Peng Z, et al. Folic acid supplementation, preconception body mass index, and preterm delivery: findings from the preconception cohort data in a Chinese rural population. BMC Pregnancy Childbirth. 2015;15:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nossier SA, Naeim NE, El-Sayed NA, Abu Zeid AA. The effect of zinc supplementation on pregnancy outcomes: a double-blind, randomised controlled trial, Egypt. Br J Nutr. 2015;114:274-285. [DOI] [PubMed] [Google Scholar]
- 48. McAlpine JM, Scott R, Scuffham PA, Perkins AV, Vanderlelie JJ. The association between third trimester multivitamin/mineral supplements and gestational length in uncomplicated pregnancies. Women Birth. 2016;29:41-46. [DOI] [PubMed] [Google Scholar]
- 49. Vanderlelie J, Perkins AVA. Selenium and preeclampsia: a global perspective. Pregnancy Hypertens. 2011;1:213-224. [DOI] [PubMed] [Google Scholar]
- 50. Vincent A, Khera A, Vanderlielie J. Placental oxidative stress, selenium and pre-eclampsia. Placenta. 2014;35:A77. [Google Scholar]
- 51. Yildirim E, Derici MK, Demir E, et al. Is the concentration of cadmium, lead, mercury, and selenium related to preterm birth. Biol Trace Elem Res. 2019;191:306-312. [DOI] [PubMed] [Google Scholar]
- 52. Irwinda R, Wibowo N, Putri AS. The concentration of micronutrients and heavy metals in maternal serum, placenta, and cord blood: a cross-sectional study in preterm birth. J Pregnancy. 2019;2019:5062365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santos C, Garcia-Fuentes E, Callejon-Leblic B, et al. Selenium, selenoproteins and selenometabolites in mothers and babies at the time of birth. Br J Nutr. 2017;117:1304-1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1_-_Correlations_table_-_MONT_dietary_data_V2_xyz247585339b45f for Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia by Janelle M McAlpine, Daniel R McKeating, Lisa Vincze, Jessica J Vanderlelie and Anthony V Perkins in Nutrition and Metabolic Insights
Supplemental material, Appendix_2_-_Correlations_table_-_ICPMS_micronutrient_values_v2_xyz2475897b2fdfa_1 for Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia by Janelle M McAlpine, Daniel R McKeating, Lisa Vincze, Jessica J Vanderlelie and Anthony V Perkins in Nutrition and Metabolic Insights


