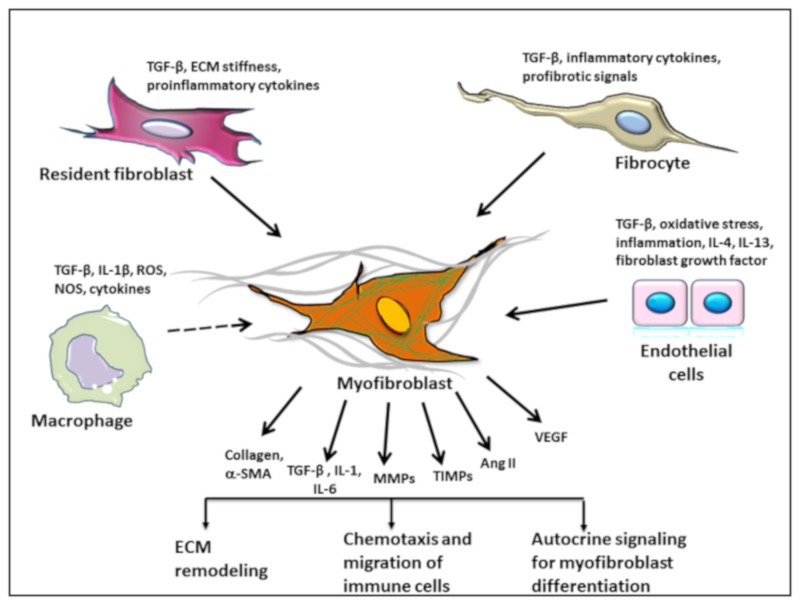
Figure 1.
Various progenitor cells lead to the origin of myofibroblasts in atherosclerosis and aortic valve sclerosis. Resident fibroblasts in the tissue and fibrocytes undergo differentiation under the influence of TGF-β, ECM stiffness, proinflammatory cytokines, and profibrotic signals. Endothelial cells undergo an endothelial to mesenchymal transition (EndMT) under the influence of oxidative stress, inflammation, TGF-β, IL-4, IL-13, and fibroblast growth factor. TGF-β, IL-1β, ROS, NOS, and cytokines facilitate the activation of macrophages, which are a major source of profibrotic cytokines, leading to an indirect myofibroblast differentiation through hematopoietic cells. The relative contribution of various cell types to the myofibroblast population is variable and depends on the degree of fibrosis. TGF-β: transforming growth factor-β; ECM: extracellular matrix; IL-4: interleukin-4; IL-13: interleukin-13; α-SMA: α-smooth muscle actin; IL-1: interleukin-1; IL-6: interleukin-6; MMPs: matrix metalloproteases; TIMPs: tissue inhibitors of MMPs; Ang II: angiotensin II; VEGF: vascular endothelial growth factor; IL-1β: interleukin-1β; ROS: reactive oxygen species; NOS: nitric oxide synthase.