Abstract
Background:
The purpose of this study was to determine the effectiveness of an adjustable sling compared with an artificial urinary sphincter (AUS) in patients with severe urinary incontinence (SUI) postprostatectomy (PP).
Methods:
This review was carried out following the Cochrane Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) declaration. We searched Medline, Embase, LILACS, and CENTRAL databases. Studies with patients older than 18 years of age with SUI PP who underwent sling or AUS intervention and had been monitored for longer than 12 months were included.
Results:
Seven studies were included, yielding a sample size of 420. Pads were reportedly dry or improved in 70% of the sling group compared with 74% in the AUS group. The Incontinence Impact Questionnaire, Short Form (IIQ-7) was the most frequently used scale and showed improvement, with a score of 82.8% in the AUS group compared with 86.1% in the sling group. When comparing interventions with nonintervention, relative risks (RRs) of 35.37 (95% confidence interval [CI]: 7.17–174.35) and 45.14 (95% CI: 11.09–183.70) were found for the adjustable sling and AUS, respectively, which were statistically significant. No significant differences were found when AUS versus adjustable sling were compared, with an RR of 0.78 (95% CI: 0.09–6.56). We found a low risk of bias in most studies.
Conclusions:
Both interventions can reduce incontinence and improve the quality of life of patients with SUI PP. The published literature is substantially limited as no randomized clinical trials are available, no consensus has been reached regarding the definition of severity of incontinence, and considerable heterogeneity exists across the outcome variables measured.
Keywords: incontinence, network meta-analysis, sling, systematic review, urinary sphincter
Introduction
Postprostatectomy (PP) urinary incontinence (UI) has significant negative effects on satisfaction and quality of life among patients,1 with most patients requiring surgical management. For severe UI (SUI), standard management consists of using an artificial urinary sphincter (AUS), however, an adjustable sling can also be an option.
The prevalence of male UI varies with age, with a rate of 4.8% in men between 45 years and 64 years of age and 8.3% in men older than 65 years of age, while SUI is reported in 2% and 4% of these populations, respectively.2 On the other hand, the prevalence of UI PP varies between 5% and 48%3 in different studies depending on the definition used, the surgical technique performed, and the PP time. Around 10% of UI PP cases may require surgical treatment.4 Symptom severity can be classified as mild (2–10 ml/h or 1–2 pads/day), moderate (11–50 ml/h or 3–4 pads/day), and severe (>50 ml/h or >5 pads/day), however no consensus has been reached regarding the definition of UI severity.5,6
Both noninvasive and invasive approaches can be applied for the management of UI. Noninvasive management consists of lifestyle changes, pelvic floor therapy, electrical stimulation, and pharmacological therapy, while invasive management involves surgical intervention, including injectables, slings, and AUSs.7 Selection of treatment type depends on the severity and duration of symptoms and the type of UI.8
The goal of surgical treatments for UI is to improve urethral strength, decrease urine leakage, and preserve normal bladder function. No standard protocol has been established for the initiation and type of surgical management in patients with UI PP based on the duration and severity of symptoms, however when symptoms last for more than 12 months, surgical management can be considered.8 Although AUS placement has been regarded as the standard treatment for SUI, slings have been shown to be safe and less invasive and can be considered as an initial treatment option prior to AUS implantation.4
Slings can be categorized as adjustable and nonadjustable. Adjustable slings include the Argus, Remeex, and ATOMS. We set out to compare the adjustable sling with the AUS given that the advantage of adjustable slings is the ability to easily provide more urethral compression if incontinence persists.9 These interventions could be comparable in patients with SUI PP but there could be a difference in the costs of devices with a similar treatment objective. Therefore, we compared the effectiveness of an adjustable sling with an AUS in patients with SUI PP.
Materials and methods
This review was carried out in accordance with the recommendations of the Cochrane Manual of Systematic Reviews of Interventions and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Declaration. The registration number in PROSPERO is CRD42018105714.
Inclusion criteria
We included observational, analytical, prospective, and retrospective longitudinal studies that included patients older than 18 years of age with SUI (> 5 pads/day) following prostatectomy (radical and benign prostatic hyperplasia [BPH] surgery) who were managed via placement of an AUS or adjustable sling. The studies were required to have at least 12 months of follow up. No adjustments or language restrictions were applied. The exclusion criteria were follow ups of less than 12 months, previous use of a sling, and UI not originating from prostatectomy. The principal outcome assessed was a decrease in pad use over 24 h. This categorical variable was classified into dry, improved, not used, or a maximum of 1 pad/24 h, a 50% decrease in pad use, and a greater than 50% decrease in pad use. Secondary outcomes included improvements in the quality-of-life scales and in readjustments or changes to the implanted device.
Information sources
The literature search was conducted in accordance with the recommendations of the Cochrane Manual of Systematic Reviews of Interventions. We used medical subject headings (MeSH), Emtree language, Descriptors in health sciences (DeCS), and text words related in a complete search strategy (Appendix 1). We searched MEDLINE (OVID), EMBASE, LILACS, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to the present. To ensure literature saturation, we scanned references from relevant articles identified through the search, conferences, thesis databases, Open Grey, Google scholar, and ClinicalTrials gov, among others. We contacted authors by email in the case of missing information.
Data collection
We reviewed each reference by title and abstract. Then we scanned full texts of relevant studies, applied prespecified inclusion and exclusion criteria, and extracted the data. Disagreements were resolved by consensus and where disagreement could not be resolved, a third reviewer settled the conflict.
Relevant data were collected in duplicate using a standardized data extraction sheet that contained the following information: author names; year of publication; title; study design; geographic location; objectives; inclusion and exclusion criteria; number of patients included; losses to follow up; timing; definition of outcomes (infection); outcomes and association measures; funding source.
Risk of bias
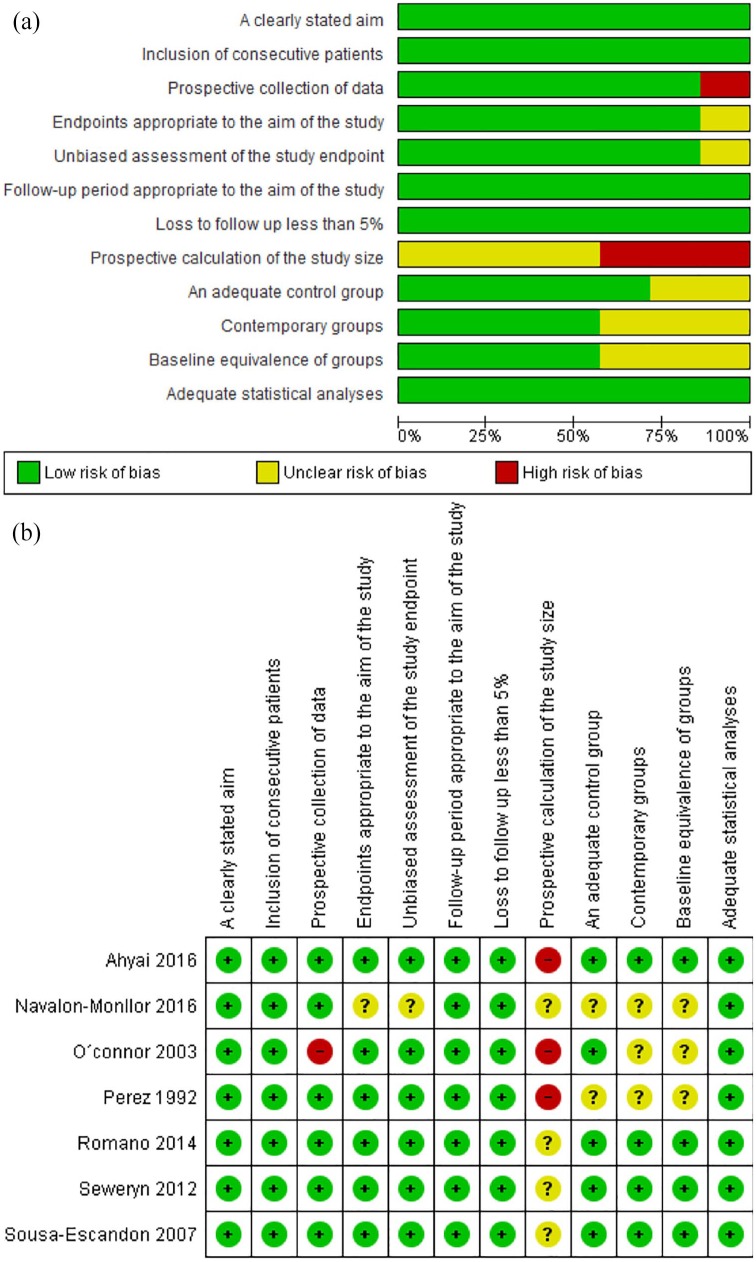
The assessment of the risk of bias for each study was made using the methodological index for nonrandomized studies (minors), which covers: a clearly stated aim; inclusion of consecutive patients; prospective collection of data; endpoints appropriate to the aim of the study; unbiased assessment of the study endpoint; follow-up period appropriate to the aim of the study; loss to follow up less than 5%; prospective calculation of study size; an adequate control group; contemporary groups; baseline equivalence of groups; adequate statistical analyses. We rated the possible risk of bias from extracted information as ‘high risk’, ‘low risk’, or ‘unclear risk’. We computed graphic representation of potential bias using RevMan 5.3 (Cochrane, London, UK).
Data analysis/synthesis of results
We performed the statistical analysis in R10 with the command netmeta. For outcomes we reported information about relative risk (RR) with 95% confidence intervals (CIs) according to the type of variable, and we pooled the information with a fixed-effect network meta-analysis according to the heterogeneity expected. The results were reported in forest plots of the estimated effects of the included studies with a 95% CI. Heterogeneity was evaluated using the I2 test. For the interpretation, it was determined that the values of 25%, 50%, and 75% in the I2 test corresponded to low, medium, and high levels of heterogeneity, respectively.
Assumption of transitivity was plausible and evaluated according to the kind of comparisons and considering the similarity of the distribution of the potential effect modifiers across the different pairwise comparisons. In addition, for every treatment, we estimated the probability of being at each possible rank to infer the relative ranking of the treatments.
Publication bias
An evaluation was conducted to identify reporting or publication bias using Egger and Begg statistical tests.
Sensitivity analysis
We performed sensitivity analysis extracting weighted studies and running the estimated effect to find differences.
Geometry of the network
We produced network diagrams to show the amount of evidence available for each outcome and the most frequent comparison. The size of the nodes was proportional to the total number of patients allocated to the treatments across all trials and the width of the lines was proportional to the total number of randomized clinical trials evaluating the comparisons.
Assessment of inconsistency
We evaluated and stated consistency within indirect and direct comparisons. We assessed statistical inconsistency (i.e. the agreement between direct and indirect evidence) by a loop-specific approach, which evaluates inconsistency in every closed loop of evidence. We ultimately found a consistent loop.
Results
Study selection
A total of 456 articles were retrieved using the search strategies, 56 complete texts were reviewed, and 7 studies were ultimately included in the analysis6,11–16 (Figure 1)
Figure 1.
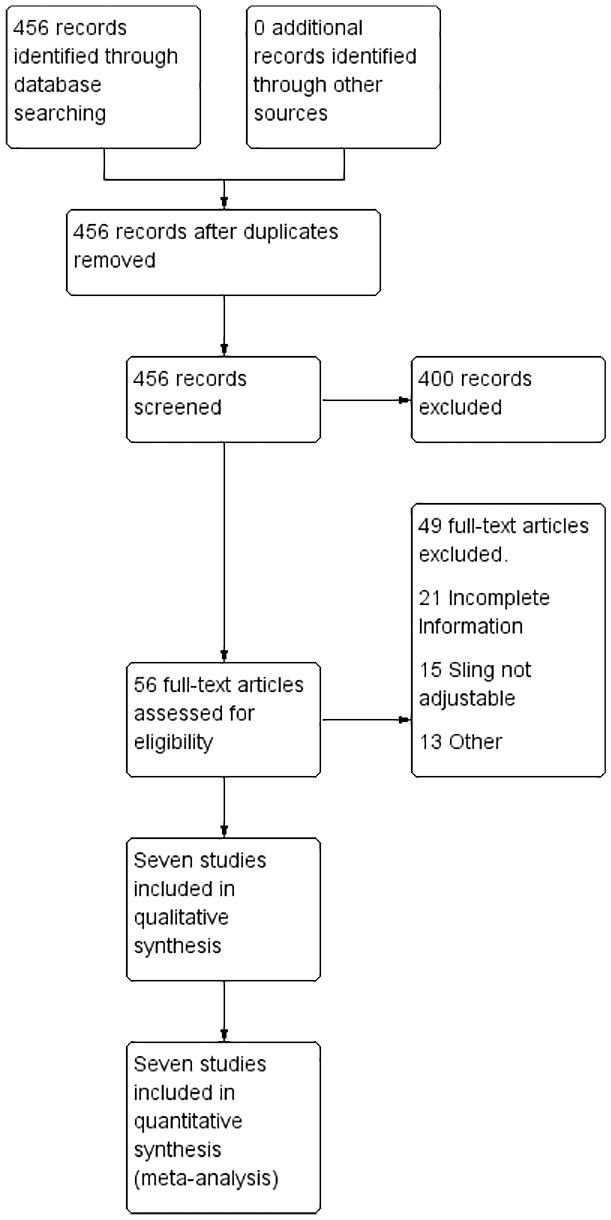
Flowchart of included studies.
Characteristics of included studies
A total of 463 participants were included, 420 of whom had SUI. The average age was 68.8 years and the mean follow up was 36 months. The definition of severity varied between studies, however, the most frequently used definition for SUI was the use of >5 pads/day (Table 1).
Table 1.
Characteristics of included studies.
| Author, year | Country | Age | Device | Participants IU Severe | Study Design | Follow- up (months) | Definition of Severity | Outcome assessment | Number of adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Perez, 1992 | United States | 67 | AUS | 77 | Prospective cohort | 43 | > 5 Pads daily | Initial postoperative continence was satisfactory (2 pads or less per day) | NA |
| O’Connor, 2003 | United States | 67.2 | AUS | 56 | Retrospective cohort | 41.3 | >5 Pads daily | IIQ-7-Pads daily-Complications | NA |
| Sousa-Escandón, 2007 | Spain Italy Greece Germany Portugal | 69 | MRS | 32 | Prospective cohort | 32 | >5 Pads daily | Dry: No pads or used security padsImprove: If the number of pads needed diminished more than 50%Failure: The number of pads diminished less than 50%IIQ7 | NA |
| Seweryn, 2012 | Austria | 70 | ATOMS | 22 | Prospective cohort | 16.9 | >5 Pads daily | Dry: 0 to 1 pads and less tan 15 ml per 24-hour pad test. Improve: More than 1 pad per 24 hours and 16 to 100 ml 24-hour pad test. Failure: More than 2 pads daily or more than 100 ml per 24-hour pad test. | 3.97 (Range 0 to 9) |
| Romano, 2014 | Argentina Brazil Austria | 70 | Argus T | 29 | Prospective cohort | 12-30 | >400 gr | - ICIQ-SF- VAS scale (from 0 no discomfort to 10 very uncomfortable).- Pads daily: Dry (no pads or one for protection), improve (one wet pad a day) or failure (two or more wet pads daily or implant removal) | 25% required adjustment |
| Ahyai, 2016 | Germany | 70 | AUS | 180 | Prospective cohort | 24 | >5 Pads daily | Continence objective. use of 0–1 security pad/day | NA |
| Navalón-Monllor, 2016 | Spain | 67 | MRS | 24 | Prospective cohort | 40.7 | >450 gr - >5 Pads daily | IIQ7 | 2.4 (Range 0 to 6) |
AUS, artificial urinary sphincter; ICIQ-SF, International consultation on Incontinence Questionnaire Short Form; IIQ-7, Incontinence Impact Questionnaire, Short Form; MRS, Male Remeex System; NA, not available; SUI, severe urinary incontinence, VAS, visual analogue scale
Excluded studies
Two studies were excluded because they did not report data regarding stratification of incontinence severity.
Summary of the network
A total of 420 patients with SUI PP received interventions, including 107 patients who received an adjustable sling and 313 who received an AUS. A total of 29 patients received the Argus T adjustable system,11 36 patients received the Remeex system,6,13 and 22 patients received the ATOMS adjustable sling.14 In three studies, 313 patients received an AUS.12,15 No comparison group was included in any of the adjustable sling studies. In the US studies, the comparator was analyzed for different implantation techniques (Figure 2).
Figure 2.
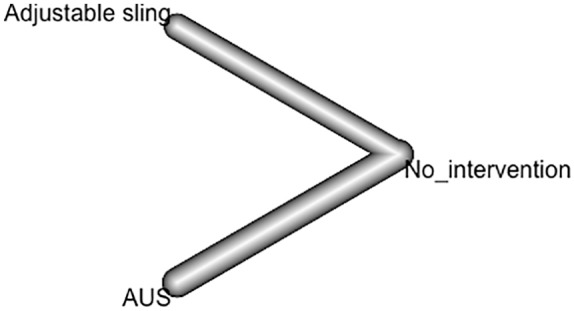
Network meta-analysis. Artificial urinary sphincter and auto-adjustable sling.
Risk of bias
We found a high risk of bias in all of the studies for the sample size calculation, which was not reported. No comparison group was included in the studies by Navalón-Monllor and colleagues,13 O’Connor and colleagues, 12 and Perez, 16 while a low risk of bias was determined for most of the remaining parameters in the nonrandomized studies (Figure 3).
Figure 3.
(a) Risk of bias across studies; (b) risk of bias within studies.
Exploration for inconsistency
For incontinence improvement, we found no heterogeneity I2 = 0% and no inconsistency p = 0.6124. The rank value (p score) was higher for the AUS (0.79) and 0.7 for the auto-adjustable sling.
Evaluation of incontinence improvement
For the outcome of decreased pad use, three articles were found for each intervention (Figure 4), and when comparing these interventions with nonintervention in a fixed-model analysis, RRs of 35.37 (95% CI: 7.17–174.35) and 45.14 (95% CI: 11.09–183.70) were calculated for the adjustable sling and AUS, respectively, which were statistically significant (Figure 5). When comparing the AUS versus the adjustable sling, an RR of 0.78 (95% CI: 0.09–6.56) was calculated, reflecting no statistically significant differences between the interventions.
Figure 4.
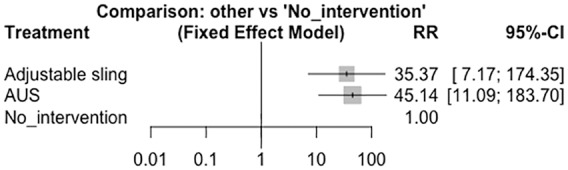
Fixed-effect model meta-analysis. Artificial urinary sphincter versus no intervention. AUS, artificial urinary sphincter; CI, confidence interval; RR, relative risk.
Figure 5.
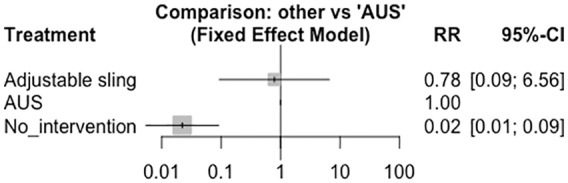
Fixed-effect model meta-analysis. Artificial urinary sphincter versus other interventions. AUS, artificial urinary sphincter; CI, confidence interval; RR, relative risk.
Secondary outcomes
The most frequently used quality-of-life scale was the Incontinence Impact Questionnaire, Short Form (IIQ-7), which was used in one study for each intervention and showed improved scores of 82.8% for the AUS compared with 86.1% for the adjustable sling. For device readjustments, improved scores of 40.3% for the AUS, 25% for the Argus T adjustable sling, and 91% for the male Remeex system adjustable sling were found.
Discussion
For decreased pad use, we found that both interventions are effective compared with nonintervention, with RRs of 35.37 (95% CI: 7.17–174.35) and 45.14 (95% CI: 11.09–183.70) for the adjustable sling and the AUS, respectively. We found no differences between the two interventions, with an RR of 0.78 (95% CI: 0.09–6.56). These results would indicate that performing an intervention compared with not intervening has statistically significant favorable results in the improvement of incontinence, so these patients would benefit from either of the two interventions. When comparing both interventions there were no significant differences. Given that the AUS is the gold standard in this type of patient and taking into account these results, the adjustable sling could be an option in the management of SUI PP, however, it is not available in all countries.
Comparison with other systematic reviews and the literature
In 2016, Alwaal and colleagues compared complications related to slings (n = 597) versus AUSs (n = 608) and found that slings were associated with a lower complication rate at 30 days (2.8% versus 5.1%, p = 0.046).17 Given these results and observing the results of this meta-analysis regarding the improvement of incontinence we could assume that the sling would be similar to the AUS and also with respect to complication rates. Belot and colleagues found that a previously unsuccessful sling influenced patients’ results after an AUS was implanted and did not find differences between patients who had previously used a sling and patients who used an AUS as a first-line treatment.18 However, Ajay and colleagues compared the results of continence among patients with UI PP treated with a rescue AUS or a transobturator sling and found that those who underwent a secondary sling procedure were up to six times more likely to have persistent incontinence compared with those who underwent AUS placement.19 Kumar and colleagues found that 75% of patients with moderate UI preferred a sling over an AUS.4 Therefore, a sling can be considered for the initial management of SUI PP given its good effectiveness and patient preference.
Regarding the results obtained in previous systematic reviews and meta-analyses, in 2013, Cerruto and colleagues included five observational studies with a mean patient age of 68.06 years (standard deviation, 1.37) at the time of surgery and a follow up of 15 months. They found a combined cure rate of 77.4% for all types of slings, which was considered high, but they could not identify reliable pre- and postoperative prognostic factors due to heterogeneity, a lack of standardized results, and a high risk of bias.20 In 2015, Van Bruwaene and colleagues reported a systematic review in which they found acceptable success rates for slings depending on the severity of symptoms, including the Remeex (78% for SUI versus 100% for mild UI), Argus (67% for SUI versus 92% for mild UI), and ATOMS (91% for SUI versus 92% for all severities). However, the lack of a consistent definition for SUI complicates comparisons of results between studies.21 With regard to our systematic review and meta-analysis compared with previous studies, there is a difference, given that we focused only on data from patients with SUI, both radical and BPH, where the standard of management was the AUS in this subgroup of patients, However, due to our results, we could consider whether the sling might be an option for the management of these patients, given similar results and lower rates of complications as referred to by Alwaal and colleagues.17
Regarding the results of our analysis, significant differences were found between both interventions and nonintervention, but no differences were found between AUSs and adjustable slings. Therefore, the interventions can be assumed to be comparable, and an adjustable sling may be suitable for the initial management of SUI PP based on similar success rates to those of the standard treatment. However, no controlled clinical trials or comparative studies have directly compared both interventions. Most of the studies included in our analysis were prospective cohort studies. Furthermore, the wide variety of definitions used in SUI studies and the large number of variables used to measure outcomes complicate extrapolation of the data to specific populations.
Strengths and limitations
One of the strengths of our study was the quality of the search strategies designed for each database, which were highly sensitive and specific in detecting articles related to the systematic review question proposed. The main characteristic of the included studies regarding SUI PP was a comparison of two specific interventions, including the standard of treatment and another potentially successful treatment, indicating that the two interventions are likely clinically comparable.
The limitations of this study include the lack of nonrandomized clinical trials comparing these interventions, the multiple types of implanted devices and the absence of comparison between them, differences in some clinical characteristics, substantial heterogeneity in the definition of SUI, and considerable variability between the outcomes measured in the studies. All of the studies were treated with single-arm cohorts, assuming a comparison group of patients with absence of incontinence. Initially in the protocol the population was proposed only for SUI after radical prostatectomy however, in the majority of the studies it was not possible to extract the data for this type of patient, so all types of prostate surgery were included.
Implications for practice
This review was carried out to address a subgroup of patients with SUI PP whose best treatment strategy can be difficult to determine. With these results, critical clinical symptoms can help to determine the best treatment option for patients with SUI PP.
Conclusion
Both interventions can effectively and comparably reduce incontinence and improve the quality of life of patients with SUI PP. However, the published literature is considerably limited, as no randomized clinical trials are available, no consensus has been reached regarding the definition of the severity of incontinence, and substantial heterogeneity exists across the outcome variables measured. Therefore, the strength of the results obtained in this study should prompt the initiation of studies that can provide a higher level of evidence for the comparison of adjustable slings and AUSs in patients with SUI PP.
Appendix 1. Search strategy
MEDLINE (Ovid):
Exp Urinary Incontinence
(urinary adj2 incontinence).mp
(urine adj2 incontinence).mp
or/
Prostatectomy/
Prostatectom$.mp
(Suprapubic adj2 prostatectom$).mp
(Retropubic adj2 prostatectom$).mp
Exp Prostatic Neoplasms
(Prostat* adj2 neoplasm$).mp
(Prostat* adj2 cancer).mp
Or/
Exp Suburethral Slings
(suburethral adj2 sling$).mp
(adjustable adj2 sling$).mp
argus.mp
remeex.mp
(transobturator adj2 tape).mp
(suburethral adj2 tape).mp
Exp Urinary Sphincter, Artificial
(Artificial adj2 urinary adj2 sphincter).mp
(urinary adj2 sphincter).mp
Or/
randomized controlled trial.pt
controlled clinical trial.pt
randomized.ab
placebo.ab
randomly.ab
trial.ab
(clinical adj2 trial).mp.
(randomi*ed adj2 controlled adj2 trial).mp.
exp double-blind method
clinical trial.pt
exp Non-Randomized Controlled Trials
(quasi adj2 experiment*).mp
exp cohort studies
cohort*.mp
or/
4 and 12 and 23 and 38
Embase
‘Urine Incontinence’/exp
(Urine NEXT/2 incontinence):ti, ab
(Urinary NEXT/2 incontinence):ti, ab
or/
Prostatectomy/exp
Prostatectom*:ti, ab
(Suprapubic NEXT/2 prostatectom*):ti, ab
(Retropubic NEXT/2 prostatectom*):ti, ab
‘Prostate tumor’/exp
‘Prostatic intraepithelial neoplasia’/exp
(Prostatic NEXT/2 neoplasm*):ti, ab
(Prostatic NEXT/2 cancer):ti, ab
(Prostate NEXT/2 neoplasm$):ti, ab
(Prostate NEXT/2 cancer):ti, ab
Or/
‘Suburethral sling’/exp
‘Suburethral sling procedure’/exp
‘transobturator tape’/exp
(suburethral NEXT/2 sling*):ti, ab
(adjustable NEXT/2 sling*):ti, ab
(argus NEXT/2 sling*):ti, ab
(remeex NEXT/2 sling*):ti, ab
(advance NEXT/2 sling*):ti, ab
(transobturator NEXT/2 tape*):ti, ab
(trans NEXT/2 obturator NEXT/2 tape*):ti, ab
(suburethral NEXT/2 tape*):ti, ab
(transobturator NEXT/2 male NEXT/2 system):ti, ab
(trans*obturator NEXT/2 tape*):ti, ab
‘Bladder sphincter prosthesis’/exp
(artificial NEXT/2 urinary next/2 sphincter):ti, ab
(artificial NEXT/2 genitourinary sphincter):ti, ab
(Bladder NEXT/2 sphincter NEXT/2 prosthesis):ti, ab
(artificial NEXT/2 bladder NEXT/2 sphincter):ti, ab
(bladder NEXT/2 sphincter):ti, ab
Or/
‘randomized controlled trial’/exp
(randomi*ed NEXT/2 controlled NEXT/2 trial):ti, ab
‘clinical trial’/exp
(clinical NEXT/2 trial):ti, ab
‘double blind procedure’/exp
‘quasi experimental study’/exp
(quasi NEXT/2 experimental NEXT/2 study):ti, ab
‘cohort analysis’/exp
cohort*:ti, ab
or/
4 and 15 and 35 and 45
Central (Ovid)
Exp Urinary Incontinence
(urinary adj2 incontinence).mp
(urine adj2 incontinence).mp
or/
Prostatectomy/
Prostatectom$.mp
(Suprapubic adj2 prostatectom$).mp
(Retropubic adj2 prostatectom$).mp
Exp Prostatic Neoplasms
(Prostat* adj2 neoplasm$).mp
(Prostat* adj2 cancer).mp
Or/
Exp Suburethral Slings
(suburethral adj2 sling$).mp
(adjustable adj2 sling$).mp
argus.mp
remeex.mp
(transobturator adj2 tape).mp
(suburethral adj2 tape).mp
Exp Urinary Sphincter, Artificial
(Artificial adj2 urinary adj2 sphincter).mp
(urinary adj2 sphincter).mp
or/
4 and 12 and 23
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Ethics statement: This is a systematic review and network meta-analysis, accordingly, we used only papers and data from them to perform the analysis. Nonetheless, we complied with all international ethical statements.
ORCID iD: Herney Andrés García-Perdomo  https://orcid.org/0000-0001-6945-8261
https://orcid.org/0000-0001-6945-8261
Contributor Information
Pedro Luis Guachetá Bomba, UROGIV Research Group, Department of Urology, Universidad del Valle, Cali, Colombia.
Ginna Marcela Ocampo Flórez, UROGIV Research Group, Universidad del Valle, Cali, Colombia, and Department of Urology, Universidad CES, Medellín, Colombia.
Fernando Echeverría García, UROGIV Research Group, Department of Urology, Universidad del Valle, Cali, Colombia.
Herney Andrés García-Perdomo, Department of Urology, Universidad del Valle, Cali-Colombia, Cll 4B #36-00, Cali 439, Colombia.
References
- 1. Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012; 62: 405–417. [DOI] [PubMed] [Google Scholar]
- 2. Shamliyan TA, Wyman JF, Ping R, et al. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol 2009; 11: 145–165. [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer RM, Gozzi C, Hübner W, et al. Contemporary management of postprostatectomy incontinence. Eur Urol 2011; 59: 985–996. [DOI] [PubMed] [Google Scholar]
- 4. Kumar A, Litt ER, Ballert KN, et al. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence – what do patients choose? J Urol 2009; 181: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 5. O’Sullivan R, Karantanis E, Stevermuer TL, et al. Definition of mild, moderate and severe incontinence on the 24-hour pad test. BJOG An Int J Obstet Gynaecol 2004; 111: 859–862. [DOI] [PubMed] [Google Scholar]
- 6. Sousa-Escandón A, Cabrera J, Mantovani F, et al. Adjustable suburethral sling (male remeex system) in the treatment of male stress urinary incontinence: a multicentric European study. Eur Urol 2007; 52: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 7. Burkhard FC, Bosch LHR, Cruz F, et al. EAU Guidelines on urinary incontinence in adults, https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urinary-Incontinence-2018-large-text.pdf (2017, accessed 15 June 2019).
- 8. Demaagd GA, Davenport TC. Management of urinary incontinence. P T 2012; 37: 345–361H, 361B–361H. [PMC free article] [PubMed] [Google Scholar]
- 9. Chung E. Contemporary surgical devices for male stress urinary incontinence: a review of technological advances in current continence surgery. Transl Androl Urol 2017; 6: S112–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 11. Romano SV, Huebner W, Rocha FT, et al. A transobturator adjustable system for male incontinence: 30-month follow-up of a multicenter study. Int Braz J Urol 2014; 40: 781–789. [DOI] [PubMed] [Google Scholar]
- 12. O’ Connor RC, Gerber GS, Avila D, et al. Comparison of outcomes after single or DOUBLE-CUFF artificial urinary sphincter insertion. Urology 2003; 62: 723–726. [DOI] [PubMed] [Google Scholar]
- 13. Navalón-Monllor V, Ordono-Domínguez V, Pallás-Costa Y, et al. Long-term follow-up for the treatment of male urinary incontinence with the remeex system. Actas Urol Esp 2016; 40: 585–591. [DOI] [PubMed] [Google Scholar]
- 14. Seweryn J, Bauer W, Ponholzer A, et al. Initial experience and results with a new adjustable transobturator male system for the treatment of stress urinary incontinence. J Urol 2012; 187: 956–961. [DOI] [PubMed] [Google Scholar]
- 15. Ahyai SA, Ludwig TA, Dahlem R, et al. Outcomes of single- vs double-cuff artificial urinary sphincter insertion in low- and high-risk profile male patients with severe stress urinary incontinence. BJU Int 2016; 118: 625–632. [DOI] [PubMed] [Google Scholar]
- 16. Perez L. Succesful outcome of artificial urinary sphincters in men with post-prostatectomy urinary incontinence despite adverse implantion features. J Urol 1992; 168: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 17. Alwaal A, Harris CR, Awad MA, et al. Comparison of complication rates related to male urethral slings and artificial urinary sphincters for urinary incontinence: national multi-institutional analysis of ACS-NSQIP database. Int Urol Nephrol 2016; 48: 1571–1576. [DOI] [PubMed] [Google Scholar]
- 18. Belot PY, Fassi-Fehri H, Crouzet S, et al. Traitement de l’incontinence urinaire d’effort après prostatectomie: résultats du sphincter urinaire artificiel après échec de bandelette sous-urétrale. Prog en Urol 2012; 22: 644–649. [DOI] [PubMed] [Google Scholar]
- 19. Ajay D, Zhang H, Gupta S, et al. The artificial urinary sphincter is superior to a secondary transobturator male sling in cases of a primary sling failure. J Urol 2015; 194: 1038–1042. [DOI] [PubMed] [Google Scholar]
- 20. Cerruto MA, D’Elia C, Artibani W. Continence and complications rates after male slings as primary surgery for post-prostatectomy incontinence: a systematic review. Archivio Italiano di Urologia e Andrologia 2013; 85: 92–95. [DOI] [PubMed] [Google Scholar]
- 21. Van Bruwaene S, De Ridder D, Van Der Aa F. The use of sling vs sphincter in post-prostatectomy urinary incontinence. BJU Int 2015; 116: 330–342. [DOI] [PubMed] [Google Scholar]