Abstract
The aim of the present study was to investigate elemental diet (ED)-induced alteration of the fecal and mucosal microbiome in mice. The control group was fed a normal chow and the ED group was fed normal chow containing 50% w/w Elental® (EA Pharma, Tokyo, Japan) for 28 days. Fecal and mucosal microbiome were analyzed using 16S rRNA gene sequencing. In the fecal samples, the observed species, an index for microbial richness, was significantly decreased in the ED group. Principal coordinate analysis revealed that there were significant compositional differences between the control and ED groups (PERMANOVA p = 0.0007 for unweighted and p = 0.002 for weighted UniFrac distance, respectively). In contrast, there was no significant difference in the overall structure of mucosal microbiome between the control and ED groups. In the fecal samples, abundance of the genera Adlercreutzia, Akkermansia, Streptococcus, Helicobacter, Coprobacillus and Coprococcus was significantly reduced in the ED group compared to the control group. Abundance of the genera Lactobacillus and Staphylococcus was significantly increased in the ED group. In a functional analysis using PICRUSt software, ED altered various pathways involved in amino acid metabolism of the gut microbiome. In conclusion, ED caused a reduction in bacterial diversity and altered metabolic functions.
Keywords: Elental, dysbiosis, Crohn’s disease
Introduction
The incidence of inflammatory bowel diseases (IBDs) is continuously growing around the world.(1,2) The global increase of IBD has been explained by the influence of a Westernized diet and environmental factors that are linked to the life style in modern, industrialized nations.(1,2) In particular, the changes to a Westernized diet is considered to be a key factor contributing to the emergence of IBD,(1,3) since one of common luminal antigens is the dietary factors which directly stimulate the mucosal immune system and induce mucosal inflammation.
The development of biologics has dramatically changed the therapeutic strategy for IBD and contributed to improvements in patients’ quality of life.(2) However, it has become clear that such drugs have some serious side effects such as vulnerability to opportunistic infection and carcinogenesis and some patients become refractory to them. Furthermore, biologics are very expensive, causing economic problems for health care providers. Along with success of biologics, the re-emergence of enteral nutrition is attracting attention in the field of IBD treatment, as enteral nutrition has fewer side effects and is much cheaper than biologics. Enteral nutrition plays a pivotal role in the clinical care of all patients with IBD and recently released clinical guidelines for IBD have stated that enteral nutrition is an option for inducing and maintaining clinical remission.(4,5) The effectiveness of enteral nutrition in patients with Crohn’s disease (CD), especially for the maintenance of clinical remission, has been recently confirmed.(6,7)
Enteral nutrition includes elemental diet (containing only amino acids, fatty acids, vitamins, minerals and nutrients that do not need digestion), semielemental diet (containing only small peptides, oligosaccharides, vitamins, minerals and medium-chain fatty acids), and polymeric diet (containing proteins, carbohydrates, medium- and long-chain fatty acids, vitamins, and trace elements).(5) The mode of action of enteral nutrition, especially of elemental diet (ED), is considered multifactorial. The ED has been shown to improve intestinal permeability which mediate easy access of luminal antigens to immune cells resident in the lamina propria.(8,9) Since the ED contains no dietary antigens, it has no ability to stimulate the immune response and reduces the workload of digestion and absorption, leading to bowel relaxation. Malnutrition is sometimes observed in patients with IBD, especially CD, and enteral nutrition has been used as an optional treatment to overcome nutritional problems.
Dysregulated host-microbial interactions play a role in initiating and perpetuating IBD.(10–13) A global alteration of the diversity and composition of the gut microbiome (dysbiosis) rather than the presence of specific pathogens likely plays a critical role.(11,14,15) Recent clinical studies have revealed that the mechanisms by which enteral nutrition reduces both physiological and metabolic markers of inflammation in CD might relate to modulation of the gut microbial structure.(16–19) However, there are no reports on precisely performs this modulatory function. In this study, we investigated ED-induced alteration of the fecal and mucosal microbiome in mice.
Materials and Methods
Animals
BALB/c mice (6 to 8 weeks-old female) were purchased from CLEA Japan Inc. (Tokyo, Japan) and housed under specific pathogen-free conditions. Mice were allowed free access to water and rodent chow (CE-2; CLEA Japan, Inc.). The elemental diet (Elental®) was provided by EA Pharma Co., Ltd. (Tokyo, Japan). The formula of Elental® contains a variety of amino acids, together with easily digestible nutrition, minerals, vitamins and a major energy source, dextrin.(20) Fat is present in a very small amount, whereas l-glutamine is present at an especially high dose (1.932 mg/package).(20) Mice were divided into two groups; the control group was fed a normal chow (n = 10) and the elemental diet group was fed normal chow containing 50% w/w Elental® (n = 10). Mice were treated for 28 days and then sacrificed under isoflurane anesthesia by quick cervical distortion. This study was approved by the Research Center for Animal Life Science and Use Committee at the Shiga University of Medical Science (Otsu, Japan) (Permit number: 2016-12-7).
DNA extraction from fecal and mucosal samples
DNA was extracted from fresh fecal and mucosal samples of the middle colon using Quick Gene DNA tissue kits (Kurabo, Osaka, Japan) as described previously.(21)
16S rRNA sequencing
The MiSeqTM System (Illumina, San Diego, CA) was used for 16S rRNA sequencing according to a previously described method.(22) Briefly, the V3–V4 hypervariable regions of 16S rRNA were amplified by polymerase chain reaction (PCR) using the universal primers 341F and 805R, followed by the second PCR to introduce a unique combination of dual indices (I5 and I7 index). The concentration of the second PCR products was normalized with a SequalPrep Normalization Plate Kit (Life Technologies, Tokyo, Japan) and concentrated using AMPure XP beads (Beckman Coulter, Tokyo, Japan). Ten pM of the library combined with phiX Control was sequenced using a 300-bp paired-end strategy according to the manufacturer’s instructions.
16S rRNA-based taxonomic analysis and statistical analysis
QIIME ver. 1.9,(23) USEARCH v9.2.64, UCHIME ver. 4.2.40(24) and VSEARCH ver. 2.4.3(25) were used for processing of sequence data including chimera check, operational taxonomic unit (OTU) definition and taxonomy assignment. Singletons were omitted. The RDP classifier v2.10.2 with the Greengenes database (published May, 2013)(26) was used for taxonomy assignment of the acquired OTUs.
Statistical analyses
The observed species, Chao-1 and Shannon phylogenetic diversity indices were calculated by the R “phyloseq” package(27) and statistically analyzed using the Wilcoxon test. β-Diversity for bacterial microbiome was estimated using the UniFrac metric. Statistical analysis was performed using permutational multivariate analysis of variance (PERMANOVA). The figures were generated using the QIIME or R “phyloseq”. The relative abundance of bacterial phyla and genera were compared by Wlech’s test using STAMP software.(28) Microbial composition was statistically analyzed by the Kruskal-Wallis test and followed by the unpaired Wilcoxon test using Linear Discriminant Analysis Effect Size (LEfSe)(29) (available at http://huttenhower.sph.harvard.edu/galaxy/).
Functional changes in the microbiome
Potential changes in the microbiome at the functional level were evaluated using PICRUSt software(30) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database release 70.0.(31) The PICRUSt software uses 16S rRNA sequence profiles to estimate metagenome content based on reference bacterial genomes and KEGG pathway database. The results were further analyzed statistically by Welch’s t test using the STAMP software.(28) Benjamini-Hochberg-corrected p values (<0.05) were used to determine any statistically significant differences between the groups.
Results
Initially, we compared the fecal and mucosal microbiome between the control and ED groups. The results of fecal samples are shown in Fig. 1 and those of mucosal samples are shown in Fig. 2.
Fig. 1.
Comparative analyses of the fecal microbial community in the control (n = 10) and elemental diet (ED)-treated mice (n = 10). (A) α-Diversity indices. *p<0.05 by Bonferroni test. (B) Unweighted and weighted PCoA of β-diversity measures of all samples. The microbial community was significantly different between the control group and ED group (p = 0.0007 and 0.002 by PERMANOVA test, respectively).
Fig. 2.
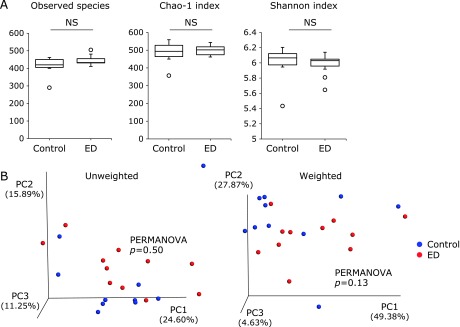
Comparative analyses of the mucosal microbial community in the control (n = 10) and elemental diet (ED)-treated mice (n = 10). (A) α-Diversity indices. NS, not significant. (B) Unweighted and weighted PCoA of β-diversity measures of all samples. There were no significant differences between the control group and ED group (p = 0.50 and 0.13 by PERMANOVA test, respectively).
In the fecal samples, there was a statistically significant difference in the observed species between the control and ED groups (Fig. 1A), although there was no significant difference in the Chao-1 and Shannon indices (Fig. 1A). The observed species and the Chao-1 index indicate OTU richness estimation and the Shannon index represents OTU evenness estimation. The overall structure of the fecal microbiome was evaluated using β-diversity indices calculated for unweighted and weighted UniFrac distance. Principal coordinate (PCo) analysis revealed that there were significant structural differences between the control and ED groups (PERMANOVA p = 0.0007 for unweighted and p = 0.002 for weighted UniFrac distance, respectively) (Fig. 1B).
As shown in Fig. 2A, in mucosal samples there were no statistically significant differences in the observed species, Chao-1 and Shannon indices between the control and ED groups. Furthermore, there was no significant difference in the overall structure of mucosal microbiome between the two groups (Fig. 2B).
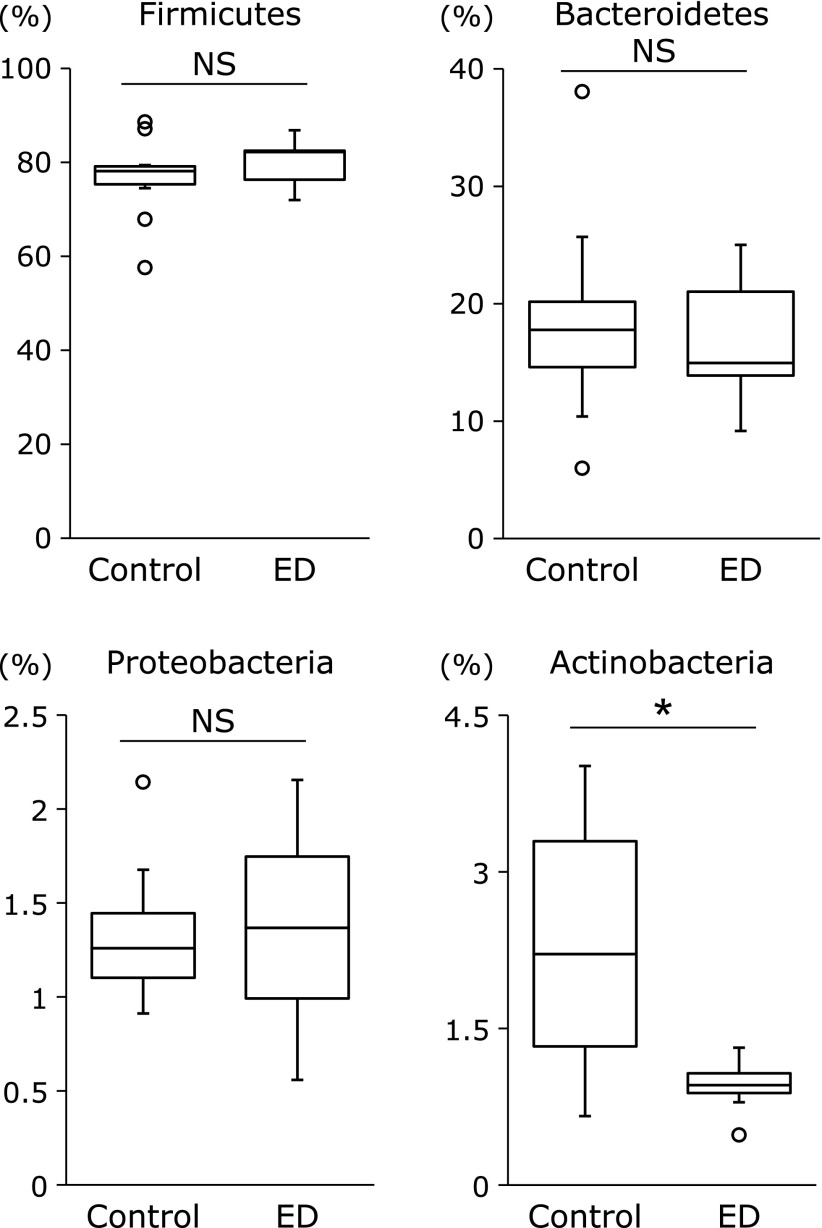
The differences in the gut microbial structure were taxonomically evaluated at the phylum level (Fig. 3). There were no significant differences in the abundance of the phyla Firmicutes, Bacterodetes, and Proteobacteria between the control and ED groups. On the other hand, the abundance of the phylum Actinobacteria was significantly reduced in the ED group compared to the control group.
Fig. 3.
Comparative analyses of the taxonomic composition of the microbial community at the phylum level. Number in bars means %. ED, elemental diet. *p<0.01.
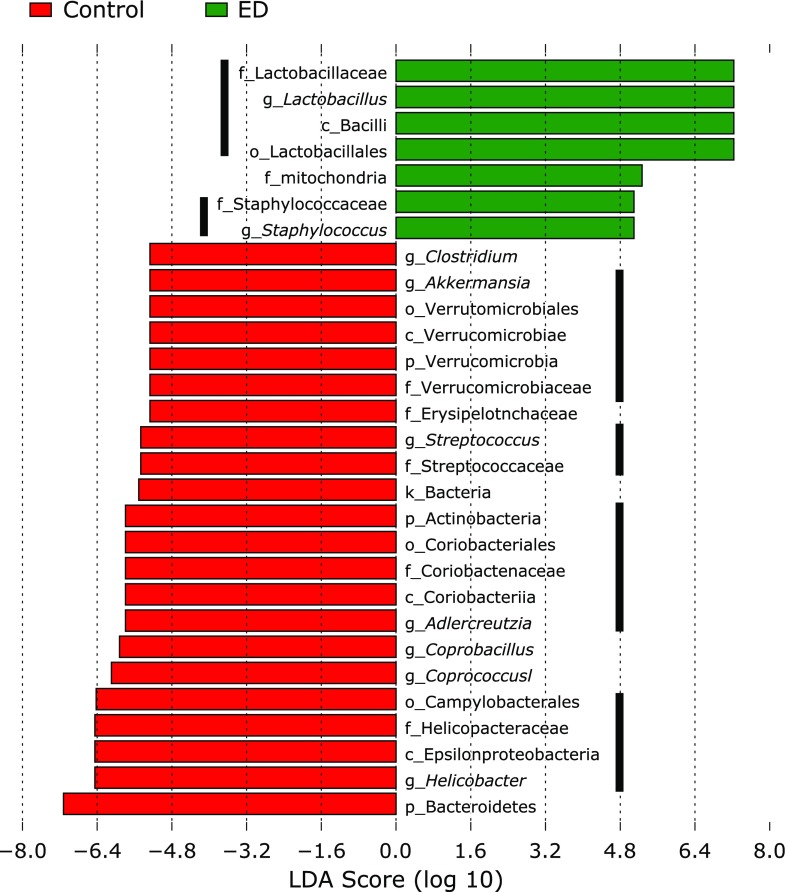
Changes in microbial composition of fecal samples were further analyzed using Linear Discriminant Analysis Effect Size (LEfSe)(29) (Fig. 4) and also demonstrated by Cladogram (Fig. 5). The significant reduction in the abundance of the phylum Actinobacteria was represented by a reduced abundance of the genus Adlercreutzia (Kruskal-Wallis test p<0.05). In addition, the abundance of the genera Akkermansia, Streptococcus, Helicobacter, Coprobacillus and Coprococcus was significantly reduced in the ED group compared to the control group. On the other hand, the abundance of the genera Lactobacillus and Staphylococcus was significantly increased in the ED group compared to the control group. A significant increase in the genus Lactobacillus was also detected in mucosal samples (data not shown).
Fig. 4.
Alteration of the relative abundance of bacteria in the control and ED groups using linear discriminant analysis effect size (LEfSe). The histogram indicates the Linear Discriminant Analysis (LDA) score. These taxa showed a statistically significant difference between the control and ED group (p<0.05 by the Wilcoxon test).
Fig. 5.
Cladogram showing a comparison of the bacterial microbial profile between the control and ED groups.
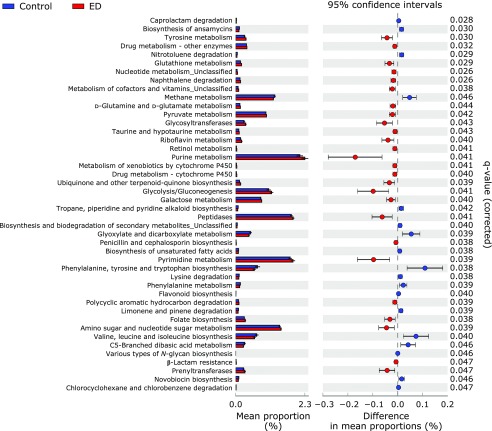
Potential differences in the function of the microbiome were evaluated using PICRUSt software(30) (Fig. 6). When compared with the control group, the proportion of genes responsible for tyrosine metabolism, taurine and hypotaurine metabolism, purine metabolism and glycolysis/gluconeogenesis was significantly increased in the ED group. In contrast, the proportion of genes responsible for methane metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, lysine degradation, phenylalanine metabolism and valine, leucine and isoleucine biosynthesis was significantly reduced in the ED group.
Fig. 6.
Functional analysis of the gut microbiome of control and ED-treated mice. PICRUSt predictions of the functional composition of metagenome using 16S rRNA gene data and a database of reference genomes.(30) The KEGG database functional categories are shown with the displayed histograms and p value determinations, as calculated by the STAMP software.(28)
Discussion
A number of studies have investigated the efficacy and mode of action of diet in IBD and nutrition is now regarded not only as a supportive therapy but also as a primary treatment to induce or maintain remission.(32) The ED is a therapeutic approach that is safe and effective for maintaining remission and preventing recurrence of Crohn’s disease. Previous clinical studies have suggested that enteral nutrition exerts its therapeutic effects on IBD through modulation of the gut microbiome.(16,17) Reported microbial changes associated with enteral nutrition included: reduced abundance of Firmicutes, Bacteroides/Prevotella, and Proteobacteria and an increase in taxa belonging to Bacteroidetes.(16) Some studies have predicted that enteral nutrition might shift dysbiosis toward a healthier state, but recent data have shown that enteral nutrition initially induces an even more dysbiotic state.(16) It has recently been shown that CD patients have distinct forms or ‘degrees’ of dysbiosis, which could impact the likelihood of achieving remission with enteral nutrition.(19) However, studies investigating the impact of ED on the gut microbiome are lacking. Interpreting detailed microbial changes across different studies is complicated by the heterogeneity of study cohorts, high inter-individual variation, differences in sample site (e.g., stool vs mucosal biopsy), and methodological differences in analysis. So, in order to gain basic data on the effects of the ED on the gut microbiome, we simply investigated its effects of ED in mice.
The current study identified some novel findings. The first is that treatment with the ED (50% w/w) for 28 days induced a marked alteration of the fecal microbiome but did not affect the mucosal microbiome. This suggests that influence of luminal microbial changes may need a much longer period (over 28 days) to alter the mucosal microbiota. In other words, the mucosal microbiota may be regulated by a different mechanism from that involved in the regulation of luminal microbiome. For example, locally secreted antimicrobial peptides and secretory immunoglobulins might directly modulate the composition of the mucosal microbiome.(33,34)
The second finding is that ED treatment induced a significant reduction of the diversity characterized by a significant decrease in the observed species. This was accompanied by a significant decrease in the abundance of the phylum Actinobacteria. At the genus level, the relative abundance of the genera Adlercreutzia, Akkermansia, Streptococcus, Helicobacter, Coprobacillus and Coprococcus were significantly reduced in the ED group. On the other hand, the genera Lactobacillus and Staphylococcus were significantly increased in the ED group. Thus, ED treatment for 28 days relatively simplified the fecal microbiome and these findings are compatible with previous clinical reports.(16) These changes may have be resulted from not only the formula containing 17.6% amino acids but also other dietary components such as low amounts of dietary fiber.
ED induced a decrease in the relative abundance of the genus Adlercreutzia. Adlercreutzia (Adlercreutzia equolifaciens) is capable of metabolizing isoflavones to equol.(35) Equol possesses great potential for human health due to its antioxidant and anti-carcinogenic activity.(36) It has been shown to be strongly associated with anticancer activity and inhibition of the epidermal growth factor receptor tyrosine kinase.(36) These characteristics suggest that a reduced abundance of the genus Adlercreutzia may lead to the decrease of equol generation, resulting in deterioration of various bioactivities related to human health.
The relative abundance of the genus Akkermansia was decreased in the ED group. The genus Akkermansia is a mucin-degrading bacterium of the phylum Verrucomicrobia.(37) This is the only genus of the phylum Verrucomicrobia found in gastrointestinal samples and utilizes mucin as the sole source of carbon, nitrogen, and energy for growth.(37) Akkermansia resides in the mucus layer of the colon, where it contributes to the maintenance of intestinal integrity. The presence of Akkermansia is therefore considered to be associated with a healthy intestine, and its decrease has been shown in several disease states. For example, Akkermansia decreased in patients with IBD and/or obese peoples.(37) Based on these previous reports, it was suggested that ED-induced decrease in the abundance of the genus Akkermansia may not be beneficial for IBD patients. ED contains relatively low amounts of dietary fiber and leads to a thinning of the mucous layer. A reduced abundance of Akkermansia may be a secondary change to this thinning.
A positive result induced by ED treatment was an increase in relative abundance of the genus Lactobacillus. This genus forms a major part of the lactic acid bacteria group that converts sugars to lactic acid and is a representative commensal bacteria.(38) In humans, it constitutes a significant component of the microbiota at a number of body sites, such as the gastrointestinal tract, urinary and genital systems.(38) Lactobacillus exhibits a mutualistic relationship with human, as it protects the host against potential invasions by pathogens, and in turn, the host provides a source of nutrients. An increased abundance of the genus Lactobacillus is therefore considered to be a desirable phenomenon.
The functional analyses of the gut microbiome using PICRUSt software revealed that ED treatment induced a significant increase in various pathways involving “amino acid metabolism” such as tyrosine metabolism, glutathione metabolism, taurine and hypotaurine metabolism. In contrast, ED lead to a decrease in phenylalanine, tyrosine and tryptophan biosynthesis, lysine degradation, phenylalanine metabolism and valine, leucine and isoleucine biosynthesis. These findings indicate that amino acid-rich formula ED altered the amino acid metabolism of the gut microbiota complicatedly. These are based on the indirect computational method for inferring metagenomics content, and direct metagenomic DNA sequencing will be required to confirm the findings of this study. This strategy will define a precise role of ED-induced alteration of amino acid metabolism in the gut microbiota.
In conclusion, it is clear that ED has a profound impact on the gut microbiome in mice. Not all changes of the gut microbiome in response to ED were desirable for IBD patients. ED caused a reduction in bacterial diversity, changes in community-level metabolic functions, and seemed to increase microbial dysbiosis. This observation suggests that ED achieves its effect by disrupting established IBD-related dysbiosis to re-colonize and re-establish microbial communities that are a more favorable to the host. Further investigation is required to improve our understanding of how ED modulates the gut microbiome.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (18K08002), a grant for the Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan, and a grant from the Smoking Research Foundation.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152: 313–321.e312. [DOI] [PubMed] [Google Scholar]
- 2.Chan HC, Ng SC. Emerging biologics in inflammatory bowel disease. J Gastroenterol 2017; 52: 141–150. [DOI] [PubMed] [Google Scholar]
- 3.Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2018; 15: 440–452. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018; 53: 305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triantafillidis JK, Vagianos C, Papalois AE. The role of enteral nutrition in patients with inflammatory bowel disease: current aspects. Biomed Res Int 2015; 2015: 197167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2018; 4: CD000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akobeng AK, Zhang D, Gordon M, MacDonald JK. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2018; 8: CD005984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis O, Varnalidis I, Paraskevas G, Botsios D. Nutritional modulation of the inflammatory bowel response. Digestion 2011; 84: 89–101. [DOI] [PubMed] [Google Scholar]
- 9.Teahon K, Smethurst P, Pearson M, Levi AJ, Bijarnason I. The effect of elemental diet on intestinal permeability and inflammation in Crohn’s disease. Gastroenterology 1991; 101: 84–89. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol 2015; 50: 495–507. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017; 152: 327–339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino K, Nishida A, Inoue R, et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J Gastroenterol 2018; 53: 95–106. [DOI] [PubMed] [Google Scholar]
- 13.Takagi T, Naito Y, Inoue R, et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol 2019; 54: 53–63. [DOI] [PubMed] [Google Scholar]
- 14.Becker C, Neurath MF, Wirtz S. The intestinal microbiota in inflammatory bowel disease. ILAR J 2015; 56: 192–204. [DOI] [PubMed] [Google Scholar]
- 15.Imai T, Inoue R, Kawada Y, et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J Gastroenterol 2019; 54: 149–159. [DOI] [PubMed] [Google Scholar]
- 16.MacLellan A, Moore-Connors J, Grant S, Cahill L, Langille MGI, Van Limbergen J. The impact of exclusive enteral nutrition (EEN) on the gut microbiome in Crohn’s disease: a review. Nutrients 2017; 9. pii: E0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong D, Yu X, Wang L, Kong L, Gong X, Dong Q. Exclusive enteral nutrition induces remission in pediatric Crohn’s disease via modulation of the gut microbiota. Biomed Res Int 2017; 2017: 8102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatti S, Galeazzi T, Franceschini E, et al. Effects of the exclusive enteral nutrition on the microbiota profile of patients with Crohn’s disease: a systematic review. Nutrients 2017; 9. pii: E832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn KA, Moore-Connors J, MacIntyre B, et al. Early changes in microbial community structure are associated with sustained remission after nutritional treatment of pediatric Crohn’s disease. Inflamm Bowel Dis 2016; 22: 2853–2862. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Yasuda T, Doi T, et al. The amino acid-rich elemental diet Elental® preserves lean body mass during chemo- or chemoradiotherapy for esophageal cancer. Oncol Rep 2016; 36: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto M, Inoue R, Tsuruta T, Hara H, Yajima T. Long-term oral administration of cows’ milk improves insulin sensitivity in rats fed a high-sucrose diet. Br J Nutr 2009; 102: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 22.Inoue R, Sakaue Y, Sawai C, et al. A preliminary investigation on the relationship between gut microbiota and gene expressions in peripheral mononuclear cells of infants with autism spectrum disorders. Biosci Biotechnol Biochem 2016; 80: 2450–2458. [DOI] [PubMed] [Google Scholar]
- 23.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26: 2460–2461. [DOI] [PubMed] [Google Scholar]
- 25.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016; 4: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006; 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8: e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 2014; 30: 3123–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013; 31: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 2014; 42 (Database issue): D199–D205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durchschein F, Petritsch W, Hammer HF. Diet therapy for inflammatory bowel diseases: the established and the new. World J Gastroenterol 2016; 22: 2179–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Zhang C, Zhang MZ, Zang S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol 2018; 18: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okai S, Usui F, Yokota S, et al. High-affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat Microbiol 2016; 1: 16103. [DOI] [PubMed] [Google Scholar]
- 35.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol 2008; 58 (Pt 5): 1221–1227. [DOI] [PubMed] [Google Scholar]
- 36.Subedi L, Ji E, Shin D, Jin J, Yeo JH, Kim SY. Equol, a dietary daidzein gut metabolite attenuates microglial activation and potentiates neuroprotection in vitro. Nutrients 2017; 9. pii: E207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms 2018; 6. pii: E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermúdez-Humarán LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact 2013; 12: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]