Abstract
Invasive alien species threaten biodiversity worldwide and contribute to biotic homogenization, especially in freshwaters, where the ability of native animals to disperse is limited. Robotics may offer a promising tool to address this compelling problem, but whether and how invasive species can be negatively affected by robotic stimuli is an open question. Here, we explore the possibility of modulating behavioural and life-history responses of mosquitofish by varying the degree of biomimicry of a robotic predator, whose appearance and locomotion are inspired by natural mosquitofish predators. Our results support the prediction that real-time interactions at varying swimming speeds evoke a more robust antipredator response in mosquitofish than simpler movement patterns by the robot, especially in individuals with better body conditions that are less prone to take risks. Through an information-theoretic analysis of animal–robot interactions, we offer evidence in favour of a causal link between the motion of the robotic predator and a fish antipredator response. Remarkably, we observe that even a brief exposure to the robotic predator of 15 min per week is sufficient to erode energy reserves and compromise the body condition of mosquitofish, opening the door for future endeavours to control mosquitofish in the wild.
Keywords: animal personality, bioengineering, biomimetics, body condition, invasive species, predation risk
1. Introduction
The presence of animal species in areas where they are not native is common across the globe, with tremendous costs for both human activities and the ecological integrity of those areas [1,2]. Despite efforts from both governmental and academic institutions, existing methods for eradicating invasive alien species (IAS) or mitigating their negative effects remain labour-intensive, economically unviable, and, often, ineffective [3].
Freshwater animals are particularly vulnerable to IAS, whereby native species are confined to smaller water bodies and their ability to disperse is limited compared to other ecosystems [4]. Mosquitofish (Gambusia affinis, Baird and Girard, and Gambusia holbrooki, Girard) are among the most widely diffused freshwater IAS in the globe, and their negative impact on indigenous animal communities (via aggressive behaviours and/or predation [5–8]) has been recognized by the International Union for Conservation of Nature that listed mosquitofish among the world's hundred worst IAS [9].
Technical efforts to eradicate mosquitofish from water bodies and mitigate their negative impact on the native fauna are, however, limited. For example, increasing the structural complexity of the environment through artificial refugia was successful in reducing mortality in barrens topminnow (Fundulus julisia, Williams and Etnier) exposed to mosquitofish under laboratory settings, but beneficial effects from artificial refugia disappeared in the wild [10]. Similarly, the use of fish toxicants to combat the spread of invasive mosquitofish resulted in detrimental consequences for native fish [11]. The utilization of floating traps to target mosquitofish near the water surface has been shown to be a successful technique, but it is a labour-intensive process that can be pursued only in small sites and for short periods of time [12].
Robotics constitutes a promising tool for addressing some of these challenges, by offering a versatile, customizable, and consistent approach to modulate the behavioural response of live animals [13–15]. Particularly relevant are experiments that have shown the possibility of eliciting behavioural responses in freshwater fish through biologically inspired robots triggering a cost–benefit decision process [16–21]. The use of robotics in the study of predator–prey interactions might afford the design of new hypotheses-driven studies that could unfold the basis of fear and anxiety in prey fish [22–25] and illuminate the evolutionary consequences of nonlethal exposure to predators [26,27]. Just as robotics might bring new scientific insight into predator–prey interactions, it also contributes to ethics in animal experimentation by minimizing potential harm to live animals.
In particular, previous research efforts from our group indicate that a robotic fish can be designed to repel mosquitofish [28] and simultaneously attract non-invasive fish under laboratory settings [19]. The possibility to isolate fish from one species to another allows safeguarding non-invasive species from the aggressive attitudes of mosquitofish, thereby providing compelling evidence for the use of biologically inspired robots as a possible method for the selective control of mosquitofish in the wild. However, the technology to deploy autonomous robotic fish in a complex ecological environment to control the behaviour of mosquitofish is still in its infancy, calling for a scientifically principled understanding of how mosquitofish interact with biologically inspired robotic stimuli.
Mosquitofish can adjust their behavioural and life-history strategies in response to varying environmental conditions, especially in the attempt to minimize risks of predation [29]. Mosquitofish are typically less prone to take risks [30] and invest less in reproduction [31] and energy reserves [32] under predation risk than in more beneficial conditions, with plastic adjustments associated with predation risks that can eventually result in the whole body morphology of mosquitofish to be reshaped [33]. Visual cues represent the predominant factor for predator recognition in most freshwater fish [34], especially mosquitofish [28,35], and a growing literature has provided convincing evidence that visual cues from animated images [36–38] and biologically inspired robots [19,28] can be used to influence mosquitofish behaviour.
While experiments comparing mosquitofish behavioural response to computer-animated and robotic stimuli are presently lacking, evidence from other freshwater fish suggests that visual stimuli associated with a biologically inspired robotic predator might elicit a stronger response than computer-animated images [23]. Experiments in [23] have compared the fear response of zebrafish (Danio rerio, Hamilton) evoked by live predator fish, a robotic replica of the predator fish, and computer-animated images of the predator fish, determining that: (a) the robot caused a robust avoidance response in zebrafish that was comparable to that observed for live predators, while computer-animated images did not, and (b) individual responses were more consistent over time when zebrafish were exposed to the robot than to live predators and computer-animated images. In addition to these methodological observations, practical considerations towards future deployment in the wild favour the use of robots over computer-animated images. In fact, practicality challenges the use of computer-animated images in the wild, where it may be unfeasible to employ computer screens or projectors. Based on these methodological and practical aspects, we favour the use of robotic stimuli in place of computer-animated images.
In this study, we sought to test whether behavioural and life-history responses of mosquitofish could be modulated through a robotic predator whose visual appearance and locomotion were inspired by mosquitofish predators, the largemouth bass (Micropterus salmoides, Lacépède; figure 1). Largemouth bass coexist with mosquitofish in the wild and constitute their most common predators [39,40], with mosquitofish representing over 80% of the fish consumed by juvenile largemouth bass in their native environments [41]. Our biologically inspired robotic predator was designed to take advantage of the innate antipredator behaviour that largemouth bass induce in mosquitofish under laboratory settings [31,42]. We repeatedly exposed mosquitofish to robotic predators varying in their degree of biomimicry to disentangle the relative contribution of the robot swimming and its interactivity on both behavioural and life-history adjustments associated with antipredator responses in mosquitofish. We hypothesized that: (a) visual stimuli from the robotic predator would repel mosquitofish, as suggested in [28,36], (b) increasing the degree of biomimicry in the motion of the robot would increase antipredator behaviours and impact life-history strategies (i.e. energy reserves) in mosquitofish, and (c) individuals would differ from each other in the extent of their antipredator responses [43], with individuals with high future expectations (i.e. individuals with high energy reserves) being more risk-averse than others [44].
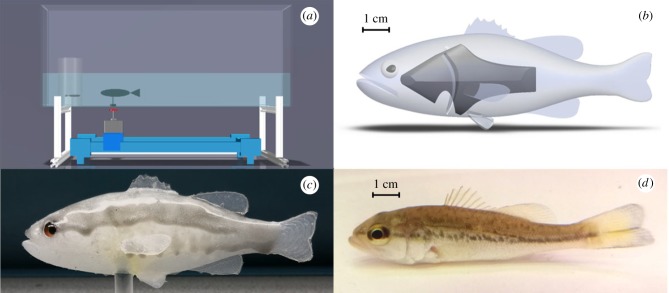
Figure 1.
Schematic for (a) the overview of experimental apparatus, (b) the three-dimensional representation of the biologically inspired predator replica, (c) the biologically inspired predator replica used for experiments and (d) a picture of a juvenile largemouth bass. (Online version in colour.)
From a methodological point of view, our study contributes to the state of the art in animal–robot interactions [13,14,45] along several research directions. First, we established a robotic platform that allows for tailoring the degree of complexity of the interaction through a closed-loop control system, integrating real-time tracking and high-precision robotics. Through this platform, we successfully varied the degree of biomimicry of the interactive robotic predator, by simulating random attacks towards the fish at either constant or increasing speed. This experimental manipulation effectively allowed for the quantification of the relative contributions of typical locomotory patterns of predators in triggering antipredator responses in mosquitofish. Second, we shied away from a rigid prototype, in favour of a soft robotic replica that incorporates a spine-like structure to promote natural oscillations that are reminiscent of body undulations, which are known to be critical for fish–robot interactions in the water [18,21]. Third, we integrated traditional means of behavioural analysis with modern elements of dynamical systems theory, through the information-theoretic framework of transfer entropy [46]. Through the lens of transfer entropy, we demonstrated an improved comprehension of the antipredator response of mosquitofish, by testing for potential cause-and-effect relationships between the motion of the robotic predator and mosquitofish antipredator response. Finally, although few recent studies have considered behavioural response of animals repeatedly confronted with robots [47,48], a detailed study of individual variation in mosquitofish behaviour was lacking, especially in the context of life-history consequences of the exposure to robotic stimuli.
Although focused on mosquitofish, the theoretical and methodological underpinnings of this work could inform research on other IAS, whose presence in the environment is also a threat to biodiversity and economy. For example, recent studies have demonstrated the possibility of using robots inspired by live predators to influence the behaviour of locusts (Locusta migratoria, Linnaeus), a major pest for human agricultural economies and ecosystems stability [49,50]. Similarly, the peregrine falcon-like robot Robird has been recently presented for deployment in the aviation industry to deter birds from flying in the vicinity of aircrafts [51].
2. Material and methods
2.1. Study organism and maintenance
A total of 150 wild-caught western mosquitofish (Gambusia affinis, Baird and Girard) were purchased from a commercial supplier (Carolina Biological Supply Co., Burlington, NC, USA) and were acclimatized for 1 day in stock tanks. Then, 75 focal individuals (average body length of 2.9 ± 0.3 cm) were randomly selected from stock tanks, with sick individuals and fish showing physical and/or behavioural anomalies excluded a priori.
Focal fish were housed individually in transparent Plexiglas cylinders (10 cm diameter), placed within a large housing tank (185 × 47 × 60 cm, length, width and height) and submerged in water for 10 cm, as in [29,52]. The lateral surface of the transparent cylinders was perforated to promote water circulation across separate cylinders, affording visual and chemical interaction among individuals despite physical isolation. This housing scheme prevented aggression, competition for resources, and sexual harassment among mosquitofish, with each cylinder marked with a unique identification code to facilitate the identification of individuals over time. The position of the cylinders was periodically randomized to allow visual and chemical interactions among all fish. Fish were acclimatized in the cylinders for one month before experiments, and they were housed in these cylinders for the whole duration of the study (approx. three months).
Fish were kept under a 12 h light/12 h dark photoperiod and fed with commercial flake food (Nutrafin max; Hagen Corp., Mansfield, MA, USA) once a day. Water parameters were checked daily, with temperature and pH maintained at 26°C and 7.2, respectively, throughout the study.
2.2. Experimental set-up
2.2.1. Experimental arena for behavioural tests
Behavioural trials were performed in an experimental arena (44 × 30 × 30 cm, length, width and height), filled with 10 cm of conditioned water (figure 1a). The walls and the bottom surface of the arena were covered with white opaque contact paper to control for external disturbance and optimize automated computer tracking of fish motion during trials. Two 38 W fluorescent tubes (All-Glass Aquarium, UK) were mounted 130 cm above ground and were used to provide homogeneous illumination to the apparatus. A high-resolution webcam (Logitech C920 webcam, Lausanne, Switzerland) was mounted 140 cm above the floor for a complete overview of the experimental arena.
2.2.2. Robotic platform and predator replica
The experimental arena was supported by aluminium T-slotted bars 29 cm above the ground to allow the placement of the robotic platform underneath (figure 1a). The platform allowed for manoeuvring the robotic replica along the 3 d.f.: 2 d.f. were controlled for in-plane translational motion of the replica and 1 d.f. served to adjust the predator body rotation. The replica was magnetically connected to the platform through a 3D-printed base made of polylactic acid filaments (3.2 cm × 1.0 cm × 0.6 cm length, width and height) containing two circular neodymium magnets (0.63 cm thick and 0.3 cm diameter) and an acrylic rod (4 cm length and 0.62 cm diameter; figure 1a–c). The in-plane translational motion was based on a Cartesian plotter (XY Plotter Robot Kit, Makeblock Co. Ltd, Shenzhen, China) and the body rotation was controlled via a stepper motor (NEMA 14, Pololu Corp., Las Vegas, NV, USA). Further details on the robotic platform are in the electronic supplementary material. The platform was originally designed in [53] to study zebrafish social behaviour and used in [54] to examine zebrafish learning.
Locomotory patterns of the predator replica were inspired by pilot tests performed on three juvenile largemouth bass (7.0 ± 0.5 cm), purchased from Teichwirtschaften Armin Kittner in Quitzdorf am See, Germany (https://www.teichwirtschaft-kittner.de/), before the beginning of the experiment (figure 1d). Live bass were placed individually in the experimental arena and their behaviour was recorded over 30 min. Swimming trajectories and swimming speeds were then obtained through an offline tracking software developed by our group [55]. Mean and maximum swimming speed measured in the pilot tests and a swimming trajectory representative of the bass behaviour in the experimental arena were used for the motion of the predator replica.
The morphology and coloration of the replica were also chosen to capture salient features of juvenile largemouth bass (figure 1b–d). Towards this aim, we took photos of the live bass from different angles and estimated their body dimensions using a dedicated software (ImageJ, National Institute of Health, Bethesda, Maryland, USA). The body morphology of the replica was accordingly modelled in Solidworks (Dassault Systèmes SolidWorks Corp., Waltham, Massachusetts, USA) to create a three-dimensional design and, then, a solid mould. A spine-like structure in polylactic acid filament material was 3D-printed and integrated within the 3D-printed mould of the predator replica together with two glass eyes, relatively smaller than in live bass (figure 1b). Then, the mould was filled with non-toxic and aquarium safe silicone (Dragon Skin 10 Medium, Smooth-On, Macungie, PA, USA) and let dry. The spine-like structure provided support to the weight of the silicone body of the replica and facilitated body oscillations during swimming. Lastly, the silicone body of the replica was hand-painted using non-toxic, aquarium safe and silicone-based light grey and silver paints (Smooth-On, Inc., Macungie, PA, USA) to mimic the characteristic coloration pattern of largemouth bass (figure 1c). Colour reflectance comparisons between live bass and its robotic replica were not performed. However, non-toxic pigments used to paint the body of the robotic replica have been shown to be effectively perceived as natural pigments in bluefin killifish (Lucania goodie, Jordan) [20], a freshwater fish with well-developed vision like mosquitofish.
The moulded silicone body with glass eyes and spine-like structure was attached to a clear acrylic rod, connected to 3D-printed base with magnets. The clear acrylic rod allowed for setting the swimming depth of the biologically inspired predator replica in the middle of the water column, i.e. where the antipredator response of mosquitofish is known to be the strongest [28].
2.2.3. Experimental conditions and live tracking
We designed a series of experimental conditions with robotic replicas varying their motion to proxy different degrees of biomimicry of live predators. In one control condition, the experimental fish were tested in the absence of the replica (no predator, NP). In a second control condition, the replica was motionless and positioned randomly within the arena before each trial started (predator motionless, PM). In the four experimental conditions where a swimming replica was employed, the replica swam on either the predetermined trajectory inspired by live bass (open-loop, OL) or it alternated between the predetermined trajectory and targeted real-time interactions (closed-loop, CL) with the focal fish. In two OL conditions, the biologically inspired predator replica followed the predetermined swimming trajectory, either at a varying speed based on the motion of the live predator (OL1) or at a constant speed (OL2). In condition OL2, the trajectory from the live bass was processed to manoeuvre the replica at a constant speed. Specifically, we locally fitted the trajectory using cubic splines (interparc, Copyright (c) 2012 John D'Errico) and placed equally spaced waypoints on the splines such that the replica would move at a constant speed. The constant speed was chosen to be 6 cm s−1 to match the mean speed observed in juvenile largemouth bass in our pilot tests and provide a dynamically rich visual stimulation for mosquitofish. The same speed was used as the mean value of the speed profile in condition OL1, consistently scaling experimental observations.
In the CL conditions, the replica, besides following swimming trajectories at a varying speed, was programmed to interact in real time with the focal fish and to perform simulated attacks at random. However, the replica always performed an attack every minute of the trial for a total of 15 attacks. During an attack, the replica either accelerated to attain a large speed (20 cm s−1; CL1) comparable to the maximum speed of live bass attacking a prey [56], or swam at a constant speed towards the fish (6 cm s−1; CL2).
When the replica was commanded to attack the focal fish, its motion was a function of the distance from the fish. For CL1 condition, if the distance between the fish and replica was less than 1 cm, the replica would only change its heading towards the direction of the focal fish and return to the original heading; for distances between 1 and 10 cm (inspection zone in [57]), the replica would change its heading, accelerate towards the fish at 20 cm s−2, and stop at approximately 1 cm from it; and for distances larger than 10 cm, the replica would change the heading, accelerate at 20 cm s−2 until reaching a speed of 20 cm s−1, and maintain this speed until stopping at 1 cm from the fish. For CL2 condition, if the distance between the fish and replica was less than 1 cm, the replica would only change its heading towards the direction of the focal fish and return to the original heading. For any distance greater than 1 cm, the replica would change its heading, and attack the fish with a constant speed of 6 cm s−1 and stop at 1 cm from the fish.
After an attack was completed, the replica returned to its original position prior to the attack and restarted swimming along the predetermined trajectory until the next attack. Notably, the region in which the robotic replica swam was smaller than the actual size of the experimental arena to allow at least 1 cm from the extremities of the replica's body (i.e. head and caudal fin) and the edges of the arena. This tolerance permitted smooth operation of the robotic platform and avoided collision with the walls of the arena. Further details on the real-time tracking system implemented for CL conditions are in the electronic supplementary material.
The custom-made software was calibrated on the exact size of an individual fish at each trial (week) separately and used to calculate the following quantities: distance moved (cm), time spent freezing (s), speed variance during swimming (cm2 s−2), mean distance from the predator replica (cm), predator inspection (counts) and time spent within one-body length from the wall (s)—i.e. thigmotaxis [58]. In particular, if a fish moved at a speed less than half of its body length per second for two consecutive seconds, it was considered as freezing [59]. Predator inspection was estimated according to standard protocols developed for guppies (Poecilia reticulata, Peters) [57], a poeciliid species closely related to mosquitofish. In particular, we counted the number of events that a fish voluntarily approached the predator replica by entering the 10 cm region around the replica while actively swimming in its direction, i.e. at an angle lower than ± 90° from the replica's head [57]. The distance from the wall used to estimate thigmotaxis was selected based on pilot tests in which mosquitofish were exposed to the same robotic predator replica used in this study. Details of data extraction and tracking system are in the electronic supplementary material.
Notably, reduced activity (in the form of short travelled distances and prolonged freezing) and large number of predator inspections, hesitancy in exploring open spaces that are unfamiliar and potentially dangerous (i.e. high thigmotaxis), and erratic swimming patterns dominated by high speed variance are typically associated with risk aversion and fearful states in animals [43], including mosquitofish [28,29,36,52,59].
2.3. Experimental procedure
Once a week over seven consecutive weeks, fish were anaesthetized in a solution of tricaine methanesulfonate (MS-222; 168 mg per 1 l H2O), sexed, and their body length (to the nearest 0.5 mm) and body weight (to the nearest 0.01 g) were measured. These measurements were conducted before the experiment started (baseline body measurements) and after the conclusion of each behavioural trial (week 1 to week 6). Fulton's condition factor K (weight length−3 104, g mm−3 104) [60] was then calculated as an index for the nutritional state (i.e. body condition) of each fish at each week.
In each trial, a mosquitofish was gently hand-netted and placed into an opaque cylinder in the experimental arena for 5 min to allow acclimatization to the set-up. During acclimatization, the motors of the robotic platform were turned off and fish had no visual contact with the apparatus outside the opaque cylinder. Then, the opaque cylinder was gently removed and the platform turned on, allowing the fish to explore the arena in either absence (NP) or presence of the biologically inspired predator replica (PM, OL1, OL2, CL1 and CL2 conditions) for 15 min. After the trial was completed, the fish was transferred back into its individual housing cylinder and the next trial was initiated.
The behaviour of each individual (n = 75) was tested once a week over six consecutive weeks, with individuals tested once per condition. An equal number of replicates were conducted for each condition. One week interval between two consecutive behavioural measurements is commonly adopted when testing individual variation in mosquitofish behaviour to minimize memory effects [29,52]. Experimental conditions were performed in a randomized order, but the NP condition was always performed last to mitigate bias on fish baseline behaviour caused by individuals being exposed to diverse degrees of predator threat, as observed in [42] for risk avoidance in mosquitofish. Fish were tested in a randomized order to exclude consistent differences in their behavioural outcome caused by hunger [61].
2.4. Statistical analysis
We initially tested whether body length, body mass and Fulton's K were correlated by estimating phenotypic correlations (i.e. the overall correlation attributable to between- and within-individual correlations) with bivariate linear mixed-effects models (LMMs), as suggested by Dingemanse & Dochtermann [62]. In these models, we specified the individual as the random effect (i.e. random intercepts) to account for repeated measures of the same individual across weeks. Body size was correlated with both mass and K, while mass and K were not correlated with each other (table S1). Therefore, we included both body mass and Fulton's K as fixed effects in the LMMs below, while body size was excluded from the models.
Since we were interested in testing whether mosquitofish antipredator response increased with an increased degree of biomimicry of the replica, we measured individual behaviour repeatedly across experimental conditions. We ran separate LMMs in which distance moved, freezing, speed variance, mean distance from the replica, predator inspection and thigmotaxis were included one-by-one as the dependent variables. In each model, individual identities were included as the random effect, while body mass, Fulton's K, sex, week and condition (i.e. the degree of biomimicry of the robotic predator) were entered as fixed effects. A significant effect of condition in a given model (or any other fixed effect included in that model) would indicate that condition explained a significant portion of the behavioural variance observed after accounting for the variation explained by the other fixed effects. The significance of individual differences was tested using both likelihood ratio tests (LRTs) and Akaike information criteria (AICs), where a full model including individual as a random effect was compared with a reduced model in which the random effect was excluded. Random intercepts represented the proportion of the total phenotypic variance not attributable to fixed effects that was explained by among-individual variance, i.e. differences in personality traits among individuals.
Building upon our previous work [15], we implemented the information-theoretic notion of transfer entropy to quantify the influence of the biologically inspired predator replica on the behaviour of the live fish and vice versa. Given two stochastic processes, transfer entropy quantifies the reduction in the uncertainty in the prediction of the future of one of the processes from its present due to additional knowledge about the other stochastic processes [63]. In this vein, a non-zero value of transfer entropy indicates a potential influence between the two processes [63]. Here, transfer entropy was computed on the time series of the speed of the replica and the mosquitofish, which were first down-sampled to 1 Hz to ensure that one time step (1 s) would suffice to encode the response time of the fish to the replica and vice versa. Therefore, a total of 904 points (904 s) were used per each trial. Then, we converted the time series into symbols depending on whether the speed increased or decreased between two consecutive time steps [64]. In agreement with [15], we computed the transfer entropy from the replica (R) to the fish (F) as follows:
where F and R are the down-sampled time series of the speeds, and Pr represents the probability mass function computed via plug-in estimation. By flipping F with R, we computed transfer entropy from the fish to the replica . Across the five experimental conditions in which the predator replica was employed, transfer entropy could only be used in OL1, CL1 and CL2, since the speed of the replica was constant in PM and OL2 and, therefore, encoded no meaningful information.
We expected information flow in OL1 to be one-directional, since the replica swam irrespective of the fish, which should be influenced by the swimming pattern of the replica. On the other hand, the information flow in CL1 and CL2 was expected to be two-directional, with the fish responding to the replica and the replica adjusting its attacks as a function of the behaviour of the fish. For each of the three conditions (OL1, CL1 and CL2), we obtained surrogate data from all the possible shuffling (74 × 74) of the identities of the fish and the replica within each condition. For each of these shuffling, we randomly selected 74 values without repetitions to obtain a mean transfer entropy value; this process was repeated 20 000 times to obtain a surrogate distribution. To ascertain an influence through transfer entropy, we tested whether the corresponding experimental value was in the right tail of the distribution. This process was conducted six times, twice for each of the three conditions to examine information flow in either direction (fish to robot or robot to fish, similar to [65]).
We then tested whether transfer entropy differed across conditions and between directions ( and ). Therefore, we built an LMM with transfer entropy as the dependent variable, the direction of information flow, condition, and their interaction as fixed effects, and both individual identities (fish identity) and pair identities (fish and replica identities) included as random intercepts. As for the LMMs on behavioural traits described above, the significance of random intercepts (both individual and pair identities) was tested using LRTs and AICs.
Lastly, we were interested in testing whether fish energy reserves (Fulton's K) varied in response to the exposure to robotic predator replicas. Towards this aim, we built an LMM with Fulton's K as the dependent variable, including individuals' identity as the random effect (i.e. random intercepts) and sex, week and condition (i.e. the degree of biomimicry of the robotic predator) as fixed effects. We then tested whether the behavioural variation observed across conditions reflected variation in Fulton's K. Based on our initial hypothesis and findings from behavioural analyses, experimental conditions were consolidated in three categories: controls (K measured before the experiment started, after tests performed in the absence of the predator replica, and after the exposure to the predator motionless; baseline, NP and PM, respectively), low degree of biomimicry (OL1, OL2 and CL2), and high degree of biomimicry (CL1). Variation in K was then tested with an LMM, in which Fulton's K was the dependent variable, individuals' identities the random effect (i.e. random intercepts), and sex, weeks and condition category the fixed effects.
Data analyses were performed in R v.3.5.1 [66] using the ‘lme4', ‘nlme', ‘lmerTest' and ‘MCMCglmm' packages [67–70], estimated marginal means (EMMs) based on univariate models and post hoc comparisons were performed with ‘emmeans' adjusted for simultaneous inference with the mvt method [71], while permutation tests for transfer entropy analysis were conducted in Matlab (R2018a; MathWorks, Natick, MA, USA [72]). Prior to all analyses, speed variance was log-transformed to normalize error distribution in the model's residuals. Except for the permutation test that is independent by error distributions, we assumed Gaussian error distributions that were confirmed for all response variables after visual inspection of model residuals. The significance level was set at α < 0.05.
3. Results
Behaviour was strongly dependent on the experimental condition in which mosquitofish were tested after controlling for variation explained by week (see results from the LMMs in table 1). The distance between the fish and the replica decreased when the replica was allowed to swim in the arena with respect to the condition PM where it was held in place (p < 0.001 in pairwise comparisons between PM and any other experimental condition; electronic supplementary material, figure S1). This was especially evident when attacks were performed in real time by accelerating towards the fish and the interactive nature of the replica buffered fish attempts to be away from it (p < 0.001 in pairwise comparisons between CL1 and OL1, OL2 and CL2; electronic supplementary material, figure S1).
Table 1.
Analysis of variance with Satterthwaite's method from linear mixed models with distance moved, freezing, speed variance, mean distance from replica, predator inspection, thigmotaxis and transfer entropy as dependent variables. Fulton's K, body mass, sex, week and condition are included in all models as fixed factors, except for transfer entropy in which condition, direction, and their interaction were included as fixed factors. The individual is included as a random effect (i.e. random intercepts) in all models, while pair (fish–robot) is included as a second random effect in the transfer entropy model, to account for repeated measures. The significance was set at α < 0.05, and significant results are indicated with * (less than 0.05), ** (less than 0.01) and *** (less than 0.001).
| fixed factors | mean square | d.f. | F | p-value |
|---|---|---|---|---|
| distance moved (cm) | ||||
| K | 2 801 434 | 1, 424 | 3.567 | 0.059 |
| mass | 239 961 | 1, 121 | 0.3055 | 0.581 |
| sex | 2 306 162 | 1, 78 | 2.936 | 0.091 |
| week | 82 525 615 | 1, 379 | 105.080 | <0.001*** |
| condition | 32 234 416 | 5, 367 | 41.044 | <0.001*** |
| freezing (s) | ||||
| K | 11 392 | 1, 340 | 0.359 | 0.549 |
| mass | 57 781 | 1, 96 | 1.821 | 0.180 |
| sex | 85 910 | 1, 75 | 2.708 | 0.104 |
| week | 3 161 017 | 1, 386 | 99.631 | <0.001*** |
| condition | 735 543 | 5, 367 | 23.183 | <0.001*** |
| speed variance (cm2 s−2) | ||||
| K | 3.376 | 1, 234 | 7.044 | 0.008** |
| mass | 0.047 | 1, 85 | 0.099 | 0.754 |
| sex | 0.027 | 1, 74 | 0.056 | 0.814 |
| week | 3.881 | 1, 389 | 8.099 | 0.005** |
| condition | 8.935 | 5, 368 | 18.644 | <0.001*** |
| distance from replica (cm) | ||||
| K | 62.46 | 1, 186 | 3.784 | 0.053 |
| mass | 0.390 | 1, 77 | 0.024 | 0.878 |
| sex | 0.06 | 1, 73 | 0.003 | 0.954 |
| week | 0.59 | 1, 315 | 0.036 | 0.850 |
| condition | 343.970 | 4, 291 | 20.837 | <0.001*** |
| predator inspection (counts) | ||||
| K | 199.50 | 1, 288 | 3.025 | 0.083 |
| mass | 66.50 | 1, 80 | 1.008 | 0.318 |
| sex | 212.90 | 1, 74 | 3.227 | 0.076 |
| week | 441.80 | 1, 314 | 6.698 | 0.010* |
| condition | 5539.30 | 4, 291 | 83.977 | <0.001*** |
| thigmotaxis (s) | ||||
| K | 59 307 | 1, 404 | 2.944 | 0.087 |
| mass | 23 814 | 1, 107 | 1.182 | 0.279 |
| sex | 104 813 | 1, 75 | 5.202 | 0.025* |
| week | 232 953 | 1, 380 | 11.562 | <0.001*** |
| condition | 291 277 | 5, 365 | 14.457 | <0.001*** |
| transfer entropy (bits) | ||||
| condition | <0.001 | 2, 146 | 0.514 | 0.599 |
| direction | <0.001 | 1, 219 | 49.516 | <0.001*** |
| condition × direction | <0.001 | 2, 219 | 5.262 | 0.006** |
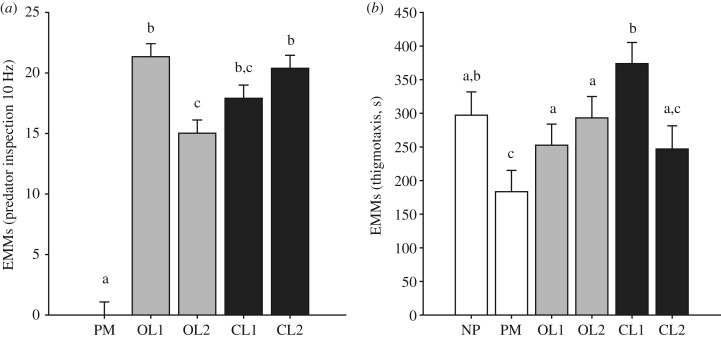
On the contrary, fish tendency to inspect the predator replica did not vary across swimming replicas, i.e. the number of inspections in CL1 was undistinguishable from OL1, OL2 and CL2 (figure 2a). Accordingly, fish swam on average longer distances, varied their swimming speed more, and froze less when exposed to a swimming replica than in control conditions (p < 0.001 in pairwise comparisons between NP and PM confronted with any other experimental condition, except for speed variance and freezing between NP and CL1 and between PM and CL1, respectively; electronic supplementary material, figure S1).
Figure 2.
Estimated marginal mean (EMM) differences represent adjusted mean differences (+s.e.) in predator inspection (a) and thigmotaxis (b) across conditions once the contribution of fixed effects included in the model (i.e. Fulton's K, body mass, sex, week) is accounted for, except sex that was excluded in EMMs for predator inspection to preserve positive values in PM condition and favour the interpretation while not altering results. White histograms correspond to control conditions (NP and PM), light grey histograms to open-loop conditions (OL1 and OL2), and dark grey histograms to closed-loop conditions (CL1 and CL2). NP condition is not shown for predator inspection (a) since fish were tested in the absence of the predator replica. Means not sharing a common superscript are significantly different. The significance was set at α < 0.05.
Thigmotaxis increased with increasing biomimicry in the replica's motion, whereby the time interval spent in the proximity of the walls was longer when fish were exposed to a replica varying its attacking speed in real time (CL1) than other replicas (p < 0.001 in pairwise comparisons between CL1 and any other experimental condition in which a robotic replica was employed), with the shortest time observed in the presence of the motionless replica (PM; figure 2b). On the other hand, behavioural responses of fish exposed to an attacking replica that swam at a constant speed (CL2) were comparable with those observed in OL conditions (OL1 and OL2), consistently across all measured traits (figure 2; electronic supplementary material, figure S1).
The variation in body condition (Fulton's K) among individuals was a significant predictor for the variation in their behavioural response across conditions (see results from the LMMs in table 1). In particular, individuals with more energy reserves varied their swimming speed more (i.e. exhibited higher speed variance) in response to the replica and an analogous role of K was also noted, albeit not significant, with respect to distance moved, distance from the replica, predator inspection and thigmotaxis (table 1). Accordingly, individuals with higher K tended to swim longer distances, maintained larger distances from the replicas, inspected the replicas less, and spent more time in the proximity of the walls. Nevertheless, we registered consistent among-individual variance in all traits after that behavioural variation explained by the model predictors was accounted for, i.e. fish differed in personality traits (see results from the LMMs in table 2), except for the mean distance from the replica and the individual intercepts for the transfer entropy.
Table 2.
Results from general linear mixed models with distance moved, freezing, speed variance, mean distance from replica, predator inspection, thigmotaxis and transfer entropy as dependent variables. Fulton's K, body mass, sex, week and condition are included in all models as fixed factors, except for transfer entropy in which condition, direction, and their interaction were included as fixed factors. Random intercepts are included for each individual in all models, while random intercepts for each pair (fish–robot) are included for transfer entropy only, which allowed variance decomposition. Within-individual variance (Vwithin), among-individual variance (Vamong) and repeatability are shown for each behavioural trait. Test statistics () and significant levels of the random effects (i.e. intercepts) were estimated using LRTs (p) and AICs between the full and the null model. Note that ΔAIC corresponds to the difference in AIC between the null models minus the AIC from the full model. The significance was set at α < 0.05, and significant results are indicated with * (less than 0.05) and *** (less than 0.001).
| variance components | Vwithin | Vamong | repeatability | ΔAIC | p-value | |
|---|---|---|---|---|---|---|
| distance moved (cm) | 785 359 | 599 257 | 0.433 | 118.770 | 120.769 | <0.001*** |
| freezing (s) | 31 727 | 9038 | 0.222 | 33.978 | 35.977 | <0.001*** |
| speed variance (cm2 s−2) | 0.479 | 0.035 | 0.069 | 2.322 | 4.322 | 0.038* |
| distance from replica (cm) | 16.507 | 0.482 | 0.028 | 1.461 | 0.539 | 0.463 |
| predator inspection (counts) | 65.960 | 19.270 | 0.226 | 25.019 | 27.019 | <0.001*** |
| thigmotaxis (s) | 20 148 | 11 185 | 0.357 | 80.800 | 82.800 | <0.001*** |
| transfer entropy—individual (bits) | <0.001 | <0.001 | 0.172 | 1.7 | 3.734 | 0.053 |
| transfer entropy—pair (bits) | <0.001 | <0.001 | 0.455 | 42.100 | 44.068 | <0.001*** |
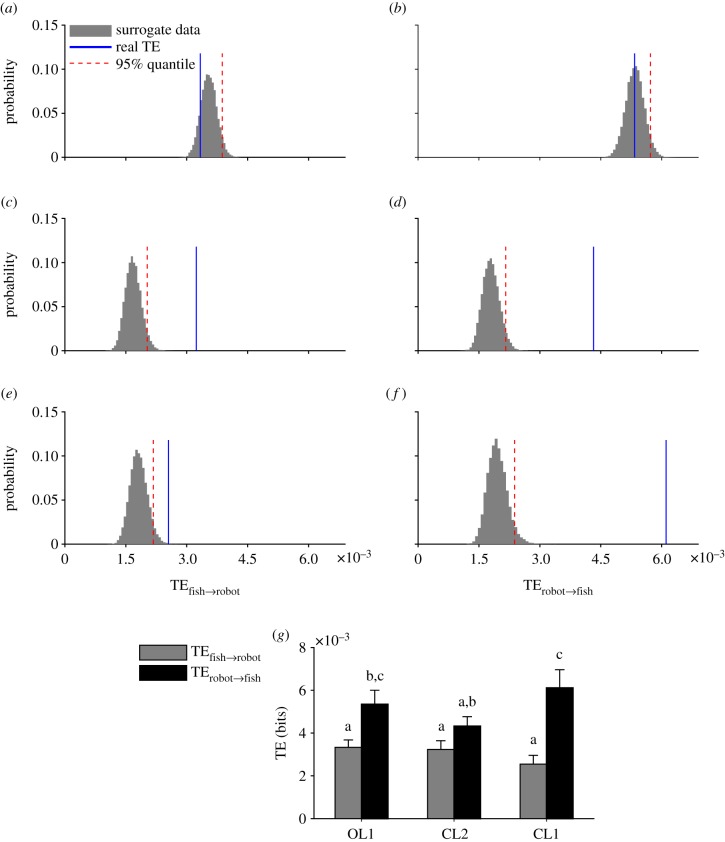
We failed to identify an information transfer flow in the OL condition OL1 in both directions (i.e. from the robot to the fish and vice versa; figure 3a,b). On the contrary, a significant information transfer was observed in both directions in CL1 (figure 3c,d) and CL2 (figure 3e,f). When comparing information transfers within conditions, we observed that transfer entropy from the robot to the fish in the OL condition OL1 was higher than from the fish to the robot (p = 0.003; figure 3g), in agreement with our expectations on the one-directional nature of the interaction in OL1. Transfer entropy in the closed-loop condition CL1 was also larger from the robot to the fish than in the opposite direction (p < 0.001), while transfer entropy in CL2 was comparable between directions (figure 3g). Importantly, the effect of the replica on fish behaviour was stronger in CL1 than in CL2 (p = 0.042; figure 3g), while other pairwise comparisons were not significant. In other words, the biologically inspired robotic predator interacting with mosquitofish in real time and accelerating towards the fish (CL1) was more effective in eliciting antipredator responses in mosquitofish than when it attacked at a constant speed (CL2).
Figure 3.
Transfer entropy between fish and robotic replicas. Transfer entropy from fish to robot is represented in (a,c,e) and from robot to fish in (b,d,f) with respect to conditions OL1 (first row), CL2 (second row) and CL1 (third row). Transfer entropy in both directions (fish-to-robot and robot-to-fish) for each of the three conditions is represented in (g) (+s.e.). Means not sharing a common superscript are significantly different. The significance was set at α < 0.05. (Online version in colour.)
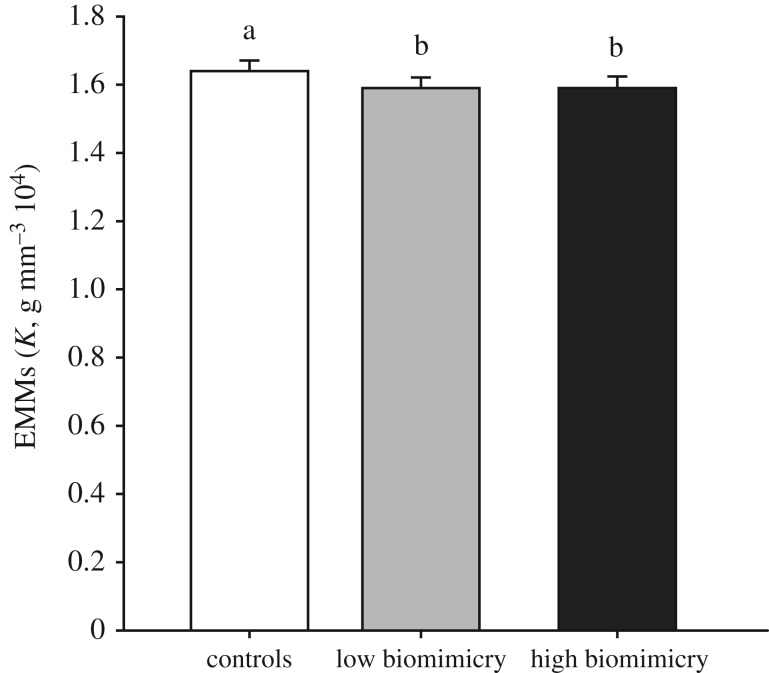
We also found that body condition (Fulton's K) varied across experimental conditions (see results from the LMM in electronic supplementary material, table S2), with K significantly lower after fish faced the predator replicas than after fish were tested in the absence of the replica (p < 0.001 in pairwise comparisons between NP and any other experimental condition; electronic supplementary material, figure S2). The decrease in K after exposure to the replica (p < 0.001 in pairwise comparisons between controls and replicas with either low and high biomimicry; figure 4) appeared, however, to be independent of the degree of biomimicry of the replica (non-significant pairwise comparison between low and high biomimicry; figure 4).
Figure 4.
Estimated marginal mean (EMM) differences represent adjusted mean differences (+s.e.) in Fulton's condition factor K across conditions once the contribution of fixed effects included in the model (i.e. sex and week) is accounted for. The white histogram corresponds to controls (baseline, NP and PM), the light grey histogram to replicas with low biomimicry (OL1, OL2 and CL2), and the dark grey histogram to replicas with high biomimicry (CL1). Means not sharing a common superscript are significantly different. The significance was set at α < 0.05.
4. Discussion
Here, we have disentangled the relative contributions of swimming pattern and closed-loop control of an interactive robotic predator on the antipredator behavioural response and life-history strategies in mosquitofish. Fish thigmotaxis increased with the degree of biomimicry in the motion of the replica, suggesting that integrating real-time feedback from mosquitofish position in the control of a replica interacting at increasing speed plays a key role in eliciting antipredator response in mosquitofish. The quantification of the information flow between the replica and fish supported the existence of a causal relationship between fish antipredator response and the motion of the biologically inspired replica. We also observed that individual behaviour was relatively predictable, with variations in energy reserves explaining a large portion of the behavioural variance observed among mosquitofish. Notably, energy reserves decreased after fish were exposed to the biologically inspired robot only 15 min per week, but variation in energy reserves did not depend on the degree of biomimicry in the motion of the replica.
After the initial detection of a potential predator, a fish typically identifies and assesses the threat based on cues from its natural predators [73]. The extent of an antipredator response is determined from the trade-off between minimizing risk of predation and energy consumption towards survival and reproduction [74], such that greater threats produce stronger avoidance [75]. Here, we provide experimental evidence that swimming patterns represent a salient source of information for predator recognition in mosquitofish that regulate the extent of their antipredator response. This evidence is based on highly controllable experiments that employ a state-of-the-art robotic predator replica, whose visual appearance and swimming pattern were inspired by measurements on juvenile largemouth bass, the main predator of mosquitofish in the wild [39–41]. Not only did the robotic replica allow for controlling the swimming speed and acceleration of the predator stimulus, but also it afforded the implementation of controlled attacks towards mosquitofish to study their antipredator response in real time. By opting for a robotics-based platform in lieu of a live predator, we were able to exclude potential correlations between antipredator response of mosquitofish and inherent biological variations in the predator behaviour (i.e. idiosyncrasies with focal individuals, fatigue and hunger) that could confound hypothesis testing.
The more robust antipredator behavioural response was registered when mosquitofish were exposed to a replica swimming at a varying speed and performing targeted, fast attacks. Reducing the degree of biomimicry towards a replica that performed attacks in real time at a constant speed resulted into a weaker antipredator behavioural response, similar to that registered with non-interactive replicas that followed predetermined swimming trajectories. This evidence aligns with prediction from the literature positing that speed and acceleration should play a key role on prey–predator interactions in fish [76]. Our information-theoretic analysis of the interaction between the robotic replica and the fish suggests the presence of a cause–effect relationship underlying the antipredator behavioural response of mosquitofish, which confirms the expected link between a predator's attacking speed and fish behavioural response [77]. More specifically, we determined that the uncertainty in the prediction of the future speed of mosquitofish from its present speed was reduced due to additional knowledge about the speed of the replica, such that the motion of the replica encoded valuable information about the behaviour of mosquitofish.
Beyond the analysis of the mean behavioural response at the population level, we discovered that a relatively short exposure to the biologically inspired robotic predator (only 15 min per week) resulted in a substantial reduction in the whole body condition of mosquitofish (index of fat reserves for a given body size; condition factor K) that did not depend on the swimming pattern of the robot. Recent evidence from multiple populations of mosquitofish in the wild has shown that the condition factor K in mosquitofish decreased on average 5.8% over a five month period in response to severe environmental challenges associated with water pollution [78]. Here, we observed that the body condition declined 3.1% over a week, after mosquitofish were exposed once to a predator replica, thereby suggesting a hidden effect of the robot on mosquitofish life-history adjustments. This finding aligns with evidence of nonlethal effects of predator–prey interactions [26], whereby costs of antipredator responses extend to ecologically relevant traits beyond behaviour, such as physiology and body condition [79].
In fact, theory predicts that stress responses affect the way animals allocate resources to fuel emergency functions [79], with animals investing relatively more energy in survival (i.e. escaping from the predator) and relatively less in future reproduction (i.e. energy reserves) with increasing predation risk [80]. With respect to mosquitofish, nonlethal effects of predator exposure have been found to lower their body condition, ultimately leading to lower fertility and fecundity rates [31]. Under this perspective, evidence from this study indicates that a relatively brief exposure to a biologically inspired robotic predator compromised the body condition of mosquitofish. Notably, the body condition increased again when mosquitofish were tested in the arena in the absence of the replica, indicating that variation in body condition resulted from the exposure to the robotic predator rather than other factors (for example, time, exposure to the arena and handling of the fish).
At the individual level, we found that fish differed consistently from each other in the extent of their antipredator response across six repeated exposures to robotic predators varying in their degree of biomimicry (i.e. fish differed in personality traits [43]). While the presence of personality variation among mosquitofish is well documented in the literature (see for example [29,52,59] and references therein), this study offers evidence that meaningful variation in antipredator response among mosquitofish can be successfully captured using robotic stimuli. Interestingly, a large portion of the variance in the antipredator response observed among mosquitofish was explained by variation in their body condition. In particular, individuals in better body conditions varied their swimming speed more in response to the robotic predator, tended to swim longer distances, maintained larger distances and inspected less the replicas, and spent more time in the proximity of the wall than mosquitofish in poorer body conditions. Individuals can trade-off survival at the cost of future reproduction, but the antipredator behavioural response of an individual should also depend on its body condition [81] as the reproductive value is condition-dependent. In this vein, our results are in agreement with predictions from the life-history theory that individuals with high future expectations (i.e. individuals with high energy reserves) should systematically be more risk-averse than others [81]. Therefore, our findings suggest that antipredator behavioural response towards robotic predator fish differs at the individual level in a relatively predictable manner.
This study contributes to the state of the art on the modulation of the behaviour of invasive and pest species through the use of predator-mimicking robotic fish [19,28], supporting the technological evolution of pest control agents, along similar line of development as insects [50] and birds [51]. Specifically, we aimed at the precise quantification of granular features of predator locomotion on antipredator responses of invasive mosquitofish through the development of a state-of-the-art robotic predator whose swimming characteristics can be controlled across a continuum range of biomimicry. Our findings build on previous research efforts on the modulation of mosquitofish behaviour through biologically inspired robots, shedding light on the role of the robot morphology on mosquitofish behaviour [28] and addressing the differential response of mosquitofish and zebrafish to robots [19]. In particular, we demonstrated that a biologically inspired robotic predator swimming at a varying speed and performing targeted attacks elicits a strong antipredator behavioural response that erodes energy reserves and compromises the body condition of mosquitofish. We propose that further efforts should test whether biologically inspired robots can effectively represent a novel, autonomous, and effective solution to contrast the negative impact of invasive mosquitofish on freshwater ecosystems worldwide [5–9].
Supplementary Material
Acknowledgements
The authors are grateful to Yanpeng Yang for his support with the setting of the robotic platform, Shinnosuke Nakayama for advising on the statistics, and the anonymous reviewers for their constructive feedback that has helped improve the work and the presentation.
Ethics
Experiments were performed in accordance with relevant guidelines and regulations and were approved by the University Animal Welfare Committee (UAWC) of New York University under the protocol number 13-1424. Notably, pilot tests on predator fish were approved through an animal care permit (G 0074/15) granted by the Landesamt für Gesundheit und Soziales Berlin (LAGeSo) and performed in Germany.
Data accessibility
All data can be found at https://figshare.com/s/1e755aeba5b781f753a9.
Authors' contributions
G.P. and M.P. conceived the research question and supervised the research. G.P. designed the experiment. G.P., V.R.S. and C.S. developed the experimental set-up. M.K., V.R.S. and C.S. conducted the experiments. G.P. and M.K. analysed the data and all authors discussed the results. G.P. and M.K. wrote a first draft of the Material and Methods section. G.P. and M.P. wrote the manuscript. All the authors reviewed the final draft.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation under grant nos. CMMI-1433670 and CMMI-1505832 and by the Forrest Research Foundation.
References
- 1.Pimentel D, Zuniga R, Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288. ( 10.1016/j.ecolecon.2004.10.002) [DOI] [Google Scholar]
- 2.Raven PH, Johnson GB. 1992. Biology, 3rd edn St Louis, MO: Mosby Year Book. [Google Scholar]
- 3.Byers JE, et al. 2002. Directing research to reduce the impacts of nonindigenous species. Conserv. Biol. 16, 630–640. ( 10.1046/j.1523-1739.2002.01057.x) [DOI] [Google Scholar]
- 4.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 5.Pyke GH. 2005. A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fisheries 15, 339–365. ( 10.1007/s11160-006-6394-x) [DOI] [Google Scholar]
- 6.Pyke G. 2008. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 39, 171–191. ( 10.1146/annurev.ecolsys.39.110707.173451) [DOI] [Google Scholar]
- 7.Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. 2008. Fish invasions in the world's river systems: when natural processes are blurred by human activities. PLoS Biol. 6, e28 ( 10.1371/journal.pbio.0060028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kats LB, Ferrer RP. 2003. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distributions 9, 99–110. ( 10.1046/j.1472-4642.2003.00013.x) [DOI] [Google Scholar]
- 9.Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world's worst invasive alien species: a selection from the global invasive species database, vol. 12 Auckland, New Zealand: Invasive Species Specialist Group. [Google Scholar]
- 10.Westhoff JT, Watts AV, Mattingly HT. 2013. Efficacy of artificial refuge to enhance survival of young Barrens topminnows exposed to western mosquitofish. Aquat. Conserv. Marine Freshwater Ecosyst. 23, 65–76. ( 10.1002/aqc.2265) [DOI] [Google Scholar]
- 11.Willis K, Ling N. 2000. Sensitivities of mosquitofish and black mudfish to a piscicide: could rotenone be used to control mosquitofish in New Zealand wetlands? N.Z. J. Zool. 27, 85–91. ( 10.1080/03014223.2000.9518214) [DOI] [Google Scholar]
- 12.Brookhouse N, Coughran J. 2010. Exploring the potential for an ecology-specific, physical control method of the exotic pest mosquitofish, Gambusia holbrooki. Ecol. Manage. Restor. 11, 226–228. ( 10.1111/j.1442-8903.2010.00556.x) [DOI] [Google Scholar]
- 13.Romano D, Donati E, Benelli G, Stefanini C. 2018. A review on animal–robot interaction: from bio-hybrid organisms to mixed societies. Biol. Cybern. 113, 201–225. ( 10.1007/s00422-018-0787-5) [DOI] [PubMed] [Google Scholar]
- 14.Krause J, Winfield AFT, Deneubourg JL. 2011. Interactive robots in experimental biology. Trends Ecol. Evol. 26, 369–375. ( 10.1016/j.tree.2011.03.015) [DOI] [PubMed] [Google Scholar]
- 15.Porfiri M. 2018. Inferring causal relationships in zebrafish-robot interactions through transfer entropy: a small lure to catch a big fish. Anim. Behav. Cogn. 5, 341–367. ( 10.26451/abc.05.04.03.2018) [DOI] [Google Scholar]
- 16.Romano D, Benelli G, Donati E, Remorini D, Canale A, Stefanini C. 2017. Multiple cues produced by a robotic fish modulate aggressive behaviour in Siamese fighting fishes. Sci. Rep. 7, 4667 ( 10.1038/s41598-017-04840-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano D, Benelli G, Hwang JS, Stefanini C. 2019. Fighting fish love robots: mate discrimination in males of a highly territorial fish by using female-mimicking robotic cues. Hydrobiologia 833, 185–196. ( 10.1007/s10750-019-3899-6) [DOI] [Google Scholar]
- 18.Polverino G, Phamduy P, Porfiri M. 2013. Fish and robots swimming together in a water tunnel: robot color and tail-beat frequency influence fish behavior. PLoS ONE 8, e77589 ( 10.1371/journal.pone.0077589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polverino G, Porfiri M. 2013. Zebrafish (Danio rerio) behavioural response to bioinspired robotic fish and mosquitofish (Gambusia affinis). Bioinspir. Biomim. 8, 044001 ( 10.1088/1748-3182/8/4/044001) [DOI] [PubMed] [Google Scholar]
- 20.Phamduy P, Polverino G, Fuller RC, Porfiri M. 2014. Fish and robot dancing together: bluefin killifish females respond differently to the courtship of a robot with varying color morphs. Bioinspir. Biomim. 9, 036021 ( 10.1088/1748-3182/9/3/036021) [DOI] [PubMed] [Google Scholar]
- 21.Marras S, Porfiri M. 2012. Fish and robots swimming together: attraction towards the robot demands biomimetic locomotion. J. R. Soc. Interface 9, 1856–1868. ( 10.1098/rsif.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neri D, Ruberto T, Cord-Cruz G, Porfiri M. 2017. Information theory and robotics meet to study predator-prey interactions. Chaos: Interdiscip. J. Nonlinear Sci. 27, 073111 ( 10.1063/1.4990051) [DOI] [PubMed] [Google Scholar]
- 23.Ladu F, Bartolini T, Panitz SG, Chiarotti F, Butail S, Macrì S, Porfiri M. 2015. Live predators, robots, and computer-animated images elicit differential avoidance responses in zebrafish. Zebrafish 12, 205–214. ( 10.1089/zeb.2014.1041) [DOI] [PubMed] [Google Scholar]
- 24.Porfiri M, Spinello C, Yang Y, Macrì S. 2019. Zebrafish adjust their behavior in response to an interactive robotic predator. Front. Robot. AI 6, 38 ( 10.3389/frobt.2019.00038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swain DT, Couzin ID, Leonard NE. 2011. Real-time feedback-controlled robotic fish for behavioral experiments with fish schools. Proc. IEEE 100, 150–163. ( 10.1109/JPROC.2011.2165449) [DOI] [Google Scholar]
- 26.Lima SL. 1998. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 27.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 28.Polverino G, Porfiri M. 2013. Mosquitofish (Gambusia affinis) responds differentially to a robotic fish of varying swimming depth and aspect ratio. Behav. Brain Res. 250, 133–138. ( 10.1016/j.bbr.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 29.Polverino G, Santostefano F, Díaz-Gil C, Mehner T. 2018. Ecological conditions drive pace-of-life syndromes by shaping relationships between life history, physiology and behaviour in two populations of eastern mosquitofish. Sci. Rep. 8, 14673 ( 10.1038/s41598-018-33047-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinen-Kay JL, Schmidt DA, Stafford AT, Costa MT, Peterson MN, Kern EM, Langerhans RB. 2016. Predicting multifarious behavioural divergence in the wild. Anim. Behav. 121, 3–10. ( 10.1016/j.anbehav.2016.08.016) [DOI] [Google Scholar]
- 31.Mukherjee S, Heithaus MR, Trexler JC, Ray-Mukherjee J, Vaudo J. 2014. Perceived risk of predation affects reproductive life-history traits in Gambusia holbrooki, but not in Heterandria formos. PLoS ONE 9, e88832 ( 10.1371/journal.pone.0088832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benejam L, Alcaraz C, Sasal P, Simon-Levert G, García-Berthou E. 2009. Life history and parasites of the invasive mosquitofish (Gambusia holbrooki) along a latitudinal gradient. Biol. Invasions 11, 2265–2277. ( 10.1007/s10530-008-9413-0) [DOI] [Google Scholar]
- 33.Langerhans RB, Layman CA, Shokrollahi A, DeWitt TJ. 2004. Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58, 2305–2318. ( 10.1111/j.0014-3820.2004.tb01605.x) [DOI] [PubMed] [Google Scholar]
- 34.Hartman EJ, Abrahams MV. 2000. Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proc. R. Soc. Lond. B 267, 571–575. ( 10.1098/rspb.2000.1039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward AJW, Mehner T. 2010. Multimodal mixed messages: the use of multiple cues allows greater accuracy in social recognition and predator detection decisions in the mosquitofish, Gambusia holbrooki. Behav. Ecol. 21, 1315–1320. ( 10.1093/beheco/arq152) [DOI] [Google Scholar]
- 36.Polverino G, Liao JC, Porfiri M. 2013. Mosquitofish (Gambusia affinis) preference and behavioral response to animated images of conspecifics altered in their color, aspect ratio, and swimming depth. PLoS ONE 8, e54315 ( 10.1371/journal.pone.0054315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casner AM, Fackelman HC, Degtyareva O, Kight SL. 2016. Do female western mosquitofish, Gambusia affinis, prefer ornaments that males lack? Ethology 122, 561–570. ( 10.1111/eth.12507) [DOI] [Google Scholar]
- 38.Chen BJ, Liu K, Zhou LJ, Gomes-Silva G, Sommer-Trembo C, Plath M. 2018. Personality differentially affects individual mate choice decisions in female and male western mosquitofish (Gambusia affinis). PLoS ONE 13, e0197197 ( 10.1371/journal.pone.0197197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Berthou E. 2002. Ontogenetic diet shifts and interrupted piscivory in introduced largemouth bass (Micropterus salmoides). Int. Rev. Hydrobiol. 87, 353–363. () [DOI] [Google Scholar]
- 40.Godinho FN, Ferreira MT, Cortes RV. 1997. The environmental basis of diet variation in pumpkinseed sunfish, Lepomis gibbosus, and largemouth bass, Micropterus salmoides, along an Iberian river basin. Environ. Biol. Fishes 50, 105–115. ( 10.1023/A:1007302718072) [DOI] [Google Scholar]
- 41.Wicker AM, Johnson WE. 1987. Relationships among fat content, condition factor, and first-year survival of Florida largemouth bass. Trans. Am. Fish. Soc. 116, 264–271. () [DOI] [Google Scholar]
- 42.Darby N, McGhee K. 2019. Boldness is affected by recent experience with predation cues and body size in mosquitofish. Behav. Processes 164, 143–149. [DOI] [PubMed] [Google Scholar]
- 43.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 44.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 45.Rossi C, Coral W, Barrientos A. 2012. Robotic fish to lead the school. In Swimming physiology of fish: towards using exercise for farming a fit fish in sustainable aquaculture (eds Palstra AP, Planas JV). New York, NY: Springer. [Google Scholar]
- 46.Schreiber T. 2000. Measuring information transfer. Phys. Rev. Lett. 85, 461 ( 10.1103/PhysRevLett.85.461) [DOI] [PubMed] [Google Scholar]
- 47.Ruberto T, Polverino G, Porfiri M. 2017. How different is a 3D-printed replica from a conspecific in the eyes of a zebrafish? J. Exp. Anal. Behav. 107, 279–293. ( 10.1002/jeab.247) [DOI] [PubMed] [Google Scholar]
- 48.Bierbach D, Landgraf T, Romanczuk P, Lukas J, Nguyen H, Wolf M, Krause J. 2018. Using a robotic fish to investigate individual differences in social responsiveness in the guppy. R. Soc. open sci. 5, 181026 ( 10.1098/rsos.181026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romano D, Benelli G, Stefanini C. 2019. Encoding lateralization of jump kinematics and eye use in a locust via bio-robotic artifacts. J. Exp. Biol. 222, jeb187427 ( 10.1242/jeb.187427) [DOI] [PubMed] [Google Scholar]
- 50.Romano D, Benelli G, Stefanini C. 2017. Escape and surveillance asymmetries in locusts exposed to a Guinea fowl-mimicking robot predator. Sci. Rep. 7, 12825 ( 10.1038/s41598-017-12941-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Folkertsma GA, Straatman W, Nijenhuis N, Venner CH, Stramigioli S. 2017. Robird: a robotic bird of prey. IEEE Rob. Autom. Mag. 24, 22–29. ( 10.1109/MRA.2016.2636368) [DOI] [Google Scholar]
- 52.Polverino G, Cigliano C, Nakayama S, Mehner T. 2016. Emergence and development of personality over the ontogeny of fish in absence of environmental stress factors. Behav. Ecol. Sociobiol. 70, 2027–2037. ( 10.1007/s00265-016-2206-z) [DOI] [Google Scholar]
- 53.DeLellis P, Cadolini E, Croce A, Yang Y, di Bernardo M, Porfiri M. Submitted. Model-based feedback control of live zebrafish behavior via interaction with a robotic replica. [DOI] [PMC free article] [PubMed]
- 54.Yang Y, Clément RJG, Ghirlanda S, Porfiri M. 2019. A comparison of individual learning and social learning in zebrafish through an ethorobotics approach. Front. Robot. AI 6, 71 ( 10.3389/frobt.2019.00071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butail S, Bartolini T, Porfiri M. 2013. Collective response of zebrafish shoals to a free-swimming robotic fish. PLoS ONE 8, e76123 ( 10.1371/journal.pone.0076123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolok AS. 1992. The swimming performances of individual largemouth bass (Micropterus salmoides) are repeatable. J. Exp. Biol. 170, 265–270. [Google Scholar]
- 57.Magurran AE, Seghers BH. 1990. Population differences in predator recognition and attack cone avoidance in the guppy Poecilia reticulata. Anim. Behav. 40, 443–452. ( 10.1016/S0003-3472(05)80524-X) [DOI] [Google Scholar]
- 58.Macrì S, Neri D, Ruberto T, Mwaffo V, Butail S, Porfiri M. 2017. Three-dimensional scoring of zebrafish behavior unveils biological phenomena hidden by two-dimensional analyses. Sci. Rep. 7, 1962 ( 10.1038/s41598-017-01990-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polverino G, Ruberto T, Staaks G, Mehner T. 2016. Tank size alters mean behaviours and individual rank orders in personality traits of fish depending on their life stage. Anim. Behav. 115, 127–135. ( 10.1016/j.anbehav.2016.03.013) [DOI] [Google Scholar]
- 60.Froese R. 2006. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol. 22, 241–253. ( 10.1111/j.1439-0426.2006.00805.x) [DOI] [Google Scholar]
- 61.Krause J, Loader SP, McDermott J, Ruxton GD. 1998. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proc. R. Soc. Lond. B 265, 2373–2379. ( 10.1098/rspb.1998.0586) [DOI] [Google Scholar]
- 62.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 63.Bossomaier T, Barnett L, Harré M, Lizier JT. 2016. An introduction to transfer entropy. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 64.Porfiri M, Ruiz Marín M. 2018. Inference of time-varying networks through transfer entropy, the case of a Boolean network model. Chaos: Interdiscip. J. Nonlinear Sci. 28, 103123 ( 10.1063/1.5047429) [DOI] [PubMed] [Google Scholar]
- 65.Zhang P, Krasner E, Peterson SD, Porfiri M. 2019. An information-theoretic study of fish swimming in the wake of a pitching airfoil. Physica D 396, 35–46. ( 10.1016/j.physd.2019.02.014) [DOI] [Google Scholar]
- 66.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 67.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-5.
- 68.Pinheiro J, Bates D, DebRoy S, Sarkar D, CoreTeam R. 2014. nlme: linear and nonlinear mixed effects models. R package version 3.1-118.
- 69.Kuznetsova A, Brockhoff PB, Christensen RHB. 2013. lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 2-6.
- 70.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 71.Lenth R. 2018. Emmeans: estimated marginal means, aka least-squares means. R package version 1.
- 72.Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25. ( 10.1002/hbm.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly JL, Magurran AE. 2003. Learned predator recognition and antipredator responses in fishes. Fish Fisheries 4, 216–226. ( 10.1046/j.1467-2979.2003.00126.x) [DOI] [Google Scholar]
- 74.Ydenberg RC, Dill LM. 1986. The economics of fleeing from predators. Adv. Study Behav. 16, 229–249. ( 10.1016/S0065-3454(08)60192-8) [DOI] [Google Scholar]
- 75.Helfman GS. 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav. Ecol. Sociobiol. 24, 47–58. ( 10.1007/BF00300117) [DOI] [Google Scholar]
- 76.Domenici P. 2001. The scaling of locomotor performance in predator-prey encounters: from fish to killer whales. Comp. Biochem. Physiol. A Mole. Integr. Physiol. 131, 169–182. ( 10.1016/S1095-6433(01)00465-2) [DOI] [PubMed] [Google Scholar]
- 77.Meager JJ, Domenici P, Shingles A, Utne-Palm AC. 2006. Escape responses in juvenile Atlantic cod Gadus morhua L.: the effects of turbidity and predator speed. J. Exp. Biol. 209, 4174–4184. ( 10.1242/jeb.02489) [DOI] [PubMed] [Google Scholar]
- 78.Rautenberg GE, Amé MV, Monferrán MV, Bonansea RI, Hued AC. 2015. A multi-level approach using Gambusia affinis as a bioindicator of environmental pollution in the middle-lower basin of Suquía River. Ecol. Indic. 48, 706–720. ( 10.1016/j.ecolind.2014.09.025) [DOI] [Google Scholar]
- 79.Hawlena D, Schmitz OJ. 2010. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 176, 537–556. ( 10.1086/656495) [DOI] [PubMed] [Google Scholar]
- 80.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 81.Clark CW. 1994. Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170. ( 10.1093/beheco/5.2.159) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data can be found at https://figshare.com/s/1e755aeba5b781f753a9.