Abstract
Background
Visceral leishmaniasis (VL), due to Leishmania infantum, is a persistent intracellular parasitic infection transmitted by the bite of infected sand flies. Symptomatic VL has been reported in U.S. soldiers with Iraq deployment. Untreated symptomatic VL can be fatal; asymptomatic VL (AVL) may establish a lifelong risk of reactivation. We report prevalence and AVL risk factors in Operation Iraqi Freedom (OIF) deployers during 2002–11.
Methods
Healthy soldiers exposed to VL endemic areas in Iraq and 50 controls who never traveled to endemic regions were recruited through military healthcare facilities (2015–17). Responses to a risk factor survey and blood samples were obtained. Leishmania research diagnostics utilized included enzyme-linked immunosorbent assay (ELISA), rk39 test strips, quantitative polymerase chain reaction (PCR), and interferon gamma release (IGRA) assays. Statistical analyses included Fisher exact test, Pearson χ2 test, Mann-Whitney U test, and logistic regression.
Results
200 deployed subjects were enrolled, mostly males (84.0%), of white ethnicity (79.0%), and median age 41 (range 24–61) years. 64% were seropositive for Phlebotomus alexandri saliva antibodies. Prevalence of AVL (any positive test result) was 39/200 (19.5%, 95% confidence interval 14.4%–25.8%). Two (1.0%) PCR, 10 (5%) ELISA, and 28 (14%) IGRA samples were positive. Travel to Ninewa governorate increased risk for AVL (P = .01).
Conclusion
AVL was identified in 19.5% of OIF deployers; travel to northwest Iraq correlated with infection. Further studies are needed to inform risk for reactivation VL in US veterans and to target additional blood safety and surveillance measures.
Keywords: visceral leishmaniasis, asymptomatic, US soldiers, deployed, Iraq
Approximately 20% of US soldiers deployed to Iraq were found to be infected with Leishmania by blood assays performed at least a decade after deployment. This disease burden and risk for reactivation in US military personnel is underrecognized.
In Iraq, a bite from an infected sand fly (Phlebotomus alexandri) can transmit Leishmania infantum, the parasite that causes visceral leishmaniasis (VL) [1, 2]. No prophylactic medicine or vaccine exists. North Americans typically have no protective immunity; the deployment of over 1 million US service members to Iraq potentially exposed many to Leishmania [3]. This parasite can persist inside human macrophages lifelong. Activated VL manifests as a chronic illness with fever, weight loss, cytopenias, and hepatosplenomegaly. Host immune responses (similar to tuberculosis) hold latent VL in check. Reported risks for activation in European/ Brazilian adults include biologic response modifying agents, human immunodeficiency virus (HIV), organ transplant, poorly controlled diabetes, and alcohol abuse [4–8]. Untreated active VL has been associated with >90% mortality but can be successfully treated with liposomal amphotericin [9, 10].
In the past decade, asymptomatic visceral leishmaniasis (AVL) has been recognized as common in endemic areas with infection to disease ratios ranging from 50–100:1 in Spain [11], 8.9:1 in India, Nepal [12, 13], 13:1 in Iran [14] and 6.5:1 to 89:1 in Brazil [15–17]. In Iraq, among asymptomatic close contacts of children with active VL, 86/250 (34%) tested rK39 and Leishmania seropositive [18]. The risk of asymptomatic infection in healthy travelers from non-endemic areas, such as deployed military, is poorly understood [19]. Although cases of cutaneous and active visceral leishmaniasis have been described in US soldiers deployed to Iraq, chronic asymptomatic visceral infection with L. infantum has not been previously recognized in US forces [20, 21]. Due to risk of AVL reactivation among soldiers with service in Iraq, we investigated 200 higher risk military persons approximately 11.3 years after Iraq deployment studying blood samples with multiple assays to include rK39 test strips, Leishmania enzyme-linked immunosorbent assay (ELISA), Leishmania interferon gamma release assay (IGRA), and quantitative polymerase chain reaction (qPCR) measuring blood parasite load.
METHODS
Ethical Considerations
This research protocol was approved by the Uniformed Services University (USU), Walter Reed National Military Medical Center (WRNMMC), William Beaumont Army Medical Center (WBAMC), and the Food and Drug Administration (FDA) Institutional Review Boards (IRB). All participants provided written informed consent.
Patient Population
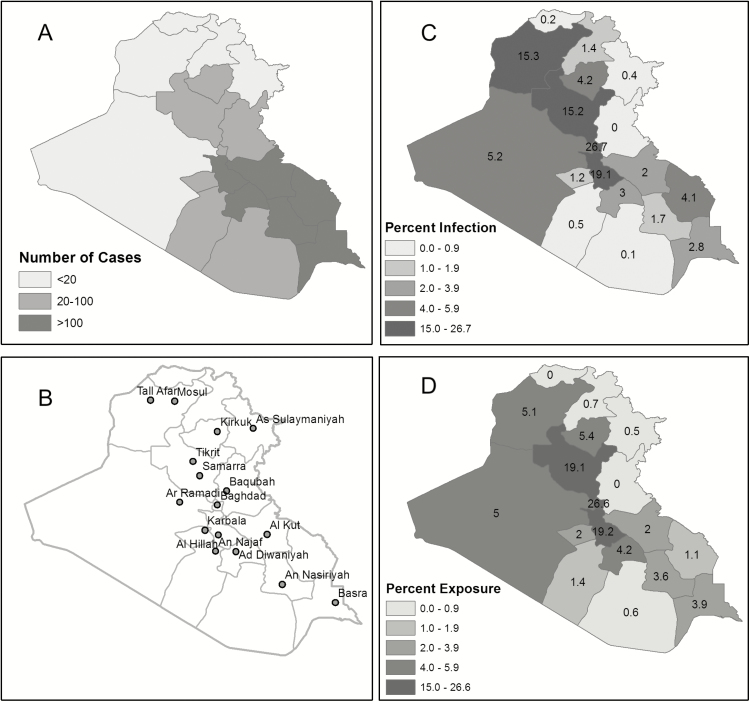
Subjects completed a risk factor survey and donated blood samples for VL research assays. Enrollment occurred at WRNMMC/USU in Bethesda MD, DiLorenzo Tricare Health Clinic-Pentagon, Washington, DC, and WBAMC in El Paso TX. Subjects were US military personnel in good health, age 18–60 years, who traveled to Iraq between 2002–11. Higher likelihood of sand fly exposure was selected by requiring at least one summer month spent in Iraq, at least three nights per week spent outdoors, and deployment to governorates where the Iraq Ministry of Health reported VL (Figure 1A courtesy of Eric Milstrey). Exclusion criteria included receiving blood transfusion since leaving Iraq and underlying immunosuppressive conditions. Controls were healthy individuals aged 18–60 years who had no life-long travel to areas endemic for leishmaniasis.
Figure 1.
Figure 1 shows distribution of reported Iraqi Visceral leishmaniasis cases in 2004 by goverante (A); major cities in Iraq (B); distribution of deployed locations (as % time spent) for US infected (n = 39) (C); and US not infected (n = 161) (D). * P = .01, Mann-Whitney U test comparing person time fractions spent in Ninewa governate (15.3 vs. 5.1).
Risk Factor Survey
Participants completed a 20-item questionnaire detailing demographic and military information, dates and details of first deployment to endemic areas of Iraq, including risk factors for exposure to sand flies. Participants were queried about other deployments to VL endemic areas, postdeployment hospital admissions, and systemic symptoms occurring in the 3 months preceding enrollment.
Serum and Risk Factor Data From the Immediate Iraq Deployment Period
Concurrent data about personal protective measures (PPM) and medications collected in the immediate postdeployment period were obtained from the Defense Medical Surveillance System (DMSS) [22]. Matched, banked serum specimens provided within 6 months of the end of the first Iraq deployment were retrieved from the Department of Defense Serum Repository (DoDSR), The Armed Forces Health Surveillance Branch, US Department of Defense, Silver Spring, MD; release dates 2015–17.
Parasite Culture and Antigen Preparation
L. infantum parasites (MHOM/BR/00/1669, courtesy Dr. M. Wilson, University of Iowa) were grown at 26° C in hemoflagellate modified minimal essential medium with 10% fetal bovine serum until reaching stationary phase [23, 24]. Soluble Leishmania antigen (SLA) was prepared as described [25]. SLA was tested for endotoxin using Limulus lysate assay (Lonza) with measured level of 0.38 EU/ug.
Interferon Gamma Release Assay
One mL of whole blood or 1 × 106 peripheral blood mononuclear cells (PBMC) were stimulated with either 20 ug/mL SLA or 10% phytohemagglutinin (PHA, Life Technologies) for 72 hours at 37° C, 5% CO2. Supernatants were collected and tested in duplicate using human interferon gamma (IFN-ɣ) ELISA kits (Ready-SET-Go, eBioscience). Assay cutoff was determined by calculating mean of IFN-ɣ in control subjects plus 2 standard deviations (SD).
Soluble Leishmania Antigen ELISA
SLA ELISA was performed as previously described [26] with modification. Coating was done with 0.5 ug/well of SLA, serum samples were diluted 1:400, and secondary antibody was 1:10 000 goat anti-human IgG-HRP (Southern Biotech). Reaction was developed in the presence of 3,3′,5,5′tetramethylbenzidine substrate for 30 minutes, then stopped (KPL, Inc.). ELISA cutoff was defined as the average optical density (OD) of control subjects plus 3 SD.
rK39
Reactivity against rK39 was tested using the immunochromatographic Kalazar Detect™ Rapid test (InBios).
L. infantum Real-time Quantitative PCR
DNA was extracted from 5 × 106 PBMC using the DNAeasy Blood & Tissue Kit (Qiagen) with modification. Samples were incubated overnight at 56○C in the presence of proteinase K and AL buffer, and DNA was eluted in 50 uL H20. The target REPL repeat was amplified from 5 uL DNA as previously described [27].
Phlebotomus (Paraphlebotomus) alexandri Collection and Salivary Gland Homogenates ELISA
Sand fly collections were conducted using CDC light traps (John W. Hock Company) at Waqqa, in the north Jordan Valley, Jordan, in August 2016. Salivary glands from Ph. alexandri females were dissected at Jordan University of Science and Technology, frozen at −20 ○C, and shipped to USU [28, 29]. Salivary gland homogenate (SGH) ELISA was performed as previously described with modification; coating was done with 0.1 ug SGH/well, sera 1:200 dilution, and secondary antibody at a 1:5000 dilution [30]. ELISA cutoff was defined as the average OD of control subjects plus 3 SD.
Statistical Analysis
A sample size of 200 deployers was chosen to determine deployed population VL prevalence within 5% bounds of a 15% point estimate. Categorical data were analyzed using Fisher exact and χ2 tests [31], whereas continuous data were analyzed using independent samples t-test or Mann-Whitney U test. The level of statistical significance was set at P < .05. Univariate logistic regression models (using SPSS 24) were constructed to screen for potential confounders among risk factors associated with VL infection at P-value threshold <.25.
RESULTS
Population
In sum, 250 volunteers were enrolled: 200 OIF deployers and 50 controls. The majority of deployers were males (84.0%) of white race or ethnicity (79.0%) with median age 41 (range 24–61) years. Most served in the Army (88.5%) as enlisted soldiers (64.5%). Average time since first deployment to Iraq was 11.3 years (range 5–14). Controls were mainly white (72.0%) males (74.0%) with median age 32 years (range 19–58). Four control subjects were excluded from the final analysis: 1 was unable to obtain blood, 1 did not reveal full travel history initially, and 2 had elevated not stimulated IFNγ levels and did not reach threshold for adequate stimulation.
Laboratory Assays
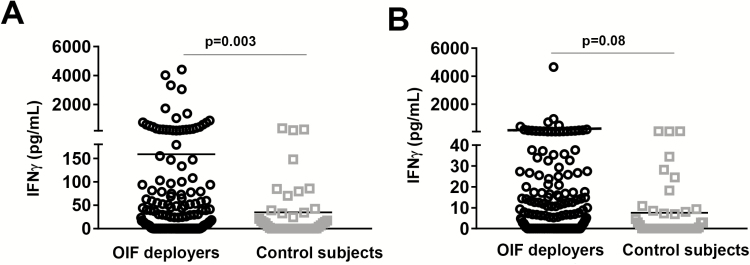
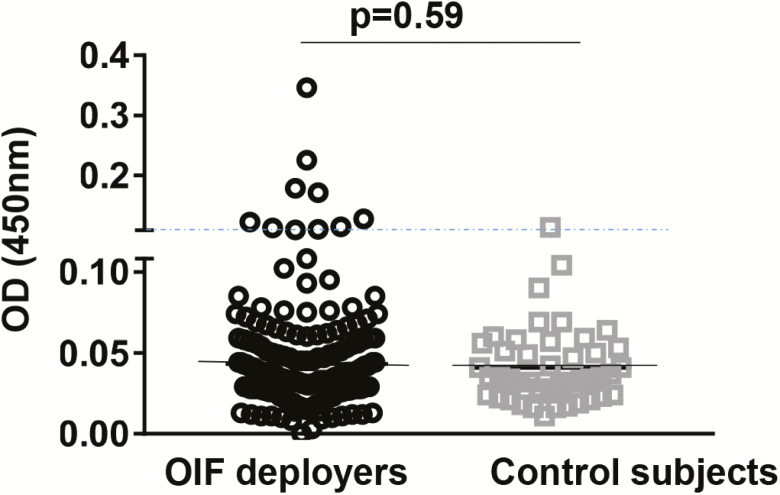
In this study, AVL was defined as a positive result on any test. Out of 200 OIF deployers, 39 (19.5%; 95% confidence interval [CI]: 14.4%, 25.8%) had positive test results using L. infantum assays conducted with blood samples obtained at enrollment (remote from OIF deployment). None had positive rK39 immunochromatographic test results. Two (1.0%) deployers were qPCR positive (quantified as 5 and 15 parasites/mL), 9 (4.5%) tested SLA ELISA positive, 27 (13.5%) tested Leishmania IGRA positive; 1 person (0.5%) was both ELISA and IGRA positive. IFN-γ levels in deployers and controls are shown in PBMC versus whole blood assays (Figure 2A and 2B). Figure 3 shows antibody levels measured by SLA ELISA comparing deployers versus controls. We cultured buffy coat samples from PCR positive individuals but were not able to isolate Leishmania. Four OIF deployers had a history of cutaneous leishmaniasis with IGRA tests positive with higher IFN-γ levels after L. infantum SLA compared to L major SLA stimulation (data not shown). Three controls were IGRA positive. One control had positive SLA ELISA; all sera with positive SLA ELISA showed no cross reactivity with ELISA for T. cruzi (Quest Diagnostics).
Figure 2.
IFN-γ levels in SLA proliferated PBMC and whole blood. IFN-γ (pg/mL) production by (A) PBMC or (B) blood stimulated with L. infantum SLA in Iraq deployers (n = 200) and not-exposed control subjects (n = 46). The assay cutoff is defined as the mean OD + 2 SDs for the values obtained from control samples (180 pg/mL for PBMC and 40 pg/mL for blood). The solid lines represent the mean. The significance of differences between groups was evaluated by the Mann-Whitney U test. P values <.05 were considered significant. Abbreviations: IFN- γ, interferon γ; PBMC, peripheral blood mononuclear cell; OD, optical density; OIF, Operation Iraqi Freedom; SD, standard deviation; SLA, soluble Leishmania antigen.
Figure 3.
Serum IgG measured using L. infantum SLA ELISA in Iraq deployers and control subjects. The dotted line shows the cutoff value, which is defined as the mean OD + 3 SDs for the values obtained with sera from nonexposed controls (0.109). Solid lines indicate mean OD values. The significance of differences between groups was evaluated by the Mann-Whitney U test. P values <.05 were considered significant. Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; OD, optical density; OIF, Operation Iraqi Freedom; SD, standard deviation; SLA, soluble Leishmania antigen.
Epidemiologic Risks for Infection
Risk characteristics of exposed OIF deployers were assessed (Table 1). No statistical association was found comparing infected versus uninfected subjects in military rank and job type, early (before 2006 with limited support infrastructure) versus late deployment, duration of deployment, night activities, attire, sleep accommodations, illness post-deployment, exposure to dogs, or use of PPM. Presence of antibodies to Ph. alexandri saliva was assessed; 77% of the AVL infected were positive for SGH antibodies compared to 61% of the noninfected, odds ratio 2.14, (95% CI: 0.95, 4.81). Evaluating geographic exposure within Iraq, deployment to Ninewa governorate was associated with infection, P = .01 (Figure 1A–1D). Using logistic regression, factors potentially associated with VL infection examined were deployment to Ninewa for ≥30 days (P = .01), occupation (combat arms, combat support, health care) (P = .10), and presence of SGH antibody (P = .06). Deployment to Ninewa remained significantly associated with infection after adjusting for job code and sand fly ELISA (OR for infection = 3.09, 95% CI 1.18–8.09). SGH antibodies and job code were not found to be associated with infection in the adjusted model.
Table 1.
Comparison of Characteristics and Potential Risk Factors of Exposed Infected versus Noninfected OIF Veterans (n = 200)
| Infected n (%) |
Not Infected n (%) |
P | |
|---|---|---|---|
| 39 (100) | 161 (100) | ||
| Mean current age (range) | 40.5 years (28–58) |
41.2 years (24–61) |
NSd |
| Sex | NSe | ||
| Male | 35 (89.7) | 133 (82.6) | |
| Female | 4 (10.3) | 28 (17.4) | |
| Race | NSe | ||
| White | 32 (82.1) | 126 (78.3) | |
| Black/African American | 5 (12.8) | 20 (12.4) | |
| Asian | 0 | 6 (3.7) | |
| Pacific Islander | 1 (2.6) | 2 (1.2) | |
| Other | 1 (2.6) | 7 (4.3) | |
| Service | NSe | ||
| Army | 34 (87.2) | 143 (88.8) | |
| Marine Corps | 2 (5.1) | 9 (5.6) | |
| Navy | 3 (7.7) | 6 (3.7) | |
| Air Force | 0 | 3 (1.9) | |
| Rank | |||
| Officer | 15 (38.5) | 56 (34.8) | NSe |
| Enlisted | 24 (61.5) | 105 (65.2) | |
| Military occupation in Iraq | NSe | ||
| Combat arms | 4 (10.3) | 37 (23.0) | |
| Aviation | 3 (7.7) | 8 (5.0) | |
| Special operationsa | 0 | 11 (6.8) | |
| Combat support | 7 (18.0) | 31 (19.3) | |
| Healthcare | 16 (41.0) | 46 (38.6) | |
| Administration | 4 (10.3) | 16 (9.9) | |
| Supply | 4 (10.3) | 4 (2.5) | |
| Mechanic | 0 | 4 (2.5) | |
| Transportation | 1 (2.6) | 4 (2.5) | |
| Median days deployedb in Iraq (range) | 361 (93–517) |
321 (30–790) |
NSf |
| Total deployments to Iraq or Afghanistan since 2001 | NSe (for trend) |
||
| 1 | 12 (30.8) | 56 (34.8) | |
| 2–4 | 22 (56.4) | 95 (59.0) | |
| 5 or more | 5 (12.8) | 10 (6.2) | |
| Summer evening outdoor activitiesc | NSe | ||
| 2–4 days/week | 7 (18.5) | 19 (11.8) | |
| Five or more days/week | 31 (81.6) | 142 (88.2) | |
| Typical summer sleepwear | NSe | ||
| Full uniform ± boots | 7 (18) | 16 (9.9) | |
| Shorts and t-shirt (physical fitness uniform) | 23 (59.0) | 87 (54.0) | |
| “Skivvies” only | 7 (18) | 46 (28.6) | |
| Other | 2 (5.1) | 11 (6.8) | |
| Summer sleep conditions | |||
| Total no. of nights (mean) | 7228 (185) | 27681 (188) | |
| Percentage of all nights on ground (vs. bed/cot) | 19.5% | 13.5% | NSe |
| Exposure to dogs | NSe | ||
| Had a local pet | 4 (10.3) | 14 (8.7) | |
| Others in unit had a pet | 14 (35.9) | 62 (38.5) | |
| Worked with military working dogs | 3 (7.7) | 12 (7.5) | |
| Little or no direct contact | 23 (59) | 89 (55.3) | |
| Use of DEET insect repellent | NSe | ||
| None | 16 (41) | 64 (39.8) | |
| Brought own brand | 4 (10.3) | 15 (9.3) | |
| Used military issued repellent | 10 (25.6) | 54 (33.5) | |
| Used both | 9 (23.1) | 28 (17.4) | |
| Frequency of repellent use | NSe | ||
| Never | 17 (43.6) | 60 (37.3) | |
| ≤ 1/week | 13 (33.3) | 69 (42.9) | |
| >1/week | 4 (10.3) | 21 (13.0) | |
| Daily | 5 (12.8) | 11 (6.8) | |
| Permethrin–treated uniforms | NSe | ||
| None | 15 (38.5) | 64 (39.8) | |
| Unknown | 2 (5.1) | 20 (12.4) | |
| Some uniforms treated | 12 (30.8) | 34 (21.1) | |
| All uniforms treated | 10 (25.6) | 43 (26.7) | |
| †Sand fly saliva antibodies detected | 30 (76.9) | 98 (60.9) | P = .06e |
| Constitutional symptoms (in previous 3 months) | NSe | ||
| Fever | 5 (12.8) | 15 (9.3) | |
| Night sweats | 9 (23.1) | 36 (22.4) | |
| Weight loss | 0 | 5 (3.1) | |
| Fatigue | 12 (30.8) | 44 (27.3) | |
| Skin rash | 10 (25.6) | 37 (23.0) | |
| Multiple symptoms reported | NSe | ||
| >3 | 4 (10.3) | 1 (.06) | |
| 3 | 1 (2.6) | 2 (1.2) | |
| 2 | 6 (15.4) | 2 (1.2) | |
| Postdeployment hospitalization | 11 (28.2) | 43 (26.7) | NSe |
Infection defined as positive by polymerase chain reaction, interferon-y release assay, or serology. Individual comparison level of significance P = .05. No correction for multiple positive comparisons was performed.
Abbreviations: DEET, N,N-diethyl-meta-toluamide; NS, nonsignificant; OIF, Operation Iraqi Freedom.
aSalivary gland homogenate ELISA antibody.
bDuring first Iraq deployment.
cNo response from one infected participant.
dMeans compared with Student’s t-test.
eProportions compared with χ2 or Fisher’s exact test.
fRank sums compared with Mann-Whitney test.
Data from DD Form 2796 (post deployment health assessment) were retrieved. In sum, 177 volunteers completed the forms; no significant differences were found in recall of PPM or symptoms immediately postdeployment compared to current responses.
DISCUSSION
Leishmaniasis has emerged as a parasitic disease of relevance to US Armed Forces in recent conflicts with reports detailing cutaneous, viscerotropic, and visceral leishmaniasis [20, 32–35]. Approximately 2040 diagnoses of leishmaniasis were reported from 2001–16 in US Armed Forces with over 1000 cutaneous and 25 visceral leishmaniasis cases reported in DMSS [20].
This study is the first to our knowledge to look at AVL in a cohort of US forces deployed during OIF; approximately 20% were found to be Leishmania infected by blood assays performed at least a decade after deployment to Iraq. Most tested positive by IGRA (14%), fewer by ELISA (5%), rarely PCR (1%), and none by rK39. Vector exposure was high with 64% testing positive for Ph. alexandri saliva antibody. Our results are similar to another deployed population: 1048 United Nations volunteers in nonendemic Austria where 4.5% persons tested serologically positive and 0.4% were PCR positive for leishmaniasis [19].
There are few well-defined diagnostic tests to detect AVL. Invasive tissue diagnostics are too aggressive, less sensitive in asymptomatic individuals, and unsuitable for surveillance [27]. Rapid tests such as ELISA and PCR are useful, but sensitivity/specificity depends on the antigen [36] or DNA target used [37]. In our study, we adapted immunological (SLA ELISA and IGRA) and molecular (real-time PCR) tools that identified a prevalence rate of 20% among OIF deployers. Because of individual variability in host immune responses, combined assays testing Th1 and Th2 responses and parasite antigen presumably enhanced the capacity to detect asymptomatic infection [38].
Prevalence of asymptomatic infection in OIF deployers was within the ranges published from endemic regions. AVL may be seen in up to 30% or more in endemic areas and seropositivity has been reported in 7% of worldwide blood donors [39, 40]. Molecular testing in two subjects showed parasite loads (mean 10, range 5–15 parasites/mL) consistent with active parasitemia since parasite DNA degrades shortly after amastigote death [41]. Parasitemia in OIF deployers appears similar to parasite loads previously reported in AVL; L. donovani cases had a median of 7.7 parasites/mL [42] and 10–56 parasites/mL (L. infantum) were reported among healthy Brazilian children [43].
Progression of AVL is determined by parasite virulence and host factors [39, 44, 45]. AVL infected persons may be at risk for clinical progression with risk ranging from 1.5% to 23% up to 3 years after AVL diagnosis in India/ Nepal versus no risk of progression after seroconversion seen in Brazil over a decade [12, 45–49]. Leishmania infection of macrophages and the leishmaniacidal activity of these cells is moderated by cell mediated immunity. Cytokine release of IFN-γ and tumor necrosis factor results in eradication and/or containment, an appropriate long term durable response paralleling immune responses in latent tuberculosis [45, 50–52]. Host antibody responses (measured in ELISA, rK39) correlate with more recent infection with waning response over months to years [47, 53]. Molecular diagnostics have shown high sensitivity and specificity and have been utilized in the diagnosis of AVL and for monitoring therapy response [54, 55]
In our study, most soldiers with AVL had positive IGRA results indicative of ongoing immunity and/or containment of infection more than a decade after exposure. The SLA ELISA results in this group (anticipated to be short-lived) could be explained by differences in individual immune responses, boosting with potential re-exposure to leishmaniasis on other deployments, or nonspecific cross reactivity of antigens used. Supporting nonspecific cross reactivity, four control participants (4/50, 8%) with no apparent exposure to leishmaniasis had positive results (3 IGRA, one ELISA). Although this proportion is excluded from the 95% CI for the prevalence in the exposed group, the 95% CIs for the proportions in the 2 groups overlap and the 95% CI for the difference in the 2 proportions includes zero.
The AVL prevalence noted in this cohort raises concerns regarding the potential for future reactivation of latent disease in the setting of acquired or iatrogenic immunosuppression. There are reports of leishmaniasis reactivation, including fatal VL, in populations given TNF antagonists and those with impaired cell mediated immunity due to HIV, transplant, lymph/hematopoietic neoplasms, and high dose corticosteroids [7, 56–59]. Extrapolating from these reports, those identified as positive for AVL would presumptively be at risk of reactivation disease if immunosuppressed.
Acquisition of cutaneous leishmaniasis in US Armed Forces in Iraq has been associated with exposure to sand fly bites in summer months, using less insect repellant, nightly activities, and inadequate PPM with a majority of cases reported in 2003–4 and subsequent decline after 2005 [20, 60]. Epidemiologic risks assessed in this study, however, showed no statistical differences between infected and noninfected in occupation, early versus later deployment (when improved infrastructure present), number of deployments, use of PPM, or animal reservoir exposure. Some differences in exposure risk compared to prior evaluations could be due to recall bias and the small sample size of this cohort. However, subject responses to similar questions on post-deployment assessments performed immediately after they returned home did not differ from their information obtained on our survey. Deployment to Ninewa governorate was positively associated with the risk for AVL. It is uncertain why deployment to Ninewa would be associated with greater risk as this Iraq region is not reported as VL endemic [2], although with increasing cases of VL in the neighboring Syrian Arab Republic [61] it is possible that differences in vector or vector activity, local reservoirs for infection, insufficient public health reporting, and soldier activity in this region contributed to this finding.
There are several limitations of this study. Issues with recall have been noted (ie, reporting exposure history more than a decade later). Despite focusing on initial deployments to Iraq, many soldiers had multiple deployments, which complicates ascertaining the exact timeframe or exposure location. Other than rK39, study assays were experimental and their performance characteristics have received limited investigation.
This study suggests the burden of VL in US military deployed to Iraq is greater than previously realized. 25 cases of overt VL were reported between 2001 and 2016 in US Armed Forces, but in this surveillance study approximately 20% of the cohort had AVL [20]. The natural course of AVL infection in healthy individuals, especially in nonendemic areas, is not well understood. Infected soldiers in our study were evaluated clinically and universally found to be asymptomatic. Unexplained splenomegaly was noted in one although no evidence of disease progression was seen. However, because of persistent intracellular infection, there is a potential threat for future VL reactivation in this population. No prospective studies are available to sufficiently gauge this risk [4]. Unrecognized AVL may also result in transmission risk when infected individuals donate blood. Our study suggests that the likelihood of an individual with AVL donating blood may be higher than anticipated [62].
Optimal management of AVL is unknown with practice guidelines advocating close monitoring of those who will be immunosuppressed and initiation of VL therapy if symptoms develop [63]. FDA-approved AVL diagnostic screening tests, a clinically available quantitative L. infantum PCR, and future prospective studies are needed to improve AVL detection and discern risks for disease progression in this population. Overall, due to the high mortality of undiagnosed/untreated overt VL, clinicians caring for US personnel who have remotely deployed to leishmaniasis endemic areas should be aware of possible AVL reactivation with immunosuppression and entertain VL as a diagnosis when their patients develop a consistent clinical syndrome.
Notes
Author contributions. All the authors contributed to data collection, analysis, interpretation, and drafting of this manuscript. N. E. A. and R. F. D. contributed to study design and had full access to the combined database set. R. M. M., J. E. S., and N. E. A. had responsibility for submission of the manuscript for publication.
Acknowledgments. We are indebted to Dr. Angelia Cost for her assistance with the DMSS and DoDSR matched data, to Penny Masuoka for her assistance with the map figure, to Saule Nurmukhambetova for laboratory assistance, to Dr. Cara Olsen for her statistical consultation, to Dr. Marc Franzos and Dr. Thomas Oliver for facilitating our project at the Pentagon and the USU Clinical Research Unit, and to Emanuel Nevarez for coordinating logistical support (including phlebotomy) at William Beaumont Army Medical Center.
Disclaimer. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force/Marine Corp., Department of Defense, or US Government. Additionally, brand name products identified are not meant for endorsement but as disclosure to ensure the study could be replicated by others.
Funding. Funding support for this project PRoMIS ID P0024_17_HS was received from the Global Emerging Infections Surveillance Section, Armed Forces Health Surveillance Branch, Department of Defense.
Potential conflicts of interest. R. C. D. reports collaborative research with Diatherix Laboratories, LLC, outside the submitted work. N. E. A. reports support from Up to Date, Elsevier, Infectious Diseases Society of America, Kuvin Center, and Hebrew University, outside the submitted work. All other authors report no conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Coleman RE, Hochberg LP, Swanson KI, et al. . Impact of phlebotomine sand flies on U.S. military operations at Tallil Air Base, Iraq: 4. Detection and identification of leishmania parasites in sand flies. J Med Entomol 2009; 46:649–63. [DOI] [PubMed] [Google Scholar]
- 2. Majeed B, Sobel J, Nawar A, Badri S, Muslim H. The persisting burden of visceral leishmaniasis in Iraq: data of the National Surveillance System, 1990–2009. Epidemiol Infect 2013; 141:443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armed Forces Press Services. Iraq by the numbers. Washington DC: Cited by Democratic Policy and Communications Center, 2011. Available at: https://wwwdpcsenategov/docs/fs-112-1-36pdf. [Google Scholar]
- 4. Weisser M, Khanlari B, Terracciano L, et al. . Visceral leishmaniasis: a threat to immunocompromised patients in non-endemic areas?Clin Microbiol Infect 2007; 13:751–3. [DOI] [PubMed] [Google Scholar]
- 5. Fletcher K, Issa R, Lockwood DN. Visceral leishmaniasis and immunocompromise as a risk factor for the development of visceral leishmaniasis: a changing pattern at the hospital for tropical diseases, London. PLoS One 2015; 10:e0121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clemente W, Vidal E, Girão E, et al. . Risk factors, clinical features and outcomes of visceral leishmaniasis in solid-organ transplant recipients: a retrospective multicenter case-control study. Clin Microbiol Infect 2015; 21:89–95. [DOI] [PubMed] [Google Scholar]
- 7. van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 2014; 20:286–99. [DOI] [PubMed] [Google Scholar]
- 8. Xynos ID, Tektonidou MG, Pikazis D, Sipsas NV. Leishmaniasis, autoimmune rheumatic disease, and anti-tumor necrosis factor therapy, Europe. Emerg Infect Dis 2009; 15:956–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyerhoff A. U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis 1999; 28:42–8; discussion 9–51. [DOI] [PubMed] [Google Scholar]
- 10. Oliveira JM, Fernandes AC, Dorval ME, et al. . Mortality due to visceral leishmaniasis: clinical and laboratory characteristics. Rev Soc Bras Med Trop 2010; 43:188–93. [DOI] [PubMed] [Google Scholar]
- 11. Moral L, Rubio EM, Moya M. A leishmanin skin test survey in the human population of l’Alacantí region (Spain): implications for the epidemiology of Leishmania infantum infection in southern Europe. Trans R Soc Trop Med Hyg 2002; 96:129–32. [DOI] [PubMed] [Google Scholar]
- 12. Ostyn B, Gidwani K, Khanal B, et al. . Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis 2011; 5:e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Topno RK, Das VN, Ranjan A, et al. . Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in bihar, India. Am J Trop Med Hyg 2010; 83:502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies CR, Mazloumi Gavgani AS. Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology 1999; 119 (Pt 3):247–57. [DOI] [PubMed] [Google Scholar]
- 15. Evans TG, Teixeira MJ, McAuliffe IT, et al. . Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis 1992; 166:1124–32. [DOI] [PubMed] [Google Scholar]
- 16. Badaró R, Jones TC, Lorenço R, et al. . A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis 1986; 154:639–49. [DOI] [PubMed] [Google Scholar]
- 17. Maia Z, Viana V, Muniz E, et al. . Risk factors associated with human visceral leishmaniasis in an urban area of Bahia, Brazil. Vector Borne Zoonotic Dis 2016; 16:368–76. [DOI] [PubMed] [Google Scholar]
- 18. Hantosh HA, Al Lami FH. Prevalence of asymptomatic visceral leishmaniasis among under 5 years contacts of confirmed cases in Thiqar Governorate, 2012. Infect Dis Ther 2013; 1:122. doi:10.4172/2332-0877.1000122 [Google Scholar]
- 19. Poeppl W, Herkner H, Tobudic S, et al. . Seroprevalence and asymptomatic carriage of Leishmania spp. in Austria, a non-endemic European country. Clin Microbiol Infect 2013; 19:572–7. [DOI] [PubMed] [Google Scholar]
- 20. Stahlman S, Williams VF, Taubman SB. Incident diagnoses of leishmaniasis, active and reserve components, U.S. Armed Forces, 2001–2016. MSMR 2017; 24:2–7. [PubMed] [Google Scholar]
- 21. Aronson NE. Leishmaniasis in American soldires: parasites from the front. In: Scheld M, Hooper DC, Hughes JM, ed. Emerging infectious disease 7, 7th ed. Washington, DC: ASM Press, 2007:325–42. [Google Scholar]
- 22. Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health 2002; 92:1900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berens RL, Brun R, Krassner SM. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol 1976; 62:360–5. [PubMed] [Google Scholar]
- 24. Ramer-Tait AE, Lei SM, Bellaire BH, Beetham JK. Differential surface deposition of complement proteins on logarithmic and stationary phase Leishmania chagasi promastigotes. J Parasitol 2012; 98:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of soluble promastigote extract. J Immunol 1987; 139:3118–25. [PubMed] [Google Scholar]
- 26. Maalej IA, Chenik M, Louzir H, et al. . Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am J Trop Med Hyg 2003; 68:312–20. [PubMed] [Google Scholar]
- 27. Vallur AC, Duthie MS, Reinhart C, et al. . Biomarkers for intracellular pathogens: establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis. Clin Microbiol Infect 2014; 20:O374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lane RP. The Sandflies of Egypt (Diptera, Phlebotominae). Bull Br Mus Nat Hist 1986; 52:35. [Google Scholar]
- 29. Kakarsulemankhel JK. Taxonomic review of sand flies of the subgenus Paraphlebotomus Theodor (Diptera: Psychodidae). Pakistan Entomologisr 2010; 32:125–47. [Google Scholar]
- 30. Oliveira F, Rowton E, Aslan H, et al. . A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med 2015; 7:290ra90. [DOI] [PubMed] [Google Scholar]
- 31. Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist 1947; 18:50–60. [Google Scholar]
- 32. Magill AJ, Grögl M, Gasser RA Jr, Sun W, Oster CN. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N Engl J Med 1993; 328:1383–7. [DOI] [PubMed] [Google Scholar]
- 33. Weina PJ, Neafie RC, Wortmann G, Polhemus M, Aronson NE. Old world leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin Infect Dis 2004; 39:1674–80. [DOI] [PubMed] [Google Scholar]
- 34. Aronson NE, Sanders JW, Moran KA. In harm’s way: infections in deployed American military forces. Clin Infect Dis 2006; 43:1045–51. [DOI] [PubMed] [Google Scholar]
- 35. Myles O, Wortmann GW, Cummings JF, et al. . Visceral leishmaniasis: clinical observations in 4 US army soldiers deployed to Afghanistan or Iraq, 2002-2004. Arch Intern Med 2007; 167:1899–901. [DOI] [PubMed] [Google Scholar]
- 36. Singh OP, Sundar S. Developments in diagnosis of visceral leishmaniasis in the elimination era. J Parasitol Res 2015; 2015:239469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol 2004; 42:5249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Gouvêa Viana L, de Assis TS, Orsini M, et al. . Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Trans R Soc Trop Med Hyg 2008; 102:548–55. [DOI] [PubMed] [Google Scholar]
- 39. Michel G, Pomares C, Ferrua B, Marty P. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop 2011; 119:69–75. [DOI] [PubMed] [Google Scholar]
- 40. Asfaram S, Fakhar M, Mohebali M, et al. . Asymptomatic human blood donors carriers of Leishmania infantum: potential reservoirs for visceral leishmaniasis in northwestern Iran. Transfus Apher Sci 2017; 56:474–9. [DOI] [PubMed] [Google Scholar]
- 41. Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes Infect 2007; 9:1307–15. [DOI] [PubMed] [Google Scholar]
- 42. Kaushal H, Bhattacharya SK, Verma S, Salotra P. Serological and molecular analysis of Leishmania infection in healthy individuals from two districts of West Bengal, India, endemic for visceral leishmaniasis. Am J Trop Med Hyg 2017; 96:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. dos Santos Marques LH, Gomes LI, da Rocha IC, et al. . Low parasite load estimated by qPCR in a cohort of children living in urban area endemic for visceral leishmaniasis in Brazil. PLoS Negl Trop Dis 2012; 6:e1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 2011; 9:604–15. [DOI] [PubMed] [Google Scholar]
- 45. Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clin Infect Dis 2014; 58:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeronimo SM, Teixeira MJ, Sousa Ad, Thielking P, Pearson RD, Evans TG. Natural history of Leishmania (Leishmania) chagasi infection in Northeastern Brazil: long-term follow-up. Clin Infect Dis 2000; 30:608–9. [DOI] [PubMed] [Google Scholar]
- 47. Silva LA, Romero HD, Nogueira Nascentes GA, Costa RT, Rodrigues V, Prata A. Antileishmania immunological tests for asymptomatic subjects living in a visceral leishmaniasis-endemic area in Brazil. Am J Trop Med Hyg 2011; 84:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirve S, Boelaert M, Matlashewski G, et al. . Transmission dynamics of visceral leishmaniasis in the Indian Subcontinent: a systematic literature review. PLoS Negl Trop Dis 2016; 10:e0004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saha P, Ganguly S, Chatterjee M, et al. . Asymptomatic leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLoS Negl Trop Dis 2017; 11:e0005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gidwani K, Jones S, Kumar R, Boelaert M, Sundar S. Interferon-gamma release assay (modified QuantiFERON) as a potential marker of infection for Leishmania donovani, a proof of concept study. PLoS Negl Trop Dis 2011; 5:e1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schnorr D, Muniz AC, Passos S, et al. . IFN-γ production to Leishmania antigen supplements the Leishmania skin test in identifying exposure to L. braziliensis infection. PLoS Negl Trop Dis 2012; 6:e1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodrigues V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasit Vectors 2016; 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cunningham J, Hasker E, Das P, et al. ; WHO/TDR Visceral Leishmaniasis Laboratory Network. A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clin Infect Dis 2012; 55:1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silva LA, Romero HD, Fagundes A, et al. . Use of the polymerase chain reaction for the diagnosis of asymptomatic Leishmania infection in a visceral leishmaniasis-endemic area. Rev Inst Med Trop Sao Paulo 2013; 55:101–4. [DOI] [PubMed] [Google Scholar]
- 55. Sakkas H, Gartzonika C, Levidiotou S. Laboratory diagnosis of human visceral leishmaniasis. J Vector Borne Dis 2016; 53:8–16. [PubMed] [Google Scholar]
- 56. Kubar J, Marty P, Lelièvre A, et al. . Visceral leishmaniosis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS 1998; 12:2147–53. [DOI] [PubMed] [Google Scholar]
- 57. Kopterides P, Mourtzoukou EG, Skopelitis E, Tsavaris N, Falagas ME. Aspects of the association between leishmaniasis and malignant disorders. Trans R Soc Trop Med Hyg 2007; 101:1181–9. [DOI] [PubMed] [Google Scholar]
- 58. Zanger P, Gabrysch S. Leishmaniasis in the era of tumor necrosis factor alpha antagonist therapy—a research agenda for Europe. Euro Surveill 2013; 18:20542. [DOI] [PubMed] [Google Scholar]
- 59. Herrador Z, Gherasim A, Jimenez BC, Granados M, San Martin JV, Aparicio P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997–2011: raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl Trop Dis 2015; 9:e0003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gambel JM, Brundage JF, Kuschner RA, Kelley PW. Deployed US Army soldiers’ knowledge and use of personal protection measures to prevent arthropod-related casualties. J Travel Med 1998; 5:217–20. [DOI] [PubMed] [Google Scholar]
- 61. Al-Nahhas S, Shabaan M, Hammoud L, Al-Taweel A, Al-Jorf S. Visceral leishmaniasis in the Syrian Arab Republic: early detection using rK39. East Mediterr Health J 2003; 9:856–62. [PubMed] [Google Scholar]
- 62. Cardo LJ. Leishmania: risk to the blood supply. Transfusion 2006; 46:1641–5. [DOI] [PubMed] [Google Scholar]
- 63. Aronson N, Herwaldt BL, Libman M, et al. . Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63:e202–64. [DOI] [PubMed] [Google Scholar]