Abstract
Background: Breast cancer diagnosis and treatment affect quality of life and stress and are associated with fatigue. Meditation interventions are effective strategies for patients with breast cancer but are often limited by poor access, high cost, substantial time commitment, and poor adherence. In this feasibility study, we investigated the use of a portable, wearable, electroencephalographic device for guided meditation practices by breast cancer patients during the period from breast cancer diagnosis until 3 months after surgical treatment. Methods: We enrolled women (age = 20-75 years) who had received a recent diagnosis of breast cancer and planned to undergo surgical treatment. Participants were randomly assigned to perform guided meditation with the device (intervention group) or receive CD-based stress-reduction education (control group). Surveys were used to measure stress, quality of life, and fatigue at baseline, within 4 days before surgery, up to 14 days after surgery, and at 3 months after surgery. Results: In the intervention group, 15 of 17 participants (88.2%) completed the study; in the control group, 13 of 13 participants completed the study (100%). Participants in both groups had less fatigue and stress and improved quality of life at 2 weeks and 3 months after surgery compared with baseline, but there were no significant intergroup differences at any time point. Conclusion: The use of this wearable electroencephalographic device for meditation is a feasible strategy for patients with breast cancer.
Keywords: biofeedback, breast cancer, breast surgery, fatigue, integrative medicine, meditation, quality of life
Introduction
After patients receive a diagnosis of breast cancer, their stress often increases and quality of life (QOL) decreases. Distress is common in patients with cancer and occurs in more than half of these patients at the time of breast cancer diagnosis.1 Fatigue is a common treatment-related symptom after breast cancer treatment and contributes to lower overall QOL.2 Inadequately addressing the psychosocial aspects of cancer care compromises patient health and treatment adherence and effectiveness.3
Initiating mind-body therapies at the time of cancer diagnosis and during cancer care can be difficult. Many studies have evaluated therapies delivered after the completion of active treatment or therapies for specific symptoms, such as persistent cancer-related fatigue.4-6 Still, distress increases after they receive a cancer diagnosis, and they often feel angst in the days or weeks before cancer treatment begins. Thus, patients would benefit from interventions that alleviate symptoms and improve QOL soon after breast cancer is diagnosed, particularly if such interventions were easy to implement and integrate into patient care.
Recently, distress management tools for patients with breast cancer have received more attention. The Society of Integrative Oncology published guidelines for the use of integrative medicine modalities for the treatment and supportive care of women with breast cancer,7 which were subsequently endorsed by the American Society of Clinical Oncology.8 In the systematic review–based guidelines of the Society of Integrative Medicine, meditation had the highest level of evidence (grade A) for the management of stress and anxiety.
Randomized controlled trials have shown that meditation can reduce anxiety and improve QOL for patients with breast cancer, but data on the effects of meditation at diagnosis or during early treatment are sparse.9-15 In addition, meditation intervention groups often require in-person attendance at several sessions held over 8 to 12 weeks. This time commitment for patients is a barrier to providing effective therapy more broadly. In addition, traditional instruction in mind-body therapies requires qualified instructors, thereby increasing the cost of providing this service. Finally, continued adherence to meditation practice after face-to-face interactions is difficult to track and to ensure.
Currently, many platforms are marketed to provide mind-body training to patients in a more accessible and less costly manner. For example, Mindfulness-Based Cancer Recovery, a program that provides digital, online instruction, has been evaluated for feasibility.16 However, because patients do not receive feedback, this approach is limited by unidirectional interactions with the program and the lack of accountability to participation.
In contrast, the portable, interactive, electroencephalographic (EEG) Muse headband (InteraXon Inc, Toronto, Ontario) could be used to provide feedback and accountability. The Muse headband is meant to encourage mindfulness practices, to guide the user toward a calm state, and to reduce stress and anxiety. The Muse device measures brain activity by detecting electrical impulses with 7 sensors that provide 4 channels of EEG data. Participants receive meditation instruction through the Muse application (app) on a smartphone or tablet and are led through a series of attention-focusing sessions that train them to reach a meditative or calm state. Each session lasts a recommended minimum of 3 minutes and can be done in any quiet place, including the participant’s home. The Muse headband monitors the brain state and measures EEG signals at frontal and temporal locations. These signals are converted to measures of the brain state, and by using a proprietary algorithm, the device can distinguish between active and calm states. The Muse device provides immediate, real-time feedback about the participant’s brain state through a series of audible, typically weather-related, cues. In addition, the participant’s data are collected directly by the device, and immediate feedback is available about the participant’s personal history of sessions. The Muse app can also provide reminders to participate in sessions on a regular basis.
In this randomized controlled trial, we used the Muse headband to measure the calm state of the brain and its potential effects on fatigue, QOL, and stress in patients with newly diagnosed breast cancer who were scheduled to undergo surgical treatment. Our primary goal was to evaluate the feasibility of using this intervention to facilitate meditation as a means of improving symptoms through practice, feedback, and accountability. Secondary aims were to compare fatigue, QOL, and stress between patients using the Muse device and patients receiving stress-management education and to assess the association between the amount of Muse use and changes in outcomes.
Methods
Participants
We decided to enroll 30 participants after considering the resources needed to conduct this pilot study. Participants eligible for inclusion in this trial were women aged 20 to 75 years with newly diagnosed breast cancer who were planning to undergo surgical treatment and could complete at least 7 days of intervention before the presurgical visit. Participants had to be able to provide informed consent and had to be willing to complete all aspects of the trial. Exclusion criteria included currently participating in mindfulness practice or receiving integrative medicine therapy, including acupuncture, mindfulness or stress-reduction programs, massage, and energy therapies; being enrolled in another clinical research program evaluating fatigue, QOL, or stress; being pregnant or breastfeeding; or receiving neoadjuvant chemotherapy.
Enrollment
We used flyers and provider referral to recruit patients through the Breast Diagnostic Clinic at Mayo Clinic in Rochester, Minnesota. A study coordinator contacted potential participants for a scripted prescreening telephone interview. Eligible, interested patients completed a baseline visit in which informed consent was obtained. Patients without access to an iOS device were lent an iPad mini (Apple Inc, Cupertino, CA) to use during the study.
At baseline, participants were randomly assigned in a 1:1 ratio with a computer-generated program to receive the Muse device intervention or stress-reduction education with a practice-based CD for home use. Eligible participants were enrolled in the study. Participants received $50 (prorated per visit) for completion of all 3 follow-up visits. Participants in the intervention group were allowed to keep the Muse device. In addition, Muse devices were provided to those in the control group at completion of the study. If borrowed, the iPad mini was returned to the study team.
Interventions
Participants were randomly assigned to 1 of 2 treatment groups (Muse or CD) with a computerized dynamic allocation algorithm written by the Division of Biostatistics and Informatics.
Muse Intervention Group
The participants in the Muse intervention group received a Muse headband, instructions for use, and guidance for installing the Muse app on their smartphone or tablet. The study team checked the fit of the headband to ensure proper positioning and signal capture, after which the participant completed an introductory 3-minute meditation session.
The Muse device collected and uploaded deidentified data to a secure, Health Insurance Portability and Accountability Act–approved cloud server. Each episode of use was recorded by the Muse device, and the date, time, and duration of the session with a calculated percent calm score were provided directly back to the participant. The deidentified data were available in aggregate to the study coordinator and statistician. Adherence data were obtained directly from the Muse device.
Control Group
After being randomly assigned to the control group, participants received stress-management materials from a study team member, who reviewed them with the participant during the 60-minute baseline visit. A 3-minute stress-reduction CD was provided, and participants were encouraged to use it daily. Adherence was monitored with a written log completed by the participant.
Outcomes
Outcomes were measured by the study coordinator with surveys administered at 4 time points: baseline, presurgery (up to 4 days before the procedure), postsurgery (up to 14 days after the procedure), and 3 months postsurgery (86-94 days after the procedure). Visits were done in person or over the phone if the participant was unable to meet in person. Validated, standardized scales were used to assess fatigue, QOL, and stress.
Fatigue
The validated, 30-statement Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) was administered to assess participants’ fatigue in the prior week.17 Participants were asked to rate their symptoms from 0 (“not at all”) to 4 (“extremely”), and a score (range = 0-24) was reported for each of the 5 subscales: general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor. A higher score on the vigor subscale indicates less fatigue; lower scores on all other subscales, less fatigue. The total score is the sum of the 4 subscale scores for fatigue (general, physical, emotional, and mental) minus the subscale score for vigor (total score range = −24 to 96).18-20
Quality of Life
Quality of life was assessed with the multidimensional, cancer-specific Functional Assessment of Cancer Therapy–General (FACT-G), a validated and reliable tool for assessing QOL in cancer patients enrolled in clinical research studies.21 The FACT-G results include an overall score and 4 subscale scores: physical well-being (7 items; score range = 0-28), social/family well-being (7 items; score range = 0-28), emotional well-being (6 items; score range = 0-24), and functional well-being (7 items; score range = 0-28). The total FACT-G score is the sum of the scores on these subscales (score range = 0-108).
Stress
Stress was evaluated with the Perceived Stress Scale (PSS). The PSS is a 10-item Likert-type scale used to measure global life stress by assessing the degree to which life events are appraised as uncontrollable or unpredictable. Responses are scored on a 5-point scale from 0 (“never”) to 4 (“very often”). A higher total score indicates greater stress (total score range = 0-40). The PSS correlates well with life experiences, stress measures, and social anxiety, and has adequate reliability.22
Participant Feedback
At the 3-month postsurgical visit, participants additionally completed the Was It Worth It (WIWI) questionnaire, which was administered to collect themes about participant satisfaction with the study.23
Statistical Methods
The participant characteristics and scores were summarized as number (percentage) or mean (standard deviation), as appropriate. The baseline characteristics of the participants who completed follow-up assessments, along with responses to the WIWI questionnaire, were compared between study groups with the Fisher exact test (for categorical variables) or the Wilcoxon rank sum test (for ordinal variables). The WIWI items were also compared according to surgery type (lumpectomy vs mastectomy). We used the Wilcoxon signed rank test to compare the subscale and total scores of MFSI-SF, FACT-G, and PSS at each follow-up visit with the baseline scores within each study group. Score differences (presurgery vs baseline, postsurgery vs baseline, and 3-months postsurgery vs baseline) were compared between groups with the Wilcoxon rank sum test, including comparisons according to study group (Muse intervention group vs control group), use (high vs low Muse use and high vs low CD use), and surgery type (lumpectomy vs mastectomy). P values less than .05 were considered significant. Because this study evaluated the feasibility of the Muse intervention, no adjustment for multiple statistical testing was applied. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
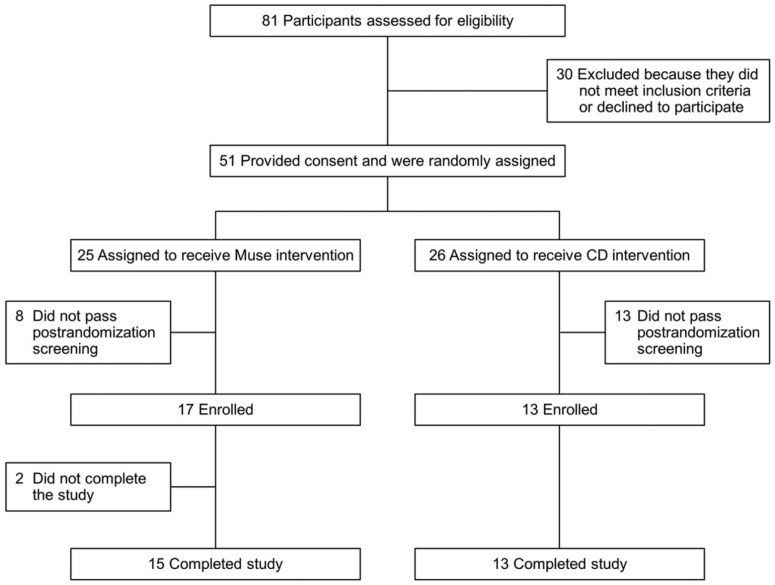
The participant enrollment period was from September 1, 2015, through June 27, 2016, and follow-up continued through September 2, 2016. Of 81 patients considered for enrollment in this trial, 30 did not meet the inclusion criteria or were not interested in participating (Figure 1). The remaining 51 participants provided consent and were randomly assigned to the Muse group (n = 25) or the control group (n = 26), but 8 in the Muse group and 13 in the control group did not meet additional criteria, and another 2 in the Muse group did not complete the study owing to changes in their surgical management. The final analysis included 15 participants in the Muse group and 13 participants in the control group who completed the study and all assessments.
Figure 1.
CONSORT flowchart showing patient enrollment. CONSORT, Consolidated Standards of Reporting Trials.
The baseline demographic and clinical characteristics of the participants were similar between the study groups (Table 1). Participants in the control group had a slightly higher (but not significantly different) level of education compared with the participants in the Muse group (84.6% of participants in the control group had a 4-year college, graduate, or professional degree vs 53.3% of participants in the Muse group; P =.05). The median (interquartile range) number of sessions completed by participants in the Muse group was 29 (14-35; range = 0-116); in the control group, 14 (9-23; range = 0-35). High use was defined as use of the intervention more than the median number for each group. In the Muse group, 7 of 15 participants completed 30 or more sessions and were therefore considered high Muse users; in the control group, 7 of 13 participants used the CD 14 times or more and were therefore considered high CD users.
Table 1.
| Characteristic | Muse (N = 15) | CD (N = 13) | P |
|---|---|---|---|
| Age in years | 55.9 (11.1) [39.0-73.0] | 55.8 (10.8) [35.0-70.0] | >.99 |
| Race/ethnicity | >.99 | ||
| American Indian/Alaskan Native, not Hispanic | 1 (6.7) | 0 (0) | |
| White, not Hispanic | 14 (93.3) | 13 (100) | |
| Marital status | .67 | ||
| Never married | 1 (6.7) | 0 (0) | |
| Separated or divorced | 2 (13.3) | 1 (7.7) | |
| Widowed | 2 (13.3) | 0 (0) | |
| Married | 9 (60.0) | 11 (84.6) | |
| Other | 1 (6.7) | 1 (7.7) | |
| Education | .05 | ||
| Some high school | 1 (6.7) | 0 (0) | |
| High school degree | 3 (20.0) | 0 (0) | |
| Some college, technical, or vocational school | 3 (20.0) | 2 (15.4) | |
| 4-year college degree | 4 (26.7) | 4 (30.8) | |
| Graduate or professional degree | 4 (26.7) | 7 (53.8) | |
| Current stress levelc,d | 6.2 (2.4) [2.0-10.0] | 6.2 (1.3) [3.0-7.0] | .65 |
| Ever had a period of time lasting several days or longer when most of the day you felt sad, empty, or depressed?d | .25 | ||
| No | 6 (40.0) | 8 (66.7) | |
| Yes | 9 (60.0) | 4 (33.3) | |
| Current level of activityd | .91 | ||
| Sedentary | 2 (13.3) | 2 (16.7) | |
| Moderately active | 11 (73.3) | 8 (66.7) | |
| Vigorously active | 1 (6.7) | 2 (16.7) | |
| Extremely active | 1 (6.7) | 0 (0) | |
| Initial surgery | .48 | ||
| Lumpectomye | 8 (53.3)f | 5 (38.5) | |
| Mastectomy | 7 (46.7)g | 8 (61.5) |
Abbreviation: SD, standard deviation.
Values are reported as n (%) or mean (SD) [range].
Data are shown for participants who completed baseline and follow-up assessments.
Reported with a scale from 1 (no stress) to 10 (highest stress).
Data are shown for 12 participants in the CD group.
Three patients who initially underwent lumpectomy received follow-up with mastectomy 1 to 3 months later (2 in the CD group and 1 in the Muse group).
One lumpectomy also involved an implant exchange.
All except 1 mastectomy involved reconstruction.
In the Muse group, the mean MFSI-SF scores for the emotional and mental fatigue and vigor subscales and the total MFSI-SF score improved significantly from baseline to postsurgery and from baseline to 3 months postsurgery (Table 2). Muse participants had significant improvements in FACT-G scores for emotional well-being between baseline and the postsurgical visit and between baseline and the 3-month postsurgical visit, but the mean scores for physical and functional well-being were significantly worse from baseline to the postsurgical visit. Mean PSS scores for the Muse group improved significantly between baseline and the postsurgical visits and between baseline and the 3-month postsurgical visits.
Table 2.
Survey Results, Stratified by Follow-up Visit and Intervention Group.
| Survey | Muse (N = 15), Mean (SD) |
CD (N = 13), Mean (SD) |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Presurgery | Postsurgery | 3-Months Postsurgery | Baseline | Presurgery | Postsurgery | 3-Months Postsurgery | |
| MFSI-SF | ||||||||
| Generala | 9.9 (6.4) | 7.7 (5.5) | 7.3 (5.6) | 6.3 (6.6) | 9.6 (6.8) | 9.0 (5.4) | 9.5 (4.8) | 10.0 (7.6) |
| Physicala | 3.4 (4.4) | 2.5 (3.2) | 2.7 (3.6) | 4.0 (4.9) | 4.8 (4.3) | 4.0 (4.4) | 3.8 (4.5) | 5.0 (5.6) |
| Emotionala | 9.4 (5.1) | 7.9 (5.7) | 3.8 (2.9)b | 3.5 (3.3)b | 7.8 (3.8) | 9.5 (5.1) | 5.9 (4.9) | 3.9 (3.7)b |
| Mentala | 6.2 (4.8) | 5.0 (4.6) | 3.5 (2.5)c | 3.3 (3.4)c | 7.2 (5.9) | 6.2 (4.4) | 4.8 (4.7)b | 4.7 (3.8)b |
| Vigord | 8.4 (5.0) | 10.0 (5.8) | 11.9 (5.5)b | 13.5 (5.1)b | 9.1 (4.8) | 9.8 (4.1) | 11.1 (4.2) | 11.5 (6.3)b |
| Totala | 20.5 (21.5) | 13.2 (20.9) | 5.4 (15.5)c | 3.6 (18.7)b | 20.4 (21.5) | 18.9 (18.9) | 12.8 (20.8) | 12.1 (22.5) |
| FACT-Ge | ||||||||
| PWB | 25.2 (2.3) | 24.5 (3.0) | 21.9 (4.7)c | 23.7 (4.9) | 22.5 (4.9) | 23.6 (2.9) | 19.4 (4.7) | 20.6 (5.5) |
| SWB | 24.3 (4.0) | 24.9 (4.1) | 24.6 (4.0) | 23.8 (4.3) | 22.4 (4.5) | 23.6 (4.7) | 23.4 (5.3) | 22.3 (5.6) |
| EWB | 17.3 (4.0) | 17.6 (4.8) | 20.2 (3.3)b | 20.9 (2.5)b | 16.8 (2.8) | 16.8 (4.5) | 18.3 (4.9) | 21.2 (2.7)b |
| FWB | 22.0 (4.9) | 20.3 (6.1) | 17.7 (6.1)b | 20.3 (5.6) | 19.4 (5.1) | 17.8 (5.2) | 15.8 (5.7)c | 20.2 (6.4) |
| Total | 88.8 (11.1) | 86.3 (14.7) | 84.4 (14.7) | 88.7 (13.1) | 81.2 (14.4) | 81.9 (14.4) | 77.0 (17.9) | 84.3 (16.0) |
| PSSf | 16.0 (6.7) | 13.0 (7.4) | 9.6 (5.0)b | 10.1 (5.4)b | 17.3 (4.7) | 14.9 (5.1)c | 13.9 (7.5)c | 11.1 (5.1)b |
Abbreviations: EWB, emotional well-being; FACT-G, Functional Assessment of Cancer Therapy–General; FWB, functional well-being; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; PSS, Perceived Stress Scale; PWB, physical well-being; SD, standard deviation; SWB, social/family well-being.
Lower scores indicate less fatigue.
Significantly different from the baseline score at P < .01.
Significantly different from the baseline score at P < .05.
Higher scores indicate less fatigue.
Higher scores indicate better well-being.
Lower scores indicate less stress.
For the control group, the mean MFSI-SF mental fatigue score improved significantly from baseline to the postsurgical visit, and the mean emotional and mental fatigue and vigor scores improved significantly from baseline to the 3-month postsurgical visit. Mean FACT-G scores for control participants were significantly worse from baseline to the postsurgical visit for functional well-being, but the mean emotional well-being score was better at the 3-month postsurgical visit. The mean PSS scores for control participants improved significantly between baseline and each of the other 3 visits.
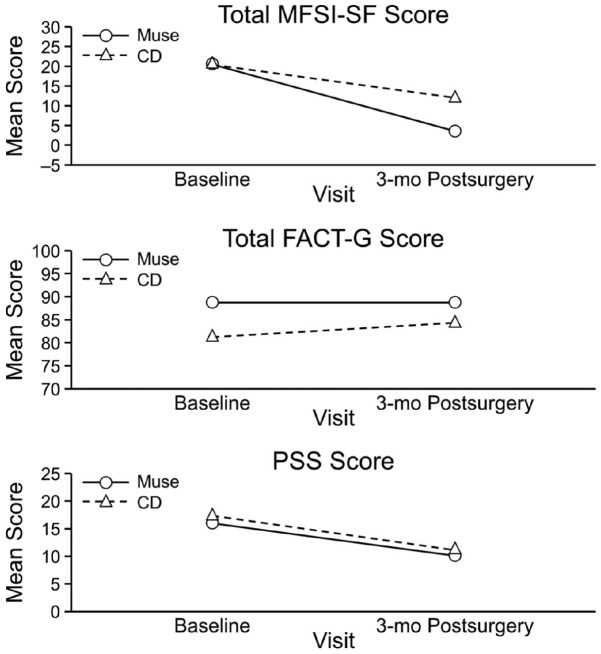
Although the changes in scores from baseline to each of the 3 visits were not significantly different between the Muse and control groups, some differences were notable. In the Muse group, the mean total MFSI-SF score decreased from 20.5 at baseline to 3.6 at the 3-month postsurgical visit; in the control group, from 20.4 to 12.1. In the Muse group, the mean total FACT-G score slightly decreased from 88.8 at baseline to 88.7 at the 3-month postsurgical visit; in the control group, mean total FACT-G increased from 81.2 to 84.3. In the Muse group, the mean PSS score decreased from 16.0 at baseline to 10.1 at the 3-month postsurgical visit; in the control group, from 17.3 to 11.1 (Figure 2).
Figure 2.
Changes in total mean scores. FACT-G, Functional Assessment of Cancer Therapy–General; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; PSS, Perceived Stress Scale.
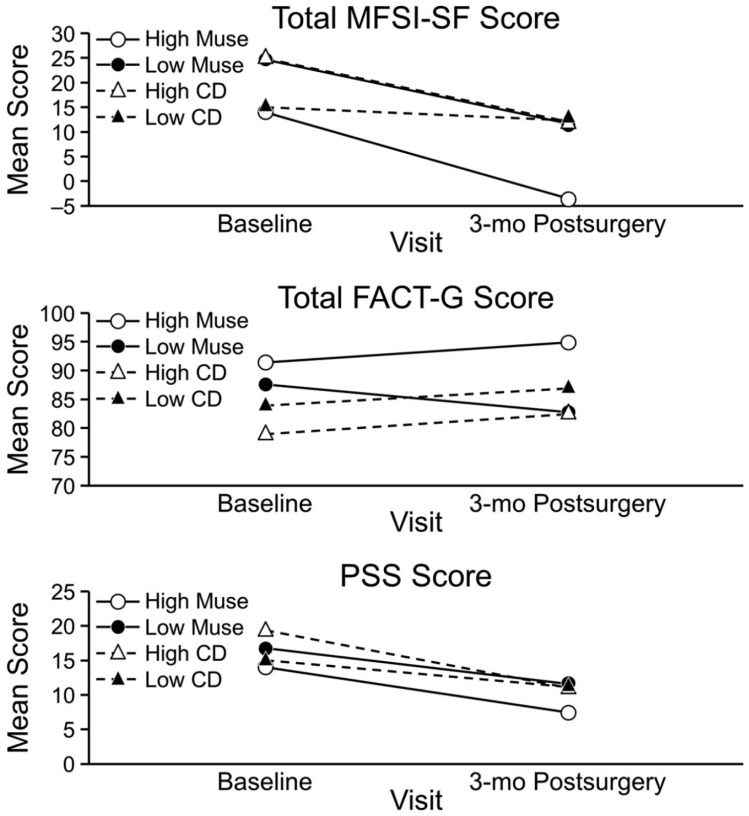
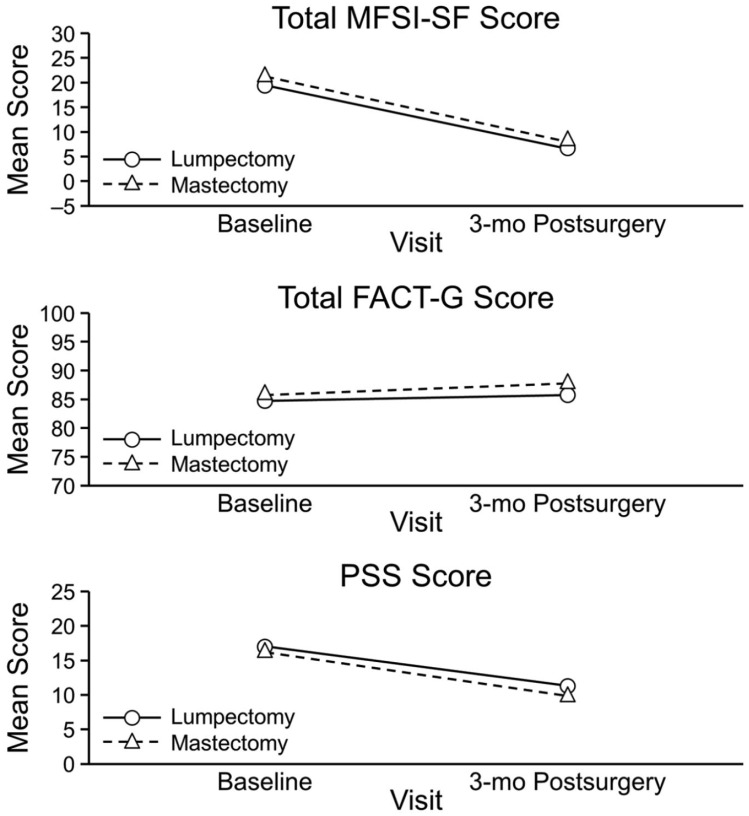
High Muse users (n = 7) and low Muse users (n = 8) showed significant improvements in the emotional fatigue subscores and emotional well-being scores from baseline to the 3-month postsurgical visits. High Muse users showed slightly more improvement in PSS scores compared with the low Muse users, but this difference was not significant (Figure 3). For high CD users, mean scores improved significantly from baseline to 3 months postsurgery for fatigue (MFSI-SF emotional, mental, and vigor subscales), FACT-G emotional well-being, and PSS stress; for low CD users, none of the differences were significant. When we compared changes within study groups by use, only the scores on the MFSI-SF emotional fatigue subscale were significantly different between CD users: high CD users had significantly less fatigue compared with low CD users. Data were combined from the Muse and control groups to compare outcomes according to type of surgery (lumpectomy [n = 13] or mastectomy [n = 15]; Figure 4). Improvement was similar from baseline to the 3-month postsurgical visit for MFSI-SF fatigue (emotional, mental, and vigor subscale scores and total score), QOL (FACT-G emotional subscale), and PSS stress scores within each surgical group, and improvement was similar from baseline to the 3-month postsurgical visit between the surgical groups.
Figure 3.
Changes in mean scores, stratified by device use. FACT-G, Functional Assessment of Cancer Therapy–General; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; PSS, Perceived Stress Scale.
Figure 4.
Changes in mean scores, stratified by surgery type. FACT-G, Functional Assessment of Cancer Therapy–General; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; PSS, Perceived Stress Scale.
The WIWI survey was completed by 15 participants in the Muse group and 12 participants in the control group (Table 3). Although 9 participants (75%) in the control group rated the experience of participating in the study as “the same as I expected,” 9 participants (60%) in the Muse group rated the experience as “better than I expected.”
Table 3.
Responses to the Was It Worth It Questionnairea.
| Question | Muse (N = 15), n (%) | CD (N = 12), n (%) | P |
|---|---|---|---|
| Was it worthwhile for you to participate in this research study? | |||
| Yes | 11 (73.3) | 10 (83.3) | >.99 |
| No | 1 (6.7) | 0 (0) | |
| Unsure | 3 (20.0) | 2 (16.7) | |
| If you had to do it over, would you participate in this research study again? | |||
| Yes | 14 (93.3) | 11 (91.7) | .70 |
| No | 1 (6.7) | 0 (0) | |
| Unsure | 0 (0) | 1 (8.3) | |
| Would you recommend participating in this research study to others? | |||
| Yes | 13 (86.7) | 10 (83.3) | >.99 |
| Unsure | 2 (13.3) | 2 (16.7) | |
| Overall, did your quality of life change by participating in this research study? | |||
| It improved | 9 (60.0) | 5 (41.7) | .45 |
| It stayed the same | 6 (40.0) | 7 (58.3) | |
| Overall, how was your experience of participating in this research study? | |||
| Better than I expected | 9 (60.0) | 3 (25.0) | .12 |
| The same as I expected | 6 (40.0) | 9 (75.0) | |
Study utilizing the Was It Worth It Questionnaire published in abstract form in Chauhan et al.23
The themes of the comments from the Muse group included satisfaction with the Muse device, a need for additional training with the device, interest in communicating with other participants, and a sense that the intervention was more rewarding for those interested in meditation. The themes from the control group included a preference for more advanced technology and tracking, a need for additional training, interest in having digital reminders, and dissatisfaction with the CD sound quality. None of the participants reported intervention-related adverse effects.
Discussion
This feasibility study shows that, despite previous limited evidence of efficacy, use of the Muse device may improve fatigue, QOL, and stress in patients with newly diagnosed breast cancer. Use of the Muse device in this population is feasible24 and effective. Muse is a relatively low-cost, low-risk, practical device that requires little or no training to use. Implementation of the intervention is easy because the Muse device is simple to set up and connect to a freely downloadable app on the user’s own smartphone or tablet. These results also indicated that the Muse device was easily integrated, acceptable, and well received.
Our findings of improved measures of fatigue, QOL, and stress with mind-body therapies are consistent with the results of studies of similar interventions for patients with breast cancer.25,26 Functional well-being was significantly worse at the postsurgical visit than at baseline, but this was expected for patients who have undergone major surgery. Compared with other studies of mindfulness interventions for patients with breast cancer, our outcomes showed lasting effects for QOL and stress at 3 months, with substantially less patient training and face-to-face time (1 hour vs 6-8 weeks for mindfulness-based stress reduction).27 Inability to commit to a lengthy, intensive training period has been cited as a reason for not participating in mind-body therapies.28
In our study, the Muse device was used more times than the stress-management CD. Participants’ comments at the 3-month postsurgical visit also indicated that the participants had more positive impressions of the Muse interface and less positive impressions of the noninteractive, nonmodifiable CD. These findings are comparable to previous findings of patients’ preferences for and interests in interacting with high-tech interfaces to change behavior.29,30 Another feature that may increase the effectiveness of Muse over other options is the availability of direct feedback data, which may encourage the participant to use the Muse device and may increase time spent in the “calm” brain state.
Most participants believed that their experience in this study was worthwhile and that the Muse intervention improved their QOL. Poststudy feedback indicated that 60.0% of participants felt that their overall QOL improved with use of the Muse device and 93.3% would be willing to participate in this study again. The high rate of utilization also indicates that Muse can be used by most individuals. Muse also has a low potential for harm.
The primary limitation of this study is the small sample size, which limited the amount of data for comparison of clinical outcomes. In addition, participants in the control group received a stress-management resource that is not consistently a part of standard care. Thus, this study cannot distinguish a real effect of the intervention from a placebo effect. Finally, the control group had a slightly greater proportion of participants with an advanced-education degree than the intervention group. Women with a higher level of education tend to favor integrative medicine therapies, and therefore, this may have contributed to their willingness to participate in mind-body practices and may have reduced fatigue, improved QOL, and reduced stress.
In conclusion, use of a wearable, EEG device such as the Muse by patients with newly diagnosed breast cancer is feasible and improves fatigue, QOL, and stress. Such devices have the potential to be efficacious and effective tools to reduce the stress of breast cancer patients before surgery, and a larger trial is warranted. Future research, including larger randomized controlled studies, of low-cost, easily accessible devices are needed to investigate the psychological outcomes of breast cancer patients and other patient populations.
Acknowledgments
InteraXon Inc provided the Muse devices for this study but did not participate in the study design or analysis of the results.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was received from the Ralph A. Stump & Radene G. Stump Foundation.
Authors’ Note: Mayo Clinic does not endorse specific products or services included in this article.
ORCID iD: Sandhya Pruthi  https://orcid.org/0000-0002-2235-4542
https://orcid.org/0000-0002-2235-4542
References
- 1. Ng CG, Mohamed S, Kaur K, et al. ; MyBCC Study Group. Perceived distress and its association with depression and anxiety in breast cancer patients. PLoS One. 2017;12:e0172975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Syrowatka A, Motulsky A, Kurteva S, et al. Predictors of distress in female breast cancer survivors: a systematic review. Breast Cancer Res Treat. 2017;165:229-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler NE, Page AEK, eds Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 4. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juvet LK, Thune I, Elvsaas IKO, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166-177. [DOI] [PubMed] [Google Scholar]
- 6. Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. 2015;121:1873-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655. [DOI] [PubMed] [Google Scholar]
- 9. Kim YH, Kim HJ, Ahn SD, Seo YJ, Kim SH. Effects of meditation on anxiety, depression, fatigue, and quality of life of women undergoing radiation therapy for breast cancer. Complement Ther Med. 2013;21:379-387. [DOI] [PubMed] [Google Scholar]
- 10. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18:1261-1272. [DOI] [PubMed] [Google Scholar]
- 11. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49:1365-1373. [DOI] [PubMed] [Google Scholar]
- 12. Morris KT, Johnson N, Homer L, Walts D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg. 2000;179:407-411. [DOI] [PubMed] [Google Scholar]
- 13. Lengacher CA, Kip KE, Barta M, et al. A pilot study evaluating the effect of mindfulness-based stress reduction on psychological status, physical status, salivary cortisol, and interleukin-6 among advanced-stage cancer patients and their caregivers. J Holist Nurs. 2012;30:170-185. [DOI] [PubMed] [Google Scholar]
- 14. Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300:1350-1352. [DOI] [PubMed] [Google Scholar]
- 16. Zernicke KA, Campbell TS, Speca M, McCabe-Ruff K, Flowers S, Carlson LE. A randomized wait-list controlled trial of feasibility and efficacy of an online mindfulness-based cancer recovery program: the eTherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76:257-267. [DOI] [PubMed] [Google Scholar]
- 17. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005-4015. [DOI] [PubMed] [Google Scholar]
- 18. Donovan KA, Jacobsen PB. The Fatigue Symptom Inventory: a systematic review of its psychometric properties. Support Care Cancer. 2010;19:169-185. [DOI] [PubMed] [Google Scholar]
- 19. Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301-310. [DOI] [PubMed] [Google Scholar]
- 20. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the Multidimensional Fatigue Symptom Inventory–Short Form. J Pain Symptom Manage. 2004;27:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez R, Ballesteros M, Arnold BJ. Validation of the FACT-G scale for evaluating quality of life in cancer patients in Colombia. Qual Life Res. 2011;20:19-29. [DOI] [PubMed] [Google Scholar]
- 22. Taylor JM. Psychometric analysis of the ten-item Perceived Stress Scale. Psychol Assess. 2015;27:90-101. [DOI] [PubMed] [Google Scholar]
- 23. Chauhan C, Atherton PJ, Satele D, et al. Patient satisfaction with participation in phase II/III NCCTG clinical trials: Was it worth it? (N0392). J Clin Oncol. 2012;30(15 suppl):6133. [Google Scholar]
- 24. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reich RR, Lengacher CA, Alinat CB, et al. Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage. 2017;53:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stoerkel E, Bellanti D, Paat C, et al. Effectiveness of a self-care toolkit for surgical breast cancer patients in a military treatment facility. J Altern Complement Med. 2018;24:916-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haller H, Winkler MM, Klose P, Dobos G, Kummel S, Cramer H. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol. 2017;56:1665-1676. [DOI] [PubMed] [Google Scholar]
- 28. Eyles C, Leydon GM, Hoffman CJ, et al. Mindfulness for the self-management of fatigue, anxiety, and depression in women with metastatic breast cancer: a mixed methods feasibility study. Integr Cancer Ther. 2015;14:42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd GR, Oza S, Kozey-Keadle S, et al. Breast cancer survivors’ beliefs and preferences regarding technology-supported sedentary behavior reduction interventions. AIMS Public Health. 2016;3:592-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips SM, Conroy DE, Keadle SK, et al. Breast cancer survivors’ preferences for technology-supported exercise interventions. Support Care Cancer. 2017;25:3243-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]