ABSTRACT
Background
Insertional tendinopathy is likely caused by different pathologies. This variation could account for the recalcitrant nature of this condition to treatment. Ultrasound imaging may assist in identifying underlying pathology to inform patient management.
Hypothesis/Purpose
The primary purpose of this study was to quantify the presence of underlying pathology using ultrasound in individuals with a clinical diagnosis of insertional Achilles tendinopathy. Secondarily, we sought to examine the relationship of abnormal ultrasound findings to age and body mass index (BMI).
Study Design
Cross-sectional study
Methods
Fifty-six individuals with insertional tendinopathy were included in this study. B-mode ultrasound imaging was used to descriptively and quantitatively describe tendon pathology.
Results
A greater proportion of bone defect (p<0.001), intratendinous calcifications (p = 0.01) and midportion tendinosis (p<0.001) were observed on the injured side compared to the uninjured side. Higher BMI was associated with presence of bone deformity, intratendinous calcifications and distal tendinosis (p = 0.001-0.04); adding age did not significantly improve the regression model.
Conclusion
Patients with insertional tendinopathy present with multiple underlying pathologies. This may account for variable response to treatment. It may be helpful to include imaging to better identify underlying pathology when trying to determine an appropriate treatment strategy.
Level of Evidence
Level 3
Keywords: ankle, imaging, lower leg, tendinopathy, tendinosis, tendon, movement system
INTRODUCTION
Achilles tendinopathy, by definition, is pain in the area of the Achilles tendon.1 Achilles tendinopathy can be broken down into three categories – midportion tendinopathy, which accounts for 66% of Achilles tendinopathy; insertional tendinopathy, which accounts for up to 25% of Achilles tendinopathy; and other conditions, such as chronic bursitis.2,3 Clinically, insertional tendinopathy is characterized by pain at the posterior aspect of the heel, but it can serve as a “catch all” term for a variety of underlying pathologies.4 These pathologies have been suggested to include degenerative changes in the tendon, enlargement of the retrocalcaneal bursa, and Haglund's deformity.5
Patients with midportion and insertional Achilles tendinopathy present with similar magnitude of pain and functional complaints with walking and recreational activity,4 however, insertional Achilles tendinopathy is notoriously recalcitrant to treatment. It has been reported that up to 75%6,7 of individuals with insertional Achilles tendinopathy will not respond to exercise-based intervention, compared to only 18% of individuals with midportion tendinopathy.7,8 Furthermore, 47% of individuals with insertional Achilles tendinopathy will go on to surgical treatment.9
One explanation for these suboptimal outcomes is that individuals with insertional Achilles tendinopathy present with different underlying pathology, which may partially account for differences in responsiveness to treatment.4,6,9-11 Tendinosis, or tendon degeneration, has been associated with an increased risk of surgical intervention,9 but has also been found to respond to exercise-based intervention including heavy loading.12,13 At the tendon-bone interface, or enthesis, alterations in compressive strain have been suggested to result in cartilaginous and bony defects.14,15 Therefore, interventions such as heel wedges have been aimed at reducing compressive strain.14 Calcifications within the tendon, but not tendinosis, have also been found to respond to interventions like shockwave treatment.10 This may explain why shockwave treatment in conjunction with exercise has been associated with improved symptomatic relief.16,17 Lastly, irritation of the retrocalcaneal bursa may respond better to treatment aimed at decreasing bursal compression.18 In summary, the pathophysiology underlying insertional Achilles tendinopathy is likely different between individuals and seems to respond differently to intervention.
Diagnostic ultrasound imaging may be useful in assisting with the differential diagnosis of insertional Achilles tendinopathy. Similar to strength or range of motion measures, imaging in the context of a comprehensive clinical exam can help the clinician in confirming treatment targets. Prior studies have validated real-time musculoskeletal ultrasound imaging against magnetic resonance imaging (MRI)19 and X-ray20 for identification of tendon pathology and boney changes at the tendon-bone interface, respectively. Prior studies have also found abnormal findings in the Achilles tendons of 3.8% of asymptomatic individuals in a general population21 and 11% of an elite, athletic population.22 It is important, therefore, to consider ultrasound imaging a component of the clinical exam rather than a stand-alone imaging modality.
Given the clinical utility of ultrasound imaging and the potentially complex nature of insertional Achilles tendinopathy, the primary purpose of this study was to quantify the presence of underlying pathology using ultrasound in individuals with a clinical diagnosis of insertional Achilles tendinopathy. Secondarily, we sought to examine the relationship of abnormal ultrasound findings to age and body mass index (BMI).
METHODS
This is a retrospective analysis of a subgroup of individuals with insertional Achilles tendinopathy at a single time point included in a larger, single-group, prospective longitudinal parent study of individuals with a variety of Achilles tendon conditions. Participants in the parent study were recruited from local orthopaedic, podiatry, and physical therapy clinics as well as through newspaper advertisements. This study was approved by the University of Delaware Institutional Review Board and all participants gave their written informed consent. All data collections for the parent study were performed in our laboratory. Data for this analysis was collected during a baseline visit occurring between November 2014 and April 2017.
To be included in this subgroup analysis, participants needed to have a primary diagnosis of insertional Achilles tendinopathy based on subjective report of posterior heel pain and pain on palpation to the posterior heel and retrocalcaneal bursa, per previously established diagnostic criteria.23,24 Participants were excluded if they had a history of Achilles tendon rupture. Individuals with midportion tendinosis were not excluded as long as their primary complaint was insertional tendinopathy. At the time of analysis, there were 149 participants in the parent study, and 56 met the inclusion criteria for the present study.
Participants’ demographic information was collected, including sex, age, and BMI. A subjective history was taken to establish the injured side and confirm the clinical diagnosis of insertional Achilles tendinopathy. The Victorian Institute of Sport Assessment – Achilles questionnaire (VISA-A)25 was used to quantitatively assess self-reported symptom severity. When participants reported bilateral symptoms, they were asked to report a more symptomatic side. If unable to identify a more symptomatic side, the participant completed a VISA-A for each side individually, and the side with the lower score was designated as the “injured” side for analysis. The asymptomatic or less symptomatic side was designated the “contralateral” side. Participant's self-reported activity level was measured for descriptive purposes using a 6-point physical activity scale (PAS).26
Descriptive and Quantitative Tendon Assessment
Once the clinical diagnosis of Achilles tendinopathy was confirmed, Achilles tendon and peritendinous structures were assessed using B-mode ultrasound imaging. All ultrasound measurements were taken using at a frequency of 10MHz and depth of 3.5 cm using a GE Logiq e ultrasound scanner (GE LOGIQ e, GE Healthcare, Chicago, IL). All images were taken with the participant prone with the feet hanging off the edge of the treatment table. Ultrasound images were reviewed for descriptive analysis by a physician who is board-certified in sports medicine with eight years of diagnostic ultrasound imaging experience, along with additional certification by the Alliance for Physician Certification & Advancement in musculoskeletal imaging. The physician reviewing the images was aware of the study purpose (identifying the frequency of pathology on ultrasound in individuals with insertional Achilles tendinopathy), however, was blinded to all participant information including presenting complaints and injured side. Blinding was maintained in order to ensure that the individual reviewing images would not be biased to identify more pathology on the symptomatic side.
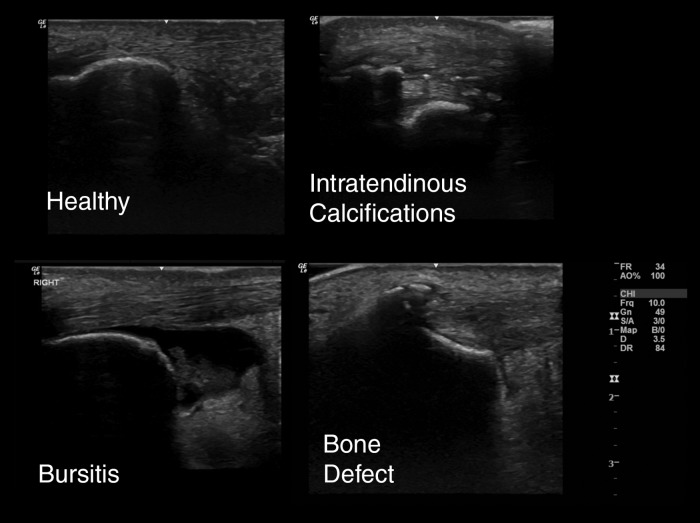
Six pathology categories were included, with participants scoring as “present” or “absent.” Pathology categories consisted of bone defect (i.e. bone defect at the enthesis or Haglund's deformity, which is a bony enlargement of the posterosuperior calcaneus), intratendinous calcifications, distal (insertional) tendinosis, midportion tendinosis, bursitis, and isolated paratenonitis (not associated with tendinosis) (Figures 1 and 2). Participants were able to be classified into multiple pathology categories. Both the injured side and contralateral side were examined. Prior studies have reported ultrasound imaging to be as good if not superior to MRI for tendon pathology27-33 as well as good agreement between radiograph and ultrasound for presence of bony defects at the enthesis.20
Figure 1.
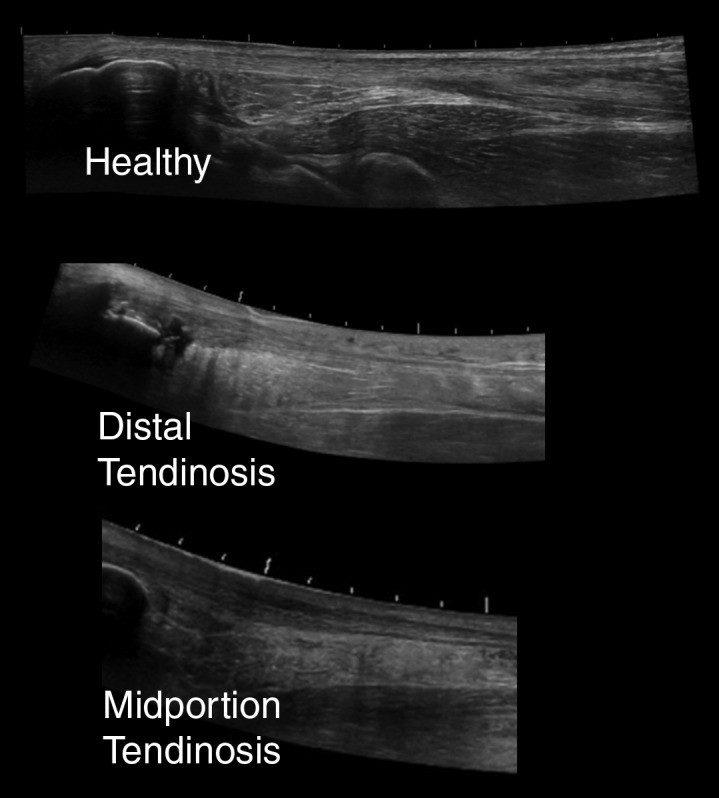
Representative figures of tendinosis on ultrasound using extended field of view settings.
Figure 2.
Representative figures of insertional pathology.
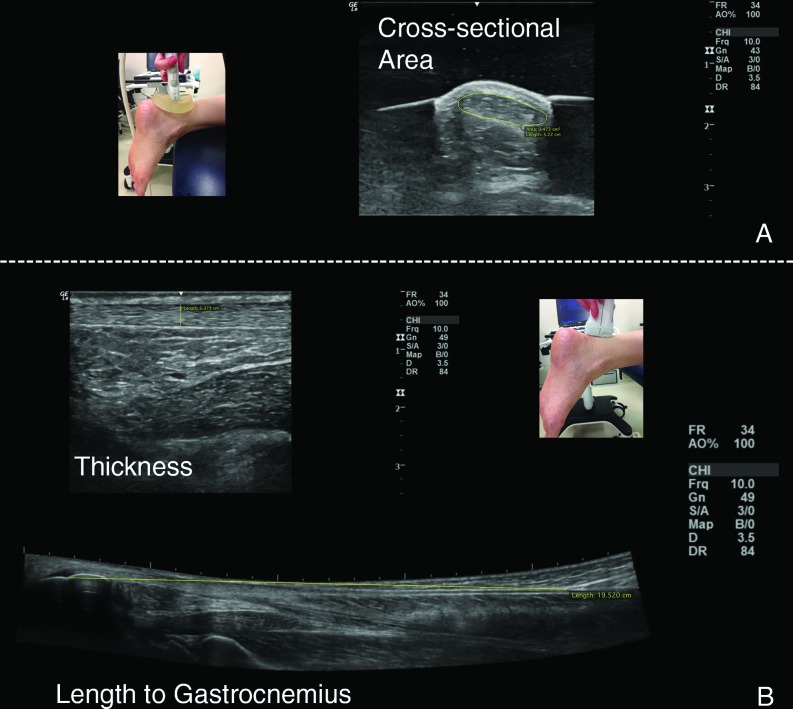
Quantitative analysis of tendon structure included three measures – tendon length, thickness, and cross sectional area (Figure 3). Tendon length was measured from the calcaneal notch to the gastrocnemius myotendinous junction using extended field of view settings.34,35 Both tendon thickness and cross sectional area were assessed at the area of the free tendon with greatest tendon thickness if tendinosis was present or at an area immediately distal to the soleus myotendinous junction if no tendinosis was present. Tendon thickness was measured in long axis from superior to deep fascial lines of the tendon,34 and cross sectional area was measured in short axis (Figure 3).
Figure 3.
Quantitative ultrasound measures (pictured is healthy tendon). A. Probe orientation and representative image of cross-sectional area. B. Probe orientation and representative images for thickness and length.
A prior study reported test-retest reliability for tendon length and thickness measures, with an Intraclass Correlation Coefficient (95% Confidence Interval) [ICC(95% CI)] of 0.944(0.852-0.979) and 0.898(0.728-0.962); standard error of measurement (SEM) of 0.7cm and 0.01cm; and group Minimal Detectible Change (MDC95%) of 0.43 cm and 0.01 cm respectively.34 The same study reported between limb SEM of 0.67 cm for tendon length and 0.02 cm for tendon thickness.34 Our lab has conducted test-retest reliability for tendon cross sectional area measures in 20 healthy individuals tested less than 30 minutes apart, showing an ICC(95% CI) of 0.986(0.964-0.994), SEM of 0.129 cm2, and group MDC95% of 0.009 cm2.
STATISTICAL METHODS
Descriptive statistics of the measures collected are reported, including means and standard deviations for continuous variables (i.e. VISA-A, BMI) and frequencies/percent of total sample for categorical variables (i.e. presence of a specific pathology). A chi-squared test was to compare the proportion of pathology identified for a tendon between the injured side and the contralateral side, which served as an internal control. Quantitative tendon morphology measures were compared between injured and contralateral sides using a paired t-test. Logistic regression was used to identify whether there was a relationship between BMI and age with a specific pathology. A priori level of significance was set at 0.05.
RESULTS
Fifty-six participants (25 male, 31 female) were included in this study. Demographic and symptomatology data are shown in Table 1. Thirteen participants (23%) reported bilateral symptoms. Seventy-five percent (42/56) of participants reported needing to change their activity level due to their Achilles tendon injury.
Table 1.
Participant demographics and symptomatology (Data from 56 participants unless otherwise noted).
| Variable | Mean(SD); Range |
|---|---|
| Age | 51.9(16.3); 19-79 years |
| Body Mass Index (n = 54) | 27.8(8.1); 17-52 kg/m2 |
| VISA-A Score (n = 55) | 49.2(21.8); 13-100 of 100 total points |
| Physical Activity Scale Score | 4.1(1.5); 1-6 of 6 total points |
VISA-A = Victorian Institute of Sport Assessment – Achilles questionnaire.
Descriptive and Quantitative Tendon Assessment
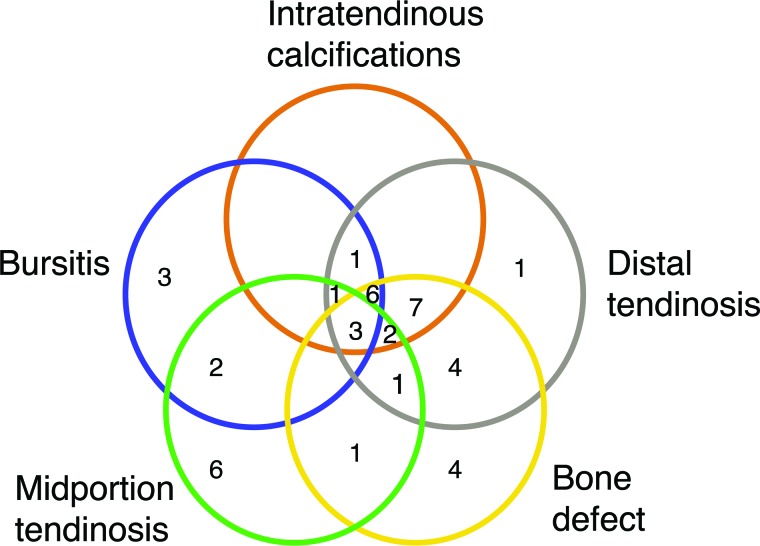
The frequency of pathology and results of chi-square test comparing proportion of pathology by side is presented in Table 2. Distribution of participants by pathology subgroup is displayed in Figure 4.
Table 2.
Number of participants with ultrasound pathology and quantitative ultrasound measures on injured and contralateral sides. Total number of participants = 56.
| Pathology | Injured Side | Contralateral Side | BothSides | NeitherSide | p-value |
|---|---|---|---|---|---|
| Bone Defect | 13 | 2 | 16 | 25 | <0.001 |
| Intratendinous Calcifications | 13 | 3 | 7 | 33 | 0.013 |
| Distal Tendinosis | 20 | 7 | 9 | 20 | 0.672 |
| Midportion Tendinosis | 8 | 1 | 8 | 39 | <0.001 |
| Bursitis | 16 | 4 | 3 | 33 | 0.594 |
| Quantitative Ultrasound Measures | |||||
| Tendon Length (cm) | 20.2(3.0) | 19.8(2.9) | - | - | 0.04 |
| Tendon Cross-sectional Area (cm2) | 0.85(0.38) | 0.72(0.39) | - | - | 0.04* |
| Tendon Thickness (cm) | 0.68(0.23) | 0.57(0.16) | - | - | < 0.001* |
Bolded values indicate p < 0.05. * indicates difference between sides that exceeds reported MDC of the measure.
Figure 4.
Venn diagram of combined pathologies on the injured side. Not pictured due to figure constraints: no pathology – 11/56; combined distal tendinosis and bursitis – 2/56; combined bone defect, distal tendinosis, and bursitis – 1/56. Where no value is displayed, the value is 0/56. Note that many participants presented with multiple, concomitant pathologies.
Quantitatively, the Achilles tendon on the injured side was thicker (mean difference = 0.11 cm, p < 0.001) and had greater cross sectional area (mean difference = 0.14 cm2, p = 0.042, n = 55) than the contralateral side (Table 1). While tendons on the injured side were significantly longer (mean difference = 0.38, p = 0.042), the mean difference between sides did not exceed the SEM and therefore may not be clinically meaningful (Table 2).
Relationship of BMI and Age on Tendon Pathology
Sequential logistic regression was used to test if BMI and age predict presence of bone deformity, intratendinous calcifications, and distal tendinosis. In the first block, BMI was entered into the model first, followed by age in the second block, and then their interaction term (age*BMI) in the third. For all outcomes, there was no statistically significant improvement in model fit by the addition of age or the interaction term. Results of the logistic regression are displayed in Table 3.
Table 3.
Results of logistic regression for presence of pathology predicted by BMI and age.
| Outcome variable | Predictor variables (p-value for variable in model) | p-value for model | Nagelkerke R2 | Percentage correctly classified | B |
|---|---|---|---|---|---|
| Bone deformity | BMI (p = 0.005) | 0.001 | 0.258 | 75.9 | 0.14 |
| Intratendinous calcifications | BMI (p = 0.020) | 0.013 | 0.149 | 70.4 | 0.09 |
| Distal tendinosis | BMI (p = 0.060) | 0.044 | 0.096 | 64.8 | 0.07 |
Bolded values indicate p < 0.05.
DISCUSSION
Individuals with insertional Achilles tendinopathy demonstrate more pathological findings on the injured side compared to the contralateral side. Furthermore, there are variations of underlying pathology, which presented concomitantly in 55% of cases. Presence of bone-related pathology at the insertion of the Achilles seems to be related to higher BMI. Despite just over half of participants having had imaging, there was an apparent mismatch in treatment strategy and underlying pathology.
The relationship between pathological findings on imaging of tendons and symptomatology has been widely debated, often citing concerns about abnormal findings in healthy individuals.36 The intention of the present study was to establish the frequency and types of pathology in symptomatic individuals, as an accurate diagnosis is important for appropriate management.11,37 To address concerns regarding presence of pathology in asymptomatic individuals, it was found that there were differences in the proportion of individuals with abnormal findings on their injured compared to contralateral sides. Additionally, 80% of the individuals included in this study had abnormal findings on their injured side and 48% on their contralateral side, compared to rates of 3-11%21,22 in asymptomatic individuals. It seems that symptoms and presence of pathology are connected within an individual.
The frequency of bone deformity in this study aligns well with a recent study investigating frequencies of pathology on MRI in individuals with Achilles-related complaints.19 On MRI, 59% of individuals were reported to have Haglund's deformity, compared to 52% with bone deformity in the present study. Higher frequencies of retrocalcaneal bursitis (63%19 compared to 24% in the present study) and lower rates of distal tendinosis (37%19 compared to 52% in the present study) were reported in the MRI study. Differences in rates of pathology may be due to inconsistencies in inclusion criteria between studies, as pathological findings on MRI were required for inclusion in that study.19 A prior study38 has also reported tendon thickening in individuals with insertional tendinopathy, which is consistent with the findings of the quantitative ultrasound measures included in the present study.
It does seem that both BMI and age play a role in demonstrating abnormalities on ultrasound in the context of insertional Achilles tendinopathy, with bone deformity, intratendinous calcifications, and distal tendinosis occurring more frequently in individuals with higher BMI and older age. Systematic review-level evidence has reported BMI to be an important factor contributing to Achilles tendinopathy,39 however, isolating the role of BMI is challenging. A study by Scott et al.,40 reported BMI and age to be the most significant factors in this population of individuals. In the current study, individuals with higher BMI also tended to be older, however, it seems that BMI has a stronger relationship to underlying pathology than age alone.
There are several limitations to this study. This study was of cross-sectional design and intended to capture the wide range of individuals affected by Achilles tendinopathy. Therefore, cause-effect relationships with regard to driving factors for pathology cannot be determined. While the observed rates of pathology well above what has been reported in asymptomatic individuals, it is possible that the pathology seen on ultrasound was not the primary cause of symptoms in these individuals. Individuals with bilateral symptoms were not excluded. While this complicates the interpretation of the study findings, it does represent the heterogenic population of individuals with insertional Achilles complaints. Finally, the ultrasound scans were not done in such a way to evaluate for plantaris involvement.
CONCLUSIONS
In summary, the findings of this study suggest that patients with insertional Achilles tendinopathy present with more abnormal findings on their injured side. Patients may present with multiple underlying pathologies and may, therefore, respond differently to treatment. From a treatment standpoint it may be beneficial to identify the underlying pathology to more appropriately tailor physical therapy and alternative treatment.34 Ultrasound may be a high quality, less expensive alternative to other imaging modalities. Physical therapists are uniquely situated to be able to use diagnostic ultrasound imaging during the course of a clinical examination – a clarification of scope of physical therapist practice recently supported by the American Institute of Ultrasound in Medicine. Further studies are needed to help clarify which of these subpathologies of Achilles tendinopathy respond best to physical therapy or are likely to need further intervention. In research settings, it may be helpful to differentiate participants based on underlying pathology to better understand response to interventional strategies. In clinical settings, it may be beneficial to incorporate ultrasound imaging as part of a comprehensive, clinical patient assessment in order to appropriately diagnose the affected structure and align treatment accordingly.
REFERENCES
- 1.Maffulli N Khan KM Puddu G. Time to change a confusing terminology. Arthrosopy. 1998;14(8):840-843. [DOI] [PubMed] [Google Scholar]
- 2.Paavola M Kannus P Järvinen T. Achilles tendinopathy. J Bone Jt Surg. 2002;84-A(11):2062-2076. [DOI] [PubMed] [Google Scholar]
- 3.Li H-Y Hua Y-H. Achilles tendinopathy: Current concepts about the basic science and clinical treatments. Biomed Res Int. 2016. 10.1155/2016/6492597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfredson H Spang C. Clinical presentation and surgical management of chronic Achilles tendon disorders - A retrospective observation on a set of consecutive patients being operated by the same orthopedic surgeon. Foot Ankle Surg. 2018;24(6):490-494. [DOI] [PubMed] [Google Scholar]
- 5.Solan M, Davies M. Management of insertional tendinopathy of the Achilles tendon. Foot Ankle Clin. 2007;12(4):597-615. [DOI] [PubMed] [Google Scholar]
- 6.Wiegerinck JI Kerkhoffs GM van Sterkenburg MN Sierevelt IN van Dijk CN. Treatment for insertional Achilles tendinopathy: A systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2013;21:1345-1355. [DOI] [PubMed] [Google Scholar]
- 7.Fahlström M Jonsson P Lorentzon R Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surgery, Sport Traumatol Arthrosc. 2003;11(5):327-333. [DOI] [PubMed] [Google Scholar]
- 8.Mafi N Lorentzon R Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sport Traumatol Arthrosc. 2001;9:42-47. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson CW Berlet GC Lee TH. Prediction of the success of nonoperative treatment of insertional Achilles tendinosis based on MRI. Foot Ankle Int. 2007;28(4):472-477. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y Zhang J Cai Y. Utility of ultrasonography in assessing the effectiveness of extracorporeal shock wave therapy in insertional Achilles tendinopathy. Biomed Res Int. 2016;2016. 10.1155/2016/2580969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caudell GMM. Insertional Achilles tendinopathy. Clin Podiatr Med Surg. 2017;34:195-205. [DOI] [PubMed] [Google Scholar]
- 12.Malliaras P Barton CJ Reeves ND Langberg H. Achilles and patellar tendinopathy loading programmes: A systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sport Med. 2013;43:267-286. [DOI] [PubMed] [Google Scholar]
- 13.Langberg H Ellingsgaard H Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sport. 2007;17:61-66. [DOI] [PubMed] [Google Scholar]
- 14.Chimenti RL Flemister AS Ketz J Bucklin M Buckley MR Richards MS. Ultrasound strain mapping of Achilles tendon compressive strain patterns during dorsiflexion. J Biomech. 2015;49(1):39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chimenti RL Bucklin M Kelly M, et al. Insertional Achilles tendinopathy associated with altered transverse compressive and axial tensile strain during ankle dorsiflexion. J Orthop Res. 2017;35(4):910-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rompe JD Nafe B Furia JP Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: A randomized controlled trial. Am J Sports Med. 2007;35(3):374-383. [DOI] [PubMed] [Google Scholar]
- 17.Rompe JD Furia J Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy: A randomized, controlled trial. J Bone Jt Surg. 2008;90(1):52-61. [DOI] [PubMed] [Google Scholar]
- 18.Wiegerinck JI Kok AC Van Dijk CN. Surgical treatment of chronic retrocalcaneal bursitis. Arthrosc - J Arthrosc Relat Surg. 2012;28(2):283-293. [DOI] [PubMed] [Google Scholar]
- 19.Bullock MJ Mourelatos J Mar A. Achilles impingement tendinopathy on magnetic resonance imaging. J Foot Ankle Surg. 2017;56(3):555-563. [DOI] [PubMed] [Google Scholar]
- 20.Chimenti RL Chimenti PC Buckley MR Houck JR Flemister AS. Utility of ultrasound for imaging osteophytes in patients with insertional Achilles tendinopathy. Arch Phys Med Rehabil. 2016;97(7):1206-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph F. M Anderson M. J Trojian H. T, et al. Incidence of morphologic changes in asymptomatic Achilles tendons in an active young adult population. J Sport Rehabil. 2012;21(3):249-252. [DOI] [PubMed] [Google Scholar]
- 22.Fredberg U Bolvig L. Significance of ultrasonographically detected asymptomatic tendinosis in the patellar and Achilles tendons of elite soccer players. Am J Sports Med. 2002;30(4):488-491. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk CN van Sterkenburg MN Wiegerinck JI Karlsson J Maffulli N. Terminology for Achilles tendon related disorders. Knee Surgery, Sport Traumatol Arthrosc. 2011;19:835-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinfeld SBB. Achilles tendon disorders. Med Clin North Am. 2014;98(2):331-338. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JM Cook JL Purdam C, et al. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimby G. Physcial activity and muscle training in the elderly. Acta Med Scand. 1986;711:233-237. [DOI] [PubMed] [Google Scholar]
- 27.Forney MC Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med. 2018;85(4):283-300. [DOI] [PubMed] [Google Scholar]
- 28.De Miguel E. New perspectives in enthesis ultrasound: Validation of enthesis fibrocartilage. Int J Clin Rheumtol. 2011;6(1):11-14. [Google Scholar]
- 29.De Miguel E Cobo T Muñoz-Femández S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68(2):169-174. [DOI] [PubMed] [Google Scholar]
- 30.Ficjan A Husic R Gretler J, et al. Ultrasound composite scores for the assessment of inflammatory and structural pathologies in psoriatic arthritis (PsASon-Score). Arthritis Res Ther. 2014;16(476). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balint P V. Terslev L Aegerter P, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann Rheum Dis. 2018;77:1730-1735. [DOI] [PubMed] [Google Scholar]
- 32.Tom S Zhong Y Cook R Aydin SZ Kaeley G Eder L. Development of a preliminary ultrasonographic enthesitis score in psoriatic arthritis — GRAPPA ultrasound working group. J Rheumatol. 2018. 10.3899/jrheum.171465. [DOI] [PubMed] [Google Scholar]
- 33.Jackson JB Chu CH Williams KA Bornemann PH. Normal ultrasonographic parameters of the posterior tibial, peroneal, and Achilles tendons. Foot Ankle Spec. 2018. 10.1177/1938640018800785. [DOI] [PubMed] [Google Scholar]
- 34.Silbernagel KG Shelley K Powell S Varrecchia S. Extended field of view ultrasound imaging to evaluate Achilles tendon length and thickness: A reliability and validity study. Muscles Ligaments Tendons J. 2016;6(1):104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan ED Rosenberg JG Scharville MJ Sobolewski EJ Thompson BJ King GE. Test-retest reliability and the minimal detectable change for Achilles tendon length: A panoramic ultrasound assessment. Ultrasound Med Biol. 2013;39(12):2488-2491. [DOI] [PubMed] [Google Scholar]
- 36.Ryan M Bisset L Newsham-West R. Should we care about tendon structureϿ. The disconnect between structure and symptoms in tendinopathy. J Orthop Sport Phys Ther. 2015;45(11):823-825. [DOI] [PubMed] [Google Scholar]
- 37.Wong GNL Tan TJ. MR imaging as a problem solving tool in posterior ankle pain: A review. Eur J Radiol. 2016;85(12):2238-2256. [DOI] [PubMed] [Google Scholar]
- 38.Chimenti RL Flemister AS Tome J, et al. Altered tendon characteristics and mechanical properties associated with insertional achilles tendinopathy. J Orthop Sports Phys Ther. 2014;44(9):680-689. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi F Papalia R Paciotti M, et al. Obesity as a risk factor for tendinopathy: A systematic review. Int J Endocrinol. 2014;2014:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott RT Hyer CF Granata A. The correlation of Achilles tendinopathy and body mass index. Foot Ankle Spec. 2013;6:283-285. [DOI] [PubMed] [Google Scholar]