Abstract
Salivary alpha-amylase (sAA) is a stress biomarker in human diseases, but there are no reports of sAA measurements in diseased dogs. This study measured the sAA and serum alpha-amylase (AA) levels in 16 healthy dogs and 31 diseased dogs using a kinetic enzyme assay to assess the stress status. The sAA and serum AA levels were significantly higher in the diseased dogs than in healthy dogs (p < 0.05), but there was no correlation between the 2 groups (r = 0.251, p = 0.089). This suggests that sAA can be useful as a stress biomarker in diseased dogs.
Keywords: Alpha-amylase, dogs, saliva, serum, stress
Salivary alpha-amylase (sAA) has been reported as a stress biomarker because it is produced by the acinar cells of the salivary glands when stimulated by stress [1,2]. Two mechanisms for this have been suggested: the sympathetic nervous adreno-medullary (SAM) response to acute stress and the hypothalamic-pituitary-adrenocortical response to chronic stress [3]. The sAA activity correlates with plasma norepinephrine produced after acute psychosocial stress because SAM stimulation increases the level of salivary protein secretion within a few min [4]. Therefore, it responds with greater speed and sensitivity than cortisol [5]. The sAA activity has been studied in stressful situations in animals and humans [6,7,8], but the sAA levels have not been measured in diseased animals. Because disease itself is stressful, it was suggested that the sAA levels would increase in disease. This study compared the sAA activity between healthy and diseased dogs and evaluated its potential as a non-invasive biomarker for detecting stress in disease.
The serum alpha-amylase (AA) and sAA levels were measured in 16 healthy Beagle dogs and 31 diseased dogs visiting the Veterinary Medical Teaching Hospital College of Chungnam National University and Daegu Small Animal Medical Center from May to October 2017. Thirty-one dogs with a range of diseases were selected randomly for this study, and the owners were informed. All dogs underwent physical examinations, complete blood counts (Advia 2120; Siemens Healthcare Diagnostics, USA), and biochemistry profiles (Mindray BS-300; Mindray Bio-Medical Electronics, China). The healthy dogs were housed individually in 1 m3 cages under a 12-h light/dark cycle and fed commercial canine food with water available ad libitum. The sampling procedures were performed within one day.
The dogs were fasted for more than 1 h prior to sampling. Saliva was collected using small cotton rolls around the mouth for over 2 min, which were then placed in Eppendorf tubes and centrifuged at 1,500 × g for 15 min. The saliva samples were transferred into new Eppendorf tubes, and stored at −70°C. Blood samples were collected through venipuncture of the jugular vein and centrifuged at 3,000 × g for 10 min at room temperature. The serum was transferred into Eppendorf tubes, and aliquots were stored as described. Almost all samples were collected between 10 AM and 3 PM to minimize the errors caused by the change in collection time. The sample collection protocol was approved by the Institutional Animal Care and Use Committee at Chungnam National University (approval No. CNU-00950).
The sAA and AA activities were measured using a commercial enzyme kit (Salivary α-Amylase; Salimetrics, USA) that uses 2-chloro-p-nitrophenol linked with maltotriose as the substrate. All aliquots were thawed, mixed thoroughly, and assayed in duplicate. The analysis was performed manually according to the manufacturer's instructions. The absorbance was detected at a wavelength of 405 nm (Infinite M200; Tecan, Austria).
Statistical analyses were performed using SPSS (SPSS statistics 22; IBM, USA). Both parametric and non-parametric variables were analyzed. Therefore, the median (25th percentile and 75th percentile) was set to the representative values. The Kolmogorov-Smirnov and Shapiro-Wilk tests determined the normal distribution of the data. The sAA activity between the 2 groups was evaluated using the Mann-Whitney U test. The Spearman correlation was used to analyze the associations between sAA and serum AA. A p value < 0.05 was considered significant.
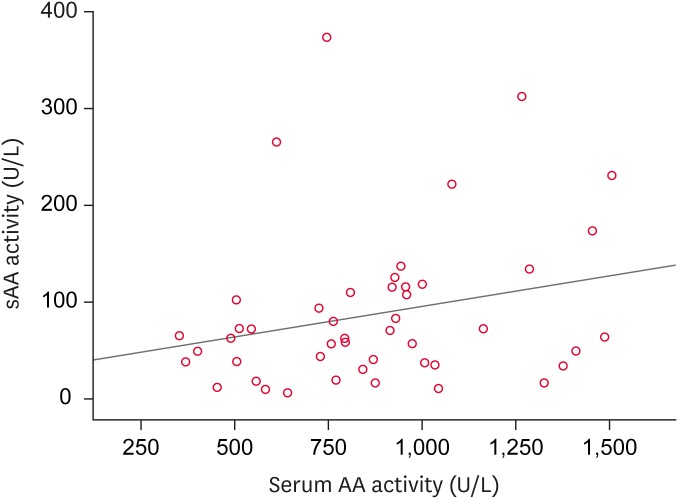
The sAA activity was evaluated in 47 dogs, including 16 healthy Beagles and 31 diseased dogs. Of these, 20 dogs were male, 17 of which were castrated, and 11 were females, 9 of which were spayed. In the diseased group, 16 dogs were purebreds of different breeds and 1 dog was a mixed-breed. The underlying conditions were tumors (n = 7), renal diseases (n = 5), pancreatitis (n = 4), cardiovascular diseases (n = 3), post-surgery (n = 3), infectious diseases (n = 2), dermatologic diseases (n = 2), endocrine diseases (n = 2), respiratory disease (n = 1), neurologic disease (n = 1), and immune-mediated disease (n = 1). Significant difference in serum and salivary AA activity were observed between the healthy and diseased dogs (p < 0.05) (Table 1). No correlation was observed between the sAA and serum AA activities (r = 0.251, p = 0.089) (Fig. 1).
Table 1. Alpha-amylase activity (U/L) in the saliva and serum samples from healthy and diseased dogs analyzed using an enzyme kit.
| Samples | Healthy dogs | Diseased dogs | p value |
|---|---|---|---|
| Saliva | 37.25 (15.40–52.68) | 72.71 (57.15–122.06) | < 0.05 |
| Serum | 725.995 (501.79–870.92) | 942.97 (776.34–1,121.14) | < 0.05 |
Significant differences were expressed as p values by comparing the group of healthy dogs with that of diseased dogs using the Mann-Whitney U test.
Fig. 1. Relationship between the sAA and serum AA in all dogs. No correlation was observed between the alpha-amylase activities of the serum and saliva according to Spearman correlation analysis (r = 0.251, p = 0.089).
sAA, salivary alpha-amylase; AA, alpha-amylase.
Overall, the sAA activity increased in dogs with various diseases compared to that in healthy dogs. To the best of the authors' knowledge, this is the first report confirming a high sAA concentration in diseased dogs. In a human salivary biomarker study, stress increased the sAA levels rapidly within 15 min and was moderately correlated with the State-Trait Anxiety Inventory score, whereas the other biomarkers, including chromogranin-A and cortisol, did not [9]. Therefore, the sAA activity is a better indicator of stress. In the present study, the median sAA activity (72.71 U/L) in diseased dogs was similar to that of dogs after sympathetic activation in an earlier study (89.5 U/L) [7]. Based on this, the sAA levels increased in the diseased dogs due to stress. Earlier human studies reported that the sAA activity increased due to stress, occurring in a range of diseases, such as pulmonary disease and diabetes [10,11]. Therefore, the sAA activity may be a potential disease biomarker. Further studies will be needed to calculate the difference between the range of sAA concentrations for disease-free and disease-induced stressful conditions.
Salivary sampling has several distinct advantages in veterinary medicine. The disadvantages, however, are that the sAA activity is lower than the blood amylase activity and it is difficult to collect saliva from aggressive or dehydrated patients [12]. Needle injections can induce stress and increase sAA activity [6]. Because saliva can be collected without a needle, its sampling is easier and less stressful than blood or urine collection and it can be performed outside the hospital with minimal technical training [1,12]. Moreover, saliva can be multi-sampled and is delivered easily [12], making it ideal for owners unable to visit the hospital and for animals that are too small or anemic for blood sampling. Therefore, measurement of the sAA activity might be an effective, noninvasive, and minimally stressful screening test prior to blood testing.
The serum AA activity was significantly higher in the diseased group compared to that in the healthy group. This result is similar to that of humans with pancreatitis, mumps, and pancreatic cancer [13], and may be due to the AA produced in the pancreas and salivary glands. The serum AA levels have not been investigated in other diseases. In the present study, the serum AA was high in diseased dogs. The cause is yet unknown, but it is estimated that animals with diseases can easily become dehydrated and the pancreas is susceptible to hypoperfusion. On the other hand, there was no significant correlation between the sAA and serum AA levels (r = 0.251, p = 0.089). Thus, the increased serum AA activity could be due to other unknown pathways in dogs.
This study had some limitations. First, the sAA activity was not compared with that of other stress hormones, such as norepinephrine and cortisol. Second, a larger study population is required to establish the normal range of sAA activity and assess the sAA sensitivity in diseased dogs. Third, the factors affecting the composition of saliva, such as psychological stress, degree of hydration, body composition, and circadian rhythm, could not be controlled, and validation of the sAA enzymatic assay was not validated. Continual measurements from the same dogs are recommended if the sAA levels are to be used as a biomarker for monitoring the treatment and disease prognosis.
In conclusion, the AA activity increases in both canine serum and saliva due to disease-induced stress, even though there was no significant correlation between them. Therefore, sAA can be considered a less invasive, convenient, and relatively reliable method to detect stressful disease conditions in dogs.
Footnotes
Funding: This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) with funding from the Ministry of Science & ICT to Y.J.K (NRF-2014M3A9D7070779) and supported by “Cooperative Research Program of Center for Companion Animal Research (Project No. PJ014045022018): Rural Development Administration, Republic of Korea.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Seo KW.
- Data curation: Hong HR, Oh YI.
- Formal analysis: Hong HR, Kim YJ, Seo KW.
- Funding acquisition: Seo KW, Kim YJ.
- Supervision: Seo KW.
- Validation: Kim YJ, Seo KW.
- Visualization: Hong HR, Oh YI.
- Writing - original draft: Hong HR.
- Writing - review & editing: Oh YI, Seo KW.
References
- 1.Rohleder N, Nater UM. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34:469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Baum BJ. Principles of saliva secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol. 2012;91:342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes-Rubio M, Fuentes F, Otal J, Quiles A, Hevia ML. Validation of an assay for quantification of alpha-amylase in saliva of sheep. Can J Vet Res. 2016;80:197–202. [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras-Aguilar MD, Tecles F, Martínez-Subiela S, Escribano D, Bernal LJ, Cerón JJ. Detection and measurement of alpha-amylase in canine saliva and changes after an experimentally induced sympathetic activation. BMC Vet Res. 2017;13:266. doi: 10.1186/s12917-017-1191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh D, Ng V, Naing L. Alpha amylase as a salivary biomarker of acute stress of venepuncture from periodic medical examinations. Front Public Health. 2014;2:121. doi: 10.3389/fpubh.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101:1873–1876. doi: 10.1213/01.ANE.0000184196.60838.8D. [DOI] [PubMed] [Google Scholar]
- 10.Yigla M, Berkovich Y, Nagler RM. Oxidative stress indices in COPD--Broncho-alveolar lavage and salivary analysis. Arch Oral Biol. 2007;52:36–43. doi: 10.1016/j.archoralbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Aydin S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol. 2007;40:29–35. doi: 10.5483/bmbrep.2007.40.1.029. [DOI] [PubMed] [Google Scholar]
- 12.Vangipuram S, Jha A, Bhashyam M. The diagnostic applications of saliva- a review. IOSR J Dent Med Sci. 2016;15:96–101. [Google Scholar]
- 13.Mandal N, Bhattacharjee M, Chattopadhyay A, Bandyopadhyay D. Point-of-care-testing of α-amylase activity in human blood serum. Biosens Bioelectron. 2019;124-125:75–81. doi: 10.1016/j.bios.2018.09.097. [DOI] [PubMed] [Google Scholar]