Short abstract
Objective
Critical care capabilities needed for the management of septic patients, such as continuous vital sign monitoring, are largely unavailable in most emergency departments (EDs) in low- and middle-income country (LMIC) settings. This study aimed to assess the feasibility and accuracy of using a wireless wearable biosensor device for continuous vital sign monitoring in ED patients with suspected sepsis in an LMIC setting.
Methods
This was a prospective observational study of pediatric (≥2 mon) and adult patients with suspected sepsis at the Kigali University Teaching Hospital ED. Heart rate, respiratory rate and temperature measurements were continuously recorded using a wearable biosensor device for the duration of the patients’ ED course and compared to intermittent manually collected vital signs.
Results
A total of 42 patients had sufficient data for analysis. Mean duration of monitoring was 32.8 h per patient. Biosensor measurements were strongly correlated with manual measurements for heart rate (r = 0.87, p < 0.001) and respiratory rate (r = 0.75, p < 0.001), although were less strong for temperature (r = 0.61, p < 0.001). Mean (SD) differences between biosensor and manual measurements were 1.2 (11.4) beats/min, 2.5 (5.5) breaths/min and 1.4 (1.0)°C. Technical or practical feasibility issues occurred in 12 patients (28.6%) although were minor and included biosensor detachment, connectivity problems, removal for a radiologic study or exam, and patient/parent desire to remove the device.
Conclusions
Wearable biosensor devices can be feasibly implemented and provide accurate continuous heart rate and respiratory rate monitoring in acutely ill pediatric and adult ED patients with sepsis in an LMIC setting.
Keywords: Sepsis, low- and middle-income country, wearable technology, biosensor, critical care, resource-limited, continuous vital signs, wearable device, Rwanda, emergency medicine
Introduction
Sepsis is one of the leading causes of mortality worldwide, responsible for over 5 million deaths annually, with the vast majority of global sepsis deaths occurring in low- and middle-income countries (LMICs).1–4 As sepsis mortality declines in high-income countries (HICs), this progress has yet to be translated to LMICs, where outcomes remain far poorer due to lack of resources.1,3 Healthcare workers in LMICs often face major barriers to implementing recommended sepsis guidelines due to the prohibitive investments in infrastructure, material and human resources needed to develop critical care capabilities analogous to those in HICs.4–7
Continuous vital sign monitoring, a component of care widely available in HICs and highly valuable for management of septic patients, is largely unavailable in LMIC hospitals, with vital signs often being measured as infrequently as once per day due to human resource constraints.8,9
Purely vital sign–driven protocols have shown promise for reducing mortality in hypotensive intensive care unit patients in Tanzania, and heart rate (HR) normalization remains one of the most reliable signs of shock resolution in children.10,11
Innovative, cost-effective methods for monitoring critically ill patients utilizing relatively simple mobile health and wearable technology may be pivotal for healthcare providers in LMICs to detect early signs of patient deterioration, better guide interventions, and thereby save lives. Despite a growing number of recent studies validating the use of wireless wearable devices for a wide range of applications, prior studies have been performed nearly exclusively in HIC settings and in stable, ambulatory patients.12–14 There remains a stark lack of research on the use of these devices in LMICs and in acutely ill patients, where they may in fact find their greatest potential.
Adequate clinical monitoring of septic patients continues to be a major challenge for healthcare providers at the Kigali University Teaching Hospital (KUTH) emergency department (ED) in Kigali, Rwanda with intermittent vital sign measurements being obtained for the majority of septic patients in the ED. The purpose of this study was to assess the feasibility and accuracy of continuous vital sign monitoring using a wireless wearable biosensor device in adult and pediatric ED patients with suspected sepsis in an LMIC setting.
Materials and methods
Study design and setting
This prospective, observational study took place over an 8-wk period from August–October 2018. KUTH is an academic tertiary care hospital located in an urban setting, and functions as the primary referral center in the region. KUTH has separate adult and pediatric EDs, with approximately 30 adult ED beds and 10 pediatric ED beds. Median ED length of stay is 1 d, although this can vary considerably due to overcrowding and boarding.15 The adult ED is separated into zones by level of acuity: red (highest acuity), orange, yellow, and triage. Vital signs are typically measured two to four times per day (depending on the patients’ zone) by an ED nurse, with documentation of vital signs in a standardized paper ED medical chart.
Study participants
All adult and pediatric patients over the age of 2 mon (using corrected gestational age) with suspected sepsis presenting to the KUTH ED when a research nurse was on-site (7 a.m.–11 p.m. daily) were screened for inclusion in the study. Exclusion criteria included history of allergy to skin adhesive, presence of an implantable cardiac device, and inability to give consent. Suspected sepsis was defined as having two or more systemic inflammatory response syndrome criteria with suspected source of infection, and confirmation of suspected sepsis with the patients’ treating physician.16 Verbal and written informed consent were obtained from the patient and/or their parent/guardian after explanation of all study procedures by the research nurse. Child assent was also obtained for all children over the age of 7 y. Participants and their families were advised that they could have the wearable biosensor device removed at any time at their request.
Staff training and oversight
Six Rwandan ED nurses fluent in Kinyarwanda and English with 7–18 y of clinical experience were hired as research nurses. Research nurses underwent two half-day training sessions in study procedures, research ethics, use of the biosensor device monitoring system, and standardized measurement of manual vital signs. The research nurses were supervised by three study physicians (GM, CU and FRT) and data collection supervised by two study coordinators (VKS and JM) based in Rwanda. None of the Rwanda-based study team had prior experience in the clinical use of wearable devices. The biosensor devices, smartphones, technical support and data analysis were provided by personalized physiology analytics company physIQ, Inc. (Naperville, IL, USA). Overall study activities were coordinated by investigators at Brown University (Providence, RI, USA). Ethical approval was obtained from the University of Rwanda College of Medicine and Human Sciences Institutional Review Board, the KUTH Institutional Review Board and the Rhode Island Hospital Institutional Review Board.
Wearable biosensor device monitoring system
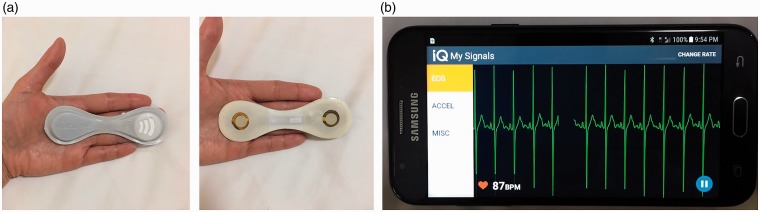
The VitalPatch (VP; VitalConnect, San Jose, CA, USA) was used for all study activities (Figure 1). The VP is a wireless wearable biosensor device approved by the United States Food and Drug Administration for up to 5 d (120 h) of continuous monitoring of the following measurements: skin temperature, HR, HR variability, respiratory rate (RR), body posture, fall detection, and activity. The VP is water-resistant, flexible and disposable with dimensions 1.15 × 3.6 × 0.8 cm and consists of an adhesive patch containing two electrocardiogram (ECG) electrodes, a thermistor, zinc-air coin battery, Bluetooth transceiver and 3-axis accelerometer. The accelerometer capabilities of the VP were not evaluated in this study. The VP was selected due to its battery life, prior use in hospitalized patients and the authors’ familiarity with the device.17,18 Data from the VP was continuously transmitted through Bluetooth connection to a Samsung Galaxy J3 smartphone running physIQ’s mobile application and subsequently transmitted from the smartphone via 3/4G or wireless internet connection to secure servers managed by physIQ. The estimated Bluetooth connection range is 10 m and the VP can store up to 18 h of data if the connection is lost.
Figure 1.
The VitalPatch (a) and display screen showing a participant's electrocardiogram signal on a Samsung Galaxy J3 smartphone running the physIQ mobile application (b).
Study procedures
After obtaining informed consent, research nurses cleaned the participant’s skin with an alcohol swab and placed a biosensor device on the participant’s left upper chest wall and connected the biosensor device to the smartphone via Bluetooth. Smartphones were secured using a cable lock at the ward nursing station and intermittently removed for charging in the staff workroom (approximately 2-h charge every 24 h). After placement of the biosensor device, an initial set of manual vital signs was obtained by the research nurse. HR and RR were counted manually using radial or carotid pulse over 1 min and RR by counting breaths over 1 min. Although the biosensor is designed to measure skin temperature, an assessment of correlation with body temperature was undertaken using a digital infrared tympanic thermometer obtained specifically for the research study. A tympanic thermometer was used due to ease of use, more reliable measurement of body temperature in comparison to oral/axillary measurements, and lack of need for associated disposable materials such as thermometer probe covers. Vital signs taken by the research nurses were measured and documented separately in addition to the standard of care vital signs documented by non-study ED nurses in the standard paper ED medical chart. Research nurse and ED nurse vital sign measurements were documented on a de-identified study case report form and abstracted into a customized research electronic data capture (REDCap, Vanderbilt University, USA) platform. Research nurses collected vital signs according to the standard vital sign monitoring schedule for each patients’ ED ward location (two to four times per day). As this was a purely observational study, vital signs collected from the biosensor or by the research nurses were not available for use by non-study staff to influence clinical care; however, research staff were instructed to notify participants’ treating providers if they had concerns regarding participants’ safety at any time. Research nurses obtained manual vital signs after first documenting the time using the clock on the phone’s locked screen (no vital sign measurements are displayed on this screen). All ED staff physicians and nurses were briefed on the study and instructed that they could remove the biosensor device if it was interfering with patient care at any time. Connectivity was monitored in real time by research staff using remote monitoring through the online platform, as well as during routine checks several times per shift. The biosensor device was removed by the research nurse for performance of any radiologic studies, upon discharge from the ED, transfer to operating room or another ward, death, or when the battery of the biosensor device ran out (approximately 120 h).
As the vital sign measurements obtained by the research nurses were used as the standard to which the biosensor vital signs were compared, an assessment of the reliability of the measurements obtained by the research nurses was undertaken using a sample of 12 study participants. These participants had a full set of vital signs obtained simultaneously and independently by two research nurses under direct observation by the research coordinator. Each research nurse recorded their measurements on a log book hidden from their colleague and was immediately given to the research coordinator for later analysis.
Statistical analysis
Participant characteristics, including age, sex, past medical history, presumed source of sepsis, ED disposition and mortality status at ED discharge, were summarized using standard descriptive statistics; results are presented as means and standard deviations (SDs) for continuous variables and frequencies and percentages for categorical data.
A target sample size was calculated based on a Pearson’s correlation coefficient effect size of 0.7 (based on data from a study using a similar biosensor device in an Ebola treatment unit in Sierra Leone) with a 95% confidence interval (CI) width of 0.2, requiring at least 178 vital sign measurements using standard calculations from the statistical literature.19,20 Assuming each participant had approximately four vital sign measurements during their ED course, a total of 45 patients was required to ensure significance.
Algorithms based on the ECG waveform data were used to generate HR and RR measurements at 1-min intervals. The percentage of useable data from the biosensor device was calculated using the duration of time that the biosensor device was able to provide a non-zero measurement over the duration of the participants’ entire data collection period. Useable data was determined using an ECG signal quality index (SQI) calculation which assesses the quality of the ECG signal via detection of QRS complexes. The SQI algorithm examines the distribution of samples in 15-s windows to make a determination of signal quality from 0–100% based on statistical characteristics of the distribution (mean, kurtosis, skewness); the window then moves forward 5 s and repeats in order to create a 1-min SQI based on SQIs calculated every 5 s. If the SQI fell below 98% the 1-min data window was rejected.
The agreement between vital sign measurements obtained from the biosensor and those manually collected by the research nurse at the same timepoint was assessed using Pearson’s product-moment correlation coefficient and Bland–Altman analysis. The Pearson’s correlation coefficient was calculated over the entire study set of recorded measurements for HR, RR and temperature. Bland–Altman analysis was used to calculate the mean difference (bias) between the two methods of measurement, which were plotted against the difference between the measurements, and 95% limits of agreement (±1.96 SD) calculated for HR, RR and temperature.21 The intraclass correlation coefficient (ICC), using a one-way random-effects model, was calculated to assess the reliability between research nurse measurements, and mean differences between observers were calculated for each vital sign measurement. Technical and practical feasibility issues were assessed using a one-page questionnaire completed by the research nurses after completion of each participant’s study involvement. Signs of allergic reaction to the device adhesive were monitored by the research nurses during every vital sign measurement encounter.
A secondary aim of this study was to evaluate the potential for continuous vital sign monitoring to identify signs indicative of clinical deterioration earlier than standard intermittent vital signs. For this aim, the difference (in minutes) between the timepoint at which KUTH ED nurses documented a clinically significant vital sign abnormality (as compared to an immediately preceding measurement within normal reference range) and the timepoint at which the biosensor registered an equivalent change in vital signs was calculated (see Table 1 for the criteria used). The criteria for clinically significant vital sign abnormalities were based on those in commonly used early warning score systems.22–24 In order to avoid bias from transient vital sign abnormalities (due to artifact or brief triggers such as pain or patient movement), only abnormal vital signs sustained over a continuous 5-min period were included for the purpose of this analysis. Temperature was not assessed in this analysis as KUTH ED patients have temperature checked less frequently than other vital signs. All statistical analyses were conducted using Stata 14 (StataCorp, College Station, TX) and R (http://cran.r-project.org) statistical packages.
Table 1.
Criteria for clinically significant abnormal heart rate or respiratory rate.
| Age | Heart rate (beats/min) | Respiratory rate (breaths/min) |
|---|---|---|
| <1 y | >150 | >50 |
| 1–4 y | >120 | >40 |
| 5–12 y | >110 | >30 |
| 13–17 y | >100 | >16 |
| ≥18 y | <50 or >100 | <9 or >15 |
Results
Enrollment and baseline characteristics
A total of 45 patients were enrolled in the study after informed consent was obtained. Three participants were excluded from further analysis as they left the ED within 1 h of enrollment (two admitted to medical ward, one to gynecology ward) shortly after the biosensor was placed, leaving 42 patients for analysis. The characteristics of the study population are shown in Table 2. The mean (SD) ages of pediatric and adult participants were 6.8 (5.1) y (range 5 mon–16 y) and 46.5 (19.6) y (range 18–87 y), respectively. ED length of stay ranged from less than 1 h to greater than 7 d.
Table 2.
Characteristics of study participants (n = 42).
| Characteristics | n (%) |
|---|---|
| Age group | |
| Adult (≥18 y) | 19 (45.2) |
| Pediatric (<18 y) | 23 (54.8) |
| Age (y), mean ± SD (range) | |
| All participants | 24.8 ± 24.2 (0.41–87) |
| Adult (≥18 y) | 46.5 ± 19.6 (18–87) |
| Pediatric (<18 y) | 6.8 ± 5.1 (0.41–16) |
| Sex | |
| Male | 23 (54.8) |
| Female | 19 (45.2) |
| Past medical history | |
| Hypertension | 2 (4.8) |
| Diabetes | 1 (2.4) |
| HIV | 3 (7.1) |
| Tuberculosis | 1 (2.4) |
| Malaria or other parasitic infection | 2 (4.8) |
| Malignancy | 2 (4.8) |
| Other | 8 (19.0) |
| None known | 24 (57.1) |
| Source(s) of sepsis | |
| Respiratory | 14 (33.3) |
| Gastrointestinal | 9 (21.4) |
| Central nervous system | 2 (4.8) |
| Skin or soft tissue | 4 (9.5) |
| Genitourinary | 2 (4.8) |
| Other source | 6 (14.3) |
| Unknown source | 8 (19.0) |
| Mortality | |
| Deceased at discharge/transfer from ED | 1 (2.4) |
| Deceased at inpatient day 7 | 7 (16.7) |
| Unknown status at day 7 | 3 (7.1) |
ED: emergency department; HIV: human immunodeficiency virus; SD: standard deviation.
Vital sign measurements
A total of 254 manual vital sign measurements were obtained by the research nurses for comparison with those obtained from the biosensor device with a mean of 6.1 measurements obtained per participant. A total of 1345 h of biosensor monitoring time was recorded with a mean of 32.8 h of data obtained per participant. One participant had more than 120 h of data as the participant left to go to the operating room and returned to the ED and had two different biosensors placed (135 h of data recorded).
A total of 191 vital sign measurements from standard ED medical charts obtained by non-study ED nurses were documented with a mean of 4.5 measurements obtained per participant. Evidence of RR estimation (a common practice in busy clinical settings) by non-study ED nurses was noted, with >50% of all adult RR measurements documented as 20 breaths/min (interquartile range 20–22 breaths/min) in the paper medical charts. Similar evidence of estimation of RR was not noted from the research nurse measurements. Mean values over the study population and stratified by age group (adult or pediatric) for HR, RR and temperature by method of measurement are shown in Table 3.
Table 3.
Vital sign measurements in study population by method of measurement.
| Research nurse | Biosensor | ED nurse | |
|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | |
| Heart rate (beats/min) | 108.6 ± 22.2 (60–180) | 109.4 ± 21.3 (33–219) | 111.5 ± 24.6 (54–192) |
| Adult | 96.6 ± 21.8 (50–160) | 97.5 ± 20.0 (54–174) | 99.2 ± 22.4 (61–154) |
| Pediatric | 115.1 ± 20.9 (68–180) | 113.1 ± 20.3 (32–219) | 116.5 ± 23.8 (54–192) |
| Respiratory rate (breaths/min) | 33.1 ± 10.7 (14–64) | 27.0 ± 7.2 (10–52) | 29.2 ± 10.5 (16–60) |
| Adult | 29.1 ± 7.1 (18–48) | 26.2 ± 7.6 (10–49) | 21.6 ± 5.1 (16–42) |
| Pediatric | 39.3 ± 11.3 (14–64) | 27.2 ± 7.1 (10–53) | 32.6 ± 10.5 (18–60) |
| Temperature (°C)a | 37.4 ± 1.2 (32.5–41.7) | 36.1 ± 1.2 (32–39) | 37.2 ± 1.3 (32.7–40.2) |
| Adult | 37.5 ± 1.0 (35.5–39.5) | 35.9 ± 1.2 (33–39) | 37.1 ± 1.0 (36–39.7) |
| Pediatric | 37.4 ± 1.3 (32.5–41.7) | 36.1 ± 1.3 (32–39) | 37.2 ± 1.3 (32.7–40.2) |
Adult: 18 y. Pediatric: <18 y.
Temperature was measured using tympanic thermometer (research nurses), skin measurement (biosensor) and axillary thermometer (ED nurses).
ED: emergency department; SD: standard deviation.
Accuracy of wearable biosensor device measurements
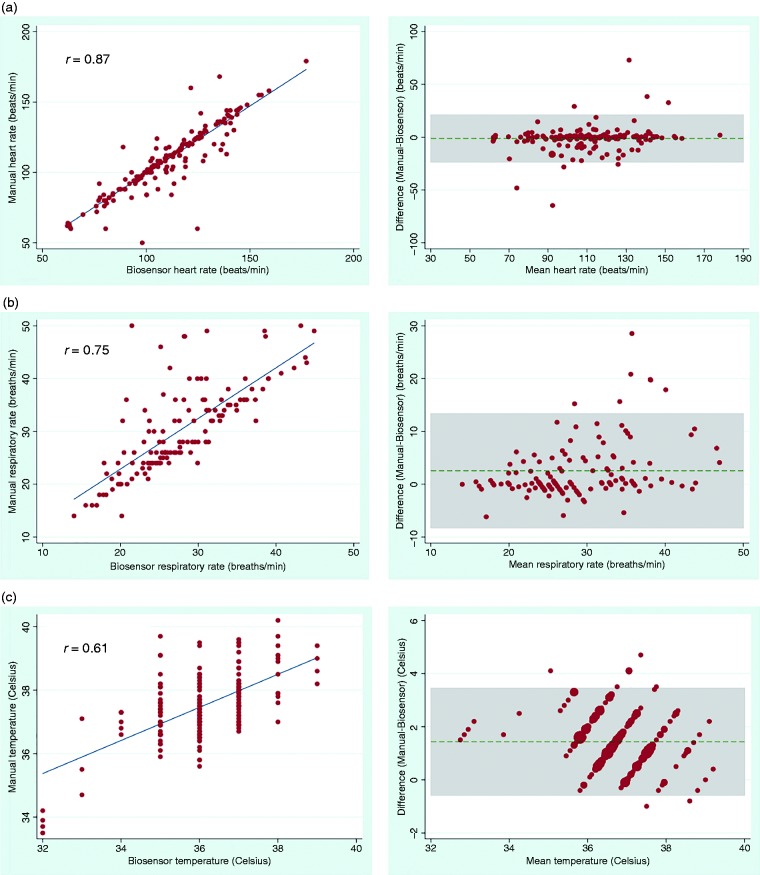
A total of 179 sets of HR, 129 sets of RR and 192 sets of temperature measurements were used for analysis of agreement between vital signs from the biosensor and those manually collected by research nurses. Correlation between the biosensor and manual measurements was strong for HR (r = 0.87, p < 0.001) and RR (r = 0.75, p < 0.001). Correlation was less strong for temperature (r = 0.61, p < 0.001), which was anticipated as the biosensor measures skin temperature whereas the tympanic thermometer measures body temperature. The mean (SD) differences between modes of measurement were 1.2 (11.4) beats/min for HR and 2.5 (5.5) breaths/min for RR, although had wide limits of agreement (Table 4). Mean difference for temperature was 1.4 (1.0) °C between biosensor skin temperature and tympanic thermometer body temperature measurements. Scatterplots showing biosensor measurements plotted against manual measurements, and Bland–Altman plots showing mean differences and limits of agreement are shown in Figure 2. A sensitivity analysis assessing the agreement between the two modes of measurement based on the age group (adult or pediatric) and stratification of the pediatric cohort into age ranges (<2 y, 2–5 y, and >5 y) was also undertaken and is shown in Table 4.
Table 4.
Bland–Altman analysis of vital sign measurements comparing manual measurements obtained by research nurses with those obtained via the biosensor device.
| Mean difference (bias) | SD | 95% LoA | |
|---|---|---|---|
| Heart rate(beats/min) | |||
| All participants | –1.2 | 11.4 | (–23.6, 21.1) |
| Adult (all) | –1.6 | 10.6 | (–22.2, 19.1) |
| Pediatric (all) | –1.1 | 11.7 | (–24.1, 21.9) |
| <2 y | –0.2 | 1.7 | (–3.5, 3.1) |
| 2–5 y | –0.5 | 5.1 | (–10.4, 9.4) |
| >5 y | –1.5 | 14.4 | (–29.7, 26.7) |
| Respiratory rate (breaths/min) | |||
| All participants | 2.5 | 5.5 | (–8.3, 13.4) |
| Adult (all) | 1.1 | 4.8 | (–8.8, 10.9) |
| Pediatric (all) | 3.4 | 5.6 | (–7.7, 14.4) |
| <2 y | 3.2 | 4.3 | (–5.2, 11.6) |
| 2–5 y | 3.2 | 4.6 | (–5.7, 12.2) |
| >5 y | 3.5 | 6.4 | (–9.1, 16.0) |
| Temperature (°C) | |||
| All participants | 1.4 | 1.0 | (–0.6, 3.5) |
| Adult (all) | 1.6 | 1.1 | (–0.5, 3.7) |
| Pediatric (all) | 1.4 | 1.0 | (–0.6, 3.4) |
| <2 y | 1.3 | 0.8 | (–0.3, 2.9) |
| 2–5 y | 1.5 | 1.0 | (–0.5, 3.5) |
| >5 y | 1.3 | 1.0 | (–0.7, 3.4) |
LoA: limits of agreement; SD: standard deviation.
Figure 2.
Correlation scatterplots (left) and Bland–Altman plots (right) for (a) heart rate, (b) respiratory rate and (c) temperature comparing biosensor measurements and manual measurements obtained by a research nurse. Horizontal dashed lines in the Bland–Altman plots indicate the mean differences (bias) and shaded areas indicate 95% limits of agreement.
Reliability of manual vital sign measurements
Each research nurse obtained two sets of simultaneous vital signs for assessment of the reliability of manual vital sign measurements with a total of 12 pairs of vital signs for analysis (Table 5). The ICC was 0.97 (95% CI 0.90–0.99) for HR, 0.85 (95% CI 0.54–0.96) for RR and 0.79 (95% CI 0.44–0.94) for temperature, indicating good to excellent reliability for all vital sign measurements.
Table 5.
Reliability of manual vital sign measurements obtained on participant simultaneously by two research nurses.
| MDO (SD) | ICC (95% CI) | |
|---|---|---|
| Heart rate (beats/min) | 1.1 (3.9) | 0.97 (95% CI 0.90–0.99) |
| Respiratory rate (breaths/min) | 2.1 (3.9) | 0.85 (95% CI 0.54–0.96) |
| Temperature (°C) | 0.22 (0.55) | 0.79 (95% CI 0.44–0.94) |
CI: confidence interval; ICC: intraclass correlation coefficient; MDO: mean difference between observers; SD: standard deviation.
Technical and practical feasibility
There was successful transmission of 1312 h of useable HR data (97.6% of total), and 1340 h of useable RR data (99.6% of total). A total of 32 h of HR and 4.9 h of RR data were not transmitted due to connectivity problem or artifact and were excluded from use in the correlation analysis. Connectivity problems, in which vital signs from the VP were not displayed on the smartphone and/or server, were reported in eight participants. In five participants, the biosensor was replaced after troubleshooting (moving the phone closer to the participant, restarting the smartphone, reconnecting Bluetooth) did not solve the connectivity problem; in these five participants, once the biosensor was replaced, no further connectivity problems were noted.
A single biosensor was used for the entire duration of the participants’ study involvement for 71% of participants. Reasons for biosensor removal or replacement are shown in Table 6. Notably, one participant (9-mon-old male) had the biosensor removed by the treating physician in order to perform a physical exam; after the exam the mother requested the biosensor not be replaced as the removal appeared to be uncomfortable (no skin damage or harm was noted by the research or staff physician). In three participants, the biosensor unintentionally detached: in one participant after the mother bathed the 5-y-old child; in two participants, the biosensor only partially detached but thereafter had poor data quality so was replaced. In one participant, a possible allergic reaction was noted (skin irritation and redness at site of the biosensor adhesive). This participant had the biosensor device in place longer than any patient (135 h) so it was unclear if this was truly due to allergy or skin irritation from prolonged use of the biosensor. No other adverse events were noted.
Table 6.
Technical and practical feasibility of biosensor devices.
| n (%) | |
|---|---|
| Duration of biosensor vital sign recording | |
| Hours of study biosensor recording | 1344 |
| Hours of biosensor recording per patient, mean ± SD (range) | 32.8 ± 31.0 (0.5–135) |
| % useable data | |
| Heart rate | 97.6% |
| Respiratory rate | 99.6% |
| Biosensor removed or replaced | |
| Removed | 2 (4.8) |
| Replaced | 10 (23.8) |
| Neither removed nor replaced | 30 (71.4) |
| Source of biosensor removal/replacement | |
| Patient | 1 (2.4) |
| Family member | 1 (2.4) |
| Physician | 1 (2.4) |
| Research nurse | 9 (21.4) |
| Reasons for biosensor removal/replacement | |
| Accidental removal/detachment | 3 (7.1) |
| Need to perform radiologic study | 2 (4.8) |
| Need to perform physical exam | 1 (2.4) |
| Poor connection with smartphone | 5 (11.9) |
| Patient preference | 1 (2.4) |
| Connectivity problems | |
| Yes | 8 (19.0) |
| No | 34 (81.0) |
| Presence of allergic reaction | |
| Yes | 1 (2.4) |
| No | 41 (97.6) |
SD: standard deviation.
Time to detection of clinically significant vital sign changes
A total of nine clinically significant vital sign changes, as determined using the pre-defined criteria, were documented in study participants’ standard ED paper charts. Participants with initially abnormal vital signs that remained abnormal or normalized throughout their ED course were not included as the ability to detect changes suggestive of clinical deterioration (i.e. normal to abnormal) was the focus of this analysis. The mean (SD) difference between the time when the biosensor recorded an equivalent and sustained measurement to that documented in the paper chart was 331.6 (268.5) min (range 63–798 min) or 5.5 h (range 1–13.3 h).
Discussion
To the authors’ knowledge, this is the first clinical study to evaluate the feasibility and accuracy of a wireless wearable device for vital sign monitoring in either ED or pediatric patients in an LMIC setting. The results of this study show that continuous HR and RR measurements from a wireless wearable device are reliable and accurate compared to those obtained by an experienced nurse in acutely ill adult and pediatric ED patients, and are highly congruent to prior studies’ findings, including those from HIC using continuous telemetry monitors as a reference standard.25–28 In addition, clinically relevant vital sign changes were detected by the biosensor device many hours on average before documentation in the clinical chart using standard vital sign monitoring. Minor technical or practical feasibility issues occurred in approximately one-third of participants, and over 97% of data from the biosensor was successfully transmitted. Furthermore, the biosensor system proved capable of monitoring the majority of participants for the entirety of their ED course, and was easily implemented after a brief training session by providers without prior experience using similar devices. Lessons learned from this pilot study strongly support further implementation and evaluation of such wearable systems in acutely ill patients in LMIC settings.
Continuous monitoring of vital signs may allow for earlier detection of clinical deterioration, and thereby allow healthcare providers to deliver timelier interventions and reduce adverse events.29–31 In this study, continuous biosensor vital sign measurements were able to detect signs of potential clinical deterioration an average of 5.5 h earlier than standard vital sign monitoring; even greater gains are expected in other hospitals where vital signs are measured less frequently than at this study site.9 These findings are similar to a 2019 study in the Netherlands which showed that measurements from a wearable device in a general ward detected high modified early warning scores (MEWS) up to 10 h earlier than during standard nurse MEWS measurements.27
A review of the current literature shows that only four studies have evaluated any form of wireless wearable technology in LMIC settings: one pilot study (conducted by two of this study’s authors) evaluating the use of a wearable device in patients with suspected Ebola virus disease in Sierra Leone; and three studies in Uganda—two in a maternity ward and one in a general medical ward.20,26,32,33 While no prior studies have evaluated wireless wearable devices for pediatric monitoring in an LMIC setting, the findings of this study urge further investigation of these devices in pediatric patients who constitute a large burden of critically ill patients in LMICs. A recent study among pediatric inpatients admitted to district-level hospitals in Kenya found that the frequency of vital sign measurements was far lower than the stated goal benchmark of three sets of vital sign measurements in a 24-h period.34 Additionally, numerous studies have described the difficulty in obtaining reliable RR measurements, even in the most controlled settings.35,36 As diagnosis and management of pneumonia, the leading cause of child mortality worldwide, is largely based on RR in LMICs (using Integrated Management of Childhood Illness criteria), there is a critical need for context-appropriate tools to accurately measure RR for children.37–39 Further benefits of wireless wearable devices for children include the lack of intrusive wires and the ability for caregivers to hold and bathe young children while monitoring is taking place. As new sensor technology for clinical applications continues to advance, it is also important to note that non-contact sensors, such as image-based complementary metal oxide semiconductor camera infrared thermography and medical radar systems, offer even further possibilities for remote vital sign monitoring and reduction of nosocomial infectious risks.40,41
Several technical and practical challenges existed in implementing the biosensor device monitoring system that were not studied systematically but which may be relevant to future implementation of similar systems. First, patients were at times far from the nursing station due to the frequent use of hallway or corner spaces as the ED wards in KUTH are large, open rooms. This required occasional moving of a patient and/or a smartphone to ensure a good connection. Additionally, due to the time difference between Rwanda and the United States (6–7 h), troubleshooting of the biosensor devices was largely done in the small period of overlapping work hours. Nearly all of the replacements were due to poor connectivity, with a lack of vital sign display on the smartphone (usually after a period when the patient was temporarily out of Bluetooth range). Later in the course of the study, it was discovered that much of the data was actually still being transmitted to the servers despite the vital signs not immediately appearing on the smartphone. These issues became less frequent as the study progressed due to the research team’s increasing confidence and familiarity with the biosensor devices.
The strengths of this study include its validation in a real-world clinical setting representative of LMIC hospitals where such devices are intended to be used. The vast majority of prior studies evaluating wearable devices have taken place in highly controlled laboratory conditions in stable, healthy volunteers.12,14 This study’s participants had highly varying ages and clinical acuity—including quite stable patients (often boarding while waiting for an inpatient bed) as well as the critically ill (including one mechanically ventilated patient)—reflecting daily clinical practice. Despite this clinical variability, the accuracy of the biosensor performed well even at extremes of human physiology. Interestingly, there was closer agreement between biosensor and manual HR measurements in the youngest cohort of participants (<5 y). This was unanticipated as extreme HR and RR measurements that are more common in young children are often more difficult to measure manually, and algorithms tend to be less accurate at these high rates. Reasons for this are unclear and may be due to lack of mobility (young children were often the most ill of all study participants) or may simply be due to their relatively small number in the study. The findings are intriguing and warrant further investigation. In terms of practicality and logistical feasibility, the devices performed well in an often hectic ED setting. Nearly all technical and logistical troubleshooting was done in real time by research team members who, although had no prior biosensor experience, used their clinical experience to develop context-appropriate solutions to problems.
Limitations
This study is limited by using data collected from a single large, urban medical center as well as use of a single type of wearable device; the results therefore may not be generalizable to all ED patients with suspected sepsis or to other similar wearable devices. Notably, the KUTH EDs have implemented specialist residency training programs as well as quality improvement projects in recent years and as a result offer a high standard of care and level of organization, which may underestimate logistical issues for other LMIC hospitals.42,43 Furthermore, the KUTH ED has a reliable electrical power supply with at least one outlet per patient ward. However, as the smartphone requires only 2 h charging per day, this system could still be feasible even in many lower level or rural facilities. Solar charging stations may also present a viable option.
Accuracy of the biosensor was assessed in comparison to manual measurements obtained by experienced research nurses as a reference standard. It is possible that the manual measurements were inaccurate as compared to other reference standards, such as continuous ECG monitoring or capnography. However, use of such sophisticated systems was not feasible and comparison to manual measurements was pragmatic, as this represents the reference standard for the great majority of patients in LMICs. Additionally, the assessment of research nurse measurements showed high reliability. The measurements were weakest for temperature, which may indicate fault with the thermometer, or variations in ambient temperature in the ward.
As the biosensor was not designed to measure body temperature, and ambient temperatures in non-temperature-controlled wards may vary significantly, the biosensor cannot currently be recommended as an accurate measurement of body temperature. However, detection of trends in skin temperature measurement may prove valuable, and conversion of skin temperature to body temperature may also be possible.44 There are few studies that have investigated the use of wearable sensors for continuous temperature measurement, with highly variable reports of accuracy (mean bias ranging between 0.1 and 1.0°C).45–48 Additionally, the majority of existing studies have been largely experimental and/or conducted with small numbers of patients in controlled laboratory settings; further clinical studies investigating the most reliable methods of continuous temperature measurement are needed.
Future directions
Use of continuous vital sign monitoring must balance the ability to detect earlier signs of clinical deterioration with the potential for increased provider workload and alarm fatigue. Novel tools incorporating machine-learning algorithms using vital signs from wearable devices may be used to create patient-specific early warning systems and thereby allow providers to better direct scarce resources. The algorithms may further take advantage of non-conventional vital signs, such as HR variability, which have shown promising potential in predicting deterioration and mortality in septic patients.49,50 Studies investigating use of these tools are currently being planned by this study’s authors.
Qualitative assessments of patient and provider perspectives on wearable technology should also be conducted before large-scale implementation of similar technology can be undertaken in any context, LMIC or otherwise. Cost-effectiveness was not studied as part of this pilot, and this needs to be thoroughly evaluated prior to use of this technology in LMIC settings. Given concerns regarding nosocomial infection transmission, reusable devices were not used for this pilot study; however, if these devices are intended to be used in LMIC settings, cost-effectiveness is likely to be greatest with reusable and sterilizable options.
Conclusion
Wearable biosensor devices can be feasibly implemented and provide accurate continuous HR and RR measurements in acutely ill pediatric and adult ED patients with suspected sepsis in an LMIC setting. Differences in accuracy of the devices in pediatric compared to adult patients warrants further study especially in children less than 2 y. Cost-effectiveness of the use of similar devices in these settings are needed, as well as prospective studies evaluating the impact of biosensor devices on improving clinical outcomes for septic and other critically ill patients.
Authors’ note
Christian Umuhoza is also affiliated with Department of Pediatrics, University Teaching Hospital of Kigali, Kigali, Rwanda.
Acknowledgements
We would like to thank the KUTH pediatric and adult ED staff, research study team and nurses, without whom this study would not have been possible. We also thank the University Emergency Medicine Foundation for providing the support for this work. We thank the patients and their families who participated in this study.
Contributorship
SCG, GM, CU, SLR, SWW, NM, KDM and ACL were involved in the initial study design and planning. SCG, GM, CU, FRT, JM, KDM and VKS were involved in the conduct and supervision of the study. SCG, OT, JM, VKS and SWW were involved in data quality assurance, data analysis and writing of the manuscript. All authors read and approved the final manuscript.
Conflict of interests
The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SWW is an employee of physIQ, Inc.
Ethical approval
Ethical approval for the study was obtained from the University of Rwanda College of Medicine and Human Sciences Institutional Review Board, the University Teaching Hospital of Kigali Institutional Review Board, and the Rhode Island Hospital Institutional Review Board.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Brown Emergency Medicine (formerly University Emergency Medicine Foundation), which is the employer for SCG, KDM and ACL.
Guarantor
SCG.
Peer review
This manuscript was reviewed by three individuals who have chosen to remain anonymous.
References
- 1.Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193: 259–272. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AC, West TE, Limmathurotsakul D, et al. Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med 2008; 5: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado FR, Angus DC. Trying to improve sepsis care in low-resource settings. JAMA 2017; 318: 1225–1227. [DOI] [PubMed] [Google Scholar]
- 4.Dünser MW, Festic E, Dondorp A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med 2012; 38: 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy S, Adhikari NK. Global health care of the critically ill in low-resource settings. Ann Am Thorac Soc 2013; 10: 509–513. [DOI] [PubMed] [Google Scholar]
- 6.Baelani I, Jochberger S, Laimer T, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit care 2011; 15: R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob ST, Lim M, Banura P, et al. Integrating sepsis management recommendations into clinical care guidelines for district hospitals in resource-limited settings: the necessity to augment new guidelines with future research. BMC Med 2013; 11: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papali A, McCurdy MT, Calvello EJB. A “three delays” model for severe sepsis in resource-limited countries. J Crit Care 2015; 30: 861.e9–861.e14. [DOI] [PubMed] [Google Scholar]
- 9.Asiimwe SB, Okello S, Moore CC. Frequency of vital signs monitoring and its association with mortality among adults with severe sepsis admitted to a general medical ward in Uganda. PLoS One 2014; 9: e89879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker T, Schell CO, Lugazia E, et al. Vital signs directed therapy: improving care in an intensive care unit in a low-income country. PLoS One 2015; 10: e0144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khilnani P, Singhi S, Lodha R, et al. Pediatric Sepsis Guidelines: Summary for resource-limited countries. Indian J Crit Care Med 2010; 14: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AM, Selvaraj N, Ferdosi N, et al. Wireless patch sensor for remote monitoring of heart rate, respiration, activity, and falls. Conf Proc IEEE Eng Med Biol Soc; 2013; 14: 6115–6118. [DOI] [PubMed] [Google Scholar]
- 13.Pipke RM, Wegerich SW, Saidi A, et al. Feasibility of personalized nonparametric analytics for predictive monitoring of heart failure patients using continuous mobile telemetry. In: Proceedings of the 4th Conference on Wireless Health Baltimore, Maryland, USA, 1–3 November 2013, article no. 7. New York: ACM.
- 14.Wang R, Blackburn G, Desai M, et al. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol 2017; 2: 104–106. [DOI] [PubMed] [Google Scholar]
- 15.Aluisio AR, Barry MA, Martin KD, et al. Impact of emergency medicine training implementation on mortality outcomes in Kigali, Rwanda: an interrupted time-series study. Afr J Emerg Med 2019; 9: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 17.Weenk M, van Goor H, Frietman B, et al. Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR Mhealth Uhealth 2017; 5: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breteler MJM, Huizinga E, van Loon K, et al. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: a clinical validation study. BMJ Open 2018; 8: e020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonett DG, Wright TA. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 2000; 65: 23–28. [Google Scholar]
- 20.Steinhubl SR, Feye D, Levine AC, et al. Validation of a portable, deployable system for continuous vital sign monitoring using a multiparametric wearable sensor and personalised analytics in an Ebola treatment centre. BMJ Glob Health 2016; 1: e000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 327: 307–310. [PubMed] [Google Scholar]
- 22.Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified Early Warning Score in medical admissions. QJM 2001; 94: 521–526. [DOI] [PubMed] [Google Scholar]
- 23.Akre M, Finkelstein M, Erickson M, et al. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics 2010; 125: e763–e769. [DOI] [PubMed] [Google Scholar]
- 24.Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care 2006; 21: 271–278. [DOI] [PubMed] [Google Scholar]
- 25.Kroll RR, Boyd JG, Maslove DM. Accuracy of a wrist-worn wearable device for monitoring heart rates in hospital inpatients: a prospective observational study. J Med Internet Res 2016; 18: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngonzi J, Boatin A. A functionality and acceptability study of wireless maternal vital sign monitor in a tertiary university teaching hospital in rural Uganda. J Womens Health Gynecol 2017; 1: 1. [Google Scholar]
- 27.Weenk M, Koeneman M, van de Belt TH, et al. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation 2019; 136: 47–53. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Silveira M, Ahmed K, Ang S-S, et al. Assessment of the feasibility of an ultra-low power, wireless digital patch for the continuous ambulatory monitoring of vital signs. BMJ Open 2015; 5: e006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown H, Terrence J, Vasquez P, et al. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med 2014; 127: 226–232. [DOI] [PubMed] [Google Scholar]
- 30.Taenzer AH, Pyke JB, McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010; 112: 282–287. [DOI] [PubMed] [Google Scholar]
- 31.Cardona-Morrell M, Prgomet M, Turner RM, et al. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta‐analysis. Int J Clin Pract 2016; 70: 806–824. [DOI] [PubMed] [Google Scholar]
- 32.Byakika-Kibwika P, Muwonge M, Watts W, et al. Lessons learned from implementing a rapid test of a technology device in a tertiary hospital in Uganda. Ann Glob Health 2015; 81: 725–730. [DOI] [PubMed] [Google Scholar]
- 33.Mugyenyi GR, Atukunda EC, Ngonzi J, et al. Functionality and acceptability of a wireless fetal heart rate monitoring device in term pregnant women in rural southwestern Uganda. BMC Pregnancy Childbirth 2017; 17: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogero M, Ayieko P, Makone B, et al. An observational study of monitoring of vital signs in children admitted to Kenyan hospitals: an insight into the quality of nursing care? J Glob Health 2018; 8: 010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amirav I, Masumbuko CK, Hawkes MT. Poor agreement and imprecision of respiratory rate measurements in children in a low-income setting. Am J Respir Crit Care Med 2018; 198: 1462–1463. [DOI] [PubMed] [Google Scholar]
- 36.Edmonds ZV, Mower WR, Lovato LM, et al. The reliability of vital sign measurements. Ann Emerg Med 2002; 39: 233–237. [DOI] [PubMed] [Google Scholar]
- 37.Mulholland K. Problems with the WHO guidelines for management of childhood pneumonia. Lancet Glob Health 2018; 6: e8–e9. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Integrated Management of Childhood Illness chart booklet. WHO, 2017. [Google Scholar]
- 39.Ginsburg AS, Lenahan JL, Izadnegahdar R, et al. A systematic review of tools to measure respiratory rate in order to identify childhood pneumonia. Am J Respir Crit Care Med 2018; 197: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 40.Sun G, Okada M, Nakamura R, et al. Twenty-four-hour continuous and remote monitoring of respiratory rate using a medical radar system for the early detection of pneumonia in symptomatic elderly bedridden hospitalized patients. Clin Case Rep 2018; 7: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun G, Nakayama Y, Dagdanpurev S, et al. Remote sensing of multiple vital signs using a CMOS camera-equipped infrared thermography system and its clinical application in rapidly screening patients with suspected infectious diseases. Int J Infect Dis 2017; 55: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mbanjumucyo G, DeVos E, Pulfrey S, et al. State of emergency medicine in Rwanda 2015: an innovative trainee and trainer model. Int J Emerg Med 2015; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byiringiro JC, Wong R, Davis C, et al. Applying quality improvement principles to improve accident and emergency department overcrowding and flow in Rwanda: a case study. J Hosp Adm 2015; 4: 47–51. [Google Scholar]
- 44.Niedermann R, Wyss E, Annaheim S, et al. Prediction of human core body temperature using non-invasive measurement methods. Int J Biometeorol 2014; 58: 7–15. [DOI] [PubMed] [Google Scholar]
- 45.Tamura T, Huang M, Togawa T. Current developments in wearable thermometers. Adv Biomed Eng 2018; 7: 88–99. [Google Scholar]
- 46.Saurabh K, Rao H, Amrutur B, et al. Continuous core body temperature estimation via surface temperature measurements using wearable sensors is it feasible. In: BIODEVICES 2014, 7th International Conference on Biomedical Electronics and Systems, Esio, Angers, Loire Valley, France, 2014, pp.181–186.
- 47.Popovic Z, Momenroodaki P, Scheeler R. Toward wearable wireless thermometers for internal body temperature measurements. IEEE Commun Mag 2014; 52: 118–125. [Google Scholar]
- 48.Chen W, Dols S, Oetomo SB, et al. Monitoring body temperature of newborn infants at neonatal intensive care units using wearable sensors. In: Proceedings of the Fifth International Conference on Body Area Networks ACM, 2010, pp.188–194.
- 49.Bohanon FJ, Mrazek AA, Shabana MT, et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am J Surg 2015; 210: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Castilho FM, Ribeiro ALP, da Silva JLP, et al. Heart rate variability as predictor of mortality in sepsis: a prospective cohort study. PLoS One 2017; 12: e0180060. [DOI] [PMC free article] [PubMed] [Google Scholar]