Abstract
Aim:
To examine the relationship between survival and diastolic blood pressure (DBP) throughout resuscitation from paediatric asphyxial cardiac arrest.
Methods:
Retrospective, secondary analysis of 200 swine resuscitations. Swine underwent asphyxial cardiac arrest and were resuscitated with predefined periods of basic and advanced life support (BLS and ALS, respectively). DBP was recorded every 30 s. Survival was defined as 20-min sustained return of spontaneous circulation (ROSC).
Results:
During BLS, DBP peaked between 1–3 min and was greater in survivors (20.0 [11.3, 33.3] mmHg) than in non-survivors (5.0 [1.0, 10.0] mmHg; p<0.001). After this transient increase, the DBP in survivors progressively decreased but remained greater than that of non-survivors after 10 min of resuscitation (9.0 [6.0, 13.8] versus 3.0 [1.0, 6.8] mmHg; p<0.001). During ALS, the magnitude of DBP change after the first adrenaline (epinephrine) administration was greater in survivors (22.0 [16.5, 36.5] mmHg) than in non-survivors (6.0 [2.0, 11.0] mmHg; p<0.001). Survival rate was greater when DBP improved by ≥26 mmHg after the first dose of adrenaline (20/21; 95%) than when DBP improved by ≤10 mmHg (1/99; 1%). The magnitude of DBP change after the first adrenaline administration correlated with the time to ROSC (r=−0.54; p<0.001).
Conclusions:
Survival after asphyxial cardiac arrest is associated with a higher DBP throughout resuscitation, but the difference between survivors and non-survivors was reduced after prolonged BLS. During ALS, response to adrenaline administration correlates with survival and time to ROSC. If confirmed clinically, these findings may be useful for titrating adrenaline during resuscitation and prognosticating likelihood of ROSC.
Institutional Protocol Numbers:
SW14M223 and SW17M101
Keywords: cardiopulmonary resuscitation, cardiac arrest, pediatrics, diastolic blood pressure, survival, swine
INTRODUCTION
Cardiac arrest occurs in 2–3% of children admitted to the paediatric intensive care unit.1,2 Although outcomes have improved over the last decade, less than half of these children survive to hospital discharge.3–5 Sufficient myocardial perfusion pressure is an important determinant of return of spontaneous circulation (ROSC) after cardiac arrest.6–8 When invasive hemodynamic monitoring is present, the diastolic blood pressure (DBP) can be used as a surrogate marker of myocardial perfusion during cardiopulmonary resuscitation (CPR).9 Mean,6 pre-shock,10 and maximal11 DBP during CPR have been reported to be greater in survivors than in non-survivors. Several preclinical studies demonstrated improved survival using haemodynamic thresholds to titrate chest compression delivery and vasoactive medication administration.12–17 Further, the use of physiologic parameters to guide CPR quality is associated with both improved haemodynamics and likelihood of ROSC in adults.18,19
In a consensus statement and in the most recent paediatric guidelines, the American Heart Association (AHA) recommends that DBP be used, when available, to guide chest compression delivery during CPR.20,21 However, evidence is insufficient to ascertain whether mean or improving values are more prognostic; the relative importance of initial, mean, and pre-defibrillation values; and how vasoactive medications affect their interpretation. Target values for mean DBP during resuscitation of infants (≥25 mmHg) and children (≥30 mmHg) have recently been described based on analysis of mean values early in cardiac arrest and in a majority of patients already receiving vasoactive infusions.22 An enhanced understanding of the prognostic value of DBP associated with the phases of resuscitation as well as the impact of vasoactive medication administration could be useful.
In this study, we retrospectively reviewed 200 Yorkshire swine resuscitations and examined the association between survival and DBP throughout the course of resuscitation from asphyxial cardiac arrest. Based on our preliminary observations, we hypothesized that survivors would have higher DBP early in the resuscitation and a greater improvement in DBP after the initial administration of adrenaline (epinephrine) than would non-survivors.
METHODS
Overall study design
We performed a retrospective, secondary analysis of several preclinical swine asphyxial cardiac arrest experiments that were originally performed to compare survival among various resuscitation strategies.23,24
Animal preparation
All studies were approved by the Animal Care and Use Committee at the Johns Hopkins University and complied with the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals. The protocols have been described previously.23,24 Briefly, 7–14-day-old Yorkshire swine (3–4 kg) were housed in cages in groups of three and fasted overnight with provision of water. The next morning, they were anesthetized with 2% isoflurane, 70% nitrous oxide, and 30% oxygen. A 4.0 cuffed tracheal tube was secured via tracheostomy. The femoral artery was cannulated and a catheter was advanced to the mid-thoracic aorta for continuous haemodynamic monitoring and arterial blood gas measurements. A femoral venous catheter was advanced to the mid-thoracic vena cava for administration of sedation prior to asphyxia and adrenaline during resuscitation. In some experiments, a pacing wire was placed through the contralateral femoral vein and advanced until ventricular irritation confirmed right ventricular placement. Pressure-controlled mechanical ventilation was adjusted to maintain a PaCO2 of 35–45 torr at a rate of 20 breaths per minute during surgery and stabilization. The FiO2 was decreased from 0.30 to 0.21 for at least 10 min to ensure normoxia prior to protocol initiation. After the surgeries were completed, isoflurane and nitrous oxide were discontinued and fentanyl (10 mcg/kg) and vecuronium (0.3 mg/kg) were administered for animal comfort. Once the asphyxia began, no further anaesthetics were administered.
Experimental protocols
Asphyxial arrest was induced by clamping the endotracheal tube for 11–23 min depending on the assigned protocol. Some piglets were assigned to an asphyxial-fibrillatory arrest and 50 mA of alternating current was applied via the femoral pacing wire for the final 6 min of arrest.23,24 At the end of asphyxia, paediatric basic life support (BLS) with chest compressions and mechanical ventilation was initiated. Pressure-controlled mechanical ventilation was resumed with an FiO2 of 1.0 at the pre-arrest rate (20 breaths/minute), inspiratory pressure, and expiratory pressure settings. The ventilation rate of 20 breaths per minute is higher than that recommended by the AHA, but was intended to offset the reduced tidal volumes achieved when using pressure-limited ventilation during chest compression delivery. Chest compressions were performed with a two-thumb-encircling hands technique, and compressors switched every 2 min per AHA guidelines. Most groups received BLS for 10 min, but some pigs received BLS for 6 min according to their assigned protocols. After completion of BLS, resuscitators initiated advanced life support (ALS), which included administration of adrenaline (300 mcg) and defibrillation attempts (30 joules), if indicated. No additional medications nor fluid resuscitation was provided during BLS or ALS. Physiologic data were recorded at baseline and every 30 seconds during asphyxia and resuscitation.
Pigs were randomized to one of three resuscitation strategies: (1) standard, (2) end-tidal carbon dioxide (ETCO2)-guided, and (3) algorithm. In the standard group, chest compressions were delivered at a rate of 100 compressions per minute and a depth of 1/3 the anteroposterior diameter (achieved via a visual marker) per the AHA guidelines.20 Full release between compressions was ensured in real time by a coach. In the ETCO2-guided group, resuscitators adjusted the rate, depth, force, and thumb position to maximize the ETCO2 level. The ETCO2 level was the only physiologic data available to the resuscitator and there was no specific goal. In both the standard and ETCO2 groups, pigs received adrenaline every 4 min during ALS. In the algorithm group, the chest compression rate was titrated to a target ETCO2 > 30 torr by sequentially increasing the chest compression rate by 10 per minute for each minute that the ETCO2 was < 30 torr. In this group, adrenaline was administered every 2 min if the ETCO2 was less than 30 torr during ALS. None of the groups used DBP as a metric for compression quality. Resuscitation in all groups was continued until either ROSC or 20 min of CPR, at which point the experiment was terminated. Survival was defined as sustained ROSC with only fluid administration for 20 min.
Statistical analysis
Normality of continuous variables was assessed with the D’Agostino-Pearson test. Physiologic data are presented as mean ± standard error of the mean (SEM) for normally distributed data and median with interquartile ranges for non-normally distributed data. Baseline characteristics of survivors and non-survivors were compared by unpaired t-test or Mann-Whitney test as appropriate. Differences in DBP values between survivors and non-survivors at specific time points during resuscitation were compared with the Mann-Whitney test, and two sided p values ≤ 0.05 were considered significant. Only DBP values during active resuscitation were analysed. DBP values during the 30-s interval in which ROSC occurred and thereafter were excluded from the analyses. The DBP response to adrenaline was measured at 90 s after administration based on prior work identifying this time point as the maximal DBP response to adrenaline in this model. Correlation between variables was calculated with Spearman’s rank correlation coefficient. All data were analysed in GraphPad Prism 8.0.0 (GraphPad Software, La Jolla, CA, USA) or SPSS 25.0 (Statistics for Windows, Armonk, NY, USA).
RESULTS
Two hundred pigs underwent asphyxial cardiac arrest. Seventy pigs were included in protocols with 6 min of BLS, whereas 130 received 10 min of BLS. The overall rate of ROSC with 20-min survival was 33% (65/200). Some animals had ROSC before administration of adrenaline (n=20) and were therefore excluded from analyses to evaluate the effect of adrenaline. Table 1 shows baseline characteristics of survivors and non-survivors. All parameters were within normal limits for paediatric swine.
Table 1.
Baseline characteristics of survivors and non-survivors.
| Parameter | Survivors (n = 65) |
Non-survivors (n = 135) |
p |
|---|---|---|---|
| Weight (kg) | 3.6 (0.1) | 3.6 (0.1) | 0.83 |
| HR (beats/min) | 252 (202, 273) | 226 (198, 265) | 0.20 |
| MAP (mmHg) | 87 (2) | 85 (1) | 0.45 |
| DBP (mmHg) | 71 (62, 84) | 70 (61, 82) | 0.58 |
| ETCO2 (torr)a | 47.1 (0.5) | 48.0 (0.3) | 0.73 |
| ICP (mmHg) | 11 (9, 12) | 12 (9, 14) | 0.08 |
| Temperature (°C) | 38.7 (38.5, 38.9) | 38.7 (38.4, 39.0) | 0.40 |
| pHa | 7.39 (0.01) | 7.39 (0.01) | 0.73 |
| PaO2 (torr) | 84 (75, 107) | 87 (69, 114) | 0.82 |
| PaCO2 (torr)a | 40 (38, 43) | 41 (39, 43) | 0.10 |
| Hb (g/dL) | 10.1 (0.2) | 9.9 (0.1) | 0.33 |
Data were collected prior to asphyxia and are presented as mean (SEM) or median (interquartile range).
DBP, diastolic blood pressure; ETCO2, end-tidal carbon dioxide; Hb, hemoglobin; HR, heart rate; ICP, intracranial pressure; MAP, mean arterial blood pressure; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen partial pressure; pH, arterial pH.
n=154 because animals subjected to pre-arrest hypercarbia as part of an experimental protocol were excluded.
Diastolic blood pressure during BLS and ALS
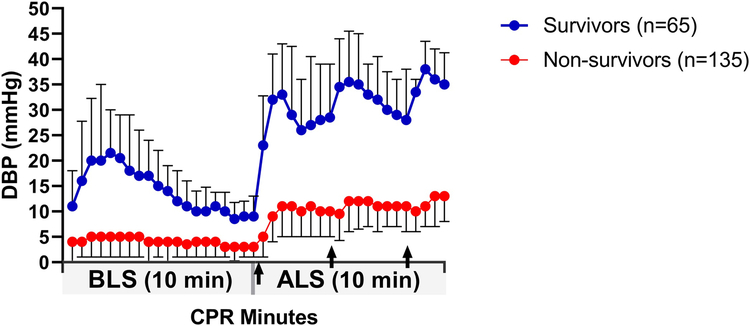
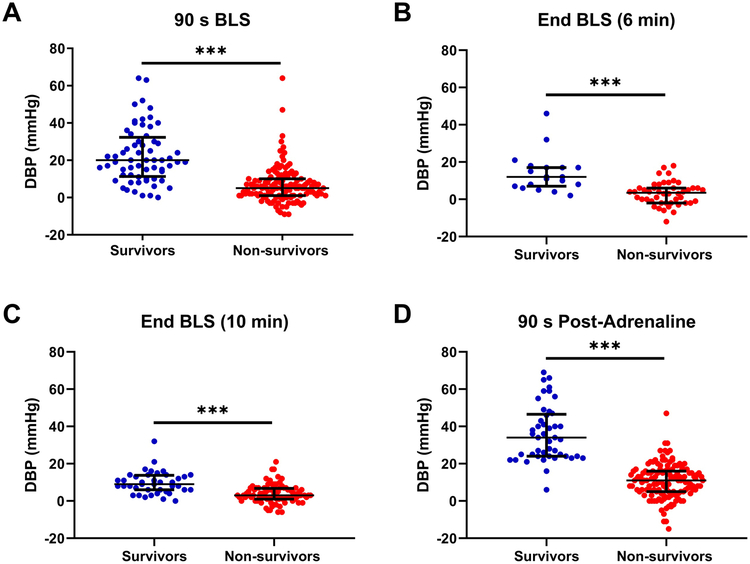
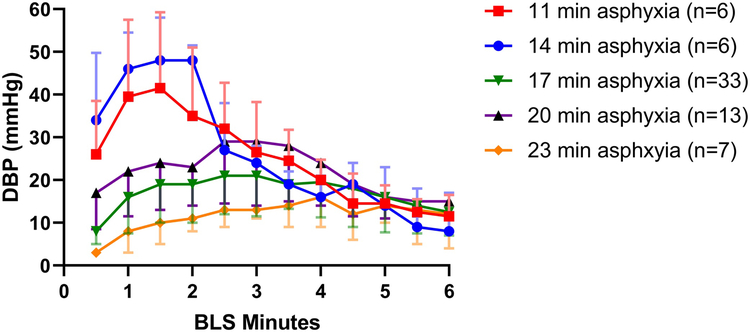
Figure 1 shows the changes in DBP in survivors (n=65) and non-survivors (n=135) over the 20-min resuscitation period. A transient increase in the DBP was observed in survivors over the first 3 min of BLS. Thirty seconds after the initiation of chest compressions, survivors had higher DBP than did non-survivors (10.1 [5.0, 18.1] versus 4.0 [0.0, 8.0] mmHg, p<0.001; Fig 1). After 90 s of BLS, the DBP was significantly higher in survivors than in non-survivors (20.0 [11.3, 33.3] versus 5.0 [1.0, 10.0] mmHg, p<0.001; Fig. 2A). The transient increase in the DBP at the beginning of BLS in survivors appeared to occur earlier and to be increased in protocols with shorter asphyxial durations (Fig. 3). After the transient increase, the DBP progressively decreased in survivors and approached levels near that of non-survivors by 7 min of chest compressions (~10 mmHg, Fig. 1). In the animals that received 6 min of BLS (n=65), the mean DBP at the end of BLS in survivors was 12.0 (7.0, 17.0) mmHg versus 3.5 (−2.0, 6.0) mmHg in non-survivors (p<0.001; Fig. 2B). In the animals that received 10 min of BLS (n=128), the DBP at the end of BLS was 9.0 (6.0, 13.8) mmHg in survivors versus 3.0 (1.0, 6.8) mmHg in non-survivors (p<0.001; Fig. 2C).
Fig. 1.
Diastolic blood pressure (DBP) in survivors (n=65) and non-survivors (n=135) over 20 min of cardiopulmonary resuscitation (CPR). Each point is a 30-s interval during 10 min of basic life support (BLS) followed by 10 min of advanced life support (ALS). Arrows indicate administration of adrenaline at 0, 4, and 8 min of ALS. Data are shown as median with interquartile range.
Fig. 2.
Diastolic blood pressure (DBP) at 4 time points during resuscitation in survivors and non-survivors. (A) DBP at 90 s of basic life support (BLS; n=199). (B and C) DBP at the end of BLS in the animals that received 6 min of BLS (n=65) and those that received 10 min of BLS (n=128), respectively. (D) DBP at 90 s after the administration of adrenaline (n=179). Median and interquartile ranges for each group are presented. Each circle represents one piglet. ***p<0.001.
Fig. 3.
Diastolic blood pressure (DBP) during the first 6 min of basic life support (BLS) in survivors. Groups that underwent five different pre-determined durations of asphyxia (ranging from 11–23 min) are graphed separately. Median with interquartile ranges are presented.
During ALS, the DBP peaked 90 s after the administration of adrenaline (Fig. 1) and was significantly greater in survivors than in non-survivors (34.0 [24.0, 46.5] versus 11.0 [5.0, 16.0] mmHg, p<0.001; Fig. 2D). The increase in DBP produced by the adrenaline was sustained, and the DBP gradually increased with subsequent administrations in the survivors (Fig. 1).
Magnitude of DBP response to adrenaline
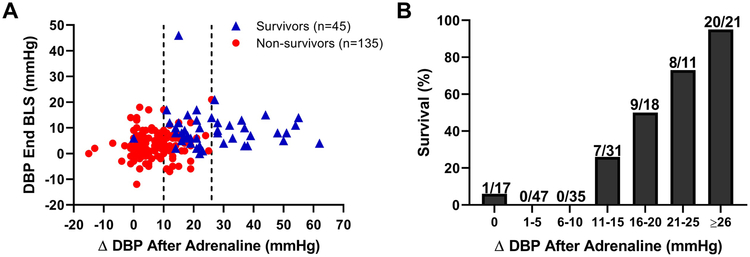
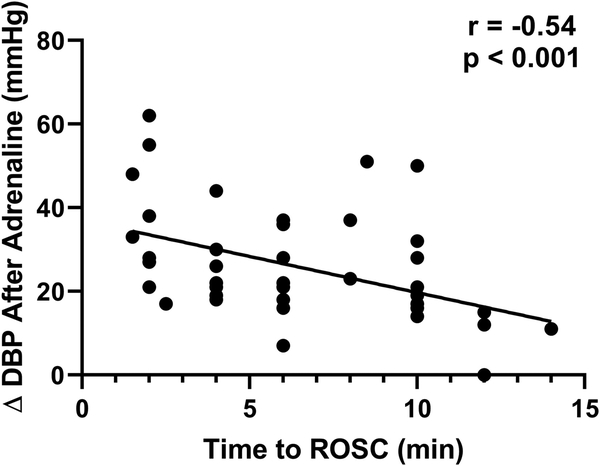
The magnitude of increase in DBP after adrenaline administration was greater in survivors than in non-survivors (22.0 [16.5, 36.5] versus 6.0 [2.0, 11.0] mmHg, p<0.001). The change in DBP after adrenaline was more predictive of survival than the DBP prior to adrenaline administration (Fig. 4A). When the improvement in DBP after of the first dose of adrenaline was ≤ 10 mmHg, survival was only 1% (1 out of 99, Fig. 4B). Conversely, when the improvement in DBP after the first dose of adrenaline was ≥ 26 mmHg, 95% of pigs survived (20 out of 21, Fig. 4B). Finally, there was a significant linear correlation between the magnitude of DBP change after the first dose of adrenaline and the time to ROSC (r=−0.54, p<0.001; Fig. 5).
Fig. 4.
Relationships among diastolic blood pressure (DBP) at the end of basic life support (BLS), the magnitude of change in DBP after the first dose of adrenaline, and survival. (A) Survival was more closely associated with the magnitude of DBP change after adrenaline than with the DBP at the end of BLS. Each point in the scatter plot represents one individual animal (n=179). Survival was 1% when the change in DBP was ≤ 10 mmHg after the first dose of adrenaline (left vertical dashed line) and 95% when the change in DBP was ≥ 26 mmHg (right vertical dashed line). (B) Bar graph showing survival with each 5 mmHg increase in the magnitude of change in DBP after the first dose of adrenaline. The number of survivors and the total number of animals in each bin are shown.
Fig. 5.
Time to return of spontaneous circulation (ROSC) and the magnitude of change in diastolic blood pressure (DBP) after the first dose of adrenaline in survivors (n=43). A large improvement in the DBP was associated with a shorter time-to-ROSC (r=−0.54, p< 0.001).
DISCUSSION
In this neonatal swine model, we show a consistent pattern in which the DBP is associated with survival during the phases of CPR. First, survivors had higher DBP than non-survivors during BLS. Survivors exhibited a transient rise in the DBP to > 20 mmHg during the first 3 min of chest compressions that was not observed in non-survivors. This increase in DBP during BLS may be associated with the response of the vasculature to the circulation of endogenous adrenaline released by the adrenal glands and sympathetic nerve terminals during cardiac arrest. The levels of plasma catecholamines increase 50–100 fold during early resuscitation from cardiac arrest in both animal models25–27 and in humans.28 In swine, a previous study showed the peak in endogenous adrenaline levels occurred one minute after the initiation of chest compressions following a five minute ventricular fibrillation arrest, and correlated with a peak in DBP early in BLS.25 Therefore, we hypothesize the early peak in DBP observed in survivors in this study may be due to either increased levels of endogenous catecholamines or a more robust vascular response. We believe that our study is the first to indicate that the height and timing of this peak in blood pressure related to endogenous adrenaline circulation is affected by the duration of asphyxial arrest (Fig. 3). The intensity and the acuity of the peak in DBP appear to be inversely related to the duration of asphyxia. This may indicate that a maximal endogenous adrenaline response occurred in all five asphyxial durations, but that the distribution of the endogenous adrenaline or the response to the distribution were less robust in the more severely asphyxiated animals. Interestingly, the association with survival is maintained even as the endogenous adrenaline and DBP fall later in BLS. However, the use of DBP thresholds to predict survival at this stage of the resuscitation may be less helpful because both survivors and non-survivors had DBP < 10 mmHg and the difference in DBP between the groups was only 6 mmHg.
The second phase of CPR in which DBP is highly associated with survival is with the first dose of adrenaline at the beginning of ALS. The DBP increased to > 35 mmHg in survivors with the initial adrenaline administration, and the magnitude of change was significantly greater than that of non-survivors. Also noted was that throughout ALS, survivors maintained a mean DBP greater than that of non-survivors. Additionally, in survivors the size of the response in the DBP to the first dose of adrenaline correlated with the time-to-ROSC. This finding supports assumptions that the vascular response to adrenaline is a key determinate of ROSC and it will be interesting to see if other studies confirm that the degree of improvement in DBP after the initial adrenaline dose correlates with both percent ROSC and the time-to-ROSC.
These observations support previous findings that the DBP is an important surrogate for coronary perfusion pressure and is associated with ROSC.6,7,9 Similar findings in an adult swine model of ventricular fibrillation showed that the magnitude of improvement in coronary perfusion pressure after vasopressor administration was significantly greater in survivors than in non-survivors.29 Our study was not intended to identify numeric targets for DBP during paediatric CPR. However, our observation that survivors maintained a DBP > 30 mmHg during ALS is congruent with previous studies. In a paediatric swine model of haemodynamically guided resuscitation, mean DBP prior to the first defibrillation attempt was 36 mmHg in survivors and was significantly greater than that of non-survivors.15 Additionally, maintenance of mean DBP ≥ 25 mmHg in infants and ≥ 30 mmHg in children was associated with both survival and survival with favourable neurologic outcome after cardiac arrest in the paediatric intensive care unit.22
The arterial vascular response to asphyxia appears to differ in survivors and non-survivors. Despite the acidosis, hypoxia, and hypercarbia produced by prolonged asphyxia (11–23 min), survivors showed a degree of systemic vascular resistance and responsiveness to both endogenous adrenaline at the initiation of chest compressions and exogenous adrenaline administered during ALS. The explanation for this is unknown, but may include innate differences in the response of vascular smooth muscle cells or in cellular anaerobic metabolism. Additional investigation into the mechanisms of vascular responsiveness in these animals is warranted.
The applicability of these findings to clinical practice remains to be determined. A robust improvement in the DBP after adrenaline administration during resuscitation might portend ROSC and support more frequent dosing of adrenaline, or other vasopressors, to maintain DBP above goal. Alternatively, if there is minimal change in the DBP after adrenaline administration, the clinician might focus on improving the quality of chest compression delivery, ruling out reversible causes, and activating extracorporeal life support earlier.
Limitations to our study include that it was retrospective in nature and involved a variety of CPR delivery methods and arrest conditions. However, none of the CPR methods involved targeted feedback from DBP. Experiments had variable asphyxial times, but the association of higher DBP with survival over variable and prolonged asphyxial periods may improve the generalizability. Evaluation of DBP during CPR requires vascular access and may only be relevant to patients with pre-existing arterial catheters who suffer a cardiac arrest in the intensive care unit or operating room. However, over 90% of paediatric in-hospital cardiac arrests occur in the intensive care unit30 and approximately 50% of infants and children who suffer cardiac arrest have an arterial catheter at the time of the event.31 The finding that the DBP was < 10 mmHg in both survivors and non-survivors (and of less prognostic value) when BLS went beyond 7 min may have limited clinical relevance as it would be unusual for a child to have arterial monitoring during CPR but not have access to early adrenaline administration. Ours is a preclinical model, though the pig’s chest has a shape, size, and compliance similar to that of a human.32 The age range of this model favours a cardiac pump mechanism for blood flow during CPR, and our findings may be relevant only to neonates and infants who are most likely to have equally compliant chests. Despite these limitations, our study provokes interest in clinical comparisons of the DBP trends and the response to the initial adrenaline administration in paediatric survivors and non-survivors of cardiac arrest.
CONCLUSIONS
In this neonatal model of asphyxial cardiac arrest, we found variations in the association between DBP and survival during prolonged resuscitation. The strongest associations between DBP and survival in this model were early in BLS and after the first dose of adrenaline. If confirmed clinically, these findings may be helpful to bedside providers regarding estimation of the severity of no-flow injury, titration of adrenaline administration during resuscitation, prognostication of the likelihood of ROSC, and activation of extracorporeal life support.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to Claire Levine, MS, ELS, for her editorial assistance. Dr. O’Brien’s institution received funding from the National Institutes of Health (NIH). Dr. Lee received support for article research from the NIH, and she received funding from Medtronic. Dr. Hunt received support for article research from the NIH, and her institution received grant funding from the Laerdal Foundation for Acute Care Medicine and from the Hartwell Foundation. She also has served as a consultant for Zoll Medical Corporation, which has supplied honoraria and travel expenses for speaking engagements. Dr. Hunt and colleagues have been awarded patents for developing several educational simulation technologies for which Zoll Medical Corporation has a non-exclusive license with the potential for royalties. Dr. Koehler received support for article research from the NIH. Dr. Shaffner’s institution received funding from the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Dr. Shaffner received funding from Wolters Kluwer and support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest. Dr. Hunt has served as a consultant for Zoll Medical Corporation, which has supplied honoraria and travel expenses for speaking engagements. Dr. Hunt and colleagues have been awarded patents for developing several educational simulation technologies for which Zoll Medical Corporation has a non-exclusive license with the potential for royalties. The remaining authors have disclosed that they do not have any potential conflicts of interest.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
REFERENCES
- 1.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics 2002;109:200–9. [DOI] [PubMed] [Google Scholar]
- 2.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation 2006;71:310–8. [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes 2013;6:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Castillo J, López-Herce J, Cañadas S, et al. Cardiac arrest and resuscitation in the pediatric intensive care unit: a prospective multicenter multinational study. Resuscitation 2014;85:1380–6. [DOI] [PubMed] [Google Scholar]
- 5.Sutton RM, Reeder RW, Landis W, et al. Chest compression rates and pediatric in-hospital cardiac arrest survival outcomes. Resuscitation 2018;130:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med 1984;12:871–3. [DOI] [PubMed] [Google Scholar]
- 7.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation 1988;16:241–50. [DOI] [PubMed] [Google Scholar]
- 8.Halperin HR, Lee K, Zviman M, et al. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med 2010;28:195–202. [DOI] [PubMed] [Google Scholar]
- 9.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med 1985;14:521–8. [DOI] [PubMed] [Google Scholar]
- 10.Morgan RW, French B, Kilbaugh TJ, et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation 2016;104:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990;263:1106–13. [PubMed] [Google Scholar]
- 12.Sutton RM, Friess SH, Naim MY, et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med 2014;190:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation 2013;84:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med 2013;41:2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RW, Kilbaugh TJ, Shoap W, et al. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation 2017;111:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naim MY, Sutton RM, Friess SH, et al. Blood pressure- and coronary perfusion pressure-targeted cardiopulmonary resuscitation improves 24-hour survival from ventricular fibrillation cardiac arrest. Crit Care Med 2016;44:e1111–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friess SH, Sutton RM, French B, et al. Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation 2014;85:1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton RM, French B, Meaney PA, et al. Physiologic monitoring of CPR quality during adult cardiac arrest: A propensity-matched cohort study. Resuscitation 2016;106:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning JE. Feasibility of blind aortic catheter placement in the prehospital environment to guide resuscitation in cardiac arrest. J Trauma Acute Care Surg 2013;75:S173–7. [DOI] [PubMed] [Google Scholar]
- 20.de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S526–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation 2013;128:417–35. [DOI] [PubMed] [Google Scholar]
- 22.Berg RA, Sutton RM, Reeder RW, et al. Association Between Diastolic Blood Pressure During Pediatric In-Hospital Cardiopulmonary Resuscitation and Survival. Circulation 2018;137:1784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamrick JT, Hamrick JL, Bhalala U, et al. End-tidal CO2-guided chest compression delivery improves survival in a neonatal asphyxial cardiac arrest model. Pediatr Crit Care Med 2017;18:e575–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrick JL, Hamrick JT, O’Brien CE, et al. The Effect of Asphyxia Arrest Duration on a Pediatric End-Tidal CO2-Guided Chest Compression Delivery Model. Pediatr Crit Care Med 2019;20:e352–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoffstall JM, Spivey WH, Davidheiser S, Fuhs L, Kirkpatrick R. Endogenous and exogenous plasma catecholamine levels in cardiac arrest in swine. Resuscitation 1990;19:241–51. [DOI] [PubMed] [Google Scholar]
- 26.Foley PJ, Tacker WA, Wortsman J, Frank S, Cryer PE. Plasma catecholamine and serum cortisol responses to experimental cardiac arrest in dogs. Am J Physiol 1987;253:E283–9. [DOI] [PubMed] [Google Scholar]
- 27.Kern KB, Elchisak MA, Sanders AB, Badylak SF, Tacker WA, Ewy GA. Plasma catecholamines and resuscitation from prolonged cardiac arrest. Crit Care Med 1989;17:786–91. [DOI] [PubMed] [Google Scholar]
- 28.Little RA, Frayn KN, Randall PE, et al. Plasma catecholamines in patients with acute myocardial infarction and in cardiac arrest. Q J Med 1985;54:133–40. [PubMed] [Google Scholar]
- 29.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care 2010;14:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med 2013;41:2292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics 2006;118:2424–33. [DOI] [PubMed] [Google Scholar]
- 32.Neurauter A, Nysaether J, Kramer-Johansen J, et al. Comparison of mechanical characteristics of the human and porcine chest during cardiopulmonary resuscitation. Resuscitation 2009;80:463–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.